Unveiling the Role of Protein Kinase C θ in Porcine Epidemic Diarrhea Virus Replication: Insights from Genome-Wide CRISPR/Cas9 Library Screening
Abstract
1. Introduction
2. Results
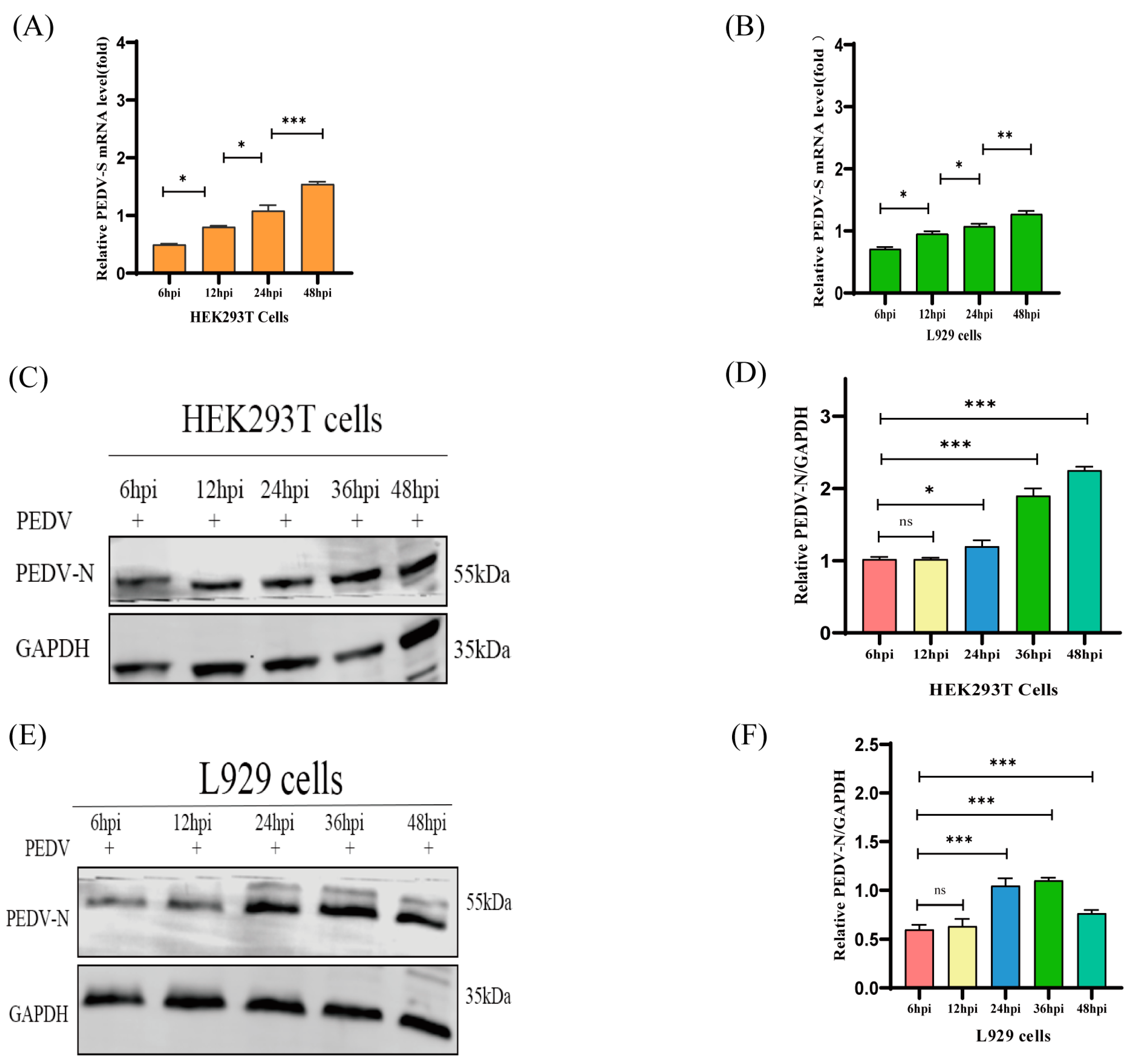
2.1. HEK293T and L929 Cells Are More Susceptible to PEDV Than Vero and IPEC-J2 Cells
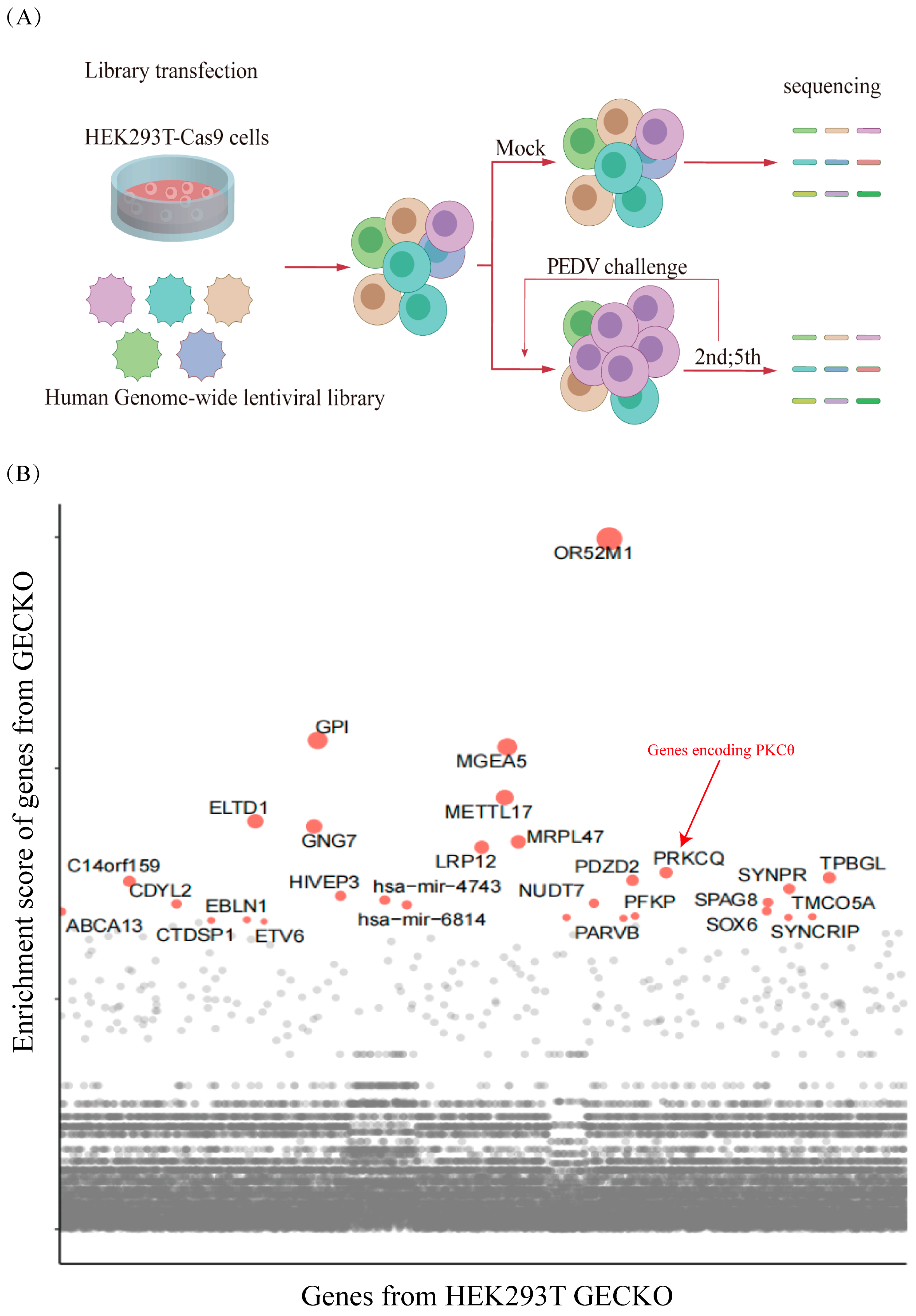
2.2. Genome-Scale CRISPR Screens Have Identified the Host Factors Associated with PEDV Infection
2.3. PEDV Infection Is Positively Correlated with PKCθ Expression and Phosphorylation
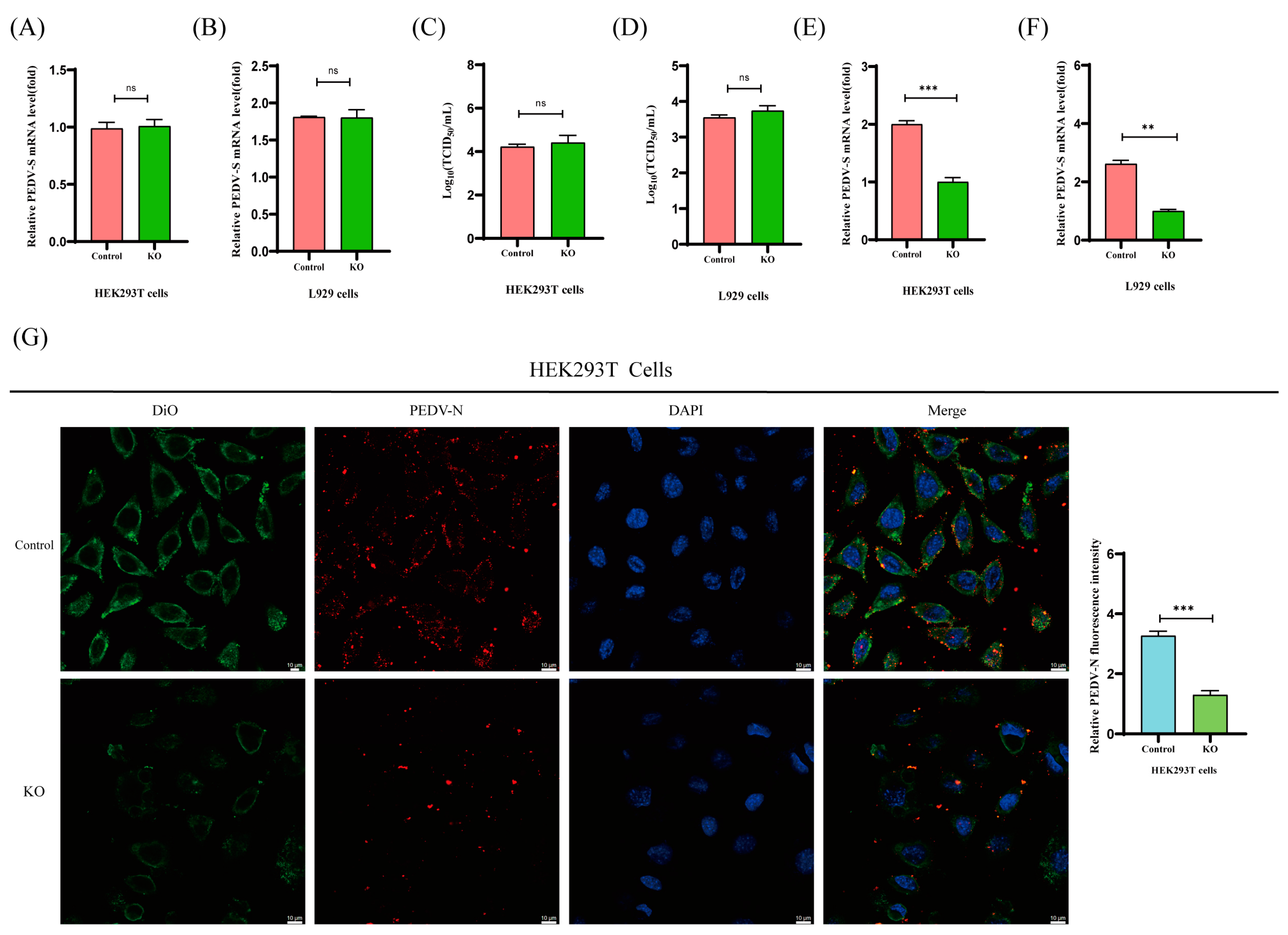
2.4. PKCθ Determines PEDV Replication
2.5. PKCθ Is Involved in PEDV Endocytosis Rather Than Absorption
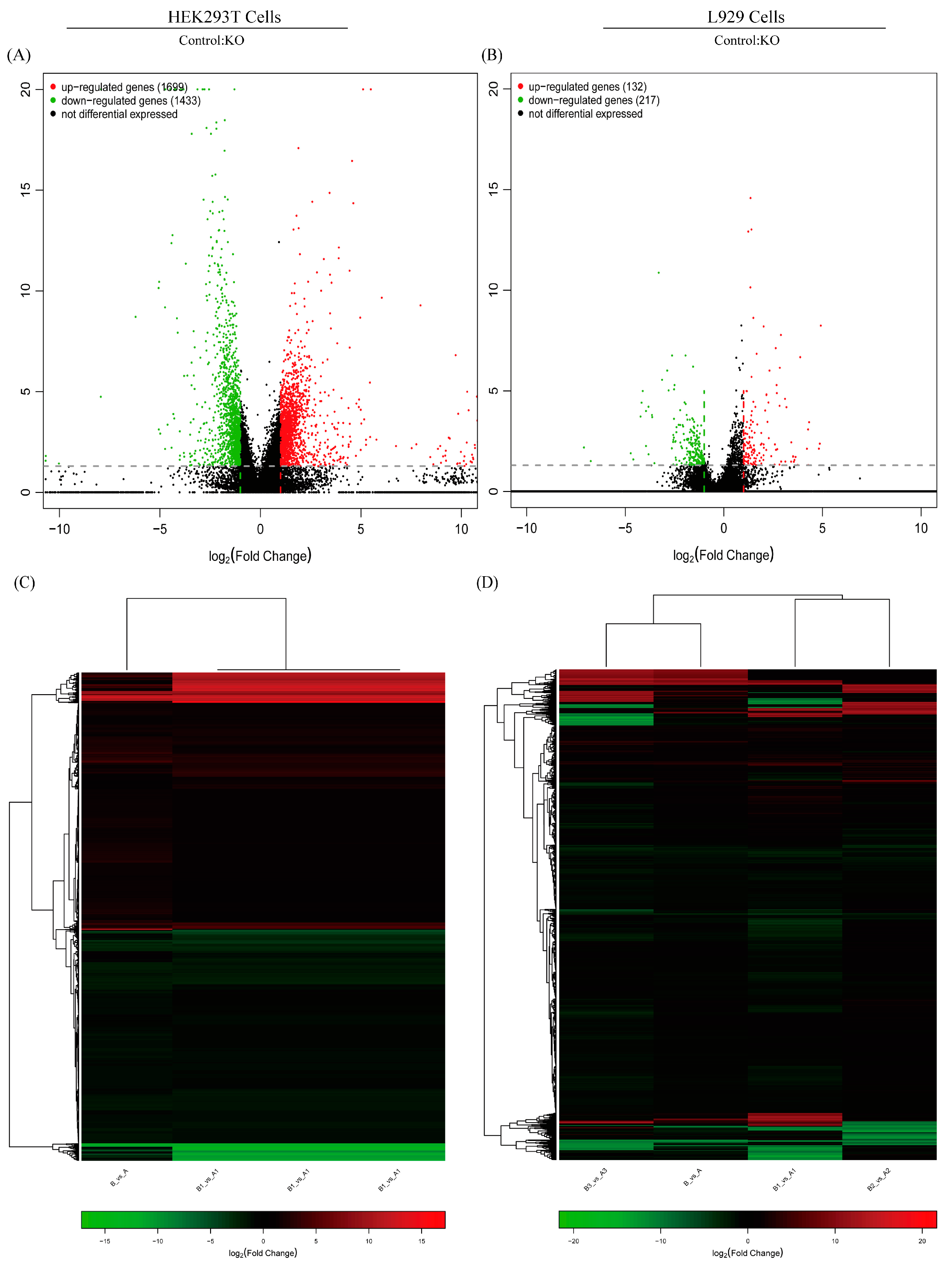
2.6. BOK Plays a Positive Role in PEDV Replication
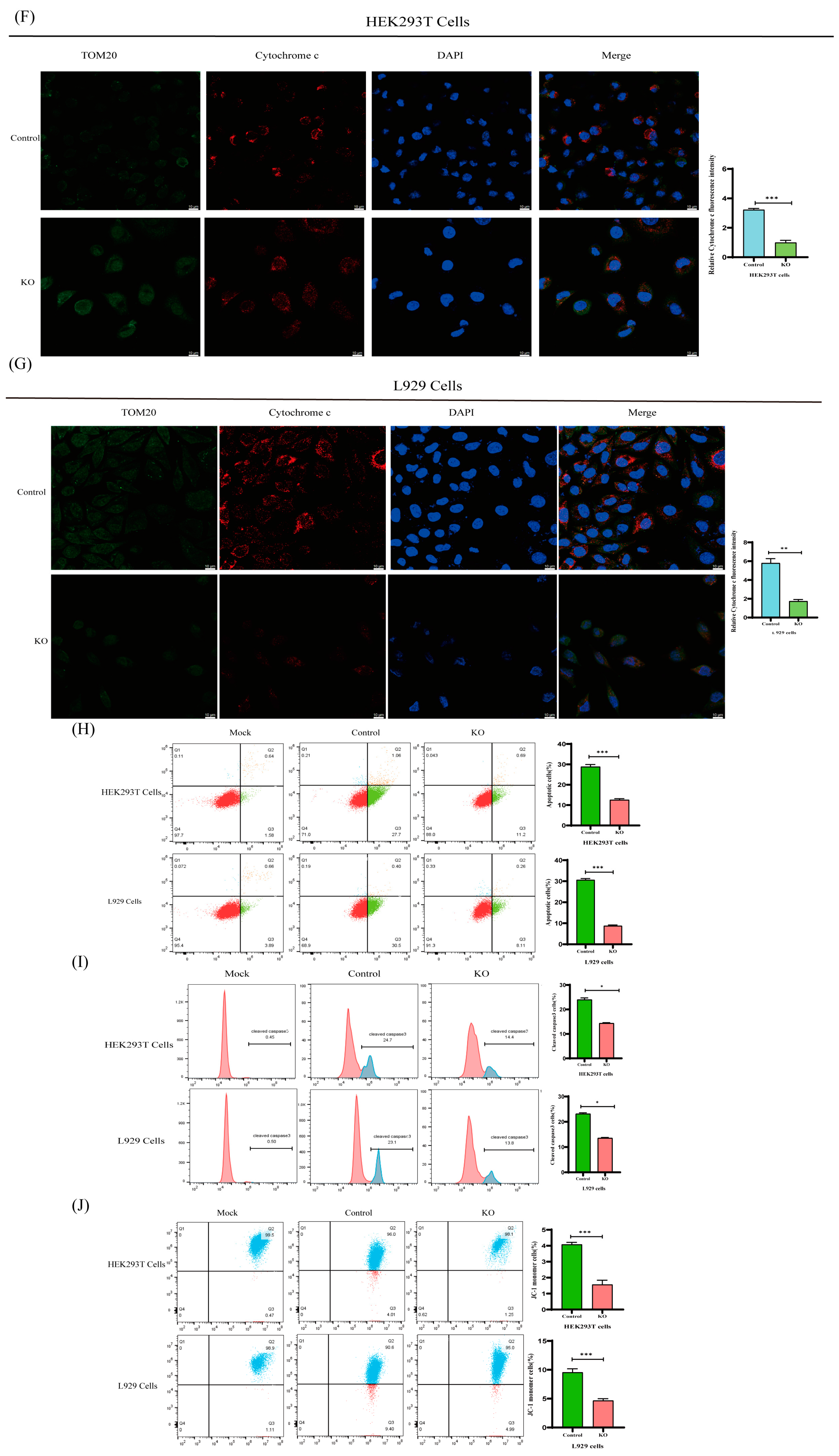
2.7. BOK Affects the Mitochondrial Apoptotic Pathway in PEDV-Infected Cells
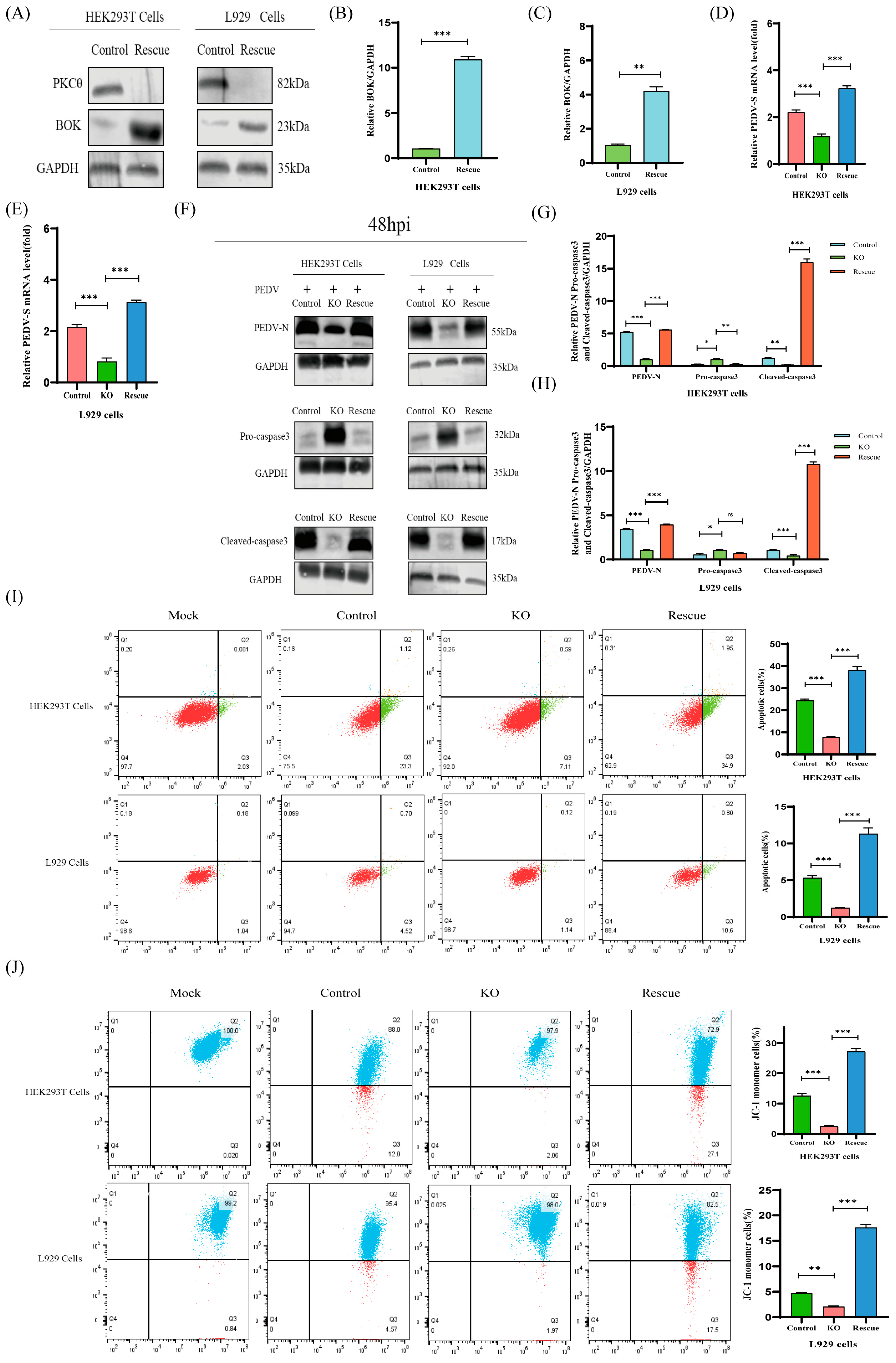
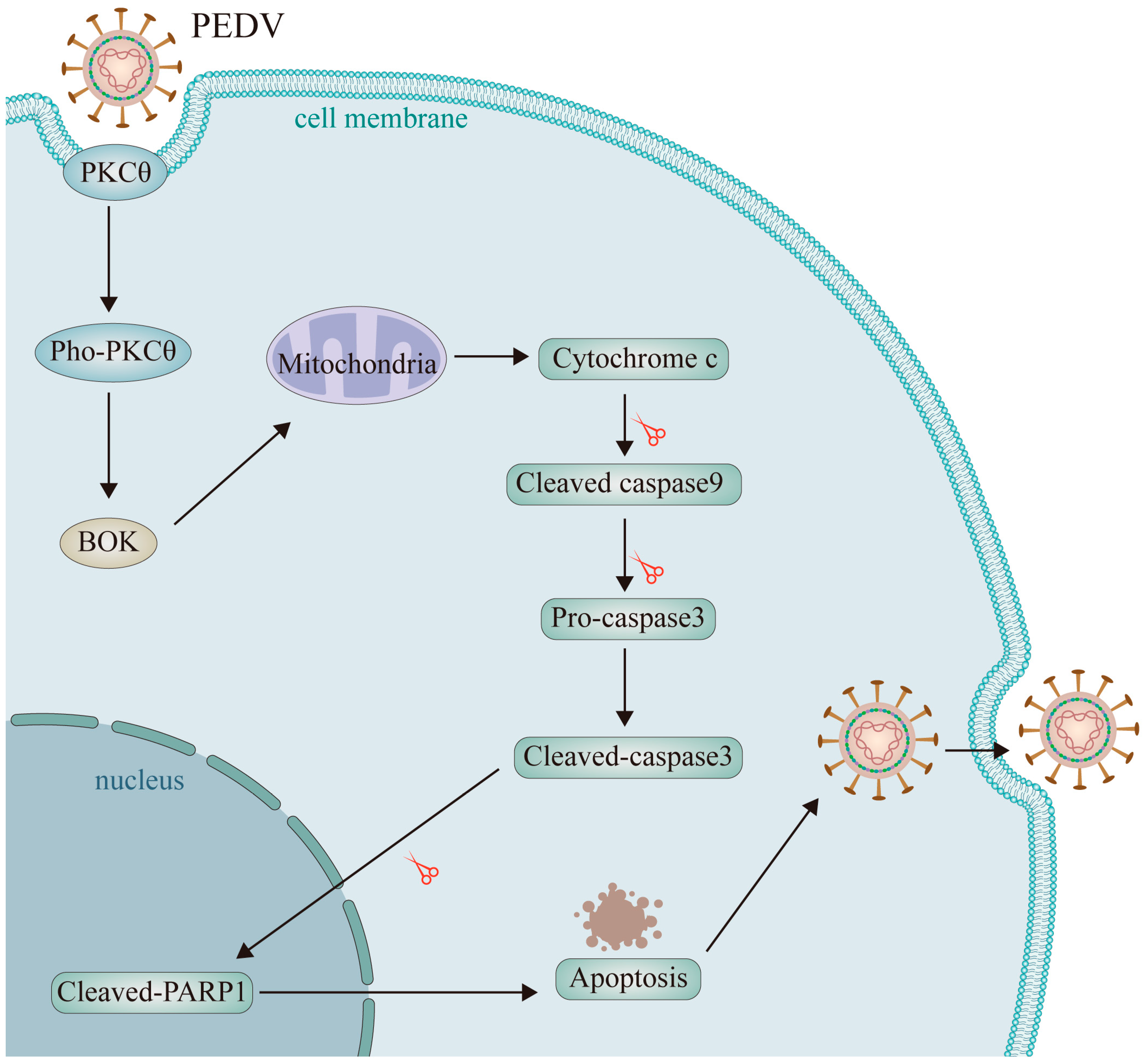
2.8. The PKCθ-BOK Axis Is Essential for PEDV Replication via Mitochondrial Apoptosis
3. Discussion
4. Materials and Methods
4.1. Cell Lines, Culture Conditions, and Viruses
4.2. HEK293T Library Generation and PEDV Screening
4.3. Generation of KO, Overexpression, and Rescued Overexpression Cell Lines
4.4. RNA Extraction and Quantitative RT-PCR
4.5. Western Blot
4.6. TCID50 and Plaque Assays
4.7. Indirect Immunofluorescence (IF) Staining
4.8. Ribonucleic Acid Sequencing (RNA-Seq)
4.9. Apoptosis Assay
4.10. Mitochondrial Membrane Potential (ΔΨm) Assay
4.11. Caspase 3 (CASP) Activity Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, R.Q.; Cai, R.J.; Chen, Y.Q.; Liang, P.S.; Chen, D.K.; Song, C.X. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg. Infect. Dis. 2012, 18, 161–163. [Google Scholar] [CrossRef]
- Jung, K.; Saif, L.J.; Wang, Q. Porcine epidemic diarrhea virus (PEDV): An Update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 286, 198045. [Google Scholar] [CrossRef] [PubMed]
- Wood, E.N. An apparently new syndrome of porcine epidemic diarrhoea. Vet. Rec. 1977, 100, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Okada, K.; Ohshima, K. An outbreak of swine diarrhea of a new-type associated with coronavirus-like particles in Japan. Jpn. J. Vet. Sci. 1983, 45, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, G.; Stasko, J.; Thomas, J.T.; Stensland, W.R.; Pillatzki, A.E.; Gauger, P.C.; Schwartz, K.J.; Madson, D.; Yoon, K.J.; et al. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J. Clin. Microbiol. 2014, 52, 234–243. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Liu, Y.; Pan, Y.; Deng, F.; Song, Y.; Tang, X.; He, Q. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg. Infect. Dis. 2012, 18, 1350–1353. [Google Scholar] [CrossRef]
- Sekhon, S.S.; Nguyen, P.L.; Ahn, J.Y.; Lee, K.A.; Lee, L.; Kim, S.Y.; Yoon, H.; Park, J.; Ko, J.H.; Kim, Y.H. Porcine epidemic diarrhea (PED) infection, diagnosis and vaccination: A mini review. Toxicol. Environ. Health Sci. 2016, 8, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Kong, N.; Zhang, Y.; Li, Y.; Sun, D.; Qin, W.; Zhai, H.; Zhai, X.; Yang, X.; Ye, C.; et al. TARDBP Inhibits Porcine Epidemic Diarrhea Virus Replication through Degrading Viral Nucleocapsid Protein and Activating Type I Interferon Signaling. J. Virol. 2022, 96, e0007022. [Google Scholar] [CrossRef] [PubMed]
- Emeny, J.M.; Morgan, M.J. Regulation of the interferon system: Evidence that Vero cells have a genetic defect in interferon production. J. Gen. Virol. 1979, 43, 247–252. [Google Scholar] [CrossRef]
- Chen, J.; Cui, Y.; Wang, Z.; Liu, G. Identification and characterization of PEDV infection in rat crypt epithelial cells. Vet. Microbiol. 2020, 249, 108848. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, C.; Zhang, N.; Liu, G. Porcine endemic diarrhea virus infection regulates long noncoding RNA expression. Virology 2019, 527, 89–97. [Google Scholar] [CrossRef]
- Lin, H.; Li, B.; Chen, L.; Ma, Z.; He, K.; Fan, H. Differential Protein Analysis of IPEC-J2 Cells Infected with Porcine Epidemic Diarrhea Virus Pandemic and Classical Strains Elucidates the Pathogenesis of Infection. J. Proteome Res. 2017, 16, 2113–2120. [Google Scholar] [CrossRef]
- Brosnahan, A.J.; Brown, D.R. Porcine IPEC-J2 intestinal epithelial cells in microbiological investigations. Vet. Microbiol. 2012, 156, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wen, Y.; Guo, X. CRISPR/Cas9 for genome editing: Progress, implications and challenges. Hum. Mol. Genet. 2014, 23, R40–R46. [Google Scholar] [CrossRef] [PubMed]
- Shalem, O.; Sanjana, N.E.; Hartenian, E.; Shi, X.; Scott, D.A.; Mikkelson, T.; Heckl, D.; Ebert, B.L.; Root, D.E.; Doench, J.G.; et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 2014, 343, 84–87. [Google Scholar] [CrossRef]
- Wei, J.; Alfajaro, M.M.; DeWeirdt, P.C.; Hanna, R.E.; Lu-Culligan, W.J.; Cai, W.L.; Strine, M.S.; Zhang, S.M.; Graziano, V.R.; Schmitz, C.O.; et al. Genome-wide CRISPR Screens Reveal Host Factors Critical for SARS-CoV-2 Infection. Cell 2021, 184, 76–91.e13. [Google Scholar] [CrossRef]
- Wang, X.; Jin, Q.; Xiao, W.; Fang, P.; Lai, L.; Xiao, S.; Wang, K.; Fang, L. Genome-Wide CRISPR/Cas9 Screen Reveals a Role for SLC35A1 in the Adsorption of Porcine Deltacoronavirus. J. Virol. 2022, 96, e0162622. [Google Scholar] [CrossRef]
- Han, J.; Perez, J.T.; Chen, C.; Li, Y.; Benitez, A.; Kandasamy, M.; Lee, Y.; Andrade, J.; tenOever, B.; Manicassamy, B. Genome-wide CRISPR/Cas9 Screen Identifies Host Factors Essential for Influenza Virus Replication. Cell Rep. 2018, 23, 596–607. [Google Scholar] [CrossRef]
- Steinberg, S.F. Structural basis of protein kinase C isoform function. Physiol. Rev. 2008, 88, 1341–1378. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Altman, A. Protein kinase C theta (PKCtheta): A key player in T cell life and death. Pharmacol. Res. 2007, 55, 537–544. [Google Scholar] [CrossRef]
- Bermejo, M.; López-Huertas, M.R.; Hedgpeth, J.; Mateos, E.; Rodríguez-Mora, S.; Maleno, M.J.; Plana, M.; Swindle, J.; Alcamí, J.; Coiras, M. Analysis of protein kinase C theta inhibitors for the control of HIV-1 replication in human CD4+ T cells reveals an effect on retrotranscription in addition to viral transcription. Biochem. Pharmacol. 2015, 94, 241–256. [Google Scholar] [CrossRef]
- Gonnella, R.; Granato, M.; Farina, A.; Santarelli, R.; Faggioni, A.; Cirone, M. PKC theta and p38 MAPK activate the EBV lytic cycle through autophagy induction. Biochim. Biophys. Acta 2015, 1853, 1586–1595. [Google Scholar] [CrossRef]
- Mondal, A.; Dawson, A.R.; Potts, G.K.; Freiberger, E.C.; Baker, S.F.; Moser, L.A.; Bernard, K.A.; Coon, J.J.; Mehle, A. Influenza virus recruits host protein kinase C to control assembly and activity of its replication machinery. eLife 2017, 6, e26910. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, L.; Zhang, Y.; Li, J.; Xu, L.; Xiao, Y.; Zhang, Q.; Bai, L.; Zhao, S.; Liu, E.; et al. Porcine reproductive and respiratory syndrome virus induces HMGB1 secretion via activating PKC-delta to trigger inflammatory response. Virology 2018, 518, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ennaciri, J.; Cordeiro, P.; El Bassam, S.; Menezes, J. Herpes simplex virus-1 up-regulates IL-15 gene expression in monocytic cells through the activation of protein tyrosine kinase and PKC zeta/lambda signaling pathways. J. Mol. Biol. 2007, 367, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, T.; Czabotar, P.E. BAX, BAK, and BOK: A Coming of Age for the BCL-2 Family Effector Proteins. Cold Spring Harb. Perspect. Biol. 2020, 12, a036319. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Y.; Meng, X.; Wang, Z.; Younis, M.; Liu, Y.; Wang, P.; Huang, X. SARS-CoV-2 membrane protein causes the mitochondrial apoptosis and pulmonary edema via targeting BOK. Cell Death Differ. 2022, 29, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Danthi, P. Viruses and the Diversity of Cell Death. Annu. Rev. Virol. 2016, 3, 533–553. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, C.; Shu, J.; Feng, H.; He, Y.; Chen, J.; Shu, J. Porcine Epidemic Diarrhea Virus Induces Vero Cell Apoptosis via the p53-PUMA Signaling Pathway. Viruses 2021, 13, 1218. [Google Scholar] [CrossRef]
- Benmerzoug, S.; Rose, S.; Bounab, B.; Gosset, D.; Duneau, L.; Chenuet, P.; Mollet, L.; Le Bert, M.; Lambers, C.; Geleff, S.; et al. STING-dependent sensing of self-DNA drives silica-induced lung inflammation. Nat. Commun. 2018, 9, 5226. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, C. Porcine epidemic diarrhea virus induces caspase-independent apoptosis through activation of mitochondrial apoptosis-inducing factor. Virology 2014, 460–461, 180–193. [Google Scholar] [CrossRef]
- Thepparit, C.; Khongwichit, S.; Ketsuwan, K.; Libsittikul, S.; Auewarakul, P.; Smith, D.R. Dengue virus requires apoptosis linked gene-2-interacting protein X (ALIX) for viral propagation. Virus Res. 2019, 261, 65–71. [Google Scholar] [CrossRef]
- Ge, M.; Zhang, Y.; Liu, Y.; Liu, T.; Zeng, F. Propagation of field highly pathogenic porcine reproductive and respiratory syndrome virus in MARC-145 cells is promoted by cell apoptosis. Virus Res. 2016, 213, 322–331. [Google Scholar] [CrossRef]
- Ampomah, P.B.; Lim, L.H.K. Influenza A virus-induced apoptosis and virus propagation. Apoptosis 2020, 25, 1–11. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, L.; Xu, Y.; Yang, L.; Shi, H.; Feng, L.; Wang, Y. Characterization of porcine epidemic diarrhea virus infectivity in human embryonic kidney cells. Arch. Virol. 2017, 162, 2415–2419. [Google Scholar] [CrossRef]
- Ye, G.; Liu, H.; Zhou, Q.; Liu, X.; Huang, L.; Weng, C. A Tug of War: Pseudorabies Virus and Host Antiviral Innate Immunity. Viruses 2022, 14, 547. [Google Scholar] [CrossRef]
- Phetsouphanh, C.; Kelleher, A.D. The Role of PKC-θ in CD4+ T Cells and HIV Infection: To the Nucleus and Back Again. Front. Immunol. 2015, 6, 391. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, X.; Sun, Y.; Zeng, W.; Li, Y.; Zhao, F.; Wu, K.; Fan, S.; Zhao, M.; Chen, J.; et al. Swine Enteric Coronavirus: Diverse Pathogen-Host Interactions. Int. J. Mol. Sci. 2022, 23, 3953. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Zamzami, N.; Marchetti, P.; Castedo, M.; Zanin, C.; Vayssière, J.L.; Petit, P.X.; Kroemer, G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J. Exp. Med. 1995, 181, 1661–1672. [Google Scholar] [CrossRef]
- Jung, K.; Vasquez-Lee, M.; Saif, L.J. Replicative capacity of porcine deltacoronavirus and porcine epidemic diarrhea virus in primary bovine mesenchymal cells. Vet. Microbiol. 2020, 244, 108660. [Google Scholar] [CrossRef]
- Wang, X.; Fang, L.; Liu, S.; Ke, W.; Wang, D.; Peng, G.; Xiao, S. Susceptibility of porcine IPI-2I intestinal epithelial cells to infection with swine enteric coronaviruses. Vet. Microbiol. 2019, 233, 21–27. [Google Scholar] [CrossRef]
- Nath, P.R.; Isakov, N. PKCθ-regulated signalling in health and disease. Biochem. Soc. Trans. 2014, 42, 1484–1489. [Google Scholar] [CrossRef]
- Nicolle, A.; Zhang, Y.; Belguise, K. The Emerging Function of PKCtheta in Cancer. Biomolecules 2021, 11, 221. [Google Scholar] [CrossRef]
- Vazquez-Ortiz, G.; García, J.A.; Ciudad, C.J.; Noé, V.; Peñuelas, S.; López-Romero, R.; Mendoza-Lorenzo, P.; Piña-Sánchez, P.; Salcedo, M. Differentially expressed genes between high-risk human papillomavirus types in human cervical cancer cells. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2007, 17, 484–491. [Google Scholar] [CrossRef]
- Fei, Y.; Han, M.; Chu, X.; Feng, Z.; Yu, L.; Luo, Y.; Lu, L.; Xu, D. Transcriptomic and proteomic analyses reveal new insights into the regulation of immune pathways during cyprinid herpesvirus 2 infection in vitro. Fish Shellfish Immunol. 2020, 106, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Petkov, S.; Herrera, C.; Else, L.; Lebina, L.; Opoka, D.; Seiphetlo, T.B.; Pillay, A.A.; Mugaba, S.; Namubiru, P.; Odoch, G.; et al. Short-term oral pre-exposure prophylaxis against HIV-1 modulates the transcriptome of foreskin tissue in young men in Africa. Front. Immunol. 2022, 13, 1009978. [Google Scholar] [CrossRef] [PubMed]
- Si, F.; Hu, X.; Wang, C.; Chen, B.; Wang, R.; Dong, S.; Yu, R.; Li, Z. Porcine Epidemic Diarrhea Virus (PEDV) ORF3 Enhances Viral Proliferation by Inhibiting Apoptosis of Infected Cells. Viruses 2020, 12, 214. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, Y.; Gao, J.; Shi, X.; Yan, Y.; Yang, N.; Wang, Q.; Zhang, Q. GRAMD4 regulates PEDV-induced cell apoptosis inhibiting virus replication via the endoplasmic reticulum stress pathway. Vet. Microbiol. 2023, 279, 109666. [Google Scholar] [CrossRef]
- Xie, S.; Liang, Z.; Yang, X.; Pan, J.; Yu, D.; Li, T.; Cao, R. Japanese Encephalitis Virus NS2B-3 Protein Complex Promotes Cell Apoptosis and Viral Particle Release by Down-Regulating the Expression of AXL. Virol. Sin. 2021, 36, 1503–1519. [Google Scholar] [CrossRef]
- Despouy, G.; Joiner, M.; Le Toriellec, E.; Weil, R.; Stern, M.H. The TCL1 oncoprotein inhibits activation-induced cell death by impairing PKCtheta and ERK pathways. Blood 2007, 110, 4406–4416. [Google Scholar] [CrossRef]
- Dubielecka, P.M.; Grzybek, M.; Kolondra, A.; Jaźwiec, B.; Draga, A.; Aleksandrowicz, P.; Kołodziejczyk, M.; Serwotka, A.; Dolińska-Krajewska, B.; Warchoł, J.; et al. Aggregation of spectrin and PKCtheta is an early hallmark of fludarabine/mitoxantrone/dexamethasone-induced apoptosis in Jurkat T and HL60 cells. Mol. Cell. Biochem. 2010, 339, 63–77. [Google Scholar] [CrossRef]







| Human Gene Stable ID | Mouse Gene Stable ID | Gene Names | Score Ordering |
|---|---|---|---|
| ENSG00000176720 | ENSMUSG00000026278 | BOK | 1 |
| ENSG00000100300 | ENSMUSG00000041736 | TSPO | 2 |
| ENSG00000127663 | ENSMUSG00000024201 | KDM4B | 3 |
| ENSG00000124145 | ENSMUSG00000017009 | SDC4 | 4 |
| ENSG00000141959 | ENSMUSG00000020277 | PFKL | 5 |
| ENSG00000100292 | ENSMUSG00000005413 | HMOX1 | 6 |
| ENSG00000171388 | ENSMUSG00000037010 | APLN | 7 |
| ENSG00000171227 | ENSMUSG00000050777 | TMEM37 | 8 |
| ENSG00000007255 | ENSMUSG00000002043 | TRAPPC6A | 9 |
| ENSG00000173153 | ENSMUSG00000024955 | ESRRA | 10 |
| ENSG00000176171 | ENSMUSG00000078566 | BNIP3 | 11 |
| ENSG00000160325 | ENSMUSG00000015488 | CACFD1 | 12 |
| ENSG00000072682 | ENSMUSG00000018906 | P4HA2 | 13 |
| ENSG00000129355 | ENSMUSG00000096472 | CDKN2D | 14 |
| ENSG00000060971 | ENSMUSG00000036138 | ACAA1 | 15 |
| ENSG00000109107 | ENSMUSG00000017390 | ALDOC | 16 |
| ENSG00000111674 | ENSMUSG00000004267 | ENO2 | 17 |
| ENSG00000172183 | ENSMUSG00000039236 | ISG20 | 18 |
| ENSG00000162522 | ENSMUSG00000050390 | KIAA1522 | 19 |
| ENSG00000186352 | ENSMUSG00000050914 | ANKRD37 | 20 |
| ENSG00000179403 | ENSMUSG00000042116 | VWA1 | 21 |
| ENSG00000136068 | ENSMUSG00000025278 | FLNB | 22 |
| ENSG00000168101 | ENSMUSG00000022516 | NUDT16L1 | 23 |
| ENSG00000135622 | ENSMUSG00000000627 | SEMA4F | 24 |
| ENSG00000168273 | ENSMUSG00000058351 | UQCC5 | 25 |
| ENSG00000162461 | ENSMUSG00000040740 | SLC25A34 | 26 |
| ENSG00000143416 | ENSMUSG00000068874 | SELENBP1 | 27 |
| Human Gene Stable ID | Mouse Gene Stable ID | Gene Names | Score Ordering |
|---|---|---|---|
| ENSG00000198763 | ENSMUSG00000064345 | MT-ND2 | 1 |
| ENSG00000228253 | ENSMUSG00000064356 | MT-ATP8 | 2 |
| ENSG00000198157 | ENSMUSG00000031245 | HMGN5 | 3 |
| ENSG00000205571 | ENSMUSG00000021645 | SMN2 | 4 |
| ENSG00000136866 | ENSMUSG00000028389 | ZFP37 | 5 |
| ENSG00000172062 | ENSMUSG00000021645 | SMN1 | 6 |
| sgRNA Names | sgRNA Sequences |
|---|---|
| sgRNA-1 | 5′-GACAAGCCAATCCGAAGAAA-3′ |
| sgRNA-2 | 5′-GGGTGGGTACATGGTAGGCT-3 |
| sgRNA-3 | 5′-CCTCATCTCAGAAACAACC-3′ |
| sgRNA-4 | 5′-TGTGGGTTGAGGGAAAAAGG-3′ |
| NCBI Gene ID | Primer Names | Primer Sequences |
|---|---|---|
| 5588 (PKCθ-human) | 5588-RT-F | GCAAAAACGTGGACCTCATCT |
| 5588-RT-R | CAAAGAAGCCTTCCGTCTCAAA | |
| 18761 (PKCθ-mouse) | 18761-RT-F | TATCCAACTTTGACTGTGGGACC |
| 18761-RT-R | CCCTTCCCTTGTTAATGTGGG | |
| 935184 (PEDV-S) | 935184-RT-F | TGCCAATGTATTTGCCACT |
| 935184-RT-R | TGACAGTAGGAGGTAAAACAGCC | |
| 666 (BOK-human) | 666-RT-F | GTCTTCGCTGCGGAGATCAT |
| 666-RT-R | CATTCCGATATACGCTGGGAC | |
| 51800 (BOK-mouse) | 51800-RT-F | TGTCTTTGCAGCGGAGATCAT |
| 51800-RT-R | TCCCGGCCTAGTGCCTTAG | |
| 2597 (GAPDH-human) | 2597-RT-F | GGAGCGAGATCCCTCCAAAAT |
| 2597-RT-R | GGCTGTTGTCATACTTCTCATGG | |
| 14433 (GAPDH-mouse) | 14433-RT-F | AGGTCGGTGTGAACGGATTTG |
| 14433-RT-R | GGGGTCGTTGATGGCAACA |
| Antibodies’ Names | Catalog. No | Dilution Ratio | Sources |
|---|---|---|---|
| Anti-PKCθ antibody | ab302891 | 1:1000 | Abcam, Cambridge, UK |
| Anti-phosphorylated PKCθ antibody | T538 | 1:1000 | Cell Signaling, Danvers, MA, USA |
| Anti-PEDV-N antibody | SD-2-1 | 1:1000 | Medgenes, Brookings, SD, USA |
| Anti-BOK antibody | ab233072 | 1:1000 | Abcam, Cambridge, UK |
| Anti Caspase 9 antibody | 9509T | 1:1000 | Cell Signaling, Danvers, MA, USA |
| Anti-Cleaved Caspase 3 antibody | 9664T | 1:1000 | Cell Signaling, Danvers, MA, USA |
| Anti-Cleaved PARP1 antibody | ab225715 | 1:100 | Abcam, Cambridge, UK |
| Anti-GAPDH antibody | 60004-1-Ig | 1:10,000 | Proteintech, Shanghai, China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Feng, Z.; Lv, D.; Wang, D.; Sang, K.; Liu, Z.; Guo, D.; Shen, Y.; Chen, Q. Unveiling the Role of Protein Kinase C θ in Porcine Epidemic Diarrhea Virus Replication: Insights from Genome-Wide CRISPR/Cas9 Library Screening. Int. J. Mol. Sci. 2024, 25, 3096. https://doi.org/10.3390/ijms25063096
Zhou J, Feng Z, Lv D, Wang D, Sang K, Liu Z, Guo D, Shen Y, Chen Q. Unveiling the Role of Protein Kinase C θ in Porcine Epidemic Diarrhea Virus Replication: Insights from Genome-Wide CRISPR/Cas9 Library Screening. International Journal of Molecular Sciences. 2024; 25(6):3096. https://doi.org/10.3390/ijms25063096
Chicago/Turabian StyleZhou, Jinglin, Zhihua Feng, Deyang Lv, Duokai Wang, Kai Sang, Zhihao Liu, Dong Guo, Yangkun Shen, and Qi Chen. 2024. "Unveiling the Role of Protein Kinase C θ in Porcine Epidemic Diarrhea Virus Replication: Insights from Genome-Wide CRISPR/Cas9 Library Screening" International Journal of Molecular Sciences 25, no. 6: 3096. https://doi.org/10.3390/ijms25063096
APA StyleZhou, J., Feng, Z., Lv, D., Wang, D., Sang, K., Liu, Z., Guo, D., Shen, Y., & Chen, Q. (2024). Unveiling the Role of Protein Kinase C θ in Porcine Epidemic Diarrhea Virus Replication: Insights from Genome-Wide CRISPR/Cas9 Library Screening. International Journal of Molecular Sciences, 25(6), 3096. https://doi.org/10.3390/ijms25063096





