A Zebrafish-Based Platform for High-Throughput Epilepsy Modeling and Drug Screening in F0
Abstract
1. Introduction
2. Results
2.1. Tyrosinase Loss of Function Allows the Selection of Crispants Carrying High Rate of Mutations in Targeted Genes
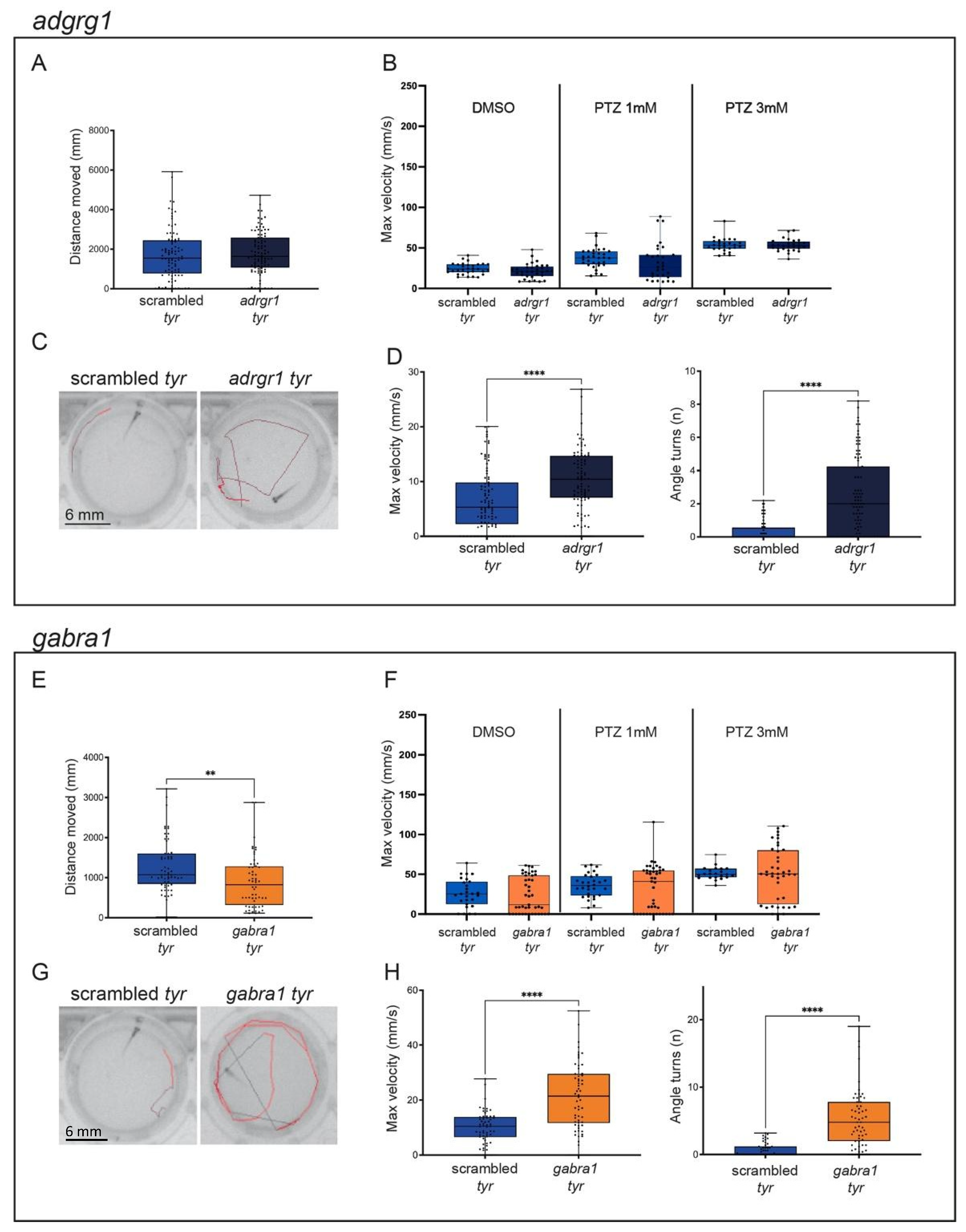
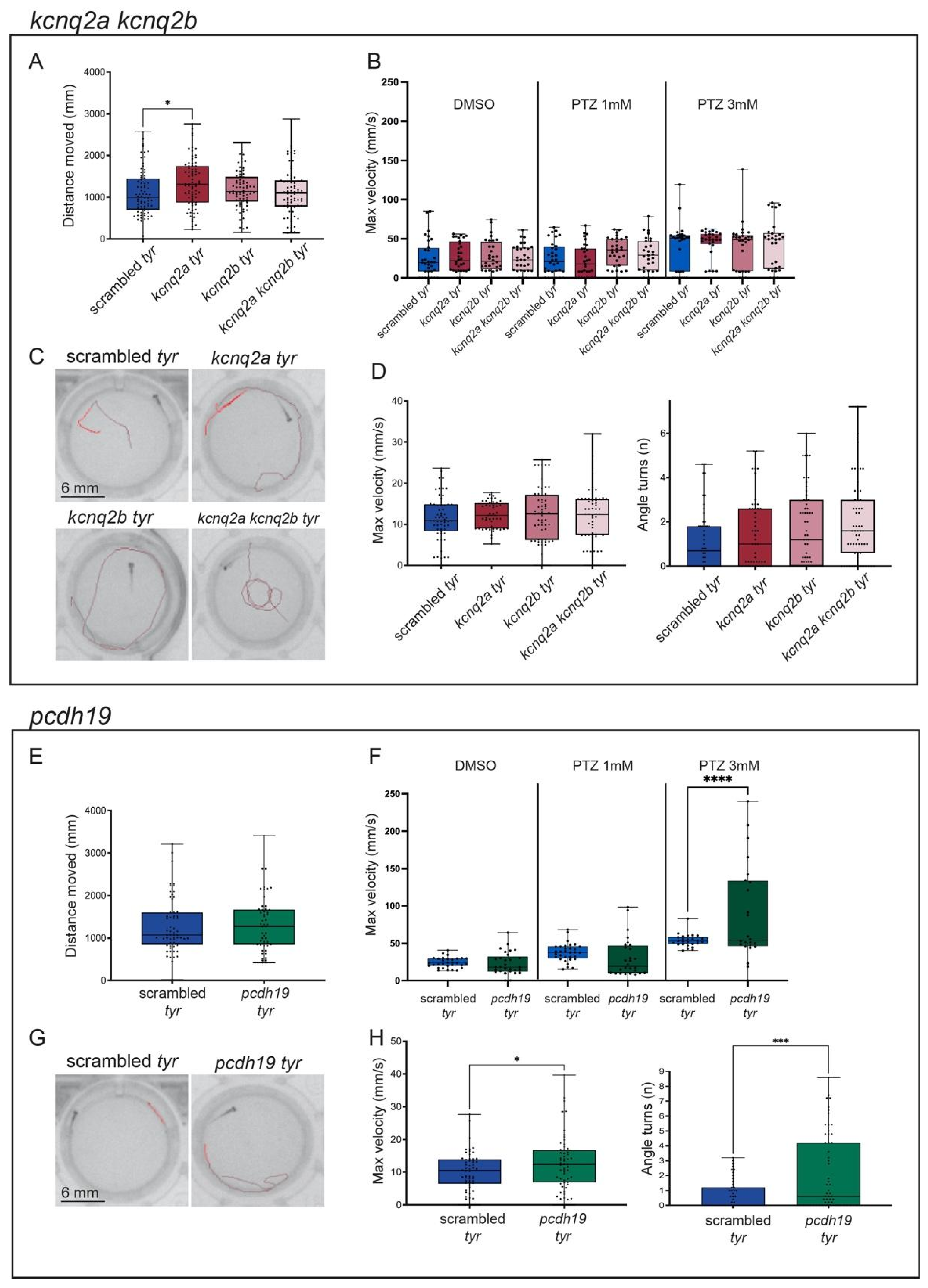
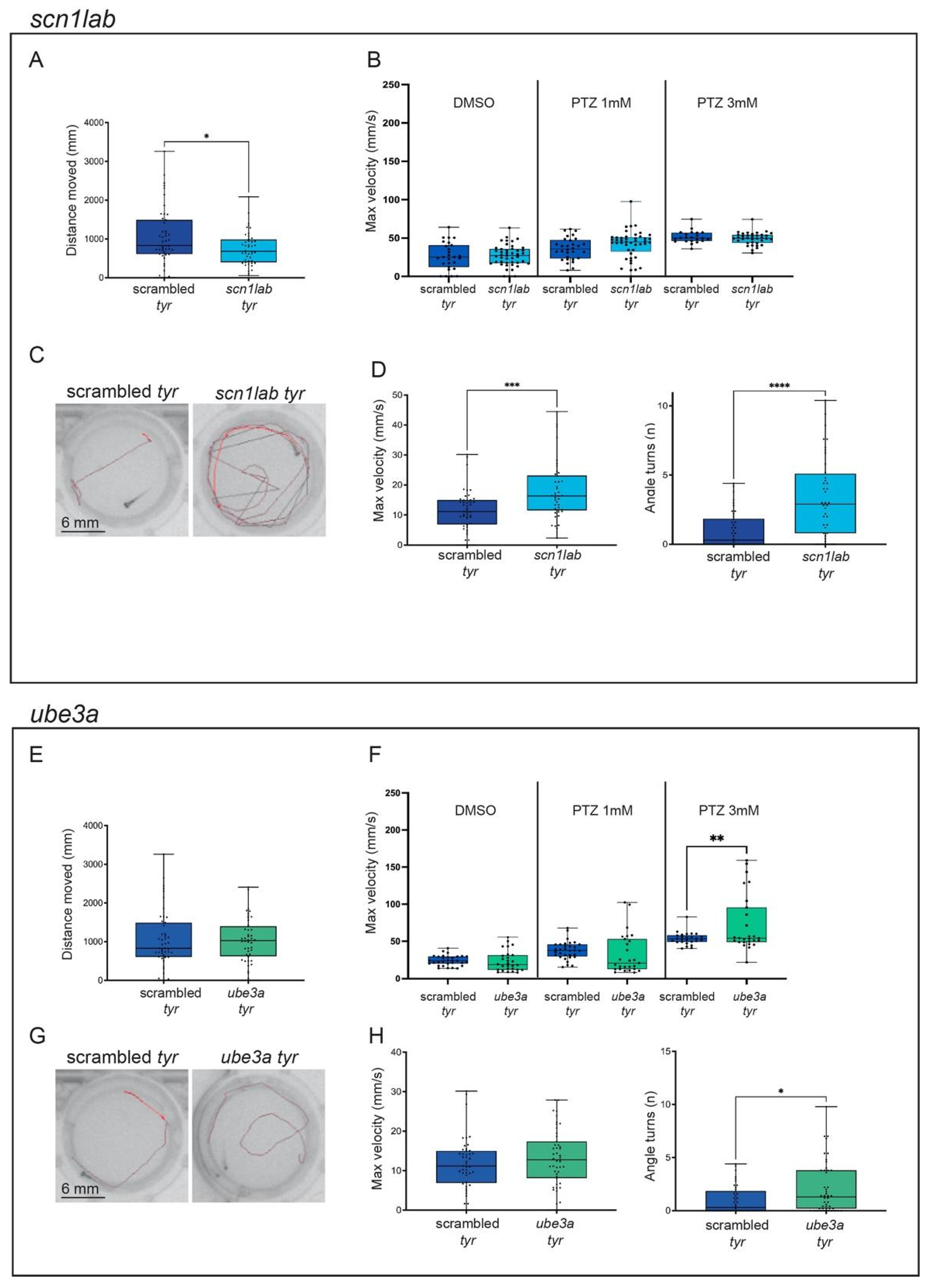
2.2. adgrg1, gabra1, pcdh19, scn1lab and ube3a Crispants Show Epilepsy-like Behavior
2.3. Multiparametric Analysis of Behavioral Response to Light Flashes Allows Fine Characterization of Photosensitive Epilepsy
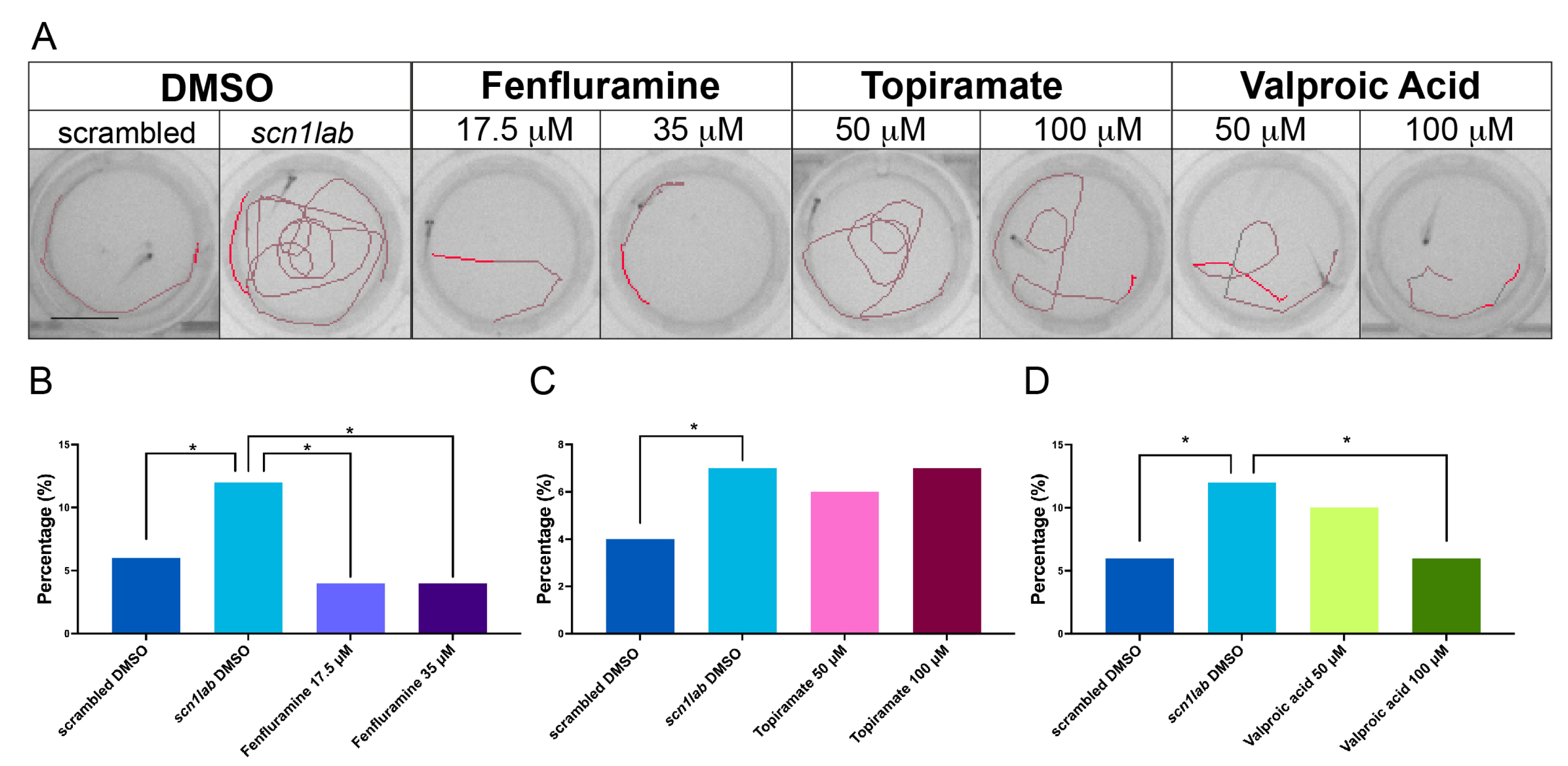
2.4. The Incubation of scn1lab Crispants with Antiepileptic Compounds Protects against Seizure-like Events
3. Discussion
3.1. An Optimized Workflow for Genetic Target Validation in Zebrafish F0 Larvae
3.2. Addressing the Phenotypic Heterogeneity of Epilepsy Combining Morphological and Behavioral Assays
3.3. The Characterization of Photosensitive Epilepsy with In-Depth Analysis of Larval Behavior
3.4. A High-Throughput Platform for ASM Drug Screening
3.5. Concluding Remarks
4. Materials and Methods
4.1. Zebrafish Husbandry and Breeding
4.2. Generation of Crispants
4.3. The Rate of Mutations Analysis
4.4. Morphological Analysis
- Plate A: scrambled, scn1lab and ube3a
- Plate B: scrambled, pcdh19 and gabra1
- Plate C: scrambled, kcnq2a, kcnq2b and kcnq2ab
- Plate D: scrambled and adgrg1
4.5. Behavioral Experiments
4.5.1. Locomotion Activity Assessment
- Plate A: scrambled, scn1lab and ube3a
- Plate B: scrambled, pcdh19 and gabra1
- Plate C: scrambled, kcnq2a, kcnq2b and kcnq2ab
- Plate D: scrambled and adgrg1
4.5.2. Light-Induced Epilepsy Behavior
- Plate A: scrambled, scn1lab and ube3a
- Plate B: scrambled, pcdh19 and gabra1
- Plate C: scrambled, kcnq2a, kcnq2b and kcnq2ab
- Plate D: scrambled and adgrg1
4.5.3. Pharmacological-Induced Epilepsy Behavior
- Plate A: scrambled, adgrg1, pcdh19 and ube3a
- Plate B: scrambled, scn1lab and gabra1
- Plate C: scrambled, kcnq2a, kcnq2b and kcnq2ab
4.6. Behavioral Analysis
4.7. Principal Component Analysis and Mahalanobis Distance Calculations
4.8. Pharmacology with Antiepileptic Compounds
- -
- Topiramate: 50 µM and 100 µM, treatment 180′ before the behavioral assay.
- -
- Valproic acid: 50 µM and 100 µM, treatment 180′ before the behavioral assay.
- -
- Fenfluramine: 17.5 µM and 35 µM, treatment 24 h before the behavioral assay.
4.9. The Statistics of the Pharmacology
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish Disease Models in Drug Discovery: From Preclinical Modelling to Clinical Trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef]
- Cully, M. Zebrafish Earn Their Drug Discovery Stripes. Nat. Rev. Drug Discov. 2019, 18, 811–813. [Google Scholar] [CrossRef]
- Rubbini, D.; Cornet, C.; Terriente, J.; Di Donato, V. CRISPR Meets Zebrafish: Accelerating the Discovery of New Therapeutic Targets. SLAS Discov. 2020, 25, 552–567. [Google Scholar] [CrossRef]
- Baraban, S.C.; Dinday, M.T.; Hortopan, G.A. Drug Screening in Scn1a Zebrafish Mutant Identifies Clemizole as a Potential Dravet Syndrome Treatment. Nat. Commun. 2013, 4, 2410. [Google Scholar] [CrossRef]
- Kawahara, G.; Karpf, J.A.; Myers, J.A.; Alexander, M.S.; Guyon, J.R.; Kunkel, L.M. Drug Screening in a Zebrafish Model of Duchenne Muscular Dystrophy. Proc. Natl. Acad. Sci. USA 2011, 108, 5331–5336. [Google Scholar] [CrossRef]
- Esplin, E.D.; Oei, L.; Snyder, M.P. Personalized Sequencing and the Future of Medicine: Discovery, Diagnosis and Defeat of Disease. Pharmacogenomics 2014, 15, 1771–1790. [Google Scholar] [CrossRef]
- Kotani, H.; Taimatsu, K.; Ohga, R.; Ota, S.; Kawahara, A. Efficient Multiple Genome Modifications Induced by the crRNAs, tracrRNA and Cas9 Protein Complex in Zebrafish. PLoS ONE 2015, 10, e0128319. [Google Scholar] [CrossRef]
- Burger, A.; Lindsay, H.; Felker, A.; Hess, C.; Anders, C.; Chiavacci, E.; Zaugg, J.; Weber, L.M.; Catena, R.; Jinek, M.; et al. Maximizing Mutagenesis with Solubilized CRISPR-Cas9 Ribonucleoprotein Complexes. Development 2016, 143, 2025–2037. [Google Scholar] [CrossRef]
- Shah, A.N.; Davey, C.F.; Whitebirch, A.C.; Miller, A.C.; Moens, C.B. Rapid Reverse Genetic Screening Using CRISPR in Zebrafish. Zebrafish 2016, 13, 152–153. [Google Scholar] [CrossRef]
- Winter, M.J.; Ono, Y.; Ball, J.S.; Walentinsson, A.; Michaelsson, E.; Tochwin, A.; Scholpp, S.; Tyler, C.R.; Rees, S.; Hetheridge, M.J.; et al. A Combined Human in Silico and CRISPR/Cas9-Mediated in Vivo Zebrafish Based Approach to Provide Phenotypic Data for Supporting Early Target Validation. Front. Pharmacol. 2022, 13, 827686. [Google Scholar] [CrossRef]
- Masselink, W. Crispants Take the Spotlight. Lab Anim. 2021, 50, 95–96. [Google Scholar] [CrossRef]
- Kroll, F.; Powell, G.T.; Ghosh, M.; Gestri, G.; Antinucci, P.; Hearn, T.J.; Tunbak, H.; Lim, S.; Dennis, H.W.; Fernandez, J.M.; et al. A Simple and Effective F0 Knockout Method for Rapid Screening of Behaviour and Other Complex Phenotypes. eLife 2021, 10, e59683. [Google Scholar] [CrossRef]
- Francés, L.; Quintero, J.; Fernández, A.; Ruiz, A.; Caules, J.; Fillon, G.; Hervás, A.; Soler, C.V. Current State of Knowledge on the Prevalence of Neurodevelopmental Disorders in Childhood According to the DSM-5: A Systematic Review in Accordance with the PRISMA Criteria. Child Adolesc. Psychiatry Ment. Health 2022, 16, 27. [Google Scholar] [CrossRef]
- Cristino, A.S.; Williams, S.M.; Hawi, Z.; An, J.-Y.; Bellgrove, M.A.; Schwartz, C.E.; Costa, L.D.F.; Claudianos, C. Neurodevelopmental and Neuropsychiatric Disorders Represent an Interconnected Molecular System. Mol. Psychiatry 2014, 19, 294–301. [Google Scholar] [CrossRef]
- Parenti, I.; Rabaneda, L.G.; Schoen, H.; Novarino, G. Neurodevelopmental Disorders: From Genetics to Functional Pathways. Trends Neurosci. 2020, 43, 608–621. [Google Scholar] [CrossRef]
- Bozzi, Y.; Casarosa, S.; Caleo, M. Epilepsy as a Neurodevelopmental Disorder. Front. Psychiatry 2012, 3, 19. [Google Scholar] [CrossRef]
- Turrini, L.; Sorelli, M.; De Vito, G.; Credi, C.; Tiso, N.; Vanzi, F.; Pavone, F.S. Multimodal Characterization of Seizures in Zebrafish Larvae. Biomedicines 2022, 10, 951. [Google Scholar] [CrossRef]
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in Adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef]
- Roberts, A.C.; Chornak, J.; Alzagatiti, J.B.; Ly, D.T.; Bill, B.R.; Trinkeller, J.; Pearce, K.C.; Choe, R.C.; Campbell, C.S.; Wong, D.; et al. Rapid Habituation of a Touch-Induced Escape Response in Zebrafish (Danio Rerio) Larvae. PLoS ONE 2019, 14, e0214374. [Google Scholar] [CrossRef]
- Fisher, R.S.; Acharya, J.N.; Baumer, F.M.; French, J.A.; Parisi, P.; Solodar, J.H.; Szaflarski, J.P.; Thio, L.L.; Tolchin, B.; Wilkins, A.J.; et al. Visually Sensitive Seizures: An Updated Review by the Epilepsy Foundation. Epilepsia 2022, 63, 739–768. [Google Scholar] [CrossRef]
- Miguel Sanz, C.; Martinez Navarro, M.; Caballero Diaz, D.; Sanchez-Elexpuru, G.; Di Donato, V. Toward the Use of Novel Alternative Methods in Epilepsy Modeling and Drug Discovery. Front. Neurol. 2023, 14, 1213969. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, P.; Yan, F.; Luo, Y.; Zhao, G. Animal Models of Epilepsy: A Phenotype-Oriented Review. Aging Dis. 2022, 13, 215. [Google Scholar] [CrossRef]
- Guo, S. Using Zebrafish to Assess the Impact of Drugs on Neural Development and Function. Expert Opin. Drug Discov. 2009, 4, 715–726. [Google Scholar] [CrossRef]
- Koseki, N.; Deguchi, J.; Yamashita, A.; Miyawaki, I.; Funabashi, H. Establishment of a Novel Experimental Protocol for Drug-Induced Seizure Liability Screening Based on a Locomotor Activity Assay in Zebrafish. J. Toxicol. Sci. 2014, 39, 579–600. [Google Scholar] [CrossRef]
- Baraban, S.C.; Taylor, M.R.; Castro, P.A.; Baier, H. Pentylenetetrazole Induced Changes in Zebrafish Behavior, Neural Activity and c-Fos Expression. Neuroscience 2005, 131, 759–768. [Google Scholar] [CrossRef]
- Wong, K.; Stewart, A.; Gilder, T.; Wu, N.; Frank, K.; Gaikwad, S.; Suciu, C.; DiLeo, J.; Utterback, E.; Chang, K.; et al. Modeling Seizure-Related Behavioral and Endocrine Phenotypes in Adult Zebrafish. Brain Res. 2010, 1348, 209–215. [Google Scholar] [CrossRef]
- Gupta, P.; Khobragade, S.B.; Shingatgeri, V.M. Effect of Various Antiepileptic Drugs in Zebrafish PTZ- Seizure Model. Indian J. Pharm. Sci. 2014, 76, 157–163. [Google Scholar]
- Dinday, M.T.; Baraban, S.C. Large-Scale Phenotype-Based Antiepileptic Drug Screening in a Zebrafish Model of Dravet Syndrome. Eneuro 2015, 2, ENEURO.0068-15.2015. [Google Scholar] [CrossRef]
- Jao, L.-E.; Wente, S.R.; Chen, W. Efficient Multiplex Biallelic Zebrafish Genome Editing Using a CRISPR Nuclease System. Proc. Natl. Acad. Sci. USA 2013, 110, 13904–13909. [Google Scholar] [CrossRef]
- Jarque, S.; Rubio-Brotons, M.; Ibarra, J.; Ordoñez, V.; Dyballa, S.; Miñana, R.; Terriente, J. Morphometric Analysis of Developing Zebrafish Embryos Allows Predicting Teratogenicity Modes of Action in Higher Vertebrates. Reprod. Toxicol. 2020, 96, 337–348. [Google Scholar] [CrossRef]
- Haigis, A.-C.; Ottermanns, R.; Schiwy, A.; Hollert, H.; Legradi, J. Getting More out of the Zebrafish Light Dark Transition Test. Chemosphere 2022, 295, 133863. [Google Scholar] [CrossRef]
- MacPhail, R.C.; Brooks, J.; Hunter, D.L.; Padnos, B.; Irons, T.D.; Padilla, S. Locomotion in Larval Zebrafish: Influence of Time of Day, Lighting and Ethanol. Neurotoxicology 2009, 30, 52–58. [Google Scholar] [CrossRef]
- Bahi-Buisson, N.; Poirier, K.; Boddaert, N.; Fallet-Bianco, C.; Specchio, N.; Bertini, E.; Caglayan, O.; Lascelles, K.; Elie, C.; Rambaud, J.; et al. GPR56-Related Bilateral Frontoparietal Polymicrogyria: Further Evidence for an Overlap with the Cobblestone Complex. Brain 2010, 133, 3194–3209. [Google Scholar] [CrossRef]
- Johannesen, K.; Marini, C.; Pfeffer, S.; Møller, R.S.; Dorn, T.; Niturad, C.E.; Gardella, E.; Weber, Y.; Søndergård, M.; Hjalgrim, H.; et al. Phenotypic Spectrum of GABRA1: From Generalized Epilepsies to Severe Epileptic Encephalopathies. Neurology 2016, 87, 1140–1151. [Google Scholar] [CrossRef]
- Castaldo, P.; Del Giudice, E.M.; Coppola, G.; Pascotto, A.; Annunziato, L.; Taglialatela, M. Benign Familial Neonatal Convulsions Caused by Altered Gating of KCNQ2/KCNQ3 Potassium Channels. J. Neurosci. 2002, 22, RC199. [Google Scholar] [CrossRef]
- Samanta, D. PCDH19-Related Epilepsy Syndrome: A Comprehensive Clinical Review. Pediatr. Neurol. 2020, 105, 3–9. [Google Scholar] [CrossRef]
- Depienne, C.; Trouillard, O.; Saint-Martin, C.; Gourfinkel-An, I.; Bouteiller, D.; Carpentier, W.; Keren, B.; Abert, B.; Gautier, A.; Baulac, S.; et al. Spectrum of SCN1A Gene Mutations Associated with Dravet Syndrome: Analysis of 333 Patients. J. Med. Genet. 2008, 46, 183–191. [Google Scholar] [CrossRef]
- Fang, P. The Spectrum of Mutations in UBE3A Causing Angelman Syndrome. Hum. Mol. Genet. 1999, 8, 129–135. [Google Scholar] [CrossRef]
- Tiedeken, J.A.; Ramsdell, J.S. Embryonic Exposure to Domoic Acid Increases the Susceptibility of Zebrafish Larvae to the Chemical Convulsant Pentylenetetrazole. Environ. Health Perspect. 2007, 115, 1547–1552. [Google Scholar] [CrossRef]
- Ellis, L.D.; Seibert, J.; Soanes, K.H. Distinct Models of Induced Hyperactivity in Zebrafish Larvae. Brain Res. 2012, 1449, 46–59. [Google Scholar] [CrossRef]
- Griffin, A.; Carpenter, C.; Liu, J.; Paterno, R.; Grone, B.; Hamling, K.; Moog, M.; Dinday, M.T.; Figueroa, F.; Anvar, M.; et al. Phenotypic Analysis of Catastrophic Childhood Epilepsy Genes. Commun. Biol. 2021, 4, 680. [Google Scholar] [CrossRef]
- Yang, X.; Lin, J.; Peng, X.; Zhang, Q.; Zhang, Y.; Guo, N.; Zhou, S.; Li, Q. Effects of Picrotoxin on Zebrafish Larvae Behaviors: A Comparison Study with PTZ. Epilepsy Behav. 2017, 70, 224–231. [Google Scholar] [CrossRef]
- Devinsky, O.; Vezzani, A.; O’Brien, T.J.; Jette, N.; Scheffer, I.E.; De Curtis, M.; Perucca, P. Epilepsy. Nat. Rev. Dis. Primers 2018, 4, 18024. [Google Scholar] [CrossRef]
- Fisher, R.S.; Cross, H.; D’Souza, C.; French, J.A.; Haut, S.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Peltola, J.; Moshé, S.L.; et al. 2017 International League Against Epilepsy Classifications of Seizures and Epilepsy Are Steps in the Right Direction. Epilepsia 2019, 60, 1040–1044. [Google Scholar] [CrossRef]
- Keezer, M.R.; Sisodiya, S.M.; Sander, J.W. Comorbidities of Epilepsy: Current Concepts and Future Perspectives. Lancet Neurol. 2016, 15, 106–115. [Google Scholar] [CrossRef]
- Owens, M.J.; Nemeroff, C.B. Pharmacology of Valproate. Psychopharmacol. Bull. 2003, 37, 17–24. [Google Scholar]
- Löscher, W. Basic Pharmacology of Valproate: A Review after 35 Years of Clinical Use for the Treatment of Epilepsy. CNS Drugs 2002, 16, 669–694. [Google Scholar] [CrossRef]
- Rosenberg, G. The Mechanisms of Action of Valproate in Neuropsychiatric Disorders: Can We See the Forest for the Trees? Cell Mol. Life Sci. 2007, 64, 2090–2103. [Google Scholar] [CrossRef]
- Mula, M.; Cavanna, A.E.; Monaco, F. Psychopharmacology of Topiramate: From Epilepsy to Bipolar Disorder. Neuropsychiatr. Dis. Treat. 2006, 2, 475–488. [Google Scholar] [CrossRef]
- Sourbron, J.; Lagae, L. Fenfluramine: A Plethora of Mechanisms? Front. Pharmacol. 2023, 14, >1192022. [Google Scholar] [CrossRef]
- Samanta, D. Fenfluramine: A Review of Pharmacology, Clinical Efficacy, and Safety in Epilepsy. Children 2022, 9, 1159. [Google Scholar] [CrossRef]
- Gogou, M.; Cross, J.H. Fenfluramine as Antiseizure Medication for Epilepsy. Dev. Med. Child Neurol. 2021, 63, 899–907. [Google Scholar] [CrossRef]
- Martens, G.; Van Loo, K. Genetic and Environmental Factors in Complex Neurodevelopmental Disorders. Curr. Genom. 2007, 8, 429–444. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Z.-J.; Liu, L.; Xu, H.-Q.; Shi, Y.-W.; Yi, Y.-H.; He, N.; Liao, W.-P. Epilepsy-Associated Genes. Seizure 2017, 44, 11–20. [Google Scholar] [CrossRef]
- Consortium, E. A Roadmap for Precision Medicine in the Epilepsies. Lancet Neurol. 2015, 14, 1219–1228. [Google Scholar] [CrossRef]
- Consortium, E. De Novo Mutations in SLC1A2 and CACNA1A Are Important Causes of Epileptic Encephalopathies. Am. J. Hum. Genet. 2016, 99, 287–298. [Google Scholar] [CrossRef]
- Cornet, C.; Di Donato, V.; Terriente, J. Combining Zebrafish and CRISPR/Cas9: Toward a More Efficient Drug Discovery Pipeline. Front. Pharmacol. 2018, 9, 703. [Google Scholar] [CrossRef]
- Porto, E.M.; Komor, A.C.; Slaymaker, I.M.; Yeo, G.W. Base Editing: Advances and Therapeutic Opportunities. Nat. Rev. Drug Discov. 2020, 19, 839–859. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-Replace Genome Editing without Double-Strand Breaks or Donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef]
- Dauncey, M.; Ingram, D. Effect of Propranolol on the Metabolism of Thyroid Hormones. Horm. Metab. Res. 1987, 19, 93–95. [Google Scholar] [CrossRef]
- Marini, C.; Porro, A.; Rastetter, A.; Dalle, C.; Rivolta, I.; Bauer, D.; Oegema, R.; Nava, C.; Parrini, E.; Mei, D.; et al. HCN1 Mutation Spectrum: From Neonatal Epileptic Encephalopathy to Benign Generalized Epilepsy and Beyond. Brain 2018, 141, 3160–3178. [Google Scholar] [CrossRef]
- Hewson, S.; Puka, K.; Mercimek-Mahmutoglu, S. Variable Expressivity of a Likely Pathogenic Variant in KCNQ2 in a Three-generation Pedigree Presenting with Intellectual Disability with Childhood Onset Seizures. Am. J. Med. Genet. A 2017, 173, 2226–2230. [Google Scholar] [CrossRef]
- Grone, B.P.; Qu, T.; Baraban, S.C. Behavioral Comorbidities and Drug Treatments in a Zebrafish Scn1lab Model of Dravet Syndrome. Eneuro 2017, 4, ENEURO.0066-17.2017. [Google Scholar] [CrossRef]
- Ding, J.; Li, X.; Tian, H.; Wang, L.; Guo, B.; Wang, Y.; Li, W.; Wang, F.; Sun, T. SCN1A Mutation—Beyond Dravet Syndrome: A Systematic Review and Narrative Synthesis. Front. Neurol. 2021, 12, 743726. [Google Scholar] [CrossRef]
- Eimon, P.M.; Ghannad-Rezaie, M.; De Rienzo, G.; Allalou, A.; Wu, Y.; Gao, M.; Roy, A.; Skolnick, J.; Yanik, M.F. Brain Activity Patterns in High-Throughput Electrophysiology Screen Predict Both Drug Efficacies and Side Effects. Nat. Commun. 2018, 9, 219. [Google Scholar] [CrossRef]
- Samarut, É.; Swaminathan, A.; Riché, R.; Liao, M.; Hassan-Abdi, R.; Renault, S.; Allard, M.; Dufour, L.; Cossette, P.; Soussi-Yanicostas, N.; et al. γ-Aminobutyric Acid Receptor Alpha 1 Subunit Loss of Function Causes Genetic Generalized Epilepsy by Impairing Inhibitory Network Neurodevelopment. Epilepsia 2018, 59, 2061–2074. [Google Scholar] [CrossRef]
- Robens, B.K.; Yang, X.; McGraw, C.M.; Turner, L.H.; Robens, C.; Thyme, S.; Rotenberg, A.; Poduri, A. Mosaic and Non-Mosaic Protocadherin 19 Mutation Leads to Neuronal Hyperexcitability in Zebrafish. Neurobiol. Dis. 2022, 169, 105738. [Google Scholar] [CrossRef]
- Helbig, I.; Abou Tayoun, A.N. Understanding Genotypes and Phenotypes in Epileptic Encephalopathies. Mol. Syndromol. 2016, 7, 172–181. [Google Scholar] [CrossRef]
- Covanis, A. Photosensitivity in Idiopathic Generalized Epilepsies. Epilepsia 2005, 46, 67–72. [Google Scholar] [CrossRef]
- Hawkins, N.A.; Jurado, M.; Thaxton, T.T.; Duarte, S.E.; Barse, L.; Tatsukawa, T.; Yamakawa, K.; Nishi, T.; Kondo, S.; Miyamoto, M.; et al. Soticlestat, a Novel Cholesterol 24-hydroxylase Inhibitor, Reduces Seizures and Premature Death in Dravet Syndrome Mice. Epilepsia 2021, 62, 2845–2857. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book, A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th ed.; University of Oregon: Eugene, OR, USA, 2000. [Google Scholar]
- Padilla, S.; Hunter, D.L.; Padnos, B.; Frady, S.; MacPhail, R.C. Assessing Locomotor Activity in Larval Zebrafish: Influence of Extrinsic and Intrinsic Variables. Neurotoxicol. Teratol. 2011, 33, 624–630. [Google Scholar] [CrossRef]
- Aggarwal, C.C. Outlier Analysis, 2nd ed.; Springer: Cham, Switzerland, 2017; ISBN 978-3-319-47577-6. [Google Scholar]
- DeOliveira-Mello, L.; Baronio, D.; Panula, P. Zebrafish Embryonically Exposed to Valproic Acid Present Impaired Retinal Development and Sleep Behavior. Autism Res. 2023, 16, 1877–1890. [Google Scholar] [CrossRef]
- Rajesh, V.; Deepan, N.; Anitha, V.; Kalaiselvan, D.; Jayaseelan, S.; Sivakumar, P.; Ganesan, V. Heart Malformation Is an Early Response to Valproic Acid in Developing Zebrafish. Naunyn-Schmiedebergs Arch. Pharmacol. 2020, 393, 2387–2409. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Ding, Y.-J.; Moses, D.; Chen, Y.-H. Teratogenic Effects of Topiramate in a Zebrafish Model. Int. J. Mol. Sci. 2017, 18, 1721. [Google Scholar] [CrossRef]
- Moog, M.; Baraban, S.C. Clemizole and Trazodone Are Effective Antiseizure Treatments in a Zebrafish Model of STXBP1 Disorder. Epilepsia Open 2022, 7, 504–511. [Google Scholar] [CrossRef]
- Zhang, Y.; Kecskés, A.; Copmans, D.; Langlois, M.; Crawford, A.D.; Ceulemans, B.; Lagae, L.; De Witte, P.A.M.; Esguerra, C.V. Pharmacological Characterization of an Antisense Knockdown Zebrafish Model of Dravet Syndrome: Inhibition of Epileptic Seizures by the Serotonin Agonist Fenfluramine. PLoS ONE 2015, 10, e0125898. [Google Scholar] [CrossRef]
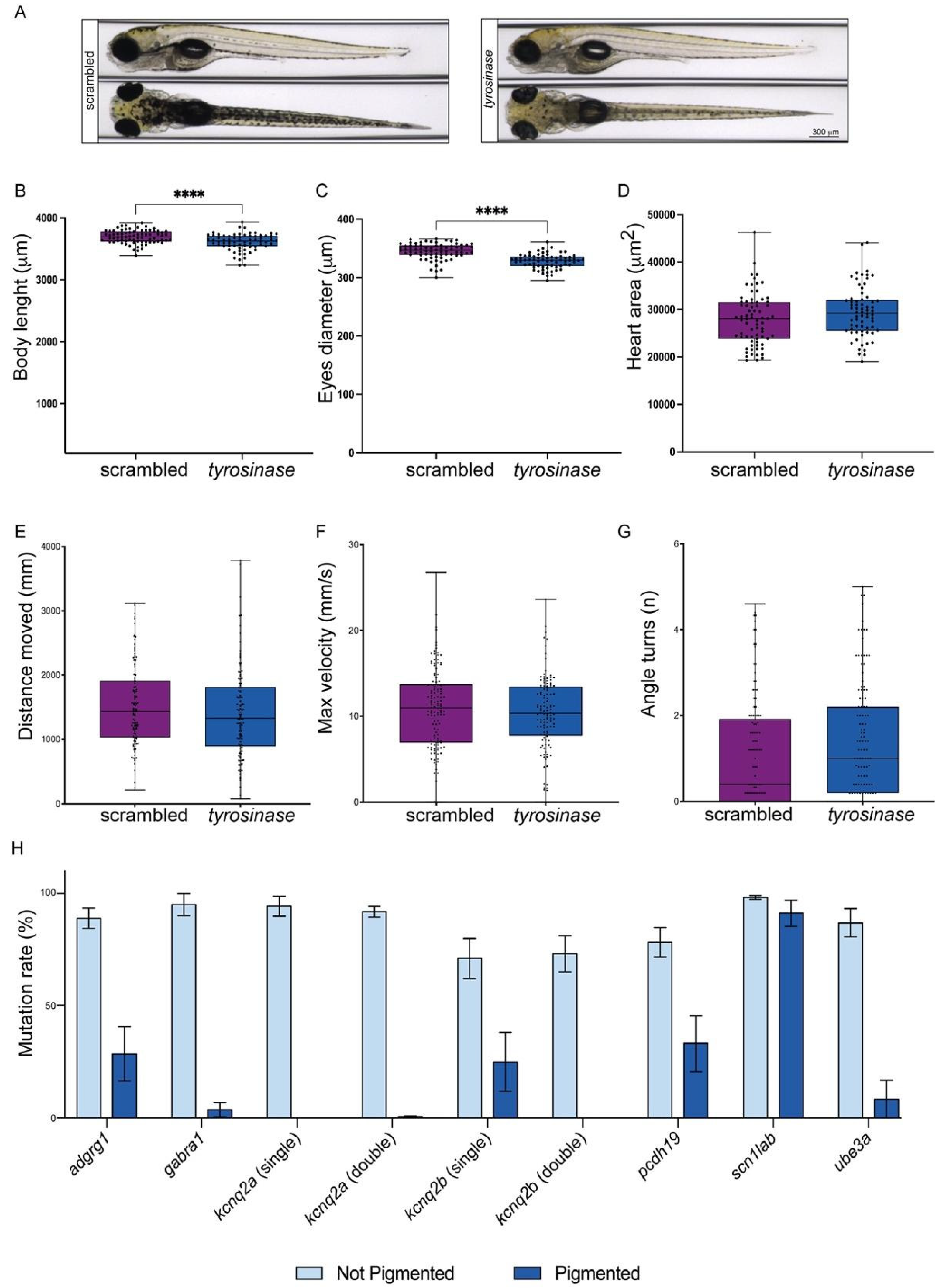

| Scrambled | Tyrosinase | |||||
|---|---|---|---|---|---|---|
| Number of Positive | Total Number | % of Positive | Number of Positive | Total Number | % of Positive | |
| Body curvature | 0 | 75 | 0 | 1 | 68 | 0.01 |
| Snout jaw defects | 2 | 75 | 0.03 | 0 | 68 | 0 |
| Yolk edema | 2 | 75 | 0.03 | 0 | 68 | 0 |
| Necrosis | 1 | 75 | 0.01 | 2 | 68 | 0.03 |
| Tail bending | 0 | 75 | 0 | 1 | 68 | 0.01 |
| Notochord defects | 0 | 75 | 0 | 0 | 68 | 0 |
| Craniofacial edema | 2 | 75 | 0.03 | 0 | 68 | 0 |
| Fin absence | 0 | 75 | 0 | 0 | 68 | 0 |
| Scoliosis | 0 | 75 | 0 | 0 | 68 | 0 |
| Human Gene | Disease | Zebrafish Orthologue |
|---|---|---|
| ADGRG1 | Bilateral frontoparietal polymicrogyria [33] | adgrg1 |
| GABRA1 | Different epileptic disorders [34] | gabra1 |
| KCNQ2 | Benign familial neonatal seizures [35] | kcnq2a; kcnq2b |
| PCDH19 | PCDH19 Epilepsy [36] | pcdh19 |
| SCN1A | Dravet syndrome [37] | scn1lab |
| UBE3A | Angelman syndrome [38] | ube3a |
| Target Gene | Number of Larvae | Number of Flash Responses | Percentage of High-Activity Responses |
|---|---|---|---|
| scrambled | 116 | 617 | 5.1% |
| adgrg1 | 86 | 282 | 8.9% |
| gabra1 | 56 | 212 | 10.2% |
| kcnq2a | 57 | 219 | 6% |
| kcnq2b | 55 | 181 | 3% |
| kcnq2a/kcnq2b | 53 | 208 | 0.6% |
| pcdh19 | 50 | 157 | 8.5% |
| scn1lab | 46 | 183 | 21.6% |
| ube3a | 44 | 150 | 6.1% |
| Zebrafish Gene | Primers | sgRNAs | Targeted Exon |
|---|---|---|---|
| adgrg1 | FW—5′-GTCATTTCTGTGTGTTCTGGGAG-3′ | 5′-CGGTGCAGCAGGTTCCTTGA-3′; | Exon 2 |
| RV—5′-GGTGATGTTGTGATGCATGGTA-3′ | 5′-GTCAAAGGTGATATCATCAC-3′ | ||
| gabra1 | FW—5′-TATTCCTTTGCACTGGCTGAGA-3′ | 5′-CTGCCTGAAGAACACATCTA-3′ | Exon 5 |
| RV—5′-CGAACACAGACACCAACGAAAT-3′ | 5′-CCCGACACGTTCTTCCACAA-3′ | ||
| kcnq2a | FW—5′-CCGCCAACGGGGAAGTTTA-3′ | 5′-GGTAAATGAACGCCCAGCCG-3′; | Exon 1 |
| RV—5′-AGTTTGAGCATTCTGGGCGG-3′ | 5′-TCTGGAGCGACCCCGCGGCT-3′ | ||
| kcnq2b | FW—5′-CCAGAACAAGTTCTCCAGGGA-3′ | 5′-TGCTCGCACCTGCTGTAGGG-3′; | Exon 1 |
| RV—5′-AATTCTGCAGGCGTCGGTAA-3′ | 5′-TTTCTCGGCCTGCGGGGCGG-3′ | ||
| pcdh19 | FW—5′-GGACTGGAGTCGATGCCG-3′ | 5′-TAACCCGCAAATAAGGCTGT-3′; | Exon 1 |
| RV—5′-GTGTACCGAGACTGCGTTTCT-3′ | 5′-CGGTACCAGATTTCCCCTAG-3′ | ||
| scn1lab | FW—5′-CATCAGCTCCCAGAGTGACC-3′ | 5′-TGCGATGCGTTGCTCGATAG-3′; | Exon 1 |
| RV—5′-CCTTCAGGTAGTCCTACAGCCT-3′ | 5′-TCTCCGTAGATGAACGGCAG-3′ | ||
| ube3a | FW—5′-ATCACAATGTGTACGCCGCT-3′ | 5′-GTTGTGGTCGCAGTACGGTG-3′; | Exon 2 |
| RV—5′-GATCCACTCGAGGGCCTTTT-3′ | 5′-AGCTCGCTGGACTCAGGGAT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Locubiche, S.; Ordóñez, V.; Abad, E.; Scotto di Mase, M.; Di Donato, V.; De Santis, F. A Zebrafish-Based Platform for High-Throughput Epilepsy Modeling and Drug Screening in F0. Int. J. Mol. Sci. 2024, 25, 2991. https://doi.org/10.3390/ijms25052991
Locubiche S, Ordóñez V, Abad E, Scotto di Mase M, Di Donato V, De Santis F. A Zebrafish-Based Platform for High-Throughput Epilepsy Modeling and Drug Screening in F0. International Journal of Molecular Sciences. 2024; 25(5):2991. https://doi.org/10.3390/ijms25052991
Chicago/Turabian StyleLocubiche, Sílvia, Víctor Ordóñez, Elena Abad, Michele Scotto di Mase, Vincenzo Di Donato, and Flavia De Santis. 2024. "A Zebrafish-Based Platform for High-Throughput Epilepsy Modeling and Drug Screening in F0" International Journal of Molecular Sciences 25, no. 5: 2991. https://doi.org/10.3390/ijms25052991
APA StyleLocubiche, S., Ordóñez, V., Abad, E., Scotto di Mase, M., Di Donato, V., & De Santis, F. (2024). A Zebrafish-Based Platform for High-Throughput Epilepsy Modeling and Drug Screening in F0. International Journal of Molecular Sciences, 25(5), 2991. https://doi.org/10.3390/ijms25052991








