Genome-Wide Identification and Characterization of the Sweet Orange (Citrus sinensis) GATA Family Reveals a Role for CsGATA12 as a Regulator of Citrus Bacterial Canker Resistance
Abstract
1. Introduction
2. Results
2.1. Systematic Analyses Identified 24 CsGATAs in the C. sinensis Genome
2.2. CsGATA Family Chromosomal Locations and Phylogenetic Relationships
2.3. Analyses of CsGATA Collinearity, Conserved Motifs, and Gene Structures
2.4. CsGATA12 Is an Xcc-Inducible Protein Localized in the Nucleus
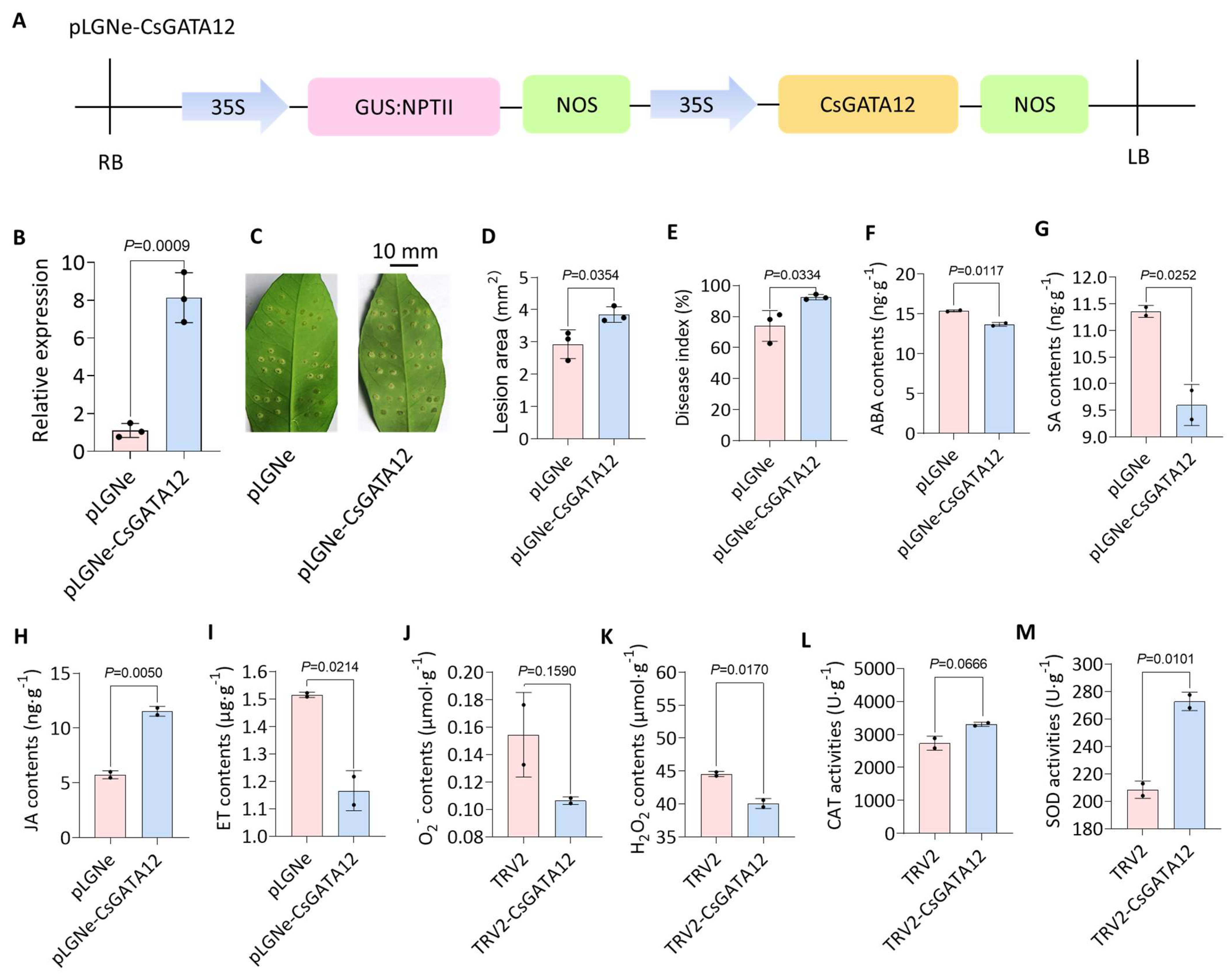
2.5. Transiently Overexpressing CsGATA12 Sensitizes Wanjincheng Plants to CBC
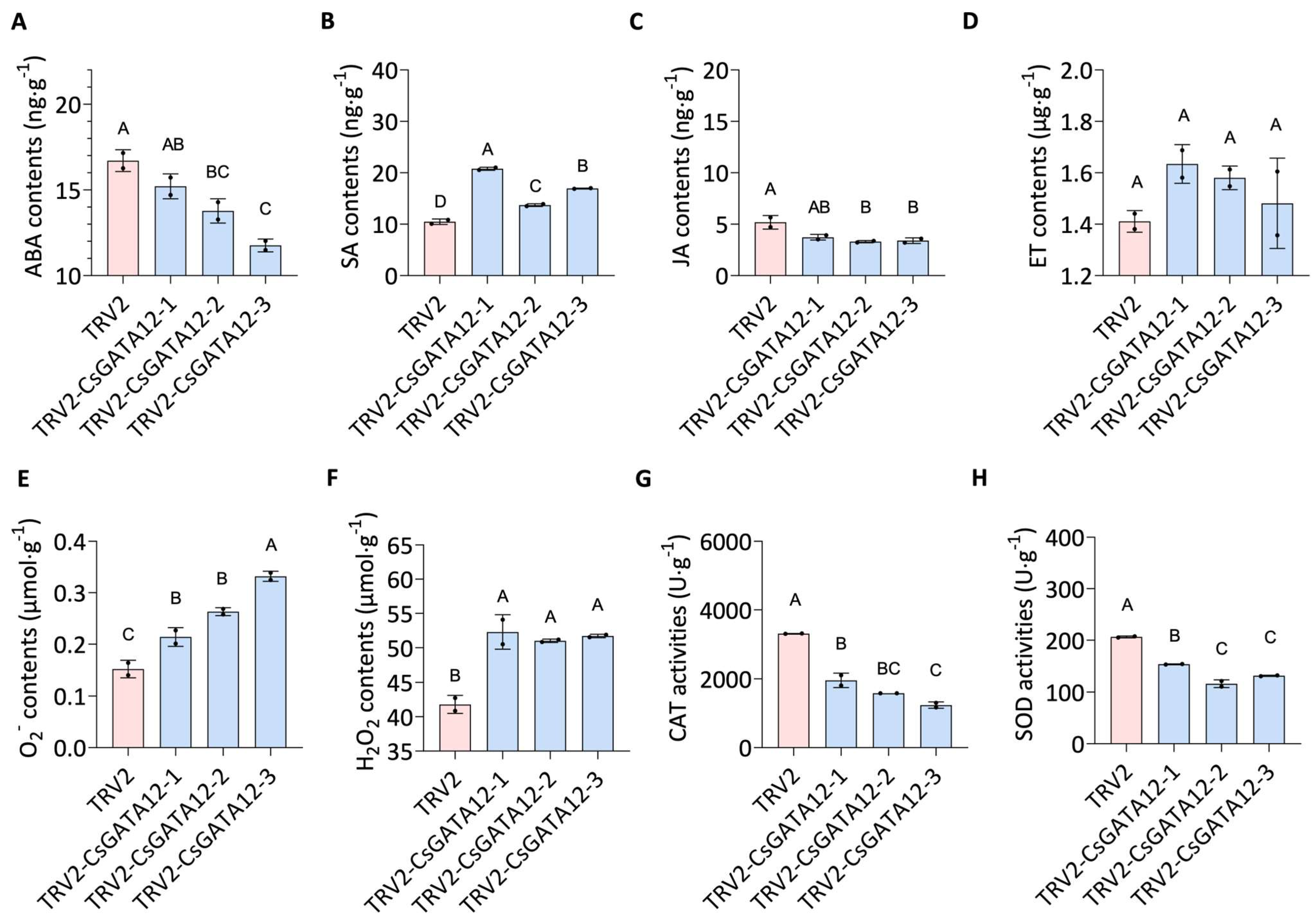
2.6. VIGS-Mediated CsGATA12 Knockdown Enhances CBC Resistance
2.7. CsGATA12 Silencing Alters Phytohormones and ROS Levels
2.8. The Silencing of CsGATA12 Alters the Expression of Phytohormone Biosynthesis and Signaling-Related Genes
3. Discussion
4. Materials and Methods
4.1. Plant and Bacterial Materials
4.2. CsGATA Family Identification and Annotation
4.3. In Silico CsGATA Characterization
4.4. Xcc Inoculation
4.5. Subcellular Localization Analyses
4.6. Transient Citrus Transformation Assays
4.7. Virus-Induced Gene Silencing
4.8. CBC Resistance Analyses
4.9. Biochemical Index Measurements
4.10. RNA Sequencing
4.11. qPCR
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, M.; Huang, Q.; Wang, Y.; Wang, C.; Zhu, R.; Zhang, S.; Kai, G. Genome-wide survey of the GATA gene family in camptothecin-producing plant Ophiorrhiza pumila. BMC Genom. 2022, 23, 256. [Google Scholar] [CrossRef]
- Kim, M.; Xi, H.; Park, J. Genome-wide comparative analyses of GATA transcription factors among 19 Arabidopsis ecotype genomes: Intraspecific characteristics of GATA transcription factors. PLoS ONE 2021, 16, e0252181. [Google Scholar] [CrossRef]
- Lai, D.; Yao, X.; Yan, J.; Gao, A.; Yang, H.; Xiang, D.; Ruan, J.; Fan, Y.; Cheng, J. Genome-wide identification, phylogenetic and expression pattern analysis of GATA family genes in foxtail millet (Setaria italica). BMC Genom. 2022, 23, 549. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, X.; Wei, X.; Lu, C.; Shen, F.; Zhang, X.; Zhang, Z. The wheat LLM-domain-containing transcription factor TaGATA1 positively modulates host immune response to Rhizoctonia cerealis. J. Exp. Bot. 2020, 71, 344–355. [Google Scholar] [CrossRef]
- Reyes, J.C.; Muro-Pastor, M.I.; Florencio, F.J. The GATA family of transcription factors in Arabidopsis and rice. Plant Physiol. 2004, 134, 1718–1732. [Google Scholar] [CrossRef]
- Davierwala, A.P.; Ramakrishna, W.; Chowdari, V.; Ranjekar, P.K.; Gupta, V.S. Potential of (GATA)n microsatellites from rice for inter- and intra-specific variability studies. BMC Evol. Biol. 2001, 1, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, C.; Hou, Y.; Hao, Q.; Chen, H.; Chen, L.; Yuan, S.; Shan, Z.; Zhang, X.; Yang, Z.; Qiu, D.; et al. Genome-wide survey of the soybean GATA transcription factor gene family and expression analysis under low nitrogen stress. PLoS ONE 2015, 10, e0125174. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Guo, Y.; Chen, Y.; Wu, D.; Jiang, L. Genome-wide identification, phylogenetic and expression pattern analysis of GATA family genes in Brassica napus. BMC Plant Biol. 2020, 20, 543. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, Y.; Lou, S.; Wei, W.; Zhao, Z.; Ren, Y.; Lin, C.; Ma, L. Genome-wide characterization and gene expression analyses of GATA transcription factors in Moso Bamboo. Int. J. Mol. Sci. 2019, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Duan, S.; Jian, Y.; Xu, J.; Hu, J.; Zhang, Z.; Lin, T.; Cheng, F.; Li, G. Genome-wide identification and gene expression analysis of the 14-3-3 gene family in potato (Solanum tuberosum L.). BMC Genom. 2022, 23, 811. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Lu, Y.; Sun, H.; Duan, W.; Hu, Y.; Yan, Y. Genome-Wide analysis of wheat GATA transcription factor genes reveals their molecular evolutionary characteristics and involvement in salt and drought tolerance. Int. J. Mol. Sci. 2022, 24, 27. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Schröder, P.M.; Blaby-Haas, C.E. Plant GATA factors: Their biology, phylogeny, and phylogenomics. Annu. Rev. Plant Biol. 2022, 73, 123–148. [Google Scholar] [CrossRef] [PubMed]
- Bastakis, E.; Hedtke, B.; Klermund, C.; Grimm, B.; Schwechheimer, C. LLM-domain B-GATA transcription factors play multifaceted roles in controlling greening in Arabidopsis. Plant Cell 2018, 30, 582–599. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, Y.; Xiao, Z.; Yang, H.; Hao, Q.; Yuan, S.; Chen, H.; Chen, L.; Chen, S.; Zhou, X.; et al. A GATA transcription factor from Soybean (Glycine max) regulates chlorophyll biosynthesis and suppresses growth in the transgenic Arabidopsis thaliana. Plants 2020, 9, 1036. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhou, Y.; Han, X.; Shen, C.; Wang, S.; Liu, C.; Yin, W.; Xia, X. The GATA transcription factor GNC plays an important role in photosynthesis and growth in poplar. J. Exp. Bot. 2020, 71, 1969–1984. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.A.; Sabir, I.A.; Shah, I.H.; Wang, H.; Yu, Z.; Rasool, F.; Mazhar, M.Z.; Younas, S.; Abdullah, M.; Cai, Y. Comprehensive comparative analysis of the GATA transcription factors in four rosaceae species and phytohormonal response in chinese pear (Pyrus bretschneideri) fruit. Int. J. Mol. Sci. 2021, 22, 12492. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Li, Y.; Zhu, X.; Liu, X.; Ye, X.; Zhou, M.; Zhang, Z. The GATA transcription factor TaGATA1 recruits demethylase TaELF6-A1 and enhances seed dormancy in wheat by directly regulating TaABI5. J. Integr. Plant Biol. 2023, 65, 1262–1276. [Google Scholar] [CrossRef]
- Bi, Y.M.; Zhang, Y.; Signorelli, T.; Zhao, R.; Zhu, T.; Rothstein, S. Genetic analysis of Arabidopsis GATA transcription factor gene family reveals a nitrate-inducible member important for chlorophyll synthesis and glucose sensitivity. Plant J. 2005, 44, 680–692. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, T.; Li, Z.; Huang, K.; Kim, N.E.; Ma, Z.; Kwon, S.W.; Jiang, W.; Du, X. OsGATA16, a GATA transcription factor, confers cold tolerance by repressing OsWRKY45-1 at the seedling stage in rice. Rice 2021, 14, 42. [Google Scholar] [CrossRef]
- Bonthala, V.S.; Mayes, K.; Moreton, J.; Blythe, M.; Wright, V.; May, S.T.; Massawe, F.; Mayes, S.; Twycross, J. Identification of gene modules associated with low temperatures response in Bambara groundnut by network-based analysis. PLoS ONE 2016, 11, e0148771. [Google Scholar] [CrossRef]
- Bhardwaj, A.R.; Joshi, G.; Kukreja, B.; Malik, V.; Arora, P.; Pandey, R.; Shukla, R.N.; Bankar, K.G.; Katiyar-Agarwal, S.; Goel, S.; et al. Global insights into high temperature and drought stress regulated genes by RNA-Seq in economically important oilseed crop Brassica juncea. BMC Plant Biol. 2015, 15, 9. [Google Scholar] [CrossRef]
- Graham, J.H.; Gottwald, T.R.; Cubero, J.; Achor, D.S. Xanthomonas axonopodis pv. citri: Factors affecting successful eradication of citrus canker. Mol. Plant Pathol. 2004, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Schubert, T.S.; Rizvi, S.A.; Sun, X.; Gottwald, T.R.; Graham, J.H.; Dixon, W.N. Meeting the challenge of eradicating citrus canker in florida-again. Plant Dis. 2001, 85, 340–356. [Google Scholar] [CrossRef]
- Brunings, A.M.; Gabriel, D.W. Xanthomonas citri: Breaking the surface. Mol. Plant Pathol. 2003, 4, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Thomma, B.P.; Eggermont, K.; Penninckx, I.A.; Mauch-Mani, B.; Vogelsang, R.; Cammue, B.P.; Broekaert, W.F. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA 1998, 95, 15107–15111. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Hückelhoven, R.; Kogel, K.H. Reactive oxygen intermediates in plant-microbe interactions: Who is who in powdery mildew resistance? Planta 2003, 216, 891–902. [Google Scholar] [CrossRef]
- Balmer, D.; de Papajewski, D.V.; Planchamp, C.; Glauser, G.; Mauch-Mani, B. Induced resistance in maize is based on organ-specific defence responses. Plant J. 2013, 74, 213–225. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, H.; Li, R.; Han, D.; Wang, L.; Wu, J.; Xu, P.; Zhang, S. GmBTB/POZ, a novel BTB/POZ domain-containing nuclear protein, positively regulates the response of soybean to Phytophthora sojae infection. Mol. Plant Pathol. 2019, 20, 78–91. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhao, Y.; Han, G.; Zhu, S. Systematic analysis of maize class III peroxidase gene family reveals a conserved subfamily involved in abiotic stress response. Gene 2015, 566, 95–108. [Google Scholar] [CrossRef]
- Catinot, J.; Buchala, A.; Abou-Mansour, E.; Métraux, J.P. Salicylic acid production in response to biotic and abiotic stress depends on isochorismate in Nicotiana benthamiana. FEBS Lett. 2008, 582, 473–478. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef]
- de Torres Zabala, M.; Bennett, M.H.; Truman, W.H.; Grant, M.R. Antagonism between salicylic and abscisic acid reflects early host-pathogen conflict and moulds plant defence responses. Plant J. 2009, 59, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Hill, L.; Crooks, C.; Doerner, P.; Lamb, C. Abscisic acid has a key role in modulating diverse plant-pathogen interactions. Plant Physiol 2009, 150, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Xie, Y.; He, Y.; Li, Q.; Zou, X.; Chen, S. Abscisic acid promotes jasmonic acid accumulation and plays a key role in citrus canker development. Front. Plant Sci. 2019, 10, 1634. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yu, Q.; Zhang, C.; Xian, B.; Fan, J.; Huang, X.; Yang, W.; Zou, X.; Chen, S.; Su, L.; et al. CsAP2-09 confers resistance against citrus bacterial canker by regulating CsGH3.1L-mediated phytohormone biosynthesis. Int. J. Biol. Macromol. 2023, 229, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qin, X.; Qi, J.; Dou, W.; Dunand, C.; Chen, S.; He, Y. CsPrx25, a class III peroxidase in Citrus sinensis, confers resistance to citrus bacterial canker through the maintenance of ROS homeostasis and cell wall lignification. Hortic. Res. 2020, 7, 192. [Google Scholar] [CrossRef] [PubMed]
- Delaney, T.P.; Uknes, S.; Vernooij, B.; Friedrich, L.; Weymann, K.; Negrotto, D.; Gaffney, T.; Gut-Rella, M.; Kessmann, H.; Ward, E.; et al. A central role of salicylic acid in plant disease resistance. Science 1994, 266, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D. Plant pathology. Paranoid plants have their genes examined. Curr. Biol. 1994, 4, 749–751. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, L.L.; Ruan, X.; Chen, D.; Zhu, A.; Chen, C.; Bertrand, D.; Jiao, W.B.; Hao, B.H.; Lyon, M.P.; et al. The draft genome of sweet orange (Citrus sinensis). Nat. Genet. 2013, 45, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, D.; Lei, Y.; Chang, J.W.; Hao, B.H.; Xing, F.; Li, S.; Xu, Q.; Deng, X.X.; Chen, L.L. Citrus sinensis annotation project (CAP): A comprehensive database for sweet orange genome. PLoS ONE 2014, 9, e87723. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, X.; Liu, S.; Huang, Y.; Guo, Y.X.; Xie, W.Z.; Tahir Ul Qamar, M.; Xu, Q.; Chen, L.L. Citrus Pan-Genome to Breeding Database (CPBD): A comprehensive genome database for citrus breeding. Mol. Plant 2022, 15, 1503–1505. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.A.; Prochnik, S.; Jenkins, J.; Salse, J.; Hellsten, U.; Murat, F.; Perrier, X.; Ruiz, M.; Scalabrin, S.; Terol, J.; et al. Sequencing of diverse mandarin, pummelo and orange genomes reveals complex history of admixture during citrus domestication. Nat. Biotechnol. 2014, 32, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic. Acids. Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qi, J.L.; Qin, X.J.; Dou, W.F.; Lei, T.G.; Hu, A.H.; Jia, R.R.; Jiang, G.J.; Zou, X.P.; Long, Q.; et al. CitGVD: A comprehensive database of citrus genomic variations. Hortic. Res. 2020, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [PubMed]
- Yu, C.S.; Chen, Y.C.; Lu, C.H.; Hwang, J.K. Prediction of protein subcellular localization. Proteins 2006, 64, 643–651. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic. Acids. Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Li, Q.; Hu, A.H.; Qi, J.J.; Dou, W.F.; Qin, X.J.; Zou, X.P.; Xu, L.Z.; Chen, S.C.; He, Y.R. CsWAKL08, a pathogen-induced wall-associated receptor-like kinase in sweet orange, confers resistance to citrus bacterial canker via ROS control and JA signaling. Hortic. Res. 2020, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, M.; Liu, X.; Xu, Y.; Zhu, S.; Shen, W.; Zhao, X. Identification of putative genes involved in limonoids biosynthesis in citrus by comparative transcriptomic analysis. Front. Plant Sci. 2017, 8, 782. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol. J. 2017, 15, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Mafra, V.; Kubo, K.S.; Alves-Ferreira, M.; Ribeiro-Alves, M.; Stuart, R.M.; Boava, L.P.; Rodrigues, C.M.; Machado, M.A. Reference genes for accurate transcript normalization in citrus genotypes under different experimental conditions. PLoS ONE 2012, 7, e31263. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Wang, S.; Dong, L.; Qu, R.; Zheng, L.; He, Y.; Chen, S.; Zou, X. Overexpression of a “Candidatus Liberibacter Asiaticus” effector gene CaLasSDE115 contributes to early colonization in Citrus sinensis. Front. Microbiol. 2021, 12, 797841. [Google Scholar] [CrossRef] [PubMed]





| Name | CPBD ID | No. of AA | MW (Da) | pI | Subcellular Loci |
|---|---|---|---|---|---|
| CsGATA01 | Cs_ont_1g006010.1 | 187 | 21,109.18 | 9.33 | Nuclear |
| CsGATA02 | Cs_ont_1g006030.1 | 176 | 19,944.61 | 8.67 | Nuclear |
| CsGATA03 | Cs_ont_1g009190.1 | 293 | 32,054.5 | 6.15 | Nuclear |
| CsGATA04 | Cs_ont_1g017330.1 | 334 | 36,666.63 | 5.72 | Nuclear |
| CsGATA05 | Cs_ont_1g026740.1 | 255 | 28,332.15 | 8.3 | Nuclear |
| CsGATA06 | Cs_ont_1g027500.1 | 381 | 41,862.23 | 7.17 | Nuclear |
| CsGATA07 | Cs_ont_2g034040.1 | 263 | 28,955.71 | 7.68 | Nuclear |
| CsGATA08 | Cs_ont_2g034050.1 | 259 | 28,697.63 | 6.23 | Nuclear |
| CsGATA09 | Cs_ont_3g012160.1 | 313 | 34,021.14 | 8.57 | Nuclear |
| CsGATA10 | Cs_ont_3g012170.1 | 372 | 40,633.73 | 4.68 | Nuclear |
| CsGATA11 | Cs_ont_4g004550.1 | 372 | 41,198.54 | 6.21 | Nuclear |
| CsGATA12 | Cs_ont_4g005450.1 | 319 | 35,283.36 | 9.36 | Nuclear |
| CsGATA13 | Cs_ont_4g005460.1 | 314 | 35,014.26 | 9.56 | Nuclear |
| CsGATA14 | Cs_ont_4g010540.1 | 306 | 33,789.07 | 9.34 | Nuclear |
| CsGATA15 | Cs_ont_5g001200.1 | 187 | 21,238.65 | 10.17 | Nuclear |
| CsGATA16 | Cs_ont_5g007570.1 | 135 | 14,885.36 | 10.07 | Nuclear |
| CsGATA17 | Cs_ont_5g015080.1 | 321 | 35,288.43 | 6.66 | Nuclear |
| CsGATA18 | Cs_ont_5g043090.1 | 542 | 60,299.05 | 6.08 | Nuclear |
| CsGATA19 | Cs_ont_7g006300.1 | 341 | 37,446.96 | 6.27 | Nuclear |
| CsGATA20 | Cs_ont_8g005160.1 | 277 | 31,232.25 | 8.96 | Nuclear |
| CsGATA21 | Cs_ont_8g017440.1 | 375 | 41,116.14 | 4.77 | Nuclear |
| CsGATA22 | Cs_ont_8g020340.1 | 316 | 35,204.05 | 5.41 | Nuclear |
| CsGATA23 | Cs_ont_9g001910.1 | 147 | 15,909.97 | 9.63 | Nuclear |
| CsGATA24 | Cs_ont_9g011520.1 | 238 | 26,444.29 | 9.6 | Nuclear |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Xian, B.; Huang, X.; Yu, Q.; Zhang, M.; Zhang, C.; Jia, R.; Chen, S.; He, Y.; Li, Q. Genome-Wide Identification and Characterization of the Sweet Orange (Citrus sinensis) GATA Family Reveals a Role for CsGATA12 as a Regulator of Citrus Bacterial Canker Resistance. Int. J. Mol. Sci. 2024, 25, 2924. https://doi.org/10.3390/ijms25052924
Fan J, Xian B, Huang X, Yu Q, Zhang M, Zhang C, Jia R, Chen S, He Y, Li Q. Genome-Wide Identification and Characterization of the Sweet Orange (Citrus sinensis) GATA Family Reveals a Role for CsGATA12 as a Regulator of Citrus Bacterial Canker Resistance. International Journal of Molecular Sciences. 2024; 25(5):2924. https://doi.org/10.3390/ijms25052924
Chicago/Turabian StyleFan, Jie, Baohang Xian, Xin Huang, Qiyuan Yu, Miao Zhang, Chenxi Zhang, Ruirui Jia, Shanchun Chen, Yongrui He, and Qiang Li. 2024. "Genome-Wide Identification and Characterization of the Sweet Orange (Citrus sinensis) GATA Family Reveals a Role for CsGATA12 as a Regulator of Citrus Bacterial Canker Resistance" International Journal of Molecular Sciences 25, no. 5: 2924. https://doi.org/10.3390/ijms25052924
APA StyleFan, J., Xian, B., Huang, X., Yu, Q., Zhang, M., Zhang, C., Jia, R., Chen, S., He, Y., & Li, Q. (2024). Genome-Wide Identification and Characterization of the Sweet Orange (Citrus sinensis) GATA Family Reveals a Role for CsGATA12 as a Regulator of Citrus Bacterial Canker Resistance. International Journal of Molecular Sciences, 25(5), 2924. https://doi.org/10.3390/ijms25052924







