Abstract
Hyaluronic acid (HA) is a remarkably multifaceted biomacromolecule, playing a role in regulating myriad biological processes such as wound healing, tissue regeneration, anti-inflammation, and immunomodulation. Crosslinked high- and low-molecular-weight hyaluronic acid hydrogels achieve higher molar concentrations, display slower degradation, and allow optimal tissue product diffusion, while harnessing the synergistic contribution of different-molecular-weight hyaluronans. A recent innovation in the world of hyaluronic acid synthesis is represented by NAHYCO® Hybrid Technology, a thermal process leading to hybrid cooperative hyaluronic acid complexes (HCC). This review summarizes the current literature on the in vitro studies and in vivo applications of HCC, from facial and body rejuvenation to future perspectives in skin wound healing, dermatology, and genitourinary pathologies.
1. Introduction: Pleiotropic Signalling of Hyaluronan in Cell Biology and Molecular Weight Specificity
HA is a linear non-sulfated high-molecular-weight glycosaminoglycan occurring naturally in all mammalian species. This linear polysaccharide is constituted by a disaccharide structure of d-glucuronic acid and N-acetyl-d-glucosamine repeats, linked by alternating beta-1,4 and beta-1,3 glycosidic bonds [1]. Its lack of sulfate substitutions but presence of highly charged residues on the sugar moieties makes the molecule notoriously hydrophilic [2]. Presumably because its structure is highly conserved among the different species, HA is poorly immunogenic in several animal species as opposed to the highly allergenic collagens [3]. In humans, HA is synthesized by three transmembrane enzymes called hyaluronan synthases (HAS1, HAS2, and HAS3) and extruded directly into the extracellular matrix (ECM) through the cell plasma membrane [1]. Hyaluronidase (HYAL) or reactive oxygen and nitrogen species (ROS/RNS) catabolize HA; the size of the resultant oligomers has a significant impact on their biological roles [4].
In its native form, hyaluronan is known as high-molecular-weight HA (H-HA), to distinguish it from the smaller, low-molecular-weight fragments (L-HA) resulting from its turnover [5]. H-HA (1000–6000 kDa) modulates cellular behaviour with different mechanisms of action as compared to L-HA (10–250 kDa) and HA oligomers (o-HA). H-HA promotes anti-inflammatory, anti-proliferative, and anti-angiogenic effects. It is also a key regulator of wound healing and embryogenesis. On the other hand, HA fragments (2–10 disaccharides) trigger signalling cascades that lead to a variety of cellular responses, such as inflammation, endothelial cell proliferation, and angiogenesis. Although increased levels of endogenous HA have been described in several types of cancer, L-HA (100–300 kDa) and o-HA (3–12 disaccharides) are able to downregulate signalling pathways that regulate cancer cell proliferation, migration, and metastasis [6]. These molecular-weight-dependent differences in action have been attributed to different modes of interaction of HA with its receptors and competition with endogenous HA for binding to said receptors [7]. The most well-known HA receptor is CD44; other notable receptors include receptor for HA-mediated motility (RHAMM), HA receptor for endocytosis (HARE), and Human Lymphatic Vessel Endothelial Hyaluronic Acid Receptor 1 (LYVE-1). Upon activation, the intracellular CD44 domain binds the cytoskeletal linker protein ankyrin and ezrin, radixin, and moesin (ERM) linkers, to regulate cell functions. The binding of H-HA to RHAMM regulates Ras/ERK/12 activities and initiates PI3K-dependent Rac activation and increased migration of arterial smooth muscle cells. The binding of L-HA to toll-like receptors (TLR) induces MyD88, downstream MAP-kinase activation, and Nf-κβ nuclear translocation to enhance the inflammatory response. HARE is responsible for the systemic clearance of HA, but L-HA binding initiates NF-κB-dependent gene expression. LYVE-1 is likewise involved in HA clearance, as well as in the endothelial transmigration of lymphocytes. An LYVE-1-dependent interaction between macrophages and the pericellular HA matrix of smooth muscle cells enhances MMP-9-dependent inhibition of arterial stiffness [8].
Besides its biological functions, due to its physicochemical properties, HA can act as a structural molecule affecting tissue hydration, ECM organization, and osmotic balance [9,10]. In terms of its role in skin homeostasis, HA has been recognized as a key molecule in skin aging, given its paramount role in determining and maintaining skin moisture [11]. One of the most notable histochemical changes occurring in senescent skin is the disappearance of epidermal HA, and a progressive reduction in the size of HA polymers in the skin [12]. In the dermis, hyaluronans display increased avidity for tissue structures, with a consequent loss of HA extractability [13]. The result is the characteristic dehydration, atrophy, and loss of elasticity of aged skin. Photoexposed skin, and therefore extrinsic skin aging, is characterized by a significant decrease in the expression of HA and an increased expression of HYAL; notwithstanding, the reasons for such changes in HA homeostasis with aging are still to be fully clarified [14].
2. NAHYCO® Hybrid Technology
HA hydrogels can be generated via chemical or physical crosslinking, which increases their elasticity and decreases viscosity [15]. Chemical crosslinked injectable hydrogels are produced by crosslinking HA with agents such as 1,4-butanediol diglycidyl ether (BDDE) and poly (ethylene glycol) diglycidyl ether (PEGDE) to acquire desired properties. On the other hand, physically crosslinked injectable hydrogels have been created using ionic, hydrophobic, or hydrogen bonding, or guest–host interactions, most commonly through temperature-responsive and ionic interactions. The main advantage of using physical crosslinking methods is biomedical safety due to the absence of chemical crosslinking agents, which avoids potential cytotoxicity [15]. Thermal processes, such as NAHYCO® Hybrid Technology [NaHYCO (Sodium Hyaluronate Hybrid Complex) technology, patent WO 2012/032151], are a recent innovation in physical hydrogel synthesis.
2.1. Creation of Hybrid Cooperative Hyaluronic Acid Complexes
HA molecules exhibit cooperative interactions upon being introduced into a solution, which stem from the development of hydrophobic interactions and hydrogen bonds. Although typically viewed as weak forces, these interactions can become highly stable when working together in a cooperative manner [16]. Moreover, the cooperativeness and therefore the strength of these interactions are strictly dependent on the length, molecular weight, and concentration of HA chains added in solution [17]. In particular, H-HAs are able to give stable interactions among all the hydrogen molecules, creating a three-dimensional network among the different chains. On the other hand, L-HAs interact in clusters, forming less-stable interactions, which do not simultaneously involve all the hydrogen molecules in the L-HA chains [18].
The variations in hydrogen bond formation affect the rheological behaviour of HA hydrogels, leading to a decrease in viscosity over time based on HA molecular weight and concentration. This results in limitations for various clinical applications [18].
Thanks to NAHYCO® Hybrid Technology [NaHYCO (Sodium Hyaluronate Hybrid Complex) technology, patent WO 2012/032151], it is possible to create, in solution, stable cooperative hybrids of equal concentration of L-HA and H-HA through a predetermined thermal cycle, leading to the formation of a new HA molecule characterized by unique rheological parameters and unaffected viscosity over time.
Importantly, to successfully obtain HCC of L-HA and H-HA, four parameters are critical: the simultaneous presence in solution of both L-HA and H-HA; the specific molecular weight of HA chains used in the process; an equal concentration in solution of L-HA and H-HA; and the thermal cycle profile performed to stabilize the new molecules.
The process leading to the HCC formation starts when a mixture of L-HA (80–100 kDa) and H-HA (1100–1400 kDa) is added in solution at room temperature. Then, the thermal cycle starts when the mixture is first heated to temperatures between 80 °C and 160 °C, preferably 100–120 °C (Figure 1), and maintained at this temperature for a short period (10 min). When the solution reaches high temperatures, the rise in the temperature creates the energy conditions to induce the rupture of the hydrogen bonds formed between the molecules of L-HA and H-HA chains. Indeed, in this condition, the weak hydrogen bonds are not able to interact in a cooperative way anymore and the polymer HA chains remain in solution independent of each other. After the heating process, the H-LA and H-HA mixture is cooled rapidly to room temperature. At this step, interchain interactions and hydrogen bonds start to rebuild, in this case, developing randomly between all the molecules of L-HA and H-HA and creating the HCC molecules (Figure 1). In this regard, HCC formation is linked to temperature and exposure time: a higher temperature and long exposure time lead to more effective hybrid formation. At the end of the thermal treatment cycle, HCC molecules are stabilized at room temperature when the increasing number of weak intermolecular bonds determines that the cooperativeness and mode of interaction among L-HA and H-HA molecules do not change over time. When the reaction process is completed, HCC molecules present new rheological characteristics that are stable over time. Surprisingly, HCC molecules are characterized by a notable decrease in viscosity compared to linear H-HA, which is stable over time. In particular, the new HCC rheological parameters are strictly related to the molecular weight of HA and to the stoichiometric ratio of L-HA and H-HA used for the reaction. The higher the difference in terms of molecular weight and stoichiometric ratio between L-HA and H-HA, the higher the decrease in terms of viscosity.
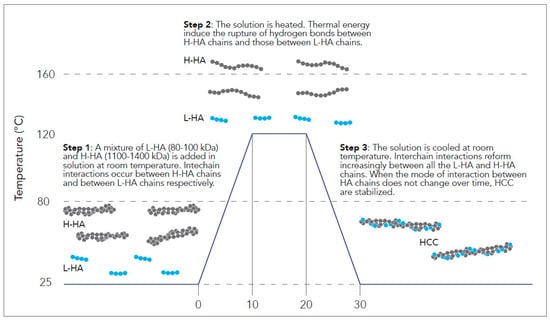
Figure 1.
NAHYCO® Hybrid Technology HCC production process.
Thanks to these characteristics, HCCs exhibit low G′ (<100 Pa) values and tanδ > 1; these properties reflect a predominance of flowability, confirmed by the dynamic viscosity values and the mean extrusion forces, allowing the gel to spread easily and homogenously during extrusion through the needle and after injection in dermal tissue. Moreover, HCCs high cohesivity scores indicate their ability to maintain their shape when injected and to remain in the injection site, ensuring no surface irregularity.
2.2. Hydrolytic Degradation and Mechanical Stability
Despite the lack of chemical modification, HCCs showed a significantly higher resistance to hyaluronidase action than H-HA in vitro [19]; additionally, in vivo murine studies employing high-frequency ultrasound showed that HCCs remained detectable for 10 weeks (32 mg/mL), and up to 29 weeks for higher molar concentrations (45 mg/mL), exhibiting a slow volumetric degradation, similar to that of chemically crosslinked gel [20]. The correlation between in vivo permanence data with in vitro enzymatic degradation results shows that HCCs at 45 mg/mL evidence a comparable duration in vivo with respect to the cross-linked HA 25 mg/mL filler (29 weeks vs. 33 weeks, respectively). However, at the same time, interestingly, degradability by hyaluronidase was enhanced, mainly at the early stage of the in vitro kinetics study [20]. At 45 mg/mL, HCCs only had freely soluble HA in them, and during the in vitro degradation study, the average molecular weight kept decreasing. On the other hand, cross-linked HA 25 mg/mL, according to its cross-linked nature, contained around 13% of the soluble HA fraction. During enzymatic degradation, its soluble fraction increased up to 70–74%, reaching a plateau after 1 h. Only once the soluble fraction plateau was reached did the average molecular weight begin to decrease [20]. For its elastic and viscous moduli, HCCs at 45 mg/mL retained around 77% and 87% of their initial values after 5 min of incubation, while H-HAs only retained 50% and 68% of their initial values. This demonstrates that when hyaluronidase and other enzymes attack and break down linear HA, the hybrid stabilized complex is better able to maintain the viscoelastic characteristics [21].
3. The Functional Impact of HCCs: In Vitro Studies
Combining different-MW HA has benefits in terms of their synergistic contribution to tissue regeneration. In vitro studies have helped elucidate the role of HCCs across human tissue types (Figure 2).
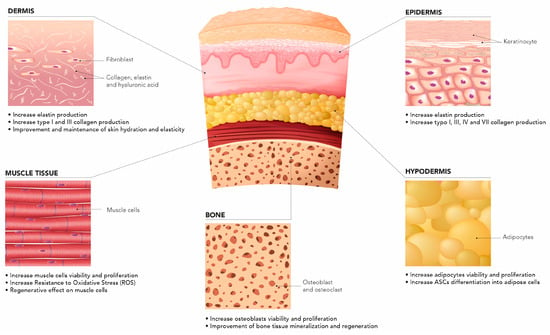
Figure 2.
HCCs across different tissue types.
3.1. Epidermic and Dermic Layers
Stellavato et al. evaluated the cellular and molecular changes both in keratinocytes and fibroblasts following the use HCCs in comparison with H-HA and L-HA gels in both in vitro cell culture assays and in a 3D skin model formed by multilayers of epidermal keratinocytes and dermal fibroblasts. Compared to untreated cells and to the H-HA and L-HA treatments, they noted that HCCs caused an increase in collagen and elastin expression levels, suggesting a bioremodeling effect on the tissue in the presence of HCCs. This was probably due to the long-lasting release and the concurrent action of the two HA components [19]. Specifically, exposure to HCCs resulted in the increased synthesis of type I and III collagens by both keratinocytes and fibroblasts, but production of type IV and VII collagens was mostly stimulated in keratinocytes [19]. This has important effects on wound repair, where both H-HA and L-HA play a pivotal role and simultaneously occur at an injury site in vivo. D’Agostino et al. observed how HCCs promote wound healing of human keratinocyte monolayers in scratch tests at twice the speed of H-HA and L-HA alone [22]. Further studies by the same group utilizing in vitro human keratinocyte/dermal fibroblast co-cultures observed that HCCs reduced the inflammatory biomarkers TGF-β, TNF-α, IL-6, and IL-8, and accelerated the healing process as confirmed by the modulation of metalloproteases and elastin, associated with a prospectively reduced risk of scar formation [22]. Furthermore, they observed higher expression of the antimicrobial peptide defensin-2 in HCC-treated samples, suggesting a potential increase in antibacterial and immunomodulatory functions.
3.2. Subcutaneous Tissue
The same group also observed the effect of HCCs on adipose-derived stem cells (ASC), which have widespread use in regenerative medicine, including fat grafting, recovery from local tissue ischemia, and scar remodelling [23]. HCC-based formulations were observed to clearly enhance adipogenic differentiation and proliferation via the upregulation of adipogenic genes and related proteins, when compared with linear HA and cross-linked hyaluronans. The authors hypothesized that the product’s low viscosity and elevated HA content allow for its easier binding to ASC receptors for differentiation and proliferation as compared with other formulations, and that injection of HCCs in the subdermal fat compartment may recruit and differentiate stem cells in adipocytes, considerably improving fat tissue renewal. At a high concentration of 45 mg/mL, the HCCs exhibited the predicted viscous liquid characteristics, with increased entanglement compared to HCCs at 32 mg/mL, thus displaying a potentially better mechanical performance and reducing deformation under the influence of external forces. This discovery has significant therapeutic significance since it enhances the product’s use for specific purposes such as face fat pad replenishment and breast regenerative medicine. Notably, the HCCs at a high concentration showed superior effectiveness to induce in vitro human adipose stromal cell differentiation towards the adipose phenotype, as shown by adipogenic markers such as PPAR-γ, adiponectin, and leptin [21].
HA is found in the extracellular matrix of the stem cell niche environment and is being studied as a crucial element for the in vitro development of stem cells [24]. Mesenchymal stromal cells (MSCs) are of great regenerative interest given their immunomodulatory properties and their capacity to sustain tissue repair and homeostasis by differentiating into osteocytes, chondrocytes, adipocytes, and smooth muscle cells. Alessio et al. observed HCCs’ efficacy in delaying senescence in mesenchymal stromal cells subjected to stressful conditions, compared to L-HA and H-HA controls [24]. This occurred without alteration of the cell cycle, cytotoxicity, or apoptosis. HCCs were also noted to promote adipogenic and chondrogenic differentiation. Stellavato et al. analysed the beneficial effect of HCCs on cells subjected to oxidative stress in vitro and on the recovery of muscle atrophy [25]. HCCs were proven to have a greater potential than L-HAs in promoting cell proliferation, in reducing ROS damage and atrophic biomarkers, and in preserving the muscle phenotype and viability in a skeletal muscle disorder model. Among MSCs, La Noce and colleagues focused on the effect of HCCs on human dental pulp stem cells (hDPSC), which is of great interest in terms of regenerative medicine given their remarkable suitability for bone–endothelium co-differentiation. All hyaluronans were observed to promote hDPSC bone differentiation in vitro, but HCCs were the main inducers of osteogenesis and the overexpression of bone-related markers as compared with linear HA [26].
Taken collectively (Table 1), the above in vitro evidence supports HCCs’ potential as a medical device in both aesthetic and regenerative medicine.

Table 1.
HCCs’ effect by cell type.
4. Medical Aesthetics Applications
In terms of clinical application, HA-based injectable dermal fillers have become the most popular agents for soft tissue contouring and volumizing [27]. In 2019, over 4.3 million HA-based aesthetic operations were carried out, a 15.7% rise from the previous year, according to the International Society of Aesthetic Plastic Surgery. Although HA plays a central role in the inflammatory process, the use of HA-based fillers has shown significant clinical and cosmetic advantages in lupus and scleroderma patients under treatment. They also have a strong safety record and might potentially be a novel therapeutic option for patients with systemic scleroderma when combined with PEP [28]. However, HA-based fillers still present some limitations, such as the use of chemical cross-linking reagents and the maximum concentration of HA used for this product (25 mg/mL). Thanks to their advantages, HCCs can be employed in several applications of the aesthetic medicine field, such as the treatment of face and neck laxity, as well as for body laxity and acne scar treatment (Figure 3) [29]. Importantly, HCCs can facilitate extracellular matrix homeostasis and sustain cellular vitality, thus reversing the signs of skin laxity through a bioremodeling action. Bioremodeling is the process that reverses the signs of skin laxity, facilitating extracellular matrix homeostasis by the improvement of the vitality of different cell types including fibroblasts, keratinocytes, adipocytes, and myocytes. The peculiar mechanism of action of HCCs significantly differs from other existing products, whose activity is based on either biorevitalisation or biostimulation. The first consists of restoring the loss in skin nourishment by directly providing vital components (e.g., amino acids, vitamins, nucleotides, linear HA, collagen) to fibroblasts and keratinocytes, while the latter improves extracellular matrix hydration and elasticity through an immune-mediated response able to stimulate the synthesis of HA, collagen, and elastin by fibroblasts. A biostimulating activity is attributed to substances such as poly-L-lactic acid (PLLA), calcium hydroxylapatite (CaHa), polycaprolactone (PCL), and carboxymethylcellulose (CMC). These differences make the NAHYCO® Hybrid Technology product represent a unique point of reference in the market, playing a central role in regenerative processes. Of note, a recent safety assessment of HCCs (Profhilo®), as derived from worldwide post-marketing data, confirmed the high tolerability and safety of such a product [30].
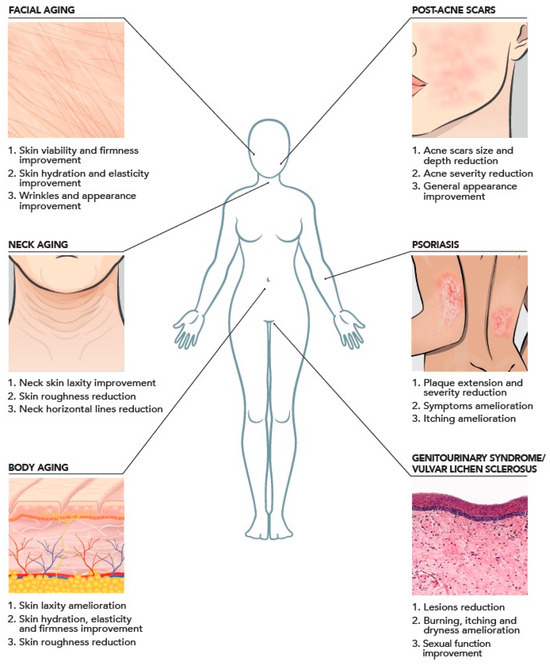
Figure 3.
Clinical evidence of intradermal injection of HCCs based on the literature with aesthetic indications and promising therapeutic applications.
4.1. Face
For the treatment of facial aging, HCCs are an excellent treatment option to restore the vitality and turgor of skin. Indeed, three independent in vivo studies, performing two facial subcutaneous injections with a 4-week time lapse between them, resulted in a significant improvement in facial face hydration, elasticity, and transepidermal water loss (TEWL), as well as in high patient satisfaction and optimal physician evaluations [30,31,32,33]. Importantly, HCC benefits were present in the absence of local side effects, as demonstrated by post-marketing surveillance data [31] and by long-term safety studies [34]. Moreover, treatment with HCCs proved to be effective not only for White populations, but also for different ethnicities. Goltsova et al. [35] and Satardinova et al. [36] demonstrated significative amelioration of skin texture and hydration in the Russian and Oriental Mongolian population. Furthermore, a recent clinical study also proved that HCC treatment is effective in improving the facial appearance of Asian women, even earlier compared to White women [37,38].
Furthermore, HCCs have been employed in the field of lipofilling to improve adipose tissue engraftment and obtain a better volumizing result months after surgery. Tateo et al. added HCCs to autologous fat grafts employed for lipofilling and noted an increase in the stromal vascular fraction of adipose stem cells (ASCs) and of the metabolic viability of adipocytes in samples with added HCCs, as compared with control samples [39]. As a preliminary tolerability and efficacy test of the association, they also treated a small number of patients with facial lipofilling with autologous adipose tissue followed by HCC injection and noted good skin tone and trophism after the procedure, with no complications in a 6-month follow-up period. HCCs used at higher concentrations (45 mg/mL) also proved to best integrate into adipose tissue and to be able to specifically restore the fat compartment, leading to a marked improvement in skin firmness and a reduction in superficial and deep wrinkles [40]. Only 4 out of 50 participants had mild bruises at the injection sites, which vanished entirely within 5–10 days. Cassuto et al. also demonstrated an increase in tissue thickness evaluated by US scan when HCCs (45 mg/mL) were injected into the preauricular area. Taken together, the data showed a clear improvement of the fat compartment affected by the aging process, leading to a final lipo-lifting effect and amelioration of subjects’ facial appearance [41].
4.2. Neck
Paganelli et al. obtained similar promising data on the use of HCCs combined with plasma exeresis for neck rejuvenation, with an improvement in both patient- and physician-assigned neck skin laxity scores, excellent tolerability evaluations, and only transient and mild side effects such as erythema and oedema [42]. Sparavigna et al. reported amelioration after injection of HCCs in the neck area in terms of skin roughness and laxity based on clinical, instrumental, and subjective evaluations [43]. The clinical study showed a statistically significant improvement in neck skin laxity, which was already detectable at 1 month after the first aesthetic procedure and further increased at 4 months. This improvement was associated with a reduction in the IBSA Neck Laxity Scale of at least 1 grade in more than half of the subjects [43]. Comparable to research on face rejuvenation, HCCs were proven to be effective for neck rejuvenation not only on in White populations, but also in different ethnicities (Asian women) [37].
4.3. Body
Two studies have been performed to evaluate the impact of HCCs for the treatment of body laxity, targeting the inner arm, abdomen, and knees [44,45]. The treatment consisted of 3 mL injections of Profhilo Body® for each brachial zone, for the abdomen, and for each upper part of the knees, performed with a 29G needle into the middle-deep dermis. Subjects showed a significant improvement in their skin laxity clinical score for each analysed anatomical area. Specifically, most subjects had an improvement of at least 1 grade in the evaluation scale in all investigated areas. A monocentric, open-label, not-controlled, exploratory study was also designed to evaluate the performance and tolerability of HCC treatment in the back of the hands (dorsum) over a 4-month period. The use of the medical device resulted in statistically significant improvements in skin laxity, resistance to pinching, roughness, wrinkle depth, immediate extensibility, viscoelasticity, and immediate elastic recovery in the hand dorsum [46]. Histological analysis of postobese patients who received HCC injections in one of the two arms before undergoing a bilateral brachioplasty has shown that the main mechanism responsible for reversing skin laxity is the deposition of uniform elastin globules onto microfibrils and preventing uncontrolled aggregation. In the HCC-treated samples, the study found that there were more elastin fibres and a more even distribution of elastic fibre architecture. The contralateral untreated area showed an irregular structure with elastosis and elastolysis [47]. Table 2 summarises the results of the above studies on the use of HCCs for the medical aesthetic treatments of facial and body laxity.

Table 2.
Clinical outcomes of HCC treatments according to the product indications.
5. Role in Regenerative Medicine
5.1. Regenerative Medicine and Treatment Approaches
In the last few decades, regenerative medicine has gained a fundamental role in several medicine branches, including the aesthetic field [48,49,50]. Regenerative medicine is defined as the area of medicine that aims to restore organs or tissues that are impaired by pathological conditions or by trauma [51,52,53]. In this regard, potential therapeutic strategies to counteract the action of several inflammatory conditions and diseases are represented by novel procedures based on cellular therapies, principally involving the use of stem cells [54,55] or by several biomaterial formulations [56,57]. In particular, skin defects induced by trauma, burns, and unhealing chronic wound are a potential target to be treated thanks to the use of both cell-based and cell-free therapies [58,59]. Regenerative medicine approaches for skin regeneration can be also used to address the facial and body ageing process and they are also becoming of fundamental importance in the aesthetic medicine field of application [60]. Regenerative aesthetics is a relatively new concept in aesthetic applications and it is nowadays defined as the branch of regenerative medicine that focuses on the restoration of soft tissues damaged or lost as a consequence of the ageing process [60]. In a similar manner to the other therapeutic strategies used in regenerative medicine, potential treatments for regenerative aesthetics are represented by cell-based therapies and by the use of biomaterials [61]. For the latter, several studies have showed the potentiality of different synthetic biomaterials, such as PLLA, CaHa, and CMC, to induce collagen and elastin production when injected in the dermis layer of the skin [62,63]. However, all these products exert their effect through a biostimulatory action. The biostimulation process induced by synthetic biomaterials, such as PLLA products, usually involves an immune-mediated response that consequently may induce the establishment of a fibrotic pathway [64]. Some studies suggest that CaHa-based fillers can stimulate tissue regeneration without causing inflammation [65,66]. However, scientific literature commonly acknowledges that these products are biostimulatory, triggering collagen production through an immune-mediated response [67,68]. Therefore, these biostimulatory molecules cannot be classified as regenerative approaches, which instead should exert their functions through more physiological pathways.
Currently, exosomes are also considered as a promising treatment for regenerative medicine [69]. Exosomes are small extracellular nanovesicles that are released by cells, acting as help in intercellular signaling molecules and maintaining tissue homeostasis [69]. Their potential role in regenerative medicine has been explored in different applications, including dermatological diseases [70] and skin rejuvenation [71]. Although this approach has gained recent popularity especially in aesthetic medicine [71], several limitations are still present to consider this technique as effective. In particular, the use of exosomes in regenerative medicine is still limited by inefficient preparation methods, difficulties characterization and lack of specific biomarkers [72]. More importantly, these treatments are mainly not still officially classified by regulatory agencies such as FDA or EMA [70].
On the other hand, HCCs have the potentiality to play a key role in the regenerative approaches thanks to its bioremodeling action, which is able to induce tissue restoration through a physiological improvement of extracellular matrix homeostasis and cellular viability. The importance of HCCs is also confirmed by the patent-protected technology, which further assures the uniqueness of this product and of its mode of action. In this regard, HCCs have already proved their efficacy to restore damaged skin or connective tissues in several applications. A recently published paper interestingly demonstrated the efficacy of HCCs to induce an amelioration in terms of skin quality, skin elasticity, and quality of life in patients affected by scleroderma when perioral injections have been performed [73]. Moreover, several studies also demonstrated the efficacy of HCCs to repair damaged skin in psoriasis [74] or acne scars [75,76,77] demonstrating the pivotal role of HCCs in regenerative medicine.
5.2. Future Perspectives of HCCs in Regenerative Medicine
HCC applications in regenerative medicine extend beyond aesthetic purposes to include skin wound healing, atopic dermatitis, psoriasis, acne scars, and genitourinary pathologies (Figure 3). These applications are supported by their demonstrated wound healing and bioremodeling effects in laboratory settings, which are indicated by decreased levels of inflammatory biomarkers, regulation of metalloprotease and elastin, and promotion of antimicrobial peptides in keratinocyte/fibroblast co-culture models [19].
Siquier-Dameto et al. employed injectable HCCs in psoriasis by performing two monthly intra- and peri-lesional deep-dermal bolus injections and observed amelioration in both the extension and severity of psoriasis, as well as in pruritus [74]. Scar contractures also benefited from intralesional HA injection, as described by Cassuto et al., in a small case series in which pneumatically injected HCCs improved scar texture, laxity, and appearance, as well as range of motion and pruritus in two patients [75]. Post-acne scars can also be successfully treated with HCCs, alone or in combination with non-ablative laser or subcision, as demonstrated by several independent investigators [76,77,78,79]. Tedesco et al. employed HCC infiltrations for the treatment of vulvar lichen sclerosus, which were found to be safe and tolerable as well as significantly effective in reducing symptoms of itching, pain, and burning sensation [80]. The usefulness of HCCs in treating menopausal women who experience pain during or after sexual activity was also investigated in a prospective study. Without any negative side effects, HCC injections improved the lubricating and orgasmic aspects of sexual function and showed promise in reducing sensations of dryness [81]. Vestibular injections of HCCs were employed by Garavaglia et al. to treat vulvovaginal atrophy, one of the symptoms of genitourinary syndrome, with a significant improvement in genital symptoms and sexual function, and high patient-reported satisfaction levels [82]. While HA–SC has mostly been studied as a medical device in the areas of orthopaedics, in vitro findings also point to the innovative formulation as a possible new therapy in several dermatological, cosmetic, and regenerative medicine applications [83]. Compared to HHC without chondroitin, the combined formula (HA–SC) works more efficiently for wound reparation, collagen, and elastin expression [84]. Inflammation is acknowledged as an internal element in the aging process, and reducing inflammation might be a viable approach for anti-aging [85]. An in vitro model using spheroids created by cytokines has shown that HA–SC may suppress the activation of NF-kB, a key factor in inflammation, hence reducing the production of pro-inflammatory cytokines including IL-6 and TNF-α [86,87].
6. Conclusions
HA-based materials have long been recognized as the ideal products for cutaneous rejuvenation thanks to their long-lasting, fully reversible effects, as well as their ease of performance and low allergic potential. With the vast potential of hyaluronan, HA-based materials could transform regenerative medicine, aiming to restore diseased and injured tissues and whole organs. Several cases showed the successful use of HA as a compound in the fabrication of HA-based products such as hydrogels, nanofibers, and 3D materials, which have been applied in bone and tissue regeneration, topical gels for wound healing, and cancer treatment via HA-loaded drug delivery approaches [88]. Advances in crosslinking techniques, such as thermal stabilizing processes that do not require chemical biolinkers, have further allowed the diverse bioactivity of L-HA and H-HA to be fully harnessed. Furthermore, HCCs’ anti-inflammatory and antimicrobial properties have benefited conditions other than cutaneous aging, including keloids and atrophic scars and genitourinary pathologies. Far from being a mere filling product, HA’s ability to promote healing and bioremodeling is now ensuring its broader application in new and exciting fields such as regenerative medicine, tissue engineering, and nanomedicine.
Author Contributions
D.H., B.M. and G.S. have reviewed the results. All authors have revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The medical writing has been supported by IBSA Farmaceutici Italia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors are grateful to Alba Sommerschield for her help with writing the manuscript.
Conflicts of Interest
C.C., G.B., & F.G. are employees of IBSA Farmaceutici Italia Srl. The other authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Abruzzese, F.; Basoli, F.; Constantini, M.; Giannitelli, S.; Gori, M.; Mozetic, P.; Rainer, A.; Trombetta, M. Hyaluronan: An Overview. J. Biol. Regul. Homeost. Agents 2017, 31 (4 Suppl. 2), 9–22. [Google Scholar]
- Maytin, E.V. Hyaluronan: More than Just a Wrinkle Filler. Glycobiology 2016, 26, 553–559. [Google Scholar] [CrossRef]
- Gilbert, E.; Hui, A.; Meehan, S.; Waldorf, H.A. The Basic Science of Dermal Fillers: Past and Present Part II: Adverse Effects. J. Drugs Dermatol. 2012, 11, 1069–1076. [Google Scholar]
- Stern, R. Hyaluronan Catabolism: A New Metabolic Pathway. Eur. J. Cell Biol. 2004, 83, 317–325. [Google Scholar] [CrossRef]
- Litwiniuk, M.; Krejner, A.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration|Wounds Research. Wounds 2016, 28, 78–88. [Google Scholar] [PubMed]
- Tavianatou, A.G.; Piperigkou, Z.; Barbera, C.; Beninatto, R.; Masola, V.; Caon, I.; Onisto, M.; Franchi, M.; Galesso, D.; Karamanos, N.K. Molecular Size-Dependent Specificity of Hyaluronan on Functional Properties, Morphology and Matrix Composition of Mammary Cancer Cells. Matrix Biol. Plus 2019, 3, 100008. [Google Scholar] [CrossRef] [PubMed]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular Size-Dependent Signaling and Biological Functions in Inflammation and Cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef]
- Berdiaki, A.; Neagu, M.; Spyridaki, I.; Kuskov, A.; Perez, S.; Nikitovic, D. Hyaluronan and Reactive Oxygen Species Signaling—Novel Cues from the Matrix? Antioxidants 2023, 12, 824. [Google Scholar] [CrossRef] [PubMed]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and Function. Biomolecules 2020, 10, 1525. [Google Scholar] [CrossRef]
- Humzah, D.; Romagnoli, M.; Tateo, A.; Bellia, G. Hyaluronic Acid: A Strategic Molecule for Rejuvenating Procedures. Esperienze Dermatol. 2020, 22, 36–41. [Google Scholar] [CrossRef]
- Meyer, L.J.M.; Stern, R. Age-Dependent Changes of Hyaluronan in Human Skin. J. Investig. Dermatol. 1994, 102, 385–389. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic Acid: A Key Molecule in Skin Aging. Dermatoendocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Tzellos, T.G.; Klagas, I.; Vahtsevanos, K.; Triaridis, S.; Printza, A.; Kyrgidis, A.; Karakiulakis, G.; Zouboulis, C.C.; Papakonstantinou, E. Extrinsic Ageing in the Human Skin Is Associated with Alterations in the Expression of Hyaluronic Acid and Its Metabolizing Enzymes. Exp. Dermatol. 2009, 18, 1028–1035. [Google Scholar] [CrossRef]
- Cassuto, D.; Bellia, G.; Schiraldi, C. An Overview of Soft Tissue Fillers for Cosmetic Dermatology: From Filling to Regenerative Medicine. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1857–1866. [Google Scholar] [CrossRef]
- Giubertoni, G.; Koenderink, G.H.; Bakker, H.J. Direct Observation of Intrachain Hydrogen Bonds in Aqueous Hyaluronan. J. Phys. Chem. A 2019, 123, 8220–8225. [Google Scholar] [CrossRef]
- Dong, Q.; Guo, X.; Li, L.; Yu, C.; Nie, L.; Tian, W.; Zhang, H.; Huang, S.; Zang, H. Understanding hyaluronic acid induced variation of water structure by near-infrared spectroscopy. Sci. Rep. 2020, 10, 1387. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef] [PubMed]
- Stellavato, A.; Corsuto, L.; D’Agostino, A.; La Gatta, A.; Diana, P.; Bernini, P.; De Rosa, M.; Schiraldi, C. Hyaluronan Hybrid Cooperative Complexes as a Novel Frontier for Cellular Bioprocesses Re-Activation. PLoS ONE 2016, 11, e0163510. [Google Scholar] [CrossRef] [PubMed]
- Scrima, M.; Merola, F.; Vito, N.; Pacchioni, D.; Vecchi, G.; Melito, C.; Iorio, A.; Giori, A.M.; Ferravante, A. Elucidations on the Performance and Reversibility of Treatment with Hyaluronic Acid Based Dermal Fillers: In Vivo and in Vitro Approaches. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2629. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, V.; Di Meo, C.; Alessio, N.; La Gatta, A.; Ferraro, G.A.; Nicoletti, G.F.; Schiraldi, C. Highly Concentrated Stabilized Hybrid Complexes of Hyaluronic Acid: Rheological and Biological Assessment of Compatibility with Adipose Tissue and Derived Stromal Cells towards Regenerative Medicine. Int. J. Mol. Sci. 2024, 25, 2019. [Google Scholar] [CrossRef]
- D’Agostino, A.; Stellavato, A.; Busico, T.; Papa, A.; Tirino, V.; Papaccio, G.; La Gatta, A.; De Rosa, M.; Schiraldi, C. In Vitro Analysis of the Effects on Wound Healing of High- and Low-Molecular Weight Chains of Hyaluronan and Their Hybrid H-HA/L-HA Complexes. BMC Cell Biol. 2015, 16, 19. [Google Scholar] [CrossRef]
- Stellavato, A.; La Noce, M.; Corsuto, L.; Pirozzi, A.V.A.; De Rosa, M.; Papaccio, G.; Schiraldi, C.; Tirino, V. Hybrid Complexes of High and Low Molecular Weight Hyaluronans Highly Enhance Hascs Differentiation: Implication for Facial Bioremodeling. Cell. Physiol. Biochem. 2017, 44, 1078–1092. [Google Scholar] [CrossRef] [PubMed]
- Alessio, N.; Stellavato, A.; Squillaro, T.; Del Gaudio, S.; Di Bernardo, G.; Peluso, G.; De Rosa, M.; Schiraldi, C.; Galderisi, U. Hybrid Complexes of High and Low Molecular Weight Hyaluronan Delay in Vitro Replicative Senescence of Mesenchymal Stromal Cells: A Pilot Study for Future Therapeutic Application. Aging 2018, 10, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Stellavato, A.; Abate, L.; Vassallo, V.; Donniacuo, M.; Rinaldi, B.; Schiraldi, C. An in Vitro Study to Assess the Effect of Hyaluronan-Based Gels on Muscle-Derived Cells: Highlighting a New Perspective in Regenerative Medicine. PLoS ONE 2020, 15, e0236164. [Google Scholar] [CrossRef] [PubMed]
- La Noce, M.; Stellavato, A.; Vassallo, V.; Cammarota, M.; Laino, L.; Desiderio, V.; Del Vecchio, V.; Nicoletti, G.F.; Tirino, V.; Papaccio, G.; et al. Hyaluronan-Based Gel Promotes Human Dental Pulp Stem Cells Bone Differentiation by Activating YAP/TAZ Pathway. Cells 2021, 10, 2899. [Google Scholar] [CrossRef]
- Bravo, B.; Correia, P.; Gonçalves Junior, J.E.; Sant’Anna, B.; Kerob, D. Benefits of topical hyaluronic acid for skin quality and signs of skin aging: From literature review to clinical evidence. Dermatol. Ther. 2022, 35, e15903. [Google Scholar] [CrossRef]
- Pieretti, G.; Rafaniello, C.; Fraenza, F.; Donniacuo, M.; Cuomo, R.; Lanzano, G.; Ciccarelli, F.; Capuano, A.; Nicoletti, G. Hyaluronic acid-based fillers in patients with autoimmune inflammatory diseases. J. Cosmet. Dermatol. 2023, 22, 2420–2423. [Google Scholar] [CrossRef]
- Agolli, E.; Diffidenti, B.; Di Zitti, N.; Massidda, E.; Patella, F.; Santerini, C.; Beatini, A.; Bianchini, M.; Bizzarri, S.; Camilleri, V.; et al. Hybrid Cooperative Complexes of High and Low Molecular Weight Hyaluronans (Profhilo®): Review of the Literature and Presentation of the VisionHA Project. Esperienze Dermatol. 2021, 20, 5–14. [Google Scholar] [CrossRef]
- Cassuto, D.; Delledonne, M.; Zaccaria, G.; Illiano, I.; Giori, A.M.; Bellia, G. Safety Assessment of High- and Low-Molecular-Weight Hyaluronans (Profhilo®) as Derived from Worldwide Postmarketing Data. Biomed. Res. Int. 2020, 2020, 8159047. [Google Scholar] [CrossRef]
- Laurino, C.; Palmieri, B.; Coacci, A. Efficacy, Safety, and Tolerance of a New Injection Technique for High- and Low-Molecular-Weight Hyaluronic Acid Hybrid Complexes. Eplasty 2015, 15, e46. [Google Scholar]
- Sparavigna, A.; Tenconi, B. Efficacy and Tolerance of an Injectable Medical Device Containing Stable Hybrid Cooperative Complexes of High- and Low-Molecular-Weight Hyaluronic Acid: A Monocentric 16 Weeks Open-Label Evaluation. Clin. Cosmet. Investig. Dermatol. 2016, 9, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Abascal, M.; Saldana Fernandez, M. Bio-Remodelación Facial Mediante Inyección Intradérmica de Un Complejo Híbrido Estabilizado de Ácido Hialurónico de Alto y Bajo Peso Molecular: Estudio Prospectivo En 30 Pacientes. Eur. Aesthetic Plast. Surg. J. 2015, 5, 123–131. [Google Scholar]
- Sparavigna, A. Long Term Efficacy and Tolerance Of High-And Low-Molecular-Weight Hyaluronans (Profhilo®) Intradermal Injections. J. Plast. Pathol. Dermatol. 2022, 18, 185–191. [Google Scholar]
- Goltsova, E.; Shemonaeva, O. Hybrid Cooperative Complexes of H-HA and L-HA (Profhilo®) and the BAP Technique for Facial Skin Bioremodeling: A Clinical Experience at the NEO-Clinic (Tyumen, Russia). Esperienze Dermatol. 2019, 21, 47–53. [Google Scholar] [CrossRef]
- Satardinova, E. Hybrid Cooperative Complexes of High and Low Molecular Weight Hyaluronans for Facial Skin Rejuvenation in the Oriental Mongoloid Face: A Case Series. Aesthetic Med. 2019, 5, 14–19. [Google Scholar]
- Sparavigna, A.; Lualdi, R.; Cicerone, M.; Giori, A.M.; Bellia, G. A Pilot Study Testing Efficacy and Tolerability of Hybrid Cooperative Complexes of Hyaluronic Acid Intradermal Injections in Chinese Women. Aesthetic Med. Plast. Surg. 2023, 1, 5–12. [Google Scholar]
- Sparavigna, A.; Cigni, C.; Grimolizzi, F.; Bellia, G. Neck and face rejuvenation outcomes with Profhilo®: A comparative analysis between Chinese and Caucasian cohorts. Gazz. Med. Ital.-Arch. Sci. Med. 2023, 182, 628–633. [Google Scholar] [CrossRef]
- Tateo, A.; Sofo, G. Optimization of the Lipofilling Procedure with Hybrid Cooperative Complexes of High and Low Molecular Weight Hyaluronic Acid: Preliminary Experiments. Esperienze Dermatol. 2019, 21, 22–28. [Google Scholar] [CrossRef]
- Sparavigna, A.; Grimolizzi, F.; Cigni, C.; Lualdi, R.; Bellia, G. Efficacy and tolerability of Profhilo® Structura intended to restore lateral cheek fat compartment: An observational pilot study. Health Sci. Rep. 2024, 7, e1743. [Google Scholar] [CrossRef]
- Cassuto, D.; Cigni, C.; Bellia, G.; Schiraldi, C. Restoring Adipose Tissue Homeostasis in Response to Aging: Initial Clinical Experience with Profhilo Structura®. Gels 2023, 9, 614. [Google Scholar] [CrossRef]
- Paganelli, A.; Mandel, V.D.; Pellacani, G.; Rossi, E. Synergic Effect of Plasma Exeresis and Non-Cross-Linked Low and High Molecular Weight Hyaluronic Acid to Improve Neck Skin Laxities. J. Cosmet. Dermatol. 2020, 19, 55–60. [Google Scholar] [CrossRef]
- Sparavigna, A.; Bombelli, L.; Giori, A.M.; Bellia, G. Efficacy and Tolerability of Hybrid Complexes of High- and Low-Molecular-Weight Hyaluronan Intradermal Injections for the Treatment of Skin Roughness and Laxity of the Neck. Sci. World J. 2022, 2022, 4497176. [Google Scholar] [CrossRef] [PubMed]
- Sparavigna, A.; Musella, D.; Cicerone, M.; Giori, A.M.; Bellia, G. Hybrid cooperative complexes of high and low molecular weight hyaluronans (96 mg/3 mL) for the treatment of skin laxity of the inner arm and abdomen. Gazz. Med. Ital. Arch. Sci. Med. 2022, 181, 487–495. [Google Scholar] [CrossRef]
- Sparavigna, A.; Grimolizzi, F.; Cigni, C.; Lualdi, R.; Bellia, G. Profhilo Body® for Tackling Skin Roughness and Laxity of Inner Arm, Abdomen and Knees. J. Plast. Pathol. Dermatol. 2023, 19, 31–38. [Google Scholar]
- Sparavigna, A.; Grimolizzi, F.; Cigni, C.; Lualdi, R.; Bellia, G. Use of intradermic injection of hybrid cooperative complexes of hyaluronic acid to counteract Skin Roughness and Laxity on the Back of the Hands. J. Plast. Pathol. Dermatol. 2023, 19, 7–13. [Google Scholar]
- Margara, A.; Haykal, D.; Musella, D.; Bellia, G.; Boriani, F. Hyaluronan Hybrid Cooperative Complexes Injection as a Biostimulation for Postobese Skin Laxity in the Arm: A Histopathologic Study. Aesthetic Surg. J. Open Forum 2024, 6, ojad110. [Google Scholar] [CrossRef]
- Ntege, E.H.; Sunami, H.; Shimizu, Y. Advances in regenerative therapy: A review of the literature and future directions. Regen. Ther. 2020, 14, 136–153. [Google Scholar] [CrossRef]
- Zarbafian, M.; Fabi, S.G.; Dayan, S.; Goldie, K. The Emerging Field of Regenerative Aesthetics—Where We Are Now. Dermatol. Surg. 2022, 48, 101–108. [Google Scholar] [CrossRef]
- Petrosyan, A.; Martins, P.N.; Solez, K.; Uygun, B.E.; Gorantla, V.S.; Orlando, G. Regenerative medicine applications: An overview of clinical trials. Front. Bioeng. Biotechnol. 2022, 10, 942750. [Google Scholar] [CrossRef]
- McKinley, K.L.; Longaker, M.T.; Naik, S. Emerging frontiers in regenerative medicine. Science 2023, 380, 796–798. [Google Scholar] [CrossRef]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef]
- Iismaa, S.E.; Kaidonis, X.; Nicks, A.M.; Bogush, N.; Kikuchi, K.; Naqvi, N.; Harvey, R.P.; Husain, H.; Graham, R.M. Comparative regenerative mechanisms across different mammalian tissues. npj Regen. Med. 2018, 3, 6. [Google Scholar] [CrossRef]
- Tatullo, M.; Zavanx, B.; Piattelli, A. Critical Overview on Regenerative Medicine: New Insights into the Role of Stem Cells and Innovative Biomaterials. Int. J. Mol. Sci. 2023, 24, 7936. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, S.; Yu, Q.; Chen, T.; Liu, D. Application of stem cells in regeneration medicine. MedComm. 2023, 4, e291. [Google Scholar] [CrossRef]
- Mahheidari, N.; Kamalabadi-Farahani, M.; Nourani, M.R. Biological study of skin wound treated with Alginate/Carboxymethyl cellulose/chorion membrane, diopside nanoparticles, and Botox A. npj Regen. Med. 2024, 9, 9. [Google Scholar] [CrossRef]
- Nii, T.; Katayama, Y. Biomaterial-Assisted Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8657. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, L.; Zhao, H.; Li, Z.; Chen, J.; Cen, Y.; Zhang, Z. The Therapeutic Role of ADSC-EVs in Skin Regeneration. Front. Med. 2022, 9, 858824. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ntege, E.H.; Sunami, H. Current regenerative medicine-based approaches for skin regeneration: A review of literature and a report on clinical applications in Japan. Regen. Ther. 2022, 21, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Goldie, K. The evolving field of regenerative aesthetics. J. Cosmet. Dermatol. 2023, 22 (Suppl. 1), 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khunger, N. Regenerative medicine in aesthetic surgery: Hope or hype? J Cutan Aesthet Surg. 2014, 7, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Ta, H.T. Investigating the Effect of Biomaterials Such as Poly-(l-Lactic Acid) Particles on Collagen Synthesis In Vitro: Method Is Matter. J. Funct. Biomater. 2020, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Khan, N.R.; Basit, H.M.; Mahmood, S. Physico-chemical based mechanistic insight into surfactant modulated sodium carboxymethylcellulose film for skin tissue regeneration applications. J. Polym. Res. 2019, 27, 20. [Google Scholar] [CrossRef]
- Vleggaar, D.; Fitzgerald, R.; Lorenc, Z.P. Composition and mechanism of action of poly-L-lactic acid in soft tissue augmentation. J. Drugs Dermatol. 2014, 13 (Suppl. 4), S29–S31. [Google Scholar]
- Nowag, B.; Schäfer, D.; Hengl, T.; Corduff, N.; Goldie, K. Biostimulating fillers and induction of inflammatory pathways: A preclinical investigation of macrophage response to calcium hydroxylapatite and poly-L lactic acid. J. Cosmet. Dermatol. 2024, 23, 99–106. [Google Scholar] [CrossRef]
- Aguilera, S.B.; McCarthy, A.; Khalifian, S.; Lorenc, Z.P.; Goldie, K.; Chernoff, W.G. The Role of Calcium Hydroxylapatite (Radiesse) as a Regenerative Aesthetic Treatment: A Narrative Review. Aesthet. Surg. J. 2023, 43, 1063–1090. [Google Scholar] [CrossRef]
- Funt, D.K. Treatment of Delayed-onset Inflammatory Reactions to Hyaluronic Acid Filler: An Algorithmic Approach. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4362. [Google Scholar] [CrossRef]
- Gonzaga da Cunha, M.; Engracia, M.; Gasques de Souza, L.; D’Apparecida Machado Filho, C. Biostimulators and their mechanisms of action. Surg. Cosm. Derm. 2020, 12, 109–117. [Google Scholar] [CrossRef]
- Muthu, S.; Bapat, A.; Jain, R.; Jeyaraman, N.; Jeyaraman, M. Exosomal therapy-a new frontier in regenerative medicine. Stem Cell Investig. 2021, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Shah, D.; Rai, D.; Parra, D.C.; Pathikonda, S.; Kurilova, S.; Cili, A. Therapeutic Values of Exosomes in Cosmetics, Skin Care, Tissue Regeneration, and Dermatological Diseases. Cosmetics 2023, 10, 65. [Google Scholar] [CrossRef]
- Olumesi, K.R.; Goldberg, D.J. A review of exosomes and their application in cutaneous medical aesthetics. J. Cosmet. Dermatol. 2023, 22, 2628–2634. [Google Scholar] [CrossRef]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef]
- Shir-az, O.; Berl, A.; Mann, D.; Bilal, B.S.; Levy, Y.; Shalom, A. Treatment of Scleroderma-Related Microstomia Using Hyaluronic Acid: An Interventional Study. Life 2023, 13, 2176. [Google Scholar] [CrossRef]
- Siquier-Dameto, G.; Solalinde, L. Positive Effect of Hybrid Stable Cooperative Complexes of High- and Low-Molecular-Weight Hyaluronic Acid in Psoriasis: A Clinical Series. Esperienze Dermatol. 2021, 22, 53–55. [Google Scholar] [CrossRef]
- Cassuto, D.; Yuri, V. Treatment of Scar Contracture with Intralesional Jet-Assisted Injection of Hyaluronic Acid. J. Dermatol. Res. Ther. 2020, 6, 94–98. [Google Scholar]
- Artzi, O.; Cohen, S.; Koren, A.; Niv, R.; Friedman, O. Dual-Plane Hyaluronic Acid Treatment for Atrophic Acne Scars. J. Cosmet. Dermatol. 2020, 19, 69–74. [Google Scholar] [CrossRef]
- Dastgheib, M.; Heidari, S.; Azizipour, A.; Kavyani, M.; Lajevardi, V.; Ehsani, A.H.; Teimourpour, A.; Daneshpazhooh, M.; Kashani, M.N.; Balighi, K. Investigating the impact of added Profhilo mesogel to subcision versus subcision monotherapy in treating acne scars; a single-blinded, split-face randomized trial. J. Cosmet. Dermatol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Akerman, L.; Mimouni, D.; Nosrati, A.; Hilewitz, D.; Solomon-Cohen, E. A Combination of Non-Ablative Laser and Hyaluronic Acid Injectable for Postacne Scars: A Novel Treatment Protocol. J. Clin. Aesthet. Dermatol. 2022, 15, 53. [Google Scholar]
- Mohammed, G.F.; Al-Dhubaibi, M.S. Triple Steps Acne Scar Revision Technique: A New Combination Therapeutic Modality for Atrophic Acne Scars. J. Cosmet. Dermatol. 2022, 21, 4659–4668. [Google Scholar] [CrossRef]
- Tedesco, M.; Garelli, V.; Elia, F.; Sperati, F.; Biondi, F.; Mosiello, L.; Morrone, A.; Migliano, E. Efficacy of Injecting Hybrid Cooperative Complexes of Hyaluronic Acid for the Treatment of Vulvar Lichen Sclerosus: A Preliminary Study. J. Cosmet. Dermatol. 2023, 22, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, M.; Alei, L.; Bonadies, A.; Pallara, T.; Parisi, P.; Latini, A.; Bellei, B.; Sperati, F.; Migliano, E. Hybrid cooperative complexes to decrease VAS score and enhance sexual function in women with vulvar lichen sclerosus. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 814–821. [Google Scholar] [PubMed]
- Garavaglia, E.; Sala, C.; Busato, M.; Bellia, G.; Tamburlin, N.; Massirone, A. First Use of Thermal Stabilized Hyaluronic Acid Injection in One-Year Follow-Up Patients with Genitourinary Syndrome. Med. Devices 2020, 13, 399–410. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, A.; La Gatta, A.; Stellavato, A.; Cimini, D.; Corsuto, L.; Cammarota, M.; D’Agostino, M.; Schiraldi, C. Potential of Biofermentative Unsulfated Chondroitin and Hyaluronic Acid in Dermal Repair. Int. J. Mol. Sci. 2022, 23, 1686. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Sig Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, V.; Di Meo, C.; Toro, G.; Alfano, A.; Iolascon, G.; Schiraldi, C. Hyaluronic Acid-Based Injective Medical Devices: In Vitro Characterization of Novel Formulations Containing Biofermentative Unsulfated Chondroitin or Extractive Sulfated One with Cyclodextrins. Pharmaceuticals 2023, 16, 1429. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Park, S.J.; Santosh, S.S.; Ganeshalingam, A.; Thiripuranathar, G.; Sathiyaseelan, A.; Vijayasarathy, S.; Swaminathan, A.; Priya, V.V.; Wang, M.H. Application of Hyaluronic Acid in Tissue Engineering, Regenerative Medicine, and Nanomedicine: A Review. Int. J. Biol. Macromol. 2022, 222 Pt B, 2744–2760. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Valachová, K.; Šoltés, L. Hyaluronan as a Prominent Biomolecule with Numerous Applications in Medicine. Int. J. Mol. Sci. 2021, 22, 7077. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).