Abstract
Zebrafish are an emergent animal model to study human diseases due to their significant genetic similarity to humans, swift development, and genetic manipulability. Their utility extends to the exploration of the involvement of inflammation in host defense, immune responses, and tissue regeneration. Additionally, the zebrafish model system facilitates prompt screening of chemical compounds that affect inflammation. This study explored the diverse roles of inflammatory pathways in zebrafish development and aging. Serving as a crucial model, zebrafish provides insights into the intricate interplay of inflammation in both developmental and aging contexts. The evidence presented suggests that the same inflammatory signaling pathways often play instructive or beneficial roles during embryogenesis and are associated with malignancies in adults.
Keywords:
Danio rerio; zebrafish; inflammation; aging; development; immunity; immune signaling; hematopoiesis; inflammaging 1. Zebrafish as Inflammation Model
The zebrafish has become a prominent vertebrate animal model. Zebrafish are useful for studying human diseases as they share homology with more than 70% of human genes [1], and most pathways, cell types, and tissues involved in human diseases are conserved in zebrafish. Together with unquestionable advantages compared to other vertebrate models, such as rapid development, high fecundity, transparency, and ease of gene editing, the zebrafish is a powerful animal model to study a great variety of human diseases.
Some of the fields in which zebrafish have proven particularly valuable are the study of the role of inflammation in host defense, immune responses, development, tissue regeneration, and aging [2,3,4,5,6,7]. Inflammation can be triggered by several sources, including biological, chemical, and mechanical, and these have been largely employed in zebrafish models to address molecular and cellular questions, particularly in pathogenic conditions.
Because of the early emergence of innate immune cells [8] and the ease of assessing local and systemic microinjections, zebrafish have been established as a powerful model for numerous bacterial, viral, and fungal pathogens [9,10,11]. These infection models have advanced our understanding of host-pathogen interactions, cellular immunity, and emergency granulopoiesis, as well as the bases of pathogenesis and cell biology [11,12].
Zebrafish has also been demonstrated to be a reliable model system for studying human viral pathologies, including the most recent coronavirus disease 2019 [13]. For example, using an adult zebrafish model, it was observed that intranasally delivered SARS-CoV-2 S spike protein caused severe olfactory histopathology, providing key insights for the evaluation of the mechanisms of action and side effects of human intranasally delivered vaccines against SARS-CoV-2 [14]. Due to the unique characteristics of zebrafish, such as their small size, optical transparency, and simple external development, the zebrafish model system allows for the rapid screening of chemical compounds that affect inflammation. Likewise, Zebrafish has been employed to assess inflammation and toxicity upon chemical damage, including exposure to alcohol [15], nicotine [16], neuropeptides [17], and acids [18], enabling the identification of potential lead compounds for anti-inflammatory therapies.
Moreover, zebrafish fin injury models are robust tools for dissecting the role of inflammation in tissue damage and assessing regeneration and tissue repair. Indeed, the tailfin injury zebrafish model allows for evaluating the molecular mechanisms underlying the inflammatory response and drug discovery studies [19]. Furthermore, zebrafish fin injury models, in combination with zebrafish transgenic reporter lines of different cell types and/or chemical mediators of wound inflammation, also allow exploring the role of inflammation in the wound regenerative process and the impact of the immune cell migration machinery on the trajectory of inflammation [20,21,22,23]. Indeed, the alteration of leukocyte dynamics is considered an inflammation parameter [24,25], and tailfin injury in zebrafish represents a powerful physiological setup for qualitative and quantitative live-cell dynamics in inflamed tissues.
Owing to the toolkits and putative applications listed above, zebrafish have been largely exploited for the assessment of inflammation cues and pathways in different setups. This review focuses on the use of zebrafish as a model to explore the role of inflammation in both development and aging.
2. Inflammatory Pathways Are Involved in Zebrafish Development and Aging
Inflammation implies the activation of a molecular and cellular response. The most significant mediators of the inflammatory response are cells of the innate immune system, such as macrophages and neutrophils. The immune system is highly conserved between zebrafish and humans [26,27,28]. Orthologs of the main proinflammatory cytokines involved in inflammatory responses, such as interleukin IL-6, tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β), are all found in zebrafish [29]. The innate branch of the immune system matures first during zebrafish development [8]. Thereafter, the lymphoid lineage begins to mature in the thymus and in the kidney marrow, which is the equivalent of bone marrow in mammals [30,31]. The complete maturation of the adaptive immune system occurs at the juvenile stage [32], which means that zebrafish offer a unique and powerful model for assessing inflammation driven by innate immunity independently of adaptive responses at early stages. The following provides an overview of the molecular pathways and cytokines involved in sterile inflammation, as well as recent research findings that have shed light on the role of these signaling pathways in both development and aging.
2.1. NF-κB
The nuclear factor-κB (NF-κB) family is a group of inducible transcription factors that control both innate and adaptive immune responses, the expression of proinflammatory genes, the activation of inflammasomes [33], and the activation, differentiation, and effector function of inflammatory T cells [34]. The NF-κB family consists of five protein monomers (NF-κB1 p50, NF-κB2 p52, p65 [RelA], Rel B, and c-Rel), which can form up to 15 different homo- or heterodimers with distinct functions. Some of these exhibit transcriptional activity (NF-κB1 p50-p65 and NF-κB2 p52-RelB) [35]. These homo- or heterodimers normally exist as components of inactive cytoplasmic complexes bound by members of the inhibitor of κB (IκB) family. NF-κB activation occurs via two main signaling pathways: canonical and non-canonical [36]. Upon infection, the canonical NF-κB pathway or NF-κB essential modulator (NEMO, also known as IKKγ)-dependent pathway is activated. Canonical NF-κB family members include NF-κB1 p50, p65, and c-Rel. In most cells, p65 is the predominant NF-κB transcription factor and consequently drives the expression of many proinflammatory genes that control immunity and immune functions.
In addition to regulating immune and inflammatory responses, NF-κB is essential for embryogenesis, including dorsal–ventral patterning and limb, liver, skin, lung, neural, notochord, muscle, skeletal, and hematopoietic formation [37]. Defects in these components can lead to embryonic lethality [38,39] or developmental abnormalities [40,41]. Interestingly, although the NF-κB pathway is well conserved in mammals and fish [42], mice deficient in p65 die after 15–16 days of embryogenesis due to massive liver destruction [38], whereas p65-null zebrafish survive [43]. Therefore, p65-null zebrafish provide a crucial tool for addressing the specific function of NF-κB during embryogenesis, which has not been addressed in other animal systems.
NF-κB signaling has been shown to be important for other functions during the larval stages of zebrafish. For example, NF-κB-dependent activation in phagocytes occurs rapidly after myelin injury and is required for myelin debris degradation, inflammation resolution, and the initiation of the generation of new oligodendrocytes [44]. Moreover, blockage of NF-κB activity by overexpression of the dominant-negative form of the murine IκBα gene affects embryonic dorsalization and leads to notochord deformities [42].
In adults and aging, using zebrafish lines expressing the beta-cell-specific fluorescence ubiquitination cell cycle indicator (FUCCI), it has been shown that NF-kB signaling is activated in beta cells. These findings indicate that the activation of NF-kB is associated with a gradual decrease in beta cell proliferation as individuals age, accompanied by immune cell infiltration in the islets of Langerhans [6]. This study showed that zebrafish is a novel model for investigating the interconnections between aging, beta cell biology, the innate immune system, and diabetes.
Moreover, knockout of the longevity gene sirt1 in zebrafish results in oxidative damage, chronic inflammation, and a shortened lifespan [45]. The expression of inflammation-related genes, such as il1b, il6, nfkb, tnfa, and inos, was increased in adult zebrafish with the sirt1 mutation, showing that this upregulation occurs through NF-kB activation in the aorta during aging. Additionally, these mutant zebrafish exhibit inflammatory cell infiltration in neighboring organs, including the pancreas, intestine, and spleen.
In addition to NF-κB, other inflammatory pathways have been described in zebrafish developmental and aging studies. For example, complement-mediated inflammation has recently been shown to be involved in zebrafish myelogenesis [46]. The small GPI-anchored protein Cd59, a cell surface protein known to suppress complement-mediated inflammation, modulates Schwann cell (SC) proliferation during development [46].
2.2. TNF-α
TNF-α is a pleiotropic inflammatory cytokine that is produced mainly by activated macrophages and neutrophils. It is involved in a broad range of cellular activities, including proliferation, survival, differentiation, and apoptosis. The TNF ligand family functions via interactions with its cognate membrane receptors, including the TNF receptor (TNF-R) family [47,48].
Two specific cell surface receptors, tumor necrosis factor receptor superfamily members 1A and 1B (TNFRSF1A and TNFRSF1B), can initiate distinct or overlapping signal transduction pathways, resulting in a large spectrum of cellular responses, including normal functions such as immune responses, hematopoiesis, morphogenesis, and cell death [49]. TNFRSF1A and TNFRSF1B, once stimulated, recruit accessory proteins via interactions with their cytoplasmic domains, including TNFR-associated factors (TRAFs), FAS-associated death domains (FADDs), and TNFR-associated death domains (TRADDs). Different signal transduction pathways activate NF-κB [50].
TNF-α, TNFRSF1, TNFRSF2, TNF-N, CD40L, FASL, and 4-1BBL have all been identified in zebrafish. Zebrafish TNF-α is required for several functions, such as larval fin and spinal cord regeneration [21,51,52], and development of the retina [53], liver [54], and blood vessels [55]. TNF-α promotes oligodendrogenesis after myelin injury in a NF-κB–dependent manner, and zebrafish mutants for myeloid differentiation factor 88 (Myd88), a key adaptor for the activation of NF-κB signaling, result in reduced generation of TNF-α in lesions [44]. TNF-α plays a key inflammatory role in Duchenne muscular dystrophy (DMD). In a well-characterized DMD zebrafish embryo model [56], 1,3-1,6 β-glucans showed a significant effect on the inflammatory state of DMD, as shown by the downregulation of the TNF-α cytokine [57].
The proinflammatory effects of zebrafish TNF-α can be mediated through the activation of endothelial cells [28], and a balance of signaling mediated by TNFRSF1A or TNFRSF1B receptors is required for endothelial cell integrity [55]. Genetic depletion of TNFRSF1B in zebrafish embryos results in the induction of apoptosis, which is rescued by simultaneous depletion of TNFRSF1A or activation of NF-κB [55].
The dual effects of TNF-α have also been described in the context of tuberculosis [12,58,59]. Excess TNF-α, via induction of reverse electron transport (RET) in mitochondrial complex I, drives the production of mitochondrial reactive oxygen species (mROS) in Mycobacterium marinum-infected macrophages, which rapidly triggers necrosis [12,59]. These findings in zebrafish larvae infected with Mycobacterium marinum have revealed the mechanisms underlying host resistance versus susceptibility to TNF-α.
A study on the toxicity of heavy metals in adult zebrafish revealed that the expression of the tnfa and il1b genes increased progressively following exposure to heavy metals [60]. It was also shown that exposure to acute combined severe stress in adult zebrafish results in persistent behavioral changes that become apparent one week later, along with elevated cortisol levels and increased expression of gene markers in the brain related to activated neuroglia (M1/M2 and A1/A2 imbalance), proinflammatory cytokines including tnfa, apoptosis, and key epigenetic enzymes [61].
2.3. IL-1β
IL-1β is a potent proinflammatory cytokine produced as an inactive precursor that is processed by caspase-1 and inflammasomes to produce mature IL-1β. Inflammasomes are multimeric complexes that generally comprise one member of a family of Nod-like pattern recognition receptors (NLR), the adapter protein Pycard, and Caspase-1, which autoactivates and cleaves IL1β in response to a variety of microbial and host-derived stimuli [62,63,64]. In particular, inflammasome activation takes place in two sequential steps: a “priming” signal, usually mediated by Toll-like receptor (TLR) induction, resulting in transcriptional activation of NFκB and synthesis of IL1β and NLRs, and a second “activation” signal, which is responsible for the assembly and activation of caspase-1 to cleave IL1β into its active form [62,63]. Once secreted, mature IL-1β binds to the Interleukin-1 receptor, type 1, or IL1R1, whose conformational change leads to the binding of the co-receptor IL-1 receptor accessory protein, or IL1RAP. The resulting trimeric complex induces a strong proinflammatory signal [65].
Inflammasome-forming proteins are present in zebrafish [66,67,68], and zebrafish Caspase a (Caspa) has the ability to cleave IL1β [69]. Moreover, utilizing a genetic zebrafish model of Il-1β-induced inflammation [70] in combination with in silico analyses, it was recently identified that functional zebrafish Il1r1 has predicted protein structures highly similar to human IL1R1 [71].
The ease of genetic manipulation in zebrafish has given rise to several transgenic models that help to dissect the role of IL-1β. Examples include heat-shock-inducible mature IL-1β [72], cell-specific (pancreatic β cells) expression of mature Il-1β [73], and recently, a doxycycline-inducible model driving expression of il1b together with other two inflammatory cytokines: tnfa and ifng1 [74]. These systems highlight IL-1β as a key factor for the recruitment of neutrophils, but not macrophages, to the injury-induced inflammatory site [72] as an inducer of islet inflammation [75]. IL-1β and TNF-α are involved in spinal cord regeneration, tissue injury, and regeneration [76,77].
In adult zebrafish, it has been demonstrated that IL-1β is involved not only in infection but also in the sterile inflammatory response [78]. The zebrafish liver exhibits an accumulation of lipids and displays abnormalities when exposed to ethanol in water, which is accompanied by an increase in il1b expression, suggesting that inflammatory signaling is a key factor in hepatic steatosis [79].
2.4. Notch
Some of the same inflammatory stimuli, such as IL-1β and TNF-α, which are responsible for the activation of inflammatory signaling, can also induce Notch signaling. The latter does not have a specific pro- or anti-inflammatory effect but can lead to the activation of inflammatory signaling. Indeed, the crosstalk between Notch and the inflammatory compartment has been reported to be key in both development and aging [80,81,82].
Notch is a transmembrane receptor, and its signaling is established through cell-cell contact. One of the four transmembrane Notch receptors (Notch1, Notch2, Notch3, and Notch4 in mice; Notch1a, Notch1b, Notch2, and Notch3 in zebrafish) in a signal-receiving cell binds to Jagged and Delta ligands in a signal-emitting cell and undergoes two cleavage events. First, the Notch receptor is cleaved by members of the ADAM TACE metalloproteases at the S2 site, followed by γ-secretase at the S3 site to release a Notch intracellular domain (NICD) that translocates to the nucleus to modulate the transcription of Notch target genes [83].
Zebrafish Notch1a and Notch1b receptors are evolutionary paralogues of mammalian Notch1 [84], and, as in mammals, zebrafish Notch signaling is involved in both organ formation and morphogenesis and in cell-cell communication [85,86]. Thus, the signals exchanged between neighboring cells through the Notch pathway can modulate differentiation, proliferation, and apoptosis, thereby influencing cell fate and tissue homeostasis [87].
Zebrafish Notch signaling was described to be involved in larval zebrafish heart regeneration [81] and in the establishment of arterial system development and arterial-venous identity [88]. The Notch pathway controls cell fate decisions during embryonic development [89,90]; activation of Notch is required for initiating endothelial cell and Hemogenic Endothelium formation in the dorsal aorta [91] and for regulating goblet cell numbers in the developing zebrafish intestine [92].
In adult aging and regeneration, the endocardium stimulates myocardial regeneration by delivering negative and positive proliferative signals through the Serpine1 and Notch pathways, respectively [93]. Some studies have used adult zebrafish as animal models to discern the role of Notch in eye regeneration. In a zebrafish model of inherited retinal dystrophy, inhibition of the Notch pathway stimulated photoreceptor regeneration in models of progressive degeneration, and immunosuppression prevented photoreceptor loss [82]. Another study reported that Notch signaling is a key pathway for Muller-Glia reprogramming in zebrafish [94]. These findings provide valuable insights into the regenerative processes that rely on Müller glia and the impact of the inflammation-Notch pathway axis on photoreceptor degeneration.
In general, there are inflammatory signals that, in the context of development, are beneficial, but in adults, they are mainly detrimental (Figure 1). Table 1 summarizes the studies reported above that were performed on larvae and adult zebrafish classified by the inflammatory pathway affected.
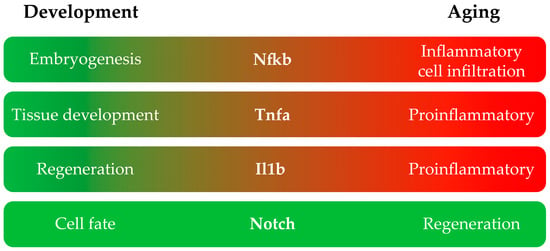
Figure 1.
Main roles of each inflammation pathway in the development and adult stages of zebrafish.

Table 1.
Inflammation pathways involved in the development and adult stages of zebrafish.
3. Inflammation as a Developmental Mechanism for HSPC Emergence
Hematopoiesis is the process that gives rise to blood cells of different lineages throughout normal life. The hematopoietic system is organized as a hierarchy of cell types that gradually lose multiple alternate potentials while committing to lineage fate. Hematopoietic stem and progenitor cells (HSPCs) can commit to either myeloid or lymphoid lineages, giving rise to innate and adaptive branches of the immune system, respectively [95,96,97,98].
The ontogeny of the hematopoietic system relies on a specific sequence of events and spatial and temporal cellular interactions throughout development, which control stem cell properties and balance self-renewal, quiescence, and lineage commitment [99,100]. As such, hematopoiesis is a complex and accurate developmental process that provides a good platform to identify the genetic requirements of specific critical stages and characterize the molecular mechanisms and cellular mediators of developmental inflammation [101,102,103].
Zebrafish have provided a superb model system to dissect a variety of inflammatory cues specifying HSPCs during the early stages of embryonic development [89,103,104,105], as summarized in Figure 2.
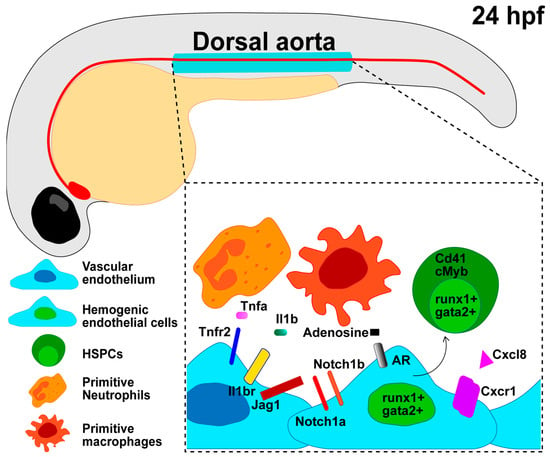
Figure 2.
Developmental inflammation regulates the endothelial cell transition into HSPCs. Zebrafish HSPCs emerge around 24 h post-fertilization (hpf) from a specialized endothelial cell termed the hemogenic endothelium (HE) in the ventral wall of the dorsal aorta. Notch, Nfkb, and other inflammatory signaling pathways can induce a hemogenic cell fate (runx1+; gata2+) in the vascular endothelium (kdrl+; fli1+) and further HSPC commitment (runx1+; gata2+; Cd41, cMyb). Sources of developmental inflammation include cytokines released by primitive macrophages or neutrophils that interact with the vascular endothelium and HSPCs, as well as glucose exposure, the release of adenosine from ATP, repetitive transcript elements, and R-loops.
3.1. Zebrafish Hematopoiesis
Zebrafish are at the forefront of vertebrate model systems for studying vertebrate developmental hematopoiesis. The high homology of cellular components, core regulators of hematopoiesis, and conserved expression and function of genes for both myeloid/lymphoid fate determination and terminal differentiation between humans and zebrafish make them a powerful model for studying the developmental process of hematopoiesis [101,106,107,108,109,110]. Moreover, defects in zebrafish hematopoiesis reliably phenocopy human blood disorders, including leukemia, making them an attractive model for defining critical regulators of these blood disorders [111,112]. Given these advantages, studying zebrafish hematopoiesis has led to fundamental knowledge and crucial new discoveries regarding different aspects of normal and pathological blood development.
HSPCs arise from the posterior lateral mesoderm (PLM) [113], emerging from specialized endothelial cells (ECs) that compose the hemogenic endothelium (HE) found within the ventral wall of the dorsal aorta (DA) (equivalent to the AGM in mammals). The process of HSPC conversion from ECs is termed the endothelial-to-hematopoietic transition (EHT) and involves the budding of HSPCs/HPCs from the aortic endothelium [114,115]. Nascent HSPCs then enter circulation to migrate from the DA to the caudal hematopoietic tissue [116,117], where they expand and differentiate into mature blood cells. The majority of HSPCs then re-enter circulation and seed their final destination in the thymus or kidney marrow (equivalent to the bone marrow in mammals).
3.2. Sources of Developmental Inflammation in HSPC Emergence and Specification
The consensus dictates that the developmental inflammation responsible for HSPC emergence and specification is sterile. In the absence of any pathogen or pathological condition, sources of inflammatory signals during development are, at least in part, hematopoietic cells derived from the earlier waves of hematopoiesis, primarily myeloid cells such as primitive macrophages or neutrophils [118,119]. These primitive myeloid cells can induce a hemogenic cell fate (runx1+; gata2+) in the vascular endothelium (kdrl+; fli1+) and are in close proximity to emerging HSPCs in the HE of zebrafish, mice, and humans [120,121,122]. Other sources of inflammatory signals in sterile setups have recently been summarized [101]. Among these, metabolic alterations due to glucose exposure are responsible for the activation of the NLRP3 inflammasome complex, which in turn elicits sterile inflammation and HSPC emergence [102]. Elevated glucose levels accelerate the induction of HSPCs from the hemogenic endothelium via a Hif1α-regulated signaling axis [123,124]. HSPC generation is also induced by the release of adenosine from ATP, and during shear stress generated by blood flow [125], zebrafish mutants of the adenosine receptor have decreased Cxcl8/Cxcr1 signaling, resulting in reduced EHT and diminished HSPC output [102].
Repetitive element transcripts and R-loops also participate in the induction of developmental inflammation [101]. Transposable elements expressed in the zebrafish hemogenic endothelium can induce NF-κB and IFN-mediated inflammatory pathways by activating retinoic acid-inducible gene 1-like receptors (RLRs). Indeed, Rig-1 and Mda5 zebrafish RLRs mutants exhibit impaired HSPC production [126]. Finally, loss of the DEAD-box helicase Ddx41 triggers an R-loop-mediated sterile inflammatory cascade, characterized by the accumulation of R loops or RNA:DNA hybrids and the induction of the cGAS-STING inflammatory pathway, leading to increased numbers of hemogenic endothelium and HSPCs [127].
3.3. Cytokines Role in HSPC Emergence
Primitive macrophages and neutrophils are the main source of proinflammatory cytokines, such as IL-1β and TNF-α, as previously described. These cytokines act mainly downstream of Notch and NF-κB. Other cytokines involved in HSPC specification and emergence include IFN-γ [128], IL6 [129], TGFβ [130], and Gcsfa/Gcsfb [131].
It has been previously established that glucose metabolism expands HSPC formation in zebrafish embryos through mitochondria-derived ROS-mediated stimulation of hypoxia-inducible factor 1α (hif1α) [123]. Recently, it was reported that metabolic alterations promote inflammasome-induced IL1β signaling to enhance HSPC production. Macrophages are the main source of this cytokine, and they promote the production of Il1rl1+ HSPCs via inflammasome action, with loss of inflammasome function inhibiting HSPC production in zebrafish embryos [102].
3.4. NF-κB Signaling in HSPC Emergence
Canonical NF-κB activation is required for HSPC specification during embryogenesis [118]; indeed, the specific impairment of nuclear NF-κB translocation in the hemogenic endothelium impairs NF-κB activation and further HSPC emergence [118,119]. Mechanistically, NF-κB regulates hemogenic endothelium-derived HSPC development signaling by promoting Notch activity [119], and primitive neutrophils produce TNF-α, which in turn stimulates NFκB-dependent expression of the Notch ligand jagged1, jag1, in tnfr2-expressing endothelial cells of DA. Jag1 binds to its receptor Notch1a on neighboring hemogenic endothelial cells lining the DA floor, which in turn activates runx1, enforcing hematopoietic cell fate [118]. Conversely, in the absence of jagged1, zebrafish fail to produce HSPCs [132] and display an established arterial fate, but have compromised HE and HSPC formation [130].
The involvement of NF-kB in EC priming towards the fate of HE has been described recently. Cheng and colleagues found that the non-inflammasome-forming NLR Nod1 programs the endothelium to become hemogenic, initiating the HE commitment, which is a prerequisite for HSPC specification. They also observed a remarkable downregulation of NF-kB activity in the DA at 22 h post-fertilization in embryos deficient for either Nod1, Ripk2, or Rac1. Further characterization of this pathway led them to identify the axis Rac1-Nod1-Ripk2 to signal through NF-kB in order to initiate HE commitment [133].
3.5. Notch Signaling in HSPC Emergence
One essential requirement for HSPC emergence in both mammals and zebrafish is signaling through the Notch pathway [83,89,134,135,136]. Here, we summarize some studies concerning the role of Notch in HSPC development. However, given the strong connection with both stimuli and targets of the inflammation compartment, we believe further studies should address the crosstalk and integration of Notch with other signaling pathways in order to provide a better understanding of the developmental signalosome in the hematopoietic niche.
In zebrafish, both Notch1a and Notch1b receptors are expressed in the DA during the window of HSPC emergence [88], and the induction of Runt-related transcription factor 1, or Runx1, one of the earliest markers of HSPCs [114,137], relies on Notch1a/b receptor-mediated signaling in the HE [83].
Runx1 is highly expressed in endothelial cells and is positive for the Notch reporter line Tp1:GFP, which expresses GFP under the control of tandem Notch responsive elements [138]. Interestingly, HE identity may be established by Notch signaling much earlier than the onset of runx1 expression [83,89,139].
The requirement for Notch-Runx1 signaling is also a key feature distinguishing HSPCs from erythro-myeloid hematopoietic progenitors (EMPs) [140,141]. HSPCs are unable to form in the absence of Notch-Runx1 signaling; in contrast, EMPs do not express the Notch receptor, and their generation is not affected under these conditions [140,141]. Moreover, induction of the Notch intracellular domain (NICD) in wild-type zebrafish embryos leads to upregulation of HSPCs without inducing ectopic arterial gene expression [110,140,142]. When the interaction of Notch ligands DeltaC and DeltaD with Jam1a and Jam2a on early vascular progenitors is missing, arterial specification is unaffected, but HE is lost [143], further proving evidence of the Notch-specific role in HSPC generation from the HE.
3.6. Demand-Driven Hematopoiesis Influences HSPC Lineage Commitment
The role of developmental inflammation in HSPC lineage commitment and blood cell maturation and differentiation is still poorly characterized. Whether baseline inflammatory signaling might favor one lineage over another is not explored. However, once in demand, that is, in response to pathogens or any external cue, inflammatory signals might change the programmed fate of HSPCs by subverting the proliferation and/or differentiation of HSPCs or progenitor cells. This is the case of emergency myelopoiesis or lymphopoiesis [144,145].
4. Zebrafish as a Model to Study Inflammaging
Life expectancy has increased over the past few decades. According to the World Health Organization, more than 10% of the world’s population is over 60 years old, and by 2050, it will nearly double. Zebrafish is a promising model in the field to study different age-related diseases due to its genetic and physiological similarities to humans [5,146,147].
It has been described that the aging process is usually accompanied by a decline in immune function, causing chronic inflammation in older organisms. Age-related inflammation is called “inflammaging” [148,149,150]. Recent studies using zebrafish have shown that microglial density is higher in aged zebrafish than in young adults [151]. In addition, 18-month-old animals reported fewer microglia and macrophages in the optic nerve at four days post-myelin damage compared to young adults [152].
4.1. Telomeres
Telomeres are hexanucleotide tandem repeats of DNA and associated proteins that form dynamic structures at the ends of chromosomes, which can maintain genomic stability and integrity. Telomeres are maintained by the telomerase ribonucleoprotein, and loss of telomerase function leads to telomere shortening and chromosomal instability, ultimately leading to aging and cancer [153,154]. It was shown that zebrafish can replicate human telomere and telomerase biology [7,155]. Zebrafish lacking telomerase are valuable models for investigating telomere-driven aging. Furthermore, it is an outstanding vertebrate model for the development of new therapies that can temporarily restore telomerase expression in individuals with diseases with partial telomerase deficiency, such as dyskeratosis congenita and aplastic anemia [156,157].
Age-associated telomere shortening affects the adaptive immune system, leading to immunosenescence [158]. Despite the decline in immune function, research has shown that older organisms often experience a persistent proinflammatory state, known as inflammaging. This condition is characterized by elevated levels of proinflammatory markers in cells and tissues as well as chronic activation of the innate immune system, which can occur even in the absence of infection or other risk stimuli [149].
Other studies using telomerase retrotranscriptase (tert)-mutant zebrafish have shown that telomere shortening increases senescence and systemic inflammation, facilitating melanoma dissemination [159]. As a result, similar to the aging process in humans, telomere shortening creates a persistent inflammatory environment, which, in turn, contributes to a higher incidence of cancer. There have been described extracurricular roles of the internal RNA template component of the telomerase complex (TR or terc), regulating the levels of the cytoquines Gcsfa/Gcsfb and the key transcription factors spi1 and gata1, which are responsible for controlling the production of myeloid and erythroid cells, respectively [160]. Additionally, telomerase RNA-based aptamers can restore defective myelopoiesis in zebrafish models of congenital neutropenic syndromes [161]. Aplastic anemia and dyskeratosis congenita patients present mutations in TR and TERT [162,163]. These models of tert and terc deficiencies in zebrafish may be useful for identifying potential therapeutic approaches for treating these diseases.
4.2. TERRA
In recent years, long fragments of non-coding RNA expressed from telomeres, called TERRA, have been identified [164,165]. These form part of the nucleoprotein complex, promoting chromosomal stability and the replication of telomeric repeats [166,167]. It has recently been reported that telomere dysfunction can lead to the activation of inflammatory signaling via secretion into the extracellular environment of TERRA within exosomes, known as cfTERRA [168].
Activation of the telomerase maintenance mechanism is an essential step in cancer progression to escape senescence and apoptosis and is routinely performed by telomerase retrotranscriptase. Alternative telomere lengthening (ALT) associated with increased TERRA expression has been observed in certain malignant tumors. The expression of tert has been shown to reverse ALT characteristics and normalize TERRA expression levels in zebrafish [169]. These findings indicate that TERRA and telomerase exhibit opposing functions in telomere maintenance through several mechanisms, as the reintroduction of tert in brain tumors promotes the formation of heterochromatin and decreases the levels of TERRA, leading to enhanced survival in fish and opening up possibilities for future treatments for ALT brain tumors.
4.3. Senescence
Another zebrafish aging model is the rag1 mutant. Recombination-activating gene 1 (RAG1) plays an essential role in adaptive immunity, orchestrating DNA rearrangements that generate an enormous variety of immunoglobulins [170]. Zebrafish have one functional rag1 gene, and its loss of function leads to a deficiency in the functional adaptive immune system [171]. Unlike mammals, rag1 mutant zebrafish do not show increased susceptibility to infection and respond more quickly to viral infections [172]. However, rag−/− zebrafish exhibit early signs of aging and a reduced lifespan [173]. It has been shown that rag1-deficient zebrafish have increased immune-related gene expression and oxidative stress due to a decline in antioxidant activity and increased oxidative cell damage. Moreover, the number of senescent cells increased, and telomere length was shorter than that in wild-type zebrafish. The use of antioxidant and senolytic drugs, which induce apoptosis in senescent cells, reduced interleukin 1b and senescence gene expression in rag−/− zebrafish.
Recently, it was reported that senolytic drugs have anti-inflammatory effects in zebrafish models of chronic skin inflammation and in a high-cholesterol diet [174,175,176,177,178,179]. Taken together, these characteristics suggest that rag mutant zebrafish offer a valuable platform for investigating and seeking treatments for senescence in vivo, as well as for the aging process induced by chronic inflammation (Figure 3).
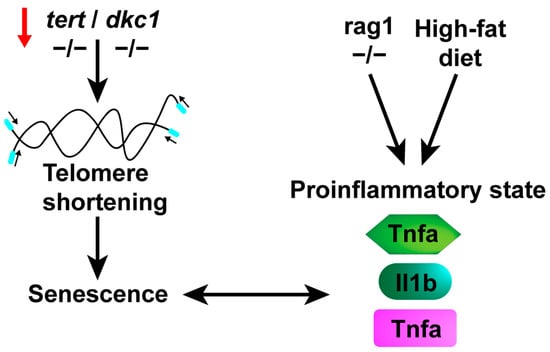
Figure 3.
A proinflammatory state is triggered by stimuli such as a high-cholesterol diet and/or the aging process. Telomere shortening, associated with aging or various diseases affecting telomerase components, leads to cellular senescence. This progression results in an elevation of proinflammatory markers, giving rise to a proinflammatory state. Additionally, congenital deficiencies or a high-cholesterol diet can also induce this state, ultimately culminating in senescence.
Despite all these advances, more research is needed to understand the complex interactions among metabolism, the immune system, and aging, and zebrafish is emerging as a key model for studies. In a broader context, these studies underscore the significance of using zebrafish to assess drug efficacy across various disease models. A summary of the different disease models developed in the zebrafish is presented in Table 2.

Table 2.
Zebrafish models of inflammatory, developmental, and aging diseases.
5. Conclusions
This review provides a comprehensive overview of the significance and utility of the zebrafish model system for investigating inflammation throughout the developmental and aging processes. Here, we review the main inflammatory pathways examined in zebrafish and summarize the available knowledge on their function in both development and aging. Specifically, we present examples illustrating how different cell entities rely on inflammatory signals to enable their specification and function during development. For example, modeling developmental inflammation in larval zebrafish has provided unique insights into the role of inflammation in the regulation of steady-state hematopoiesis. In contrast, in aging, inflammation is often a chronic condition fostering a pathological onset, called inflammaging, and could be linked to age-related diseases. These studies provide evidence that the same inflammatory signaling pathways often have instructive/beneficial roles during embryogenesis, whereas they are linked to malignancies in adults. Thus, the dichotomy of whether the same stimuli and signaling pathways are instructive or malignant depends on the developmental stage. As reviewed here, zebrafish offers a powerful toolkit to answer these questions and is a unique, reliable system for drug discovery.
Author Contributions
Conceptualization, F.J.M.-N. and M.M.; figures’ creation, F.J.M.-N. and M.M.; writing—original draft preparation, F.J.M.-N. and M.M.; writing—review and editing, F.J.M.-N., M.M., T.V.B. and M.L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study has been funded by the Instituto de Salud Carlos III (ISCIII) through the projects PI19/00188 and PI22/00861 and co-funded by the European Union (grant to MLC). F.J.M.N. was supported by the ISCIII through a Sara Borrell Fellowship. M.M. was supported by an American-Italian Cancer Foundation Post-Doctoral Research Fellowship, the Ceriale Post Doc Fellowship Award, and the Paul S. Frenette Scholar Awards Program of the Ruth L. and David S. Gottesman Institute for Stem Cell Research and Regenerative Medicine. T.V.B. was supported by funds from NIH R01DK121738, NIH R01DK131445, and the Edward P. Evans Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Xie, Y.; Meijer, A.H.; Schaaf, M.J.M. Modeling Inflammation in Zebrafish for the Development of Anti-Inflammatory Drugs. Front. Cell Dev. Biol. 2020, 8, 620984. [Google Scholar] [CrossRef] [PubMed]
- Zanandrea, R.; Bonan, C.D.; Campos, M.M. Zebrafish as a model for inflammation and drug discovery. Drug Discov. Today 2020, 25, 2201–2211. [Google Scholar] [CrossRef] [PubMed]
- Iribarne, M. Inflammation induces zebrafish regeneration. Neural Regen. Res. 2021, 16, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Kijima, Y.; Wantong, W.; Igarashi, Y.; Yoshitake, K.; Asakawa, S.; Suzuki, Y.; Watabe, S.; Kinoshita, S. Age-Associated Different Transcriptome Profiling in Zebrafish and Rats: An Insight into the Diversity of Vertebrate Aging. Mar. Biotechnol. 2022, 24, 895–910. [Google Scholar] [CrossRef]
- Janjuha, S.; Singh, S.P.; Tsakmaki, A.; Mousavy Gharavy, S.N.; Murawala, P.; Konantz, J.; Birke, S.; Hodson, D.J.; Rutter, G.A.; Bewick, G.A.; et al. Age-related islet inflammation marks the proliferative decline of pancreatic beta-cells in zebrafish. eLife 2018, 7, e32965. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.C.; de Castro, I.P.; Ferreira, M.G. Telomeres in aging and disease: Lessons from zebrafish. Dis. Models Mech. 2016, 9, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Herbomel, P.; Thisse, B.; Thisse, C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development 1999, 126, 3735–3745. [Google Scholar] [CrossRef]
- Gratacap, R.L.; Wheeler, R.T. Utilization of zebrafish for intravital study of eukaryotic pathogen-host interactions. Dev. Comp. Immunol. 2014, 46, 108–115. [Google Scholar] [CrossRef]
- Masud, S.; Torraca, V.; Meijer, A.H. Modeling Infectious Diseases in the Context of a Developing Immune System. Curr. Top. Dev. Biol. 2017, 124, 277–329. [Google Scholar] [CrossRef]
- Torraca, V.; Mostowy, S. Zebrafish Infection: From Pathogenesis to Cell Biology. Trends Cell Biol. 2018, 28, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Roca, F.J.; Ramakrishnan, L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 2013, 153, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Tyrkalska, S.D.; Candel, S.; Pedoto, A.; García-Moreno, D.; Alcaraz-Pérez, F.; Sánchez-Ferrer, Á.; Cayuela, M.L.; Mulero, V. Zebrafish models of COVID-19. FEMS Microbiol. Rev. 2023, 47, fuac042. [Google Scholar] [CrossRef] [PubMed]
- Kraus, A.; Huertas, M.; Ellis, L.; Boudinot, P.; Levraud, J.P.; Salinas, I. Intranasal delivery of SARS-CoV-2 spike protein is sufficient to cause olfactory damage, inflammation and olfactory dysfunction in zebrafish. Brain Behav. Immun. 2022, 102, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Sun, C.; Chen, Z.; Yang, D.; Zhou, Z.; Peng, X.; Tang, C. Alcohol Induces Zebrafish Skeletal Muscle Atrophy through HMGB1/TLR4/NF-κB Signaling. Life 2022, 12, 1211. [Google Scholar] [CrossRef]
- Onyenwoke, R.U.; Leung, T.; Huang, X.; Parker, D.; Shipman, J.G.; Alhadyan, S.K.; Sivaraman, V. An assessment of vaping-induced inflammation and toxicity: A feasibility study using a 2-stage zebrafish and mouse platform. Food Chem. Toxicol. 2022, 163, 112923. [Google Scholar] [CrossRef] [PubMed]
- Kasica-Jarosz, N.; Podlasz, P.; Kaleczyc, J. Pituitary adenylate cyclase-activating polypeptide (PACAP-38) plays an inhibitory role against inflammation induced by chemical damage to zebrafish hair cells. PLoS ONE 2018, 13, e0198180. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Wang, B.; Lu, G.; Liu, J.; Wu, D.; Yan, Z. Toxicity comparison of perfluorooctanoic acid (PFOA), hexafluoropropylene oxide dimer acid (HFPO-DA), and hexafluoropropylene oxide trimer acid (HFPO-TA) in zebrafish gut. Aquat. Toxicol. 2023, 262, 106655. [Google Scholar] [CrossRef]
- Naomi, R.; Bahari, H.; Yazid, M.D.; Embong, H.; Othman, F. Zebrafish as a Model System to Study the Mechanism of Cutaneous Wound Healing and Drug Discovery: Advantages and Challenges. Pharmaceuticals 2021, 14, 1058. [Google Scholar] [CrossRef]
- Ellett, F.; Pase, L.; Hayman, J.W.; Andrianopoulos, A.; Lieschke, G.J. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 2011, 117, e49–e56. [Google Scholar] [CrossRef]
- Nguyen-Chi, M.; Laplace-Builhe, B.; Travnickova, J.; Luz-Crawford, P.; Tejedor, G.; Lutfalla, G.; Kissa, K.; Jorgensen, C.; Djouad, F. TNF signaling and macrophages govern fin regeneration in zebrafish larvae. Cell Death Dis. 2017, 8, e2979. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Chi, M.; Laplace-Builhe, B.; Travnickova, J.; Luz-Crawford, P.; Tejedor, G.; Phan, Q.T.; Duroux-Richard, I.; Levraud, J.P.; Kissa, K.; Lutfalla, G.; et al. Identification of polarized macrophage subsets in zebrafish. eLife 2015, 4, e07288. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, S.; Lopez-Munoz, A.; Martinez-Navarro, F.J.; Galindo-Villegas, J.; Mulero, V.; Calado, A. Cxcl8-l1 and Cxcl8-l2 are required in the zebrafish defense against Salmonella Typhimurium. Dev. Comp. Immunol. 2015, 49, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Anderson, R.; Mirmira, R.G. A zebrafish tailfin injury assay protocol for quantifying immune cell migration and infiltration. STAR Protoc. 2022, 3, 101196. [Google Scholar] [CrossRef] [PubMed]
- Michael, C.; de Oliveira, S. Exploring the dynamic behavior of leukocytes with zebrafish. Curr. Opin. Cell Biol. 2023, 85, 102276. [Google Scholar] [CrossRef] [PubMed]
- Boehm, T.; Iwanami, N.; Hess, I. Evolution of the immune system in the lower vertebrates. Annu. Rev. Genom. Hum. Genet. 2012, 13, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Boehm, T. Evolution of vertebrate immunity. Curr. Biol. 2012, 22, R722–R732. [Google Scholar] [CrossRef]
- Roca, F.J.; Mulero, I.; López-Muñoz, A.; Sepulcre, M.P.; Renshaw, S.A.; Meseguer, J.; Mulero, V. Evolution of the inflammatory response in vertebrates: Fish TNF-alpha is a powerful activator of endothelial cells but hardly activates phagocytes. J. Immunol. 2008, 181, 5071–5081. [Google Scholar] [CrossRef]
- Zou, J.; Secombes, C.J. The Function of Fish Cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef]
- Langenau, D.M.; Ferrando, A.A.; Traver, D.; Kutok, J.L.; Hezel, J.P.; Kanki, J.P.; Zon, L.I.; Look, A.T.; Trede, N.S. In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc. Natl. Acad. Sci. USA 2004, 101, 7369–7374. [Google Scholar] [CrossRef]
- Davidson, A.J.; Zon, L.I. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene 2004, 23, 7233–7246. [Google Scholar] [CrossRef]
- Lam, S.H.; Chua, H.L.; Gong, Z.; Lam, T.J.; Sin, Y.M. Development and maturation of the immune system in zebrafish, Danio rerio: A gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 2004, 28, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Sutterwala, F.S.; Haasken, S.; Cassel, S.L. Mechanism of NLRP3 inflammasome activation. Ann. N. Y Acad. Sci. 2014, 1319, 82–95. [Google Scholar] [CrossRef]
- Visekruna, A.; Volkov, A.; Steinhoff, U. A Key Role for NF-κB Transcription Factor c-Rel in T-Lymphocyte-Differentiation and Effector Functions. Clin. Dev. Immunol. 2012, 2012, 239368. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared Principles in NF-κB Signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Espín-Palazón, R.; Traver, D. The NF-κB family: Key players during embryonic development and HSC emergence. Exp. Hematol. 2016, 44, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Beg, A.A.; Sha, W.C.; Bronson, R.T.; Ghosh, S.; Baltimore, D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 1995, 376, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Beg, A.A.; Sha, W.C.; Bronson, R.T.; Baltimore, D. Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev. 1995, 9, 2736–2746. [Google Scholar] [CrossRef]
- Takeda, K.; Takeuchi, O.; Tsujimura, T.; Itami, S.; Adachi, O.; Kawai, T.; Sanjo, H.; Yoshikawa, K.; Terada, N.; Akira, S. Limb and skin abnormalities in mice lacking IKKα. Science 1999, 284, 313–316. [Google Scholar] [CrossRef]
- Hu, Y.; Baud, V.; Delhase, M.; Zhang, P.; Deerinck, T.; Ellisman, M.; Johnson, R.; Karin, M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science 1999, 284, 316–320. [Google Scholar] [CrossRef]
- Correa, R.G.; Tergaonkar, V.; Ng, J.K.; Dubova, I.; Izpisua-Belmonte, J.C.; Verma, I.M. Characterization of NF-κΒ/IκΒ proteins in zebra fish and their involvement in notochord development. Mol. Cell. Biol. 2004, 24, 5257–5268. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.; Liao, Q.; Zhang, D.; Rong, F.; Cai, X.; Fan, S.; Zhu, J.; Wang, J.; Liu, X.; Liu, X.; et al. Zebrafish NF-κB/p65 Is Required for Antiviral Responses. J. Immunol. 2020, 204, 3019–3029. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.I.; Su, M.; Cantuti-Castelvetri, L.; Müller, S.A.; Schifferer, M.; Djannatian, M.; Alexopoulos, I.; van der Meer, F.; Winkler, A.; van Ham, T.J.; et al. Pro-inflammatory activation following demyelination is required for myelin clearance and oligodendrogenesis. J. Exp. Med. 2020, 217, e20191390. [Google Scholar] [CrossRef]
- Kim, D.H.; Jung, I.H.; Kim, D.H.; Park, S.W. Knockout of longevity gene Sirt1 in zebrafish leads to oxidative injury, chronic inflammation, and reduced life span. PLoS ONE 2019, 14, e0220581. [Google Scholar] [CrossRef]
- Wiltbank, A.T.; Steinson, E.R.; Criswell, S.J.; Piller, M.; Kucenas, S. Cd59 and inflammation regulate Schwann cell development. eLife 2022, 11, e76640. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chang, L.; Huang, A.; Liu, X.; Liu, X.; Zhou, H.; Liang, J.G.; Liang, P. Functional Detection of TNF Receptor Family Members by Affinity-Labeled Ligands. Sci. Rep. 2017, 7, 6944. [Google Scholar] [CrossRef]
- Su, Z.; Wu, Y. A computational model for understanding the oligomerization mechanisms of TNF receptor superfamily. Comput. Struct. Biotechnol. J. 2020, 18, 258–270. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef]
- MacEwan, D.J. TNF receptor subtype signalling: Differences and cellular consequences. Cell. Signal. 2002, 14, 477–492. [Google Scholar] [CrossRef]
- Tsarouchas, T.M.; Wehner, D.; Cavone, L.; Munir, T.; Keatinge, M.; Lambertus, M.; Underhill, A.; Barrett, T.; Kassapis, E.; Ogryzko, N.; et al. Dynamic control of proinflammatory cytokines Il-1β and Tnf-α by macrophages in zebrafish spinal cord regeneration. Nat. Commun. 2018, 9, 4670. [Google Scholar] [CrossRef] [PubMed]
- Bohaud, C.; Johansen, M.D.; Varga, B.; Contreras-Lopez, R.; Barthelaix, A.; Hamela, C.; Sapède, D.; Cloitre, T.; Gergely, C.; Jorgensen, C.; et al. Exploring Macrophage-Dependent Wound Regeneration during Mycobacterial Infection in Zebrafish. Front. Immunol. 2022, 13, 838425. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.D.; Sun, Y.; Cai, S.J.; Fang, Y.W.; Cui, J.L.; Li, Y.H. Role of tumor necrosis factor-alpha in zebrafish retinal neurogenesis and myelination. Int. J. Ophthalmol. 2016, 9, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Song, J.; Yang, H.; Gao, W.; Liu, N.A.; Zhang, B.; Lin, S. Mmp23b promotes liver development and hepatocyte proliferation through the tumor necrosis factor pathway in zebrafish. Hepatology 2010, 52, 2158–2166. [Google Scholar] [CrossRef] [PubMed]
- Espín, R.; Roca, F.J.; Candel, S.; Sepulcre, M.P.; González-Rosa, J.M.; Alcaraz-Pérez, F.; Meseguer, J.; Cayuela, M.L.; Mercader, N.; Mulero, V. TNF receptors regulate vascular homeostasis in zebrafish through a caspase-8, caspase-2 and P53 apoptotic program that bypasses caspase-3. Dis. Models Mech. 2013, 6, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.I.; Bryson-Richardson, R.J.; Daggett, D.F.; Gautier, P.; Keenan, D.G.; Currie, P.D. Dystrophin is required for the formation of stable muscle attachments in the zebrafish embryo. Development 2003, 130, 5851–5860. [Google Scholar] [CrossRef] [PubMed]
- Brogi, L.; Marchese, M.; Cellerino, A.; Licitra, R.; Naef, V.; Mero, S.; Bibbiani, C.; Fronte, B. β-Glucans as Dietary Supplement to Improve Locomotion and Mitochondrial Respiration in a Model of Duchenne Muscular Dystrophy. Nutrients 2021, 13, 1619. [Google Scholar] [CrossRef]
- Tobin, D.M.; Roca, F.J.; Oh, S.F.; McFarland, R.; Vickery, T.W.; Ray, J.P.; Ko, D.C.; Zou, Y.; Bang, N.D.; Chau, T.T.; et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell 2012, 148, 434–446. [Google Scholar] [CrossRef]
- Roca, F.J.; Whitworth, L.J.; Prag, H.A.; Murphy, M.P.; Ramakrishnan, L. Tumor necrosis factor induces pathogenic mitochondrial ROS in tuberculosis through reverse electron transport. Science 2022, 376, eabh2841. [Google Scholar] [CrossRef]
- Yin, J.; Wang, A.P.; Li, W.F.; Shi, R.; Jin, H.T.; Wei, J.F. Time-response characteristic and potential biomarker identification of heavy metal induced toxicity in zebrafish. Fish. Shellfish. Immunol. 2018, 72, 309–317. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Wang, D.; Hu, G.; Liu, Z.; Yan, D.; Serikuly, N.; Alpyshov, E.T.; Demin, K.A.; Strekalova, T.; et al. Delayed behavioral and genomic responses to acute combined stress in zebrafish, potentially relevant to PTSD and other stress-related disorders: Focus on neuroglia, neuroinflammation, apoptosis and epigenetic modulation. Behav. Brain Res. 2020, 389, 112644. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.-K.; Kim, J.K.; Shin, D.-M.; Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2016, 13, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Masumoto, J.; Zhou, W.; Chen, F.F.; Su, F.; Kuwada, J.Y.; Hidaka, E.; Katsuyama, T.; Sagara, J.; Taniguchi, S.; Ngo-Hazelett, P.; et al. Caspy, a zebrafish caspase, activated by ASC oligomerization is required for pharyngeal arch development. J. Biol. Chem. 2003, 278, 4268–4276. [Google Scholar] [CrossRef]
- Kuri, P.; Schieber, N.L.; Thumberger, T.; Wittbrodt, J.; Schwab, Y.; Leptin, M. Dynamics of in vivo ASC speck formation. J. Cell Biol. 2017, 216, 2891–2909. [Google Scholar] [CrossRef]
- Li, J.Y.; Gao, K.; Shao, T.; Fan, D.D.; Hu, C.B.; Sun, C.C.; Dong, W.R.; Lin, A.F.; Xiang, L.X.; Shao, J.Z. Characterization of an NLRP1 Inflammasome from Zebrafish Reveals a Unique Sequential Activation Mechanism Underlying Inflammatory Caspases in Ancient Vertebrates. J. Immunol. 2018, 201, 1946–1966. [Google Scholar] [CrossRef]
- Vojtech, L.N.; Scharping, N.; Woodson, J.C.; Hansen, J.D. Roles of inflammatory caspases during processing of zebrafish interleukin-1beta in Francisella noatunensis infection. Infect. Immun. 2012, 80, 2878–2885. [Google Scholar] [CrossRef]
- Lanham, K.A.; Nedden, M.L.; Wise, V.E.; Taylor, M.R. Genetically inducible and reversible zebrafish model of systemic inflammation. Biol. Open 2022, 11, bio058559. [Google Scholar] [CrossRef] [PubMed]
- Sebo, D.J.; Fetsko, A.R.; Phipps, K.K.; Taylor, M.R. Functional identification of the zebrafish Interleukin-1 receptor in an embryonic model of Il-1β-induced systemic inflammation. Front. Immunol. 2022, 13, 1039161. [Google Scholar] [CrossRef]
- Yan, B.; Han, P.; Pan, L.; Lu, W.; Xiong, J.; Zhang, M.; Zhang, W.; Li, L.; Wen, Z. IL-1β and reactive oxygen species differentially regulate neutrophil directional migration and Basal random motility in a zebrafish injury-induced inflammation model. J. Immunol. 2014, 192, 5998–6008. [Google Scholar] [CrossRef]
- Delgadillo-Silva, L.F.; Tsakmaki, A.; Akhtar, N.; Franklin, Z.J.; Konantz, J.; Bewick, G.A.; Ninov, N. Modelling pancreatic β-cell inflammation in zebrafish identifies the natural product wedelolactone for human islet protection. Dis. Models Mech. 2019, 12, dmm036004. [Google Scholar] [CrossRef]
- Ibrahim, S.; Harris-Kawano, A.; Haider, I.; Mirmira, R.G.; Sims, E.K.; Anderson, R.M. A novel Cre-enabled tetracycline-inducible transgenic system for tissue-specific cytokine expression in the zebrafish: CETI-PIC3. Dis. Models Mech. 2020, 13, dmm042556. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Maddison, L.A.; Zaborska, K.E.; Dai, C.; Yin, L.; Tang, Z.; Zang, L.; Jacobson, D.A.; Powers, A.C.; Chen, W. RIPK3-mediated inflammation is a conserved β cell response to ER stress. Sci. Adv. 2020, 6, eabd7272. [Google Scholar] [CrossRef] [PubMed]
- Ogryzko, N.V.; Hoggett, E.E.; Solaymani-Kohal, S.; Tazzyman, S.; Chico, T.J.; Renshaw, S.A.; Wilson, H.L. Zebrafish tissue injury causes upregulation of interleukin-1 and caspase-dependent amplification of the inflammatory response. Dis. Models Mech. 2014, 7, 259–264. [Google Scholar] [CrossRef]
- Hasegawa, T.; Hall, C.J.; Crosier, P.S.; Abe, G.; Kawakami, K.; Kudo, A.; Kawakami, A. Transient inflammatory response mediated by interleukin-1β is required for proper regeneration in zebrafish fin fold. eLife 2017, 6, e22716. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Harper, S.L.; Yun, S.I. In Vivo toxicological assessment of biologically synthesized silver nanoparticles in adult Zebrafish (Danio rerio). J. Hazard. Mater. 2016, 301, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.C.; Gregorio, C.; Uribe-Cruz, C.; Guizzo, R.; Malysz, T.; Faccioni-Heuser, M.C.; Longo, L.; da Silveira, T.R. Chronic exposure to ethanol causes steatosis and inflammation in zebrafish liver. World J. Hepatol. 2017, 9, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Kemble, S.; Croft, A.P. Critical Role of Synovial Tissue-Resident Macrophage and Fibroblast Subsets in the Persistence of Joint Inflammation. Front. Immunol. 2021, 12, 715894. [Google Scholar] [CrossRef]
- Bruton, F.A.; Kaveh, A.; Ross-Stewart, K.M.; Matrone, G.; Oremek, M.E.M.; Solomonidis, E.G.; Tucker, C.S.; Mullins, J.J.; Lucas, C.D.; Brittan, M.; et al. Macrophages trigger cardiomyocyte proliferation by increasing epicardial vegfaa expression during larval zebrafish heart regeneration. Dev. Cell 2022, 57, 1512–1528.e1515. [Google Scholar] [CrossRef]
- Fogerty, J.; Song, P.; Boyd, P.; Grabinski, S.E.; Hoang, T.; Reich, A.; Cianciolo, L.T.; Blackshaw, S.; Mumm, J.S.; Hyde, D.R.; et al. Notch Inhibition Promotes Regeneration and Immunosuppression Supports Cone Survival in a Zebrafish Model of Inherited Retinal Dystrophy. J. Neurosci. 2022, 42, 5144–5158. [Google Scholar] [CrossRef]
- Kim, A.D.; Melick, C.H.; Clements, W.K.; Stachura, D.L.; Distel, M.; Panáková, D.; MacRae, C.; Mork, L.A.; Crump, J.G.; Traver, D. Discrete Notch signaling requirements in the specification of hematopoietic stem cells. EMBO J. 2014, 33, 2363–2373. [Google Scholar] [CrossRef]
- Kortschak, D.R.; Tamme, R.; Lardelli, M. Evolutionary analysis of vertebrate Notch Genes Dev. Genes Evol. 2001, 211, 350–354. [Google Scholar] [CrossRef]
- Lai, E.C. Notch signaling: Control of cell communication and cell fate. Development 2004, 131, 965–973. [Google Scholar] [CrossRef]
- Sharma, P.; Saraswathy, V.M.; Xiang, L.; Furthauer, M. Notch-mediated inhibition of neurogenesis is required for zebrafish spinal cord morphogenesis. Sci. Rep. 2019, 9, 9958. [Google Scholar] [CrossRef] [PubMed]
- Fazio, C.; Ricciardiello, L. Inflammation and Notch signaling: A crosstalk with opposite effects on tumorigenesis. Cell Death Dis. 2016, 7, e2515. [Google Scholar] [CrossRef] [PubMed]
- Quillien, A.; Moore, J.C.; Shin, M.; Siekmann, A.F.; Smith, T.; Pan, L.; Moens, C.B.; Parsons, M.J.; Lawson, N.D. Distinct Notch signaling outputs pattern the developing arterial system. Development 2014, 141, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Butko, E.; Pouget, C.; Traver, D. Complex regulation of HSC emergence by the Notch signaling pathway. Dev. Biol. 2016, 409, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Thambyrajah, R.; Bigas, A. Notch Signaling in HSC Emergence: When, Why and How. Cells 2022, 11, 358. [Google Scholar] [CrossRef]
- Lee, C.Y.; Vogeli, K.M.; Kim, S.H.; Chong, S.W.; Jiang, Y.J.; Stainier, D.Y.; Jin, S.W. Notch signaling functions as a cell-fate switch between the endothelial and hematopoietic lineages. Curr. Biol. 2009, 19, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Morales, R.A.; Rabahi, S.; Diaz, O.E.; Salloum, Y.; Kern, B.C.; Westling, M.; Luo, X.; Parigi, S.M.; Monasterio, G.; Das, S.; et al. Interleukin-10 regulates goblet cell numbers through Notch signaling in the developing zebrafish intestine. Mucosal Immunol. 2022, 15, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Münch, J.; Grivas, D.; González-Rajal, Á.; Torregrosa-Carrión, R.; de la Pompa, J.L. Notch signalling restricts inflammation and serpine1 expression in the dynamic endocardium of the regenerating zebrafish heart. Development 2017, 144, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Luodan, A.; Huang, X.; Chen, X.; Xu, H. Muller Glia-Mediated Retinal Regeneration. Mol. Neurobiol. 2021, 58, 2342–2361. [Google Scholar] [CrossRef] [PubMed]
- Chapple, R.H.; Tseng, Y.J.; Hu, T.; Kitano, A.; Takeichi, M.; Hoegenauer, K.A.; Nakada, D. Lineage tracing of murine adult hematopoietic stem cells reveals active contribution to steady-state hematopoiesis. Blood Adv. 2018, 2, 1220–1228. [Google Scholar] [CrossRef]
- Hofer, T.; Busch, K.; Klapproth, K.; Rodewald, H.R. Fate Mapping and Quantitation of Hematopoiesis In Vivo. Annu. Rev. Immunol. 2016, 34, 449–478. [Google Scholar] [CrossRef]
- Kondo, M. Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. Immunol. Rev. 2010, 238, 37–46. [Google Scholar] [CrossRef]
- Lai, A.Y.; Kondo, M. Asymmetrical lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. J. Exp. Med. 2006, 203, 1867–1873. [Google Scholar] [CrossRef]
- Cumano, A.; Godin, I. Ontogeny of the hematopoietic system. Annu. Rev. Immunol. 2007, 25, 745–785. [Google Scholar] [CrossRef]
- Orkin, S.H.; Zon, L.I. Hematopoiesis: An evolving paradigm for stem cell biology. Cell 2008, 132, 631–644. [Google Scholar] [CrossRef]
- Ketharnathan, S.; Rajan, V.; Prykhozhij, S.V.; Berman, J.N. Zebrafish models of inflammation in hematopoietic development and disease. Front. Cell Dev. Biol. 2022, 10, 955658. [Google Scholar] [CrossRef] [PubMed]
- Frame, J.M.; Kubaczka, C.; Long, T.L.; Esain, V.; Soto, R.A.; Hachimi, M.; Jing, R.; Shwartz, A.; Goessling, W.; Daley, G.Q.; et al. Metabolic Regulation of Inflammasome Activity Controls Embryonic Hematopoietic Stem and Progenitor Cell Production. Dev. Cell 2020, 55, 133–149.e136. [Google Scholar] [CrossRef] [PubMed]
- Espin-Palazon, R.; Weijts, B.; Mulero, V.; Traver, D. Proinflammatory Signals as Fuel for the Fire of Hematopoietic Stem Cell Emergence. Trends Cell Biol. 2018, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Crosier, P.; Crosier, K. Inflammatory cytokines provide both infection-responsive and developmental signals for blood development: Lessons from the zebrafish. Mol. Immunol. 2016, 69, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Nik, S.; Weinreb, J.T.; Bowman, T.V. Developmental HSC Microenvironments: Lessons from Zebrafish. Adv. Exp. Med. Biol. 2017, 1041, 33–53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Traver, D.; Herbomel, P.; Patton, E.E.; Murphey, R.D.; Yoder, J.A.; Litman, G.W.; Catic, A.; Amemiya, C.T.; Zon, L.I.; Trede, N.S. The zebrafish as a model organism to study development of the immune system. Adv. Immunol. 2003, 81, 253–330. [Google Scholar]
- Paik, E.J.; Zon, L.I. Hematopoietic development in the zebrafish. Int. J. Dev. Biol. 2010, 54, 1127–1137. [Google Scholar] [CrossRef]
- Jing, L.; Zon, L.I. Zebrafish as a model for normal and malignant hematopoiesis. Dis. Models Mech. 2011, 4, 433–438. [Google Scholar] [CrossRef]
- Jagannathan-Bogdan, M.; Zon, L.I. Hematopoiesis. Development 2013, 140, 2463–2467. [Google Scholar] [CrossRef]
- Gore, A.V.; Pillay, L.M.; Venero Galanternik, M.; Weinstein, B.M. The zebrafish: A fintastic model for hematopoietic development and disease. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e312. [Google Scholar] [CrossRef]
- Potts, K.S.; Bowman, T.V. Modeling Myeloid Malignancies Using Zebrafish. Front. Oncol. 2017, 7, 297. [Google Scholar] [CrossRef]
- Berman, J.; Payne, E.; Hall, C. The Zebrafish as a Tool to Study Hematopoiesis, Human Blood Diseases, and Immune Function. Adv. Hematol. 2012, 2012, 425345. [Google Scholar] [CrossRef][Green Version]
- Ho, R.K.; Kimmel, C.B. Commitment of Cell Fate in the Early Zebrafish Embryo. Science 1993, 261, 109–111. [Google Scholar] [CrossRef]
- Kissa, K.; Herbomel, P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 2010, 464, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, J.Y.; Chi, N.C.; Santoso, B.; Teng, S.; Stainier, D.Y.; Traver, D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 2010, 464, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Murayama, E.; Kissa, K.; Zapata, A.; Mordelet, E.; Briolat, V.; Lin, H.F.; Handin, R.I.; Herbomel, P. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity 2006, 25, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Kissa, K.; Murayama, E.; Zapata, A.; Cortés, A.; Perret, E.; Machu, C.; Herbomel, P. Live imaging of emerging hematopoietic stem cells and early thymus colonization. Blood 2008, 111, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Espín-Palazón, R.; Stachura, D.L.; Campbell, C.A.; García-Moreno, D.; Del Cid, N.; Kim, A.D.; Candel, S.; Meseguer, J.; Mulero, V.; Traver, D. Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell 2014, 159, 1070–1085. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhang, C.; Wang, L.; Zhang, P.; Ma, D.; Lv, J.; Liu, F. Inflammatory signaling regulates hematopoietic stem and progenitor cell emergence in vertebrates. Blood 2015, 125, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Travnickova, J.; Tran Chau, V.; Julien, E.; Mateos-Langerak, J.; Gonzalez, C.; Lelièvre, E.; Lutfalla, G.; Tavian, M.; Kissa, K. Primitive macrophages control HSPC mobilization and definitive haematopoiesis. Nat. Commun. 2015, 6, 6227. [Google Scholar] [CrossRef]
- Yuan, H.; Gao, S.; Chen, H.; Liu, X.; Zhou, J.; de The, H.; Zhu, J. Primitive macrophages are dispensable for HSPC mobilization and definitive hematopoiesis. Blood 2019, 134, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Wattrus, S.J.; Smith, M.L.; Rodrigues, C.P.; Hagedorn, E.J.; Kim, J.W.; Budnik, B.; Zon, L.I. Quality assurance of hematopoietic stem cells by macrophages determines stem cell clonality. Science 2022, 377, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M.; Esain, V.; Frechette, G.M.; Harris, L.J.; Cox, A.G.; Cortes, M.; Garnaas, M.K.; Carroll, K.J.; Cutting, C.C.; Khan, T.; et al. Glucose metabolism impacts the spatiotemporal onset and magnitude of HSC induction in vivo. Blood 2013, 121, 2483–2493. [Google Scholar] [CrossRef]
- Lim, S.-E.; Esain, V.; Kwan, W.; Theodore, L.N.; Cortes, M.; Frost, I.M.; Liu, S.Y.; North, T.E. HIF1α-induced PDGFRβ signaling promotes developmental HSC production via IL-6 activation. Exp. Hematol. 2017, 46, 83–95.e86. [Google Scholar] [CrossRef]
- Yegutkin, G.; Bodin, P.; Burnstock, G. Effect of shear stress on the release of soluble ecto-enzymes ATPase and 5′-nucleotidase along with endogenous ATP from vascular endothelial cells. Br. J. Pharmacol. 2000, 129, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Lefkopoulos, S.; Polyzou, A.; Derecka, M.; Bergo, V.; Clapes, T.; Cauchy, P.; Jerez-Longres, C.; Onishi-Seebacher, M.; Yin, N.; Martagon-Calderón, N.A.; et al. Repetitive Elements Trigger RIG-I-like Receptor Signaling that Regulates the Emergence of Hematopoietic Stem and Progenitor Cells. Immunity 2020, 53, 934–951.e939. [Google Scholar] [CrossRef]
- Weinreb, J.T.; Ghazale, N.; Pradhan, K.; Gupta, V.; Potts, K.S.; Tricomi, B.; Daniels, N.J.; Padgett, R.A.; De Oliveira, S.; Verma, A.; et al. Excessive R-loops trigger an inflammatory cascade leading to increased HSPC production. Dev. Cell 2021, 56, 627–640.e625. [Google Scholar] [CrossRef]
- Sawamiphak, S.; Kontarakis, Z.; Stainier, D.Y. Interferon gamma signaling positively regulates hematopoietic stem cell emergence. Dev. Cell 2014, 31, 640–653. [Google Scholar] [CrossRef]
- Tie, R.; Li, H.; Cai, S.; Liang, Z.; Shan, W.; Wang, B.; Tan, Y.; Zheng, W.; Huang, H. Interleukin-6 signaling regulates hematopoietic stem cell emergence. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Monteiro, R.; Pinheiro, P.; Joseph, N.; Peterkin, T.; Koth, J.; Repapi, E.; Bonkhofer, F.; Kirmizitas, A.; Patient, R. Transforming Growth Factor β Drives Hemogenic Endothelium Programming and the Transition to Hematopoietic Stem Cells. Dev. Cell 2016, 38, 358–370. [Google Scholar] [CrossRef]
- Stachura, D.L.; Svoboda, O.; Campbell, C.A.; Espin-Palazon, R.; Lau, R.P.; Zon, L.I.; Bartunek, P.; Traver, D. The zebrafish granulocyte colony-stimulating factors (Gcsfs): 2 paralogous cytokines and their roles in hematopoietic development and maintenance. Blood 2013, 122, 3918–3928. [Google Scholar] [CrossRef]
- Robert-Moreno, A.; Guiu, J.; Ruiz-Herguido, C.; López, M.E.; Inglés-Esteve, J.; Riera, L.; Tipping, A.; Enver, T.; Dzierzak, E.; Gridley, T.; et al. Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. EMBO J. 2008, 27, 1886–1895. [Google Scholar] [CrossRef]
- Cheng, X.; Barakat, R.; Pavani, G.; Usha, M.K.; Calderon, R.; Snella, E.; Gorden, A.; Zhang, Y.; Gadue, P.; French, D.L.; et al. Nod1-dependent NF-kB activation initiates hematopoietic stem cell specification in response to small Rho GTPases. Nat. Commun. 2023, 14, 7668. [Google Scholar] [CrossRef]
- Gering, M.; Patient, R. Notch signalling and haematopoietic stem cell formation during embryogenesis. J. Cell. Physiol. 2010, 222, 11–16. [Google Scholar] [CrossRef]
- Bigas, A.; Guiu, J.; Gama-Norton, L. Notch and Wnt signaling in the emergence of hematopoietic stem cells. Blood Cells Mol. Dis. 2013, 51, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Lomelí, H.; Castillo-Castellanos, F. Notch signaling and the emergence of hematopoietic stem cells. Dev. Dyn. 2020, 249, 1302–1317. [Google Scholar] [CrossRef]
- Sood, R.; English, M.A.; Belele, C.L.; Jin, H.; Bishop, K.; Haskins, R.; McKinney, M.C.; Chahal, J.; Weinstein, B.M.; Wen, Z.; et al. Development of multilineage adult hematopoiesis in the zebrafish with a runx1 truncation mutation. Blood 2010, 115, 2806–2809. [Google Scholar] [CrossRef]
- Parsons, M.J.; Pisharath, H.; Yusuff, S.; Moore, J.C.; Siekmann, A.F.; Lawson, N.; Leach, S.D. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech. Dev. 2009, 126, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Perlin, J.R.; Robertson, A.L.; Zon, L.I. Efforts to enhance blood stem cell engraftment: Recent insights from zebrafish hematopoiesis. J. Exp. Med. 2017, 214, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.E.; Traver, D.; Mayhall, E.; Shepard, J.L.; Zon, L.I. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005, 19, 2331–2342. [Google Scholar] [CrossRef]
- Bertrand, J.Y.; Cisson, J.L.; Stachura, D.L.; Traver, D. Notch signaling distinguishes 2 waves of definitive hematopoiesis in the zebrafish embryo. Blood 2010, 115, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.I.; Price, E.N.; Boatman, S.; Hagedorn, E.J.; Trompouki, E.; Satishchandran, S.; Carspecken, C.W.; Uong, A.; DiBiase, A.; Yang, S.; et al. Angiopoietin-like proteins stimulate HSPC development through interaction with notch receptor signaling. eLife 2015, 4, e05544. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, I.; Kobayashi-Sun, J.; Kim, A.D.; Pouget, C.; Fujita, N.; Suda, T.; Traver, D. Jam1a-Jam2a interactions regulate haematopoietic stem cell fate through Notch signalling. Nature 2014, 512, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, B.H.; Scott, M.L.; Cherry, S.R.; Bronson, R.T.; Baltimore, D. Failure of lymphopoiesis after adoptive transfer of NF-kappaB-deficient fetal liver cells. Immunity 1997, 6, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, C.; Zhang, Y.; Lin, C.; Zhang, Y.; Shu, L.; Luo, L.; Zhuo, J.; Li, L. Macrophage-Derived IL-1β Regulates Emergency Myelopoiesis via the NF-κB and C/ebpβ in Zebrafish. J. Immunol. 2020, 205, 2694–2706. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, M.L.; Claes, K.B.M.; Ferreira, M.G.; Henriques, C.M.; van Eeden, F.; Varga, M.; Vierstraete, J.; Mione, M.C. The Zebrafish as an Emerging Model to Study DNA Damage in Aging, Cancer and Other Diseases. Front. Cell Dev. Biol. 2018, 6, 178. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Fu, Y.; Ma, J.; Li, X.; Lu, C.; Zhang, R. Functional alterations and transcriptomic changes during zebrafish cardiac aging. Biogerontology 2020, 21, 637–652. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2023, 64, 109–122. [Google Scholar] [CrossRef]
- Van Houcke, J.; Bollaerts, I.; Geeraerts, E.; Davis, B.; Beckers, A.; Van Hove, I.; Lemmens, K.; De Groef, L.; Moons, L. Successful optic nerve regeneration in the senescent zebrafish despite age-related decline of cell intrinsic and extrinsic response processes. Neurobiol. Aging 2017, 60, 1–10. [Google Scholar] [CrossRef]
- Munzel, E.J.; Becker, C.G.; Becker, T.; Williams, A. Zebrafish regenerate full thickness optic nerve myelin after demyelination, but this fails with increasing age. Acta Neuropathol. Commun. 2014, 2, 77. [Google Scholar] [CrossRef]
- Blasco, M.A.; Lee, H.W.; Hande, M.P.; Samper, E.; Lansdorp, P.M.; DePinho, R.A.; Greider, C.W. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 1997, 91, 25–34. [Google Scholar] [CrossRef]
- Hayashi, M.T. Telomere biology in aging and cancer: Early history and perspectives. Genes Genet. Syst. 2018, 92, 107–118. [Google Scholar] [CrossRef]
- Anchelin, M.; Alcaraz-Perez, F.; Martinez, C.M.; Bernabe-Garcia, M.; Mulero, V.; Cayuela, M.L. Premature aging in telomerase-deficient zebrafish. Dis. Models Mech. 2013, 6, 1101–1112. [Google Scholar] [CrossRef]
- Blasco, M.A. Telomeres and human disease: Ageing, cancer and beyond. Nat. Rev. Genet. 2005, 6, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Calado, R.T.; Ly, H.; Kajigaya, S.; Baerlocher, G.M.; Chanock, S.J.; Lansdorp, P.M.; Young, N.S. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N. Engl. J. Med. 2005, 352, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G. Hallmarks of human “immunosenescence”: Adaptation or dysregulation? Immun. Ageing 2012, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Lex, K.; Maia Gil, M.; Lopes-Bastos, B.; Figueira, M.; Marzullo, M.; Giannetti, K.; Carvalho, T.; Ferreira, M.G. Telomere shortening produces an inflammatory environment that increases tumor incidence in zebrafish. Proc. Natl. Acad. Sci. USA 2020, 117, 15066–15074. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz-Perez, F.; Garcia-Castillo, J.; Garcia-Moreno, D.; Lopez-Munoz, A.; Anchelin, M.; Angosto, D.; Zon, L.I.; Mulero, V.; Cayuela, M.L. A non-canonical function of telomerase RNA in the regulation of developmental myelopoiesis in zebrafish. Nat. Commun. 2014, 5, 3228. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Balsalobre, E.; Garcia-Castillo, J.; Garcia-Moreno, D.; Naranjo-Sanchez, E.; Fernandez-Lajarin, M.; Blasco, M.A.; Alcaraz-Perez, F.; Mulero, V.; Cayuela, M.L. Telomerase RNA-based aptamers restore defective myelopoiesis in congenital neutropenic syndromes. Nat. Commun. 2023, 14, 5912. [Google Scholar] [CrossRef]
- Kirwan, M.; Dokal, I. Dyskeratosis congenita, stem cells and telomeres. Biochim. Biophys. Acta 2009, 1792, 371–379. [Google Scholar] [CrossRef]
- Trahan, C.; Dragon, F. Dyskeratosis congenita mutations in the H/ACA domain of human telomerase RNA affect its assembly into a pre-RNP. RNA 2009, 15, 235–243. [Google Scholar] [CrossRef]
- Schoeftner, S.; Blasco, M.A. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat. Cell Biol. 2008, 10, 228–236. [Google Scholar] [CrossRef]
- Azzalin, C.M.; Reichenbach, P.; Khoriauli, L.; Giulotto, E.; Lingner, J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 2007, 318, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, M.; Chartrand, P. TERRA, a Multifaceted Regulator of Telomerase Activity at Telomeres. J. Mol. Biol. 2020, 432, 4232–4243. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and Telomere Length: A General Overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Deng, Z.; Dahmane, N.; Tsai, K.; Wang, P.; Williams, D.R.; Kossenkov, A.V.; Showe, L.C.; Zhang, R.; Huang, Q.; et al. Telomeric repeat-containing RNA (TERRA) constitutes a nucleoprotein component of extracellular inflammatory exosomes. Proc. Natl. Acad. Sci. USA 2015, 112, E6293–E6300. [Google Scholar] [CrossRef] [PubMed]
- Idilli, A.I.; Cusanelli, E.; Pagani, F.; Berardinelli, F.; Bernabe, M.; Cayuela, M.L.; Poliani, P.L.; Mione, M.C. Expression of tert Prevents ALT in Zebrafish Brain Tumors. Front. Cell Dev. Biol. 2020, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Bassing, C.H.; Swat, W.; Alt, F.W. The mechanism and regulation of chromosomal V(D)J recombination. Cell 2002, 109, S45–S55. [Google Scholar] [CrossRef] [PubMed]
- Wienholds, E.; Schulte-Merker, S.; Walderich, B.; Plasterk, R.H. Target-selected inactivation of the zebrafish rag1 gene. Science 2002, 297, 99–102. [Google Scholar] [CrossRef]
- Garcia-Valtanen, P.; Martinez-Lopez, A.; Lopez-Munoz, A.; Bello-Perez, M.; Medina-Gali, R.M.; Ortega-Villaizan, M.D.; Varela, M.; Figueras, A.; Mulero, V.; Novoa, B.; et al. Zebra Fish Lacking Adaptive Immunity Acquire an Antiviral Alert State Characterized by Upregulated Gene Expression of Apoptosis, Multigene Families, and Interferon-Related Genes. Front. Immunol. 2017, 8, 121. [Google Scholar] [CrossRef]
- Novoa, B.; Pereiro, P.; Lopez-Munoz, A.; Varela, M.; Forn-Cuni, G.; Anchelin, M.; Dios, S.; Romero, A.; Martinez-Lopez, A.; Medina-Gali, R.M.; et al. Rag1 immunodeficiency-induced early aging and senescence in zebrafish are dependent on chronic inflammation and oxidative stress. Aging Cell 2019, 18, e13020. [Google Scholar] [CrossRef]
- Michael, C.; Martinez-Navarro, F.J.; de Oliveira, S. Analysis of Liver Microenvironment during Early Progression of Non-Alcoholic Fatty Liver Disease-Associated Hepatocellular Carcinoma in Zebrafish. J. Vis. Exp. 2021. [Google Scholar] [CrossRef]
- Vergroesen, J.E.; Thee, E.F.; de Crom, T.O.E.; Kiefte-de Jong, J.C.; Meester-Smoor, M.A.; Voortman, T.; Klaver, C.C.W.; Ramdas, W.D. The inflammatory potential of diet is associated with the risk of age-related eye diseases. Clin. Nutr. 2023, 42, 2404–2413. [Google Scholar] [CrossRef]
- Rocha, I.; Torrinhas, R.; Fonseca, D.; Lyra, C.O.; de Sousa Alves Neri, J.L.; Balmant, B.D.; Callado, L.; Charlton, K.; Queiroz, N.; Waitzberg, D.L. Pro-Inflammatory Diet Is Correlated with High Veillonella rogosae, Gut Inflammation and Clinical Relapse of Inflammatory Bowel Disease. Nutrients 2023, 15, 4148. [Google Scholar] [CrossRef] [PubMed]
- Ganbaatar, G.; Okami, Y.; Kadota, A.; Ganbaatar, N.; Yano, Y.; Kondo, K.; Harada, A.; Okuda, N.; Yoshita, K.; Okamura, T.; et al. Association of Pro-Inflammatory Diet with Long-Term Risk of All-Cause and Cardiovascular Disease Mortality: NIPPON DATA80. J. Atheroscler. Thromb. 2023. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Silva, D.; Canton-Sandoval, J.; Martinez-Navarro, F.J.; Perez-Sanchez, H.; de Oliveira, S.; Mulero, V.; Alcaraz-Perez, F.; Cayuela, M.L. Senescence-Independent Anti-Inflammatory Activity of the Senolytic Drugs Dasatinib, Navitoclax, and Venetoclax in Zebrafish Models of Chronic Inflammation. Int. J. Mol. Sci. 2022, 23, 10468. [Google Scholar] [CrossRef] [PubMed]
- Mathias, J.R.; Dodd, M.E.; Walters, K.B.; Rhodes, J.; Kanki, J.P.; Look, A.T.; Huttenlocher, A. Live imaging of chronic inflammation caused by mutation of zebrafish Hai1. J. Cell Sci. 2007, 120, 3372–3383. [Google Scholar] [CrossRef] [PubMed]
- Carney, T.J.; von der Hardt, S.; Sonntag, C.; Amsterdam, A.; Topczewski, J.; Hopkins, N.; Hammerschmidt, M. Inactivation of serine protease Matriptase1a by its inhibitor Hai1 is required for epithelial integrity of the zebrafish epidermis. Development 2007, 134, 3461–3471. [Google Scholar] [CrossRef]
- Martinez-Navarro, F.J.; Martinez-Menchon, T.; Mulero, V.; Galindo-Villegas, J. Models of human psoriasis: Zebrafish the newly appointed player. Dev. Comp. Immunol. 2019, 97, 76–87. [Google Scholar] [CrossRef]
- Martinez-Navarro, F.J.; Martinez-Morcillo, F.J.; Lopez-Munoz, A.; Pardo-Sanchez, I.; Martinez-Menchon, T.; Corbalan-Velez, R.; Cayuela, M.L.; Perez-Oliva, A.B.; Garcia-Moreno, D.; Mulero, V. The vitamin B6-regulated enzymes PYGL and G6PD fuel NADPH oxidases to promote skin inflammation. Dev. Comp. Immunol. 2020, 108, 103666. [Google Scholar] [CrossRef]
- Martinez-Morcillo, F.J.; Canton-Sandoval, J.; Martinez-Navarro, F.J.; Cabas, I.; Martinez-Vicente, I.; Armistead, J.; Hatzold, J.; Lopez-Munoz, A.; Martinez-Menchon, T.; Corbalan-Velez, R.; et al. NAMPT-derived NAD+ fuels PARP1 to promote skin inflammation through parthanatos cell death. PLoS Biol. 2021, 19, e3001455. [Google Scholar] [CrossRef]
- Solman, M.; Blokzijl-Franke, S.; Piques, F.; Yan, C.; Yang, Q.; Strullu, M.; Kamel, S.M.; Ak, P.; Bakkers, J.; Langenau, D.M.; et al. Inflammatory response in hematopoietic stem and progenitor cells triggered by activating SHP2 mutations evokes blood defects. eLife 2022, 11, e73040. [Google Scholar] [CrossRef]
- Progatzky, F.; Sangha, N.J.; Yoshida, N.; McBrien, M.; Cheung, J.; Shia, A.; Scott, J.; Marchesi, J.R.; Lamb, J.R.; Bugeon, L.; et al. Dietary cholesterol directly induces acute inflammasome-dependent intestinal inflammation. Nat. Commun. 2014, 5, 5864. [Google Scholar] [CrossRef]
- Kulkarni, P.; Yellanki, S.; Medishetti, R.; Sriram, D.; Saxena, U.; Yogeeswari, P. Novel Zebrafish EAE model: A quick in vivo screen for multiple sclerosis. Mult. Scler. Relat. Disord. 2017, 11, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Imamura, S.; Uchiyama, J.; Koshimizu, E.; Hanai, J.; Raftopoulou, C.; Murphey, R.D.; Bayliss, P.E.; Imai, Y.; Burns, C.E.; Masutomi, K.; et al. A non-canonical function of zebrafish telomerase reverse transcriptase is required for developmental hematopoiesis. PLoS ONE 2008, 3, e3364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Morimoto, K.; Danilova, N.; Zhang, B.; Lin, S. Zebrafish models for dyskeratosis congenita reveal critical roles of p53 activation contributing to hematopoietic defects through RNA processing. PLoS ONE 2012, 7, e30188. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).