Abstract
The human skeleton is a metabolically active system that is constantly regenerating via the tightly regulated and highly coordinated processes of bone resorption and formation. Emerging evidence reveals fascinating new insights into the role of sphingolipids, including sphingomyelin, sphingosine, ceramide, and sphingosine-1-phosphate, in bone homeostasis. Sphingolipids are a major class of highly bioactive lipids able to activate distinct protein targets including, lipases, phosphatases, and kinases, thereby conferring distinct cellular functions beyond energy metabolism. Lipids are known to contribute to the progression of chronic inflammation, and notably, an increase in bone marrow adiposity parallel to elevated bone loss is observed in most pathological bone conditions, including aging, rheumatoid arthritis, osteoarthritis, and osteomyelitis. Of the numerous classes of lipids that form, sphingolipids are considered among the most deleterious. This review highlights the important primary role of sphingolipids in bone homeostasis and how dysregulation of these bioactive metabolites appears central to many chronic bone-related diseases. Further, their contribution to the invasion, virulence, and colonization of both viral and bacterial host cell infections is also discussed. Many unmet clinical needs remain, and data to date suggest the future use of sphingolipid-targeted therapy to regulate bone dysfunction due to a variety of diseases or infection are highly promising. However, deciphering the biochemical and molecular mechanisms of this diverse and extremely complex sphingolipidome, both in terms of bone health and disease, is considered the next frontier in the field.
1. Introduction
Lipids are fundamental building blocks ubiquitous in all cells and are increasingly becoming recognized as critical components of major signaling and regulatory pathways in human biology, physiology, and pathophysiology. Remarkably, the eukaryotic lipidome is comprised of an extremely heterogeneous body of molecules, and emerging analyses reveal unprecedented and unanticipated complexity, whereby many stimuli and species affect one or more enzymes [1]. Due to the large number of varying biochemical transformations that occur during their biosynthesis, lipid structures are generally much more complex than linear protein combinations [2]. As such, lipid diversity is considered of a magnitude that contends with the proteome, where together, cells express tens of thousands and hundreds of lipids and proteins, respectively, together tightly regulating metabolism, energy storage, and transport [2,3]. Using the LIPIDs MAPS classification system, lipids have been divided into eight categories: fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, sterol lipids, prenol lipids, saccharolipids, and polyketides, with each containing distinct classes and subclasses of biomolecules, thereby delivering differing effects [2,4,5].
The concept of bioactive lipids and their role in human physiology and pathophysiology has increasingly evolved over recent decades and continues to gain considerable traction [6]. Bioactive lipids are functionally defined as lipid species able to react and respond following the delivery of specific stimuli, thereby in turn mediating definitive downstream effectors and targets via their contribution to various signaling pathways [7,8]. As such, bioactive lipids are distinguishable from other lipids with known structural and/or energetic functions. Recent research has shown that sphingolipids, a major class of eukaryotic lipids containing 4000 distinct chemical entities [9], are highly bioactive and activate distinct protein targets, including lipases, phosphatases, kinases, and other membrane receptors and enzymes, thereby confirming distinct cellular functions beyond energy metabolism [1,10]. In this respect, sphingolipids have been shown to be critical in mediating cellular processes, including regulating the actin cytoskeleton, endocytosis, cell cycle, apoptosis [11], and cell stress responses, including programmed cell death [12], senescence [13], cell survival, migration, adhesion, inflammation, vesicular trafficking, and phagocytosis [1,14,15,16]. Although sphingolipids represent a small proportion of the total cellular lipid pool, their aberrant ectopic accumulation within tissues not equipped for fat storage is reported to drive cellular dysfunction and damage [17,18]. To this end, sphingolipids have been theorized to contribute essential roles in cancer [19], obesity [20], type 2 diabetes, cardiovascular and immune dysfunction, Gaucher’s disease, and neurodegeneration [1,18,21,22]. However, and despite being a rapidly expanding area of high significance, their potential role as biomarkers of disease, diagnostic markers differentiating between bacterial and viral infections, or use towards the development of novel therapeutic approaches remains elusive. To this end, a major unresolved challenge is the necessity to identify and decode their structures and biochemical and molecular mechanisms of action.
1.1. A Summary of Eukaryotic Sphingolipid Metabolism
Sphingolipids are ubiquitous and involved in a diverse number of cell functions, making them essential components within all eukaryotic membranes [1]. The synthesis and metabolism of sphingolipids are not presented here but have been previously and comprehensively reviewed [1,20,21,23,24,25,26]. In brief, sphingolipids are characterized by the presence of a long-chained sphingoid base together with a 2-amino group amide linked to a fatty acid (FA) and a polar head group. This combination forms ceramide, the core unit. As such, ceramides constitute a family of closely related molecules and form the precursor and metabolic hub of all complex sphingolipids. In brief, ceramide occupies a central position within a highly coordinated system that interconnects several pathways in both sphingolipid biosynthesis and catabolism. Fundamentally, the family of sphingolipids is defined by the type of FA, carbon length, polar head group, degree of unsaturation, and hydroxylation, among other characterizations [10].
The first step in the de novo sphingolipid biosynthetic pathway begins in the endoplasmic reticulum (ER) with condensation of an amino acid, most frequently serine, typically together with the 16-carbon saturated FA palmitate, which is activated following a reaction with fatty acyl-CoA [20] (Figure 1). This reaction is catalyzed by the enzyme serine palmitoyltransferase (SPT), a membrane-bound component of the ER, to form transient 3-ketodihydrosphingosine. The 3-ketodihydrosphingosine is then rapidly reduced to dihydrosphingosine followed by N-acylation by one of six ceramide synthases to form dihydroceramides [27,28]. Dihydroceramide desaturase (DES1 or DES2) subsequently yields ceramide, as shown in Figure 1 [29,30]. Notably, as the enzyme SPT is the only entrance point into the sphingolipid network, it uniquely serves as a critical node for regulating the steady state and rate-limiting fluctuations in sphingolipid metabolism [31]. To this end, the abundance of the sphingolipid pool is primarily dictated via feedback from a family of three highly homologous ER membrane-bound ORMDL proteins. These homeostatic regulators of SPT are able to sense ceramide levels, and when beyond physiologic thresholds, the ORMDL proteins subsequently inhibit SPT activity [20,31,32]. Notably, another protein has also been found essential in regulating SPT activity. A more recent investigation highlighted the important contribution of Nogo-B, a membrane protein of the ER, which also contributes direct effects to SPT activity [33]. However, its interactions with both SPT and ORMDL proteins remain poorly understood.
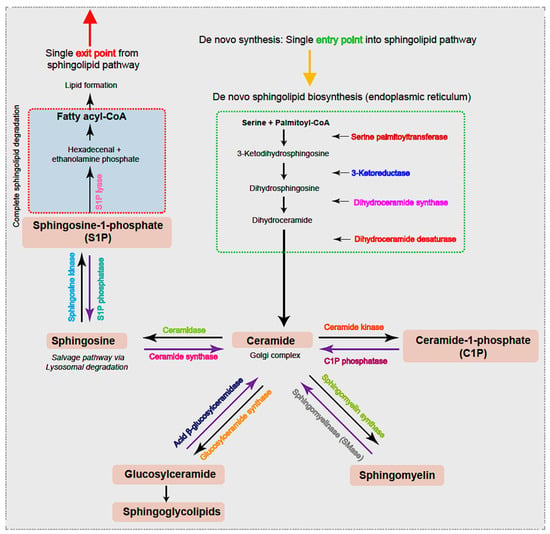
Figure 1.
Ceramide, the hub of the sphingolipid pathway, can be generated via different pathways. De novo synthesis, i.e., the single entry point into the cycle, begins in the ER with condensation of typically serine and palmitoyl-CoA to form 3-ketosphingosine. The 3-ketodihydrosphingosine is subsequently and rapidly converted to dihydroxyceramide prior to acylation to dihydroceramides by the action of dihydroceramide synthase. Dihydroceramide is then dehydrated by dihydrodesaturase (DES) to form ceramide, which is translocated to the Golgi complex. Ceramides can also be generated via the sphingomyelinase (SMase) pathway that degrades sphingomyelin or via the catabolic pathway that generates ceramides from sphingosine by ceramide synthase from glucosylceramides by acid β-glucosylceramidase or by C1P via the action of C1P phosphatase. Similarly, and in parallel, the reactions are reversible, and ceramides generate sphingomyelin through the activity of sphingomyelin synthase, sphingosine through the enzyme ceramidase within the lysosome, and C1P via ceramide kinase activity. Further, sphingosine can instead be converted to S1P via sphingosine kinase or instead irreversibly cleaved by ER-localized S1P lyase, leading to complete sphingolipid degradation, the single exit route within the sphingolipid pathway. The various enzymes involved within the pathway are highlighted in color.
Following both vesicular- and protein-mediated transportation from the ER and predominantly within the Golgi complex, ceramides serve as a substrate for sphingolipid metabolism. Through modifications located at the 1-hydroxyl position, ceramides are incorporated into various complex sphingolipids that fall within four primary categories. First, ceramide phosphorylation occurs via the enzyme ceramide kinase and generates the sphingolipid ceramide-1-phosphate (C1P) [34]. In the second category, the addition of a sugar molecule leads to glycosylation by glucosyl or galactosyl ceramide synthases to form glycoceramides and, subsequently, glycosphingolipids [35]. Third, and via sphingomyelin synthases, ceramides can receive a phosphocholine head group from phosphatidylcholine to form sphingomyelin (SM), the most abundant mammalian sphingolipid [1,36]. The fourth category is formed within the lysosome where the acyl chain may be removed from the ceramide substrate to produce the lyso-sphingolipid sphingosine and via action of the enzyme ceramidase [37]. Notably, sphingosine can be salvaged and recycled to ceramide for reutilization by the action of ceramide synthase or, alternatively, phosphorylated by sphingosine kinases (SPHK1 and SPHK2) to form sphingosine-1-phosphate (S1P). S1P can then be irreversibly cleaved by ER-localized S1P lyase, forming the single exit route, a pathway that results in complete sphingolipid degradation. Alternatively, S1P can be dephosphorylated by S1P phosphatase and returned to form sphingosine followed by ceramide. Notably, ceramide (proapoptotic) and S1P (antiapoptotic) display opposing effects, and it has been suggested that the ratio of these two molecules is crucial to understanding their pathological roles [20]. Further, SM, C1P, and glucosylceramides can also be salvaged and recycled to ceramide for reutilization via the action of sphingomyelinase (SMase), C1P phosphatase, and acid β-glucosylceramidase activity, respectively.
Together, these reactions regulate extra- and intra-cellular sphingolipid concentrations prior to their being shipped to other membranes, where complex cellular trafficking subsequently directs their distribution to initiate a myriad of signaling pathways [18,38]. Thus, the sphingolipidome is highly complex, where each can interconnect and interconvert one bioactive lipid into others. Thus, functionally distinct pools of chemically equivalent sphingolipids may be generated by either de novo synthesis or by the recycling and reutilization of existing complex sphingolipids. It is highly conceivable that even minor alterations in the species or enzyme produced can result in a ripple effect, thereby dictating subsequent metabolite levels, enzyme activities, and specific cellular end-functions [39].
1.2. The Basics of Healthy Bone Homeostasis
Bone is a highly dynamic and metabolically active tissue. Throughout life, it undergoes synchronous events that together orchestrate and tightly regulate cycles of bone resorption and formation. When in balance, this essential sequence of events enables the repair, restoration, and maintenance of a healthy bone tissue microenvironment, as well as the integrity of a mechanically functional structure. Traditionally, the critical cells involved include bone-forming osteoblasts and their precursors (i.e., bone marrow-derived mesenchymal stem cells (BMSCs)), bone-resorbing osteoclasts and their precursor cells, and the overarching regulatory contribution of osteocytes. In brief, bone formation or resorption occurs through complex interactions between (i) the transmembrane receptor activator of nuclear factor-κB (NF-κB (also known as RANK)), (ii) the receptor activator of nuclear factor κB ligand (RANKL), and (iii) the decoy protein osteoprotegerin (OPG), which together upregulate or downregulate osteoclast activity (Figure 2) [40,41,42,43]. Fundamentally, the up- or downregulation of bone formation or resorption is maintained via fluctuations in the RANKL:OPG ratio, where increased RANKL predisposes to increased osteoclastic activity, while a higher abundance of OPG is associated with increased osteoblastic activity and bone formation. Furthermore, emerging studies highlight the important osteoimmunological contribution. For example, macrophages directly regulate bone turnover through release of either pro-inflammatory cytokines (e.g., interleukin 1β (IL-1β) and IL-6), resulting in bone loss, or anti-inflammatory cytokines (e.g., IL-10 and IL-13) that promote bone formation and repair [44,45,46]. Further, mature B-lymphocytes produce >50% of total bone marrow-derived OPG, and mice lacking both T- and B-lymphocytes develop osteoporosis, although the critical role of T-cells in bone homeostasis is less clear [47,48]. Further, megakaryocytes derived from hematopoietic cells express both RANKL and OPG, and secrete anti-osteoclastic and bone anabolic factors [49,50]. Mechanical signals (e.g., compression, tensile strain, and shear stress), as well as hormonal cues (e.g., calcitonin, parathyroid hormone, 1,25(OH)2 vitamin D3, and estrogen) and growth factors (e.g., insulin-like growth factor (IGF), transforming growth factor beta (TGFβ), fibroblast growth factors (FGFs), epidermal growth factor (EGF), Wingless-related integration sites (Wnts), and bone morphogenetic proteins (BMPs)) are critical activators of bone formation or loss [51,52,53]. Also among the essential mechanisms for healthy bone homeostasis is the coordinated migration and trafficking of cells between the bone marrow and blood, as well as the regulated migration of precursor and mature cells to distinct regions on the bone surface for remodeling or repair. However, the intrinsic spatial, biomolecular, and mechanotransduction complexities are nevertheless still debated [52].
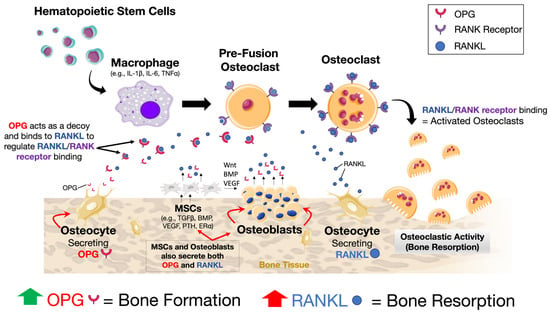
Figure 2.
A schematic showing the relationship between RANK, RANKL, and OPG in activating or inhibiting osteoclastic activity and subsequent bone resorption. The binding of RANKL to the membrane-bound receptor RANK results in the activation of osteoclastic activity. However, OPG acts as a decoy and, if present, will bind to RANKL, thereby preventing RANK–RANKL interaction and osteoclastic activation. Thus, bone formation versus resorption is fundamentally dependent on the RANKL:OPG ratio, where increased RANKL expression results in bone resorption and increased OPG in bone formation. Many cells secrete either or both OPG or RANKL or both (e.g., osteocytes, osteoblasts, BMSCs, B-lymphocytes, and megakaryocytes). Regulation of the cellular response occurs via mechanical, hormonal, and growth factor-induced signaling, among others, making the overall governance of this system highly complex.
1.3. Role of Lipids in Bone Turnover
Lipids are an important nutrient and contribute a critical component to bone homeostasis, regeneration, and bone disease. In terms of homeostasis, adipocytes regulate bone formation via the inhibition or promotion of osteoblast and osteoclast differentiation [54]. This is achieved in part through the expression and secretion of peptides derived from white adipose tissue, including leptin, adiponectin, omentin-1, vesfatin, and resistin, as well as via adipocytokines such as tumor necrosis factor alpha (TNFα), IL-6, and IL-1β [55,56,57,58]. Fatty acids [59,60], cholesterol [61,62], phospholipids [63], and several endogenous lipid metabolites, including prostaglandins [64] and oxysterols [65], are also reported to stimulate bone cell function and activity. The lineage commitment of BMSCs towards either an osteoblastic or adipogenic fate are closely related, and preferential engagement along one axis over the other contributes an essential role in regulating bone mass [54]. This is likely due to several extracellular signaling proteins possessing overlapping functions, thereby contributing a key role in determining adipogenic versus osteogenic fate. These include the master adipogenic transcription factor peroxisome proliferator activated receptor gamma (PPARγ), osteoblast-specific transcription factors Runx2 and Osterix, as well as other proteins, including BMP-2, BMP-4, BMP-6, BMP-7, BMP-9, IGF, and FGFs [54,66]. It has been generally assumed that lipids are present within the bone marrow only and are largely absent from the mineralized bone structure itself. However, evidence suggests the mineral component contains small amounts of lipids that may play a pivotal role in bone physiology [67]. In this respect, lipids are not only located within bone cells as loosely bound and easily utilized biomolecules but also form tightly associated complexes with proteins and minerals within the mineralized tissue matrix. The presence of lipid within the porous compartments of trabecular bone is reported to restrict its hydraulic permeability, thereby influencing the transport of nutrients and waste products and thus the metabolic function of the cells located within [68]. Further, van Gastel et al. [69] showed that the decreased availability of extracellular lipids resulted in chondrogenic over osteogenic differentiation of skeletal progenitor cells.
Our interest in the role of lipids in bone turnover is increasingly expanding due to recent evidence that indicates a reciprocal relationship between bone mass and bone marrow adiposity. To this end, an increase in bone marrow adiposity has been observed in most bone loss conditions, including aging [55,70], osteoarthritis [71,72], obesity [73], osteomyelitis [74], and various other pathological conditions [55,75,76,77]. Further, adipokines contribute to the progression of chronic inflammation [78], which is also a key cause of multiple pathological bone loss conditions [79,80,81,82,83]. Together, this is of high significance, as our aging population and obesity continue to rise globally. As such, the treatment and care of conditions, including osteoporosis, osteoarthritis, and other chronic inflammatory-associated health conditions, will undoubtedly bear a huge health–economic burden in all regions of the world [84,85,86]. Obesity plays an important role in immunity and is considered a state of prolonged inflammation. Recent clinical and experimental studies have determined a correlation between the progress of obesity and some infections, prompting emergence of the “infectobesity theory” [87]. As such, bacterial-targeted adipose dysfunction and use as a reservoir for pathogens could promote virulence, exacerbate infection, and ultimately result in bone loss. To this end, a switch in lineage commitment toward adipogenic differentiation at the expense of osteoblastogenesis and increased bone marrow adiposity has also been identified during bacterial infection [74,88]. Notably, bacteria including Staphylococcus aureus and Escherichia coli are able to adhere and internalize within adipose-derived MSCs, and the subsequent up- or downregulation of osteogenic versus adipogenic differentiation was observed to be dependent on the type of bacterial strain [89]. Finally, exposure to ionizing radiation (IR) is critical during radiotherapy but does cause osteoradionecrosis, osteoporosis, pathologic insufficiency fractures, and nonunions, causing significant pain and morbidity in cancer survivors [90,91,92,93]. A switch in lineage commitment toward adipogenic differentiation at the expense of osteogenic differentiation, thereby leading to increased bone marrow adiposity, is reported following IR-induced bone injury [65]. Additionally, the transdifferentiation of osteoblasts into adipocytes [66] has also been identified. Together, these studies highlight the important role of the osteo-adipogenic axis in bone disease and infection, conditions that currently affect a substantial number of individuals and for which effective treatment strategies remain to be discovered.
To date, it remains unclear whether the increase in marrow adiposity is a result of or the cause of bone loss [54]. Notably, osteoblasts produce and secrete various forms of lipids (e.g., triglycerides, cholesterol, and phospholipids [94,95]), as well as initiate the nonenzymatic oxidation of lipoproteins, which results in a reactive oxygen species-induced inflammatory microenvironment and osteopathogenesis [96,97,98]. Of the numerous classes of lipids that accrue, sphingolipids are considered among the most deleterious. Here, we review and summarize evidence that dysregulation of sphingolipid metabolism, in particular, ceramide, C1P, S1P, acid sphingomyelinase, and SM, correlates with the pathogenesis of bone disease, osteoporosis, and dysfunction due to bacterial infection.
2. Sphingolipids in Bone Homeostasis and Disease
2.1. Ceramides
Ceramides are signals of lipid excess and have been reported to confer many pathological effects. For example, ceramides promote the expression of genes that facilitate the incorporation of free FAs into harmful triglycerides and expedite their storage in lipid droplets [29,99]. Additionally, ceramides inhibit the uptake of both glucose [100,101] and amino acids [102,103], leading to the preferential utilization of FAs for energy. Further, ceramides decrease mitochondrial efficiency, inhibiting oxygen consumption via the electron-transport chain, thereby leading to decreased adenosine triphosphate (ATP) produced per FA molecule [104,105]. Finally, ceramides slow lipolysis by blocking activation of hormone-sensitive lipase [29]. Together, these ceramide actions promote the use and storage of FAs. Remarkably, preclinical rodent studies have shown that the inhibition of ceramide biosynthesis resulted in the amelioration of hypertriglyceridemia [29,106,107], type 2 diabetes [106], insulin resistance [29,106], hepatic steatosis [29,106,107], atherosclerosis [108,109], and heart failure [110,111]. It is now apparent that several metabolic disorders, including inflammation, oxidative stress, hormonal triggers, and the gut microbiome, influence ceramide synthesis and degradation [20]. To this end, ceramide production is elevated on exposure to excessive ROS [112], pro-inflammatory cytokine release [113,114], and alterations in gut bacteria of the phylum Bacteroidetes [115] (Figure 3). Further, elevated ceramide levels induce BMSC, fibroblast senescence, and death [13,116]. In the context of bone tissue, together, oxidative stress, pro-inflammatory cytokine release, and an altered gut microbiome are primary drivers associated with promoting bone resorption while impairing bone formation, thereby progressing the pathogenesis of bone disease and osteoporosis [117,118,119,120,121]. However, the direct effect of ceramide on bone remains largely unexplored.
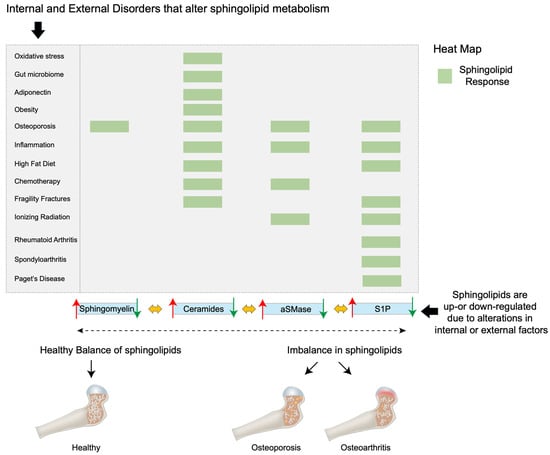
Figure 3.
A heat map showing how biomolecules, the gut microbiome, diet, disease, and external factors (e.g., chemotherapy and ionizing radiation) can alter sphingolipid metabolism. When in balance, bone tissue function and structure are maintained. However, when sphingolipid levels, including sphingomyelin, ceramide, aSMase, and S1P, are either up- or downregulated, this can result in, for example, osteoporosis, fragility fractures, inflammation, Paget’s disease, osteoarthritis, rheumatoid arthritis, and spondyloarthritis.
At the cellular level, lower ceramide concentrations (≤10−7 M) are reported to promote osteoblast viability; however, levels ≥ 2 × 10−6 M significantly reduced viability and increased apoptosis in a dose- and time-dependent manner [122]. Alsahli et al. [123] investigated the osteoblast response to increased concentrations of palmitic acid, and similarly reported that ceramide accumulation inhibited osteoblast function in vitro and bone formation markers in a high-fat diet murine model in vivo. Further, endogenous cellular ceramide concentrations have been demonstrated to increase osteoblastic apoptosis following TNFα treatment and via the NF-κβ pathway [124]. Nevertheless, physiological concentrations of ceramide appear to be essential in bone development. Cilia, the mechanosensory antenna on many cell types, are present on osteoblasts and their precursors are dependent on the sphingolipid ceramide for their genesis [125]. Primary cilia are required for osteoblast and osteocyte polarity and alignment during bone development [126,127], and ciliopathies result in various skeletal abnormalities, including dysplasias [128,129]. Notably, ceramide depletion via neutral sphingomyelinase 2 (nSMase2), abolished primary cilia distribution and differentiation [130], and ceramide located at the base of cilia were shown to be critical in maintaining bone mass via β-catenin signaling in osteoblasts [127].
When stimulated, BMSCs mobilize from the bone marrow into the peripheral bloodstream and towards the site of bone injury, where they assist in regeneration and repair. The migratory “homing” of BMSCs is facilitated by factors including C1P [131,132], stromal cell-derived factor-1 (SDF-1) [133,134], substance P (SP) [135,136], and TGFβ [137]. A recent study by Yu et al. [138] demonstrated that intracellular ceramide kinase is also essential for BMSC migration for this purpose. Similarly, ceramides contribute to bone resorption, and the influence of ceramide on osteoclast apoptosis was also demonstrated to occur in a dose- and time-dependent manner [122]. To this end, ceramides have been demonstrated to mediate pro-apoptotic pathways and an anti-inflammatory response [6,139] while enhancing osteoclast survival and reducing osteoclastic activity via inhibition of F-actin ring formation [140]. Contrarily, increased levels of lactosylceramide, a glycosphingolipid, increased RANKL expression in osteoclasts, and data suggested that lactosylceramide is necessary for the initiation step of RANKL-induced osteoclastogenesis [141]. Several pro-inflammatory cytokines, including IL-1, increase cellular ceramide levels [6], and TNFα has also been shown to activate sphingomyelinases, thereby acutely liberating ceramides [12,142,143]. In terms of IL-1 activation, studies on hepatocytes have suggested that the ceramide formed is generated following the degradation of SM via the activation of neutral or acidic SMases to IL-1 [144,145]. Further, emerging evidence indicates that alterations in ORMDL protein levels confer a regulatory mechanism for the formation of sphingolipids. As described above, it has been widely considered that ORMDL proteins are negative regulators of SPT, where increased expression of ORMDL protein would further inhibit de novo sphingolipid synthesis [31]. However, and notably, overexpression of ORMDL3 increased SPT activity and ceramide levels in RAW264.7 macrophages, which promoted chronic inflammation in vitro and within a murine model in vivo [146]. Further, sphingolipid signaling during an IL-1-mediated inflammatory response has also been shown to increase ceramide levels via ORMDL protein expression during the de novo sphingolipid biosynthesis pathway [147]. Together, these data suggest a key concentration-dependent role for ceramides in driving both bone formation and bone resorption in bone metabolism.
Furthermore, human plasma levels of ceramide have been shown to increase with age in women, and are negatively correlated with serum estradiol concentrations [148]. To this end, Kim et al. [149] reported that ceramides directly increased osteoclastogenesis, the expression of osteoclast differentiation markers, and bone resorption in vitro. Further, the study also demonstrated increased levels of ceramide within the peripheral bloodstream of patients of increasing age. Notably, patients who presented with higher levels of ceramide were predisposed to increased fragility fractures of the hip and presented with increased bone resorption markers within blood and bone marrow aspirate biopsies. Notably, the de novo synthesis of ceramide and subsequent cellular apoptosis is enhanced in response to some chemotherapeutic agents, including etoposide and daunorubicin [150,151]. Finally, C1P, a product of ceramide phosphorylation, is widely reported to be proinflammatory via the NF-κβ pathway [152]. More recently, C1P was also found to potently activate cell growth and survival and regulate cell migration, as well as deliver anti-inflammatory properties to some cell types and tissues [153,154,155]. Additionally, C1P plays an important role in phagocytosis and macrophage chemotaxis [15,156].
2.2. Sphingosine-1-Phosphate
The lipid mediator S1P is the most studied and can act directly on intracellular targets or alternatively via an intracellular second messenger or extracellular signal molecule by binding membrane receptors called G protein-coupled receptors, namely, S1PR1 to S1PR5 [67]. As such, S1P has both intracellular, S1PR-independent actions (e.g., calcium release [155], TNFα signaling [157], or PPARγ [158]) and S1PR-dependent extracellular functions in a variety of tissues. S1P has been shown to serve as a key mediator in the regulation of cell apoptosis [159], proliferation [160], migration, and death [161], as well as cell adhesion, motility, and platelet aggregation [162] in a variety of cells. In terms of bone tissue, S1PR1, S1PR2, and SIPR3 are predominantly expressed by mature osteoblasts [163], osteoclast precursors [164], and MSCs [165]. Sphingosine-1-phosphate is enriched within circulating blood compared with bone marrow and is reported to regulate hematopoietic stem cell (HSC) and osteoclast precursor migration between the bone marrow and peripheral blood stream [166]. The inhibition of S1P degradation or downregulation of S1PR1 by S1P lyase has been shown to dissipate the S1P gradient between blood and bone marrow and subsequently reduces the number of circulating HSCs [167]. Here, and at low S1P concentrations (<10−7 M), osteoclast precursors, as modeled using RAW 264.7 macrophages, expeditiously migrated towards the S1P chemoattractant in vitro, while at higher concentrations (10−6 M), S1P led to the chemorepulsion of macrophage migration, resulting in stationary cells [168]. This suggests the S1P gradient is important in osteoclast precursor trafficking to the bone surface, where they have been shown to undergo cell fusion to form terminal differentiated osteoclasts [164]. Similarly, BMSCs also display a dose-dependent migratory response to S1P. Using a Transwell migration assay, Kong et al. [169] showed that human-derived BMSCs migrated towards low concentrations (1–10 nM) of S1P, whereas no migration was measured when investigated at higher concentrations (50–1000 nM). Notably, Golan et al. [170] demonstrated that S1P induced SDF-1 secretion from BMSCs, which occurred via ROS signaling. Further, S1P has been shown to stimulate BMSC cell chemotaxis via Janus kinase (JAK)/signal transducers and activators of transcription (STAT) and focal adhesion kinase (FAK)/phosphatidylinositol 3-kinase (P13K)/protein kinase B (AKT) signaling and through both S1P1 and S1P2 [165]. However, the migratory BMSC response likely differs depending on whether downstream signaling is supported by S1PR1, S1PR2, or S1PR3 [165,169]. S1P has also been reported to be secreted by osteoclasts, where it promotes survival [171], and osteoblasts, predominantly through ATP-independent spinster 2 transporter activity [172,173]. Further, it has been reported that through S1P secretion, osteoclasts recruit osteoblast precursor cells and promote osteoblast survival, proliferation [163,174,175], and migration [176] via Wnt/BMP [177] and mitogen-activated protein kinase (MAPK) signaling [178]. Further, the generation of S1P is associated with the activity of 17β-estradiol, and both S1P and estrogen are reported to augment osteoblast proliferation [174]. In the study, Tantikanlayaporn et al. reported that estrogen increased SPHK1 protein expression in human osteoblasts, and notably, S1P upregulated estrogen receptor (ER)-β mRNA expression, but not ERα, as well as increased levels of SPHK1 and S1PR1. Keller et al. [172] investigated S1P via the bone resorption inhibitor calcitonin and revealed S1P as an osteoanabolic molecule. In support of this, Weske et al. [179] increased S1P levels through the inhibition of S1P lyase in a murine model. Notably, data showed decreased levels of white adipose tissue formation alongside increased bone formation, mass, and strength. Further, Ishii et al. [164] showed that in a murine model, daily injection of the nonselective S1P receptor agonist FTY720 (fingolimod; 2-amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diol hydrochloride), a novel immunosuppressive drug, reduced ovariectomy-induced osteoporosis. The study confirmed a decrease in the number of mature osteoclasts in contact with the bone surface, suggesting the potential of S1P as a therapeutic agent for osteoporosis. Conversely, S1P has also been shown to augment osteoclastogenesis. All S1P receptors with the exception of S1PR5 have been detected in bone marrow-derived macrophages and differentiating osteoclasts [171,172]. Ryu et al. [171] investigated the effect of S1P on the osteoclastogenesis of bone marrow-derived macrophages. The study showed that RANKL upregulated sphingosine kinase activity, thereby increasing intracellular S1P production and secretion, with no osteoclastogeneic differentiation measured. In contrast, the addition of S1P to a co-culture of osteoblasts and bone marrow-derived macrophages significantly increased osteoclastogenesis via increased COX-2 and PGE2 and, subsequently, RANKL production in osteoblasts and T cells. Further, S1P was also demonstrated to activate osteoblast migration and survival, together suggesting that secreted S1P attracts and activates both osteoblasts and T cells to augment osteoclastogenesis, but appeared to have no direct effect on bone marrow-derived macrophage osteoclastogenesis itself. To this end, Xiao et al. [180] unveiled the regulatory role of the sphingosine kinase/S1PR1/RANKL axis in increasing inflammatory bone loss. The study showed that stimulated macrophages induced sphingosine kinase activity, which led to the activation of S1PR1 in BMSCs and the production of RANKL. Notably, S1PR1 blockage abolished this affect, and the authors proposed this cell-signaling pathway as a potential future therapeutic target. As such, S1P facilitates the proliferation, migration, and survival of osteoblasts, and couples osteoblast–osteoclast communication and induces RANKL-induced osteoclastogenesis, osteoclastic activation, and bone resorption (Figure 4) [171].
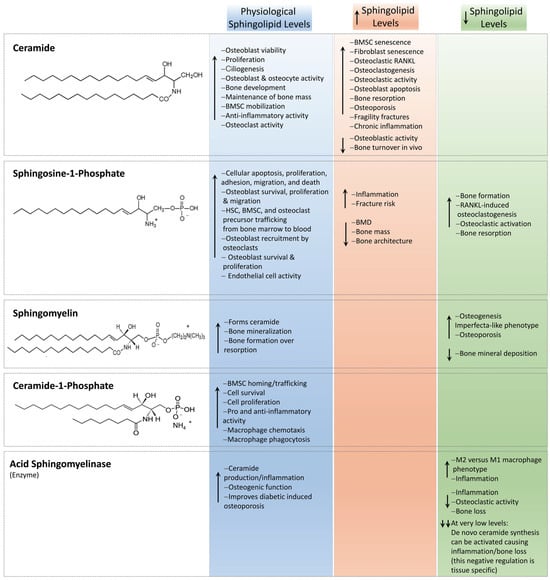
Figure 4.
A schematic demonstrating the known effects of ceramides, C1P, sphingomyelin, aSMase, and S1P to the key cells within bone. The effect of physiological, increased, and decreased levels of each sphingolipid are described. Representative chemical structures are presented. The ↑ arrow indicates sphingolipid, biomolecule, cellular, or tissue upregulation, while the ↓ arrow highlights their downregulation.
Notably, inflammation is reported to be associated with high levels of S1P in humans [181]. To this end, high plasma levels of S1P correlated with decreased bone mineral density (BMD) [181,182,183], bone mass and architecture [184], and parathyroid hormone levels [179,181], and a 9.33-fold [182] and 9.89-fold [183] increased risk of vertebral fracture in post-menopausal women. Further, incident fractures have also been reported to occur more frequently in post-menopausal women who presented with increased plasma S1P levels [185]. Importantly, Ardawi et al. [183] reported that plasma S1P levels and their association with fracture risk were independent of BMD and other established clinical risk factors. Further, Lee et al. [181] confirmed that high S1P plasma levels consistently identified as a significant risk factor in patients with osteopenia and that this was independent of the established fracture risk assessment tools developed by the World Health Organization. These data strongly support S1P as a new and independent biomarker for the risk of osteoporotic fracture [186], although the role of an S1P-tissue gradient (e.g., between bone marrow and plasma) also appears to be important when determining the fracture risk response [187,188]. It has been suggested that the major effect of S1P in regulating bone homeostasis involves the preferential recruitment of osteoclast precursors as opposed to the stimulation of an osteoanabolic response [181,184,185]. In this context, Grewe et al. [189] speculated that high S1P levels would result in the internalization of S1PR1, thereby resulting in the dominant expression of S1PR2 on the osteoclast precursor cell surface. This would subsequently drive the recruitment of osteoclast precursor cell migration into the bone marrow via S1PR2-induced chemorepulsion. However, a study by Heilamann et al. [190] found no improvement in fracture healing when FYT720 was administered daily for 10 and 21 days in a murine osteotomy model of bone fracture. Interestingly, Paget’s disease is characterized by an increased number of abnormal osteoclasts, which drive elevated levels of bone formation [191]. Notably, Nagata et al. [192] demonstrated significantly increased levels of SPHK1 secretion from osteoclasts isolated from a murine model of Paget’s disease as well as osteoclasts isolated from a human donor of the disease. The study identified a Paget’s disease-induced increase in IL-6 production by osteoclasts, subsequently increasing S1P secretion, which, together with IGF-1, promoted S1PR3 expression in osteoblasts to promote bone formation. The authors concluded that when combined, and via the upregulation of Ephrin (Eph) B2 and EphB4 in osteoclasts and osteoblasts, respectively, increased S1P via S1PR3 signaling resulted in the displayed increase in bone formation observed in Paget’s disease. The study concluded that S1P-based drugs may offer a promising anabolic treatment for bone loss.
The enhanced turnover of subchondral trabecular bone is a hallmark of rheumatoid arthritis (RA) [193]. To this end, and notably, significantly increased levels of S1P have been identified within the synovium of patients with RA (17.5 μM) compared to patients with osteoarthritis (3.5 μM) [194]. Notably, the inhibition of S1P has also been shown to alleviate osteoarthritis in a knockout Sphl1LysMCre murine model [195]. As such, therapeutics that target S1P have been proposed for use in the elderly [196]. To this end, and in a murine model of collagen-induced RA, the pharmacological or siRNA knockdown of SPHK1 significantly reduced release of the inflammatory cytokines TNFα, IL-6, IL-1β, MCP1, and MMP9 in vitro [194]. Further, the study showed that joint erosion and serum levels of inflammation were also significantly reduced in vivo. In support of this, and in a rat collagen-induced RA model, administration of the S1PR agonist FTY720 inhibited the formation of synovitis and bone erosions more effectively than prednisone [197]. Further, and in both an adjuvant-induced and collagen-induced rat model of RA, FTY720 successfully inhibited joint inflammation to equal or higher efficacy compared to mizoribine and prednisone [198]. Hutami et al. [193], using a Fas-deficient MRL/lpr mouse model that spontaneously develops autoimmune arthritis and exhibits reduced bone mass, demonstrated significantly increased S1PR1 within the condylar cartilage of the temporomandibular joint. The study revealed that Fas/S1PR1 signaling via NF-κB was necessary for S1P-induced migration of osteoclast precursor cells and that inhibition of NF-κB resulted in the reduction of SPHK1/S1P1 signaling and subchondral bone loss. Finally, and as S1P is able to prevent IL-1β-induced cartilage degradation, Stradner et al. [199] demonstrated that the S1PR agonist FTY720 significantly reduced TNFα, IL-1β, and iNOS levels in chondrocytes in vitro. Together, these studies suggest that increased levels of S1P via SPHK1 contributes a primary role in the progression of inflammatory arthritis, and SPHK1 modulation may provide a novel approach to treating autoimmune conditions such as RA. Spondyloarthritis is a group of chronic rheumatic inflammatory diseases and the second most common type of inflammatory arthritis after RA [200,201]. Bougault et al. [202] recently reported that S1P serum levels were significantly elevated in spondyloarthritis patients (6.1 μM) compared to healthy individuals (1.6 μM). Interestingly, cyclic stretch has been shown to enhance SPHK1 gene expression in cultured osteoblasts and chondrocytes, where supplementation with TNFα or IL-17 further increased stretch-induced SPHK1 upregulation [203]. The authors speculated that S1P production by chondrocytes may therefore be stimulated by both inflammation and mechanical stress in spondyloarthritis.
Notably, S1P opposes the proapoptotic function of ceramide, and the ratio of S1P to ceramide has also been described as a sphingolipid rheostat involved in pathogenesis [204]. Notably, inducible overexpression of adiponectin receptors in adipocytes enhances ceramidase activity, thereby promoting ceramide to sphingosine [205]. Thus, targeting S1P and its receptors may represent a novel route to prevent or reduce osteoporosis. However, together, these studies indicate that S1P signaling is a highly dynamic interconnecting network that involves multiple players, and as such, is substantially complex. Therefore, further mechanistic clarity is first warranted. Nevertheless, sphingolipid-based therapies may hold significant promise in beneficially regulating diseases that cause disturbances where bone resorption exceeds bone formation.
2.3. Acid Sphingomyelinase
Mechanistically, sphingomyelinases function as hydrolases of phospho-diester bonds, where their peak activity is contingent on the local pH [206]. As such, sphingomyelinases are classified as acidic, neutral, or alkaline, and are located in separate cellular sub-locations, where the activity of their products mediates specific and targeted functions [207]. Acid sphingomyelinase (aSMase) is an enzyme that catalyzes the conversion of SM into ceramide, and its deficiency results in the lysosomal storage disease known as Niemann–Pick-type A and B [208]. aSMase activation is reported to increase ceramide production via the hydrolysis of SM, and this is associated with the amplification of inflammatory signaling in macrophages, as well as cellular stress signaling pathways [209,210]. In terms of bone tissue turnover, the inhibition of aSMase by imipramine ameliorated the synergy between metabolic syndrome and Aggregatibacter actinomycetemcomitans-induced periodontitis and alveolar bone loss in a high-fat diet-fed murine model [211]. Notably, the study showed that the inhibition of aSMase reduced ceramide production and subsequently lipopolysaccharide (LPS)-induced periodontitis by reducing pro-inflammatory and pro-osteoclastogenic gene expression in macrophages in vitro and metabolic syndrome-induced periodontitis in vivo. Based on these results, the authors suggested aSMase as a potential therapeutic target. Interestingly, a previous study by this group demonstrated that in a low-fat diet-fed murine model, aSMase-deficiency instead exacerbated LPS-induced periodontitis [212]. Remarkably, both the SM and ceramide content in mice increased with aSMase deficiency, and the unexpected increase in ceramide formation occurred via de novo synthesis, which was upregulated to compensate for the experimentally induced low levels of aSMase. In support of this, Deevsaka et al. [213] also reported increased ceramide de novo synthesis within the liver of aSMase-deficient mice. Notably, de novo ceramide synthesis was found to not occur in all tissues within aSMase-deficient mice, thereby suggesting that the negative regulation of ceramide de novo synthesis is tissue-specific [212]. Roux-Biejat et al. [214] reported that aSMase regulated the expression of macrophage M1 versus M2 phenotype and that the absence of aSMase reduced inflammation in a murine muscle model. Further, the long-term use of fluoxetine, a commonly prescribed antidepressant, is increasingly being associated with increased bone fragility. Interestingly, Zhang et al. [215] showed that through the inhibition of aSMase, fluoxetine induced the disruption of sphingolipid metabolism within bone marrow adipose tissue. In contrast, the study demonstrated that a significant reduction in bone volume was observed in aSMase knockout mice. The formation of ceramide via the de novo synthesis pathway was not investigated, and here, the authors speculated that the inhibition of aSMase reduced ceramide and S1P levels within bone marrow adipocytes, leading to RANKL secretion via cyclooxygenase-2 (COX-2) and its enzymatic product, prostaglandin E2 (PGE2), in a dose-dependent manner. The overproduction of PGE2 induced the secretion of RANKL, thereby promoting osteoclastogenesis. Remarkably, clinically administered oral supplementation of L-serine (250 mg/kg/d), a precursor of SPT, and thus sphingolipid de novo biosynthesis, prevented the fluoxetine-induced accelerated bone loss in postmenopausal women with major depressive disorder, thereby providing new insights and a potential future treatment strategy. Finally, ferroptosis, an iron-catalyzed form of regulated necrosis, has been implicated in the pathological process of type 2 diabetes-induced osteoporosis. Du et al. [216] showed that osteoblastic expression of aSMase and ceramide increased when in a high glucose environment. The study showed that an increase in aSMase levels improved osteogenic function by decreasing high glucose-induced autophagy, GPX4 degradation, and ferroptosis. The authors suggested that aSMase regulation may be a promising method for the treatment of diabetes-induced osteoporosis.
Further, multiple stress stimuli, including ionizing radiation and chemotherapeutic agents (e.g., platinum, paclitaxel, and histone deacetylase inhibitors), have been demonstrated to rapidly activate aSMase [6], potentially with the simultaneous generation of ceramide [217]. Notably, both chemotherapy (e.g., methotrexate, imatinib, and taxanes) and the exposure to IR can cause significant bone loss and fragility [218,219]. The mechanisms of how stress agents activate aSMase remain elusive. However, ROS has been shown to activate aSMase [220], and, for example, IR is known to significantly upregulate oxidative stress within bone tissue [79] as well as ceramide levels [221]. Endothelial bone cell crosstalk and vessel networks are critical in bone homeostasis and repair [222], and high-dose radiotherapy (>8–10 Gy) causes expeditious endothelial cell death via the hydrolyzation of SM to ceramide via aSMase [223,224]. In contrast, low doses (<6 Gy) are reported not to biologically activate ceramide production. Notably, the pre-treatment of endothelial cells with S1P has been shown to reduce ceramide-induced IR cell death, and aSMase knockout mice are more radioresistant to high-dose IR than healthy control animals [225,226]. At the cell membrane, ceramide molecules interact and form stable and tightly packed ceramide-enriched membrane domains. These domains can spontaneously associate to form large ceramide-enriched membrane macrodomains, also called lipid rafts, ~10–200 nm in size, increasing to >300 nm if they fuse to form microscopic domains via protein–protein and protein–lipid interactions [227,228,229]. Ladjohounlou et al. [229] recently demonstrated that ceramide-enriched platforms play a significant role in both the targeted and non-targeted “bystander” effects in head and neck squamous cell carcinoma cells following exposure to IR. The study reported that the preexistence of these domains led to cellular insensitivity to the non-targeted effects of IR and, depending on the genesis and/or intrinsic nature of the cells, ceramide-enriched domains may modulate both cell survival and/or death. Notably, S1P administration prior to IR exposure has been reported to prevent gastrointestinal syndrome by inhibiting endothelium collapse in mice following exposure to 10 Gy [230]. Further, Bonnaud et al. [231] demonstrated that S1P protected human microvascular endothelial cells from ceramide-induced apoptosis following 15 Gy of IR, but not from DNA damage-induced mitotic death. Most notably, opaganib, a first-in-class inhibitor of sphingolipid metabolism via SPHK2, has been shown to deliver broad anti-inflammatory (via the downregulation of NF-κB and TNFα) and anti-cancer activity [232]. As such, opaganib acts as a sphingosine mimetic molecule and also inhibits DES1, thereby increasing levels of dihydroceramides and promoting autophagy [233]. Opaganib is reported to elevate ceramide and reduce S1P in cells, thereby increasing the antitumor efficacy of IR while suppressing inflammation. To this end, mice exposed to high-dose IR showed a dose-dependent survival advantage following the oral administration of opaganib 4 h before or 24 h after radiation exposure. Remarkably, opaganib substantially protected normal host tissue from IR-induced damage.
Together, these studies highlight complexity in the function of aSMase and its influence on bone regulation, as well as the magnitude of aSMase inhibition and the important alterations that ensue in terms of the regulation and activation of de novo biosynthesis and subsequent opposing cellular and bone tissue responses. However, it remains a promising therapeutic approach to several conditions that negatively impact bone regeneration and repair.
2.4. Sphingomyelin
Sphingomyelin constitutes a major membrane component within, for example, lipid rafts, caveolae, and clathrin-coated pits, thereby contributing to the mediation of transmembrane signaling [234]. As such, SM has been indicated to play a key role in cell survival, proliferation, migration, and inflammation [36,234]. Nevertheless, its bioactivity is considered to mainly rely on its hydrolysis and the downstream formation of ceramide and S1P [10]. In situ SM hydrolysis within bone results in the formation of the bioactive lipid metabolites phosphocholine and ceramide, and both are critical for bone mineralization. In this respect, the mineralization of matrix vesicles, which bud from the plasma membrane of mineral-forming cells such as osteoblasts, chondrocytes, and odontoblasts [63], is associated with the rapid breakdown of SM, suggesting the involvement of a sphingomyelinase [235]. Notably, deletion of the gene Smpd3, which encodes nSMase2, a membrane-bound enzyme that hydrolyses SM to phosphocholine and ceramide, results in mice displaying a severe osteogenesis imperfecta phenotype [236,237]. Further, nSMase2 is reported to be highly expressed in mature osteoblasts, and cells deficient in this enzyme reveal an impairment in their ability to deposit minerals in vitro [238]. However, the mechanisms by which nSMase2 regulates bone mineralization remain to be determined. It is possible that via the generation of phosphocholine nSMase2 may participate in the local increase in the PO43− pool within bone, thus facilitating the initiation of mineralization [67]. Further, nSMase2 activity also generates ceramide, which can be further hydrolyzed to sphingosine, while both phosphocholine and ceramide can be further phosphorylated via kinases to C1P and S1P [239]. The SM-mediated mechanisms leading to osteoporosis are incompletely understood. To this end, a study by Pekkinen et al. [240] showed that six families with rare skeletal phenotypes were all identified with a heterozygous variant in the SM synthase 2 gene, suggesting a fundamental and critical role for SM metabolism in a spectrum of conditions ranging from osteoporosis to complex skeletal dysplasia. Further, SM synthase 2-knockout was demonstrated to suppress TRAP-positive osteoclast staining via RANKL in mice, suggesting the potential likelihood of a critical contribution towards bone formation rather than bone resorption [241]. Finally, and when loaded with cholesterol, SPT and SM levels were reported to increase in macrophages [242]. Notably, the study showed that ORMDL levels were reduced with cholesterol loading, thereby potentially reducing ORMDL-dependent SPT activity. However, the mechanistic role of cholesterol in sphingolipid metabolism remains to be elucidated.
3. Sphingolipids and Infection-Induced Bone Loss
Surgical site infections and periprosthetic joint infections (PJIs) associated with bone or bone marrow are extremely challenging complications that are coupled with high morbidity and the need for complex treatment strategies [243,244]. Biofilms, communities of surface-adherent microbes (e.g., bacteria) protected by a polymeric matrix, are notoriously difficult to treat. Their formation is associated with ~1.7 million hospital-acquired infections per year in the United States, incurring an annual economic burden of ~USD 11 billion [245]. As the demand for orthopedic surgery continues to rise at an accelerated rate [246], a future infection-related healthcare and economic burden is anticipated [247,248,249,250]. Efficacious strategies to prevent and treat PJI, osteomyelitis, and its recurrence remain elusive, and innovative approaches are needed, particularly within the context of the continuous emergence of antimicrobial resistance in common etiological agents of PJI and osteomyelitis. Common pathogens associated with implants and osteomyelitis include Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, and Pseudomonas aeruginosa [251,252,253]. Here, we discuss the potential role of sphingolipids as a promising approach to combat orthopedic infection.
3.1. The Antibacterial Activity of Sphingolipids
Sphingolipids located within the stratum corneum of the skin exhibit antimicrobial activity against both Gram-positive and Gram-negative bacteria and likely contribute to the permeability and innate immunologic barriers of the skin [254,255,256,257]. Included among these lipids are free sphingosine, phytosphingosine, and dihydrosphingosine derived from epithelial sphingolipids and via the activation of hydrolytic enzymes. Sphingosine, phytosphingosine, and dihydrosphingosine deliver varying degrees of antimicrobial activity to many pathogens, including S. aureus, E. coli, and P. aeruginosa. However, the bactericidal mechanisms that underpin these properties remain poorly understood, and despite a 2019 review by Kunz et al. [258] hypothesizing an association with the Gram classification, this remains to be fully elucidated [259,260,261]. A study by Bibel et al. [261] suggested the site of activity was the cell wall. Here, electron microscopy revealed that supplementation of S. aureus with sphingolipids introduced cell wall lesions to the bacterial cell, disrupting the membrane and leading to release of its intracellular contents. Fischer et al. [256] also demonstrated that sphingolipids induced differential ultrastructural damage, accumulated within S. aureus, and induced intracellular inclusions. Further, it has been reported that bactericidal sphingosine inhibited the adherence of Streptococcus mutans onto a hydroxyapatite-coated surface and disrupted pre-formed biofilm, suggesting a role in the control of oral biofilms [262].
In respiratory epithelia, it has been shown that the increased abundance of ceramides in people with cystic fibrosis (CF) relative to those without contributes to susceptibility to endobronchial P. aeruginosa infection—a clinical presentation underpinned by inadequate acid ceramidase-coding ASAH1 gene expression and subsequent ceramide accumulation, and deficient upregulation of bactericidal sphingosine production in the presence of P. aeruginosa [263]. This imbalance was ameliorated following treatment with recombinant human acid ceramidase (rhAC), which attenuated the aberrant inflammation previously observed within the CF population in addition to increasing the proportion of sphingosine within the host plasma membrane, reducing the adherence of both S. aureus and P. aeruginosa to the apical membrane of host epithelial cells [263].
In addition to the direct interactions of host sphingolipids and bacteria, it has been suggested that the generation of ROS during the cleavage of sphingomyelin into ceramide and phosphorylcholine facilitates the apoptosis of macrophages infected with P. aeruginosa [264]. Thus, the interaction of host sphingolipids with the wider inflammasome provides an environment conducive to the clearance of pathogenic bacteria in both human and murine models. Furthermore, host glycosphingolipid β-glucosylceramide, which acts as a damage-associated molecular pattern and is the only identified ligand of macrophage-inducible C-type lectin, is associated with the formation of neutrophil extracellular traps, thus facilitating bacterial clearance by host neutrophils [265].
3.2. Sphingolipid-Mediated Host Cell Invasion
Most bacteria do not contain sphingolipids, but some have evolved mechanisms to utilize the sphingolipids produced by eukaryotic cells to promote their own virulence (Figure 5). To this end, and to ensure their own survival, pathogenic bacteria have evolved many strategies to adhere, engage, enter, and hijack host cell responses. To successfully invade host cells, pathogenic bacteria modulate host cell membrane properties, and the manipulation of lipid biogenesis and membrane stability via the sphingolipid pathway is emerging as a primary pathway in bacterial host cell control [266]. As it participates in membrane reorganization and the formation of ceramide-enriched platforms, a frequent target by pathogens is aSMase. As described, sphingosine displays antibacterial properties, and thus, decreasing sphingosine levels indirectly by activating aSMase is beneficial for pathogen survival and can occur within a few minutes after infection [267]. For example, P. aeruginosa has been shown to stimulate aSMase formation within the plasma membrane, which leads to the production and release of ceramide within sphingolipid-rich rafts [267]. Although sphingolipid-enriched rafts are essential sites of origin for cell-mediated signaling and homeostasis [268], the newly aSMase-induced ceramide subsequently reorganizes these rafts into larger ceramide-enriched signaling macrodomains or platforms. These binding platforms are used by P. aeruginosa to enter and internalize within the host cell, inducing apoptosis and regulating the cytokine response. In a murine-based study, the authors speculated that the ceramide-enriched platforms may trap receptor molecules (e.g., cystic fibrosis transmembrane conductance regulator (CFTR) and CD95), thereby biophysically inhibiting the host cell defense signaling mechanism that would otherwise respond by excluding interactions with the bacterium [268]. The formation of ceramide-enriched platforms has also been reported to induce the local accumulation of β1-integrins, which subsequently suppresses acid ceramidase activity, thereby leading to the additional accumulation of ceramide, which, in parallel, reduces beneficial and antibacterial surface levels of sphingosine [269]. Notably, S. aureus [270] and Clostridium difficile [271] infections also activate aSMase and are essential for infection, while SM is required for the entry of Helicobacter pylori [272]. Further, host cell glycosphingolipids are also reported to contribute to the adhesion of P. aeruginosa and are critical for the internalization of P. aeruginosa into nonphagocytic cells [273].
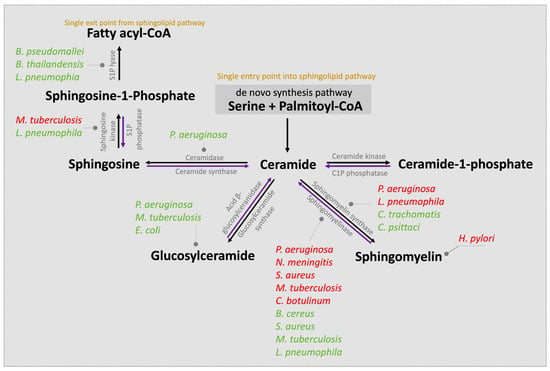
Figure 5.
Pathogenic bacteria are able to adhere, engage, enter, and hijack host cell responses via the sphingolipid pathway. In addition to S. aureus, P. aeruginosa, and E. coli, Helicobacter pylori [274], Neisseria meningitis [275,276], Clostridium botulinum [277,278], Mycobacterium tuberculosis [279,280], Chlamydia psittaci [281], Bacillus cereus [282], Burkolderia pseudomallei and Burkholderia thailandensis [283,284], and Legionella pneumophila [285] are pathogens able to infect bone or that have been reported within the bone marrow. Chlamydia trochomatis is a notorious pathogen able to avoid destruction and persist within host cells [286], and is associated with reactive RA [287]. These bacteria target host cell sphingolipid enzymes either directly (red) or indirectly (green). Image adapted from Rolando et al. [266].
An efficient host response to bacterial invasion is the fusion of late phagosomes and lysosomes, a process critical for the relocation of antibacterial lysosomal hydrolases into phagosomes and thereby facilitating the transport and elimination of pathogens from the host cell [288,289]. The role of aSMase in the efficient fusion and formation of phagolysosomes is essential [290]. Li et al. [291] showed that in S. aureus, ROS-induced aSMase formation via CD44 resulted in ceramide release, the clustering of CD44 within ceramide-enriched membrane platforms, and a rapid rearrangement of the actin cytoskeleton with cortical actin polymerization. Notably, aSMase-deficient cells abrogated these signaling events and reduced the formation of phagolysosomes and the internalization of S. aureus into macrophages by 60–80%. This thereby facilitated S. aureus survival and replication, and increased the severity of infection in vivo. Similar to P. aeruginosa, this suggests an essential role of aSMase in not only promoting ceramide-enriched rafts and entry into the cell but also in assisting S. aureus by disrupting its transport and elimination within host cell phagolysosomes.
Although their study regards a unicellular eukaryote and not a prokaryote, Ghosh et al. [292] revealed that an increase in intracellular de novo ceramide synthesis and activation of aSMase led to a decrease in the growth and survival of the obligate intracellular protozoan Leishmania donovani. The study reported that enhanced levels of ceramide within murine peritoneal macrophages were essential for the downregulation of classical protein kinase C (PKC) activity and the upregulation of calcium-independent atypical PKC-ζ expression. Further, increased ceramide impaired the phosphorylation of MAPK and suppressed the formation of reactive nitrogen species (RNS) within host macrophages, which further facilitated the survival of this parasite. Immune cells deficient in aSMase have been reported to produce RNS but were nevertheless incapable of inhibiting the intracellular proliferation of prokaryotic Listeria monocytogenes in vitro [293]. Therefore, this may suggest the limited role of RNS during pathogen invasion and further highlights the importance of aSMase and ceramides in pathogen control and infection. Together, these studies show that during severe infection and sepsis, the stress responsive enzyme aSMase is activated, and the hydrolysis and degradation of, for example, relatively inert SM into ceramide, together with enhanced de novo ceramide synthesis, unfastens the doorway for invading pathogens.
P. aeruginosa also produces and secretes sphingolipid-metabolizing enzymes. The large extracellular protease hemolytic phospholipase C (PlcH) is known to be a virulence determinant in animal models of infection [294]. Multifunctional PlcH hydrolyzes phosphatidylcholine and SM to produce diacylglycerol and ceramide, respectively, lysing erythrocyte membranes and causing severe toxicity and damage to host cells [295,296,297]. P. aeruginosa has been shown to secrete a neutral ceramidase capable of cleaving the N-acyl linkage between sphingosine and the fatty acids of ceramide [298,299]. Notably, ceramidase-deficient P. aeruginosa attenuated PlcH-induced hemolysis, confirming the primary role of bacterial ceramidase and PlcH in virulence in vitro. Further, a PlcH-mutant murine model investigated by Kida et al. [300] displayed decreased P. aeruginosa virulence, thereby confirming that bacteria utilize sphingolipid-mediated PlcH to contribute to virulence in vivo. Host-derived sphingolipids can also induce and enhance the secretion of a neutral P. aeruginosa ceramidase, which serves to further enhance PlcH-mediated hemolysis and cytotoxicity via the P. aeruginosa sphingosine (sph)-inducible transcriptional regulator sphR [298,299]. To this end, a mobility shift assay demonstrated that P. aeruginosa-located SphR specifically bound free sphingoid bases such as sphingosine, phytosphingosine, and dihydrosphingosine but not SM or ceramide. Further, in vitro data have shown that deletion of sphR is toxic for bacteria and increases their sensitivity to the antimicrobial effects of sphingosine. In support of this, the deletion of sphR resulted in reduced P. aeruginosa survival in vivo [301]. Together, this shows that P. aeruginosa is capable of intercepting and responding to host immune signaling molecules via their own sphingolipid-metabolizing enzymes and raises the question of whether sphingolipids and, in particular sphingosine, as well as SphR-inhibitor therapy, may contribute an important role in controlling virulence and infection.
Further to the above pathogen interactions with host sphingolipids, a growing body of evidence has identified several bacterial species capable of endogenous sphingolipid biosynthesis. Recent studies have demonstrated that several Gram-negative bacterial species are capable of synthesizing sphingolipids. A 2022 study by Stankevibiute et al. [302] identified Stretomyces aurantiacus as the first known Gram-positive bacterial species to possess a complete and functional sphingolipid biosynthetic pathway, likely acquired by horizontal gene transfer from Gram-negative Deltaproteobacteria. Bacterial sphingolipids differ structurally from their eukaryotic counterparts, likely as a factor of limited synthetic enzyme homology between the prokaryotic and eukaryotic domains. Additionally, biochemical research has revealed that the biosynthetic process occurs in different sequences between both domains, with further phylogenetic analyses concluding that mechanisms of ceramide synthesis in bacteria and eukaryotes are the products of convergent evolution [302].
3.3. Sphingolipids, Bacterial Inflammation, and Bone Loss
Host–pathogen interaction triggers excessive inflammation via the orchestrated release of cytokines, including IL-1β, IL-6, IL-8, and TNFα, which subsequently impairs tissue integrity [303,304]. Although highly complex, the simultaneous release and crosstalk of anti-inflammatory cytokines (e.g., IL-10) acts to counterbalance and compartmentalize the inflammatory response, reducing host tissue damage and facilitating host cell survival [305]. The extent and magnitude of the inflammatory response is related to disease severity and clinical outcomes [306,307,308]. During this inflammatory pathogenesis process, bacterial pathogens activate several cellular signaling cascades, including RANK/RANKL signaling pathways (e.g., NF-κB, MAPK, and PI3K), where upregulation activates osteoclastogenesis and, ultimately, bone resorption [309]. Monocytes and macrophages are osteoclast precursor cells and fuse to form osteoclasts, thereby contributing a critical role in facilitating bone resorption [310]. Furthermore, Porphymonas gingivalis-synthesized phosphoglycerol dihydroceramides were found to interact with non-muscle myosin IIA (Myh9), a host cytoplasmic osteoclast cell fusion-regulatory protein that generates Ras-related C3 botulinum toxin substrate 1 (Rac1) and subsequently upregulates the expression of the osteoclast fusogen dendritic cell-specific transmembrane protein (DC-STAMP). The synergistic effect of this upregulation in osteoclast fusion, paired with the inhibitory effect of P. gingivalis phosphoethanolamine dihydroceramides and phosphoglycerol dihydroceramides on osteoblast differentiation gene expression, has been shown to facilitate the alveolar bone loss observed in P. gingivalis-associated periodontitis. The associated mechanistic details of these virulence factors have been succinctly reviewed by Olsen and Nichols [311].
S1PR2 has been shown to play an essential role in modulating bacterial-induced proinflammatory cytokine release via PI3K, extracellular signal-regulated kinase, c-Jun N-terminal kinase, and NF-κB pathways [312], and also to regulate RANKL-induced osteoclastogenesis [313]. The authors showed that the inhibition of S1PR2 reduced IL-1β, TNFα, IL-6, and S1P production within murine bone marrow cells following exposure to Aggregatobacter actinomycetemcomitans; inhibited infection-induced bone marrow-derived monocyte and macrophage chemotaxis; and suppressed RANKL-induced bone resorption. It was concluded that the downregulation of S1PR2 signaling may be a novel therapeutic strategy to treat inflammatory bone loss diseases.
There is evidence showing the IL-1-induced promotion of de novo ceramide biosynthesis via SPT during an acute-phase infection response [314,315]. Further, increased ceramide production is reported to induce IL-1β and TNFα secretion by macrophages [316] and also lead to macrophage death [317]. S. aureus infection has also been shown to increase ceramide levels, which stimulate IL-1β and TNFα signaling via the release of cathepsin B and D from lysosomes. This potentially occurs through the binding of its major toxin, α-toxin, and subsequent activation of aSMase [318]. Notably, mice challenged with endotoxin generated a 2-fold increase in aSMase plasma levels and the release of pro-inflammatory cytokines [319]. Similarly, increased ceramide content within serum lipoproteins alongside a 10-fold increase in the basal level of circulating aSMase for up to 24 h was reported following LPS administration in mice [314]. Further, the study also showed that aSMase knockout mice retained the LPS-induced increase in serum ceramide, suggesting the compensatory activation of de novo ceramide synthesis within the liver. Alterations in ceramide and C1P levels have also been shown to modulate the direction of the inflammatory cascade via a TNFα-controlled response to LPS, a constituent of the cell walls of Gram-negative bacteria [320]. Finally, ceramides modulate the efficacy of macrophages to sense and respond to microbes via pattern recognition receptors, including toll-like receptors [321]. To this end, ceramide accumulation has been described as a critical component in mediating various immune cell functions, including the tailored regulation of the cell response to bacteria and other foreign invaders [322]. Further, Albeituni and Stiban [322] speculated that the ability of ceramides to induce downstream signaling for the production of proinflammatory cytokines during an infection allows macrophages to persist and orchestrate the activation of immune cells that subsequently produce survival factors, including macrophage-colony stimulating factor. Following successful pathogen clearance, ceramides subsequently assist in mediating macrophage apoptosis during the downregulation of immune cell activation and likely promoting healthy bone cell function.
IL-1β is central to the lethal effect of P. aeruginosa infection, and Grassmé et al. [267] showed that sphingolipid-enriched platforms are important for stabilizing IL-1β levels and likely other cytokines during infection. Both sphingosine and S1P also confer key roles in P. aeruginosa-induced inflammatory lung injury via SPHK2. Upon infection and mediated by PKC-ζ, SPHK2 is phosphorylated, increasing its nuclear localization and thereby upregulating S1P and increasing histone acetylation and IL-6 and TNFα secretion [323]. The study indicates a critical role of nuclear SPHK2/S1P signaling in epigenetic regulation of P. aeruginosa-mediated inflammatory lung injury, and targeting SPHK2 may represent a potential strategy to reduce lung inflammation. Although not an orthopedic application, a murine model of CF, a condition commonly challenged by P. aeruginosa, displayed a significant reduction in sphingosine alongside ceramide accumulation within the respiratory tract due to a suppression of aSMase activity [324]. Remarkably, inhalation with sphingosine, FTY720, or aSMase rescued susceptible mice from infection, and the authors suggested the use of sphingosine as a promising novel approach to prevent P. aeruginosa infection. Further, Tabazavareh et al. [325] demonstrated that CF mice were highly susceptible to S. aureus and MRSA infection compared to wild-type mice and that inhalation of sphingosine for 30–40 min before introducing the pathogen significantly reduced the incidence of pulmonary infection by these pathogens. Notably, the study also confirmed the bactericidal activity of sphingosine on S. aureus cells in vitro and showed that the inclusion of ceramide abrogated this effect. Seitz and colleagues investigated sphingosine and phytosphingosine coatings on endotracheal ventilation tubes and demonstrated that the coatings prevented pathogen surface adherence; facilitated the immediate killing of P. aeruginosa, Acinetobater baumannii, and S. aureus species; and prevented biofilm formation. Notably, sphingosine conferred no obvious side effects to tracheal epithelial cells, and importantly, the application of a sphingosine coating prevented P. aeruginosa and S. aureus pneumonia in vivo [326]. Beck et al. [327] demonstrated the bactericidal effect of sphingosine against three different strains of S. epidermidis, which represented frequent microbes involved in PJI. The study showed that coating a titanium K-wire with sphingosine prevented planktonic surface contamination and reduced biofilm formation by 99.942%. Further, the application of a sphingosine solution (1 mM) on preformed biofilm eliminated 99.999% of bacteria on titanium surfaces as well as on steel and polymethylmethacrylate surfaces.
4. Sphingolipids, Viral Host Cell Entry, and Orthopedic Infection
The impact and challenges associated with bacterial and, more rarely, fungal PJIs, are well recognized [328]. However, new and emerging studies emphasize the important need to investigate viral musculoskeletal infections, with concerns that their influence may be underestimated [329,330]. Genomic and sequencing analyses reveal the presence of viral DNA and RNA within bone tissue, but little is understood about the interaction, persistence, and pathogenesis of viruses in relation to bone disease [331]. Recent evidence suggests that viruses can cause moderate to severe arthritis and osteitis, with risk factors such as pre-existing rheumatologic disease contributing to higher disease severity and duration of symptoms [329,330]. To this end, viral agents such as human immunodeficiency virus (HIV), hepatitis B, hepatitis C, and alphavirus, among others, have been shown to cause viral arthritis [332]. Typically, viral arthritis has been interpreted as viral-induced autoimmunity and a resulting inflammatory response, rather than the result of direct viral infection with primary damage to bone and cartilage tissue [330]. However, in the case of alphavirus, direct osteoblastic infection and subsequent disruption of RANKL/OPG expression has been identified [333]. Further, Dimitriou et al. [334] recently reported the significantly increased risk of PJI in HIV-positive patients. Notably, HIV-1 directly infected osteoclasts, causing increased adhesion and osteolytic activity and leading to bone resorption and defect formation [335]. This appeared to occur via modification of the structure of the sealing zone and thus altered the resorption machinery of the osteoclast. Further, a virally induced decrease in osteoblast-related alkaline phosphatase expression was also measured. Similarly, a recent study by Haudenschild et al. [336] provided the first evidence that SARS-CoV-2 infection led to acute bone loss, increased osteoclast numbers, and thinner growth plates. This was speculated to occur via hyperinflammation and decreased mobility together with the direct effects of SARS-CoV-2 infection of bone cells. The study concluded that the bone loss measured may decrease whole-bone mechanical strength and increase the risk of fragility fractures, particularly in older patients.
Sphingolipids play an important role in the viral infection process [337], including (i) viral attachment and entry into the host cell plasma membrane. This is predominantly influenced by sphingolipid-enriched membrane microdomains where structural alterations influence and modify the fusion of the host plasma membrane with the viral membrane. These first interactions between viruses and cells subsequently effect biophysical processes such as (ii) endocytosis, where the endocytosis of viruses and their subsequent uptake and intracellular trafficking are modulated. Similar to bacteria, this occurs via the formation of condensed ceramide-enriched membrane platforms in response to sphingomyelinase activation or ceramide inhibition [338,339]. Thus, conditions that favor microdomain formation enhance viral infection. Finally, (iii) sphingolipids are involved in changes in host signaling and cellular metabolism, which directly influences the viral replication cycle [337]. Sphingomyelin and aSMase have been implicated in the early steps towards the adhesion of Ebola virus, rhinovirus, measles viruses, Japanese encephalitis virus, and SARS-CoV-2 to host cells [337,340,341,342]. Sphingomyelin was shown to be required for attachment, while aSMase was strongly associated with the interaction of viral particles on the host membrane. Evidence of a protective role of ceramides has been shown, where, for example, active influenza A virus replication resulted in a significant increase in ceramide generation via the de novo biosynthesis pathway [343]. Notably, this did not occur when a heat-inactivated virus was investigated. More thorough reviews of the viral response to sphingolipids have been published [337,344,345,346], and although much remains to be discovered, the targeting of host cell sphingolipid metabolism to limit viral adhesion, entry, trafficking, and replication within host cells may offer exciting future therapeutic avenues of exploitation to aid in addressing the challenges associated with orthopedic PJI.
5. Discussion
Regulation of the diverse sphingolipid machinery is tightly governed by the highly conserved nature of their synthesis, degradation, and modification. This review suggests an extremely complex sphingolipidome where the targeted action of each sphingolipid will depend on changes in the expression of many of the other bioactive sphingolipids present. The intricacy of this interconnected network remains to be fully understood but is considered sophisticated and convoluted, as, for example, an increased flux through the de novo pathway does not necessarily lead to ceramide accumulation unless its conversion to, e.g., SM, C1P, or glucosylceramide is also suppressed or impaired [6]. As such, the downregulation or complete inhibition of one bioactive metabolite may have varying levels of effects in terms of ultimately promoting beneficial bone tissue regeneration or, indeed, dysfunction. Furthermore, this review highlights the dynamic adaptability of this system, as, for example, when aSMase levels are pharmacologically or pathogenically inhibited, a compensatory route is activated where ceramide levels are generated via the de novo synthesis route to overcome this loss. Although under healthy bone conditions this is undoubtedly important for ensuring the critical steady state of physiological levels of ceramide, this pathway also opens the door for dysregulation during the pathogenesis of various metabolic disorders. Several bacterial pathogens actively modulate the sphingolipid machinery in host cells to promote colonization, and similarly, it is increasingly clear that sphingolipids also contribute a dual role in both assisting with the establishment of infection while also activating host defenses against the invading pathogen. Further, it is conceivable that alterations in these bioactive metabolites are not the cause but rather a symptom of dysfunctional sphingolipid metabolism, which ultimately leads to the pathophysiologies described.
In terms of their mechanism of action and co-dependent activity, much remains to be resolved. For example, five distinct ceramidases and five differing sphingomyelinases that localize to the plasma membrane, ER, Golgi complex, lysosome, mitochondria, or extracellular space have been identified. Six distinguishable ceramide synthases, which show distinct preference for interacting with varying FAs, also contribute to this complexity [6]. An additional tier of intricacy is the recent discovery of several atypical sphingolipids. For example, 1-deoxysphingolipids are formed via the alternate action of SPT on alanine instead of the substrate serine [347] in both eukaryotes and prokaryotes [348]. Further, and as they lack the essential C1-hydroxyl group, 1-deoxysphingolipids cannot be converted to complex sphingolipids and are not degraded via the canonical catabolic pathways, thereby undergoing repeated de- and re-acylation cycles. Thus, and due to their elevated cytotoxicity compared to canonical sphingolipids, pathologically increased levels of 1-deoxysphingolipids are involved in several disease conditions, including type 2 diabetes and sensory and autonomic neuropathy [347,349,350,351].
6. Conclusions
In conclusion, dysregulation within this important group of metabolites appears to be central to many chronic metabolic diseases and disorders where unmet orthopedic clinical needs remain. For example, the discovery of new technologies and approaches are urgently needed to limit bone loss and damage due to age-induced osteoporosis, rheumatoid and osteoarthritis, and radiotherapy. This is of high significance, as our aging population continues to increase globally, and the treatment and care of conditions such as osteoporosis, osteoarthritis, and cancer will undoubtedly bear a huge health–economic burden in all regions of the world [84,85,86]. Finally, the emergence of antibiotic-resistant bacterial strains is also driving the innovation and development of next-generation approaches to resolving the contemporary issues associated with orthopedic PJI and osteomyelitis. This review suggests the pivotal role of sphingolipids in not only modulating host cell–pathogen interactions but also in regulating inflammation, osteoclastic activity, and bone resorption during infection. As such, the future use of sphingolipid therapy is highly promising; however, many open questions remain, and a greater understanding of the multifaceted sphingolipidome may represent the next frontier in this regard.
Author Contributions
Conceptualization, A.S., M.H. and M.J.C.; methodology, A.S., M.H. and M.J.C.; formal analysis, A.S., M.H., F.W., A.S.P., C.N., J.R., J.D.S. and M.J.C.; investigation, A.S., M.H., F.W., A.S.P., C.N., J.R., J.D.S. and M.J.C.; resources, M.J.C.; writing—original draft preparation, A.S., M.H., F.W., A.S.P., C.N., J.R., J.D.S. and M.J.C.; writing—review and editing, A.S., M.H., F.W., A.S.P., C.N., J.R., J.D.S. and M.J.C.; visualization, F.W., C.N. and M.J.C.; supervision, M.J.C.; project administration, M.J.C.; funding acquisition, M.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. Author C.N.’s work was supported by the National Aeronautics and Space Administration [grant No. 80NSSC21M0309], issued through the NASA Office of STEM Engagement.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and Their Metabolism in Physiology and Disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid Classification, Structures and Tools. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2011, 1811, 637–647. [Google Scholar] [CrossRef]
- Muro, E.; Atilla-Gokcumen, G.E.; Eggert, U.S. Lipids in Cell Biology: How Can We Understand Them Better? Mol. Biol. Cell 2014, 25, 1819–1823. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A Comprehensive Classification System for Lipids. J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef]
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Köfeler, H.; et al. Update on LIPID MAPS Classification, Nomenclature, and Shorthand Notation for MS-Derived Lipid Structures. J. Lipid Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of Bioactive Lipid Signalling: Lessons from Sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Shimizu, T. Lipid Mediators in Health and Disease: Enzymes and Receptors as Therapeutic Targets for the Regulation of Immunity and Inflammation. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 123–150. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Maccarrone, M. Bioactive Lipids as Modulators of Immunity, Inflammation and Emotions. Curr. Opin. Pharmacol. 2016, 29, 54–62. [Google Scholar] [CrossRef]
- Merrill, A.H. De Novo Sphingolipid Biosynthesis: A Necessary, but Dangerous, Pathway. J. Biol. Chem. 2002, 277, 25843–25846. [Google Scholar] [CrossRef]
- Qi, T.; Li, L.; Weidong, T. The Role of Sphingolipid Metabolism in Bone Remodeling. Front. Cell Dev. Biol. 2021, 9, 752540. [Google Scholar] [CrossRef]
- Smith, E.R.; Merrill, A.H., Jr.; Obeid, L.M.; Hannun, Y.A. Effects of Sphingosine and Other Sphingolipids on Protein Kinase C. Methods Enzymol. 2000, 312, 361–373. [Google Scholar] [CrossRef]
- Obeid, L.M.; Linardic, C.M.; Karolak, L.A.; Hannun, Y.A. Programmed Cell Death Induced by Ceramide. Science 1993, 259, 1769–1771. [Google Scholar] [CrossRef]
- Venable, M.E.; Lee, J.Y.; Smyth, M.J.; Bielawska, A.; Obeid, L.M. Role of Ceramide in Cellular Senescence. J. Biol. Chem. 1995, 270, 30701–30708. [Google Scholar] [CrossRef]
- Hla, T. Physiological and Pathological Actions of Sphingosine 1-Phosphate. Semin. Cell Dev. Biol. 2004, 15, 513–520. [Google Scholar] [CrossRef]
- Hinkovska-Galcheva, V.; Boxer, L.A.; Kindzelskii, A.; Hiraoka, M.; Abe, A.; Goparju, S.; Spiegel, S.; Petty, H.R.; Shayman, J.A. Ceramide 1-Phosphate, a Mediator of Phagocytosis. J. Biol. Chem. 2005, 280, 26612–26621. [Google Scholar] [CrossRef]
- Mitsutake, S.; Kim, T.-J.; Inagaki, Y.; Kato, M.; Yamashita, T.; Igarashi, Y. Ceramide Kinase Is a Mediator of Calcium-Dependent Degranulation in Mast Cells. J. Biol. Chem. 2004, 279, 17570–17577. [Google Scholar] [CrossRef]
- Summers, S.A.; Chaurasia, B.; Holland, W.L. Metabolic Messengers: Ceramides. Nat. Metab. 2019, 1, 1051–1058. [Google Scholar] [CrossRef]
- Pralhada Rao, R.; Vaidyanathan, N.; Rengasamy, M.; Mammen Oommen, A.; Somaiya, N.; Jagannath, M.R. Sphingolipid Metabolic Pathway: An Overview of Major Roles Played in Human Diseases. J. Lipids 2013, 2013, 178910. [Google Scholar] [CrossRef]
- Ryland, L.K.; Fox, T.E.; Liu, X.; Loughran, T.P.; Kester, M. Dysregulation of Sphingolipid Metabolism in Cancer. Cancer Biol. Ther. 2011, 11, 138–149. [Google Scholar] [CrossRef]
- Green, C.D.; Maceyka, M.; Cowart, L.A.; Spiegel, S. Sphingolipids in Metabolic Disease: The Good, the Bad, and the Unknown. Cell Metab. 2021, 33, 1293–1306. [Google Scholar] [CrossRef]
- Huang, X.; Withers, B.R.; Dickson, R.C. Sphingolipids and Lifespan Regulation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 657–664. [Google Scholar] [CrossRef]
- Mistry, P.K.; Weinreb, N.J.; Kaplan, P.; Cole, J.A.; Gwosdow, A.R.; Hangartner, T. Osteopenia in Gaucher Disease Develops Early in Life: Response to Imiglucerase Enzyme Therapy in Children, Adolescents and Adults. Blood Cells Mol. Dis. 2011, 46, 66–72. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Many Ceramides. J. Biol. Chem. 2011, 286, 27855–27862. [Google Scholar] [CrossRef]
- Schulze, H.; Sandhoff, K. Sphingolipids and Lysosomal Pathologies. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 799–810. [Google Scholar] [CrossRef]
- Tidhar, R.; Futerman, A.H. The Complexity of Sphingolipid Biosynthesis in the Endoplasmic Reticulum. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 2511–2518. [Google Scholar] [CrossRef]
- Merrill, A.H. Sphingolipid and Glycosphingolipid Metabolic Pathways in the Era of Sphingolipidomics. Chem. Rev. 2011, 111, 6387–6422. [Google Scholar] [CrossRef]
- Perry, D.K. Serine Palmitoyltransferase: Role in Apoptotic de Novo Ceramide Synthesis and Other Stress Responses. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2002, 1585, 146–152. [Google Scholar] [CrossRef]
- Menaldino, D. Sphingoid Bases and de Novo Ceramide Synthesis: Enzymes Involved, Pharmacology and Mechanisms of Action. Pharmacol. Res. 2003, 47, 373–381. [Google Scholar] [CrossRef]
- Chaurasia, B.; Tippetts, T.S.; Mayoral Monibas, R.; Liu, J.; Li, Y.; Wang, L.; Wilkerson, J.L.; Sweeney, C.R.; Pereira, R.F.; Sumida, D.H.; et al. Targeting a Ceramide Double Bond Improves Insulin Resistance and Hepatic Steatosis. Science 2019, 365, 386–392. [Google Scholar] [CrossRef]
- Causeret, C.; Geeraert, L.; Van der Hoeven, G.; Mannaerts, G.P.; Van Veldhoven, P.P. Further Characterization of Rat Dihydroceramide Desaturase: Tissue Distribution, Subcellular Localization, and Substrate Specificity. Lipids 2000, 35, 1117–1125. [Google Scholar] [CrossRef]
- Davis, D.; Kannan, M.; Wattenberg, B. Orm/ORMDL Proteins: Gate Guardians and Master Regulators. Adv. Biol. Regul. 2018, 70, 3–18. [Google Scholar] [CrossRef]
- Cowart, L.A.; Hannun, Y.A. Selective Substrate Supply in the Regulation of Yeast de Novo Sphingolipid Synthesis. J. Biol. Chem. 2007, 282, 12330–12340. [Google Scholar] [CrossRef]
- Cantalupo, A.; Zhang, Y.; Kothiya, M.; Galvani, S.; Obinata, H.; Bucci, M.; Giordano, F.J.; Jiang, X.-C.; Hla, T.; Di Lorenzo, A. Nogo-B Regulates Endothelial Sphingolipid Homeostasis to Control Vascular Function and Blood Pressure. Nat. Med. 2015, 21, 1028–1037. [Google Scholar] [CrossRef]
- Wijesinghe, D.S.; Massiello, A.; Subramanian, P.; Szulc, Z.; Bielawska, A.; Chalfant, C.E. Substrate Specificity of Human Ceramide Kinase. J. Lipid Res. 2005, 46, 2706–2716. [Google Scholar] [CrossRef]
- Raas-Rothschild, A.; Pankova-Kholmyansky, I.; Kacher, Y.; Futerman, A.H. Glycosphingolipidoses: Beyond the Enzymatic Defect. Glycoconj. J. 2004, 21, 295–304. [Google Scholar] [CrossRef]
- Tafesse, F.G.; Ternes, P.; Holthuis, J.C.M. The Multigenic Sphingomyelin Synthase Family. J. Biol. Chem. 2006, 281, 29421–29425. [Google Scholar] [CrossRef]
- Milhas, D.; Clarke, C.J.; Hannun, Y.A. Sphingomyelin Metabolism at the Plasma Membrane: Implications for Bioactive Sphingolipids. FEBS Lett. 2010, 584, 1887–1894. [Google Scholar] [CrossRef]
- Canals, D.; Salamone, S.; Hannun, Y.A. Visualizing Bioactive Ceramides. Chem. Phys. Lipids 2018, 216, 142–151. [Google Scholar] [CrossRef]
- Brice, S.E.; Cowart, L.A. Sphingolipid Metabolism and Analysis in Metabolic Disease. Sphingolipids Metab. Dis. 2011, 721, 1–17. [Google Scholar] [CrossRef]
- Nakamura, M.; Udagawa, N.; Matsuura, S.; Mogi, M.; Nakamura, H.; Horiuchi, H.; Saito, N.; Hiraoka, B.Y.; Kobayashi, Y.; Takaoka, K.; et al. Osteoprotegerin Regulates Bone Formation through a Coupling Mechanism with Bone Resorption. Endocrinology 2003, 144, 5441–5449. [Google Scholar] [CrossRef]
- Simonet, W.; Lacey, D.; Dunstan, C.; Kelley, M.; Chang, M.-S.; Lüthy, R.; Nguyen, H.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A Novel Secreted Protein Involved in the Regulation of Bone Density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Min, H.; Morony, S.; Sarosi, I.; Dunstan, C.R.; Capparelli, C.; Scully, S.; Van, G.; Kaufman, S.; Kostenuik, P.J.; Lacey, D.L.; et al. Osteoprotegerin Reverses Osteoporosis by Inhibiting Endosteal Osteoclasts and Prevents Vascular Calcification by Blocking a Process Resembling Osteoclastogenesis. J. Exp. Med. 2000, 192, 463–474. [Google Scholar] [CrossRef]
- Oshita, K.; Yamaoka, K.; Udagawa, N.; Fukuyo, S.; Sonomoto, K.; Maeshima, K.; Kurihara, R.; Nakano, K.; Saito, K.; Okada, Y.; et al. Human Mesenchymal Stem Cells Inhibit Osteoclastogenesis through Osteoprotegerin Production. Arthritis Rheum. 2011, 63, 1658–1667. [Google Scholar] [CrossRef]
- Lee, C.-H.; Kim, Y.-J.; Jang, J.-H.; Park, J.-W. Modulating Macrophage Polarization with Divalent Cations in Nanostructured Titanium Implant Surfaces. Nanotechnology 2016, 27, 085101. [Google Scholar] [CrossRef]
- Sharaf-Eldin, W.E.; Abu-Shahba, N.; Mahmoud, M.; El-Badri, N. The Modulatory Effects of Mesenchymal Stem Cells on Osteoclastogenesis. Stem Cells Int. 2016, 2016, 1908365. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, T.-S.; Choi, Y.-W.Y.; Lorenzo, J. Osteoimmunology: Cytokines and the Skeletal System. BMB Rep. 2008, 41, 495–510. [Google Scholar] [CrossRef]
- Li, Y.; Toraldo, G.; Li, A.; Yang, X.; Zhang, H.; Qian, W.-P.; Weitzmann, M.N. B Cells and T Cells Are Critical for the Preservation of Bone Homeostasis and Attainment of Peak Bone Mass in Vivo. Blood 2007, 109, 3839–3848. [Google Scholar] [CrossRef]
- Pacifici, R. T Cells, Osteoblasts, and Osteocytes: Interacting Lineages Key for the Bone Anabolic and Catabolic Activities of Parathyroid Hormone. Ann. N. Y. Acad. Sci. 2016, 1364, 11–24. [Google Scholar] [CrossRef]
- Lorenzo, J.; Horowitz, M.; Choi, Y. Osteoimmunology: Interactions of the Bone and Immune System. Endocr. Rev. 2008, 29, 403–440. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kwak, M.K.; Moon, S.-A.; Choi, Y.J.; Baek, J.E.; Park, S.Y.; Kim, B.-J.; Lee, S.H.; Koh, J.-M. Regulation of Bone Metabolism by Megakaryocytes in a Paracrine Manner. Sci. Rep. 2020, 10, 2277. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Bolamperti, S.; Villa, I.; Rubinacci, A. Bone Remodeling: An Operational Process Ensuring Survival and Bone Mechanical Competence. Bone Res. 2022, 10, 48. [Google Scholar] [CrossRef]
- Wei, F.; Flowerdew, K.; Kinzel, M.; Perotti, L.E.; Asiatico, J.; Omer, M.; Hovell, C.; Reumers, V.; Coathup, M.J. Changes in Interstitial Fluid Flow, Mass Transport and the Bone Cell Response in Microgravity and Normogravity. Bone Res. 2022, 10, 65. [Google Scholar] [CrossRef]
- Muruganandan, S.; Sinal, C.J. The Impact of Bone Marrow Adipocytes on Osteoblast and Osteoclast Differentiation. IUBMB Life 2014, 66, 147–155. [Google Scholar] [CrossRef]
- Muruganandan, S.; Govindarajan, R.; Sinal, C.J. Bone Marrow Adipose Tissue and Skeletal Health. Curr. Osteoporos. Rep. 2018, 16, 434–442. [Google Scholar] [CrossRef]
- Shin, E.; Koo, J.S. The Role of Adipokines and Bone Marrow Adipocytes in Breast Cancer Bone Metastasis. Int. J. Mol. Sci. 2020, 21, 4967. [Google Scholar] [CrossRef]
- Thommesen, L.; Stunes, A.K.; Monjo, M.; Grøsvik, K.; Tamburstuen, M.V.; Kjøbli, E.; Lyngstadaas, S.P.; Reseland, J.E.; Syversen, U. Expression and Regulation of Resistin in Osteoblasts and Osteoclasts Indicate a Role in Bone Metabolism. J. Cell. Biochem. 2006, 99, 824–834. [Google Scholar] [CrossRef]
- Xie, H.; Xie, P.-L.; Luo, X.-H.; Wu, X.-P.; Zhou, H.-D.; Tang, S.-Y.; Liao, E.-Y. Omentin-1 Exerts Bone-Sparing Effect in Ovariectomized Mice. Osteoporos. Int. 2012, 23, 1425–1436. [Google Scholar] [CrossRef]
- Lau, B.Y.Y.; Ward, W.E.; Kang, J.X.; Ma, D.W.L. Fat-1 Gene Modulates the Fatty Acid Composition of Femoral and Vertebral Phospholipids. Appl. Physiol. Nutr. Metab. 2010, 35, 447–455. [Google Scholar] [CrossRef]
- Lucas, S.; Clabaut, A.; Ghali, O.; Haren, N.; Hardouin, P.; Broux, O. Implication of Fatty Acids in the Inhibitory Effect of Human Adipocytes on Osteoblastic Differentiation. Bone 2013, 55, 429–430. [Google Scholar] [CrossRef]
- You, L.; Sheng, Z.; Tang, C.; Chen, L.; Pan, L.; Chen, J. High Cholesterol Diet Increases Osteoporosis Risk via Inhibiting Bone Formation in Rats. Acta Pharmacol. Sin. 2011, 32, 1498–1504. [Google Scholar] [CrossRef]
- Luegmayr, E.; Glantschnig, H.; Wesolowski, G.A.; Gentile, M.A.; Fisher, J.E.; Rodan, G.A.; Reszka, A.A. Osteoclast Formation, Survival and Morphology Are Highly Dependent on Exogenous Cholesterol/Lipoproteins. Cell Death Differ. 2004, 11, S108–S118. [Google Scholar] [CrossRef]
- Anderson, H.C.; Garimella, R.; Tague, S.E. The Role of Matrix Vesicles in Growth Plate Development and Biomineralization. Front. Biosci. 2005, 10, 822–837. [Google Scholar] [CrossRef]
- Blackwell, K.A.; Raisz, L.G.; Pilbeam, C.C. Prostaglandins in Bone: Bad Cop, Good Cop? Trends Endocrinol. Metab. 2010, 21, 294–301. [Google Scholar] [CrossRef]
- Cottrill, E.; Lazzari, J.; Pennington, Z.; Ehresman, J.; Schilling, A.; Dirckx, N.; Theodore, N.; Sciubba, D.; Witham, T. Oxysterols as Promising Small Molecules for Bone Tissue Engineering: Systematic Review. World J. Orthop. 2020, 11, 328–344. [Google Scholar] [CrossRef]
- Muruganandan, S.; Roman, A.A.; Sinal, C.J. Adipocyte Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells: Cross Talk with the Osteoblastogenic Program. Cell. Mol. Life Sci. 2009, 66, 236–253. [Google Scholar] [CrossRef]
- During, A.; Penel, G.; Hardouin, P. Understanding the Local Actions of Lipids in Bone Physiology. Prog. Lipid Res. 2015, 59, 126–146. [Google Scholar] [CrossRef]
- Wen, D.; Androjna, C.; Vasanji, A.; Belovich, J.; Midura, R.J. Lipids and Collagen Matrix Restrict the Hydraulic Permeability Within the Porous Compartment of Adult Cortical Bone. Ann. Biomed. Eng. 2010, 38, 558–569. [Google Scholar] [CrossRef]
- van Gastel, N.; Stegen, S.; Eelen, G.; Schoors, S.; Carlier, A.; Daniëls, V.W.; Baryawno, N.; Przybylski, D.; Depypere, M.; Stiers, P.-J.; et al. Lipid Availability Determines Fate of Skeletal Progenitor Cells via SOX9. Nature 2020, 579, 111–117. [Google Scholar] [CrossRef]
- Moerman, E.J.; Teng, K.; Lipschitz, D.A.; Lecka-Czernik, B. Aging Activates Adipogenic and Suppresses Osteogenic Programs in Mesenchymal Marrow Stroma/Stem Cells: The Role of PPAR-Γ2 Transcription Factor and TGF-β/BMP Signaling Pathways. Aging Cell 2004, 3, 379–389. [Google Scholar] [CrossRef]
- Zapata-Linares, N.; Toillon, I.; Wanherdrick, K.; Pigenet, A.; San Martin-Uriz, P.; Louvet, L.; Calleja-Cervantes, M.E.; Ghali, O.; Vilas-Zornoza, A.; Nourissat, G.; et al. Implication of Bone Marrow Adipose Tissue in Bone Homeostasis during Osteoarthritis. Osteoarthr. Cartil. 2022, 30, S303. [Google Scholar] [CrossRef]
- Shepherd, R.F.; Allison, K.; Rhodes, B.; Kerns, J.G.; Taylor, A.M. Do Changes in the Composition of Bone Marrow Adipose Tissue Affect the Development of Osteoarthritis? FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Cohen, A.; Dempster, D.W.; Recker, R.R.; Lappe, J.M.; Zhou, H.; Zwahlen, A.; Müller, R.; Zhao, B.; Guo, X.; Lang, T.; et al. Abdominal Fat Is Associated with Lower Bone Formation and Inferior Bone Quality in Healthy Premenopausal Women: A Transiliac Bone Biopsy Study. J. Clin. Endocrinol. Metab. 2013, 98, 2562–2572. [Google Scholar] [CrossRef]
- Gursahaney, D.L.; Jesse, M.; Stoneback, J. Extraosseous Marrow Fat: An MRI Sign of Acute Aggressive Osteomyelitis. BJR Case Rep. 2019, 5, 20180050. [Google Scholar] [CrossRef]
- McGee-Lawrence, M.E.; Carpio, L.R.; Schulze, R.J.; Pierce, J.L.; McNiven, M.A.; Farr, J.N.; Khosla, S.; Oursler, M.J.; Westendorf, J.J. Hdac3 Deficiency Increases Marrow Adiposity and Induces Lipid Storage and Glucocorticoid Metabolism in Osteochondroprogenitor Cells. J. Bone Miner. Res. 2016, 31, 116–128. [Google Scholar] [CrossRef]
- Cohen, A.; Dempster, D.W.; Stein, E.M.; Nickolas, T.L.; Zhou, H.; McMahon, D.J.; Müller, R.; Kohler, T.; Zwahlen, A.; Lappe, J.M.; et al. Increased Marrow Adiposity in Premenopausal Women with Idiopathic Osteoporosis. J. Clin. Endocrinol. Metab. 2012, 97, 2782–2791. [Google Scholar] [CrossRef]
- Misra, M.; Klibanski, A. Anorexia Nervosa, Obesity and Bone Metabolism. Pediatr. Endocrinol. Rev. 2013, 11, 21–33. [Google Scholar]
- Aaron, N.; Costa, S.; Rosen, C.J.; Qiang, L. The Implications of Bone Marrow Adipose Tissue on Inflammaging. Front. Endocrinol. 2022, 13, 853765. [Google Scholar] [CrossRef]
- Wei, F.; Neal, C.J.; Sakthivel, T.S.; Fu, Y.; Omer, M.; Adhikary, A.; Ward, S.; Ta, K.M.; Moxon, S.; Molinari, M.; et al. A Novel Approach for the Prevention of Ionizing Radiation-Induced Bone Loss Using a Designer Multifunctional Cerium Oxide Nanozyme. Bioact. Mater. 2023, 21, 547–565. [Google Scholar] [CrossRef]
- Fan, Y.; Hanai, J.; Le, P.T.; Bi, R.; Maridas, D.; DeMambro, V.; Figueroa, C.A.; Kir, S.; Zhou, X.; Mannstadt, M.; et al. Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab. 2017, 25, 661–672. [Google Scholar] [CrossRef]
- Veldhuis-Vlug, A.G.; Rosen, C.J. Clinical Implications of Bone Marrow Adiposity. J. Intern. Med. 2018, 283, 121–139. [Google Scholar] [CrossRef]
- Kim, T.Y.; Schafer, A.L. Diabetes and Bone Marrow Adiposity. Curr. Osteoporos. Rep. 2016, 14, 337–344. [Google Scholar] [CrossRef]
- Wei, F.; Tuong, Z.K.; Omer, M.; Ngo, C.; Asiatico, J.; Kinzel, M.; Pugazhendhi, A.S.; Khaled, A.R.; Ghosh, R.; Coathup, M. A Novel Multifunctional Radioprotective Strategy Using P7C3 as a Countermeasure against Ionizing Radiation-Induced Bone Loss. Bone Res. 2023, 11, 34. [Google Scholar] [CrossRef]
- Dempster, D.W. Osteoporosis and the Burden of Osteoporosis-Related Fractures. Am. J. Manag. Care 2011, 17 (Suppl. S6), S164–S169. [Google Scholar]
- Fogel, H.A.; Jenis, L.G. The Economic Burden of Osteoporosis. In Vertebral Compression Fractures in Osteoporotic and Pathologic Bone; Springer International Publishing: Cham, Switzerland, 2020; pp. 21–29. [Google Scholar] [CrossRef]
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M.; et al. Global, Regional, and National Burden of Osteoarthritis, 1990–2020 and Projections to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef] [PubMed]
- López-Ortega, O.; Moreno-Corona, N.C.; Cruz-Holguin, V.J.; Garcia-Gonzalez, L.D.; Helguera-Repetto, A.C.; Romero-Valdovinos, M.; Arevalo-Romero, H.; Cedillo-Barron, L.; León-Juárez, M. The Immune Response in Adipocytes and Their Susceptibility to Infection: A Possible Relationship with Infectobesity. Int. J. Mol. Sci. 2022, 23, 6154. [Google Scholar] [CrossRef]
- Freiberger, R.N.; López, C.A.M.; Sviercz, F.A.; Cevallos, C.; Guano, A.D.; Jarmoluk, P.; Quarleri, J.; Delpino, M.V.B. Abortus Infection Promotes an Imbalance in the Adipocyte–Osteoblast Crosstalk Favoring Bone Resorption. Int. J. Mol. Sci. 2023, 24, 5617. [Google Scholar] [CrossRef]
- Fiedler, T.; Salamon, A.; Adam, S.; Herzmann, N.; Taubenheim, J.; Peters, K. Impact of Bacteria and Bacterial Components on Osteogenic and Adipogenic Differentiation of Adipose-Derived Mesenchymal Stem Cells. Exp. Cell Res. 2013, 319, 2883–2892. [Google Scholar] [CrossRef] [PubMed]
- Curi, M.M.; Cardoso, C.L.; de Lima, H.G.; Kowalski, L.P.; Martins, M.D.; de Lima, H.G.; Kowalski, L.P.; Martins, M.D. Histopathologic and Histomorphometric Analysis of Irradiation Injury in Bone and the Surrounding Soft Tissues of the Jaws. J. Oral Maxillofac. Surg. 2016, 74, 190–199. [Google Scholar] [CrossRef]
- Daniel, M.; Luby, A.O.; Buchman, L.; Buchman, S.R. Overcoming Nuclear Winter: The Cutting-Edge Science of Bone Healing and Regeneration in Irradiated Fields. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3605. [Google Scholar] [CrossRef]
- Georgiou, K.R.; Hui, S.K.; Xian, C.J. Regulatory Pathways Associated with Bone Loss and Bone Marrow Adiposity Caused by Aging, Chemotherapy, Glucocorticoid Therapy and Radiotherapy. Am. J. Stem Cells 2012, 1, 205–224. [Google Scholar]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate Decision of Mesenchymal Stem Cells: Adipocytes or Osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef]
- Wuthier, R.E. Lipids of Matrix Vesicles. Fed. Proc. 1976, 35, 117–121. [Google Scholar] [PubMed]
- Dirksen, T.R.; Marinetti, G.V. Lipids of Bovine Enamel and Dentin and Human Bone. Calcif. Tissue Res. 1970, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, M.R.; Brissette, L.; Falstrault, L.; Ouellet, P.; Moreau, R. Influence of Oxidized Low-Density Lipoproteins (LDL) on the Viability of Osteoblastic Cells. Free Radic. Biol. Med. 2008, 44, 506–517. [Google Scholar] [CrossRef]
- Almeida, M.; Ambrogini, E.; Han, L.; Manolagas, S.C.; Jilka, R.L. Increased Lipid Oxidation Causes Oxidative Stress, Increased Peroxisome Proliferator-Activated Receptor-γ Expression, and Diminished Pro-Osteogenic Wnt Signaling in the Skeleton. J. Biol. Chem. 2009, 284, 27438–27448. [Google Scholar] [CrossRef]
- Tintut, Y.; Demer, L.L. Effects of Bioactive Lipids and Lipoproteins on Bone. Trends Endocrinol. Metab. 2014, 25, 53–59. [Google Scholar] [CrossRef]
- Jiang, C.; Xie, C.; Li, F.; Zhang, L.; Nichols, R.G.; Krausz, K.W.; Cai, J.; Qi, Y.; Fang, Z.-Z.; Takahashi, S.; et al. Intestinal Farnesoid X Receptor Signaling Promotes Nonalcoholic Fatty Liver Disease. J. Clin. Investig. 2015, 125, 386–402. [Google Scholar] [CrossRef]
- Summers, S.A.; Garza, L.A.; Zhou, H.; Birnbaum, M.J. Regulation of Insulin-Stimulated Glucose Transporter GLUT4 Translocation and Akt Kinase Activity by Ceramide. Mol. Cell. Biol. 1998, 18, 5457–5464. [Google Scholar] [CrossRef]
- Wang, C.-N.; O’Brien, L.; Brindley, D.N. Effects of Cell-Permeable Ceramides and Tumor Necrosis Factor-α on Insulin Signaling and Glucose Uptake in 3T3-L1 Adipocytes. Diabetes 1998, 47, 24–31. [Google Scholar] [CrossRef]
- Hyde, R.; Hajduch, E.; Powell, D.J.; Taylor, P.M.; Hundal, H.S. Ceramide Down-regulates System A Amino Acid Transport and Protein Synthesis in Rat Skeletal Muscle Cells. FASEB J. 2005, 19, 1–24. [Google Scholar] [CrossRef]
- Guenther, G.G.; Peralta, E.R.; Rosales, K.R.; Wong, S.Y.; Siskind, L.J.; Edinger, A.L. Ceramide Starves Cells to Death by Downregulating Nutrient Transporter Proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 17402–17407. [Google Scholar] [CrossRef]
- Kogot-Levin, A.; Saada, A. Ceramide and the Mitochondrial Respiratory Chain. Biochimie 2014, 100, 88–94. [Google Scholar] [CrossRef]
- Turpin, S.M.; Nicholls, H.T.; Willmes, D.M.; Mourier, A.; Brodesser, S.; Wunderlich, C.M.; Mauer, J.; Xu, E.; Hammerschmidt, P.; Brönneke, H.S.; et al. Obesity-Induced CerS6-Dependent C16:0 Ceramide Production Promotes Weight Gain and Glucose Intolerance. Cell Metab. 2014, 20, 678–686. [Google Scholar] [CrossRef]
- Holland, W.L.; Brozinick, J.T.; Wang, L.-P.; Hawkins, E.D.; Sargent, K.M.; Liu, Y.; Narra, K.; Hoehn, K.L.; Knotts, T.A.; Siesky, A.; et al. Inhibition of Ceramide Synthesis Ameliorates Glucocorticoid-, Saturated-Fat-, and Obesity-Induced Insulin Resistance. Cell Metab. 2007, 5, 167–179. [Google Scholar] [CrossRef]
- Chen, T.-C.; Lee, R.A.; Tsai, S.L.; Kanamaluru, D.; Gray, N.E.; Yiv, N.; Cheang, R.T.; Tan, J.H.; Lee, J.Y.; Fitch, M.D.; et al. An ANGPTL4–Ceramide–Protein Kinase Cζ Axis Mediates Chronic Glucocorticoid Exposure–Induced Hepatic Steatosis and Hypertriglyceridemia in Mice. J. Biol. Chem. 2019, 294, 9213–9224. [Google Scholar] [CrossRef]
- Park, T.; Rosebury, W.; Kindt, E.; Kowala, M.; Panek, R. Serine Palmitoyltransferase Inhibitor Myriocin Induces the Regression of Atherosclerotic Plaques in Hyperlipidemic ApoE-Deficient Mice. Pharmacol. Res. 2008, 58, 45–51. [Google Scholar] [CrossRef]
- Park, T.-S.; Panek, R.L.; Mueller, S.B.; Hanselman, J.C.; Rosebury, W.S.; Robertson, A.W.; Kindt, E.K.; Homan, R.; Karathanasis, S.K.; Rekhter, M.D. Inhibition of Sphingomyelin Synthesis Reduces Atherogenesis in Apolipoprotein E–Knockout Mice. Circulation 2004, 110, 3465–3471. [Google Scholar] [CrossRef]
- Park, T.-S.; Hu, Y.; Noh, H.-L.; Drosatos, K.; Okajima, K.; Buchanan, J.; Tuinei, J.; Homma, S.; Jiang, X.-C.; Abel, E.D.; et al. Ceramide Is a Cardiotoxin in Lipotoxic Cardiomyopathy. J. Lipid Res. 2008, 49, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Akashi, H.; Drosatos, K.; Liao, X.; Jiang, H.; Kennel, P.J.; Brunjes, D.L.; Castillero, E.; Zhang, X.; Deng, L.Y.; et al. Increased de Novo Ceramide Synthesis and Accumulation in Failing Myocardium. JCI Insight 2017, 2, e82922. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Castillo, S.S.; Goldkorn, T. NSMase2 Activation and Trafficking Are Modulated by Oxidative Stress to Induce Apoptosis. Biochem. Biophys. Res. Commun. 2006, 344, 900–905. [Google Scholar] [CrossRef]
- Podbielska, M.; Szulc, Z.M.; Kurowska, E.; Hogan, E.L.; Bielawski, J.; Bielawska, A.; Bhat, N.R. Cytokine-Induced Release of Ceramide-Enriched Exosomes as a Mediator of Cell Death Signaling in an Oligodendroglioma Cell Line. J. Lipid Res. 2016, 57, 2028–2039. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Corbacho, M.J.; Canals, D.; Adada, M.M.; Liu, M.; Senkal, C.E.; Yi, J.K.; Mao, C.; Luberto, C.; Hannun, Y.A.; Obeid, L.M. Tumor Necrosis Factor-α (TNFα)-Induced Ceramide Generation via Ceramide Synthases Regulates Loss of Focal Adhesion Kinase (FAK) and Programmed Cell Death. J. Biol. Chem. 2015, 290, 25356–25373. [Google Scholar] [CrossRef]
- Heaver, S.L.; Johnson, E.L.; Ley, R.E. Sphingolipids in Host–Microbial Interactions. Curr. Opin. Microbiol. 2018, 43, 92–99. [Google Scholar] [CrossRef]
- Khayrullin, A.; Krishnan, P.; Martinez-Nater, L.; Mendhe, B.; Fulzele, S.; Liu, Y.; Mattison, J.; Hamrick, M. Very Long-Chain C24:1 Ceramide Is Increased in Serum Extracellular Vesicles with Aging and Can Induce Senescence in Bone-Derived Mesenchymal Stem Cells. Cells 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V. Oxidative Stress in Bone Remodeling: Role of Antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209. [Google Scholar] [CrossRef]
- Rao, S.-S.; Hu, Y.; Xie, P.-L.; Cao, J.; Wang, Z.-X.; Liu, J.-H.; Yin, H.; Huang, J.; Tan, Y.-J.; Luo, J.; et al. Omentin-1 Prevents Inflammation-Induced Osteoporosis by Downregulating the pro-Inflammatory Cytokines. Bone Res. 2018, 6, 9. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Yu, J.; Rho, J. Bone Loss Triggered by the Cytokine Network in Inflammatory Autoimmune Diseases. J. Immunol. Res. 2015, 2015, 832127. [Google Scholar] [CrossRef]
- Hu, M.; Xing, L.; Zhang, L.; Liu, F.; Wang, S.; Xie, Y.; Wang, J.; Jiang, H.; Guo, J.; Li, X.; et al. NAP1L2 Drives Mesenchymal Stem Cell Senescence and Suppresses Osteogenic Differentiation. Aging Cell 2022, 21, e13551. [Google Scholar] [CrossRef]
- Lyu, Z.; Hu, Y.; Guo, Y.; Liu, D. Modulation of Bone Remodeling by the Gut Microbiota: A New Therapy for Osteoporosis. Bone Res. 2023, 11, 31. [Google Scholar] [CrossRef]
- Hill, P.A.; Tumber, A. Ceramide-Induced Cell Death/Survival in Murine Osteoblasts. J. Endocrinol. 2010, 206, 225–233. [Google Scholar] [CrossRef]
- Alsahli, A.; Kiefhaber, K.; Gold, T.; Muluke, M.; Jiang, H.; Cremers, S.; Schulze-Späte, U. Palmitic Acid Reduces Circulating Bone Formation Markers in Obese Animals and Impairs Osteoblast Activity via C16-Ceramide Accumulation. Calcif. Tissue Int. 2016, 98, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.J.; Chae, S.W.; Kang, J.S.; Bang, B.G.; Cho, S.B.; Park, R.K.; So, H.S.; Kim, Y.K.; Kim, H.M.; Kim, H.R. Dexamethasone Suppresses Tumor Necrosis Factor-α-Induced Apoptosis in Osteoblasts: Possible Role for Ceramide. Endocrinology 2000, 141, 2904–2913. [Google Scholar] [CrossRef]
- Elliott, K.H.; Brugmann, S.A. Sending Mixed Signals: Cilia-Dependent Signaling during Development and Disease. Dev. Biol. 2019, 447, 28–41. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, S. Primary Cilia and Intraflagellar Transport Proteins in Bone and Cartilage. J. Dent. Res. 2016, 95, 1341–1349. [Google Scholar] [CrossRef]
- Lim, J.; Li, X.; Yuan, X.; Yang, S.; Han, L.; Yang, S. Primary Cilia Control Cell Alignment and Patterning in Bone Development via Ceramide-PKCζ-β-Catenin Signaling. Commun. Biol. 2020, 3, 45. [Google Scholar] [CrossRef]
- Brancati, F.; Dallapiccola, B.; Valente, E.M. Joubert Syndrome and Related Disorders. Orphanet J. Rare Dis. 2010, 5, 20. [Google Scholar] [CrossRef]
- Beales, P.L.; Bland, E.; Tobin, J.L.; Bacchelli, C.; Tuysuz, B.; Hill, J.; Rix, S.; Pearson, C.G.; Kai, M.; Hartley, J.; et al. IFT80, Which Encodes a Conserved Intraflagellar Transport Protein, Is Mutated in Jeune Asphyxiating Thoracic Dystrophy. Nat. Genet. 2007, 39, 727–729. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, G.; Wakade, S.; Dasgupta, S.; Dinkins, M.; Kong, J.N.; Spassieva, S.D.; Bieberich, E. Primary Cilia in Stem Cells and Neural Progenitors Are Regulated by Neutral Sphingomyelinase 2 and Ceramide. Mol. Biol. Cell 2014, 25, 1715–1729. [Google Scholar] [CrossRef]
- Granado, M.H.; Gangoiti, P.; Ouro, A.; Arana, L.; González, M.; Trueba, M.; Gómez-Muñoz, A. Ceramide 1-Phosphate (C1P) Promotes Cell Migration. Cell. Signal. 2009, 21, 405–412. [Google Scholar] [CrossRef]
- Kim, C.; Schneider, G.; Abdel-Latif, A.; Mierzejewska, K.; Sunkara, M.; Borkowska, S.; Ratajczak, J.; Morris, A.J.; Kucia, M.; Ratajczak, M.Z. Ceramide-1-Phosphate Regulates Migration of Multipotent Stromal Cells and Endothelial Progenitor Cells—Implications for Tissue Regeneration. Stem Cells 2013, 31, 500–510. [Google Scholar] [CrossRef]
- Dar, A.; Kollet, O.; Lapidot, T. Mutual, Reciprocal SDF-1/CXCR4 Interactions between Hematopoietic and Bone Marrow Stromal Cells Regulate Human Stem Cell Migration and Development in NOD/SCID Chimeric Mice. Exp. Hematol. 2006, 34, 967–975. [Google Scholar] [CrossRef]
- Ho, C.-Y.; Sanghani, A.; Hua, J.; Coathup, M.; Kalia, P.; Blunn, G. Mesenchymal Stem Cells with Increased Stromal Cell-Derived Factor 1 Expression Enhanced Fracture Healing. Tissue Eng. Part A 2015, 21, 594–602. [Google Scholar] [CrossRef]
- Hong, H.S.; Lee, J.; Lee, E.; Kwon, Y.S.; Lee, E.; Ahn, W.; Jiang, M.H.; Kim, J.C.; Son, Y. A New Role of Substance P as an Injury-Inducible Messenger for Mobilization of CD29+ Stromal-like Cells. Nat. Med. 2009, 15, 425–435. [Google Scholar] [CrossRef]
- Dubon, M.J.; Park, K.-S. The Mechanisms of Substance P-Mediated Migration of Bone Marrow-Derived Mesenchymal Stem Cell-like ST2 Cells. Int. J. Mol. Med. 2016, 37, 1105–1111. [Google Scholar] [CrossRef]
- Wan, M.; Li, C.; Zhen, G.; Jiao, K.; He, W.; Jia, X.; Wang, W.; Shi, C.; Xing, Q.; Chen, Y.; et al. Injury-Activated Transforming Growth Factor β Controls Mobilization of Mesenchymal Stem Cells for Tissue Remodeling. Stem Cells 2012, 30, 2498–2511. [Google Scholar] [CrossRef]
- Yu, J.; Kim, H.M.; Kim, K.P.; Son, Y.; Kim, M.-S.; Park, K.-S. Ceramide Kinase Regulates the Migration of Bone Marrow-Derived Mesenchymal Stem Cells. Biochem. Biophys. Res. Commun. 2019, 508, 361–367. [Google Scholar] [CrossRef]
- Kitatani, K.; Sheldon, K.; Anelli, V.; Jenkins, R.W.; Sun, Y.; Grabowski, G.A.; Obeid, L.M.; Hannun, Y.A. Acid β-Glucosidase 1 Counteracts P38δ-Dependent Induction of Interleukin-6. J. Biol. Chem. 2009, 284, 12979–12988. [Google Scholar] [CrossRef]
- Takeda, H.; Ozaki, K.; Yasuda, H.; Ishida, M.; Kitano, S.; Hanazawa, S. Sphingomyelinase and Ceramide Inhibit Formation of F-actin Ring in and Bone Resorption by Rabbit Mature Osteoclasts. FEBS Lett. 1998, 422, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Fukumoto, S.; Kanaoka, K.; Sakai, E.; Shibata, M.; Fukumoto, E.; Inokuchi, J.; Takamiya, K.; Furukawa, K.; Furukawa, K.; et al. Lactosylceramide Is Essential for the Osteoclastogenesis Mediated by Macrophage-Colony-Stimulating Factor and Receptor Activator of Nuclear Factor-ΚB Ligand. J. Biol. Chem. 2001, 276, 46031–46038. [Google Scholar] [CrossRef]
- Hannun, Y. Ceramide: An Intracellular Signal for Apoptosis. Trends Biochem. Sci. 1995, 20, 73–77. [Google Scholar] [CrossRef]
- Kolesnick, R. Ceramide: A Novel Second Messenger. Trends Cell Biol. 1992, 2, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Nikolova-Karakashian, M.; Merrill, A.H.; Morgan, E.T. Regulation of Cytochrome P450 2C11 (CYP2C11) Gene Expression by Interleukin-1, Sphingomyelin Hydrolysis, and Ceramides in Rat Hepatocytes. J. Biol. Chem. 1995, 270, 25233–25238. [Google Scholar] [CrossRef]
- Dobierzewska, A.; Shi, L.; Karakashian, A.A.; Nikolova-Karakashian, M.N. Interleukin 1β Regulation of FoxO1 Protein Content and Localization. J. Biol. Chem. 2012, 287, 44749–44760. [Google Scholar] [CrossRef]
- Oyeniran, C.; Sturgill, J.L.; Hait, N.C.; Huang, W.-C.; Avni, D.; Maceyka, M.; Newton, J.; Allegood, J.C.; Montpetit, A.; Conrad, D.H.; et al. Aberrant ORM (Yeast)–like Protein Isoform 3 (ORMDL3) Expression Dysregulates Ceramide Homeostasis in Cells and Ceramide Exacerbates Allergic Asthma in Mice. J. Allergy Clin. Immunol. 2015, 136, 1035–1046.e6. [Google Scholar] [CrossRef]
- Cai, L.; Oyeniran, C.; Biswas, D.D.; Allegood, J.; Milstien, S.; Kordula, T.; Maceyka, M.; Spiegel, S. ORMDL Proteins Regulate Ceramide Levels during Sterile Inflammation. J. Lipid Res. 2016, 57, 1412–1422. [Google Scholar] [CrossRef]
- Vozella, V.; Basit, A.; Piras, F.; Realini, N.; Armirotti, A.; Bossù, P.; Assogna, F.; Sensi, S.L.; Spalletta, G.; Piomelli, D. Elevated Plasma Ceramide Levels in Post-Menopausal Women: A Cross-Sectional Study. Aging 2019, 11, 73–88. [Google Scholar] [CrossRef]
- Kim, B.-J.; Lee, J.Y.; Park, S.J.; Lee, S.H.; Kim, S.J.; Yoo, H.J.; Rivera De Pena, S.I.; McGee-Lawrence, M.; Isales, C.M.; Koh, J.-M.; et al. Elevated Ceramides 18:0 and 24:1 with Aging Are Associated with Hip Fracture Risk through Increased Bone Resorption. Aging 2019, 11, 9388–9404. [Google Scholar] [CrossRef]
- Bose, R.; Verheij, M.; Haimovitz-Friedman, A.; Scotto, K.; Fuks, Z.; Kolesnick, R. Ceramide Synthase Mediates Daunorubicin-Induced Apoptosis: An Alternative Mechanism for Generating Death Signals. Cell 1995, 82, 405–414. [Google Scholar] [CrossRef]
- Perry, D.K.; Carton, J.; Shah, A.K.; Meredith, F.; Uhlinger, D.J.; Hannun, Y.A. Serine Palmitoyltransferase Regulates de NovoCeramide Generation during Etoposide-Induced Apoptosis. J. Biol. Chem. 2000, 275, 9078–9084. [Google Scholar] [CrossRef]
- Presa, N.; Gomez-Larrauri, A.; Rivera, I.-G.; Ordoñez, M.; Trueba, M.; Gomez-Muñoz, A. Regulation of Cell Migration and Inflammation by Ceramide 1-Phosphate. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 402–409. [Google Scholar] [CrossRef]
- Maceyka, M.; Spiegel, S. Sphingolipid Metabolites in Inflammatory Disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Muñoz, A.; Gangoiti, P.; Arana, L.; Ouro, A.; Rivera, I.-G.; Ordoñez, M.; Trueba, M. New Insights on the Role of Ceramide 1-Phosphate in Inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 1060–1066. [Google Scholar] [CrossRef]
- Chalfant, C.E.; Spiegel, S. Sphingosine 1-Phosphate and Ceramide 1-Phosphate: Expanding Roles in Cell Signaling. J. Cell Sci. 2005, 118, 4605–4612. [Google Scholar] [CrossRef] [PubMed]
- Hinkovska-Galcheva, V.T.; Boxer, L.A.; Mansfield, P.J.; Harsh, D.; Blackwood, A.; Shayman, J.A. The Formation of Ceramide-1-Phosphate during Neutrophil Phagocytosis and Its Role in Liposome Fusion. J. Biol. Chem. 1998, 273, 33203–33209. [Google Scholar] [CrossRef]
- Alvarez, S.E.; Harikumar, K.B.; Hait, N.C.; Allegood, J.; Strub, G.M.; Kim, E.Y.; Maceyka, M.; Jiang, H.; Luo, C.; Kordula, T.; et al. Sphingosine-1-Phosphate Is a Missing Cofactor for the E3 Ubiquitin Ligase TRAF2. Nature 2010, 465, 1084–1088. [Google Scholar] [CrossRef]
- Parham, K.A.; Zebol, J.R.; Tooley, K.L.; Sun, W.Y.; Moldenhauer, L.M.; Cockshell, M.P.; Gliddon, B.L.; Moretti, P.A.; Tigyi, G.; Pitson, S.M.; et al. Sphingosine 1-Phosphate Is a Ligand for Peroxisome Proliferator-Activated Receptor-γ That Regulates Neoangiogenesis. FASEB J. 2015, 29, 3638–3653. [Google Scholar] [CrossRef]
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Coso, O.A.; Gutkind, J.S.; Spiegel, S. Suppression of Ceramide-Mediated Programmed Cell Death by Sphingosine-1-Phosphate. Nature 1996, 381, 800–803. [Google Scholar] [CrossRef]
- Zhang, H.; Desai, N.N.; Olivera, A.; Seki, T.; Brooker, G.; Spiegel, S. Sphingosine-1-Phosphate, a Novel Lipid, Involved in Cellular Proliferation. J. Cell Biol. 1991, 114, 155–167. [Google Scholar] [CrossRef]
- Olivera, A.; Spiegel, S. Sphingosine-1-Phosphate as Second Messenger in Cell Proliferation Induced by PDGF and FCS Mitogens. Nature 1993, 365, 557–560. [Google Scholar] [CrossRef]
- Takuwa, Y. Subtype-Specific Differential Regulation of Rho Family G Proteins and Cell Migration by the Edg Family Sphingosine-1-Phosphate Receptors. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2002, 1582, 112–120. [Google Scholar] [CrossRef]
- Grey, A.; Xu, X.; Hill, B.; Watson, M.; Callon, K.; Reid, I.R.; Cornish, J. Osteoblastic Cells Express Phospholipid Receptors and Phosphatases and Proliferate in Response to Sphingosine-1-Phosphate. Calcif. Tissue Int. 2004, 74, 542–550. [Google Scholar] [CrossRef]
- Ishii, M.; Egen, J.G.; Klauschen, F.; Meier-Schellersheim, M.; Saeki, Y.; Vacher, J.; Proia, R.L.; Germain, R.N. Sphingosine-1-Phosphate Mobilizes Osteoclast Precursors and Regulates Bone Homeostasis. Nature 2009, 458, 524–528. [Google Scholar] [CrossRef]
- Quint, P.; Ruan, M.; Pederson, L.; Kassem, M.; Westendorf, J.J.; Khosla, S.; Oursler, M.J. Sphingosine 1-Phosphate (S1P) Receptors 1 and 2 Coordinately Induce Mesenchymal Cell Migration through S1P Activation of Complementary Kinase Pathways. J. Biol. Chem. 2013, 288, 5398–5406. [Google Scholar] [CrossRef]
- Ishii, M.; Kikuta, J.; Shimazu, Y.; Meier-Schellersheim, M.; Germain, R.N. Chemorepulsion by Blood S1P Regulates Osteoclast Precursor Mobilization and Bone Remodeling in Vivo. J. Exp. Med. 2010, 207, 2793–2798. [Google Scholar] [CrossRef]
- Bendall, L.J.; Basnett, J. Role of Sphingosine 1-Phosphate in Trafficking and Mobilization of Hematopoietic Stem Cells. Curr. Opin. Hematol. 2013, 20, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Shimazu, Y.; Nishiyama, I.; Kikuta, J.; Ishii, M. The Role of Sphingosine 1-Phosphate in Migration of Osteoclast Precursors; an Application of Intravital Two-Photon Microscopy. Mol. Cells 2011, 31, 399–403. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, H.; Lin, T.; Wang, S. Sphingosine-1-Phosphate/S1P Receptors Signaling Modulates Cell Migration in Human Bone Marrow-Derived Mesenchymal Stem Cells. Mediat. Inflamm. 2014, 2014, 565369. [Google Scholar] [CrossRef]
- Golan, K.; Vagima, Y.; Ludin, A.; Itkin, T.; Cohen-Gur, S.; Kalinkovich, A.; Kollet, O.; Kim, C.; Schajnovitz, A.; Ovadya, Y.; et al. S1P Promotes Murine Progenitor Cell Egress and Mobilization via S1P1-Mediated ROS Signaling and SDF-1 Release. Blood 2012, 119, 2478–2488. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, H.J.; Chang, E.-J.; Huang, H.; Banno, Y.; Kim, H.-H. Sphingosine 1-Phosphate as a Regulator of Osteoclast Differentiation and Osteoclast–Osteoblast Coupling. EMBO J. 2006, 25, 5840–5851. [Google Scholar] [CrossRef]
- Keller, J.; Catala-Lehnen, P.; Huebner, A.K.; Jeschke, A.; Heckt, T.; Lueth, A.; Krause, M.; Koehne, T.; Albers, J.; Schulze, J.; et al. Calcitonin Controls Bone Formation by Inhibiting the Release of Sphingosine 1-Phosphate from Osteoclasts. Nat. Commun. 2014, 5, 5215. [Google Scholar] [CrossRef]
- Brizuela, L.; Martin, C.; Jeannot, P.; Ader, I.; Gstalder, C.; Andrieu, G.; Bocquet, M.; Laffosse, J.-M.; Gomez-Brouchet, A.; Malavaud, B.; et al. Osteoblast-derived Sphingosine 1-phosphate to Induce Proliferation and Confer Resistance to Therapeutics to Bone Metastasis-derived Prostate Cancer Cells. Mol. Oncol. 2014, 8, 1181–1195. [Google Scholar] [CrossRef]
- Tantikanlayaporn, D.; Tourkova, I.L.; Larrouture, Q.; Luo, J.; Piyachaturawat, P.; Witt, M.R.; Blair, H.C.; Robinson, L.J. Sphingosine-1-Phosphate Modulates the Effect of Estrogen in Human Osteoblasts. JBMR Plus 2018, 2, 217–226. [Google Scholar] [CrossRef]
- Dziak, R.; Yang, B.; Leung, B.; Li, S.; Marzec, N.; Margarone, J.; Bobek, L. Effects of Sphingosine-1-Phosphate and Lysophosphatidic Acid on Human Osteoblastic Cells. Prostaglandins Leukot. Essent. Fat. Acids 2003, 68, 239–249. [Google Scholar] [CrossRef]
- Roelofsen, T.; Akkers, R.; Beumer, W.; Apotheker, M.; Steeghs, I.; van de Ven, J.; Gelderblom, C.; Garritsen, A.; Dechering, K. Sphingosine-1-phosphate Acts as a Developmental Stage Specific Inhibitor of Platelet-derived Growth Factor-induced Chemotaxis of Osteoblasts. J. Cell. Biochem. 2008, 105, 1128–1138. [Google Scholar] [CrossRef]
- Pederson, L.; Ruan, M.; Westendorf, J.J.; Khosla, S.; Oursler, M.J. Regulation of Bone Formation by Osteoclasts Involves Wnt/BMP Signaling and the Chemokine Sphingosine-1-Phosphate. Proc. Natl. Acad. Sci. USA 2008, 105, 20764–20769. [Google Scholar] [CrossRef]
- Carpio, L.C.; Stephan, E.; Kamer, A.; Dziak, R. Sphingolipids Stimulate Cell Growth via MAP Kinase Activation in Osteoblastic Cells. Prostaglandins Leukot. Essent. Fat. Acids 1999, 61, 267–273. [Google Scholar] [CrossRef]
- Weske, S.; Vaidya, M.; Reese, A.; von Wnuck Lipinski, K.; Keul, P.; Bayer, J.K.; Fischer, J.W.; Flögel, U.; Nelsen, J.; Epple, M.; et al. Targeting Sphingosine-1-Phosphate Lyase as an Anabolic Therapy for Bone Loss. Nat. Med. 2018, 24, 667–678. [Google Scholar] [CrossRef]
- Xiao, L.; Zhou, Y.; Zhu, L.; Yang, S.; Huang, R.; Shi, W.; Peng, B.; Xiao, Y. SPHK1-S1PR1-RANKL Axis Regulates the Interactions Between Macrophages and BMSCs in Inflammatory Bone Loss. J. Bone Miner. Res. 2018, 33, 1090–1104. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.-Y.; Lee, Y.-S.; Kim, B.-J.; Lim, K.-H.; Cho, E.-H.; Kim, S.-W.; Koh, J.-M.; Kim, G.S. Higher Circulating Sphingosine 1-Phosphate Levels Are Associated with Lower Bone Mineral Density and Higher Bone Resorption Marker in Humans. J. Clin. Endocrinol. Metab. 2012, 97, E1421–E1428. [Google Scholar] [CrossRef]
- Kim, B.-J.; Koh, J.-M.; Lee, S.-Y.; Lee, Y.-S.; Lee, S.H.; Lim, K.-H.; Cho, E.-H.; Kim, S.-W.; Kim, T.-H.; Kim, S.-Y.; et al. Plasma Sphingosine 1-Phosphate Levels and the Risk of Vertebral Fracture in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2012, 97, 3807–3814. [Google Scholar] [CrossRef]
- Ardawi, M.-S.M.; Rouzi, A.A.; Al-Senani, N.S.; Qari, M.H.; Elsamanoudy, A.Z.; Mousa, S.A. High Plasma Sphingosine 1-Phosphate Levels Predict Osteoporotic Fractures in Postmenopausal Women: The Center of Excellence for Osteoporosis Research Study. J. Bone Metab. 2018, 25, 87. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.Y.; Lim, K.-H.; Lee, Y.-S.; Kim, S.-H.; Choi, S.; Cho, S.-H.; Kim, J.S.; Koh, J.-M. Associations of Circulating Levels of Sphingosine 1-Phosphate with the Trabecular Bone Score and Bone Mineral Density in Postmenopausal Women. J. Clin. Densitom. 2021, 24, 414–421. [Google Scholar] [CrossRef]
- Bae, S.J.; Lee, S.H.; Ahn, S.H.; Kim, H.-M.; Kim, B.-J.; Koh, J.-M. The Circulating Sphingosine-1-Phosphate Level Predicts Incident Fracture in Postmenopausal Women: A 3.5-Year Follow-up Observation Study. Osteoporos. Int. 2016, 27, 2533–2541. [Google Scholar] [CrossRef]
- Garnero, P. New Developments in Biological Markers of Bone Metabolism in Osteoporosis. Bone 2014, 66, 46–55. [Google Scholar] [CrossRef]
- Ahn, S.H.; Koh, J.-M.; Gong, E.J.; Byun, S.; Lee, S.-Y.; Kim, B.-J.; Lee, S.H.; Chang, J.S.; Kim, G.S. Association of Bone Marrow Sphingosine 1-Phosphate Levels with Osteoporotic Hip Fractures. J. Bone Metab. 2013, 20, 61. [Google Scholar] [CrossRef][Green Version]
- Kim, B.-J.; Shin, K.-O.; Kim, H.; Ahn, S.H.; Lee, S.H.; Seo, C.-H.; Byun, S.-E.; Chang, J.S.; Koh, J.-M.; Lee, Y.-M. The Effect of Sphingosine-1-Phosphate on Bone Metabolism in Humans Depends on Its Plasma/Bone Marrow Gradient. J. Endocrinol. Investig. 2016, 39, 297–303. [Google Scholar] [CrossRef]
- Grewe, J.M.; Knapstein, P.-R.; Donat, A.; Jiang, S.; Smit, D.J.; Xie, W.; Keller, J. The Role of Sphingosine-1-Phosphate in Bone Remodeling and Osteoporosis. Bone Res. 2022, 10, 34. [Google Scholar] [CrossRef]
- Heilmann, A.; Schinke, T.; Bindl, R.; Wehner, T.; Rapp, A.; Haffner-Luntzer, M.; Liedert, A.; Amling, M.; Ignatius, A. Systemic Treatment with the Sphingosine-1-phosphate Analog FTY720 Does Not Improve Fracture Healing in Mice. J. Orthop. Res. 2013, 31, 1845–1850. [Google Scholar] [CrossRef]
- Zajac, A.J.; Phillips, P.E. Paget’s Disease of Bone: Clinical Features and Treatment. Clin. Exp. Rheumatol. 1985, 3, 75–88. [Google Scholar]
- Nagata, Y.; Miyagawa, K.; Ohata, Y.; Petrusca, D.N.; Pagnotti, G.M.; Mohammad, K.S.; Guise, T.A.; Windle, J.J.; David Roodman, G.; Kurihara, N. Increased S1P Expression in Osteoclasts Enhances Bone Formation in an Animal Model of Paget’s Disease. J. Cell. Biochem. 2021, 122, 335–348. [Google Scholar] [CrossRef]
- Hutami, I.R.; Izawa, T.; Mino-Oka, A.; Shinohara, T.; Mori, H.; Iwasa, A.; Tanaka, E. Fas/S1P 1 Crosstalk via NF-ΚB Activation in Osteoclasts Controls Subchondral Bone Remodeling in Murine TMJ Arthritis. Biochem. Biophys. Res. Commun. 2017, 490, 1274–1281. [Google Scholar] [CrossRef]
- Lai, W.-Q.; Irwan, A.W.; Goh, H.H.; Howe, H.S.; Yu, D.T.; Valle-Oñate, R.; McInnes, I.B.; Melendez, A.J.; Leung, B.P. Anti-Inflammatory Effects of Sphingosine Kinase Modulation in Inflammatory Arthritis. J. Immunol. 2008, 181, 8010–8017. [Google Scholar] [CrossRef]
- Cherifi, C.; Latourte, A.; Vettorazzi, S.; Tuckermann, J.; Provot, S.; Ea, H.-K.; Ledoux, A.; Casas, J.; Cuvillier, O.; Richette, P.; et al. Inhibition of Sphingosine 1-Phosphate Protects Mice against Chondrocyte Catabolism and Osteoarthritis. Osteoarthr. Cartil. 2021, 29, 1335–1345. [Google Scholar] [CrossRef]
- Kikuta, J.; Iwai, K.; Saeki, Y.; Ishii, M. S1P-Targeted Therapy for Elderly Rheumatoid Arthritis Patients with Osteoporosis. Rheumatol. Int. 2011, 31, 967–969. [Google Scholar] [CrossRef]
- Wang, F.; Tan, W.; Guo, D.; He, S. Reduction of CD4 Positive T Cells and Improvement of Pathological Changes of Collagen-Induced Arthritis by FTY720. Eur. J. Pharmacol. 2007, 573, 230–240. [Google Scholar] [CrossRef]
- Matsuura, M.; Imayoshi, T.; Okumoto, T. Effect of FTY720, a Novel Immunosuppressant, on Adjuvant- and Collagen-Induced Arthritis in Rats. Int. J. Immunopharmacol. 2000, 22, 323–331. [Google Scholar] [CrossRef]
- Stradner, M.H.; Angerer, H.; Ortner, T.; Fuerst, F.C.; Setznagl, D.; Kremser, M.-L.; Hermann, J.; Graninger, W.B. The Immunosuppressant FTY720 (Fingolimod) Enhances Glycosaminoglycan Depletion in Articular Cartilage. BMC Musculoskelet. Disord. 2011, 12, 279. [Google Scholar] [CrossRef]
- Sandler, R.D.; Dunkley, L. Osteoarthritis and the Inflammatory Arthritides. Surgery 2018, 36, 21–26. [Google Scholar] [CrossRef]
- El Jamal, A.; Bougault, C.; Mebarek, S.; Magne, D.; Cuvillier, O.; Brizuela, L. The Role of Sphingosine 1-Phosphate Metabolism in Bone and Joint Pathologies and Ectopic Calcification. Bone 2020, 130, 115087. [Google Scholar] [CrossRef]
- Bougault, C.; El Jamal, A.; Briolay, A.; Mebarek, S.; Boutet, M.-A.; Garraud, T.; Le Goff, B.; Blanchard, F.; Magne, D.; Brizuela, L. Involvement of Sphingosine Kinase/Sphingosine 1-Phosphate Metabolic Pathway in Spondyloarthritis. Bone 2017, 103, 150–158. [Google Scholar] [CrossRef]
- El Jamal, A.; Briolay, A.; Mebarek, S.; Le Goff, B.; Blanchard, F.; Magne, D.; Brizuela, L.; Bougault, C. Cytokine-Induced and Stretch-Induced Sphingosine 1-Phosphate Production by Enthesis Cells Could Favor Abnormal Ossification in Spondyloarthritis. J. Bone Miner. Res. 2019, 34, 2264–2276. [Google Scholar] [CrossRef]
- Rutherford, C.; Childs, S.; Ohotski, J.; McGlynn, L.; Riddick, M.; MacFarlane, S.; Tasker, D.; Pyne, S.; Pyne, N.J.; Edwards, J.; et al. Regulation of Cell Survival by Sphingosine-1-Phosphate Receptor S1P1 via Reciprocal ERK-Dependent Suppression of Bim and PI-3-Kinase/Protein Kinase C-Mediated Upregulation of Mcl-1. Cell Death Dis. 2013, 4, e927. [Google Scholar] [CrossRef]
- Holland, W.L.; Xia, J.Y.; Johnson, J.A.; Sun, K.; Pearson, M.J.; Sharma, A.X.; Quittner-Strom, E.; Tippetts, T.S.; Gordillo, R.; Scherer, P.E. Inducible Overexpression of Adiponectin Receptors Highlight the Roles of Adiponectin-Induced Ceramidase Signaling in Lipid and Glucose Homeostasis. Mol. Metab. 2017, 6, 267–275. [Google Scholar] [CrossRef]
- Henry, B.; Ziobro, R.; Becker, K.A.; Kolesnick, R.; Gulbins, E. Acid Sphingomyelinase. In Sphingolipids: Basic Science and Drug Development; Springer: Vienna, Austria, 2013; pp. 77–88. [Google Scholar] [CrossRef]
- Goñi, F.M.; Alonso, A. Sphingomyelinases: Enzymology and Membrane Activity. FEBS Lett. 2002, 531, 38–46. [Google Scholar] [CrossRef]
- Schuchman, E.H.; Desnick, R.J. Types A and B Niemann-Pick Disease. Mol. Genet. Metab. 2017, 120, 27–33. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, X.; Lu, Z.; Perry, D.M.; Li, Y.; Russo, S.B.; Cowart, L.A.; Hannun, Y.A.; Huang, Y. Acid Sphingomyelinase Plays a Key Role in Palmitic Acid-Amplified Inflammatory Signaling Triggered by Lipopolysaccharide at Low Concentrations in Macrophages. Am. J. Physiol. Metab. 2013, 305, E853–E867. [Google Scholar] [CrossRef]
- Zeidan, Y.H.; Hannun, Y.A. Activation of Acid Sphingomyelinase by Protein Kinase Cδ-Mediated Phosphorylation. J. Biol. Chem. 2007, 282, 11549–11561. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Z.; Zhang, L.; Kirkwood, C.L.; Kirkwood, K.L.; Lopes-Virella, M.F.; Huang, Y. Inhibition of Acid Sphingomyelinase by Imipramine Abolishes the Synergy between Metabolic Syndrome and Periodontitis on Alveolar Bone Loss. J. Periodontal Res. 2022, 57, 173–185. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Z.; Zhang, L.; Kirkwood, K.L.; Lopes-Virella, M.F.; Huang, Y. Acid Sphingomyelinase Deficiency Exacerbates LPS-induced Experimental Periodontitis. Oral Dis. 2020, 26, 637–646. [Google Scholar] [CrossRef]
- Deevska, G.M.; Rozenova, K.A.; Giltiay, N.V.; Chambers, M.A.; White, J.; Boyanovsky, B.B.; Wei, J.; Daugherty, A.; Smart, E.J.; Reid, M.B.; et al. Acid Sphingomyelinase Deficiency Prevents Diet-Induced Hepatic Triacylglycerol Accumulation and Hyperglycemia in Mice. J. Biol. Chem. 2009, 284, 8359–8368. [Google Scholar] [CrossRef]
- Roux-Biejat, P.; Coazzoli, M.; Marrazzo, P.; Zecchini, S.; Di Renzo, I.; Prata, C.; Napoli, A.; Moscheni, C.; Giovarelli, M.; Barbalace, M.C.; et al. Acid Sphingomyelinase Controls Early Phases of Skeletal Muscle Regeneration by Shaping the Macrophage Phenotype. Cells 2021, 10, 3028. [Google Scholar] [CrossRef]
- Zhang, H.; Li, K.; Zhao, Y.; Zhang, Y.; Sun, J.; Li, S.; Lin, G. Long-Term Use of Fluoxetine Accelerates Bone Loss through the Disruption of Sphingolipids Metabolism in Bone Marrow Adipose Tissue. Transl. Psychiatry 2020, 10, 138. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Y.; Sun, Y.; Xu, A. Acid Sphingomyelinase Mediates Ferroptosis Induced by High Glucose via Autophagic Degradation of GPX4 in Type 2 Diabetic Osteoporosis. Mol. Med. 2023, 29, 125. [Google Scholar] [CrossRef]
- Taniguchi, M.; Ogiso, H.; Takeuchi, T.; Kitatani, K.; Umehara, H.; Okazaki, T. Lysosomal Ceramide Generated by Acid Sphingomyelinase Triggers Cytosolic Cathepsin B-Mediated Degradation of X-Linked Inhibitor of Apoptosis Protein in Natural Killer/T Lymphoma Cell Apoptosis. Cell Death Dis. 2015, 6, e1717. [Google Scholar] [CrossRef]
- Wissing, M.D. Chemotherapy- and Irradiation-Induced Bone Loss in Adults with Solid Tumors. Curr. Osteoporos. Rep. 2015, 13, 140–145. [Google Scholar] [CrossRef]
- Mancuso, S.; Scaturro, D.; Santoro, M.; Di Gaetano, G.; Vitagliani, F.; Falco, V.; Siragusa, S.; Gonnelli, S.; Mauro, G.L. Bone Damage after Chemotherapy for Lymphoma: A Real-World Experience. BMC Musculoskelet. Disord. 2021, 22, 1024. [Google Scholar] [CrossRef]
- Castillo, S.S.; Levy, M.; Thaikoottathil, J.V.; Goldkorn, T. Reactive Nitrogen and Oxygen Species Activate Different Sphingomyelinases to Induce Apoptosis in Airway Epithelial Cells. Exp. Cell Res. 2007, 313, 2680–2686. [Google Scholar] [CrossRef]
- Yura, Y.; Masui, A.; Hamada, M. Inhibitors of Ceramide- and Sphingosine-Metabolizing Enzymes as Sensitizers in Radiotherapy and Chemotherapy for Head and Neck Squamous Cell Carcinoma. Cancers 2020, 12, 2062. [Google Scholar] [CrossRef]
- Tuckermann, J.; Adams, R.H. The Endothelium–Bone Axis in Development, Homeostasis and Bone and Joint Disease. Nat. Rev. Rheumatol. 2021, 17, 608–620. [Google Scholar] [CrossRef]
- Fuks, Z.; Kolesnick, R. Engaging the Vascular Component of the Tumor Response. Cancer Cell 2005, 8, 89–91. [Google Scholar] [CrossRef]
- Peña, L.A.; Fuks, Z.; Kolesnick, R.N. Radiation-Induced Apoptosis of Endothelial Cells in the Murine Central Nervous System: Protection by Fibroblast Growth Factor and Sphingomyelinase Deficiency. Cancer Res. 2000, 60, 321–327. [Google Scholar]
- Garcia-Barros, M.; Paris, F.; Cordon-Cardo, C.; Lyden, D.; Rafii, S.; Haimovitz-Friedman, A.; Fuks, Z.; Kolesnick, R. Tumor Response to Radiotherapy Regulated by Endothelial Cell Apoptosis. Science 2003, 300, 1155–1159. [Google Scholar] [CrossRef]
- El Kaffas, A.; Al-Mahrouki, A.; Hashim, A.; Law, N.; Giles, A.; Czarnota, G.J. Role of Acid Sphingomyelinase and Ceramide in Mechano-Acoustic Enhancement of Tumor Radiation Responses. JNCI J. Natl. Cancer Inst. 2018, 110, 1009–1018. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Becker, K.A.; Gulbins, E. Ceramide-Enriched Membrane Domains—Structure and Function. Biochim. Biophys. Acta Biomembr. 2009, 1788, 178–183. [Google Scholar] [CrossRef]
- Jin, S.; Zhou, F.; Katirai, F.; Li, P.-L. Lipid Raft Redox Signaling: Molecular Mechanisms in Health and Disease. Antioxid. Redox Signal. 2011, 15, 1043–1083. [Google Scholar] [CrossRef]
- Ladjohounlou, R.; Louati, S.; Lauret, A.; Gauthier, A.; Ardail, D.; Magne, N.; Alphonse, G.; Rodriguez-Lafrasse, C. Ceramide-Enriched Membrane Domains Contribute to Targeted and Nontargeted Effects of Radiation through Modulation of PI3K/AKT Signaling in HNSCC Cells. Int. J. Mol. Sci. 2020, 21, 7200. [Google Scholar] [CrossRef]
- Bonnaud, S.; Niaudet, C.; Legoux, F.; Corre, I.; Delpon, G.; Saulquin, X.; Fuks, Z.; Gaugler, M.-H.; Kolesnick, R.; Paris, F. Sphingosine-1-Phosphate Activates the AKT Pathway to Protect Small Intestines from Radiation-Induced Endothelial Apoptosis. Cancer Res. 2010, 70, 9905–9915. [Google Scholar] [CrossRef]
- Bonnaud, S.; Niaudet, C.; Pottier, G.; Gaugler, M.-H.; Millour, J.; Barbet, J.; Sabatier, L.; Paris, F. Sphingosine-1-Phosphate Protects Proliferating Endothelial Cells from Ceramide-Induced Apoptosis but Not from DNA Damage–Induced Mitotic Death. Cancer Res. 2007, 67, 1803–1811. [Google Scholar] [CrossRef]
- Maines, L.W.; Schrecengost, R.S.; Zhuang, Y.; Keller, S.N.; Smith, R.A.; Green, C.L.; Smith, C.D. Opaganib Protects against Radiation Toxicity: Implications for Homeland Security and Antitumor Radiotherapy. Int. J. Mol. Sci. 2022, 23, 13191. [Google Scholar] [CrossRef]
- Venant, H.; Rahmaniyan, M.; Jones, E.E.; Lu, P.; Lilly, M.B.; Garrett-Mayer, E.; Drake, R.R.; Kraveka, J.M.; Smith, C.D.; Voelkel-Johnson, C. The Sphingosine Kinase 2 Inhibitor ABC294640 Reduces the Growth of Prostate Cancer Cells and Results in Accumulation of Dihydroceramides In Vitro and In Vivo. Mol. Cancer Ther. 2015, 14, 2744–2752. [Google Scholar] [CrossRef]
- Taniguchi, M.; Okazaki, T. The Role of Sphingomyelin and Sphingomyelin Synthases in Cell Death, Proliferation and Migration—From Cell and Animal Models to Human Disorders. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 692–703. [Google Scholar] [CrossRef]
- Wu, L.N.Y.; Genge, B.R.; Kang, M.W.; Arsenault, A.L.; Wuthier, R.E. Changes in Phospholipid Extractability and Composition Accompany Mineralization of Chicken Growth Plate Cartilage Matrix Vesicles. J. Biol. Chem. 2002, 277, 5126–5133. [Google Scholar] [CrossRef]
- Aubin, I.; Adams, C.P.; Opsahl, S.; Septier, D.; Bishop, C.E.; Auge, N.; Salvayre, R.; Negre-Salvayre, A.; Goldberg, M.; Guénet, J.-L.; et al. A Deletion in the Gene Encoding Sphingomyelin Phosphodiesterase 3 (Smpd3) Results in Osteogenesis and Dentinogenesis Imperfecta in the Mouse. Nat. Genet. 2005, 37, 803–805. [Google Scholar] [CrossRef]
- Goldberg, M.; Opsahl, S.; Aubin, I.; Septier, D.; Chaussain-Miller, C.; Boskey, A.; Guenet, J.-L. Sphingomyelin Degradation Is a Key Factor in Dentin and Bone Mineralization: Lessons from the Fro/Fro Mouse. J. Dent. Res. 2008, 87, 9–13. [Google Scholar] [CrossRef]
- Khavandgar, Z.; Poirier, C.; Clarke, C.J.; Li, J.; Wang, N.; McKee, M.D.; Hannun, Y.A.; Murshed, M. A Cell-Autonomous Requirement for Neutral Sphingomyelinase 2 in Bone Mineralization. J. Cell Biol. 2011, 194, 277–289. [Google Scholar] [CrossRef]
- Airola, M.V.; Hannun, Y.A. Sphingolipid Metabolism and Neutral Sphingomyelinases. In Sphingolipids: Basic Science and Drug Development; Springer: Vienna, Austria, 2013; pp. 57–76. [Google Scholar] [CrossRef]
- Pekkinen, M.; Terhal, P.A.; Botto, L.D.; Henning, P.; Mäkitie, R.E.; Roschger, P.; Jain, A.; Kol, M.; Kjellberg, M.A.; Paschalis, E.P.; et al. Osteoporosis and Skeletal Dysplasia Caused by Pathogenic Variants in SGMS2. JCI Insight 2019, 4, e126180. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Yoshizawa, T.; Domae, E.; Hirai, Y.; Kamada, A.; Okazaki, T.; Ikeo, T. Knockdown of Sphingomyelin Synthase 2 Inhibits Osteoclastogenesis by Decreasing RANKL Expression in Mouse Primary Osteoblasts. Biomed. Res. 2019, 40, 189–196. [Google Scholar] [CrossRef]
- Wang, S.; Robinet, P.; Smith, J.D.; Gulshan, K. ORMDL Orosomucoid-like Proteins Are Degraded by Free-Cholesterol-Loading–Induced Autophagy. Proc. Natl. Acad. Sci. USA 2015, 112, 3728–3733. [Google Scholar] [CrossRef]
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic Joint Infection: Current Concepts and Outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef]
- Kapadia, B.H.; Berg, R.A.; Daley, J.A.; Fritz, J.; Bhave, A.; Mont, M.A. Periprosthetic Joint Infection. Lancet 2016, 387, 386–394. [Google Scholar] [CrossRef]
- Römling, U.; Kjelleberg, S.; Normark, S.; Nyman, L.; Uhlin, B.E.; Åkerlund, B. Microbial Biofilm Formation: A Need to Act. J. Intern. Med. 2014, 276, 98–110. [Google Scholar] [CrossRef]
- Rullán, P.J.; Deren, M.E.; Zhou, G.; Emara, A.K.; Klika, A.K.; Schiltz, N.K.; Barsoum, W.K.; Koroukian, S.; Piuzzi, N.S. The Arthroplasty Surgeon Growth Indicator. J. Bone Jt. Surg. 2023, 105, 1038–1045. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Day, J.S.; Lau, E.; Ong, K.L.; Williams, G.R.; Ramsey, M.L.; Kurtz, S.M. Prevalence and Projections of Total Shoulder and Elbow Arthroplasty in the United States to 2015. J. Shoulder Elb. Surg. 2010, 19, 1115–1120. [Google Scholar] [CrossRef]
- Sloan, M.; Premkumar, A.; Sheth, N.P. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J. Bone Jt. Surg. 2018, 100, 1455–1460. [Google Scholar] [CrossRef]
- Sloan, M.; Sheth, N. Projected Volume of Primary and Revision Total Joint Arthroplasty in the United States, 2030-2060. In Proceedings of the Annual Meeting of the American Academy of Orthopaedic Surgeons, New Orleans, LA, USA, 6–10 March 2018; p. 16. [Google Scholar]
- Rakow, A.; Perka, C.; Trampuz, A.; Renz, N. Origin and Characteristics of Haematogenous Periprosthetic Joint Infection. Clin. Microbiol. Infect. 2019, 25, 845–850. [Google Scholar] [CrossRef]
- Moran, G.J.; Krishnadasan, A.; Gorwitz, R.J.; Fosheim, G.E.; McDougal, L.K.; Carey, R.B.; Talan, D.A. Methicillin-Resistant S. Aureus Infections among Patients in the Emergency Department. N. Engl. J. Med. 2006, 355, 666–674. [Google Scholar] [CrossRef]
- Von Eiff, C.; Heilmann, C.; Peters, G. New Aspects in the Molecular Basis of Polymer-Associated Infections Due to Staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 1999, 18, 843–846. [Google Scholar] [CrossRef]
- Brogden, N.K.; Mehalick, L.; Fischer, C.L.; Wertz, P.W.; Brogden, K.A. The Emerging Role of Peptides and Lipids as Antimicrobial Epidermal Barriers and Modulators of Local Inflammation. Skin Pharmacol. Physiol. 2012, 25, 167–181. [Google Scholar] [CrossRef]
- Jungersted, J.M.; Hellgren, L.I.; Jemec, G.B.E.; Agner, T. Lipids and Skin Barrier Function—A Clinical Perspective. Contact Dermat. 2008, 58, 255–262. [Google Scholar] [CrossRef]
- Fischer, C.L.; Walters, K.S.; Drake, D.R.; Blanchette, D.R.; Dawson, D.V.; Brogden, K.A.; Wertz, P.W. Sphingoid Bases Are Taken Up by Escherichia Coli and Staphylococcus aureus and Induce Ultrastructural Damage. Skin Pharmacol. Physiol. 2013, 26, 36–44. [Google Scholar] [CrossRef]
- Fischer, C.L.; Drake, D.R.; Dawson, D.V.; Blanchette, D.R.; Brogden, K.A.; Wertz, P.W. Antibacterial Activity of Sphingoid Bases and Fatty Acids against Gram-Positive and Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2012, 56, 1157–1161. [Google Scholar] [CrossRef]
- Kunz, T.C.; Kozjak-Pavlovic, V. Diverse Facets of Sphingolipid Involvement in Bacterial Infections. Front. Cell Dev. Biol. 2019, 7, 203. [Google Scholar] [CrossRef]
- Drake, D.R.; Brogden, K.A.; Dawson, D.V.; Wertz, P.W. Thematic Review Series: Skin Lipids. Antimicrobial Lipids at the Skin Surface. J. Lipid Res. 2008, 49, 4–11. [Google Scholar] [CrossRef]
- Bibel, D.J.; Aly, R.; Shinefield, H.R. Antimicrobial Activity of Sphingosines. J. Investig. Dermatol. 1992, 98, 269–273. [Google Scholar] [CrossRef]
- Bibel, D.J.; Aly, R.; Shah, S.; Shinefield, H.R. Sphingosines: Antimicrobial Barriers of the Skin. Acta Derm. Venereol. 1993, 73, 407–411. [Google Scholar] [CrossRef]
- Cukkemane, N.; Bikker, F.J.; Nazmi, K.; Brand, H.S.; Sotres, J.; Lindh, L.; Arnebrant, T.; Veerman, E.C.I. Anti-adherence and Bactericidal Activity of Sphingolipids against Streptococcus mutans. Eur. J. Oral Sci. 2015, 123, 221–227. [Google Scholar] [CrossRef]
- Gardner, A.I.; Haq, I.J.; Simpson, A.J.; Becker, K.A.; Gallagher, J.; Saint-Criq, V.; Verdon, B.; Mavin, E.; Trigg, A.; Gray, M.A.; et al. Recombinant Acid Ceramidase Reduces Inflammation and Infection in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2020, 202, 1133–1145. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Carpinteiro, A.; Gulbins, E. Acid Sphingomyelinase Amplifies Redox Signaling in Pseudomonas aeruginosa—Induced Macrophage Apoptosis. J. Immunol. 2008, 181, 4247–4254. [Google Scholar] [CrossRef]
- Sharma, A.; Chauhan, A.; Chauhan, P.; Evans, D.L.; Szlabick, R.E.; Aaland, M.O.; Mishra, B.B.; Sharma, J. Glycolipid Metabolite β-Glucosylceramide Is a Neutrophil Extracellular Trap–Inducing Ligand of Mincle Released during Bacterial Infection and Inflammation. J. Immunol. 2022, 209, 391–400. [Google Scholar] [CrossRef]
- Rolando, M.; Buchrieser, C. A Comprehensive Review on the Manipulation of the Sphingolipid Pathway by Pathogenic Bacteria. Front. Cell Dev. Biol. 2019, 7, 168. [Google Scholar] [CrossRef]
- Grassmé, H.; Jendrossek, V.; Riehle, A.; von Kürthy, G.; Berger, J.; Schwarz, H.; Weller, M.; Kolesnick, R.; Gulbins, E. Host Defense against Pseudomonas aeruginosa Requires Ceramide-Rich Membrane Rafts. Nat. Med. 2003, 9, 322–330. [Google Scholar] [CrossRef]
- Grassme, H.; Jekle, A.; Riehle, A.; Schwarz, H.; Berger, J.; Sandhoff, K.; Kolesnick, R.; Gulbins, E. CD95 Signaling via Ceramide-Rich Membrane Rafts. J. Biol. Chem. 2001, 276, 20589–20596. [Google Scholar] [CrossRef]
- Grassmé, H.; Henry, B.; Ziobro, R.; Becker, K.A.; Riethmüller, J.; Gardner, A.; Seitz, A.P.; Steinmann, J.; Lang, S.; Ward, C.; et al. Β1-Integrin Accumulates in Cystic Fibrosis Luminal Airway Epithelial Membranes and Decreases Sphingosine, Promoting Bacterial Infections. Cell Host Microbe 2017, 21, 707–718.e8. [Google Scholar] [CrossRef]
- Becker, K.A.; Fahsel, B.; Kemper, H.; Mayeres, J.; Li, C.; Wilker, B.; Keitsch, S.; Soddemann, M.; Sehl, C.; Kohnen, M.; et al. Staphylococcus aureus Alpha-Toxin Disrupts Endothelial-Cell Tight Junctions via Acid Sphingomyelinase and Ceramide. Infect. Immun. 2018, 86, 10–1128. [Google Scholar] [CrossRef]
- Schwan, C.; Nölke, T.; Kruppke, A.S.; Schubert, D.M.; Lang, A.E.; Aktories, K. Cholesterol- and Sphingolipid-Rich Microdomains Are Essential for Microtubule-Based Membrane Protrusions Induced by Clostridium Difficile Transferase (CDT). J. Biol. Chem. 2011, 286, 29356–29365. [Google Scholar] [CrossRef]
- Foegeding, N.; Caston, R.; McClain, M.; Ohi, M.; Cover, T. An Overview of Helicobacter Pylori VacA Toxin Biology. Toxins 2016, 8, 173. [Google Scholar] [CrossRef]
- de Bentzmann, S.; Roger, P.; Dupuit, F.; Bajolet-Laudinat, O.; Fuchey, C.; Plotkowski, M.C.; Puchelle, E. Asialo GM1 Is a Receptor for Pseudomonas aeruginosa Adherence to Regenerating Respiratory Epithelial Cells. Infect. Immun. 1996, 64, 1582–1588. [Google Scholar] [CrossRef]
- Chung, Y.H.; Gwak, J.S.; Hong, S.W.; Hyeon, J.H.; Lee, C.M.; Oh, S.W.; Kwon, H. Helicobacter Pylori: A Possible Risk Factor for Bone Health. Korean J. Fam. Med. 2015, 36, 239. [Google Scholar] [CrossRef][Green Version]
- Rousseau, V.; Descours, G.; Chaker, M.; Tristan, A.; Freydière, A.-M.; Gillet, Y. Ostéoarthrite de Hanche Primitive et Myosite Multifocale à Méningocoque B Chez Un Enfant de 7 Ans. Arch. Pédiatrie 2012, 19, 1330–1333. [Google Scholar] [CrossRef]
- Sheybani, F.; Figueiredo, A.H.A.; Brouwer, M.C.; van de Beek, D. Vertebral Osteomyelitis in Bacterial Meningitis Patients. Int. J. Infect. Dis. 2021, 111, 354–359. [Google Scholar] [CrossRef]
- Carlier, J.-P.; K’ouas, G.; Lozniewski, A.; Sirveaux, F.; Cailloux, P.; Mory, F. Osteosynthesis-Associated Bone Infection Caused by a Nonproteolytic, Nontoxigenic Clostridium Botulinum-Like Strain. J. Clin. Microbiol. 2004, 42, 484–486. [Google Scholar] [CrossRef]
- Ibnoulkhatib, A.; Lacroix, J.; Moine, A.; Archambaud, M.; Bonnet, E.; Laffosse, J.-M. Post-Traumatic Bone and/or Joint Limb Infections Due to Clostridium spp. Orthop. Traumatol. Surg. Res. 2012, 98, 696–705. [Google Scholar] [CrossRef]
- Mannepalli, S.; Mitchell-Samon, L.; Guzman, N.; Relan, M.; McCarter, Y.S. Mycobacterium Tuberculosis Osteomyelitis in a Patient with Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome (HIV/AIDS): A Case Report. Cases J. 2010, 3, 67. [Google Scholar] [CrossRef]
- Mohad, N.; Dabir, A.; Vahanwala, J.; Padhye, M.; Patwardhan, J. Tuberculous Osteomyelitis in Condyle of Mandible: A Case Report. Adv. Oral Maxillofac. Surg. 2021, 2, 100064. [Google Scholar] [CrossRef]
- Bendjelloul, I.; Lourtet-Hascoët, J.; Galinier, J.-L.; Charbonneau, H.; Robinet, N.; Fourcade, C.; Bonnet, E. Chlamydia Psittaci Endocarditis: A Case Report and Literature Review. Infect. Dis. Now 2023, 53, 104687. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, J.; Li, B.; Shu, H. Bacillus Cereus Isolated from a Positive Bone Tissue Culture in a Patient with Osteolysis and High-Titer Anti-Interferon-γ Autoantibodies. Medicine 2019, 98, e17609. [Google Scholar] [CrossRef]
- Raja, N.S.; Scarsbrook, C. Burkholderia pseudomallei Causing Bone and Joint Infections: A Clinical Update. Infect. Dis. Ther. 2016, 5, 17–29. [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.; Zhou, X.; Chen, S.; Mai, W.; Huang, H.; You, Z.; Zhang, S.; Zhang, X.; Lu, B. Osteomyelitis and Septic Arthritis Due to Burkholderia pseudomallei: A 10-Year Retrospective Melioidosis Study from South China. Front. Cell. Infect. Microbiol. 2021, 11, 654745. [Google Scholar] [CrossRef]
- Schindel, C.; Siepmann, U.; Han, S.-R.; Ullmann, A.J.; Mayer, E.; Fischer, T.; Maeurer, M. Persistent Legionella Infection in a Patient after Bone Marrow Transplantation. J. Clin. Microbiol. 2000, 38, 4294–4295. [Google Scholar] [CrossRef]
- Witkin, S.S.; Minis, E.; Athanasiou, A.; Leizer, J.; Linhares, I.M. Chlamydia trachomatis: The Persistent Pathogen. Clin. Vaccine Immunol. 2017, 24, e00203-17. [Google Scholar] [CrossRef]
- Bednarek-Skublewska, A.; Majdan, M.; Dryglewska, M.; Ksiazek, A. Chlamydia trachomatis Infection in Chronically Hemodialyzed Patients. Rocz. Akad. Med. Bialymst. 2005, 50, 307–310. [Google Scholar]
- Pinto, C.; Sousa, D.; Ghilas, V.; Dardis, A.; Scarpa, M.; Macedo, M. Acid Sphingomyelinase Deficiency: A Clinical and Immunological Perspective. Int. J. Mol. Sci. 2021, 22, 12870. [Google Scholar] [CrossRef]
- Utermöhlen, O.; Herz, J.; Schramm, M.; Krönke, M. Fusogenicity of Membranes: The Impact of Acid Sphingomyelinase on Innate Immune Responses. Immunobiology 2008, 213, 307–314. [Google Scholar] [CrossRef]
- Schramm, M.; Herz, J.; Haas, A.; Krönke, M.; Utermöhlen, O. Acid Sphingomyelinase Is Required for Efficient Phago-Lysosomal Fusion. Cell. Microbiol. 2008, 10, 1839–1853. [Google Scholar] [CrossRef]
- Li, C.; Wu, Y.; Riehle, A.; Orian-Rousseau, V.; Zhang, Y.; Gulbins, E.; Grassmé, H. Regulation of Staphylococcus aureus Infection of Macrophages by CD44, Reactive Oxygen Species, and Acid Sphingomyelinase. Antioxid. Redox Signal. 2018, 28, 916–934. [Google Scholar] [CrossRef]
- Ghosh, S.; Bhattacharyya, S.; Das, S.; Raha, S.; Maulik, N.; Das, D.K.; Roy, S.; Majumdar, S. Generation of Ceramide in Murine Macrophages Infected with Leishmania Donovani Alters Macrophage Signaling Events and Aids Intracellular Parasitic Survival. Mol. Cell. Biochem. 2001, 223, 47–60. [Google Scholar] [CrossRef]
- Utermohlen, O.; Karow, U.; Lohler, J.; Kronke, M. Severe Impairment in Early Host Defense Against Listeria Monocytogenes in Mice Deficient in Acid Sphingomyelinase. J. Immunol. 2003, 170, 2621–2628. [Google Scholar] [CrossRef]
- Ostroff, R.M.; Wretlind, B.; Vasil, M.L. Mutations in the Hemolytic-Phospholipase C Operon Result in Decreased Virulence of Pseudomonas aeruginosa PAO1 Grown under Phosphate-Limiting Conditions. Infect. Immun. 1989, 57, 1369–1373. [Google Scholar] [CrossRef]
- Luberto, C.; Stonehouse, M.J.; Collins, E.A.; Marchesini, N.; El-Bawab, S.; Vasil, A.I.; Vasil, M.L.; Hannun, Y.A. Purification, Characterization, and Identification of a Sphingomyelin Synthase from Pseudomonas aeruginosa. J. Biol. Chem. 2003, 278, 32733–32743. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.-L.; Li, Y.-K.; Chen, D.-K.; He, J.-F.; Yao, N. Functions of Sphingolipids in Pathogenesis During Host–Pathogen Interactions. Front. Microbiol. 2021, 12, 701041. [Google Scholar] [CrossRef]
- Vasil, M.L.; Stonehouse, M.J.; Vasil, A.I.; Wadsworth, S.J.; Goldfine, H.; Bolcome, R.E.; Chan, J. A Complex Extracellular Sphingomyelinase of Pseudomonas aeruginosa Inhibits Angiogenesis by Selective Cytotoxicity to Endothelial Cells. PLoS Pathog. 2009, 5, e1000420. [Google Scholar] [CrossRef]
- Okino, N.; Ito, M. Molecular Mechanism for Sphingosine-Induced Pseudomonas Ceramidase Expression through the Transcriptional Regulator SphR. Sci. Rep. 2016, 6, 38797. [Google Scholar] [CrossRef]
- Okino, N.; Ito, M. Ceramidase Enhances Phospholipase C-Induced Hemolysis by Pseudomonas aeruginosa. J. Biol. Chem. 2007, 282, 6021–6030. [Google Scholar] [CrossRef]
- Kida, Y.; Shimizu, T.; Kuwano, K. Cooperation between LepA and PlcH Contributes to the In Vivo Virulence and Growth of Pseudomonas aeruginosa in Mice. Infect. Immun. 2011, 79, 211–219. [Google Scholar] [CrossRef]
- LaBauve, A.E.; Wargo, M.J. Detection of Host-Derived Sphingosine by Pseudomonas aeruginosa Is Important for Survival in the Murine Lung. PLoS Pathog. 2014, 10, e1003889. [Google Scholar] [CrossRef]
- Stankeviciute, G.; Tang, P.; Ashley, B.; Chamberlain, J.D.; Hansen, M.E.B.; Coleman, A.; D’Emilia, R.; Fu, L.; Mohan, E.C.; Nguyen, H.; et al. Convergent Evolution of Bacterial Ceramide Synthesis. Nat. Chem. Biol. 2022, 18, 305–312. [Google Scholar] [CrossRef]
- Rice, T.C.; Pugh, A.M.; Caldwell, C.C.; Schneider, B.S.P. Balance Between the Proinflammatory and Anti-Inflammatory Immune Responses with Blood Transfusion in Sepsis. Crit. Care Nurs. Clin. N. Am. 2017, 29, 331–340. [Google Scholar] [CrossRef]
- Fjell, C.D.; Thair, S.; Hsu, J.L.; Walley, K.R.; Russell, J.A.; Boyd, J. Cytokines and Signaling Molecules Predict Clinical Outcomes in Sepsis. PLoS ONE 2013, 8, e79207. [Google Scholar] [CrossRef]
- Peñaloza, H.F.; Schultz, B.M.; Nieto, P.A.; Salazar, G.A.; Suazo, I.; Gonzalez, P.A.; Riedel, C.A.; Alvarez-Lobos, M.M.; Kalergis, A.M.; Bueno, S.M. Opposing Roles of IL-10 in Acute Bacterial Infection. Cytokine Growth Factor Rev. 2016, 32, 17–30. [Google Scholar] [CrossRef]
- Slaats, J.; ten Oever, J.; van de Veerdonk, F.L.; Netea, M.G. IL-1β/IL-6/CRP and IL-18/Ferritin: Distinct Inflammatory Programs in Infections. PLOS Pathog. 2016, 12, e1005973. [Google Scholar] [CrossRef]
- Bacci, M.R.; Leme, R.C.P.; Zing, N.P.C.; Murad, N.; Adami, F.; Hinnig, P.F.; Feder, D.; Chagas, A.C.P.; Fonseca, F.L.A. IL-6 and TNF-α Serum Levels Are Associated with Early Death in Community-Acquired Pneumonia Patients. Braz. J. Med. Biol. Res. 2015, 48, 427–432. [Google Scholar] [CrossRef]
- Fernandez-Botran, R.; Uriarte, S.M.; Arnold, F.W.; Rodriguez-Hernandez, L.; Rane, M.J.; Peyrani, P.; Wiemken, T.; Kelley, R.; Uppatla, S.; Cavallazzi, R.; et al. Contrasting Inflammatory Responses in Severe and Non-Severe Community-Acquired Pneumonia. Inflammation 2014, 37, 1158–1166. [Google Scholar] [CrossRef]
- Piao, X.; Kim, J.-W.; Hyun, M.; Wang, Z.; Park, S.-G.; Cho, I.A.; Ryu, J.-H.; Lee, B.-N.; Song, J.H.; Koh, J.-T. Boeravinone B, a Natural Rotenoid, Inhibits Osteoclast Differentiation through Modulating NF-ΚB, MAPK and PI3K/Akt Signaling Pathways. BMB Rep. 2023, 56, 545–550. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Xie, X.; Gu, F.; Sui, Z.; Zhang, K.; Yu, T. Macrophage-Osteoclast Associations: Origin, Polarization, and Subgroups. Front. Immunol. 2021, 12, 778078. [Google Scholar] [CrossRef]
- Olsen, I.; Nichols, F.C. Are Sphingolipids and Serine Dipeptide Lipids Underestimated Virulence Factors of Porphyromonas Gingivalis? Infect. Immun. 2018, 86, 10–1128. [Google Scholar] [CrossRef]
- Hsu, L.-C.; Reddy, S.V.; Yilmaz, Ö.; Yu, H. Sphingosine-1-Phosphate Receptor 2 Controls Podosome Components Induced by RANKL Affecting Osteoclastogenesis and Bone Resorption. Cells 2019, 8, 17. [Google Scholar] [CrossRef]
- Yu, H. Sphingosine-1-Phosphate Receptor 2 Regulates Proinflammatory Cytokine Production and Osteoclastogenesis. PLoS ONE 2016, 11, e0156303. [Google Scholar] [CrossRef]
- Lightle, S.; Tosheva, R.; Lee, A.; Queen-Baker, J.; Boyanovsky, B.; Shedlofsky, S.; Nikolova-Karakashian, M. Elevation of Ceramide in Serum Lipoproteins during Acute Phase Response in Humans and Mice: Role of Serine–Palmitoyl Transferase. Arch. Biochem. Biophys. 2003, 419, 120–128. [Google Scholar] [CrossRef]
- Memon, R.A.; Holleran, W.M.; Moser, A.H.; Seki, T.; Uchida, Y.; Fuller, J.; Shigenaga, J.K.; Grunfeld, C.; Feingold, K.R. Endotoxin and Cytokines Increase Hepatic Sphingolipid Biosynthesis and Produce Lipoproteins Enriched in Ceramides and Sphingomyelin. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1257–1265. [Google Scholar] [CrossRef]
- JeBailey, L.; Wanono, O.; Niu, W.; Roessler, J.; Rudich, A.; Klip, A. Ceramide- and Oxidant-Induced Insulin Resistance Involve Loss of Insulin-Dependent Rac-Activation and Actin Remodeling in Muscle Cells. Diabetes 2007, 56, 394–403. [Google Scholar] [CrossRef]
- Hundal, R.S.; Gómez-Muñoz, A.; Kong, J.Y.; Salh, B.S.; Marotta, A.; Duronio, V.; Steinbrecher, U.P. Oxidized Low Density Lipoprotein Inhibits Macrophage Apoptosis by Blocking Ceramide Generation, Thereby Maintaining Protein Kinase B Activation and Bcl-XL Levels. J. Biol. Chem. 2003, 278, 24399–24408. [Google Scholar] [CrossRef]
- Ma, J.; Gulbins, E.; Edwards, M.J.; Caldwell, C.C.; Fraunholz, M.; Becker, K.A. Staphylococcus aureus α-Toxin Induces Inflammatory Cytokines via Lysosomal Acid Sphingomyelinase and Ceramides. Cell. Physiol. Biochem. 2017, 43, 2170–2184. [Google Scholar] [CrossRef]
- Wong, M.-L.; Xie, B.; Beatini, N.; Phu, P.; Marathe, S.; Johns, A.; Gold, P.W.; Hirsch, E.; Williams, K.J.; Licinio, J.; et al. Acute Systemic Inflammation Up-Regulates Secretory Sphingomyelinase in Vivo: A Possible Link between Inflammatory Cytokines and Atherogenesis. Proc. Natl. Acad. Sci. USA 2000, 97, 8681–8686. [Google Scholar] [CrossRef]
- Józefowski, S.; Czerkies, M.; Łukasik, A.; Bielawska, A.; Bielawski, J.; Kwiatkowska, K.; Sobota, A. Ceramide and Ceramide 1-Phosphate Are Negative Regulators of TNF-α Production Induced by Lipopolysaccharide. J. Immunol. 2010, 185, 6960–6973. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like Receptors: Critical Proteins Linking Innate and Acquired Immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef]
- Albeituni, S.; Stiban, J. Roles of Ceramides and Other Sphingolipids in Immune Cell Function and Inflammation. Role Bioact. Lipids Cancer Inflamm. Relat. Dis. 2019, 1161, 169–191. [Google Scholar] [CrossRef]
- Ebenezer, D.L.; Berdyshev, E.V.; Bronova, I.A.; Liu, Y.; Tiruppathi, C.; Komarova, Y.; Benevolenskaya, E.V.; Suryadevara, V.; Ha, A.W.; Harijith, A.; et al. Pseudomonas aeruginosa Stimulates Nuclear Sphingosine-1-Phosphate Generation and Epigenetic Regulation of Lung Inflammatory Injury. Thorax 2019, 74, 579–591. [Google Scholar] [CrossRef]
- Pewzner-Jung, Y.; Tavakoli Tabazavareh, S.; Grassmé, H.; Becker, K.A.; Japtok, L.; Steinmann, J.; Joseph, T.; Lang, S.; Tuemmler, B.; Schuchman, E.H.; et al. Sphingoid Long Chain Bases Prevent Lung Infection by Pseudomonas aeruginosa. EMBO Mol. Med. 2014, 6, 1205–1214. [Google Scholar] [CrossRef]
- Tavakoli Tabazavareh, S.; Seitz, A.; Jernigan, P.; Sehl, C.; Keitsch, S.; Lang, S.; Kahl, B.C.; Edwards, M.; Grassmé, H.; Gulbins, E.; et al. Lack of Sphingosine Causes Susceptibility to Pulmonary Staphylococcus aureus Infections in Cystic Fibrosis. Cell. Physiol. Biochem. 2016, 38, 2094–2102. [Google Scholar] [CrossRef]
- Seitz, A.P.; Schumacher, F.; Baker, J.; Soddemann, M.; Wilker, B.; Caldwell, C.C.; Gobble, R.M.; Kamler, M.; Becker, K.A.; Beck, S.; et al. Sphingosine-Coating of Plastic Surfaces Prevents Ventilator-Associated Pneumonia. J. Mol. Med. 2019, 97, 1195–1211. [Google Scholar] [CrossRef]
- Beck, S.; Sehl, C.; Voortmann, S.; Verhasselt, H.L.; Edwards, M.J.; Buer, J.; Hasenberg, M.; Gulbins, E.; Becker, K.A. Sphingosine Is Able to Prevent and Eliminate Staphylococcus Epidermidis Biofilm Formation on Different Orthopedic Implant Materials in Vitro. J. Mol. Med. 2020, 98, 209–219. [Google Scholar] [CrossRef]
- Papachristou, S.G.; Iosifidis, E.; Sipsas, N.V.; Gamaletsou, M.N.; Walsh, T.J.; Roilides, E. Management of Osteoarticular Fungal Infections in the Setting of Immunodeficiency. Expert Rev. Anti. Infect. Ther. 2020, 18, 461–474. [Google Scholar] [CrossRef]
- Sidiropoulos, K.; Christofilos, S.I.; Tsikopoulos, K.; Kitridis, D.; Drago, L.; Meroni, G.; Romanò, C.L.; Kavarthapu, V. Viral Infections in Orthopedics: A Systematic Review and Classification Proposal. World J. Orthop. 2022, 13, 1015–1028. [Google Scholar] [CrossRef]
- Drago, L.; Romanò, C.L.; Morelli, I.; Benzakour, T. Viral Bone Infection: A Neglected Disease? Microorganisms 2020, 8, 797. [Google Scholar] [CrossRef]
- Toppinen, M.; Perdomo, M.F.; Palo, J.U.; Simmonds, P.; Lycett, S.J.; Söderlund-Venermo, M.; Sajantila, A.; Hedman, K. Bones Hold the Key to DNA Virus History and Epidemiology. Sci. Rep. 2015, 5, 17226. [Google Scholar] [CrossRef]
- Marks, M.; Marks, J.L. Viral Arthritis. Clin. Med. 2016, 16, 129–134. [Google Scholar] [CrossRef]
- Chen, W.; Foo, S.-S.; Rulli, N.E.; Taylor, A.; Sheng, K.-C.; Herrero, L.J.; Herring, B.L.; Lidbury, B.A.; Li, R.W.; Walsh, N.C.; et al. Arthritogenic Alphaviral Infection Perturbs Osteoblast Function and Triggers Pathologic Bone Loss. Proc. Natl. Acad. Sci. USA 2014, 111, 6040–6045. [Google Scholar] [CrossRef]
- Dimitriou, D.; Ramokgopa, M.; Pietrzak, J.R.T.; van der Jagt, D.; Mokete, L. Human Immunodeficiency Virus Infection and Hip and Knee Arthroplasty. JBJS Rev. 2017, 5, e8. [Google Scholar] [CrossRef]
- Raynaud-Messina, B.; Bracq, L.; Dupont, M.; Souriant, S.; Usmani, S.M.; Proag, A.; Pingris, K.; Soldan, V.; Thibault, C.; Capilla, F.; et al. Bone Degradation Machinery of Osteoclasts: An HIV-1 Target That Contributes to Bone Loss. Proc. Natl. Acad. Sci. USA 2018, 115, E2556–E2565. [Google Scholar] [CrossRef]
- Haudenschild, A.K.; Christiansen, B.A.; Orr, S.; Ball, E.E.; Weiss, C.M.; Liu, H.; Fyhrie, D.P.; Yik, J.H.N.; Coffey, L.L.; Haudenschild, D.R. Acute Bone Loss Following SARS-CoV-2 Infection in Mice. J. Orthop. Res. 2023, 41, 1945–1952. [Google Scholar] [CrossRef]
- Avota, E.; Bodem, J.; Chithelen, J.; Mandasari, P.; Beyersdorf, N.; Schneider-Schaulies, J. The Manifold Roles of Sphingolipids in Viral Infections. Front. Physiol. 2021, 12, 715527. [Google Scholar] [CrossRef]
- Grassmé, H.; Riethmüller, J.; Gulbins, E. Biological Aspects of Ceramide-Enriched Membrane Domains. Prog. Lipid Res. 2007, 46, 161–170. [Google Scholar] [CrossRef]
- Gulbins, E.; Dreschers, S.; Wilker, B.; Grassmé, H. Ceramide, Membrane Rafts and Infections. J. Mol. Med. 2004, 82, 357–363. [Google Scholar] [CrossRef]
- Miller, M.E.; Adhikary, S.; Kolokoltsov, A.A.; Davey, R.A. Ebolavirus Requires Acid Sphingomyelinase Activity and Plasma Membrane Sphingomyelin for Infection. J. Virol. 2012, 86, 7473–7483. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural Basis for the Recognition of SARS-CoV-2 by Full-Length Human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Törnquist, K.; Asghar, M.Y.; Srinivasan, V.; Korhonen, L.; Lindholm, D. Sphingolipids as Modulators of SARS-CoV-2 Infection. Front. Cell Dev. Biol. 2021, 9, 689854. [Google Scholar] [CrossRef]
- Soudani, N.; Hage-Sleiman, R.; Karam, W.; Dbaibo, G.; Zaraket, H. Ceramide Suppresses Influenza A Virus Replication In Vitro. J. Virol. 2019, 93, 10–1128. [Google Scholar] [CrossRef]
- Thomas, S.; Samuel, S.V.; Hoch, A.; Syphurs, C.; Diray-Arce, J. The Implication of Sphingolipids in Viral Infections. Int. J. Mol. Sci. 2023, 24, 17303. [Google Scholar] [CrossRef]
- Schneider-Schaulies, J.; Schneider-Schaulies, S. Sphingolipids in Viral Infection. Biol. Chem. 2015, 396, 585–595. [Google Scholar] [CrossRef]
- Guzman, G.; Creek, C.; Farley, S.; Tafesse, F.G. Genetic Tools for Studying the Roles of Sphingolipids in Viral Infections. In Virus-Host Interactions: Methods and Protocols; Springer: New York, NY, USA, 2023; pp. 1–16. [Google Scholar] [CrossRef]
- Lone, M.A.; Santos, T.; Alecu, I.; Silva, L.C.; Hornemann, T. 1-Deoxysphingolipids. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 512–521. [Google Scholar] [CrossRef]
- Hannich, J.T.; Mellal, D.; Feng, S.; Zumbuehl, A.; Riezman, H. Structure and Conserved Function of Iso-Branched Sphingoid Bases from the Nematode Caenorhabditis Elegans. Chem. Sci. 2017, 8, 3676–3686. [Google Scholar] [CrossRef]
- Alecu, I.; Tedeschi, A.; Behler, N.; Wunderling, K.; Lamberz, C.; Lauterbach, M.R.; Gaebler, A.; Ernst, D.; Van Veldhoven, P.P.; Al-Amoudi, A.; et al. Localization of 1-Deoxysphingolipids to Mitochondria Induces Mitochondrial Dysfunction. J. Lipid Res. 2017, 58, 42–59. [Google Scholar] [CrossRef]
- Güntert, T.; Hänggi, P.; Othman, A.; Suriyanarayanan, S.; Sonda, S.; Zuellig, R.A.; Hornemann, T.; Ogunshola, O.O. 1-Deoxysphingolipid-Induced Neurotoxicity Involves N-Methyl-d-Aspartate Receptor Signaling. Neuropharmacology 2016, 110, 211–222. [Google Scholar] [CrossRef]
- Mwinyi, J.; Boström, A.; Fehrer, I.; Othman, A.; Waeber, G.; Marti-Soler, H.; Vollenweider, P.; Marques-Vidal, P.; Schiöth, H.B.; von Eckardstein, A.; et al. Plasma 1-Deoxysphingolipids Are Early Predictors of Incident Type 2 Diabetes Mellitus. PLoS ONE 2017, 12, e0175776. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).