Increased Circulating Irisin Levels in Kidney Transplant Patients: Is There a Connection with Glycaemic Control?
Abstract
1. Introduction
2. Results
2.1. Irisin Serum Levels (ISLs) in Patients before and after KTx in the Studied Group
2.2. Clinical and Biochemical Factors Analysed Comparatively in HIL and LIL of Patients after KTx
2.2.1. Factors That Are Statistically Significantly Associated with a Large Growth in ISL
- a.
- Obesity
- b.
- Duration of renal replacement therapy and subsequent KTx—second and third
- c.
- Cold ischaemia time
- d.
- Immunosuppression
2.2.2. Factors Not Statistically Significant in the Comparison of HIL and LIL Groups
2.2.3. Glycaemic Disturbances in the Studied Group of Patients in the Time soon after KTx
2.2.4. Diabetes Requiring Permanent Insulin Therapy (DM)
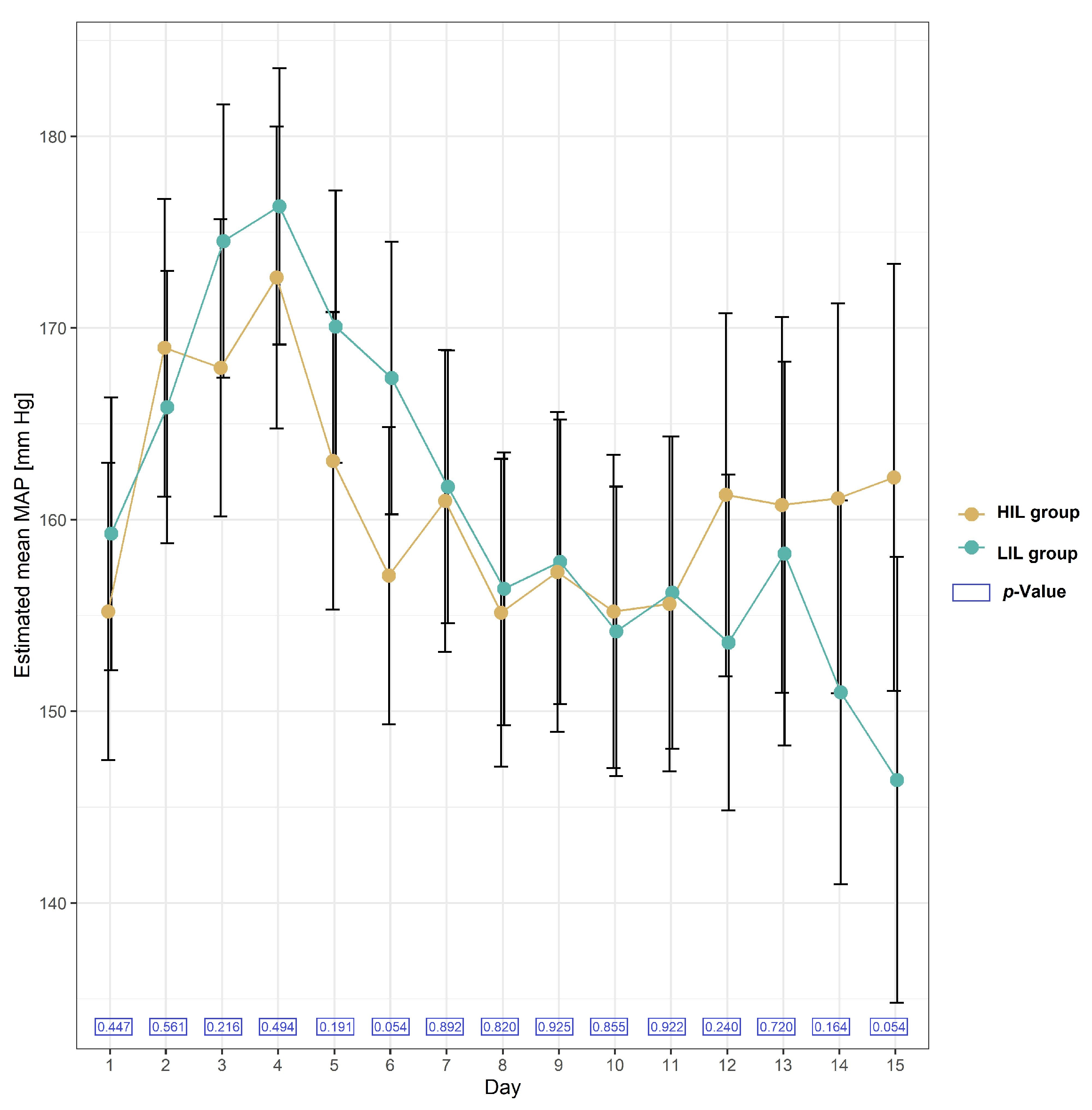
2.2.5. Blood Pressure in the Studied Group of Patients after KTx
2.2.6. Obesity and Irisin Serum Levels
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Methods
4.2.1. Irisin Serum Level (ISL)
4.2.2. Lipid Parameters, Glycaemia and Other Biochemical Parameters
4.2.3. Statistical Methodology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 2018, 175, 1756–1768. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A pgc1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Chinnam, N.; Ohashi, T.; Shah, R.S.; Erickson, H.P. The structure of irisin reveals a novel intersubunit beta-sheet fibronectin type III (FNIII) dimer: Implications for receptor activation. J. Biol. Chem. 2013, 288, 33738–33744. [Google Scholar] [CrossRef]
- Yang, X.; Enerback, S.; Smith, U. Reduced expression of FOXC2 and brown adipogenic genes in human subjects with insulin resistance. Obes. Res. 2003, 11, 1182–1191. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Wang, F.; Donelan, W.; Zona, M.C.; Li, S.; Reeves, W.; Ding, Y.; Tang, D.; Yang, L. Effects of irisin on the differentiation and browning of human visceral white adipocytes. Am. J. Transl. Res. 2019, 11, 7410–7421. [Google Scholar]
- Xin, C.; Liu, J.; Zhang, J.; Zhu, D.; Wang, H.; Xiong, L.; Lee, Y.; Ye, J.; Lian, K.; Xu, C.; et al. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int. J. Obes. 2016, 40, 443–451. [Google Scholar] [CrossRef]
- Shi, X.; Lin, M.; Liu, C.; Xiao, F.; Liu, Y.; Huang, P.; Zeng, X.; Yan, B.; Liu, S.; Li, X.; et al. Elevated circulating irisin is associated with lower risk of insulin resistance: Association and path analyses of obese Chinese adults. BMC Endocr. Disord. 2016, 16, 44. [Google Scholar] [CrossRef]
- Belviranli, M.; Okudan, N.; Çelik, F. Association of Circulating Irisin with Insulin Resistance and Oxidative Stress in Obese Women. Horm. Metab. Res. 2016, 48, 653–657. [Google Scholar] [CrossRef]
- Park, K.H.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.A.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef] [PubMed]
- Kasiske, B.L.; Snyder, J.J.; Gilbertson, D.; Matas, A.J. Diabetes mellitus after kidney transplantation in the United States. Am. J. Transplant. 2003, 3, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Ojo, A.O. Cardiovascular complications after renal transplantation and their prevention. Transplantation 2006, 82, 603–611. [Google Scholar] [CrossRef]
- Hjelmesaeth, J.; Hartmann, A.; Leivestad, T.; Holdaas, H.; Sagedal, S.; Olstad, M.; Jenssen, T. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. 2006, 69, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Boudreaux, J.P.; McHugh, L.; Canafax, D.M.; Ascher, N.; Sutherland, D.E.R.; Payne, W.; Simmons, R.L.; Najarian, J.S.; Fryd, D.S. The impact of cyclosporine and combination immunosuppression on the incidence of posttransplant diabetes in renal allograft recipients. Transplantation 1987, 44, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Hecking, M.; Haidinger, M.; Döller, D.; Werzowa, J.; Tura, A.; Zhang, J.; Tekoglu, H.; Pleiner, J.; Wrba, T.; Rasoul-Rockenschaub, S.; et al. Early basal insulin therapy decreases new-onset diabetes after renal transplantation. J. Am. Soc. Nephrol. 2012, 23, 739–749. [Google Scholar] [CrossRef]
- Chakkera, H.A.; Knowler, W.C.; Devarapalli, Y.; Weil, E.J.; Heilman, R.L.; Dueck, A.; Mulligan, D.C.; Reddy, K.S.; Moss, A.A.; Mekeel, K.L.; et al. Relationship between inpatient hyperglycemia and insulin treatment after kidney transplantation and future new onset diabetes mellitus. Clin. J. Am. Soc. Nephrol. 2010, 5, 1669–1675. [Google Scholar] [CrossRef]
- Chen, J.Q.; Huang, Y.Y.; Gusdon, A.M.; Qu, S. Irisin: A new molecular marker and target in metabolic disorder. Lipids Health Dis. 2015, 14, 2. [Google Scholar] [CrossRef]
- Porrini, E.L.; Díaz, J.M.; Moreso, F.; Mallén, P.I.D.; Torres, I.S.; Ibernon, M.; Bayés-Genís, B.; Benitez-Ruiz, R.; Lampreabe, I.; Lauzurrica, R.; et al. Clinical evolution of post-transplant diabetes mellitus. Nephrol. Dial. Transplant. 2016, 31, 495–505. [Google Scholar] [CrossRef]
- Ebert, T.; Focke, D.; Petroff, D.; Wurst, U.; Richter, J.; Bachmann, A.; Lössner, U.; Kralisch, S.; Kratzsch, J.; Beige, J.; et al. Serum levels of the myokine irisin in relation to metabolic and renal function. Eur. J. Endocrinol. 2014, 170, 501–506. [Google Scholar] [CrossRef]
- Wen, M.S.; Wang, C.Y.; Lin, S.L.; Hung, K.C. Decrease in irisin in patients with chronic kidney disease. PLoS ONE 2013, 8, e64025. [Google Scholar] [CrossRef]
- Sadeghi Shad, J.; Akbari, R.; Qujeq, D.; Hajian-Tilaki, K. Measurement of serum irisin in the different stages of chronic kidney disease. Casp. J. Intern. Med. 2019, 10, 314–319. [Google Scholar] [CrossRef]
- Gan, W.; Chen, W.; Li, T.; Shao, D.; Xu, F.; Huo, S.; Li, C.; Yang, Z.; Zeng, X. Circulating irisin level in chronic kidney disease patients: A systematic review and meta-analysis. Int. Urol. Nephrol. 2022, 54, 1295–1302. [Google Scholar] [CrossRef]
- Liu, J.J.; Wong, M.D.; Toy, W.C.; Tan, C.S.; Liu, S.; Ng, X.W.; Tavintharan, S.; Sum, C.F.; Lim, S.C. Lower circulating irisin is associated with type 2 diabetes mellitus. J. Diabetes Complicat. 2013, 27, 365–369. [Google Scholar] [CrossRef]
- Bidares, M.; Safari-Kish, B.; Abedi, M.; Malekzadeh-Shoushtari, H.; Jasemnezhad, M.; Azarbayejani, N.; Aziz, M.; Jahromi, S.P.; Fouladi, S.; Azizi-Soleiman, F. The Effect of Bariatric Surgery on Irisin Level: A Systematic Review and Meta-analysis. Obes. Surg. 2023, 33, 3256–3265. [Google Scholar] [CrossRef] [PubMed]
- Majorczyk, M.; Staszkiewicz, M.; Szklarczyk, J.; Major, P.; Pisarska, M.; Wysocki, M.; Stefura, T.; Kacprzyk, A.; Droś, J.; Hołda, M.K.; et al. The influence of bariatric surgery on serum levels of irisin and nesfatin-1. Acta Chir. Belg. 2019, 119, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernandez-Real, J.M. Irisin Is Expressed and Produced by Human Muscle and Adipose Tissue in Association With Obesity and Insulin Resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Han, Y.; Wang, J.; Liu, Y.; Zheng, S.; Zhou, L.; Jose, P.A.; Zeng, C.; Zhang, Y.; Song, H.; et al. Irisin lowers blood pressure by improvement of endothelial dysfunction via AMPK-Akt-eNOS-NO pathway in the spontaneously hypertensive rat. J. Am. Heart Assoc. 2016, 5, e003433. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.; Crujeiras, A.B.; Amil, M.; Aguera, Z.; Jiménez-Murcia, S.; Baños, R.; Botella, C.; de la Torre, R.; Estivill, X.; Fagundo, A.B.; et al. Association of irisin with fat mass, resting energy expenditure, and daily activity in conditions of extreme body mass index. Int. J. Endocrinol. 2014, 2014, 857270. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Shi, X.; Zhang, H.; Pan, L.; Ma, Z.; Liu, S.; Liu, Y.; Li, X.; Yang, S.; Li, Z. Association of serum irisin with metabolic syndrome in obese Chinese adults. PLoS ONE 2014, 9, e94235. [Google Scholar] [CrossRef]
- Sesti, G.; Andreozzi, F.; Fiorentino, T.V.; Mannino, G.C.; Sciacqua, A.; Marini, M.A.; Perticone, F. High circulating irisin levels are associated with insulin resistance and vascular atherosclerosis in a cohort of nondiabetic adult subjects. Acta Diabetol. 2014, 51, 705–713. [Google Scholar] [CrossRef]
- Brondani, L.A.; Boelter, G.; Assmann, T.S.; Leitão, C.B.; Canani, L.H.; Crispim, D. Irisin-encoding gene (FNDC5) variant is associated with changes in blood pressure and lipid profile in type 2 diabetic women but not in men. Metabolism 2015, 64, 952–957. [Google Scholar] [CrossRef] [PubMed]

| Variables | LIL Group (n = 26) | HIL Group (n = 21) | Total (n = 47) | p Value |
|---|---|---|---|---|
| Age | 0.374 | |||
| Mean (SD) | 49.885 (12.054) | 46.667 (12.431) | 48.447 (12.197) | |
| Median (Q1, Q3) | 48.500 (39.000, 58.250) | 46.000 (37.000, 57.000) | 47.000 (38.500, 58.000) | |
| Gender | 0.770 | |||
| Female | 11 (42.3%) | 8 (38.1%) | 19 (40.4%) | |
| Male | 15 (57.7%) | 13 (61.9%) | 28 (59.6%) | |
| BMI kg/m2 | 0.987 | |||
| Mean (SD) | 25.160 (3.518) | 25.142 (4.243) | 25.152 (3.815) | |
| Median (Q1, Q3) | 24.750 (23.000, 27.750) | 24.500 (22.400, 30.000) | 24.500 (22.650, 27.800) | |
| BMI > 25 | 0.821 | |||
| <25 | 14 (53.8%) | 12 (57.1%) | 26 (55.3%) | |
| >25 | 12 (46.2%) | 9 (42.9%) | 21 (44.7%) | |
| BMI > 30 | 0.041 | |||
| <30 | 25 (96.2%) | 16 (76.2%) | 41 (87.2%) | |
| >30 | 1 (3.8%) | 5 (23.8%) | 6 (12.8%) | |
| Cause of ESRD n (%) | 0.257 | |||
| GN | 14 (58.3%) | 10 (47.6%) | 24 (53.3%) | |
| ADPKD | 4 (15.4%) | 4 (19.0%) | 8 (17.0%) | |
| IN | 0 (0.0%) | 2 (9.5%) | 2 (4.3%) | |
| Not known | 6 (23.8%) | 2 (9.5%) | 8 (17.0%) | |
| HN/IschN | 2 (7.7%) | 1 (4.8%) | 3 (6.4%) | |
| Other (a/HUS) | 0 (0.0%) | 2 (9.5%) | 2 (9.5%) | |
| Dialysis modality before KTx n (%) | 0.488 | |||
| HD | 17 (65.4%) | 17 (81.0%) | 34 (72.3%) | |
| PD | 5 (19.2%) | 2 (9.5%) | 7 (14.9%) | |
| PREE | 4 (15.4%) | 2 (9.5%) | 6 (12.8%) | |
| Pretransplant dialysis time (months) | 0.005 | |||
| Mean (SD) | 26.032 (22.495) | 81.494 (92.372) | 50.813 (69.005) | |
| Median (Q1, Q3) | 23.623 (5.451, 37.443) | 51.672 (16.557, 109.311) | 29.377 (10.639, 56.590) | |
| 2nd and 3rd KTx n (%) | <0.001 | |||
| 0 (0.0%) | 8 (38.1%) | 8 (17.0%) | ||
| AR n (%) | 0.466 | |||
| 0 (0%) | 0 (0%) | 0 (0%) | ||
| DGF n (%) | 0.452 | |||
| 5 (19.2%) | 6 (28.6%) | 11 (23.4%) | ||
| Tacrolimus use n (%) | 0.466 | |||
| 24 (92.3%) | 18 (85.7%) | 42 (89.4%) | ||
| Tacrolimus blood concentration (ng/mL) on 6th day after KTx n = 42 | 0.001 | |||
| Median (Q1, Q3) | 12.650 (10.850, 16.000) | 9.000 (7.900, 10.900) | 11.000 (8.400, 13.000) | |
| Cyclosporin use n (%) | 0.466 | |||
| 2 (7.7%) | 3 (14.3%) | 5 (10.6%) | ||
| Cyclosporin blood concentration (ng/mL) on 6th day after KTx, n = 5 | 0.84 | |||
| Mean (SD) | 242.625 (40.84) | 254.083 (62.47) | 249.500 (49.07) | |
| Median (Q1, Q3) | 242.625 (213.750, 271.500) | 223.000 (213.250, 326.000) | 223.000 (213.750, 271.500) | |
| Induction use (basiliximab/anti-thymocyte globulin) n (%) | 0.108 | |||
| 23 (88.5%) | 21 (100.0%) | 44 (93.6%) | ||
| Two weeks prednisone cumulative dose (mg/kg) | 0.36 | |||
| Mean (SD) | 4.68 (0.9) | 4.97 (1.2) | 4.8 (1.1) | |
| Median (Q1, Q3) | 4.430 (4.192, 5.343) | 5.021 (3.923, 5.738) | 4.459 (3.924, 5.719) | |
| Duration of hospitalisation after KTx (days) | 0.432 * | |||
| Median (Q1, Q3) | 14.000 (12.000, 18.000) | 15.000 (12.000, 27.000) | 14.500 (12.000, 18.750) | |
| Posttransplant global mean blood glucose level (mg/dL) | 0.002 | |||
| Mean (SD) | 147.194 (24.522) | 127.499 (12.238) | 138.003 (21.935) | |
| Median (Q1, Q3) | 144.943 (130.000, 156.198) | 125.130 (120.154, 131.500) | 131.500 (123.024, 151.262) | |
| CSC one month after KTx (mg/dL) | 0.982 | |||
| Mean (SD) | 1.632 (0.522) | 1.635 (0.560) | 1.633 (0.533) | |
| Median (Q1, Q3) | 1.615 (1.162, 1.922) | 1.665 (1.350, 1.935) | 1.640 (1.200, 1.925) | |
| eGFR 4p MDRD | 0.742 | |||
| Mean (SD) | 46.762 (15.830) | 48.770 (25.168) | 47.635 (20.191) | |
| Median (Q1, Q3) | 42.750 (35.325, 59.200) | 43.700 (35.400, 49.400) | 42.750 (35.325, 57.525) | |
| eGFR CKD-EPI (mL/min/1.73 m2) | 0.589 | |||
| Mean (SD) | 47.881 (15.745) | 51.165 (25.060) | 49.309 (20.139) | |
| Median (Q1, Q3) | 44.000 (36.150, 61.475) | 44.350 (38.700, 50.300) | 44.000 (36.150, 59.850) | |
| WIT (min) | 0.848 | |||
| Mean (SD) | 32.885 (8.267) | 33.333 (7.479) | 33.085 (7.843) | |
| Median (Q1, Q3) | 31.500 (26.500, 36.500) | 33.000 (27.000, 37.000) | 32.000 (27.000, 37.000) | |
| CIT (min) | 0.055 | |||
| Mean (SD) | 840.077 (416.737) | 1116.050 (531.504) | 960.065 (484.656) | |
| Median (Q1, Q3) | 795.000 (614.500, 936.750) | 1101.000 (666.000, 1420.000) | 839.500 (627.000, 1213.250) | |
| Number of HLA mismatches | 0.202 | |||
| Mean (SD) | 2.538 (0.706) | 2.952 (1.431) | 2.723 (1.097) | |
| Median (Q1, Q3) | 2.500 (2.000, 3.000) | 3.000 (2.000, 3.000) | 3.000 (2.000, 3.000) | |
| Charlson Comorbidity Index | 0.869 | |||
| Mean (SD) | 2.808 (0.981) | 2.762 (0.889) | 2.787 (0.931) | |
| Median (Q1, Q3) | 2.500 (2.000, 3.000) | 3.000 (2.000, 3.000) | 3.000 (2.000, 3.000) | |
| Donor gender | 0.202 | |||
| F | 10 (38.5%) | 12 (57.1%) | 22 (46.8%) | |
| M | 16 (61.5%) | 9 (42.9%) | 25 (53.2%) | |
| Donor age (years) | 0.785 | |||
| Mean (SD) | 45.500 (15.943) | 46.714 (13.922) | 46.043 (14.9) | |
| Median (Q1, Q3) | 47.000 (38.250, 55.750) | 48.000 (38.000, 56.000) | 47.000 (38.000, 56.000) | |
| HbA1c before KTx (mmol/mol) | 0.454 | |||
| Mean (SD) | 5.345 (0.442) | 5.231 (0.483) | 5.297 (0.456) | |
| Median (Q1, Q3) | 5.400 (5.100, 5.600) | 5.400 (4.975, 5.500) | 5.400 (5.000, 5.575) | |
| HbA1c IFCC before KTx (%) | 0.491 | |||
| Mean (SD) | 34.805 (4.800) | 33.675 (5.140) | 34.329 (4.910) | |
| Median (Q1, Q3) | 35.350 (32.000, 38.000) | 35.350 (30.750, 36.850) | 35.350 (31.000, 37.750) | |
| Glycaemia before KTx (mg/dL) | 0.679 | |||
| Mean (SD) | 83.333 (7.418) | 84.500 (9.619) | 83.838 (8.335) | |
| Median (Q1, Q3) | 81.000 (77.000, 87.000) | 84.000 (77.750, 91.500) | 83.000 (77.000, 91.000) | |
| Insulin level before KTx (uU) | 0.399 * | |||
| Median (Q1, Q3) | 6.250 (4.325, 7.000) | 6.950 (4.000, 9.200) | 6.400 (4.125, 7.875) | |
| HOMA-IR Index before KTx | 0.434 * | |||
| Median (Q1, Q3) | 1.240 (0.800, 1.443) | 1.330 (0.952, 2.155) | 1.250 (0.871, 1.618) | |
| HOMA over the normal range before KTx > 2 n (%) | 0.214 | |||
| 3 (14.3%) | 5 (31.2%) | 8 (21.6%) | ||
| QUICKI before KTx | 0.681 | |||
| Mean (SD) | 0.374 (0.028) | 0.369 (0.045) | 0.372 (0.036) | |
| Median (Q1, Q3) | 0.368 (0.360, 0.394) | 0.366 (0.340, 0.387) | 0.368 (0.354, 0.391) | |
| QUICKI under the normal range before KTx < 0.34 n (%) | 0.230 | |||
| 2 (10.0%) | 4 (25.0%) | 6 (16.7%) | ||
| Homocysteine before KTx (umol) | 0.104 | |||
| Mean (SD) | 32.589 (20.808) | 23.439 (8.232) | 28.835 (17.268) | |
| Median (Q1, Q3) | 26.200 (19.905, 35.540) | 22.900 (18.440, 28.950) | 23.510 (19.710, 32.400) | |
| Cholesterol before KTx (mg/dL) | 0.642 | |||
| Mean (SD) | 196.826 (57.728) | 189.000 (40.229) | 193.615 (50.827) | |
| Median (Q1, Q3) | 188.000 (159.000, 215.500) | 189.500 (159.500, 208.500) | 188.000 (159.000, 215.500) | |
| Triglyceride before KTx (mg/dL) | 0.437 | |||
| Mean (SD) | 138.696 (47.266) | 126.875 (44.659) | 133.846 (45.993) | |
| Median (Q1, Q3) | 140.000 (110.000, 154.500) | 126.500 (93.750, 165.750) | 131.000 (102.000, 157.000) | |
| HDL cholesterol before KTx (mg/dL) | 0.747 | |||
| Mean (SD) | 46.773 (9.492) | 47.875 (11.342) | 47.237 (10.178) | |
| Median (Q1, Q3) | 49.000 (39.250, 51.750) | 48.500 (38.500, 55.500) | 49.000 (39.000, 54.250) | |
| LDL cholesterol before KTx (mg/dL) | 0.581 | |||
| Mean (SD) | 128.000 (55.263) | 119.250 (35.430) | 124.410 (47.777) | |
| Median (Q1, Q3) | 118.000 (91.000, 144.000) | 121.500 (100.500, 130.250) | 120.000 (91.000, 138.000) | |
| Uric acid before KTx (mg/dL) | 0.302 | |||
| Mean (SD) | 5.582 (1.985) | 4.969 (1.454) | 5.324 (1.785) | |
| Median (Q1, Q3) | 5.000 (4.075, 7.350) | 5.050 (3.950, 5.625) | 5.000 (4.000, 7.150) | |
| C-peptide before KTx (ng/mL) | 0.743 * | |||
| Median (Q1, Q3) | 5.600 (4.710, 7.750) | 6.695 (3.942, 7.795) | 5.790 (4.540, 7.750) | |
| Albumin before KTx (g/L) | 0.382 * | |||
| Median (Q1, Q3) | 38.000 (4.600, 39.500) | 29.500 (4.000, 39.250) | 37.000 (4.300, 39.500) |
| Patients with Increase in Irisin Concentration HIL Group | Patients with No Increase in Irisin Concentration LIL Group | Between-Group Change | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Days after Transplantation | Number of Patients | Fasting Glucose Serum Level; Mean (95% CI) (mg/dL) | Change from Baseline; Mean (95% CI) (mg/dL) | p-Value | Number of Patients | Fasting Glucose Serum Level; Mean (95% CI) (mg/dL) | Change from Baseline; Mean (95% CI) (mg/dL) | p-Value | Mean (95% CI) (mg/dL) | p-Value |
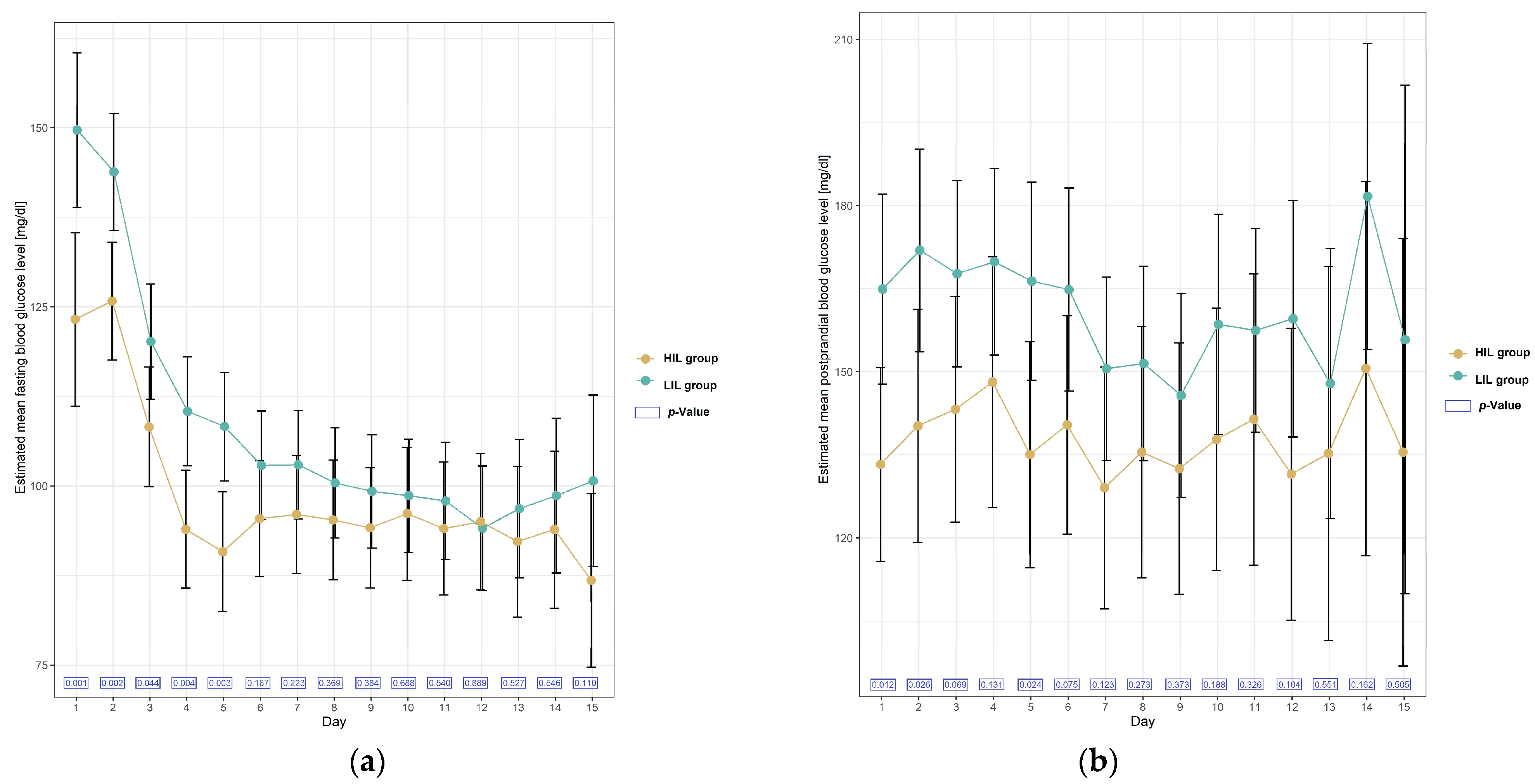
| Day 1 | 7 | 123 (111 to 135) | - | - | 9 | 150 (139 to 160) | - | - | −26 (−43 to −10) | 0.001 |
| Day 2 | 20 | 126 (118 to 134) | 3 (−19 to 24) | 0.999 | 19 | 144 (136 to 152) | −6 (−26 to −4) | 0.999 | −18 (−30 to −7) | 0.002 |
| Day 3 | 19 | 108 (100 to 117) | −15 (−37 to 7) | 0.570 | 20 | 120 (112 to 128) | −30 (−49 to −10) | <0.001 | −12 (−23 to −0.3) | 0.044 |
| Day 4 | 20 | 94 (86 to 102) | −29 (−51 to −7) | <0.001 | 24 | 110 (103 to 118) | −39 (−59 to −20) | <0.001 | −16 (−28 to −5) | 0.004 |
| Day 5 | 19 | 91 (82 to 99) | −32 (−54 to −10) | <0.001 | 24 | 108 (101 to 116) | −41 (−61 to −22) | <0.001 | −17 (−29 to −6) | 0.003 |
| Day 6 | 21 | 95 (87 to 103) | −29 (−50 to −6) | 0.001 | 24 | 102 (95 to 110) | −47 (−66 to −27) | <0.001 | −7 (−18 to 4) | 0.187 |
| Day 7 | 20 | 96 (88 to 104) | −27 (−49 to −5) | 0.002 | 24 | 103 (95 to 110) | −47 (−66 to −27) | <0.001 | −7 (−18 to 4) | 0.223 |
| Day 8 | 19 | 95.25 (87 to 104) | −28 (−50 to −6) | 0.002 | 23 | 100 (93 to 108) | −49 (−69 to −30) | <0.001 | −5 (−16 to 6) | 0.369 |
| Day 9 | 19 | 94 (86 to 103) | −29 (−51 to −7) | <0.001 | 21 | 99 (91 to 107) | −50 (−70 to −30) | <0.001 | −5 (−17 to 6) | 0.384 |
| Day 10 | 14 | 96 (87 to 105) | −27 (−50 to −4) | 0.006 | 21 | 99 (91 to 107) | −51 (−71 to −31) | <0.001 | −2 (−15 to 10) | 0.688 |
| Day 11 | 14 | 94 (85 to 103) | −29 (−52 to −6) | 0.002 | 19 | 98 (90 to 106) | −52 (−72 to −32) | <0.001 | −4 (−16 to 8) | 0.540 |
| Day 12 | 13 | 95 (85 to 104) | −28 (−52 to −5) | 0.004 | 16 | 94 (85 to 103) | −56 (−76 to −35) | <0.001 | 1 (−12 to 13) | 0.889 |
| Day 13 | 10 | 92 (82 to 103) | −31 (−56 to −6) | 0.002 | 12 | 97 (87 to 106) | −53 (−75 to −31) | <0.001 | −5 (−19 to 10) | 0.527 |
| Day 14 | 9 | 94 (83 to 105) | −29 (−54 to −4) | 0.007 | 9 | 99 (88 to 104) | −51 (−74 to −27) | <0.001 | −5 (−20 to 11) | 0.546 |
| Day 15 | 7 | 87 (75 to 99) | −36 (−63 to −10) | <0.001 | 7 | 101 (89 to 113) | −49 (−74 to −24) | <0.001 | −14 (−31 to 3) | 0.110 |
| Patients with Increase in Irisin Concentration HIL Group (n = 21) | Patients with No Increase in Irisin Concentration LIL Group (n = 26) | Between-Group Change | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Days after Kidney Transplantation | Number of Patients | Postprandial Blood Glucose Mean (95% CI) mg/dL | Change from Baseline, Mean (95% CI) mg/dL | p-Value | Number of Patients | Postprandial Blood Glucose Mean (95% CI) mg/dL | Change from Baseline, Mean (95% CI) mg/dL | p-Value | Mean (95% CI) mg/dL | p-Value |
| Day 1 | 19 | 133 (116 to 151) | - | - | 19 | 165 (148 to 182) | - | - | −32 (−56 to −7) | 0.012 |
| Day 2 | 12 | 140 (119 to 161) | 7 (−33 to 47) | 0.999 | 16 | 173 (154 to 190) | 7 (−30 to 44) | 0.999 | −32 (−60 to −4) | 0.026 |
| Day 3 | 13 | 143 (123 to 164) | 10 (−29 to 49) | 0.999 | 20 | 168 (151 to 184) | 3 (−32 to 38) | 0.999 | −24 (−51 to 2) | 0.069 |
| Day 4 | 10 | 148 (125 to 171) | 15 (−28 to 58) | 0.999 | 20 | 170 (153 to 187) | 5 (−30 to 40) | 0.999 | −22 (−50 to 6) | 0.131 |
| Day 5 | 13 | 135 (115 to 155) | 2 (−38 to 41) | 0.999 | 17 | 166 (148 to 184) | 1.42 (−35 to 38) | 0.999 | −31 (−58 to −4) | 0.024 |
| Day 6 | 14 | 140 (121 to 160) | 7 (−32 to 46) | 0.999 | 16 | 165 (146.5 to 183) | −0.07 (−37 to 37) | 0.999 | −24 (−51 to 3) | 0.075 |
| Day 7 | 11 | 129 (107 to 151) | −4 (−46 to 37) | 0.999 | 21 | 151 (134 to 167) | −14 (−49 to 20) | 0.983 | −22 (−49 to 6) | 0.123 |
| Day 8 | 10 | 136 (113 to 158) | 2 (−41 to 45) | 0.999 | 18 | 152 (134 to 169) | −14 (−49 to 22) | 0.993 | −16 (−45 to 13) | 0.273 |
| Day 9 | 10 | 133 (110 to 155) | −1 (−44 to 42) | 0.999 | 16 | 146 (127 to 164) | −19 (−56 to 18) | 0.903 | −13 (−42 to 16) | 0.373 |
| Day 10 | 9 | 138 (114 to 162) | 5 (−40 to 49) | 0.999 | 13 | 159 (139 to 178) | −7 (−45 to 33) | 0.999 | −21 (−52 to 10) | 0.188 |
| Day 11 | 7 | 141 (115 to 168) | 8 (−41 to 57) | 0.999 | 16 | 157 (139 to 176) | −8 (−44 to 29) | 0.999 | −16 (−48 to 16) | 0.326 |
| Day 12 | 7 | 132 (105 to 158) | −2 (−51 to 47) | 0.999 | 11 | 160 (138 to 182) | −5 (−47 to 36) | 0.999 | −28 (−62 to 6) | 0.104 |
| Day 13 | 4 | 135 (102 to 169) | 2 (−59 to 63) | 0.999 | 8 | 148 (124 to 172) | −17 (−63 to 29) | 0.995 | −13 (−54 to 29) | 0.551 |
| Day 14 | 4 | 151 (117 to 184) | 17 (−44 to 79) | 0.999 | 6 | 182 (154 to 209) | 17 (−35 to 68) | 0.998 | −31 (−75 to 13) | 0.162 |
| Day 15 | 3 | 136 (97 to 174) | 2 (−67 to 72) | 0.999 | 2 | 156 (110 to 202) | −9 (−91 to 73) | 0.999 | −20 (−80 to 40) | 0.505 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bzoma, B.; Kuchta, A.; Sałaga-Zaleska, K.; Krzesińska, A.; Chyła-Danił, G.; Jankowski, M.; Dębska-Ślizień, A. Increased Circulating Irisin Levels in Kidney Transplant Patients: Is There a Connection with Glycaemic Control? Int. J. Mol. Sci. 2024, 25, 2926. https://doi.org/10.3390/ijms25052926
Bzoma B, Kuchta A, Sałaga-Zaleska K, Krzesińska A, Chyła-Danił G, Jankowski M, Dębska-Ślizień A. Increased Circulating Irisin Levels in Kidney Transplant Patients: Is There a Connection with Glycaemic Control? International Journal of Molecular Sciences. 2024; 25(5):2926. https://doi.org/10.3390/ijms25052926
Chicago/Turabian StyleBzoma, Beata, Agnieszka Kuchta, Kornelia Sałaga-Zaleska, Aleksandra Krzesińska, Gabriela Chyła-Danił, Maciej Jankowski, and Alicja Dębska-Ślizień. 2024. "Increased Circulating Irisin Levels in Kidney Transplant Patients: Is There a Connection with Glycaemic Control?" International Journal of Molecular Sciences 25, no. 5: 2926. https://doi.org/10.3390/ijms25052926
APA StyleBzoma, B., Kuchta, A., Sałaga-Zaleska, K., Krzesińska, A., Chyła-Danił, G., Jankowski, M., & Dębska-Ślizień, A. (2024). Increased Circulating Irisin Levels in Kidney Transplant Patients: Is There a Connection with Glycaemic Control? International Journal of Molecular Sciences, 25(5), 2926. https://doi.org/10.3390/ijms25052926







