Downregulation of Serotonergic System Components in an Experimentally Induced Cryptorchidism in Rabbits
Abstract
1. Introduction
2. Results
2.1. Anatomical and Morphological Differences in Control and COI Testes
2.2. Determination of the Concentration Profiles of Both Serotonin and L-Kynurenine in Control and COI Testes
2.3. Expression of Transcript Genes Related to the Serotonin System
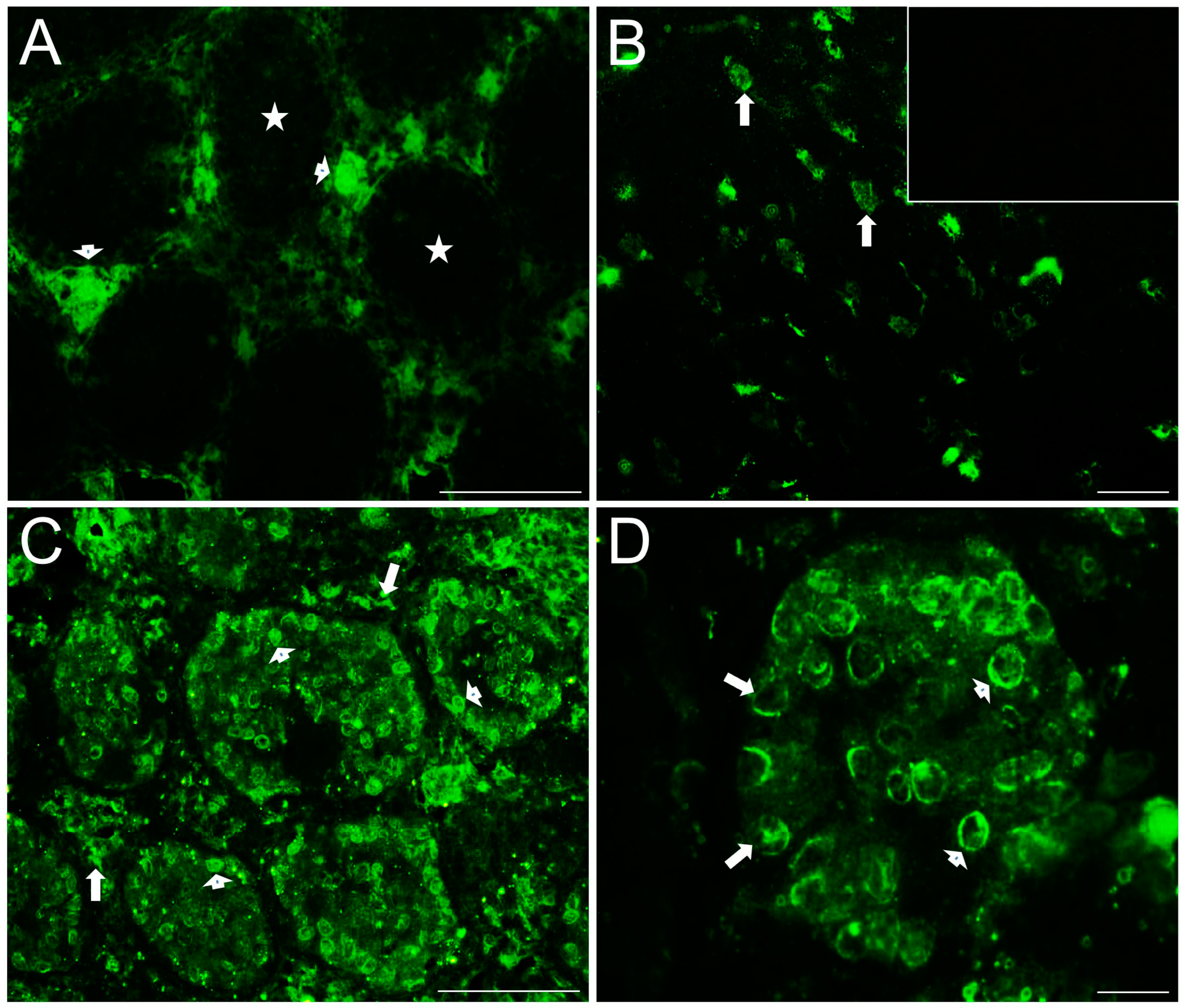
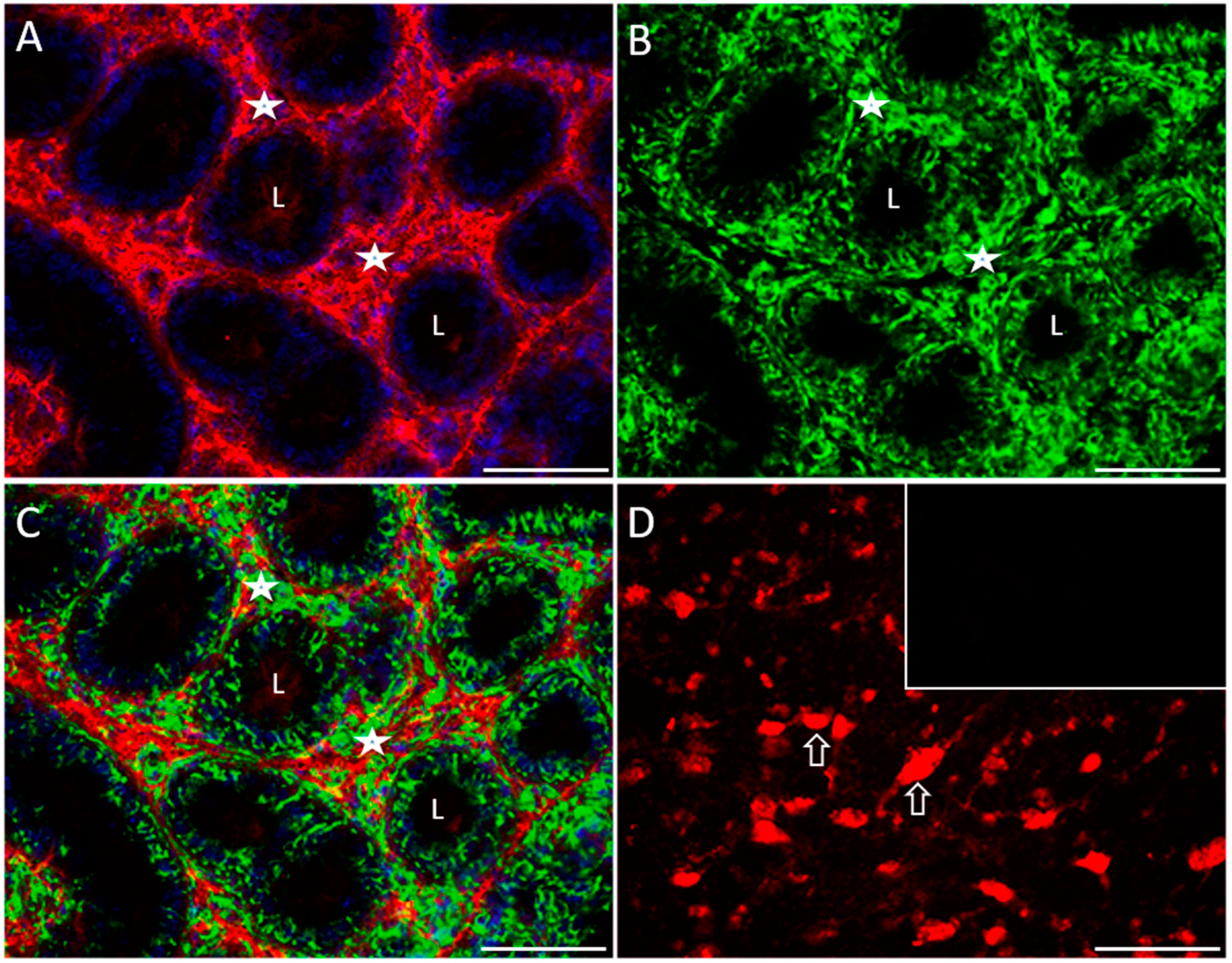
2.4. Distribution of Serotonergic System Elements in Control and COI Testes
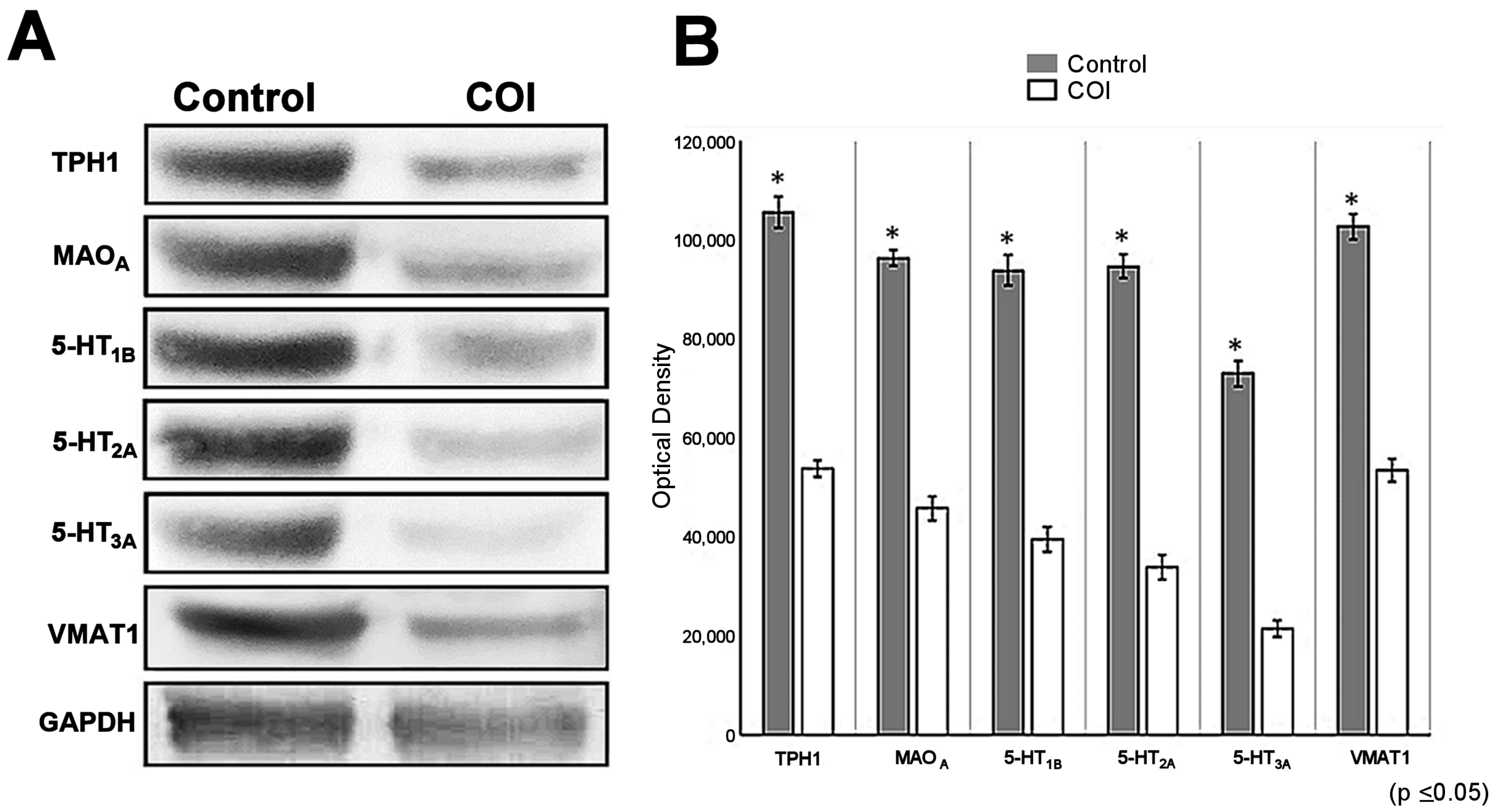
2.5. Downregulation of Proteins Measured with Immunoblotting
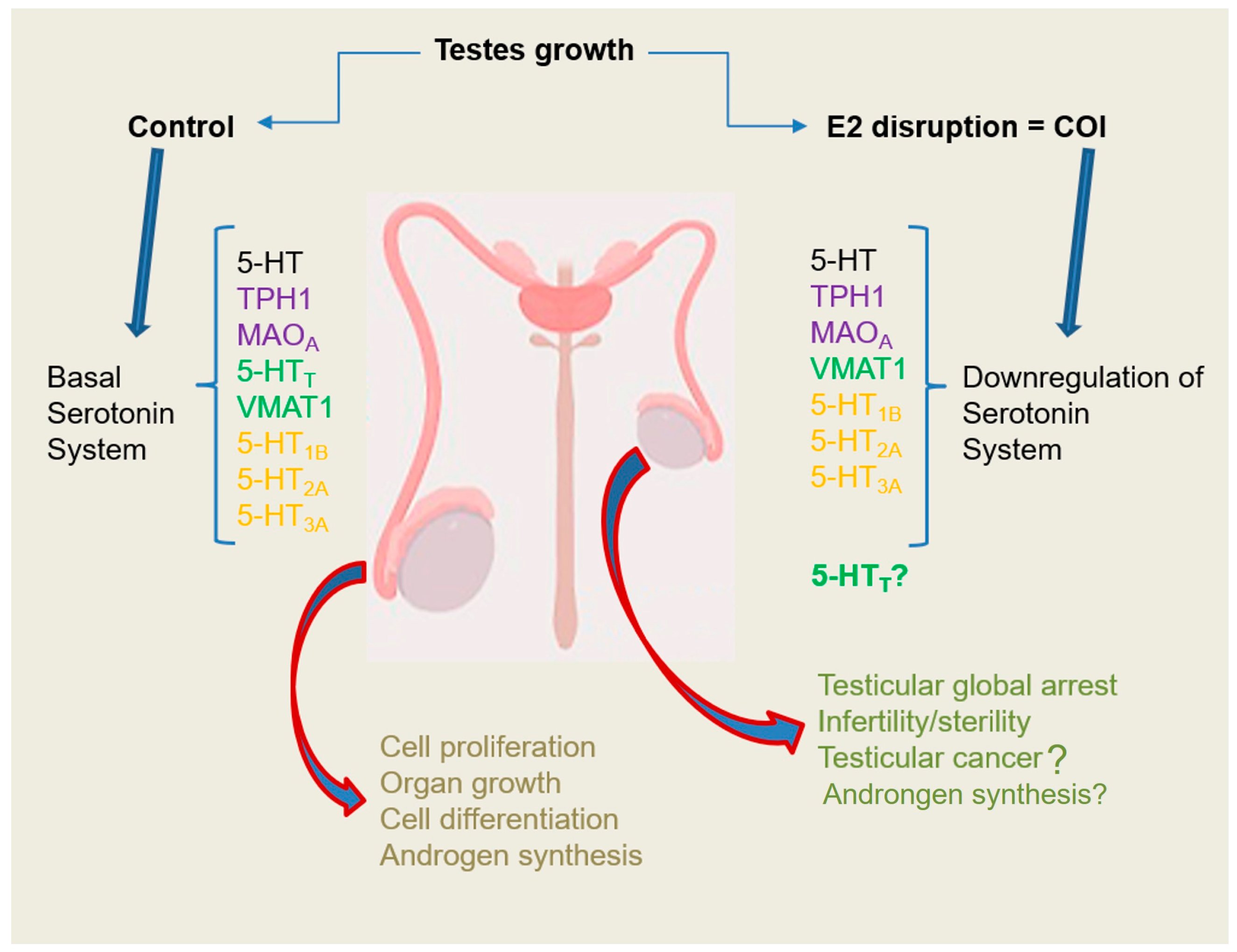
3. Discussion
4. Materials and Methods
4.1. Animals and E2 Treatment to Induce Cryptorchidism
4.2. Dissection, Collection and Preservation of Testes
4.3. Gonadosomatic Index (GSI) and Testes’ Volume (TV)
4.4. Quantitative Determination of Serotonin and L-Kynurenine in Control and COI Testes
4.5. RNA Extraction and RT-PCR
4.6. Quantitative RT-PCR
4.7. Transmission Electron Microscopy (TEM) Analysis
4.8. Immunofluorescence
4.9. Immunotransference by Western Blot
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lee, P.A.; Houk, C.P.; Ahmed, S.F.; Hughes, I.A. Consensus statement on management of intersex disorders. Pediatrics 2006, 118, e488–e500. [Google Scholar] [CrossRef]
- Skakkebæk, N.E.; Rajpert-De Meyts, E.; Main, K.M. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environmental aspects. Hum. Reprod. 2001, 16, 972–978. [Google Scholar] [CrossRef]
- Serrano, T.; Chevrier, C.; Multigner, L.; Cordier, S.; Jégou, B. International geographic correlation study of the prevalence of disorders of male reproductive health. Hum. Reprod. 2013, 28, 1974–1986. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Holley, A.M.; Crews, D. Mate choice, sexual selection, and endocrine-disrupting chemicals. Horm. Behav. 2018, 101, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Gurney, J.K.; McGlynn, K.A.; Stanley, J.; Merriman, T.; Signal, V.; Shaw, C.; Edwards, R.; Richiardi, L.; Hutson, J.; Sarfati, D. Risk factors for cryptorchidism. Nat. Rev. Urol. 2018, 14, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Hutson, J.M.; Southwell, B.R.; Li, R.; Lie, G.; Ismail, K.; Harisis, G.; Chen, N. The regulation of testicular descent and the effects of cryptorchidism. Endocr. Rev. 2013, 34, 725–752. [Google Scholar] [CrossRef] [PubMed]
- Hutson, J.M.; Li, R.; Southwell, B.R.; Petersen, B.L.; Throup, J.; Cortes, D. Germ cell development in the postnatal testis: The key to prevent malignancy in cryptorchidism? Front. Endocrinol. 2013, 3, 176. [Google Scholar] [CrossRef] [PubMed]
- Docampo, M.J.; Hadziselimovic, F. Molecular Pathology of Cryptorchidism-Induced Infertility. Sex. Dev. 2015, 9, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Abacı, A.; Çatlı, G.; Anık, A.; Böber, E. Epidemiology, classification, and management of undescended testes: Does medication have value in its treatment? J. Clin. Res. Pediatr. Endocrinol. 2013, 5, 65–72. [Google Scholar] [CrossRef]
- Cobellis, G.; Noviello, C.; Nino, F.; Romano, M.; Mariscoli, F.; Martino, A.; Parmeggiani, P.; Papparella, A. Spermatogenesis and Cryptorchidism. Front. Endocrinol. 2014, 5, 63. [Google Scholar] [CrossRef]
- Ferguson, L.; Agoulnik, A.I. Testicular cancer and cryptorchidism. Front. Endocrinol. 2013, 4, 32. [Google Scholar] [CrossRef]
- Nef, S.; Shipman, T.; Parada, L.F. A molecular basis for estrogen-induced cryptorchidism. Dev. Biol. 2000, 224, 354–361. [Google Scholar] [CrossRef][Green Version]
- United Kingdom Testicular Cancer Study Group (UK). Aetiology of testicular cancer: Association with congenital abnormalities, age at puberty, infertility, and exercise. BMJ 1994, 308, 1393–1399. [Google Scholar] [CrossRef]
- Tolszczuk, M.; Folléa, N.; Pelletier, G. Characterization and localization of β-adrenergic receptors in control and cryptorchidized rat testis by In vitro autoradiography. J. Androl. 1988, 9, 172–177. [Google Scholar] [CrossRef]
- Barthold, J.S.; Ivell, R. Perspective: A Neuro-Hormonal Systems Approach to Understanding the Complexity of Cryptorchidism Susceptibility. Front. Endocrinol. 2018, 9, 401. [Google Scholar] [CrossRef]
- Peng, J.; Shen, L.; Chen, J.; Cao, X.; Zhou, Y.; Weng, H.; Long, C.; Zhang, D.; Tu, S.; Zhang, Y.; et al. New discovery of cryptorchidism: Decreased retinoic acid in testicle. Saudi Pharm. J. 2016, 24, 279–285. [Google Scholar] [CrossRef]
- Berney, D.M.; Looijenga, L.H.J.; Idrees, M.; Oosterhuis, J.W.; Rajpert-De Meyts, E.; Ulbright, T.M.; Skakkebaek, N.E. Germ cell neoplasia in situ (GCNIS): Evolution of the current nomenclature for testicular pre-invasive germ cell malignancy. Histopathology 2016, 69, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.F.; Garcia-Hjarles, M.; Velasquez, G. Hyperprolactinaemia and hyperserotoninaemia: Their relationship to seminal quality. Andrologia 1992, 24, 95–100. [Google Scholar] [CrossRef]
- Sethi, S.; Chaturvedi, C.M. Temporal synergism of neurotransmitters (serotonin and dopamine) affects testicular development in mice. Zoology 2009, 112, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Monteiro Filho, W.O.; de Torres, S.M.; Amorim, M.J.; Andrade, A.J.; de Morais, R.N.; Tenorio, B.M.; da Silva Junior, V.A. Fluoxetine induces changes in the testicle and testosterone in adult male rats exposed via placenta and lactation. Syst. Biol. Reprod. Med. 2014, 60, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.C.; Dos Santos, A.H.; Silveira, K.M.; Kiss, A.C.; Mesquita, S.F.P.; Gerardin, D. Maternal treatment with fluoxetine promotes testicular alteration in male rat pups. Reprod. Fertil. Dev. 2015, 28, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Trejo, F.; Tapia-Rodríguez, M. Exploring the Frontiers of Serotoninomics in Male Reproduction: The Future Ahead. Single Cell Biol. 2015, 4, 3. [Google Scholar] [CrossRef]
- Jan, S.Z.; Vormer, T.L.; Jongejan, A.; Röling, M.D.; Silver, S.J.; de Rooij, D.G.; Hamer, G.; Repping, S.; van Pelt, A.M.M. Unraveling transcriptome dynamics in human spermatogenesis. Development 2017, 144, 3659–3673. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Iida, S.; Taguchi, J.; Miyajima, J.; Matsuo, M.; Tomiyasu, K.; Matsuoka, K.; Noda, S. Primary carcinoid of the testis associated with carcinoid syndrome. Int. J. Urol. 2001, 8, 522–524. [Google Scholar] [CrossRef]
- Son, H.Y.; Ra, S.W.; Jeong, J.O.; Koh, E.H.; Lee, H.I.; Koh, J.M.; Kim, W.B.; Park, J.Y.; Shong, Y.K.; Lee, K.U.; et al. Primary carcinoid tumor of the bilateral testis associated with carcinoid syndrome. Int. J. Urol. 2004, 11, 1041–1043. [Google Scholar] [CrossRef]
- Sarrouilhe, D.; Clarhaut, J.; Defamie, N.; Mesnil, M. Serotonin and cancer: What is the link? Curr. Mol. Med. 2015, 15, 62–77. [Google Scholar] [CrossRef]
- Sakamoto, H.; Saito, K.; Oohta, M.; Inoue, K.; Ogawa, Y.; Yoshida, H. Testicular volume measurement: Comparison of ultrasonography, orchidometry, and water displacement. Urology 2007, 69, 152–157. [Google Scholar] [CrossRef]
- Engmann-Hildorf, S. Clinical aspects of histological and hormonal parameters in boys with cryptorchidism. Acta Pathol. Microbiol. Scand. 2022, 130 (Suppl. 143), 1–56. [Google Scholar] [CrossRef]
- Dufau, M.L.; Tinajero, J.C.; Fabbri, A. Corticotropin-releasing factor: An antireproductive hormone of the testis. FASEB J. 1993, 7, 299–307. [Google Scholar] [CrossRef]
- Tinajero, J.C.; Fabbri, A.; Ciocca, D.R.; Dufau, M.L. Serotonin secretion from rat Leydig cells. Endocrinology 1993, 133, 3026–3029. [Google Scholar] [CrossRef]
- Aragón, M.A.; Ayala, M.E.; Marín, M.; Avilés, A.; Damián-Matsumura, P.; Domínguez, R. Serotoninergic system blockage in the prepubertal rat inhibits spermatogenesis development. Reproduction 2005, 129, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Trejo, F.; Tapia-Rodríguez, M.; Cerbón, M.; Kuhn, D.M.; Manjarrez-Gutiérrez, G.; Mendoza-Rodríguez, C.A.; Picazo, O. Evidence of 5-HT components in human sperm: Implications for protein tyrosine phosphorylation and the physiology of motility. Reproduction 2012, 144, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Frungieri, M.B.; Gonzalez-Calvar, S.I.; Rubio, M.; Ozu, M.; Lustig, L.; Calandra, R.S. Serotonin in golden hamster testes: Testicular levels, immunolocalization and role during sexual development and photoperiodic regression-recrudescence transition. Neuroendocrinology 1999, 69, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Méndez Palacios, N.; Escobar, M.E.; Mendoza, M.M.; Crispín, R.H.; Andrade, O.G.; Mélandez, J.H.; Martínez, A.A. Prepubertal male rats with high rates of germ-cell apoptosis present exacerbated rates of germ-cell apoptosis after serotonin depletion. Reprod. Fertil. Dev. 2016, 28, 806–814. [Google Scholar] [CrossRef]
- Shishkina, G.T.; Borodin, P.M. Involvement of brain serotonin in regulation of sexual maturity in male rats. Neurosci. Behav. Physiol. 1989, 19, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Shishkina, G.T.; Dygalo, N.N. Role of the serotoninergic system in the acceleration of sexual maturation in wild Norway rats selected for reduced aggressiveness toward humans. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2000, 125, 45–51. [Google Scholar] [CrossRef]
- Caldamone, A.A.; Al-Juburi, A.; Cockett, A.T. The varicocele: Elevated serotonin and infertility. J. Urol. 1980, 123, 683–685. [Google Scholar] [CrossRef]
- Shankar, G.S. Serotonin and Sexual Dysfunction. J. Autacoids Horm. 2015, 5, e129. [Google Scholar] [CrossRef]
- Bolat, M.S.; Akdeniz, E.; Saltik, F.; Sahinkaya, N.; Moral, C. Primary Carcinoid Tumor of the Testis. Case Report. Urol. Case Rep. 2015, 3, 152–154. [Google Scholar] [CrossRef][Green Version]
- Vicaut, E.; Laemmel, E.; Stücker, O. Impact of serotonin on tumour growth. Ann. Med. 2000, 32, 187–194. [Google Scholar] [CrossRef]
- Jungwirth, N.; Haeberle, L.; Schrott, K.M.; Wullich, B.; Krause, F.S. Serotonin used as prognostic marker of urological tumors. World J. Urol. 2008, 26, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Hutson, J.M.; Balic, A.; Nation, T.; Southwell, B. Cryptorchidism. Semin. Pediatr. Surg. 2010, 19, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Thorup, J.; McLachlan, R.; Cortes, D.; Nation, T.R.; Balic, A.; Southwell, B.R.; Hutson, J.M. What is new in cryptorchidism and hypospadias--a critical review on the testicular dysgenesis hypothesis. J. Pediatr. Surg. 2010, 45, 2074–2086. [Google Scholar] [CrossRef] [PubMed]
- Mamoulakis, C.; Antypas, S.; Sofras, F.; Takenaka, A.; Sofikitis, N. Testicular Descent. Hormones 2015, 14, 515–530. [Google Scholar] [CrossRef]
- Vigueras-Villaseñor, R.M.; Jiménez-Cabrera, T.; Chávez-Saldaña, M.; Jiménez Trejo, F.; Cuevas-Alpuche, O.; Rojas-Castañeda, J.C. Epigallocatechin-3-gallate protects the testis from damage generated by experimental cryptorchidism in rabbits. Histol. Histopathol. 2019, 34, 931–942. [Google Scholar] [CrossRef]
- Veeramachaneni, V.; Vandewoude, S. Interstitial cell tumour and germ cell tumour with carcinoma in situ in rabbit testes. Int. J. Androl. 1999, 22, 97–101. [Google Scholar] [CrossRef]
- Nef, S.; Parada, L.F. Cryptorchidism in mice mutant for Insl3. Nat. Genet. 1999, 22, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Schaad, O.; Descombes, P.; Chambon, P.; Vassalli, J.D.; Nef, S. Estrogen receptor alpha is a major contributor to estrogen-mediated fetal testis dysgenesis and cryptorchidism. Endocrinology 2007, 148, 5507–5519. [Google Scholar] [CrossRef]
- Bay, K.; Andersson, A.M. Human testicular insulin-like factor 3: In relation to development, reproductive hormones and andrological disorders. Int. J. Androl. 2011, 34, 97–109. [Google Scholar] [CrossRef]
- Drumond, A.L.; Meistrich, M.L.; Chiarini-Garcia, H. Spermatogonial morphology and kinetics during testis development in mice: A high-resolution light microscopy approach. Reproduction 2011, 142, 145–155. [Google Scholar] [CrossRef]
- Jiménez-Trejo, F.; Coronado-Mares, I.; Arriaga-Canon, C.; Herrera, L.A.; Roque-Rámirez, B.; Chávez-Saldaña, M.; Rojas-Castañeda, J.; Cerbón, M.; Vigueras-Villaseñor, R. Indolaminergic system in Adult Rat Testis: Evidence for a Local Serotonin System. Front. Neuroanat. 2021, 14, 570058. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Trejo, F.; Coronado-Mares, I.; Boeta, M.; González-Santoyo, I.; Vigueras-Villaseñor, R.; Tapia-Rodríguez, M. Identification of Serotoninergic System Components in Stallion Sperm. Histol. Histopathol. 2018, 33, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Tyce, G.M. Origin and metabolism of serotonin. J. Cardiovasc. Pharmacol. 1990, 16, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, P.; Cases, O.; Maroteaux, L. The developmental role of serotonin: News from mouse molecular genetics. Nature Rev. Neurosci. 2003, 4, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Daws, L.C. Unfaithful neurotransmitter transporters: Focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol. Ther. 2009, 121, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Barnes, N.M.; Ahern, G.P.; Becamel, C.; Bockaert, J.; Camilleri, M.; Chaumont-Dubel, S.; Claeysen, S.; Cunningham, K.A.; Fone, K.C.; Gershon, M.; et al. International Union of Basic Clinical Pharmacology, C.X. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function. Pharmacol. Rev. 2021, 73, 310–520. [Google Scholar] [CrossRef] [PubMed]
- Machida, T.; Iizuka, K.; Hirafuji, M. 5-hydroxytryptamine and its receptors in systemic vascular walls. Biol. Pharm. Bull. 2013, 36, 1416–1419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soslau, G. Cardiovascular serotonergic system: Evolution, receptors, transporter, and function. J. Exp. Zool. A Ecol. Integr. Physiol. 2022, 337, 115–127. [Google Scholar] [CrossRef]
- Mayerhofer, A. Human testicular peritubular cells: More than meets the eye. Reproduction 2013, 145, R107–R116. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, J.; Liu, B.; Jiang, Y.; Chen, W.; Li, J.; He, Q.; He, Z. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell Mol. Life Sci. 2019, 76, 2681–2695. [Google Scholar] [CrossRef]
- Barnes, N.M.; Hales, T.G.; Lummis, S.C.; Peters, J.A. The 5-HT3 receptor--the relationship between structure and function. Neuropharmacology 2009, 56, 273–284. [Google Scholar] [CrossRef]
- Korkhov, V.M.; Holy, M.; Freissmuth, M.; Sitte, H.H. The conserved glutamate (Glu136) in transmembrane domain 2 of the serotonin transporter is required for the conformational switch in the transport cycle. J. Biol. Chem. 2006, 281, 13439–13448. [Google Scholar] [CrossRef]
- Weihe, E.; Eiden, L.E. Chemical neuroanatomy of the vesicular amine transporters. FASEB J. 2000, 14, 2435–2449. [Google Scholar] [CrossRef]
- Edwards, R.H. The neurotransmitter cycle and quantal size. Neuron 2007, 55, 835–858. [Google Scholar] [CrossRef]
- Yaffe, D.; Forrest, L.R.; Schuldiner, S. The ins and outs of vesicular monoamine transporters. J. Gen. Physiol. 2018, 150, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Pieścikowska, I.; Lukaszyk, A.; Balcerek, M.; Filipiak, K.; Ludwiczak, H.; Butowska, W.; Wachoł, J.B. An implication to the role of testicular serotonergic innervation. An in vitro study on serotonin effects in the control of Leydig cell secretory function. Folia Morphol. 1996, 55, 414–416. [Google Scholar]
- Lombard-des Gouttes, M.N.; Falck, B.; Owman, C.H.; Rosengren, E.; Sjöberg, N.O.; Walles, B. On the question of content and distribution of amines in the rat testis during development. Endocrinology 1974, 95, 1746–1749. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.P.; Gilles, J.; Guillemin, G.J.; Brew, B.J. The Kynurenine Pathway in Stem Cell Biology. Int. J. Tryptophan Res. 2013, 6, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Jrad-Lamine, A.; Henry-Berger, J.; Gourbeyre, P.; Damon-Soubeyrand, C.; Lenoir, A.; Combaret, L.; Saez, F.; Kocer, A.; Tone, S.; Fuchs, D.; et al. Deficient Tryptophan Catabolism along the Kynurenine Pathway Reveals That the Epididymis Is in a Unique Tolerogenic State. J. Biol. Chem. 2011, 286, 8030–8042. [Google Scholar] [CrossRef] [PubMed]
- Routy, J.P.; Routy, B.; Graziani, G.M.; Mehraj, V. The Kynurenine Pathway Is a Double-Edged Sword in Immune-Privileged Sites and in Cancer: Implications for Immunotherapy. Int. J. Tryptophan Res. 2016, 9, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Vigueras-Villaseñor, R.M.; Montelongo-Solís, P.; Chávez-Saldaña, M.D.; Gutiérrez-Pérez, O.; Arteaga-Silva, M.; Rojas-Castañeda, J.C. Postnatal testicular development in the Chinchilla rabbit. Acta Histochem. 2013, 115, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Hassanin, A.; Kuwahara, S.; Nurhidayat Tsukamoto, Y.; Ogawa, K.; Hiramatsu, K.; Sasaki, F. Gonadosomatic index and testis morphology of common carp (Cyprinus carpio) in rivers contaminated with estrogenic chemicals. J. Vet. Med. Sci. 2002, 64, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Moser, D.; Zadicario, E.; Schiff, G.; Jeanmonod, D. MR-guided focused ultrasound technique in functional neurosurgery: Targeting accuracy. J. Ther. Ultrasound. 2013, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Trejo, F.; León-Galván, M.Á.; Martínez-Méndez, L.A.; Tapia-Rodríguez, M.; Mendoza-Rodríguez, C.A.; González-Santoyo, I.; López-Wilchis, R.; Vela-Hinojosa, C.; Baranda-Avila, N.; Cerbón, M. Serotonin in testes of bat Myotis velifer during annual reproductive cycle: Expression, localization, and content variations. J. Exp. Zool. A Ecol. Genet. Physiol. 2013, 319, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Peña-Lopis, S.; Brugarolas, J. Simultaneous isolation of high-quality DNA, RNA, miRNA and proteins from tissues for genomic applications. Nat. Protoc. 2013, 8, 2240–2255. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Hernández, V.; Caldelas, I.; Merchant-Larios, H. Gene expression in the supporting cells at the onset of meiosis in rabbit gonads. Sex. Dev. 2019, 13, 125–136. [Google Scholar] [CrossRef]
- Merchant-Larios, H.; Moreno-Mendoza, N.; Buehr, M. The role of the mesonephros in cell differentiation and morphogenesis of the mouse fetal testis. Int. J. Dev. Biol. 1993, 37, 407–415. [Google Scholar]
- Mándi, Y.; Stone, T.W.; Guillemin, G.J.; Vécsei, L.; Williams, R.O. Editorial: Multiple Implications of the Kynurenine Pathway in Inflammatory Diseases: Diagnostic and Therapeutic Applications. Front. Immunol. 2022, 13, 860867. [Google Scholar] [CrossRef]
- Jiménez-Trejo, F.; Tapia-Rodríguez, M.; Arriaga-Canon, C.; Herrera, L.A.; Contreras-Espinosa, L.; Jiménez-García, K.L. Expanding the Concept of Serotoninomics: Perspectives for Serotonin Studies in the 20’S of the 21ST Century. Front. Neurosci. 2023, 17, 1200370. [Google Scholar] [CrossRef]
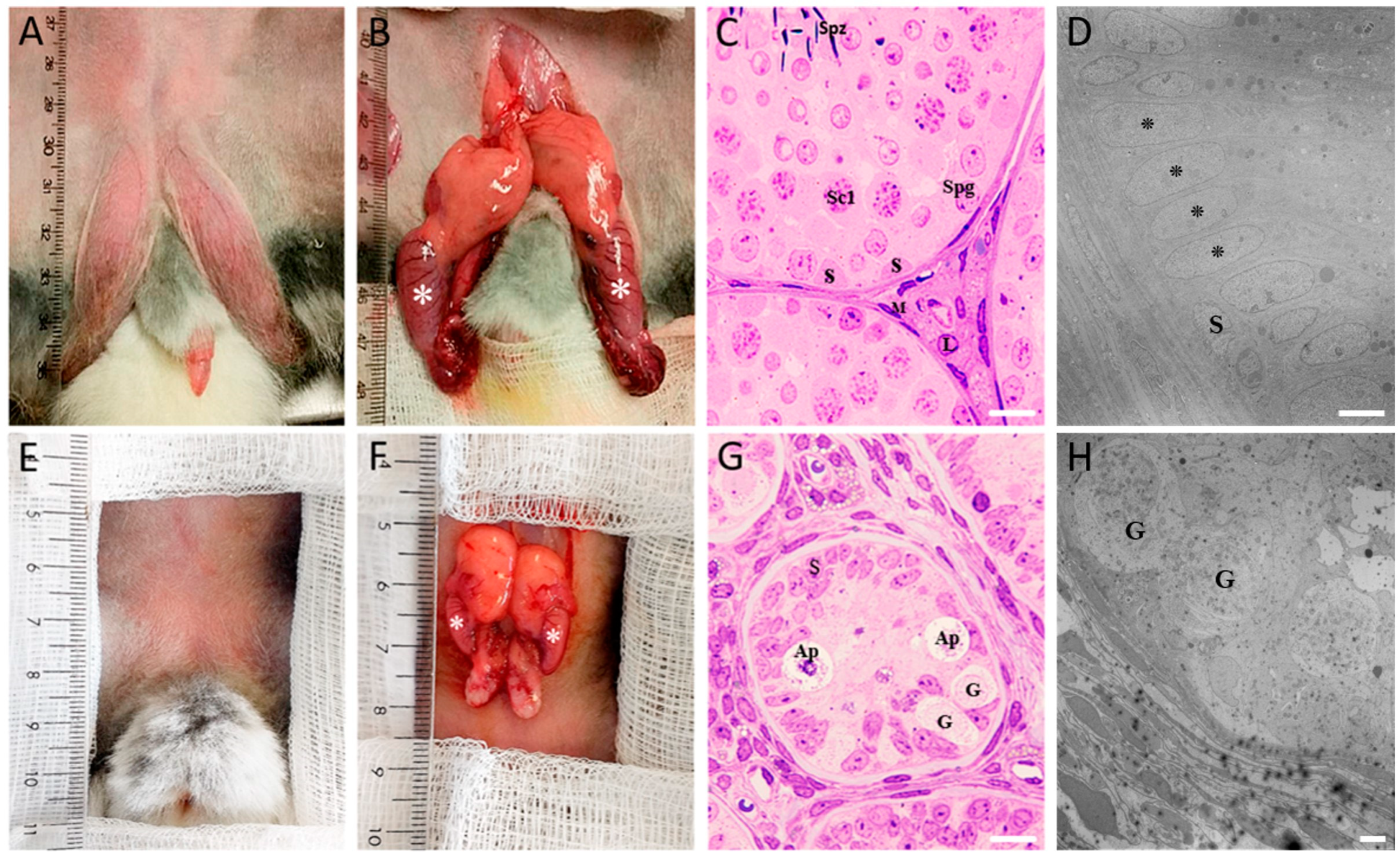
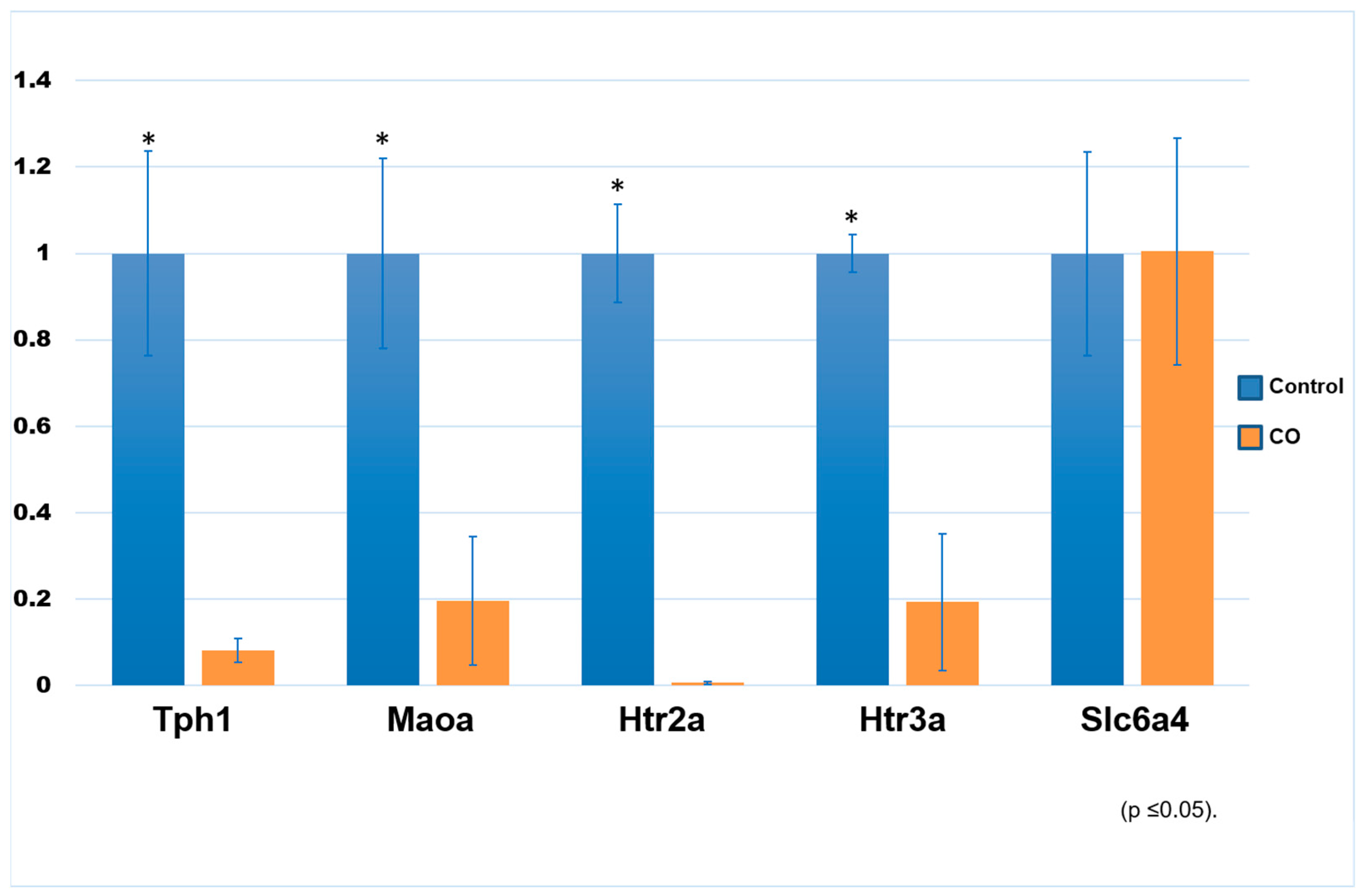
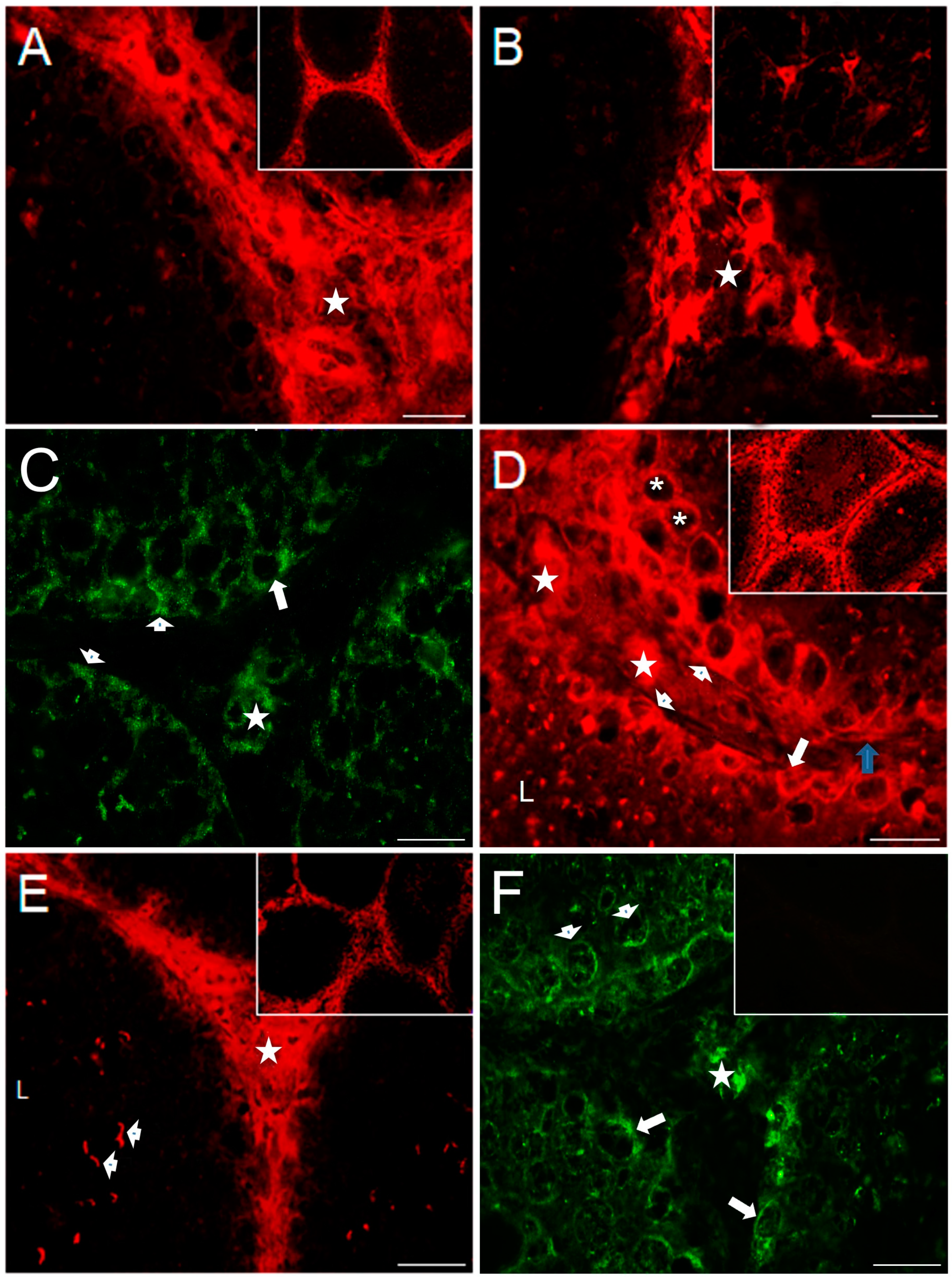
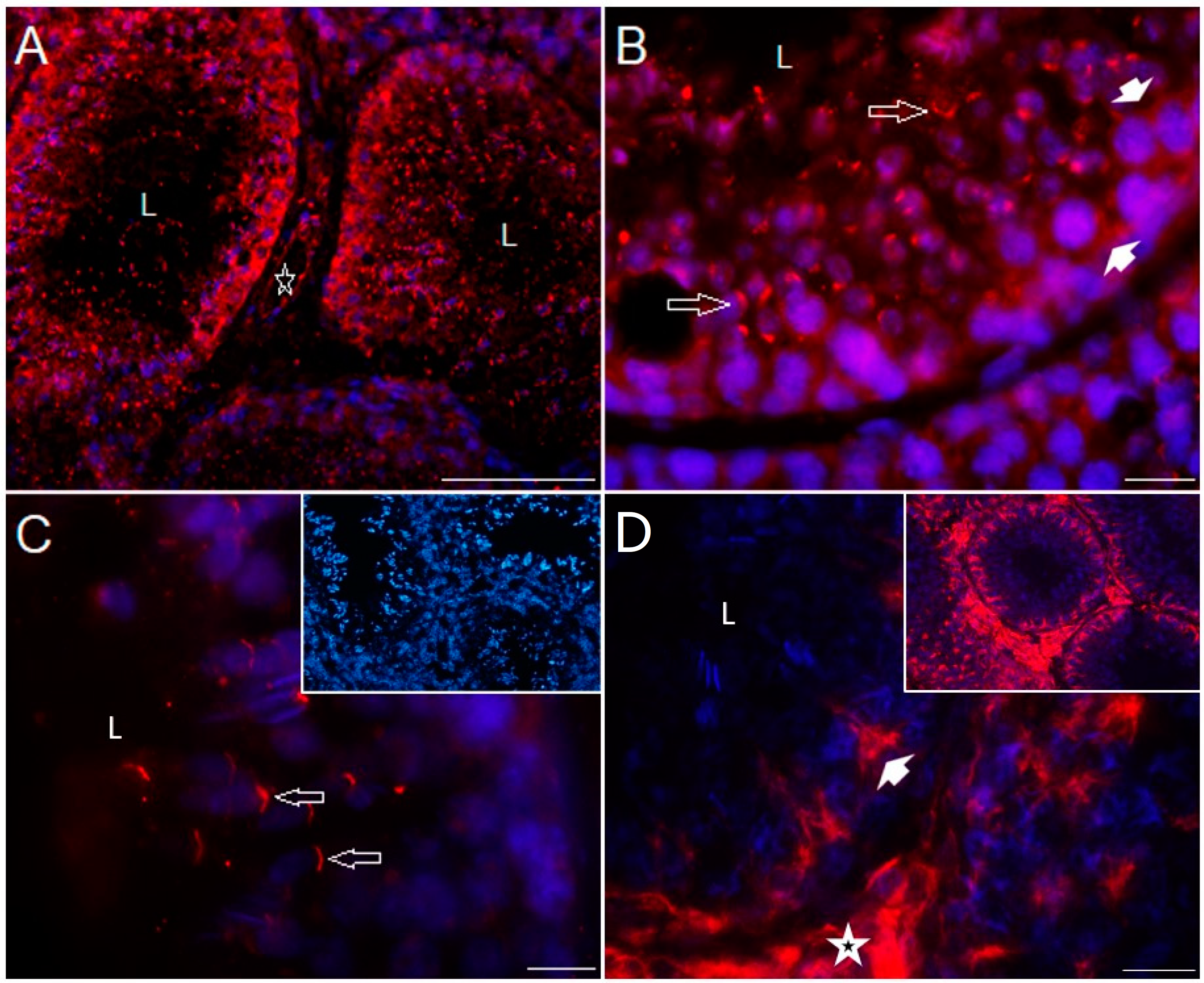

| Group | Body Weight (kg) | GSI (%) | TV (cm3) |
|---|---|---|---|
| Control | 3.144 + 133.44 | 0.104 + 0.022 * | 2.72 + 0.23 * |
| COI | 2.850 + 265.54 | 0.011 + 0.002 | 0.50 + 0.21 |
| Group | Serotonin (ng/mL) | Kynurenine (µg/mL) |
|---|---|---|
| Control | 19.02 ± 4.78 * | 123.75 ± 5.27 * |
| COI | 13.21 ± 1.97 | 76.56 ± 2.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Trejo, F.; Arriaga-Canon, C.; Herrera, L.A.; Coronado-Mares, I.; Montiel-Manríquez, R.; González-Santoyo, I.; Pérez-Báez, W.B.; Tapia-Rodríguez, M. Downregulation of Serotonergic System Components in an Experimentally Induced Cryptorchidism in Rabbits. Int. J. Mol. Sci. 2024, 25, 3149. https://doi.org/10.3390/ijms25063149
Jiménez-Trejo F, Arriaga-Canon C, Herrera LA, Coronado-Mares I, Montiel-Manríquez R, González-Santoyo I, Pérez-Báez WB, Tapia-Rodríguez M. Downregulation of Serotonergic System Components in an Experimentally Induced Cryptorchidism in Rabbits. International Journal of Molecular Sciences. 2024; 25(6):3149. https://doi.org/10.3390/ijms25063149
Chicago/Turabian StyleJiménez-Trejo, Francisco, Cristian Arriaga-Canon, Luis A. Herrera, Isabel Coronado-Mares, Rogelio Montiel-Manríquez, Isaac González-Santoyo, Wendy B. Pérez-Báez, and Miguel Tapia-Rodríguez. 2024. "Downregulation of Serotonergic System Components in an Experimentally Induced Cryptorchidism in Rabbits" International Journal of Molecular Sciences 25, no. 6: 3149. https://doi.org/10.3390/ijms25063149
APA StyleJiménez-Trejo, F., Arriaga-Canon, C., Herrera, L. A., Coronado-Mares, I., Montiel-Manríquez, R., González-Santoyo, I., Pérez-Báez, W. B., & Tapia-Rodríguez, M. (2024). Downregulation of Serotonergic System Components in an Experimentally Induced Cryptorchidism in Rabbits. International Journal of Molecular Sciences, 25(6), 3149. https://doi.org/10.3390/ijms25063149






