Role of the Gut Microbiota in Osteoarthritis, Rheumatoid Arthritis, and Spondylarthritis: An Update on the Gut–Joint Axis
Abstract
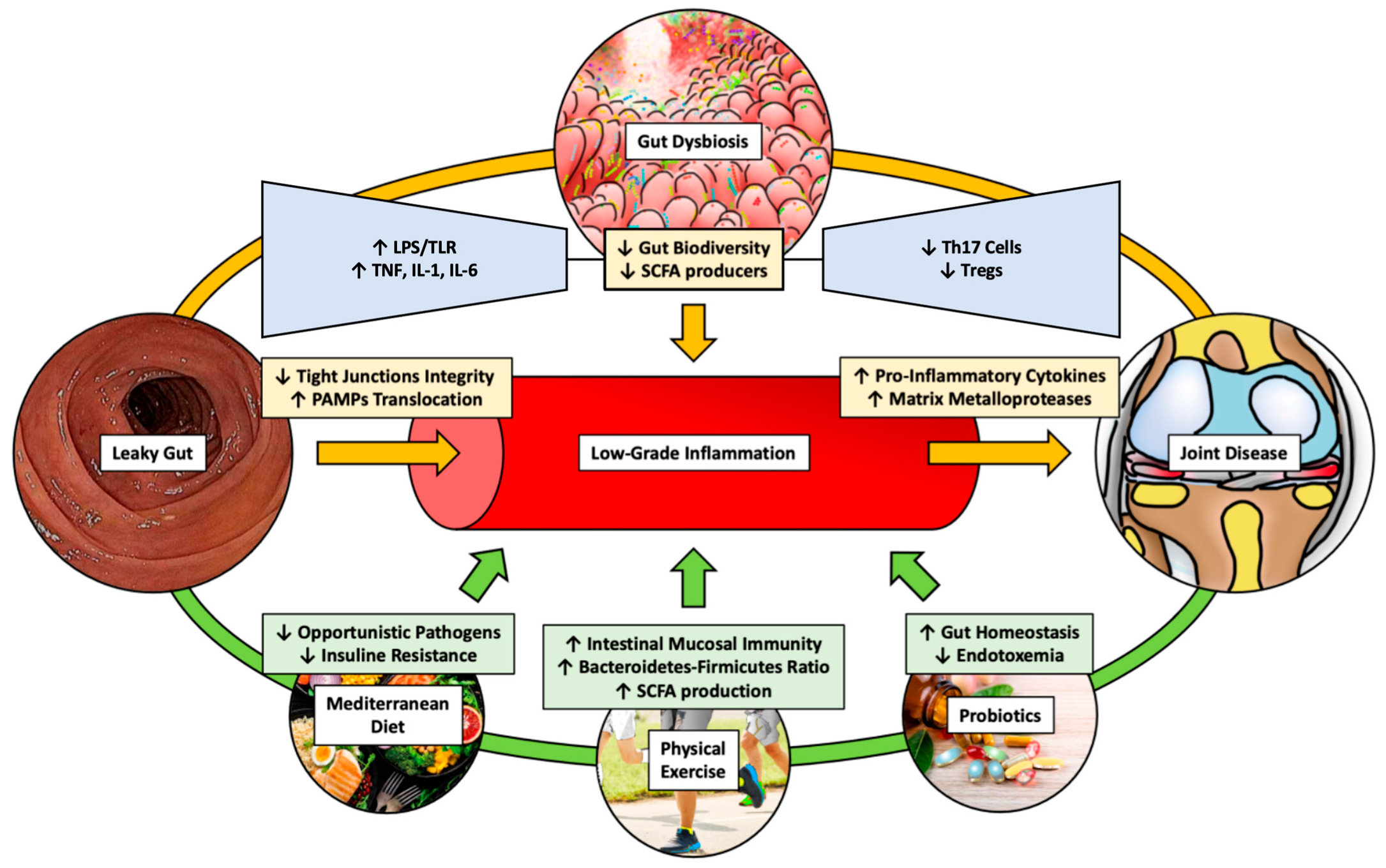
1. Introduction
2. Gut Microbiota and Arthritis
2.1. Microbiota in Osteoarthritis (OA)
2.2. Microbiota in Rheumatoid Arthritis (RA)
2.3. Microbiota in Spondylarthritis (SpA)
3. Pathogenetic Molecular Mechanisms of Osteoarthritis
3.1. Main Molecular Mechanisms Involved in Osteoarthritis
3.2. Main Molecular Mechanisms Involved in Rheumatoid Arthritis
3.3. Main Molecular Mechanisms Involved in Spondyloarthritis
4. Outcome Measures for Arthritis
4.1. Physical Functioning and Disability
4.1.1. HAQ: Health Assessment Questionnaire
4.1.2. MHAQ: Modified Health Assessment Questionnaire
4.1.3. AIMS Subscales: Arthritis Impact Measurement Scale
4.1.4. BASFI: Bath Ankylosing Spondylitis Functional Index
4.1.5. DASH: Disabilities of the Arm, Shoulder, and Hand Outcome Measure
4.1.6. GAT: Grip Ability Test
4.2. Pain
4.2.1. VAS: Visual Analog Scale
4.2.2. NRS: Numeric Scale
4.3. Fatigue and Tiredness
MAF: Multidimensional Assessment of Fatigue
5. Diagnosis of Gut Microbiota Dysbiosis and Osteoarthritis
5.1. Gut Dysbiosis
5.2. Inflammatory Joint Diseases
6. Therapeutical Options
6.1. Osteoarthritis
6.2. Rheumatoid Arthritis
6.3. Spondyloarthritis
7. Conclusions
8. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, L.; Tian, F.; Li, G.Y.; Xu, W.; Xia, R. The effects and significance of gut microbiota and its metabolites on the regulation of osteoarthritis: Close coordination of gut-bone axis. Front. Nutr. 2022, 9, 1012087. [Google Scholar] [CrossRef] [PubMed]
- Romero-Figueroa, M.D.S.; Ramírez-Durán, N.; Montiel-Jarquín, A.J.; Horta-Baas, G. Gut-joint axis: Gut dysbiosis can contribute to the onset of rheumatoid arthritis. Front. Cell. Infect. Microbiol. 2023, 13, 1092118. [Google Scholar] [CrossRef] [PubMed]
- Jacques, P.; Elewaut, D.; Mielants, H. Interactions between gut inflammation and arthritis/spondylitis. Curr. Opin. Rheumatol. 2010, 22, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Cypers, H.; Van Praet, L.; Varkas, G.; Elewaut, D. Relevance of the gut/joint axis for the management of spondyloarthritis in daily clinical practice. Curr. Opin. Rheumatol. 2014, 26, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.; Khanna, S.; Padhan, P.; Smita, S.; Raghav, S.; Gupta, B. Direct recognition of LPS drive TLR4 expressing CD8. Sci. Rep. 2017, 7, 933. [Google Scholar] [CrossRef]
- Jeong, Y.; Kim, J.W.; You, H.J.; Park, S.J.; Lee, J.; Ju, J.H.; Park, M.S.; Jin, H.; Cho, M.L.; Kwon, B.; et al. Gut Microbial Composition and Function Are Altered in Patients with Early Rheumatoid Arthritis. J. Clin. Med. 2019, 8, 693. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; de Sire, R.; Petito, V.; Masi, L.; Cisari, C.; Gasbarrini, A.; Scaldaferri, F.; Invernizzi, M. Gut-Joint Axis: The Role of Physical Exercise on Gut Microbiota Modulation in Older People with Osteoarthritis. Nutrients 2020, 12, 574. [Google Scholar] [CrossRef] [PubMed]
- Coradduzza, D.; Bo, M.; Congiargiu, A.; Azara, E.; De Miglio, M.R.; Erre, G.L.; Carru, C. Decoding the Microbiome’s Influence on Rheumatoid Arthritis. Microorganisms 2023, 11, 2170. [Google Scholar] [CrossRef]
- Hao, X.; Shang, X.; Liu, J.; Chi, R.; Zhang, J.; Xu, T. The gut microbiota in osteoarthritis: Where do we stand and what can we do? Arthritis Res. Ther. 2021, 23, 42. [Google Scholar] [CrossRef]
- Gracey, E.; Vereecke, L.; McGovern, D.; Fröhling, M.; Schett, G.; Danese, S.; De Vos, M.; Van den Bosch, F.; Elewaut, D. Revisiting the gut-joint axis: Links between gut inflammation and spondyloarthritis. Nat. Rev. Rheumatol. 2020, 16, 415–433. [Google Scholar] [CrossRef]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef]
- Qaiyum, Z.; Lim, M.; Inman, R.D. The gut-joint axis in spondyloarthritis: Immunological, microbial, and clinical insights. Semin. Immunopathol. 2021, 43, 173–192. [Google Scholar] [CrossRef]
- Zhao, T.; Wei, Y.; Zhu, Y.; Xie, Z.; Hai, Q.; Li, Z.; Qin, D. Gut microbiota and rheumatoid arthritis: From pathogenesis to novel therapeutic opportunities. Front. Immunol. 2022, 13, 1007165. [Google Scholar] [CrossRef]
- Seymour, B.J.; Allen, B.E.; Kuhn, K.A. Microbial Mechanisms of Rheumatoid Arthritis Pathogenesis. Curr. Rheumatol. Rep. 2024. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef] [PubMed]
- Chisari, E.; Wouthuyzen-Bakker, M.; Friedrich, A.W.; Parvizi, J. The relation between the gut microbiome and osteoarthritis: A systematic review of literature. PLoS ONE 2021, 16, e0261353. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, F.; Pi, G. Association Between Gut Microbiota and Osteoarthritis: A Review of Evidence for Potential Mechanisms and Therapeutics. Front. Cell. Infect. Microbiol. 2022, 12, 812596. [Google Scholar] [CrossRef] [PubMed]
- Ferrillo, M.; Giudice, A.; Marotta, N.; Fortunato, F.; Di Venere, D.; Ammendolia, A.; Fiore, P.; de Sire, A. Pain Management and Rehabilitation for Central Sensitization in Temporomandibular Disorders: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 12164. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Y.; Yao, X.; Li, S.; Wang, G.; Huang, Y.; Yang, Y.; Zhang, A.; Liu, C.; Zhu, D.; et al. Characterizations of the Gut Bacteriome, Mycobiome, and Virome in Patients with Osteoarthritis. Microbiol. Spectr. 2023, 11, e0171122. [Google Scholar] [CrossRef]
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M.; et al. Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis Rheumatol. 2016, 68, 2646–2661. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Iorio, G.C.; Ammendolia, A.; Marotta, N.; Ricardi, U.; de Sire, A. A bond between rheumatic diseases and cancer in the elderly: The interleukin-6 pathway. Int. J. Rheum Dis. 2021, 24, 1317–1320. [Google Scholar] [CrossRef]
- Xu, X.; Wang, M.; Wang, Z.; Chen, Q.; Chen, X.; Xu, Y.; Dai, M.; Wu, B.; Li, Y. The bridge of the gut-joint axis: Gut microbial metabolites in rheumatoid arthritis. Front. Immunol. 2022, 13, 1007610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, M.M.; Joyce Wu, H.J.; Mauro, D.; Schett, G.; Ciccia, F. The gut-joint axis in rheumatoid arthritis. Nat. Rev. Rheumatol. 2021, 17, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zhao, Y.; Cui, Y.; Zhong, C.; Zha, Y.; Li, S.; Cao, G.; Li, M.; Zhang, L.; Ning, K.; et al. Stage-specific roles of microbial dysbiosis and metabolic disorders in rheumatoid arthritis. Ann. Rheum. Dis. 2022, 81, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef]
- Brakenhoff, L.K.; van der Heijde, D.M.; Hommes, D.W.; Huizinga, T.W.; Fidder, H.H. The joint-gut axis in inflammatory bowel diseases. J. Crohns Colitis 2010, 4, 257–268. [Google Scholar] [CrossRef]
- Reveille, J.D.; Arnett, F.C. Spondyloarthritis: Update on pathogenesis and management. Am. J. Med. 2005, 118, 592–603. [Google Scholar] [CrossRef]
- Manasson, J.; Shen, N.; Garcia Ferrer, H.R.; Ubeda, C.; Iraheta, I.; Heguy, A.; Von Feldt, J.M.; Espinoza, L.R.; Garcia Kutzbach, A.; Segal, L.N.; et al. Gut Microbiota Perturbations in Reactive Arthritis and Postinfectious Spondyloarthritis. Arthritis Rheumatol. 2018, 70, 242–254. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Blaney Davidson, E.N.; Remst, D.F.; Vitters, E.L.; van Beuningen, H.M.; Blom, A.B.; Goumans, M.J.; van den Berg, W.B.; van der Kraan, P.M. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J. Immunol. 2009, 182, 7937–7945. [Google Scholar] [CrossRef]
- Blaney Davidson, E.N.; van der Kraan, P.M.; van den Berg, W.B. TGF-beta and osteoarthritis. Osteoarthr. Cartil. 2007, 15, 597–604. [Google Scholar] [CrossRef]
- Labinsky, H.; Panipinto, P.M.; Ly, K.A.; Khuat, D.K.; Madarampalli, B.; Mahajan, V.; Clabeaux, J.; MacDonald, K.; Verdin, P.J.; Buckner, J.H.; et al. Multiparameter Analysis Identifies Heterogeneity in Knee Osteoarthritis Synovial Responses. Arthritis Rheumatol. 2020, 72, 598–608. [Google Scholar] [CrossRef]
- Xia, B.; Chen, D.; Zhang, J.; Hu, S.; Jin, H.; Tong, P. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcif. Tissue Int. 2014, 95, 495–505. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, W.; Wang, H.L.; Dai, L.L.; Zong, W.H.; Wang, Y.Z.; Bi, J.; Han, W.; Dong, G.J. Gut microbiota and obesity-associated osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1257–1265. [Google Scholar] [CrossRef]
- Jiang, L.F.; Fang, J.H.; Wu, L.D. Role of infrapatellar fat pad in pathological process of knee osteoarthritis: Future applications in treatment. World J. Clin. Cases 2019, 7, 2134–2142. [Google Scholar] [CrossRef]
- Deane, K.D.; Demoruelle, M.K.; Kelmenson, L.B.; Kuhn, K.A.; Norris, J.M.; Holers, V.M. Genetic and environmental risk factors for rheumatoid arthritis. Best. Pract. Res. Clin. Rheumatol. 2017, 31, 3–18. [Google Scholar] [CrossRef]
- Bullock, J.; Rizvi, S.A.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med. Princ. Pract. 2018, 27, 501–507. [Google Scholar] [CrossRef]
- Mellado, M.; Martínez-Muñoz, L.; Cascio, G.; Lucas, P.; Pablos, J.L.; Rodríguez-Frade, J.M. T Cell Migration in Rheumatoid Arthritis. Front. Immunol. 2015, 6, 384. [Google Scholar] [CrossRef]
- Padyukov, L. Genetics of rheumatoid arthritis. Semin. Immunopathol. 2022, 44, 47–62. [Google Scholar] [CrossRef]
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef]
- Sokolova, M.V.; Schett, G.; Steffen, U. Autoantibodies in Rheumatoid Arthritis: Historical Background and Novel Findings. Clin. Rev. Allergy Immunol. 2022, 63, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, P.; Hämäläinen, W.; Savinainen, J.; Lehtonen, M.; Lehtiniemi, S.; Rinta-Paavola, J.; Lehenkari, P.; Kääriäinen, T.; Joukainen, A.; Kröger, H.; et al. Metabolomics of Synovial Fluid and Infrapatellar Fat Pad in Patients with Osteoarthritis or Rheumatoid Arthritis. Inflammation 2022, 45, 1101–1117. [Google Scholar] [CrossRef]
- Tsukazaki, H.; Kaito, T. The Role of the IL-23/IL-17 Pathway in the Pathogenesis of Spondyloarthritis. Int. J. Mol. Sci. 2020, 21, 6401. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Pan, F.; Gao, J.; Ge, R.; Duan, Z.; Zeng, Z.; Liao, F.; Xia, G.; Wang, S.; Xu, S.; et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin. Rheumatol. 2011, 30, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Colbert, R.A.; Tran, T.M.; Layh-Schmitt, G. HLA-B27 misfolding and ankylosing spondylitis. Mol. Immunol. 2014, 57, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Busch, R.; Kollnberger, S.; Mellins, E.D. HLA associations in inflammatory arthritis: Emerging mechanisms and clinical implications. Nat. Rev. Rheumatol. 2019, 15, 364–381. [Google Scholar] [CrossRef] [PubMed]
- Ambarus, C.A.; Yeremenko, N.; Baeten, D.L. Altered cytokine expression by macrophages from HLA-B27-positive spondyloarthritis patients without evidence of endoplasmic reticulum stress. Rheumatol. Adv. Pract. 2018, 2, rky014. [Google Scholar] [CrossRef] [PubMed]
- Deleuran, B.W.; Chu, C.Q.; Field, M.; Brennan, F.M.; Mitchell, T.; Feldmann, M.; Maini, R.N. Localization of tumor necrosis factor receptors in the synovial tissue and cartilage-pannus junction in patients with rheumatoid arthritis. Implications for local actions of tumor necrosis factor alpha. Arthritis Rheum. 1992, 35, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Falsetti, P.; Frediani, B.; Acciai, C.; Baldi, F.; Filippou, G.; Marcolongo, R. Heel fat pad involvement in rheumatoid arthritis and in spondyloarthropathies: An ultrasonographic study. Scand. J. Rheumatol. 2004, 33, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, J.J.; Boers, M.; Tugwell, P.; Clarke, M.; Williamson, P.R. Outcome measures in rheumatoid arthritis randomised trials over the last 50 years. Trials 2013, 14, 324. [Google Scholar] [CrossRef]
- Williamson, P.R.; Altman, D.G.; Bagley, H.; Barnes, K.L.; Blazeby, J.M.; Brookes, S.T.; Clarke, M.; Gargon, E.; Gorst, S.; Harman, N.; et al. The COMET Handbook: Version 1.0. Trials 2017, 18, 280. [Google Scholar] [CrossRef]
- Zochling, J. Measures of symptoms and disease status in ankylosing spondylitis: Ankylosing Spondylitis Disease Activity Score (ASDAS), Ankylosing Spondylitis Quality of Life Scale (ASQoL), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), Bath Ankylosing Spondylitis Global Score (BAS-G), Bath Ankylosing Spondylitis Metrology Index (BASMI), Dougados Functional Index (DFI), and Health Assessment Questionnaire for the Spondylarthropathies (HAQ-S). Arthritis Care Res. 2011, 63 (Suppl. S11), S47–S58. [Google Scholar] [CrossRef]
- Uhlig, T.; Haavardsholm, E.A.; Kvien, T.K. Comparison of the Health Assessment Questionnaire (HAQ) and the modified HAQ (MHAQ) in patients with rheumatoid arthritis. Rheumatology 2006, 45, 454–458. [Google Scholar] [CrossRef]
- Meenan, R.F.; Mason, J.H.; Anderson, J.J.; Guccione, A.A.; Kazis, L.E. AIMS2. The content and properties of a revised and expanded Arthritis Impact Measurement Scales Health Status Questionnaire. Arthritis Rheum. 1992, 35, 1–10. [Google Scholar] [CrossRef]
- Fusama, M.; Nakahara, H.; Hamano, Y.; Nishide, M.; Kawamoto, K.; Hosokawa, T.; Nozato, S.; Higa, S.; Igarashi, T.; Takeuchi, E.; et al. Improvement of health status evaluated by Arthritis Impact Measurement Scale 2 (AIMS-2) and Short Form-36 (SF-36) in patients with rheumatoid arthritis treated with tocilizumab. Mod. Rheumatol. 2013, 23, 276–283. [Google Scholar] [CrossRef]
- Jubair, W.K.; Hendrickson, J.D.; Severs, E.L.; Schulz, H.M.; Adhikari, S.; Ir, D.; Pagan, J.D.; Anthony, R.M.; Robertson, C.E.; Frank, D.N.; et al. Modulation of Inflammatory Arthritis in Mice by Gut Microbiota Through Mucosal Inflammation and Autoantibody Generation. Arthritis Rheumatol. 2018, 70, 1220–1233. [Google Scholar] [CrossRef]
- Franchignoni, F.; Vercelli, S.; Giordano, A.; Sartorio, F.; Bravini, E.; Ferriero, G. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (QuickDASH). J. Orthop. Sports Phys. Ther. 2014, 44, 30–39. [Google Scholar] [CrossRef]
- Bjurehed, L.; Brodin, N.; Nordenskiöld, U.; Björk, M. Improved Hand Function, Self-Rated Health, and Decreased Activity Limitations: Results After a Two-Month Hand Osteoarthritis Group Intervention. Arthritis Care Res. 2018, 70, 1039–1045. [Google Scholar] [CrossRef]
- Dellhag, B.; Bjelle, A. A Grip Ability Test for use in rheumatology practice. J. Rheumatol. 1995, 22, 1559–1565. [Google Scholar] [PubMed]
- Poole, J.L. Measures of hand function: Arthritis Hand Function Test (AHFT), Australian Canadian Osteoarthritis Hand Index (AUSCAN), Cochin Hand Function Scale, Functional Index for Hand Osteoarthritis (FIHOA), Grip Ability Test (GAT), Jebsen Hand Function Test (JHFT), and Michigan Hand Outcomes Questionnaire (MHQ). Arthritis Care Res. 2011, 63 (Suppl. S11), S189–S199. [Google Scholar] [CrossRef]
- Langley, G.B.; Sheppeard, H. The visual analogue scale: Its use in pain measurement. Rheumatol. Int. 1985, 5, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63 (Suppl. S11), S240–S252. [Google Scholar] [CrossRef]
- Hewlett, S.; Dures, E.; Almeida, C. Measures of fatigue: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF NRS) for severity, effect, and coping, Chalder Fatigue Questionnaire (CFQ), Checklist Individual Strength (CIS20R and CIS8R), Fatigue Severity Scale (FSS), Functional Assessment Chronic Illness Therapy (Fatigue) (FACIT-F), Multi-Dimensional Assessment of Fatigue (MAF), Multi-Dimensional Fatigue Inventory (MFI), Pediatric Quality Of Life (PedsQL) Multi-Dimensional Fatigue Scale, Profile of Fatigue (ProF), Short Form 36 Vitality Subscale (SF-36 VT), and Visual Analog Scales (VAS). Arthritis Care Res. 2011, 63 (Suppl. S11), S263–S286. [Google Scholar] [CrossRef]
- Ferrillo, M.; Giudice, A.; Migliario, M.; Renó, F.; Lippi, L.; Calafiore, D.; Marotta, N.; de Sire, R.; Fortunato, L.; Ammendolia, A.; et al. Oral-Gut Microbiota, Periodontal Diseases, and Arthritis: Literature Overview on the Role of Probiotics. Int. J. Mol. Sci. 2023, 24, 4626. [Google Scholar] [CrossRef] [PubMed]
- Horta-Baas, G.; Romero-Figueroa, M.D.S.; Montiel-Jarquín, A.J.; Pizano-Zárate, M.L.; García-Mena, J.; Ramírez-Durán, N. Intestinal Dysbiosis and Rheumatoid Arthritis: A Link between Gut Microbiota and the Pathogenesis of Rheumatoid Arthritis. J. Immunol. Res. 2017, 2017, 4835189. [Google Scholar] [CrossRef]
- Aa, L.X.; Fei, F.; Qi, Q.; Sun, R.B.; Gu, S.H.; Di, Z.Z.; Aa, J.Y.; Wang, G.J.; Liu, C.X. Rebalancing of the gut flora and microbial metabolism is responsible for the anti-arthritis effect of kaempferol. Acta Pharmacol. Sin. 2020, 41, 73–81. [Google Scholar] [CrossRef]
- Tang, Q.; Jin, G.; Wang, G.; Liu, T.; Liu, X.; Wang, B.; Cao, H. Current Sampling Methods for Gut Microbiota: A Call for More Precise Devices. Front. Cell. Infect. Microbiol. 2020, 10, 151. [Google Scholar] [CrossRef]
- Wei, S.; Bahl, M.I.; Baunwall, S.M.D.; Hvas, C.L.; Licht, T.R. Determining Gut Microbial Dysbiosis: A Review of Applied Indexes for Assessment of Intestinal Microbiota Imbalances. Appl. Environ. Microbiol. 2021, 87, e00395-21. [Google Scholar] [CrossRef]
- Cush, J.J. Rheumatoid Arthritis: Early Diagnosis and Treatment. Med. Clin. N. Am. 2021, 105, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Sokka, T.; Pincus, T. Quantitative joint assessment in rheumatoid arthritis. Clin. Exp. Rheumatol. 2005, 23, S58–S62. [Google Scholar]
- Deandrade, J.R.; Casagrande, P.A. A Seven-day variability study of 499 patients with peripheral rheumatoid arthritis. Arthritis Rheum. 1965, 8, 302–334. [Google Scholar] [PubMed]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E.; Longo, U.G.; Madry, H.; Marchettini, P.; Marmotti, A.; Van Assche, D.; Zanon, G.; Peretti, G.M. Non-surgical treatments for the management of early osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1775–1785. [Google Scholar] [CrossRef]
- Sconza, C.; Di Matteo, B.; Queirazza, P.; Dina, A.; Amenta, R.; Respizzi, S.; Massazza, G.; Ammendolia, A.; Kon, E.; de Sire, A. Ozone Therapy versus Hyaluronic Acid Injections for Pain Relief in Patients with Knee Osteoarthritis: Preliminary Findings on Molecular and Clinical Outcomes from a Randomized Controlled Trial. Int. J. Mol. Sci. 2023, 24, 8788. [Google Scholar] [CrossRef]
- Rabini, A.; de Sire, A.; Marzetti, E.; Gimigliano, R.; Ferriero, G.; Piazzini, D.B.; Iolascon, G.; Gimigliano, F. Effects of focal muscle vibration on physical functioning in patients with knee osteoarthritis: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2015, 51, 513–520. [Google Scholar]
- de Sire, A.; Marotta, N.; Marinaro, C.; Curci, C.; Invernizzi, M.; Ammendolia, A. Role of Physical Exercise and Nutraceuticals in Modulating Molecular Pathways of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 5722. [Google Scholar] [CrossRef]
- Longo, U.G.; Maffulli, N.; Denaro, V. Minimally invasive total knee arthroplasty. N. Engl. J. Med. 2009, 361, 633–634, author reply 634. [Google Scholar]
- Longo, U.G.; Candela, V.; Berton, A.; De Salvatore, S.; Fioravanti, S.; Giannone, L.; Marchetti, A.; De Marinis, M.G.; Denaro, V. Biosensors for Detection of Biochemical Markers Relevant to Osteoarthritis. Biosensors 2021, 11, 31. [Google Scholar] [CrossRef]
- de Sire, A.; de Sire, R.; Curci, C.; Castiglione, F.; Wahli, W. Role of Dietary Supplements and Probiotics in Modulating Microbiota and Bone Health: The Gut-Bone Axis. Cells 2022, 11, 743. [Google Scholar] [CrossRef]
- Lyu, J.L.; Wang, T.M.; Chen, Y.H.; Chang, S.T.; Wu, M.S.; Lin, Y.H.; Kuan, C.M. Oral intake of Streptococcus thermophilus improves knee osteoarthritis degeneration: A randomized, double-blind, placebo-controlled clinical study. Heliyon 2020, 6, e03757. [Google Scholar] [CrossRef]
- Lei, M.; Guo, C.; Wang, D.; Zhang, C.; Hua, L. The effect of probiotic Lactobacillus casei Shirota on knee osteoarthritis: A randomised double-blind, placebo-controlled clinical trial. Benef. Microbes 2017, 8, 697–703. [Google Scholar] [CrossRef]
- Boer, C.G.; Radjabzadeh, D.; Medina-Gomez, C.; Garmaeva, S.; Schiphof, D.; Arp, P.; Koet, T.; Kurilshikov, A.; Fu, J.; Ikram, M.A.; et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat. Commun. 2019, 10, 4881. [Google Scholar] [CrossRef]
- Bungau, S.G.; Behl, T.; Singh, A.; Sehgal, A.; Singh, S.; Chigurupati, S.; Vijayabalan, S.; Das, S.; Palanimuthu, V.R. Targeting Probiotics in Rheumatoid Arthritis. Nutrients 2021, 13, 3376. [Google Scholar] [CrossRef] [PubMed]
- Nova, E.; Gómez-Martinez, S.; González-Soltero, R. The Influence of Dietary Factors on the Gut Microbiota. Microorganisms 2022, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, G.; Letizia Mauro, G.; Fiore, P.; Cisari, C.; Benedetti, M.G.; Panella, L.; de Sire, A.; Calafiore, D.; Moretti, A.; Gimigliano, F. Can vitamin D deficiency influence muscle performance in postmenopausal women? A multicenter retrospective study. Eur. J. Phys. Rehabil. Med. 2018, 54, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, G.; Moretti, A.; de Sire, A.; Calafiore, D.; Gimigliano, F. Effectiveness of Calcifediol in Improving Muscle Function in Post-Menopausal Women: A Prospective Cohort Study. Adv. Ther. 2017, 34, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Tangestani, H.; Boroujeni, H.K.; Djafarian, K.; Emamat, H.; Shab-Bidar, S. Vitamin D and The Gut Microbiota: A Narrative Literature Review. Clin. Nutr. Res. 2021, 10, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Gleason, B.; Chisari, E.; Parvizi, J. Osteoarthritis Can Also Start in the Gut: The Gut-Joint Axis. Indian. J. Orthop. 2022, 56, 1150–1155. [Google Scholar] [CrossRef]
- Oo, W.M.; Little, C.; Duong, V.; Hunter, D.J. The Development of Disease-Modifying Therapies for Osteoarthritis (DMOADs): The Evidence to Date. Drug Des. Dev. Ther. 2021, 15, 2921–2945. [Google Scholar] [CrossRef]
- Bodkhe, R.; Balakrishnan, B.; Taneja, V. The role of microbiome in rheumatoid arthritis treatment. Ther. Adv. Musculoskelet. Dis. 2019, 11, 1759720X19844632. [Google Scholar] [CrossRef]
- Aqaeinezhad Rudbane, S.M.; Rahmdel, S.; Abdollahzadeh, S.M.; Zare, M.; Bazrafshan, A.; Mazloomi, S.M. The efficacy of probiotic supplementation in rheumatoid arthritis: A meta-analysis of randomized, controlled trials. Inflammopharmacology 2018, 26, 67–76. [Google Scholar] [CrossRef]
- Sköldstam, L.; Hagfors, L.; Johansson, G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 208–214. [Google Scholar] [CrossRef]
- Nash, P. Clinical use of Jak 1 inhibitors for rheumatoid arthritis. Rheumatology 2021, 60, ii31–ii38. [Google Scholar] [CrossRef]
- Stark, G.R.; Darnell, J.E. The JAK-STAT pathway at twenty. Immunity 2012, 36, 503–514. [Google Scholar] [CrossRef]
- Burmester, G.R.; Pope, J.E. Novel treatment strategies in rheumatoid arthritis. Lancet 2017, 389, 2338–2348. [Google Scholar] [CrossRef]
- Lee, E.B.; Fleischmann, R.; Hall, S.; Wilkinson, B.; Bradley, J.D.; Gruben, D.; Koncz, T.; Krishnaswami, S.; Wallenstein, G.V.; Zang, C.; et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N. Engl. J. Med. 2014, 370, 2377–2386. [Google Scholar] [CrossRef]
- Aaltonen, K.J.; Virkki, L.M.; Jämsen, E.; Sokka, T.; Konttinen, Y.T.; Peltomaa, R.; Tuompo, R.; Yli-Kerttula, T.; Kortelainen, S.; Ahokas-Tuohinto, P.; et al. Do biologic drugs affect the need for and outcome of joint replacements in patients with rheumatoid arthritis? A register-based study. Semin. Arthritis Rheum. 2013, 43, 55–62. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, Z.; Weng, X.S.; Liang, J.Q.; Lin, J.; Jin, J.; Qian, W.W.; Qiu, G.X. Cemented total-knee arthroplasty in rheumatoid arthritis patients aged under 60 years. Chin. Med. J. 2019, 132, 2760–2761. [Google Scholar] [CrossRef]
- Postacchini, R.; Carbone, S.; Canero, G.; Ripani, M.; Postacchini, F. Reverse shoulder prosthesis in patients with rheumatoid arthritis: A systematic review. Int. Orthop. 2016, 40, 965–973. [Google Scholar] [CrossRef]
- Wendling, D.; Lukas, C.; Paccou, J.; Claudepierre, P.; Carton, L.; Combe, B.; Goupille, P.; Guillemin, F.; Hudry, C.; Miceli-Richard, C.; et al. Recommendations of the French Society for Rheumatology (SFR) on the everyday management of patients with spondyloarthritis. Joint Bone Spine 2014, 81, 6–14. [Google Scholar] [CrossRef]
- Braun, J.; van den Berg, R.; Baraliakos, X.; Boehm, H.; Burgos-Vargas, R.; Collantes-Estevez, E.; Dagfinrud, H.; Dijkmans, B.; Dougados, M.; Emery, P.; et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann. Rheum. Dis. 2011, 70, 896–904. [Google Scholar] [CrossRef]
- Caso, F.; Costa, L.; Del Puente, A.; Di Minno, M.N.; Lupoli, G.; Scarpa, R.; Peluso, R. Pharmacological treatment of spondyloarthritis: Exploring the effectiveness of nonsteroidal anti-inflammatory drugs, traditional disease-modifying antirheumatic drugs and biological therapies. Ther. Adv. Chronic Dis. 2015, 6, 328–338. [Google Scholar] [CrossRef]
- Atteno, M.; Peluso, R.; Costa, L.; Padula, S.; Iervolino, S.; Caso, F.; Sanduzzi, A.; Lubrano, E.; Del Puente, A.; Scarpa, R. Comparison of effectiveness and safety of infliximab, etanercept, and adalimumab in psoriatic arthritis patients who experienced an inadequate response to previous disease-modifying antirheumatic drugs. Clin. Rheumatol. 2010, 29, 399–403. [Google Scholar] [CrossRef]
- Danve, A.; Deodhar, A. Treatment of axial spondyloarthritis: An update. Nat. Rev. Rheumatol. 2022, 18, 205–216. [Google Scholar] [CrossRef]
- Wolfe, F.; Zwillich, S.H. The long-term outcomes of rheumatoid arthritis: A 23-year prospective, longitudinal study of total joint replacement and its predictors in 1,600 patients with rheumatoid arthritis. Arthritis Rheum. 1998, 41, 1072–1082. [Google Scholar] [CrossRef]
| Outcome Measures and Classifications | |
|---|---|
| Physical functioning and disability | HAQ |
| MHAQ | |
| AIMS | |
| BASFI | |
| DASH | |
| GAT | |
| Pain | VAS |
| NRS | |
| Fatigue | MAF |
| Diagnosis | |
|---|---|
| Gut dysbiosis | Fecal analysis |
| Biopsy | |
| Inflammatory joint disease | Serologic tests (anti-RF and anti-CCP; ESR and CRP) |
| X-ray | |
| Genetic testing (HLA-B27) | |
| Disease activity biomarkers (TJC, RADAI, RAI) |
| Treatment | |
|---|---|
| Osteoarthritis | Lifestyle modifications |
| Probiotics | |
| Antibiotics | |
| FMT | |
| DMOADs | |
| Prosthesis implant | |
| Rheumatoid arthritis | Lifestyle modifications |
| Probiotics | |
| Corticosteroids | |
| DMARDs | |
| Prosthesis implant | |
| Spondylarthritis | Lifestyle modifications |
| NSAIDs | |
| DMARDs | |
| Anti-TNF | |
| Prosthesis implant | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, U.G.; Lalli, A.; Bandini, B.; de Sire, R.; Angeletti, S.; Lustig, S.; Ammendolia, A.; Budhiparama, N.C.; de Sire, A. Role of the Gut Microbiota in Osteoarthritis, Rheumatoid Arthritis, and Spondylarthritis: An Update on the Gut–Joint Axis. Int. J. Mol. Sci. 2024, 25, 3242. https://doi.org/10.3390/ijms25063242
Longo UG, Lalli A, Bandini B, de Sire R, Angeletti S, Lustig S, Ammendolia A, Budhiparama NC, de Sire A. Role of the Gut Microbiota in Osteoarthritis, Rheumatoid Arthritis, and Spondylarthritis: An Update on the Gut–Joint Axis. International Journal of Molecular Sciences. 2024; 25(6):3242. https://doi.org/10.3390/ijms25063242
Chicago/Turabian StyleLongo, Umile Giuseppe, Alberto Lalli, Benedetta Bandini, Roberto de Sire, Silvia Angeletti, Sebastien Lustig, Antonio Ammendolia, Nicolaas Cyrillus Budhiparama, and Alessandro de Sire. 2024. "Role of the Gut Microbiota in Osteoarthritis, Rheumatoid Arthritis, and Spondylarthritis: An Update on the Gut–Joint Axis" International Journal of Molecular Sciences 25, no. 6: 3242. https://doi.org/10.3390/ijms25063242
APA StyleLongo, U. G., Lalli, A., Bandini, B., de Sire, R., Angeletti, S., Lustig, S., Ammendolia, A., Budhiparama, N. C., & de Sire, A. (2024). Role of the Gut Microbiota in Osteoarthritis, Rheumatoid Arthritis, and Spondylarthritis: An Update on the Gut–Joint Axis. International Journal of Molecular Sciences, 25(6), 3242. https://doi.org/10.3390/ijms25063242










