Investigation of Serum Endocan Levels in SARS-CoV-2 Patients
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of COVID-19 Patients
2.2. Laboratory Findings
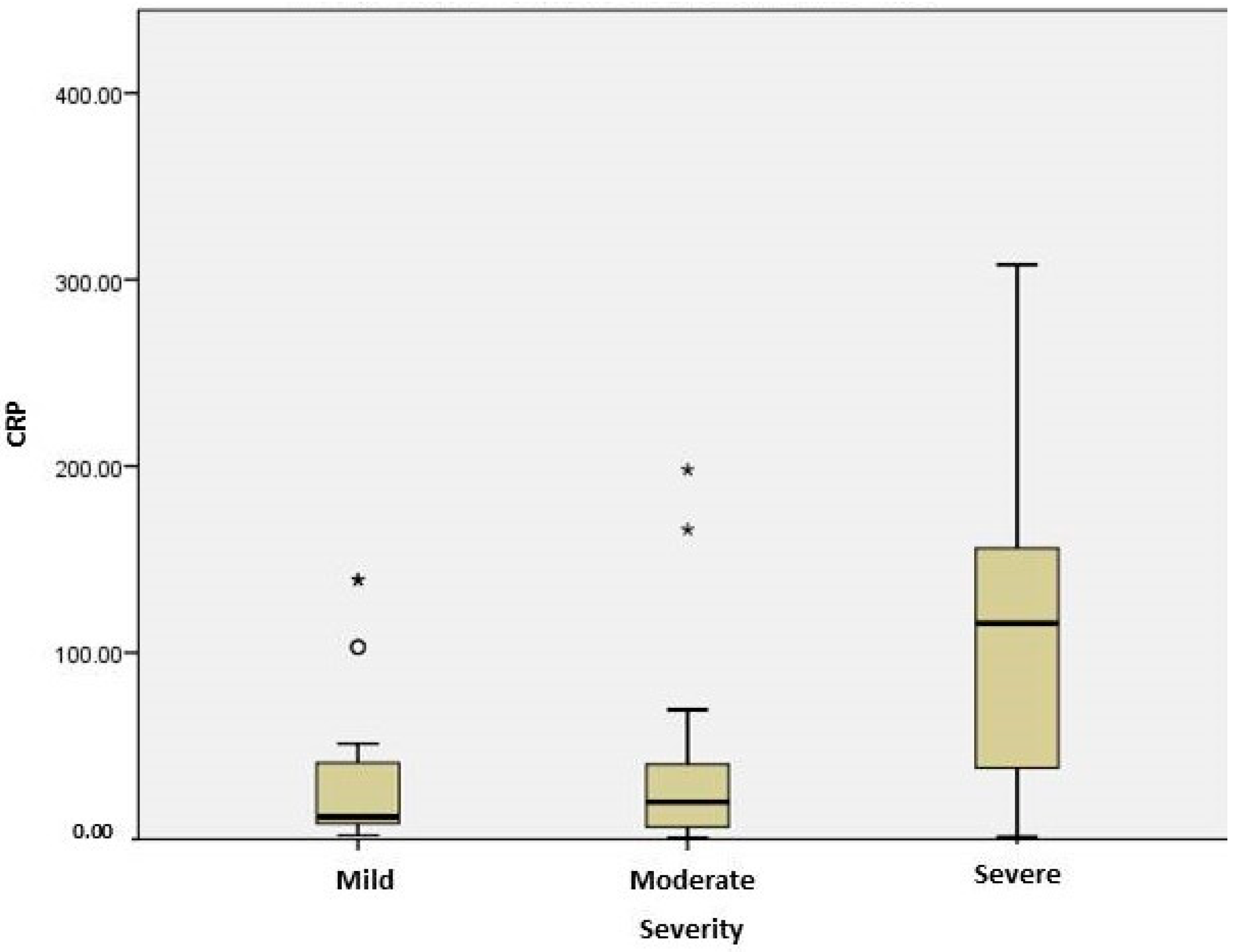
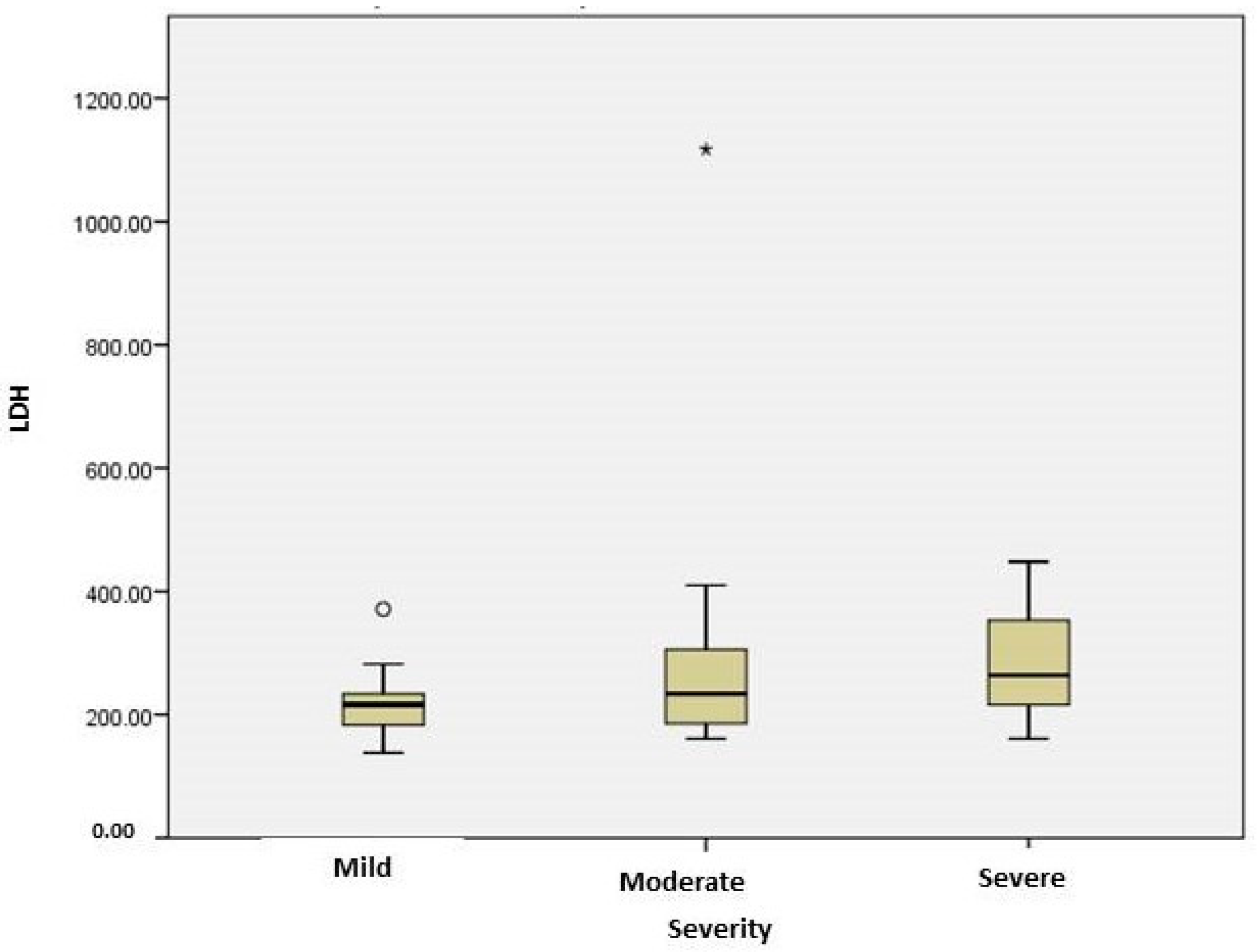
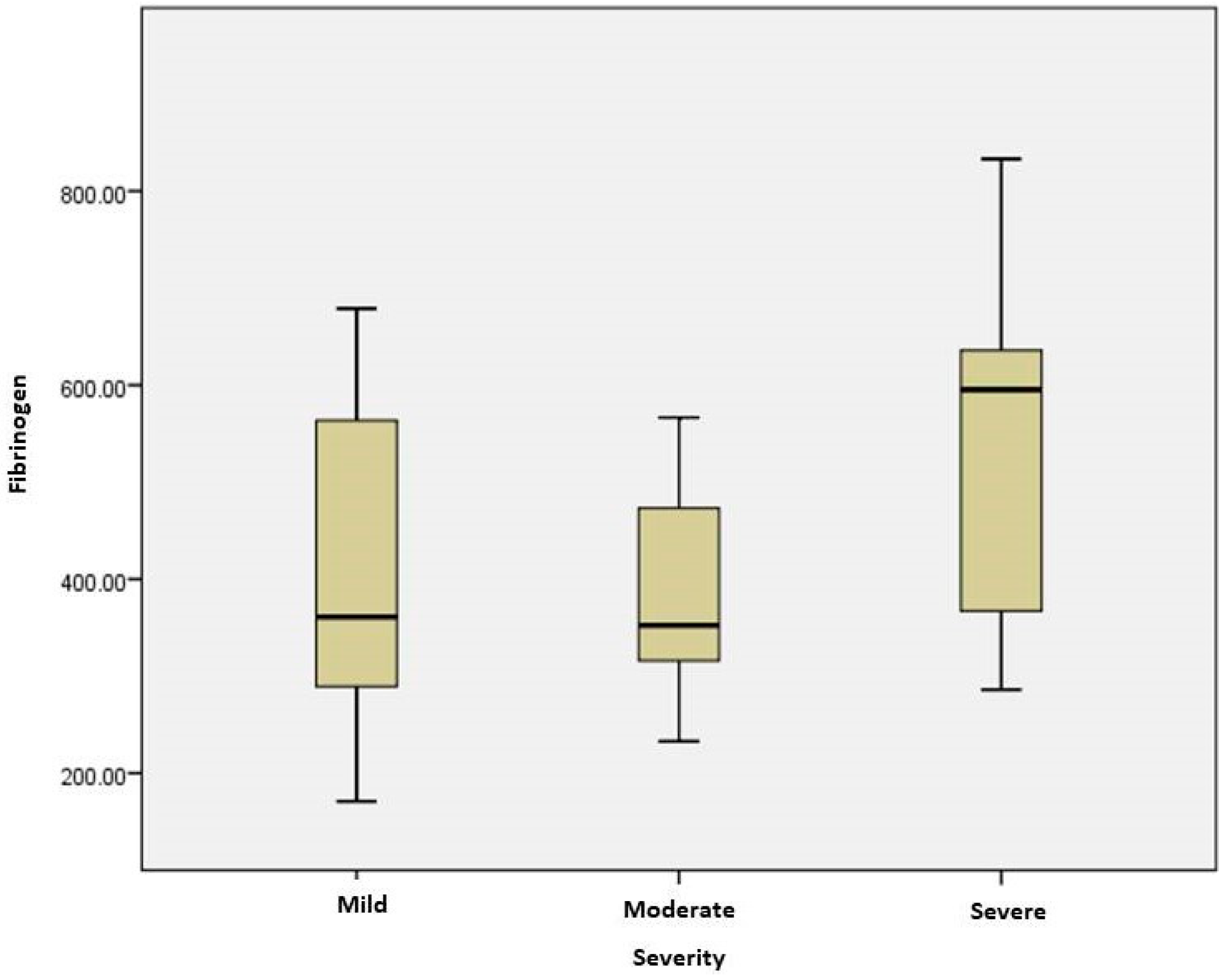
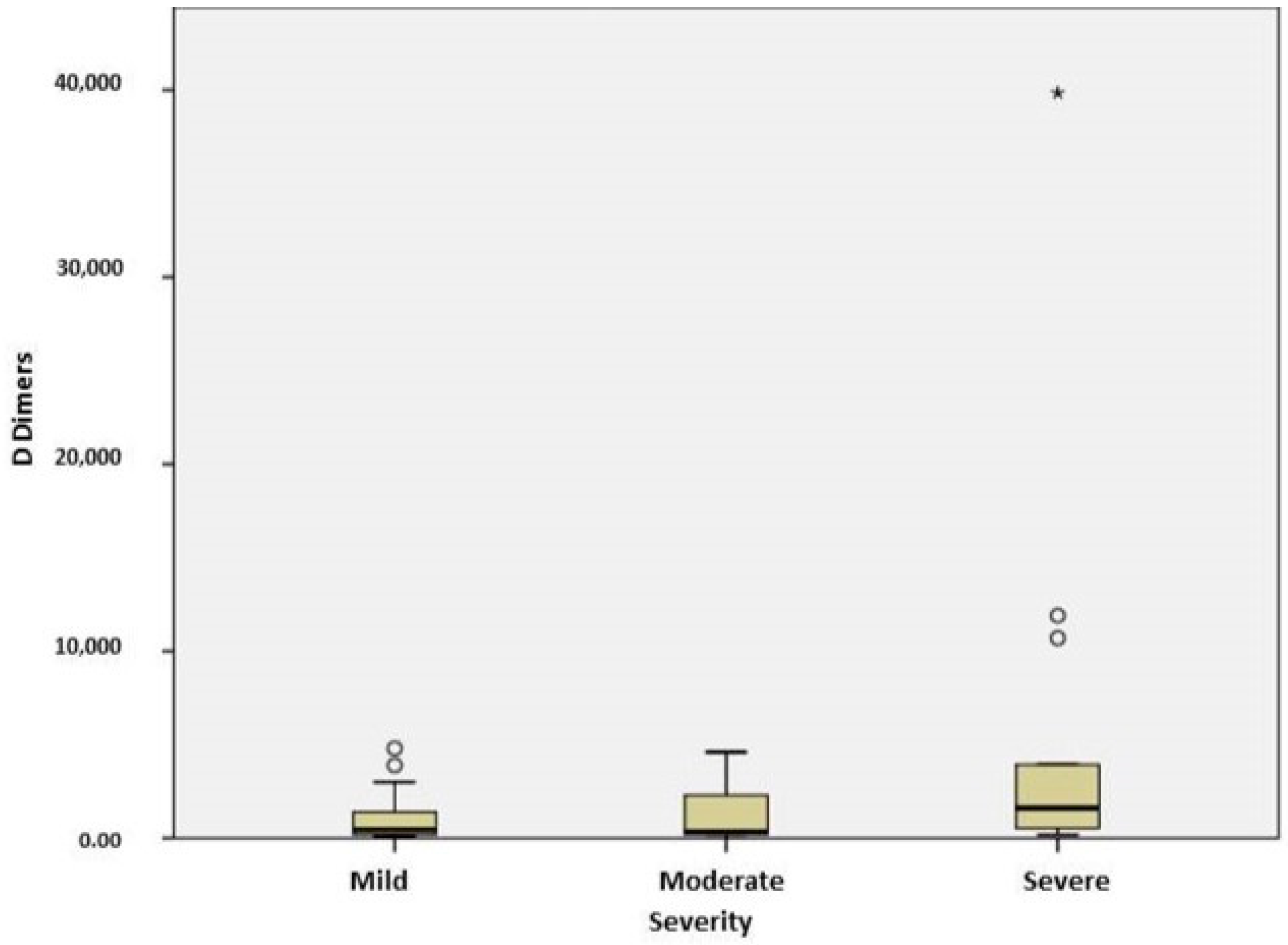
2.3. Stratification of COVID Patients
2.4. Evaluation of Serum Endocan Levels
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Measurement of Serum Endocan Levels
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonçalves, F.A.R.; Besen, B.A.M.P.; de Lima, C.A.; Corá, A.P.; Pereira, A.J.R.; Perazzio, S.F.; Gouvea, C.P.; Fonseca, L.A.M.; Trindade, E.M.; Sumita, N.M.; et al. Use and misuse of biomarkers and the role of D-dimer and C-reactive protein in the management of COVID-19: A post-hoc analysis of a prospective cohort study. Clinics 2021, 76, e3547. [Google Scholar] [CrossRef]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef]
- Baratella, E.; Bussani, R.; Zanconati, F.; Marrocchio, C.; Fabiola, G.; Braga, L.; Maiocchi, S.; Berlot, G.; Volpe, M.C.; Moro, E.; et al. Radiological–pathological signatures of patients with COVID-19-related pneumomediastinum: Is there a role for the sonic hedgehog and Wnt5a pathways? ERJ Open Res. 2021, 7, 00346-2021. [Google Scholar] [CrossRef]
- Xu, S.-W.; Ilyas, I.; Weng, J.-P. Endothelial dysfunction in COVID-19: An overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol. Sin. 2023, 44, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Petrakis, D.; Margină, D.; Tsarouhas, K.; Tekos, F.; Stan, M.; Nikitovic, D.; Kouretas, D.; Spandidos, D.A.; Tsatsakis, A. Obesity—A risk factor for increased COVID-19 prevalence, severity and lethality (Review). Mol. Med. Rep. 2020, 22, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Wool, G.D.; Miller, J.L. The Impact of COVID-19 Disease on Platelets and Coagulation. Pathobiology 2021, 88, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Sengupta, A.; Ricciotti, E.; Mrčela, A.; Mathew, D.; Mazaleuskaya, L.L.; Ghosh, S.; Brooks, T.G.; Turner, A.P.; Schanoski, A.S.; et al. Deep phenotyping of the lipidomic response in COVID-19 and non-COVID-19 sepsis. Clin. Transl. Med. 2023, 13, e1440. [Google Scholar] [CrossRef] [PubMed]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in COVID-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vetrugno, L.; Castaldo, N.; Fantin, A.; Deana, C.; Cortegiani, A.; Longhini, F.; Forfori, F.; Cammarota, G.; Grieco, D.L.; Isola, M.; et al. Ventilatory associated barotrauma in COVID-19 patients: A multicenter observational case control study (COVI-MIX-study). Pulmonology 2023, 29, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ji, W.; Yang, H.; Chen, S.; Zhang, W.; Duan, G. Endothelial activation and dysfunction in COVID-19: From basic mechanisms to potential therapeutic approaches. Signal Transduct. Target. Ther. 2020, 5, 293. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef]
- Gallo, G.; Calvez, V.; Savoia, C. Hypertension and COVID-19: Current Evidence and Perspectives. High Blood Press. Cardiovasc. Prev. 2022, 29, 115–123. [Google Scholar] [CrossRef]
- Kuluöztürk, M.; İn, E.; İlhan, N. Endocan as a marker of disease severity in pulmonary thromboembolism. Clin. Respir. J. 2019, 13, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhao, Y.; Wang, D.; Deng, W.; Li, C.; Li, Q.; Huang, S.; Shu, C. Endocan levels in peripheral blood predict outcomes of acute respiratory distress syndrome. Mediat. Inflamm. 2014, 2014, 625180. [Google Scholar] [CrossRef]
- Kosir, G.; Jug, B.; Novakovic, M.; Mijovski, M.B.; Ksela, J. Endocan is an independent predictor of heart failure-related mortality and hospitalizations in patients with chronic stable heart failure. Disease Markers 2019, 2019, 9134096. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Farooqui, T.; Sun, G.Y.; Lin, T.-N.; Teh, D.B.L.; Ong, W.-Y. COVID-19, Blood Lipid Changes, and Thrombosis. Biomedicines 2023, 11, 1181. [Google Scholar] [CrossRef]
- Görgün, S.; Cindoruk, Ş.; Özgen, E.; Yadigaroğlu, M.; Demir, M.T.; Yücel, M.; Akpınar, K.; Güzel, M. Diagnostic and Prognostic Value of Serum Endocan Levels in Patients with COVID-19. Angiology 2021, 72, 942–946. [Google Scholar] [CrossRef]
- Pascreau, T.; Tcherakian, C.; Zuber, B.; Farfour, E.; Vasse, M.; Lassalle, P. A high blood endocan profile during COVID-19 distinguishes moderate from severe acute respiratory distress syndrome. Crit. Care 2021, 25, 166. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, A.; Chenevier-Gobeaux, C.; Parmentier, E.; Delobel, J.-E.; Dubucquoi, S.; Mathieu, D.; Lassalle, P.; Caires, N.D.F. Endocan is a stable circulating molecule in ICU patients. Clin. Biochem. 2017, 50, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Güzel, A.; Duran, L.; Köksal, N.; Torun, A.; Alaçam, H.; Ekiz, B.C.; Murat, N. Evaluation of serum endothelial cell specific molecule-1 (endocan) levels as a biomarker in patients with pulmonary thromboembolism. Blood Coagul. Fibrinolysis 2014, 25, 272–276. [Google Scholar] [CrossRef]
- Chenevier-Gobeaux, C.; Ducastel, M.; Meritet, J.-F.; Ballaa, Y.; Chapuis, N.; Pene, F.; Carlier, N.; Roche, N.; Szwebel, T.-A.; Terrier, B.; et al. Plasma Endocan as a Biomarker of Thrombotic Events in COVID-19 Patients. J. Clin. Med. 2022, 11, 5560. [Google Scholar] [CrossRef]
- Tadzic, R.; Mihalj, M.; Vcev, A.; Ennen, J.; Tadzic, A.; Drenjancevic-Peric, I. The effects of arterial blood pressure reduction on endocan and soluble endothelial cell adhesion molecules (CAMs) and CAMs ligands expression in hypertensive patients on ca-channel blocker therapy. Kidney Blood Press. Res. 2013, 37, 103–115. [Google Scholar] [CrossRef]
- Lipińska-Gediga, M.; Lemańska-Perek, A.; Gozdzik, W.; Adamik, B. Changes in plasma endocan level are related to circulatory but not respiratory failure in critically ill patients with COVID-19. Sci. Rep. 2023, 13, 22307. [Google Scholar] [CrossRef] [PubMed]
- Lominadze, D.; Dean, W.L.; Tyagi, S.C.; Roberts, A.M. Mechanisms of fibrinogen-induced microvascular dysfunction during cardiovascular disease. Acta Physiol. 2010, 198, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B. C-reactive protein predicts outcome in COVID-19: Is it also a therapeutic target? Eur. Heart J. 2021, 42, 2280–2283. [Google Scholar] [CrossRef] [PubMed]
- Ungurianu, A.; Zanfirescu, A.; Grădinaru, D.; Ionescu-Tîrgoviște, C.; Miulescu, R.D.; Margină, D. Interleukins and redox impairment in type 2 diabetes mellitus: Mini-review and pilot study. Curr. Med. Res. Opin. 2022, 38, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Otoshi, R.; Hagiwara, E.; Kitayama, T.; Yamaya, T.; Higa, K.; Murohashi, K.; Sato, Y.; Tabata, E.; Shintani, R.; Okabayashi, H.; et al. Clinical characteristics of Japanese patients with moderate to severe COVID-19. J. Infect. Chemother. 2021, 27, 895–901. [Google Scholar] [CrossRef]
- Cremer, S.; Jakob, C.; Berkowitsch, A.; Borgmann, S.; Pilgram, L.; Tometten, L.; Classen, A.; Wille, K.; Weidlich, S.; Gruener, B.; et al. Elevated markers of thrombo-inflammatory activation predict outcome in patients with cardiovascular comorbidities and COVID-19 disease: Insights from the LEOSS registry. Clin. Res. Cardiol. 2021, 110, 1029–1040. [Google Scholar] [CrossRef]
- Beydoğan, E.; Atasoy, P.Y. The relationship between CRP at admission and thorax CT findings in patients diagnosed with COVID-19. Int. J. Clin. Pract. 2021, 75, e14962. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Kim, G.H.J.; Park, S.-B.; Lee, S.-I.; Koh, J.S.; Brown, M.S.; Abtin, F.; McNitt-Gray, M.F.; Goldin, J.G.; Lee, J.S. Quantitative Computed Tomography Lung COVID Scores with Laboratory Markers: Utilization to Predict Rapid Progression and Monitor Longitudinal Changes in Patients with Coronavirus 2019 (COVID-19) Pneumonia. Biomedicines 2024, 12, 120. [Google Scholar] [CrossRef]
- Henry, B.M.; Aggarwal, G.; Wong, J.; Benoit, S.; Vikse, J.; Plebani, M.; Lippi, G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am. J. Emerg. Med. 2020, 38, 1722–1726. [Google Scholar] [CrossRef]
- Malik, P.; Patel, U.; Mehta, D.; Patel, N.; Kelkar, R.; Akrmah, M.; Gabrilove, J.L.; Sacks, H. Biomarkers and outcomes of COVID-19 hospitalisations: Systematic review and meta-analysis. BMJ Evidence-Based Med. 2021, 26, 107–108. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, H.; Mu, S.; Wei, W.; Jin, C.; Tong, C.; Song, Z.; Zha, Y.; Xue, Y.; Gu, G. Lactate dehydrogenase an independent risk factor of severe COVID19 patients a retrospective and observational study. Aging 2020, 12, 11245–11258. [Google Scholar] [CrossRef]
- Suzuki, K.; Ichikawa, T.; Suzuki, S.; Tanino, Y.; Kakinoki, Y. C-reactive protein and lactate dehydrogenase are useful biomarkers for predicting the requirement for oxygen therapy in outpatients with coronavirus disease 2019. medRxiv 2021, 1–12. [Google Scholar] [CrossRef]
- Sui, J.; Noubouossie, D.F.; Gandotra, S.; Cao, L. Elevated Plasma Fibrinogen Is Associated With Excessive Inflammation and Disease Severity in COVID-19 Patients. Front. Cell. Infect. Microbiol. 2021, 11, 734005. [Google Scholar] [CrossRef]
- Kerr, R.; Stirling, D.; Ludlam, C.A. Interleukin 6 and Haemostasis. Br. J. Haematol. 2001, 115, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Dong, Y.; Wang, H.; Guo, W.; Zhou, H.; Zhang, Z.; Tian, C.; Du, K.; Zhu, R.; Wang, L.; et al. Cardiovascular disease potentially contributes to the progression and poor prognosis of COVID-19. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1061–1067. [Google Scholar] [CrossRef]
- Zhan, H.; Chen, H.; Liu, C.; Cheng, L.; Yan, S.; Li, H.; Li, Y. Diagnostic Value of D-Dimer in COVID-19: A Meta-Analysis and Meta-Regression. Clin. Appl. Thromb. 2021, 27, 10760296211010976. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Mansouritorghabeh, H. D-dimer level in COVID-19 infection: A systematic review. Expert Rev. Hematol. 2020, 13, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, L.; Bai, L.; Huang, Z.; Peng, Y. Association between D-dimer level and chest CT severity score in patients with SARS-CoV-2 pneumonia. Sci. Rep. 2021, 11, 11636. [Google Scholar] [CrossRef] [PubMed]
- Atis, O.; Keles, M.; Cankaya, E.; Dogan, H.; Aksoy, H.; Akcay, F. Vitamin D treatment effect on serum endocan and high-sensitivity c-reactive protein levels in renal transplant patients. Prog. Transplant. 2016, 26, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Kose, M.; Senkal, N.; Tukek, T.; Cebeci, T.; Atalar, S.C.; Altinkaynak, M.; Arici, H.; Genc, S.; Catma, Y.; Kocaaga, M. Severe vitamin D deficiency is associated with endothelial inflammation in healthy individuals even in the absence of subclinical atherosclerosis. Eur. Rev. Med. Pharm. Sci. 2022, 26, 7046–7052. [Google Scholar] [CrossRef]
| Demographics | |
|---|---|
| Age (years) | 71.48 ± 14.75 |
| Male n (%) | 27 (48.21%) |
| Female n (%) | 29 (51.79%) |
| Comorbidities | |
| Cardiovascular disease, n (%) | 46 (82.14%) |
| Hypertension | 36 (64.28%) |
| Heart failure | 10 (17.85%) |
| Arrhythmias | 14 (25%) |
| Overweight/Obesity | 14 (25%) |
| Diabetes mellitus | 10 (17.85%) |
| Hyperlipidemia | 10 (17.85%) |
| Chronic renal failure | 7 (12.5%) |
| Chronic obstructive pulmonary disease | 4 (7.14%) |
| Thorax CT finding | |
| Interstitial pneumonia with pleural effusion/mediastinitis | 18 (32.14%) |
| Interstitial pneumonia | 25 (44.64%) |
| No finding | 9 (16.07%) |
| Treatment at admission | |
| Oxygen therapy | 13 (23.31%) |
| Dexamethasone | 15 (26.78%) |
| Vaccinating status | |
| Vaccinated | 25 (44.64%) |
| Unvaccinated | 31 (55.36%) |
| Clinical outcome | |
| Discharged with recovery | 54 (96.42%) |
| Death | 2 (3.58%) |
| Parameters | Value |
|---|---|
| Glucose (mg/dL) | 106.5 [98; 126.25] |
| Urea (mg/dL) | 36.5 [27; 54.5] |
| Creatinine (mg/dL) | 0.9 [0.7; 1.18] |
| Bilirubin (mg/dL) | 0.6 [0.5; 0.8] |
| CKMB (U/L) | 12 [8; 19] |
| CK (U/L) | 71 [42; 116] |
| ALT (U/L) | 25 [19.25; 39.5] |
| AST (U/L) | 32 [26.25; 46] |
| LDH (U/L) | 234 [187; 305] |
| Alkaline phosphatase (U/L) | 78 [56; 94] |
| GGT (U/L) | 33 [25; 51] |
| Lipase (U/L) | 117.5 [55; 193.75] |
| CRP (mg/L) | 27.6 [7.52; 85.3] |
| Ferritin (ng/mL) | 303.25 [187.48; 539.2] |
| IL6 (pg/mL) | 71.9 [24.5; 206.1] |
| TNF-α (pg/mL) | 67.94 ± 37.17 |
| IL1 (pg/mL) | 13.9 [1.49; 40.3] |
| PAI1(pg/mL) | 267.66 ± 153.62 |
| WBC × 1000/µL | 7.4 [4.56; 9.28] |
| Neutrophil × 1000/µL | 5.5 ± 2.59 |
| Lymphocyte × 1000/µL | 0.85 [0.69; 1.28] |
| Hemoglobin (g/dL) | 12.53 ± 1.35 |
| Platelets × 1000/µL | 193 [155; 260] |
| D dimers (ng/mL) | 530 [214.5; 3100] |
| Fibrinogen (mg/dL) | 367.17 [307.5; 575.7] |
| Endocan (pg/mL) | 77.21 ± 31.4 |
| Demographics | Mild Cases | Moderate Cases | Severe Cases | p Value |
|---|---|---|---|---|
| Age (years) | 63.5 [51.25; 78] | 78.5 [78; 83] | 83 [76.75; 85.5] | |
| Males | 2 (13.33%) | 16 (64%) | 9 (56.25%) | |
| Females | 13 (86.67%) | 9 (36%) | 7 (43.75%) | |
| Comorbidities | ||||
| Cardiovascular disease | 5 (33.33%) | 15 (60%) | 16 (100%) | |
| Hypertension | 4 (26.67%) | 13 (52%) | 13 (81.25%) | |
| Overweight/Obesity | 3 (20%) | 6 (24%) | 5 (31.25%) | |
| Diabetes mellitus | 1 (6.66%) | 4 (16%) | 4 (25%) | |
| Vaccinating status | ||||
| Vaccinated | 5 (33.3%) | 14 (56%) | 6 (37.75%) | |
| Unvaccinated | 10 (66.67%) | 11 (44%) | 9 (62.25%) | |
| Treatment on admission | ||||
| Oxygen therapy | 0 | 1 (4%) | 13 (81.25%) | |
| Dexamethasone used | 3 (20%) | 2 (8%) | 9 (56.25%) | |
| Biochemical parameter | ||||
| Urea(mg/dL) | 26.5 [22.25; 37] | 36 [28.75; 50] | 56.5 [36.5; 86.5] | <0.001 |
| Creatinine (mg/dL) | 0.75 [0.7; 1] | 1 [0.78; 1.2] | 1.1 [0.78; 1.83] | 0.041 |
| LDH(U/L) | 216.5 [177.25; 236] | 234 [178; 307] | 264 [213.5; 353.5] | 0.04 |
| C reactive protein (mg/L) | 11.95 [7.57; 46.08] | 19.9 [6.4; 41.58] | 115.8 [36.78; 156.7] | 0.004 |
| IL6 (pg/mL) | 108.05 [25.9; 218.4] | 42.1 [11.4; 93.1] | 343.7 [94.43; 645.9] | 0.012 |
| Alkaline phosphatase (U/L) | 78 [61.5; 110] | 70 [55; 94.75] | 84 [55.75; 94.25] | 0.804 |
| GGT (U/L) | 32 [21.5; 56] | 33 [27.5; 73.25] | 37.5 [24.5; 51] | 0.813 |
| Red blood cells | 4.71 [4.5; 4.95] | 4.35 [3.85; 4.49] | 4.06 [3.7; 4.76] | 0.004 |
| Hemoglobin (g/dL) | 13.2 [11.63; 13.78] | 12.6 [11.68; 13.43] | 11.9 [11.2; 14] | 0.656 |
| Prothrombin time (s) | 12.05 [11.2; 13.13] | 12.1 [11.38; 14.13] | 14 [12.45; 15.58] | 0.044 |
| D dimers (ng/mL) | 448 [190; 1427.5] | 372 [190; 2525] | 1600 [464.5; 7322] | 0.046 |
| Fibrinogen (mg/dL) | 361 [283.65; 596.26] | 352.25 [307.8; 480.91] | 595.37 [356.27; 637.22] | 0.021 |
| Endocan (pg/mL) | 74.28 ± 35.97 | 87.2 ± 29.2 | 65.7 ± 24.35 | 0.117 |
| With Cardiovascular Disease (46) | Without Cardiovascular Disease (10) | p Value | |
|---|---|---|---|
| Alkaline phosphatase (U/L) | 84 [59; 112.5] | 67 [54.75; 80.75] | 0.034 |
| Lactate dehydrogenase (U/L) | 251 [202.75; 320] | 211 [160.5; 240] | 0.030 |
| C reactive protein (mg/L) | 38.2 [11.35; 121] | 10.2 [6.28; 26.5] | 0.006 |
| Fibrinogen (mg/dL) | 472.98 [332.26; 603.51] | 289.29 [257.22; 375.85] | 0.002 |
| Endocan (pg/mL) | 79 ± 30.39 | 73.72 ± 34.02 | 0.557 |
| Endocan Levels (pg/mL) | p Value | ||
|---|---|---|---|
| Absent | Present | ||
| Comorbidity | 77.08 ± 23.99 | 77.24 ± 33.08 | 0.988 |
| Diabetes | 77.2 ± 31.99 | 77.28 ± 30.31 | 0.994 |
| Cardiovascular disease | 73.72 ± 34.02 | 79 ± 30.39 | 0.557 |
| Obesity | 79.12 ± 31.27 | 70.89 ± 32.51 | 0.414 |
| Oxygen need | 78.75 ± 33.02 | 71.55 ± 25.32 | 0.424 |
| Dexamethasone treatment | 75.47 ± 32.22 | 81.96 ± 29.81 | 0.499 |
| Vaccination | 89.63 ± 28.5 | 61.81±28.41 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantin, L.; Ungurianu, A.; Streinu-Cercel, A.; Săndulescu, O.; Aramă, V.; Margină, D.; Țârcomnicu, I. Investigation of Serum Endocan Levels in SARS-CoV-2 Patients. Int. J. Mol. Sci. 2024, 25, 3042. https://doi.org/10.3390/ijms25053042
Constantin L, Ungurianu A, Streinu-Cercel A, Săndulescu O, Aramă V, Margină D, Țârcomnicu I. Investigation of Serum Endocan Levels in SARS-CoV-2 Patients. International Journal of Molecular Sciences. 2024; 25(5):3042. https://doi.org/10.3390/ijms25053042
Chicago/Turabian StyleConstantin, Laura, Anca Ungurianu, Anca Streinu-Cercel, Oana Săndulescu, Victoria Aramă, Denisa Margină, and Isabela Țârcomnicu. 2024. "Investigation of Serum Endocan Levels in SARS-CoV-2 Patients" International Journal of Molecular Sciences 25, no. 5: 3042. https://doi.org/10.3390/ijms25053042
APA StyleConstantin, L., Ungurianu, A., Streinu-Cercel, A., Săndulescu, O., Aramă, V., Margină, D., & Țârcomnicu, I. (2024). Investigation of Serum Endocan Levels in SARS-CoV-2 Patients. International Journal of Molecular Sciences, 25(5), 3042. https://doi.org/10.3390/ijms25053042







