Autoimmune Thyroiditis and Vitamin D
Abstract
1. Introduction
2. Immunomodulatory Function of Vitamin D
2.1. Vitamin D and Innate Immunity
2.2. Vitamin D and Adaptive Immunity
3. Hashimoto’s Thyroiditis
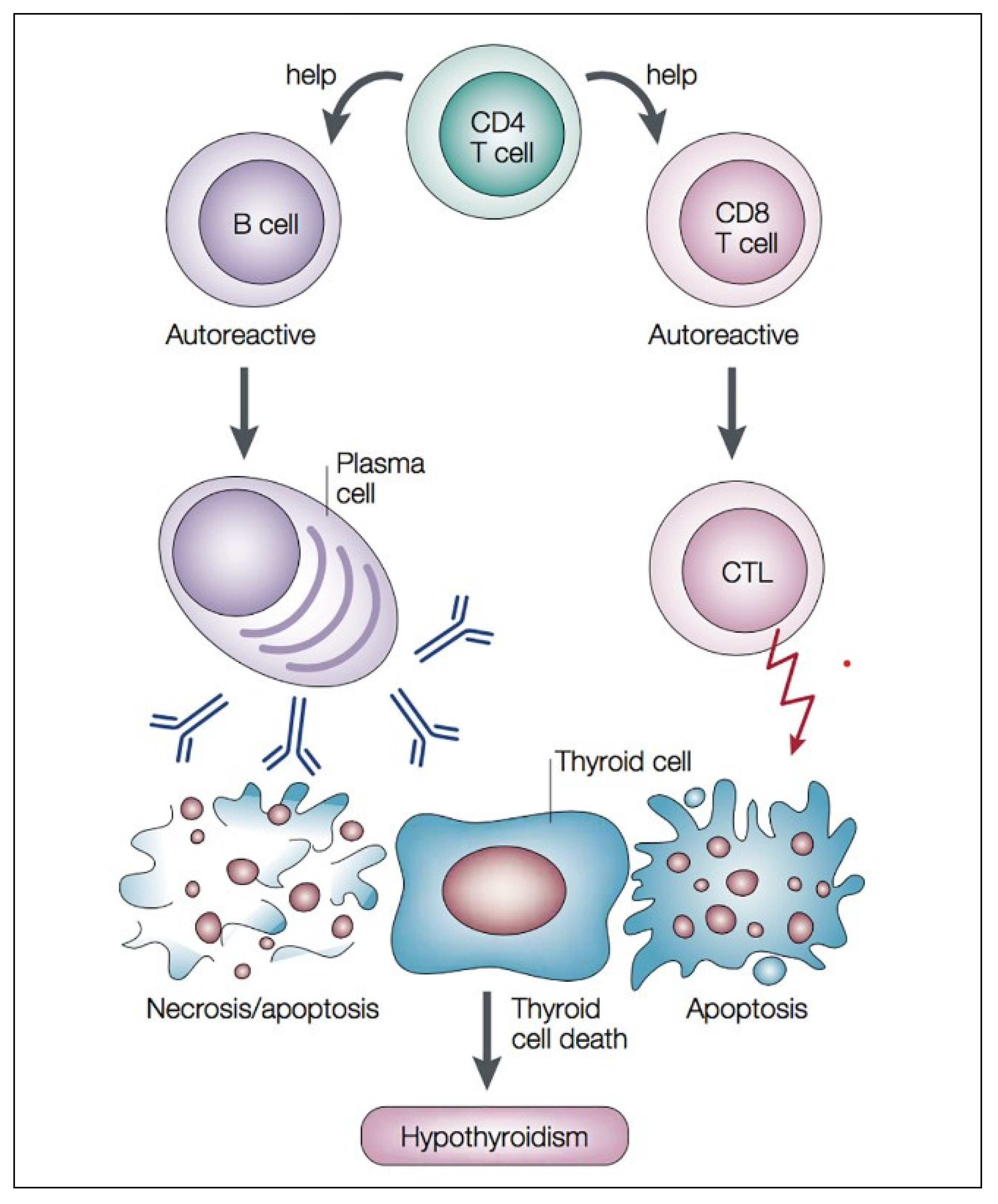
4. Autoimmune Mechanism of Autoimmune Hypothyroidism
5. Immunomodulatory Role of Vitamin D in Hashimoto’s Thyroiditis
- (a)
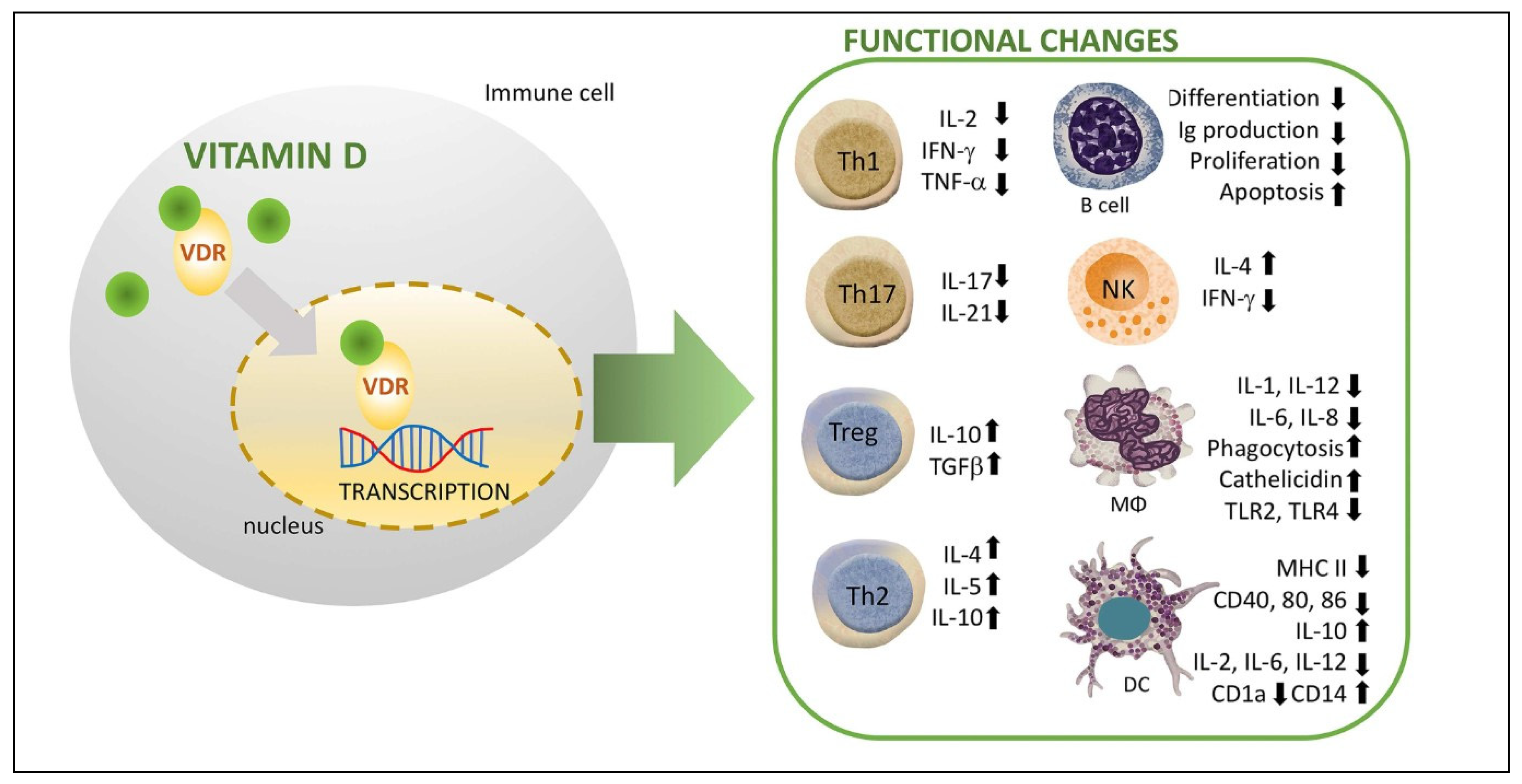
- Vitamin D inhibits the expression of various proinflammatory cytokines from DCs (IL-2, IL-6, and IL-12) that activate T cells while enhancing the expression of IL-10 (anti-inflammatory or tolerogenic cytokine); this results in a stage of insufficient immune responsiveness and, in this way, it helps avoid excessive innate responses and consequent tissue damage (systemic inflammation and/or septic shock). Additionally, vitamin D impairs DCs differentiation and maturation as evidenced by a decreased expression of MHC-II and co-stimulatory molecules (CD40, CD80, and CD86); this preservation of the immature phenotype of DCs results in a reduction in the number of antigen-presenting cells and activation of naïve T cells, thus contributing to an induction of a tolerogenic state. Vitamin D also modulates the activation and differentiation of naïve CD4+ lymphocytes after the presentation of the antigen by the DCs in the lymph nodes, resulting in a shift from a T-helper (Th)1 to a Th2 phenotype, which is an inhibition of inflammatory cytokine production (IL-2, IFN-γ, and TFN-α), and an increased production of anti-inflammatory cytokines (IL-4, IL-5, and IL-10).
- (b)
- Vitamin D may reduce MHC-II expression in the follicular thyroid cells, thus preventing T cell activation and proinflammatory cytokine response.
- (c)
- Vitamin D affects the differentiation of naïve T cells towards the Th17 phenotype, leading to a decrease in the production of inflammatory cytokines such as IL-17 (linked to organ-specific autoimmunity, inflammation, and tissue damage), and facilitates the induction of T regulatory cells (Tregs) with increased production of anti-inflammatory cytokines such as IL-10 and TGF-. Treg cells are able to suppress the proliferation and production of inflammatory cytokines by CD4+ T cells as well as the proliferation of CD8+ (cytotoxic lymphocytes) and APCs. Therefore, vitamin D contributes to the restoration of the Th17/Treg ratio (Th17 cells mainly express proinflammatory activity, which secondarily causes the development of autoimmune disorders; Tregs modulate the immune system and maintain tolerance to self-antigens, which in turn prevents autoimmunity). In this way, vitamin D would modulate cell-mediated immune responses and regulate the inflammatory activity of T cells and, consequently, have a significant role in preventing exaggerated or autoimmune responses.
- (d)
- Finally, with regard to B-lymphocyte regulation, vitamin D has an impact on B cell homeostasis in several ways. For example, it reduces naïve B cell activation and proliferation, induces apoptosis of activated B cells as well as suppresses the differentiation of B cells into plasma cells. In addition, vitamin D also inhibits memory B cell generation and reduces immunoglobulin synthesis (IgG and IgM). This control on B cell activation and proliferation may be clinically important in HT, as B cells producing autoreactive antibodies play a major role in the pathophysiology of autoimmunity.
6. Relationship between Vitamin D Status and Hashimoto’s Thyroiditis
7. Vitamin D Supplementation in Hashimoto’s Thyroiditis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DCs | dendritic cells |
| CTL | cytotoxic T lymphocytes |
| HT | Hashimoto’s thyroiditis |
| IFN-γ | interferon-gamma |
| IL | interleukin |
| Mφ | macrophage |
| MHC-II | major histocompatibility complex class II molecules |
| NK | natural killer cells |
| TG | tiroglobulin |
| TGAb | anti-thyroglobulin antibodies. |
| TGF-β | transforming growth factor beta |
| Th1 | CD4+ type 1 T helper |
| Th2 | CD4+ type 2 T helper |
| Th17 | CD4+ type 17 T helper |
| TLR | Toll-like receptors |
| TNF-α | tumor necrosis factor alfa |
| TPO | thyroid peroxidasa |
| TPOAb | anti-thyroid peroxidase antibodies |
| Treg | CD4+ T regulatory cells |
| TSH | thyrotrophin |
| VDR | vitamin D receptors |
References
- Bellan, M.; Andreoli, L.; Mele, C.; Sainaghi, P.P.; Rigamonti, C.; Piantoni, S.; De Benedittis, C.; Aimaretti, G.; Pirisi, M.; Marzullo, P. Pathophysiological Role and Therapeutic Implications of Vitamin D in Autoimmunity: Focus on Chronic Autoimmune Diseases. Nutrients 2020, 12, 789. [Google Scholar] [CrossRef]
- Ralli, M.; Angeletti, D.; Fiore, M.; D’Aguanno, V.; Lambiase, A.; Artico, M.; de Vincentiis, M.; Greco, A. Hashimoto’s Thyroiditis: An Update on Pathogenic Mechanisms, Diagnostic Protocols, Therapeutic Strategies, and Potential Malignant Transformation. Autoimmun. Rev. 2020, 19, 102649. [Google Scholar] [CrossRef]
- Hossein-Nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]
- Palermo, N.E.; Holick, M.F. Vitamin D, bone health, and other health benefits in pediatric patients. J. Pediatr. Rehabil. Med. 2014, 7, 179–192. [Google Scholar] [CrossRef]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef]
- Cyprian, F.; Lefkou, E.; Varoudi, K.; Girardi, G. Immunomodulatory Effects of Vitamin D in Pregnancy and Beyond. Front. Immunol. 2019, 10, 2739. [Google Scholar] [CrossRef] [PubMed]
- Mele, C.; Caputo, M.; Bisceglia, A.; Sama, M.T.; Zavattaro, M.; Aimaretti, G.; Pagano, L.; Prodam, F.; Marzullo, P. Immunomodulatory Effects of Vitamin D in Thyroid Diseases. Nutrients 2020, 12, 1444. [Google Scholar] [CrossRef] [PubMed]
- Boguslawska, J.; Godlewska, M.; Gajda, E.; Piekielko-Witkowska, A. Cellular and molecular basis of thyroid autoimmunity. Eur. Thyroid. J. 2022, 11, e210024. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Altieri, B.; Muscogiuri, G.; ¿Barrea, L.; Mathieu, C.; Vallone, C.V.; Mascitelli, L.; Bizzaro, G.; Altieri, V.M.; Tirabassi, G.; Balercia, G.; et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev. Endocr. Metab. Disord. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, X.; Qian, X.; Shao, S. Effects of vitamin D treatment on thyroid function and autoimmunity markers in patients with Hashimoto’s thyroiditis-A meta-analysis of randomized controlled trials. J. Clin. Pharm. Ther. 2022, 47, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Gallo, D.; Baci, D.; Kustrimovic, N.; Lanzo, N.; Patera, B.; Tanda, M.L.; Piantanida, E.; Mortara, L. How Does Vitamin D Affect Immune Cells Crosstalk in Autoimmune Diseases? Int. J. Mol. Sci. 2023, 24, 4689. [Google Scholar] [CrossRef] [PubMed]
- Rosen, Y.; Daich, J.; Soliman, I.; Brathwaite, E.; Shoenfeld, Y. Vitamin D and autoimmunity. Scand. J. Rheumatol. 2016, 45, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Song, E.; Oh, H.S.; Park, S.; Kwon, H.; Jeon, M.J.; Kim, W.B.; Shong, Y.K.; Kim, T.Y. Vitamin D deficiency affects thyroid autoimmunity and dysfunction in iodine-replete area: Korea national health and nutrition examination survey. Endocrine 2017, 58, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.H.; Rodrigues, D.; Paiva, I. Vitamin D and Autoimmune Thyroid Disease-Cause, Consequence, or a Vicious Cycle? Nutrients 2020, 12, 2791. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhang, W.; Ma, C.; Zhao, Y.; Xiong, R.; Wang, H.; Chen, W.; Zheng, S.G. Immunomodulatory Function of Vitamin D and Its Role in Autoimmune Thyroid Disease. Front. Immunol. 2021, 12, 574967. [Google Scholar] [CrossRef]
- Lebiedzinski, F.; Lisowska, K.A. Impact of Vitamin D on Immunopathology of Hashimoto’s Thyroiditis: From Theory to Practice. Nutrients 2023, 15, 3174. [Google Scholar] [CrossRef]
- Weetman, A.P. An update on the pathogenesis of Hashimoto’s thyroiditis. J. Endocrinol. Investig. 2021, 44, 883–890. [Google Scholar] [CrossRef]
- Babic Leko, M.; Jureško, I.; Rozic, I.; Pleic, N.; Gunjača, I.; Zemunik, T. Vitamin D and the Thyroid: A Critical Review of the Current Evidence. Int. J. Mol. Sci. 2023, 24, 3586. [Google Scholar] [CrossRef]
- Czarnywojtek, A.; Florek, E.; Pietronczyk, K.; Sawicka-Gutaj, N.; Ruchała, M.; Ronen, O.; Nixon, I.J.; Shaha, A.R.; Rodrigo, J.P.; Tufano, R.P.; et al. The Role of Vitamin D in utoimmune Thyroid Diseases: A Narrative Review. J. Clin. Med. 2023, 12, 1452. [Google Scholar] [CrossRef]
- Pyzik, A.; Grywalska, E.; Matyjaszek-Matuszek, B.; RoliNski, J. Immune disorders in Hashimoto’s thyroiditis: What do we know so far? J. Immunol. Res. 2015, 2015, 979167. [Google Scholar] [CrossRef] [PubMed]
- Luty, J.; Ruckemann-Dziurdzinska, K.; Witkowski, J.M.; Bryl, E. Immunological Aspects of Autoimmune Thyroid Disease—Complex Interplay between Cells and Cytokines. Cytokine 2019, 116, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Koehler, V.F.; Filmann, N.; Mann, W.A. Vitamin D Status and Thyroid Autoantibodies in Autoimmune Thyroiditis. Horm. Metab. Res. 2019, 51, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Kivity, S.; Agmon-Levin, N.; Zisappl, M.; Shapira, Y.; Nagy, E.V.; Dankó, K.; Szekanecz, Z.; Langevitz, P.; Shoenfeld, Y. Vitamin D and autoimmune thyroid diseases. Cell Mol. Immunol. 2011, 8, 243–247. [Google Scholar] [CrossRef]
- Tamer, G.; Arik, S.; Tamer, I.; Coksert, D. Relative vitamin D insufficiency in Hashimoto’s thyroiditis. Thyroid 2011, 21, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, N.C.; Karbek, B.; Ucan, B.; Sahin, M.; Cakal, E.; Ozbek, M.; Delibasi, T. The association between severity of vitamin D deficiency and Hashimoto’s thyroiditis. Endocr. Pract. 2013, 19, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Mansournia, N.; Mansournia, M.A.; Saeedi, S.; Dehghan, J. The association between serum 25OHD levels and hypothyroid Hashimoto’s thyroiditis. J. Endocrinol. Investig. 2014, 37, 473–476. [Google Scholar] [CrossRef]
- Unal, A.D.; Tarcin, O.; Parildar, H.; Cigerli, O.; Eroglu, H.; Demirag, N.G. Vitamin D deficiency is related to thyroid antibodies in autoimmune thyroiditis. Cent. Eur. J. Immunol. 2014, 39, 493–497. [Google Scholar] [CrossRef]
- Mazokopakis, E.E.; Papadomanolaki, M.G.; Tsekouras, K.C.; Evangelopoulos, A.D.; Kotsiris, D.A.; Tzortzinis, A.A. Is vitamin D related to pathogenesis and treatment of Hashimoto’s thyroiditis? Hell. J. Nucl. Med. 2015, 18, 222–227. [Google Scholar]
- Kim, D. Low vitamin D status is associated with hypothyroid Hashimoto’s thyroiditis. Hormones 2016, 15, 385–393. [Google Scholar] [CrossRef]
- Giovinazzo, S.; Vicchio, T.M.; Certo, R.; Alibrandi, A.; Palmieri, O.; Campenni, A.; Cannavò, S.; Trimarchi, F.; Ruggeri, R.M. Vitamin D receptor gene polymorphisms/haplotypes and serum 25(OH)D3 levels in Hashimoto’s thyroiditis. Endocrine 2016, 55, 599–606. [Google Scholar] [CrossRef] [PubMed]
- De Pergola, G.; Triggiani, V.; Bartolomeo, N.; Giagulli, V.A.; Anelli, M.; Masiello, M.; Candita, V.; De Bellis, D.; Silvestris, F. Low 25 Hydroxyvitamin D Levels are Independently Associated with Autoimmune Thyroiditis in a Cohort of Apparently Healthy Overweight and Obese Subjects. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Szkróbka, W.; Okopien, B. The effect of gluten-free diet on thyroid autoimmunity in drug-naïve women with Hashimoto’s thyroiditis: A pilot study. Exp. Clin. Endocrinol. Diabetes 2019, 127, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, H.S. Vitamin B12 and Vitamin D Levels in Patients with Autoimmune Hypothyroidism and Their Correlation with Anti-Thyroid Peroxidase Antibodies. Med. Princ. Pract. 2020, 29, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Appunni, S.; Rubens, M.; Ramamoorthy, V.; Saxena, A.; Tonse, R.; Veledar, E.; McGranaghan, P. Association between vitamin D deficiency and hypothyroidism: Results from the National Health and Nutrition Examination Survey (NHANES) 2007–2012. BMC Endocr. Disord. 2021, 21, 224. [Google Scholar] [CrossRef] [PubMed]
- Zaidman, V.; Maceiras, M.; Lazzati, J.; Kutasz, E.; D’Isa, G.; Chilleli, C.; Tau, C.; Viterbo, G.; Rivarola, M.A.; Belgorosky, A. High prevalence of anti-thyroid antibodies associated with a low vitamin D status in a pediatric cohort. Clin. Chem. Lab. Med. 2014, 52, e119–e122. [Google Scholar] [CrossRef]
- Camurdan, O.M.; Döger, E.; Bideci, A.; Celik, N.; Cinaz, P. Vitamin D status in children with Hashimoto thyroiditis. J. Pediatr. Endocrinol. Metab. 2012, 25, 467–470. [Google Scholar] [CrossRef]
- Evliyaoglu, O.; Acar, M.; Özcabı, B.; Erginöz, E.; Bucak, F.; Ercan, O.; Kucur, M. Vitamin D deficiency and Hashimoto’s thyroiditis in children and adolescents: A critical vitamin D level for this association? J. Clin. Res. Pediatr. Endocrinol. 2015, 7, 128–133. [Google Scholar] [CrossRef]
- Metwalley, K.A.; Farghaly, H.S.; Sherief, T.; Hussein, A. Vitamin D status in children and adolescents with autoimmune thyroiditis. J. Endocrinol. Investig. 2016, 39, 793–797. [Google Scholar] [CrossRef]
- Sönmezgöz, E.; Ozer, S.; Yilmaz, R.; Önder, Y.; Bütün, I.; Bilge, S. Hypovitaminosis D in children with Hashimoto’s thyroiditis. Rev. Med. Chil. 2016, 144, 611–616. [Google Scholar] [CrossRef]
- D’Aurizio, F.; Villalta, D.; Metus, P.; Doretto, P.; Tozzoli, R. Is vitamin D a player or not in the pathophysiology of autoimmune thyroid diseases? Autoimmun. Rev. 2015, 14, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Sun, T.; Zhang, Y.; He, L.; Wu, Q.; Liu, J.; Zha, B. 25-Hydroxyvitamin D serum level in Hashimoto’s thyroiditis, but not Graves’ disease is relatively deficient. Endocr. J. 2017, 64, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Yasmeh, J.; Farpour, F.; Rizzo, V.; Kheradnam, S.; Sachmechi, I. Hashimoto thyroiditis not associated with vitamin D deficiency. Endocr. Pract. 2016, 22, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Cvek, M.; Kalicanin, D.; Baric, A.; Vuletic, M.; Gunjac, I.; Torlak Lovric, V.; Škrabić, V.; Punda, A.; Perica, V.B. Vitamin D and Hashimoto’s Thyroiditis: Observations from CROHT Biobank. Nutrients 2021, 13, 2793. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, S.; Chen, G.; Gao, C.; He, J.; Zhong, H.; Xu, Y. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients 2015, 7, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Štefanic, M.; Tokic, S. Serum 25-hydoxyvitamin D concentrations in relation to Hashimoto’s thyroiditis: A systematic review, meta-analysis and meta-regression of observational studies. Eur. J. Nutr. 2020, 59, 859–872. [Google Scholar] [CrossRef]
- Taheriniya, S.; Arab, A.; Hadi, A.; Fadel, A.; Askari, G. Vitamin D and thyroid disorders: A systematic review and Meta-analysis of observational studies. BMC Endocr. Disord. 2021, 21, 171. [Google Scholar] [CrossRef]
- Khozam, S.A.; Sumaili, A.M.; Alflan, M.A.; Shawabketh, R.A.S. Association Between Vitamin D Deficiency and Autoimmune Thyroid Disorder: A Systematic Review. Cureus 2022, 14, e25869. [Google Scholar] [CrossRef]
- Ashok, T.; Palyam, V.; Azam, A.T.; Odeynka, O.; Alhashimi, R.; Thoota, S.; Sange, I. Relationship Between Vitamin D and Thyroid: An Enigma. Cureus 2022, 14, e21069. [Google Scholar] [CrossRef]
- Chailurkit, L.O.; Aekplakorn, W.; Ongphiphadhanakul, B. High Vitamin D Status in Younger Individuals Is Associated with Low Circulating Thyrotropin. Thyroid. 2013, 23, 25–30. [Google Scholar] [CrossRef]
- Barchetta, I.; Baroni, M.; Leonetti, F.; De Bernardinis, M.; Bertoccini, L.; Fontana, M.; Mazzei, E.; Fraioli, A.; Cavallo, M.G. TSH levels are associated with vitamin D status and seasonality in an adult population of euthyroid adults. Clin. Exp. Med. 2015, 15, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lin, H.; Wang, M. Immune intervention effects on the induction of experimental autoimmune thyroiditis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2002, 22, 343–345. [Google Scholar] [CrossRef]
- Liu, S.; Xiong, F.; Liu, E.M.; Zhu, M.; Lei, P.Y. Effects of 1,25-dihydroxyvitamin D3 in rats with experimental autoimmune thyroiditis. J. South. Med. Univ. 2010, 30, 1573–1576. [Google Scholar]
- Krysiak, R.; Szkrobka, W.; Okopien, B. The Effect of Vitamin D on Thyroid Autoimmunity in Levothyroxine-Treated Women with Hashimoto’s Thyroiditis and Normal Vitamin D Status. Exp. Clin. Endocrinol. Diabetes 2017, 125, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Dutta, D.; Kumar, M.; Saha, S.; Mondal, S.A.; Kumar, A.; Mukhopadhyay, S. Vitamin D Supplementation Reduces Thyroid Peroxidase Antibody Levels in Patients with Autoimmune Thyroid Disease: An Open-Labeled Randomized Controlled Trial. Indian. J. Endocrinol. Metab. 2016, 20, 391. [Google Scholar]
- Bhakat, B.; Pal, J.; Das, S.; Charaborty, S.K. A Prospective Study to Evaluate the Possible Role of Cholecalciferol Supplementation on Autoimmunity in Hashimoto’s Thyroiditis. Assoc. Physicians India 2023, 71, 1. [Google Scholar]
- Simsek, Y.; Cakır, I.; Yetmis, M.; Dizdar, O.S.; Baspinar, O.; Gokay, F. Effects of Vitamin D treatment on thyroid autoimmunity. J. Res. Med. Sci. 2016, 21, 85. [Google Scholar]
- Chahardoli, R.; Saboor-Yaraghi, A.A.; Amouzegar, A.; Khalili, D.; Vakili, A.Z.; Azizi, F. Can Supplementation with Vitamin D Modify Thyroid Autoantibodies (Anti-TPO Ab, Anti-Tg Ab) and Thyroid Profile (T3, T4, ¿TSH) in Hashimoto’s Thyroiditis? A Double Blind, Randomized Clinical Trial. Horm. Metab. Res. 2019, 51, 296–301. [Google Scholar] [CrossRef]
- Robat-Jazi, B.; Mobini, S.; Chahardoli, R.; Mansouri, F.; Nodehi, M.; Esfahanian, F.; Yaraghi, A.A.S. The Impact of Vitamin D Supplementation on the IFNγ-IP10 Axis in Women with Hashimoto’s Thyroiditis Treated with Levothyroxine: A Double-blind Randomized Placebo-controlled Trial. Iran. J. Allergy Asthma Immunol. 2022, 21, 407–417. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Okopień, B. The impact of vitamin D on thyroid autoimmunity and hypothalamic-pituitary-thyroid axis activity in myo-inositol-treated and myo-inositol-naïve women with autoimmune thyroiditis: A pilot study. J. Clin. Pharm. Ther. 2022, 47, 1759–1767. [Google Scholar] [CrossRef]
- Mirhosseini, N.; Brunel, L.; Muscogiuri, G.; Kimball, S. Physiological Serum 25-Hydroxyvitamin D Concentrations Are Associated with Improved Thyroid Function—Observations from a Community-Based Program. Endocrine 2017, 58, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Nodehi, M.; Ajami, A.; Izad, M.; Asgarian Omran, H.; Chahardoli, R.; Amouzegar, A.; Yekaninejad, S.; Hemmatabadi, M.; Azizi, F.; Esfahanian, F.; et al. Effects of vitamin D supplements on frequency of CD4+ T-cell subsets in women with Hashimoto’s thyroiditis: A double-blind placebo-controlled study. Eur. J. Clin. Nutr. 2019, 73, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Vondra, K.; Stárka, L.; Hampl, R. Vitamin D and thyroid diseases. Physiol. Res. 2015, 64 (Suppl. S2), S95–S100. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, K.V.; Madar, A.A.; Brekke, M.; Meyer, H.E.; Eggemoen, A.R.; Mdala, I.; Lagerløv, P. Effect of Vitamin D on Thyroid Autoimmunity: A Randomized, Double-Blind, Controlled Trial Among Ethnic Minorities. J. Endocr. Soc. 2017, 1, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Vahabi Anaraki, P.; Aminorroaya, A.; Amini, M.; Momeni, F.; Feizi, A.; Iraj, B.; Tabatabaei, A. Effect of Vitamin D deficiency treatment on thyroid function and autoimmunity markers in Hashimoto’s thyroiditis: A double-blind randomized placebo-controlled clinical trial. J. Res. Med. Sci. 2017, 22, 103. [Google Scholar]
- Wang, S.; Wu, Y.; Zuo, Z.; Zhao, Y.; Wang, K. The effect of vitamin D supplementation on thyroid autoantibody levels in the treatment of autoimmune thyroiditis: A systematic review and a meta-analysis. Endocrine 2018, 59, 499–505. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Li, H.; Li, H. Effects of Vitamin D on Thyroid Autoimmunity Markers in Hashimoto’s Thyroiditis: Systematic Review and Meta-Analysis. J. Int. Med. Res. 2021, 49, 12. [Google Scholar] [CrossRef]
- Aghili, A.; Alijanpour Aghamaleki, M.; Pornasrollah, M.; Nooreddini, H.G.; Khafri, S.; Lijanpour, S. Effect of Vitamin DTherapy on Hashimoto’s Thyroiditis in Children with Hypovitaminosis, D. Int. J. Pediatr. 2020, 8, 10889–10897. [Google Scholar]
| Author, Year, and Country | Number of Participants (F/M) | Dose of Supplementation Duration |
|---|---|---|
| Mazokopakis et al., 2015 (Greece) [29] | 173 F/13 M | 1200–4000 IU/daily for 4 months |
| Chaudhary et al., 2016 (India) [55] | 39 F/11 M | 60,000 IU weekly, 8 weeks |
| Simsek et al., 2016 (Turkey) [57] | 37 F/9M | 1000 IU/daily for 1 month |
| Krysiak et al., 2017 (Poland) [54] | 34 F | 2000 IU daily, 6 months |
| Mirhosseini et al., 2017 (Canada) [61] | 103 | Doses modified to achieve calcidiol concentration >40 ng/mL, 12 months |
| Chahardoli et al., 2019 (Iran) [58] | 42 F | 50,000 IU weekly, 3 months |
| Krysiak et al., 2022 (Poland) [60] | 42 F | 4000 IU daily for 6 months |
| Bhakat et al., 2023 (India) [56] | 50 | 60,000 IU weekly for 8 weeks |
| Robat-Jazi et al., 2022 (Iran) [59] | 40 F | 50,000 IU weekly, 3 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durá-Travé, T.; Gallinas-Victoriano, F. Autoimmune Thyroiditis and Vitamin D. Int. J. Mol. Sci. 2024, 25, 3154. https://doi.org/10.3390/ijms25063154
Durá-Travé T, Gallinas-Victoriano F. Autoimmune Thyroiditis and Vitamin D. International Journal of Molecular Sciences. 2024; 25(6):3154. https://doi.org/10.3390/ijms25063154
Chicago/Turabian StyleDurá-Travé, Teodoro, and Fidel Gallinas-Victoriano. 2024. "Autoimmune Thyroiditis and Vitamin D" International Journal of Molecular Sciences 25, no. 6: 3154. https://doi.org/10.3390/ijms25063154
APA StyleDurá-Travé, T., & Gallinas-Victoriano, F. (2024). Autoimmune Thyroiditis and Vitamin D. International Journal of Molecular Sciences, 25(6), 3154. https://doi.org/10.3390/ijms25063154






