Nitrite Attenuates the In Vitro Inflammatory Response of Immune Cells to the SARS-CoV-2 S Protein without Interfering in the Antioxidant Enzyme Activation
Abstract
1. Introduction
2. Results
2.1. Subject Characterization
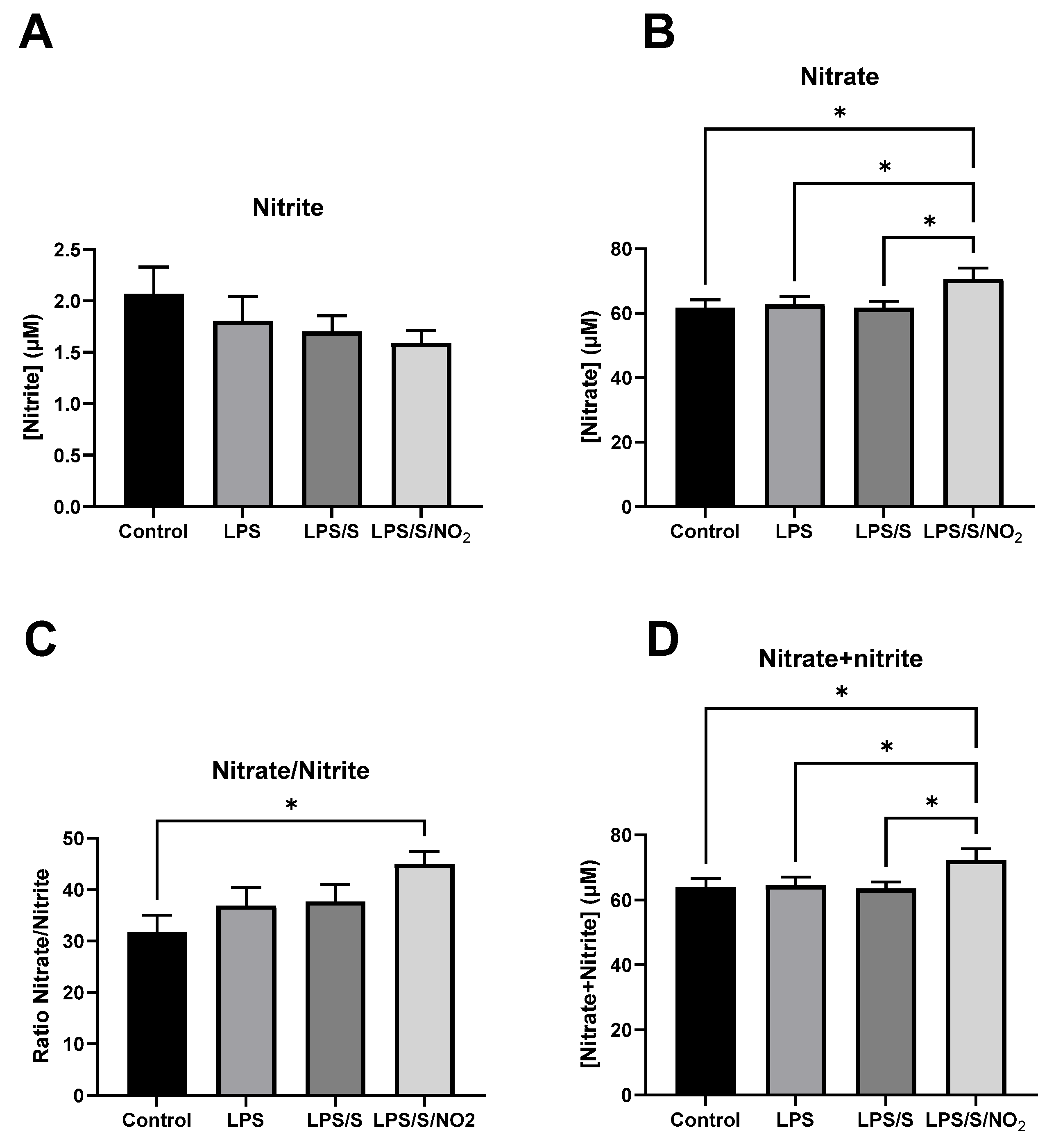
2.2. Effects of the Incubation on Nitrite and Nitrate Levels
2.3. Effects of the Incubation on the Expression of Pro-inflammatory Markers
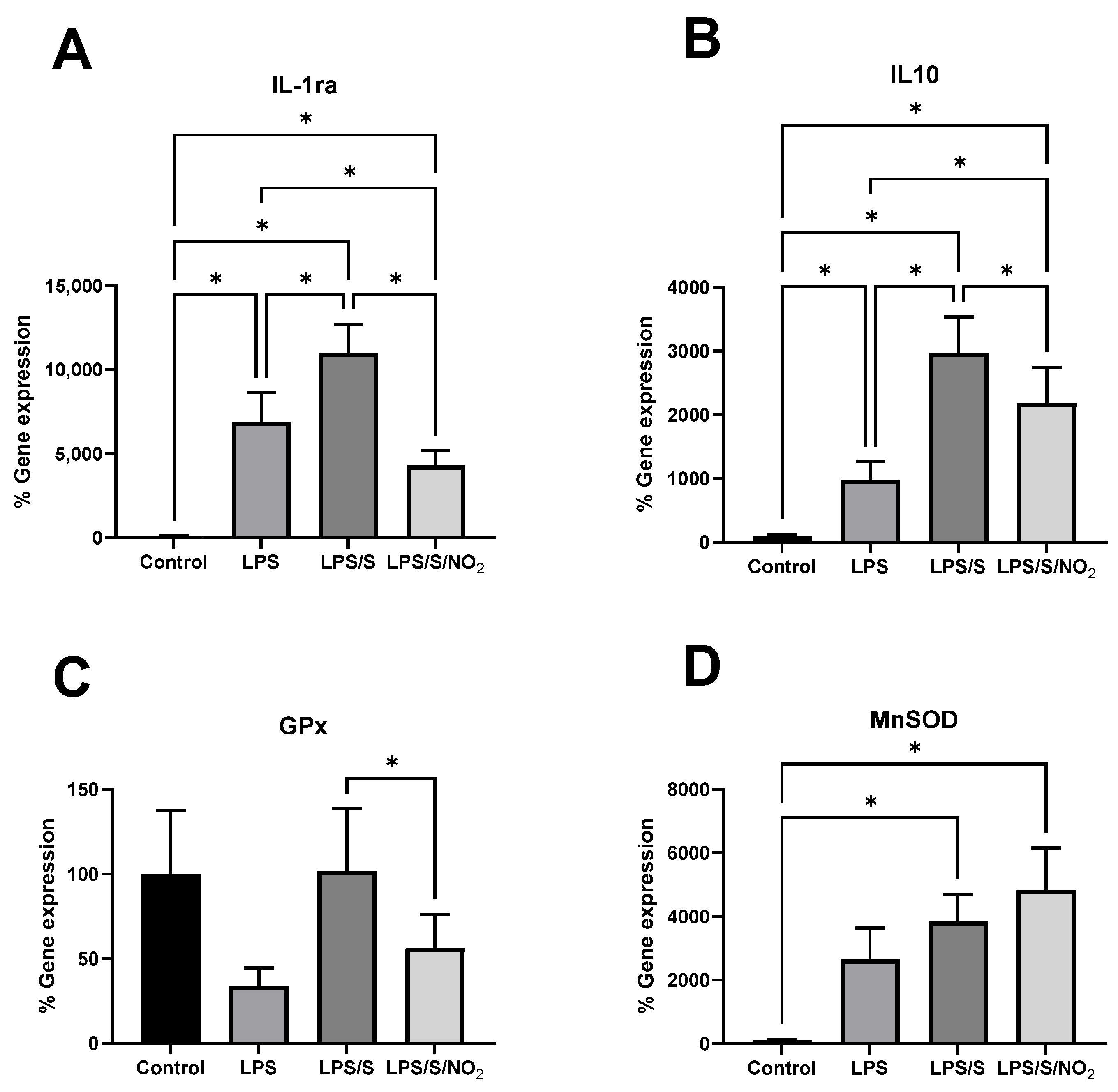
2.4. Effect of the Incubations on the Expression of Anti-Inflammatory and Antioxidant Markers
3. Discussion
4. Materials and Methods
4.1. Study Subjects and Sample Preparation
4.2. Nitrite and Nitrate Determination
4.3. RNA Isolation and Quantitative RT-PCR
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef]
- Korakas, E.; Ikonomidis, I.; Kousathana, F.; Balampanis, K.; Kountouri, A.; Raptis, A.; Palaiodimou, L.; Kokkinos, A.; Lambadiari, V. Obesity and COVID-19: Immune and metabolic derangement as a possible link to adverse clinical outcomes. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E105–E109. [Google Scholar] [CrossRef]
- Frydrych, L.M.; Bian, G.; O’Lone, D.E.; Ward, P.A.; Delano, M.J. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J. Leukoc. Biol. 2018, 104, 525–534. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Cani, P.D.; Delzenne, N.M. Interplay between obesity and associated metabolic disorders: New insights into the gut microbiota. Curr. Opin. Pharmacol. 2009, 9, 737–743. [Google Scholar] [CrossRef]
- Kruglikov, I.L.; Scherer, P.E. Preexisting and inducible endotoxemia as crucial contributors to the severity of COVID-19 outcomes. PLoS Pathog. 2021, 17, e1009306. [Google Scholar] [CrossRef]
- Petruk, G.; Puthia, M.; Petrlova, J.; Samsudin, F.; Stromdahl, A.C.; Cerps, S.; Uller, L.; Kjellstrom, S.; Bond, P.J.; Schmidtchen, A.A. SARS-CoV-2 spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity. J. Mol. Cell Biol. 2020, 12, 916–932. [Google Scholar] [CrossRef]
- Wang, F.; Nie, J.; Wang, H.; Zhao, Q.; Xiong, Y.; Deng, L.; Song, S.; Ma, Z.; Mo, P.; Zhang, Y. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J. Infect. Dis. 2020, 221, 1762–1769. [Google Scholar] [CrossRef]
- Shakoory, B.; Carcillo, J.A.; Chatham, W.W.; Amdur, R.L.; Zhao, H.; Dinarello, C.A.; Cron, R.Q.; Opal, S.M. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit. Care Med. 2016, 44, 275–281. [Google Scholar] [CrossRef]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef]
- Li, X.; Li, T.; Wang, H. Treatment and prognosis of COVID-19: Current scenario and prospects (Review). Exp. Ther. Med. 2021, 21, 3. [Google Scholar] [CrossRef]
- Hayman, T.J.; Hsu, A.C.; Kolesnik, T.B.; Dagley, L.F.; Willemsen, J.; Tate, M.D.; Baker, P.J.; Kershaw, N.J.; Kedzierski, L.; Webb, A.I.; et al. RIPLET, and not TRIM25, is required for endogenous RIG-I-dependent antiviral responses. Immunol. Cell Biol. 2019, 97, 840–852. [Google Scholar] [CrossRef]
- Saito, T.; Gale, M., Jr. Differential recognition of double-stranded RNA by RIG-I-like receptors in antiviral immunity. J. Exp. Med. 2008, 205, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- Diebold, S.S.; Kaisho, T.; Hemmi, H.; Akira, S.; Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 2004, 303, 1529–1531. [Google Scholar] [CrossRef]
- Le Goffic, R.; Pothlichet, J.; Vitour, D.; Fujita, T.; Meurs, E.; Chignard, M.; Si-Tahar, M. Cutting Edge: Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J. Immunol. 2007, 178, 3368–3372. [Google Scholar] [CrossRef]
- Hsu, A.C.-Y.; Wang, G.; Reid, A.T.; Veerati, P.C.; Pathinayake, P.S.; Daly, K.; Mayall, J.R.; Hansbro, P.M.; Horvat, J.C.; Wang, F.; et al. SARS-CoV-2 Spike protein promotes hyper-inflammatory response that can be ameliorated by Spike-antagonistic peptide and FDA-approved ER stress and MAP kinase inhibitors in vitro. bioRxiv 2020. [Google Scholar] [CrossRef]
- Schiffer, T.A.; Lundberg, J.O.; Weitzberg, E.; Carlstrom, M. Modulation of mitochondria and NADPH oxidase function by the nitrate-nitrite-NO pathway in metabolic disease with focus on type 2 diabetes. Biochim. Et Biophys. Acta Mol. Basis Dis. 2020, 1866, 165811. [Google Scholar] [CrossRef]
- Tian, R.; Peng, R.; Yang, Z.; Peng, Y.Y.; Lu, N. Supplementation of dietary nitrate attenuated oxidative stress and endothelial dysfunction in diabetic vasculature through inhibition of NADPH oxidase. Nitric Oxide 2020, 96, 54–63. [Google Scholar] [CrossRef]
- Peleli, M.; Ferreira, D.M.S.; Tarnawski, L.; McCann Haworth, S.; Xuechen, L.; Zhuge, Z.; Newton, P.T.; Massart, J.; Chagin, A.S.; Olofsson, P.S.; et al. Dietary nitrate attenuates high-fat diet-induced obesity via mechanisms involving higher adipocyte respiration and alterations in inflammatory status. Redox Biol. 2020, 28, 101387. [Google Scholar] [CrossRef]
- Capo, X.; Ferrer, M.D.; Olek, R.A.; Salaberry, E.; Suau, R.; Mari, B.; Llompart, I.; Tur, J.A.; Sureda, A.; Pons, A. Oral Administration of Sodium Nitrate to Metabolic Syndrome Patients Attenuates Mild Inflammatory and Oxidative Responses to Acute Exercise. Antioxidants 2020, 9, 596. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Carlstrom, M.; Larsen, F.J.; Weitzberg, E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc. Res. 2011, 89, 525–532. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Capo, X.; Reynes, C.; Quetglas, M.; Salaberry, E.; Tonolo, F.; Suau, R.; Mari, B.; Tur, J.A.; Sureda, A.; et al. Dietary Sodium Nitrate Activates Antioxidant and Mitochondrial Dynamics Genes after Moderate Intensity Acute Exercise in Metabolic Syndrome Patients. J. Clin. Med. 2021, 10, 2618. [Google Scholar] [CrossRef]
- A Healthy Lifestyle–WHO Recommendations. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on 7 June 2023).
- Monserrat-Mesquida, M.; Quetglas-Llabres, M.; Capo, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome is Associated with Oxidative Stress and Proinflammatory State. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef]
- Castro, A.M.; Macedo-de la Concha, L.E.; Pantoja-Meléndez, C.A. Low-grade inflammation and its relation to obesity and chronic degenerative diseases. Rev. Médica Del Hosp. Gen. De México 2017, 80, 101–105. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Bansal, R.; Gubbi, S.; Muniyappa, R. Metabolic Syndrome and COVID 19: Endocrine-Immune-Vascular Interactions Shapes Clinical Course. Endocrinology 2020, 161, bqaa112. [Google Scholar] [CrossRef]
- Zhou, Y.; Chi, J.; Lv, W.; Wang, Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (COVID-19). Diabetes/Metab. Res. Rev. 2021, 37, e3377. [Google Scholar] [CrossRef]
- Dejam, A.; Hunter, C.J.; Tremonti, C.; Pluta, R.M.; Hon, Y.Y.; Grimes, G.; Partovi, K.; Pelletier, M.M.; Oldfield, E.H.; Cannon, R.O., 3rd; et al. Nitrite infusion in humans and nonhuman primates: Endocrine effects, pharmacokinetics, and tolerance formation. Circulation 2007, 116, 1821–1831. [Google Scholar] [CrossRef]
- Hunault, C.C.; van Velzen, A.G.; Sips, A.J.; Schothorst, R.C.; Meulenbelt, J. Bioavailability of sodium nitrite from an aqueous solution in healthy adults. Toxicol. Lett. 2009, 190, 48–53. [Google Scholar] [CrossRef]
- Kapil, V.; Khambata, R.S.; Jones, D.A.; Rathod, K.; Primus, C.; Massimo, G.; Fukuto, J.M.; Ahluwalia, A. The Noncanonical Pathway for In Vivo Nitric Oxide Generation: The Nitrate-Nitrite-Nitric Oxide Pathway. Pharmacol. Rev. 2020, 72, 692–766. [Google Scholar] [CrossRef]
- Majumder, N.; Deepak, V.; Hadique, S.; Aesoph, D.; Velayutham, M.; Ye, Q.; Mazumder, M.H.H.; Lewis, S.E.; Kodali, V.; Roohollahi, A.; et al. Redox imbalance in COVID-19 pathophysiology. Redox Biol. 2022, 56, 102465. [Google Scholar] [CrossRef]
- Badawy, M.A.; Yasseen, B.A.; El-Messiery, R.M.; Abdel-Rahman, E.A.; Elkhodiry, A.A.; Kamel, A.G.; El-Sayed, H.; Shedra, A.M.; Hamdy, R.; Zidan, M.; et al. Neutrophil-mediated oxidative stress and albumin structural damage predict COVID-19-associated mortality. eLife 2021, 10, e69417. [Google Scholar] [CrossRef]
- Dominic, P.; Ahmad, J.; Bhandari, R.; Pardue, S.; Solorzano, J.; Jaisingh, K.; Watts, M.; Bailey, S.R.; Orr, A.W.; Kevil, C.G.; et al. Decreased availability of nitric oxide and hydrogen sulfide is a hallmark of COVID-19. Redox Biol. 2021, 43, 101982. [Google Scholar] [CrossRef]
- Alamdari, D.H.; Moghaddam, A.B.; Amini, S.; Keramati, M.R.; Zarmehri, A.M.; Alamdari, A.H.; Damsaz, M.; Banpour, H.; Yarahmadi, A.; Koliakos, G. Application of methylene blue -vitamin C -N-acetyl cysteine for treatment of critically ill COVID-19 patients, report of a phase-I clinical trial. Eur. J. Pharmacol. 2020, 885, 173494. [Google Scholar] [CrossRef]
- Lorente, L.; Gomez-Bernal, F.; Martin, M.M.; Navarro-Gonzalvez, J.A.; Argueso, M.; Perez, A.; Ramos-Gomez, L.; Sole-Violan, J.; Marcos, Y.R.J.A.; Ojeda, N.; et al. High serum nitrates levels in non-survivor COVID-19 patients. Med. Intensiv. 2022, 46, 132–139. [Google Scholar] [CrossRef]
- de Sousa, J.A.C.; Azul, F.; de Araujo, A.B.; Tome, R.C.; Silva, F.R.M.; de Vasconcelos, S.M.M.; Rios, F.J.; Leal, L. Epiisopiloturine, an Alkaloid from Pilocarpus microphyllus, Attenuates LPS-Induced Neuroinflammation by Interfering in the TLR4/NF-kappaB-MAPK Signaling Pathway in Microglial Cells. Oxidative Med. Cell. Longev. 2023, 2023, 4752502. [Google Scholar] [CrossRef]
- Kwon, J.; Arsenis, C.; Suessmilch, M.; McColl, A.; Cavanagh, J.; Morris, B.J. Differential Effects of Toll-Like Receptor Activation and Differential Mediation by MAP Kinases of Immune Responses in Microglial Cells. Cell. Mol. Neurobiol. 2022, 42, 2655–2671. [Google Scholar] [CrossRef]
- Somensi, N.; Rabelo, T.K.; Guimaraes, A.G.; Quintans-Junior, L.J.; de Souza Araujo, A.A.; Moreira, J.C.F.; Gelain, D.P. Carvacrol suppresses LPS-induced pro-inflammatory activation in RAW 264.7 macrophages through ERK1/2 and NF-kB pathway. Int. Immunopharmacol. 2019, 75, 105743. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef]
- Fara, A.; Mitrev, Z.; Rosalia, R.A.; Assas, B.M. Cytokine storm and COVID-19: A chronicle of pro-inflammatory cytokines. Open Biol. 2020, 10, 200160. [Google Scholar] [CrossRef]
- Capo, X.; Martorell, M.; Llompart, I.; Sureda, A.; Tur, J.A.; Pons, A. Docosahexanoic acid diet supplementation attenuates the peripheral mononuclear cell inflammatory response to exercise following LPS activation. Cytokine 2014, 69, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. CMLS 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Sureda, A.; Martorell, M.; Bibiloni, M.D.M.; Bouzas, C.; Gallardo-Alfaro, L.; Mateos, D.; Capo, X.; Tur, J.A.; Pons, A. Effect of Free Fatty Acids on Inflammatory Gene Expression and Hydrogen Peroxide Production by Ex Vivo Blood Mononuclear Cells. Nutrients 2020, 12, 146. [Google Scholar] [CrossRef]
- Fang, H.; Wang, X.; Damarla, M.; Sun, R.; He, Q.; Li, R.; Luo, P.; Liu, J.O.; Xia, Z. Dimethyl Fumarate Protects against Lipopolysaccharide- (LPS-) Induced Sepsis through Inhibition of NF-kappaB Pathway in Mice. Mediat. Inflamm. 2023, 2023, 5133505. [Google Scholar] [CrossRef]
- Yang, T.; Peleli, M.; Zollbrecht, C.; Giulietti, A.; Terrando, N.; Lundberg, J.O.; Weitzberg, E.; Carlstrom, M. Inorganic nitrite attenuates NADPH oxidase-derived superoxide generation in activated macrophages via a nitric oxide-dependent mechanism. Free Radic. Biol. Med. 2015, 83, 159–166. [Google Scholar] [CrossRef]
- Schindler, R.; Mancilla, J.; Endres, S.; Ghorbani, R.; Clark, S.C.; Dinarello, C.A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 1990, 75, 40–47. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Sureda, A.; Batle, J.M.; Capo, X.; Martorell, M.; Cordova, A.; Tur, J.A.; Pons, A. Scuba diving induces nitric oxide synthesis and the expression of inflammatory and regulatory genes of the immune response in neutrophils. Physiol. Genom. 2014, 46, 647–654. [Google Scholar] [CrossRef]
- Deon, D.; Ahmed, S.; Tai, K.; Scaletta, N.; Herrero, C.; Lee, I.H.; Krause, A.; Ivashkiv, L.B. Cross-talk between IL-1 and IL-6 signaling pathways in rheumatoid arthritis synovial fibroblasts. J. Immunol. 2001, 167, 5395–5403. [Google Scholar] [CrossRef]
- Assar, S.; Dastbaz, M.; Amini, K.; Roghani, S.A.; Lotfi, R.; Taghadosi, M.; Kafi, H.; Abdan, Z.; Allahyari, H.; Rostampour, R.; et al. Assessing the gene expression of the adenosine 5′-monophosphate-activated protein kinase (AMPK) and its relation with the IL-6 and IL-10 plasma levels in COVID-19 patients. Mol. Biol. Rep. 2023, 50, 9925–9933. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Akerstrom, T.C.; Nielsen, A.R.; Fischer, C.P. Role of myokines in exercise and metabolism. J. Appl. Physiol. 2007, 103, 1093–1098. [Google Scholar] [CrossRef]
- Chaikijurajai, T.; Tang, W.H.W. Myeloperoxidase: A potential therapeutic target for coronary artery disease. Expert Opin. Ther. Targets 2020, 24, 695–705. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Sureda, A.; Mestre, A.; Tur, J.A.; Pons, A. The double edge of reactive oxygen species as damaging and signaling molecules in HL60 cell culture. Cell. Physiol. Biochem. 2010, 25, 241–252. [Google Scholar] [CrossRef] [PubMed]

| Patient | Sex | Age | Weight (kg) | Height (cm) | BMI (kg/cm2) | Systolic Blood Pressure (mm Hg) | Diastolic Blood Pressure (mm Hg) |
|---|---|---|---|---|---|---|---|
| 1 | Male | 67 | 95.1 | 176.5 | 30.53 | 130 | 73 |
| 2 | Female | 68 | 65.8 | 155.5 | 27.21 | 170 | 93 |
| 3 | Male | 68 | 96.2 | 166 | 34.91 | 128 | 68 |
| 4 | Male | 65 | 88.1 | 170.5 | 30.31 | 122 | 77 |
| 5 | Male | 68 | 109.7 | 174.5 | 36.03 | 170 | 88 |
| 6 | Male | 63 | 89.7 | 165.5 | 32.75 | 167 | 100 |
| Reference value | <130 | <85 |
| Measurement | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Reference Value |
|---|---|---|---|---|---|---|---|
| Total cholesterol (mg/dL) | 170 | 124 | 132 | 151 | 110 | 253 | <200 |
| HDL (mg/dL) | 46 | 40 | 47 | 37 | 34 | 38 | ≥60 |
| LDL (mg/dL) | 95 | 63 | 71 | 65 | 60 | 181 | <100 |
| Triglycerides (mg/dL) | 147 | 106 | 73 | 244 | 133 | 172 | <149 |
| Erythrocytes (106/mm3) | 4.89 | 4.47 | 4.84 | 4.73 | 5.65 | 5.42 | 4.50–5.80 |
| Hematocrit (%) | 45.2 | 41.1 | 46.4 | 45.3 | 54.6 | 51 | 40.0–50.0 |
| Hb (g/dL) | 15 | 13.7 | 14.8 | 15.2 | 18 | 16.9 | 12.5–17.2 |
| Hba1c (%) | 5.9 | 6.3 | 6 | 6.7 | 6.9 | 5.5 | 3.8–6.2 |
| Leucocytes (103/mm3) | 7.1 | 6.75 | 7.37 | 10.5 | 5.56 | 6.74 | 4.00–11.00 |
| Neutrophils (103/mm3) | 4.12 | 3.87 | 3.71 | 6.28 | 3.04 | 3.89 | 1.8–7.5 |
| Lymphocytes (103/mm3) | 1.89 | 1.95 | 2.33 | 3.3 | 1.93 | 2.07 | 1.0–4.5 |
| Monocytes (103/mm3) | 0.84 | 0.59 | 0.72 | 0.64 | 4 | 0.55 | 2.5–13.0 |
| Eosinophils (103/mm3) | 0.23 | 0.29 | 0.53 | 0.25 | 0.12 | 0.17 | 0.5–7 |
| Basophils (103/mm3) | 0.02 | 0.05 | 0.07 | 0.07 | 0.07 | 0.06 | 0.0–2.0 |
| Platelets (103/mm3) | 231 | 231 | 190 | 190 | 196 | 214 | 150.0–400.0 |
| Variable | IL-6 | IL-1β | IL-10 | TLR4 | IL-1ra | TNFα | GPx | MnSOD |
|---|---|---|---|---|---|---|---|---|
| IL-6 | 1 | 0.537 ** | 0.724 ** | 0.246 | 0.742 ** | 0.732 ** | 0.485 ** | 0.357 * |
| IL-1β | 1 | 0.645 ** | 0.062 | 0.547 ** | 0.497 ** | 0.475 ** | 0.644 ** | |
| IL-10 | 1 | 0.520 ** | 0.797 ** | 0.658 ** | 0.444 ** | 0.558 ** | ||
| TLR4 | 1 | 0.611 ** | 0.337 * | 0.147 | 0.388 * | |||
| IL-1ra | 1 | 0.557 | 0.312 | 0.508 ** | ||||
| TNFα | 1 | 0.572 ** | 0.543 ** | |||||
| GPx | 1 | 0.523 ** | ||||||
| MnSOD | 1 |
| Gene | RV Sequence | FW Sequence | Cycle Conditions |
|---|---|---|---|
| 18S rRNA | 95 °C 10 s | ||
| 5′-GTGTAATCCGTCTCCACAGA | 5′-ATGTGAAGTCACTGTGCCAG | 60 °C 10 s | |
| 72 °C 15 s | |||
| TNFα | 95 °C 10 s | ||
| 5′-CTGGTTATCTCTCAGCTCCACGCCATT | 5′-CCCAGGCAGTCAGATCATCTTCTCGAA | 59 °C 10 s | |
| 72 °C 15 s | |||
| IL-6 | 95 °C 10 s | ||
| 5′-GTGTAATCCGTCTCCACAGA | 5′- ATGTGAAGTCACTGTGCCAG | 63 °C 10 s | |
| 72 °C 15 s | |||
| IL-1β | 95 °C 10 s | ||
| 5′-GGCAGACTCAAATTCCAGCT | 5′-GGACAGGATATGGAGCAACA | 58 °C 10 s | |
| 72 °C 15 s | |||
| GPx | 95 °C 10 s | ||
| 5′-TTCACCTCGCACTTCTCGAA | 5′-TTCCCGTGCAACCAGTTTG | 63 °C 10 s | |
| 72 °C 15 s | |||
| MnSOD | 95 °C 10 s | ||
| 5′-TGAACGTCACCGAGGAGAAG | 5′-CGTGCTCCCACACATCAATC | 60 °C 10 s | |
| 72 °C 12 s | |||
| TLR4 | 95 °C 10 s | ||
| 5′-TCAGAGGTCCATCAAACATCAC | 5′-GGTCACCTTTTCTTGATTCCA | 60 °C 10 s | |
| 72 °C 15 s | |||
| IL-10 | 95 °C 10 s | ||
| 5′-CCACGGCCTTGCTCTTGTT | 5′-AGAACCTGAAGACCCTCAGGC | 58 °C 10 s | |
| 72 °C 15 s | |||
| IL-1ra | 95 °C 10 s | ||
| 5′-CGCTCAGGTCAGTGATGTTAA | 5’-GAAGATGTGCCTGTCCTGTGT | 56 °C 10 s | |
| 72 °C 15 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrer, M.D.; Reynés, C.; Jiménez, L.; Malagraba, G.; Monserrat-Mesquida, M.; Bouzas, C.; Sureda, A.; Tur, J.A.; Pons, A. Nitrite Attenuates the In Vitro Inflammatory Response of Immune Cells to the SARS-CoV-2 S Protein without Interfering in the Antioxidant Enzyme Activation. Int. J. Mol. Sci. 2024, 25, 3001. https://doi.org/10.3390/ijms25053001
Ferrer MD, Reynés C, Jiménez L, Malagraba G, Monserrat-Mesquida M, Bouzas C, Sureda A, Tur JA, Pons A. Nitrite Attenuates the In Vitro Inflammatory Response of Immune Cells to the SARS-CoV-2 S Protein without Interfering in the Antioxidant Enzyme Activation. International Journal of Molecular Sciences. 2024; 25(5):3001. https://doi.org/10.3390/ijms25053001
Chicago/Turabian StyleFerrer, Miguel D., Clara Reynés, Laura Jiménez, Gianluca Malagraba, Margalida Monserrat-Mesquida, Cristina Bouzas, Antoni Sureda, Josep A. Tur, and Antoni Pons. 2024. "Nitrite Attenuates the In Vitro Inflammatory Response of Immune Cells to the SARS-CoV-2 S Protein without Interfering in the Antioxidant Enzyme Activation" International Journal of Molecular Sciences 25, no. 5: 3001. https://doi.org/10.3390/ijms25053001
APA StyleFerrer, M. D., Reynés, C., Jiménez, L., Malagraba, G., Monserrat-Mesquida, M., Bouzas, C., Sureda, A., Tur, J. A., & Pons, A. (2024). Nitrite Attenuates the In Vitro Inflammatory Response of Immune Cells to the SARS-CoV-2 S Protein without Interfering in the Antioxidant Enzyme Activation. International Journal of Molecular Sciences, 25(5), 3001. https://doi.org/10.3390/ijms25053001











