Monocytes as Targets for Immunomodulation by Regional Citrate Anticoagulation
Abstract
1. Introduction
2. Results
2.1. Cohort Characteristics
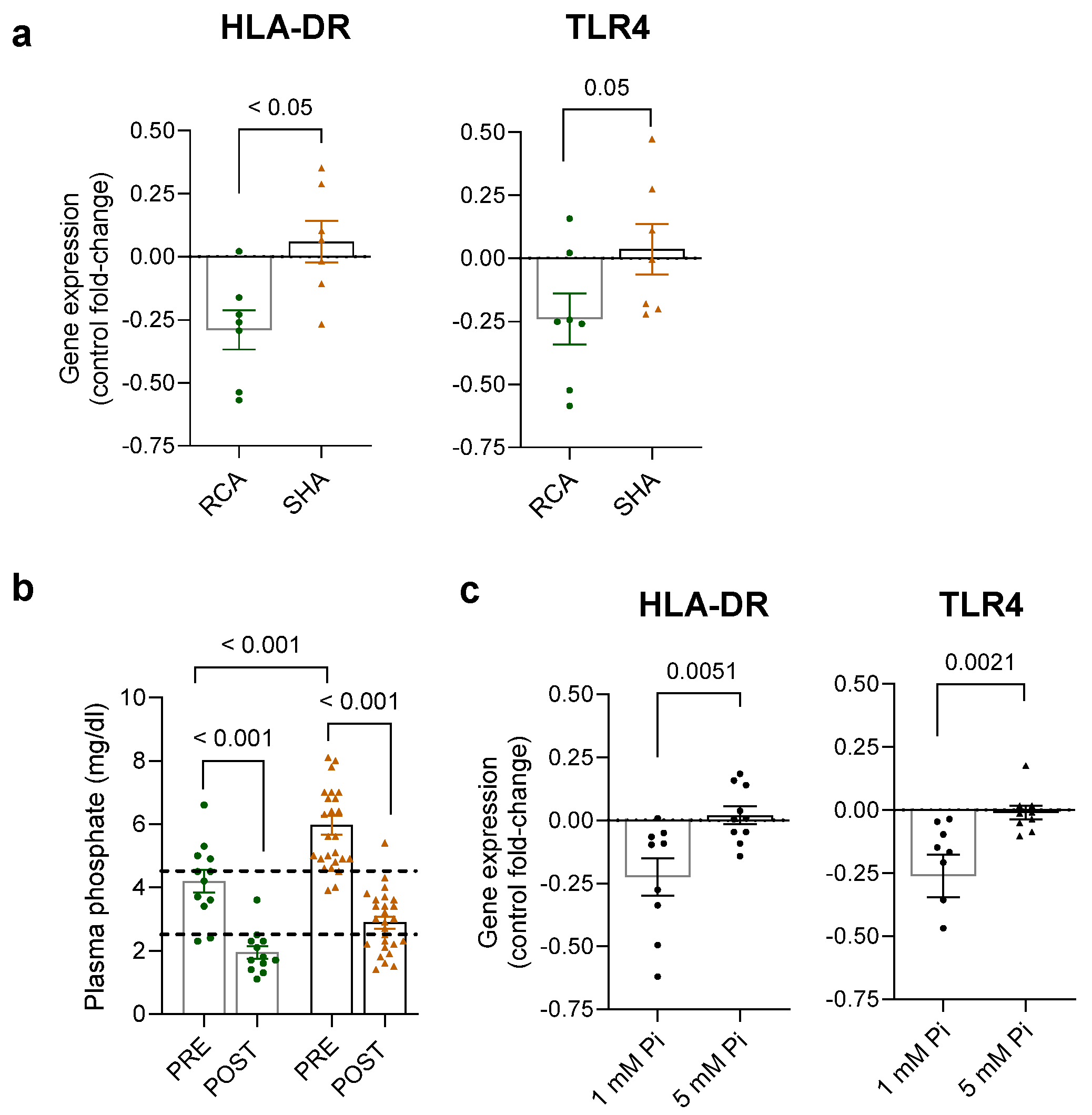
2.2. Differential Expression of Activation Markers on iHD Patient Monocytes Respective to the Anticoagulation Modality
2.3. Transcriptional Changes in Monocytes of Patients Receiving RCA and SHA
2.4. Systemic Changes Associated with the Anticoagulation Modalities
3. Discussion
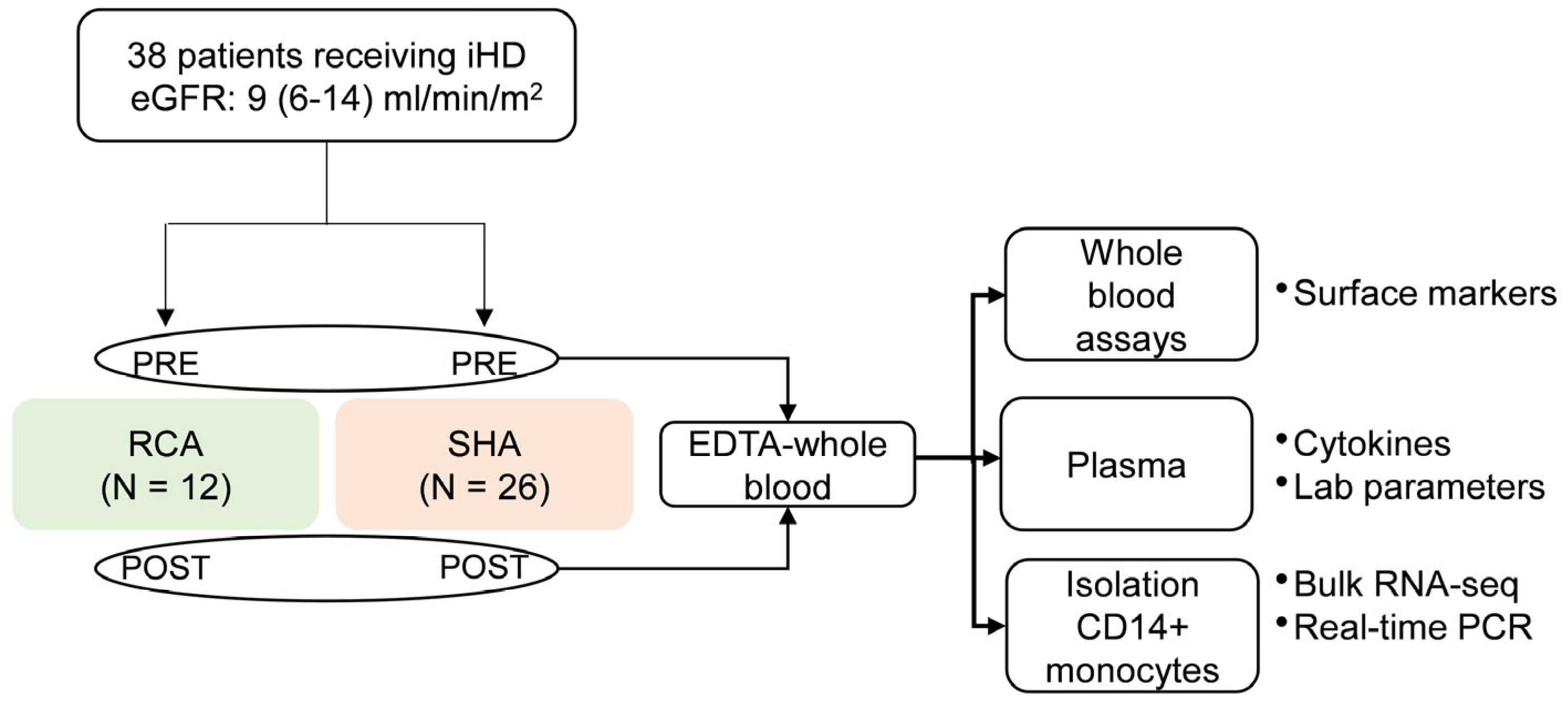
4. Materials and Methods
4.1. Study Population
4.2. Intermittent Hemodialysis and Anticoagulation Procedure
4.3. Sample and Data Collection
4.4. Characterization of Monocytes in Whole Blood Using Flow Cytometry
4.5. Monocyte Isolation
4.6. Determination of Cytokines and Circulating Proteins
4.7. RNA Isolation and Real-Time Quantitative PCR
4.8. RNA Sequencing
4.9. Functional Enrichment Analysis
4.10. THP-1 Cells
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kato, S.; Chmielewski, M.; Honda, H.; Pecoits-Filho, R.; Matsuo, S.; Yuzawa, Y.; Tranaeus, A.; Stenvinkel, P.; Lindholm, B. Aspects of immune dysfunction in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1526–1533. [Google Scholar] [CrossRef]
- Rossaint, J.; Oehmichen, J.; Van Aken, H.; Reuter, S.; Pavenstädt, H.J.; Meersch, M.; Unruh, M.; Zarbock, A. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J. Clin. Investig. 2016, 126, 962–974. [Google Scholar] [CrossRef]
- Girndt, M.; Trojanowicz, B.; Ulrich, C. Monocytes in Uremia. Toxins 2020, 12, 340. [Google Scholar] [CrossRef]
- Cohen, G. Immune Dysfunction in Uremia 2020. Toxins 2020, 12, 439. [Google Scholar] [CrossRef]
- Girndt, M.; Sester, U.; Sester, M.; Kaul, H.; Kohler, H. Impaired cellular immune function in patients with end-stage renal failure. Nephrol. Dial. Transpl. 1999, 14, 2807–2810. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.H.; Kireta, S.; Russ, G.R.; Coates, P.T. Uremia impairs blood dendritic cell function in hemodialysis patients. Kidney Int. 2007, 71, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, V.; Jeron, A.; Shah, A.; Bruder, D.; Mertens, P.R.; Gorny, X. Hemodialysis-related changes in phenotypical features of monocytes. Sci. Rep. 2018, 8, 13964. [Google Scholar] [CrossRef] [PubMed]
- Campo, S.; Lacquaniti, A.; Trombetta, D.; Smeriglio, A.; Monardo, P. Immune System Dysfunction and Inflammation in Hemodialysis Patients: Two Sides of the Same Coin. J. Clin. Med. 2022, 11, 3759. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.B.; Stinghen, A.E.M.; Kato, S.; Bucharles, S.; Aita, C.; Yuzawa, Y.; Pecoits-Filho, R. Characteristics and causes of immune dysfunction related to uremia and dialysis. Perit. Dial. Int. 2008, 28, S183–S187. [Google Scholar] [CrossRef] [PubMed]
- Goet, E.; Wentz, B.; Frank, R.D. Regional citrate anticoagulation protocol suitable for intermittent hemodialysis and post-dilution hemodiafiltration. Clin. Nephrol. 2021, 96, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.M.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J.; et al. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar] [CrossRef]
- Leroy, C.; Pereira, B.; Soum, E.; Bachelier, C.; Coupez, E.; Calvet, L.; Bachoumas, K.; Dupuis, C.; Wouweine, B.; Lautrette, A. Comparison between regional citrate anticoagulation and heparin for intermittent hemodialysis in ICU patients: A propensity score-matched cohort study. Ann. Intensive Care 2021, 11, 13. [Google Scholar] [CrossRef]
- Lin, T.; Song, L.; Huang, R.; Huang, Y.; Tang, S.; Lin, Q.; Zhang, Y.; Wu, X.; Liang, H.; Wu, Y.; et al. Modified regional citrate anticoagulation is optimal for hemodialysis in patients at high risk of bleeding: A prospective randomized study of three anticoagulation strategies. BMC Nephrol. 2019, 20, 472. [Google Scholar] [CrossRef]
- Zarbock, A.; Küllmar, M.; Kindgen-Milles, D.; Wempe, C.; Gerss, J.; Brandenburger, T.; Dimski, T.; Tyczynski, B.; Jahn, M.; Mülling, N.; et al. Effect of Regional Citrate Anticoagulation vs Systemic Heparin Anticoagulation during Continuous Kidney Replacement Therapy on Dialysis Filter Life Span and Mortality among Critically Ill Patients with Acute Kidney Injury: A Randomized Clinical Trial. JAMA 2020, 324, 1629–1639. [Google Scholar] [CrossRef]
- Kim, O.Y.; Monsel, A.; Bertrand, M.; Coriat, P.; Cavaillon, J.M.; Adib-Conquy, M. Differential down-regulation of HLA-DR on monocyte subpopulations during systemic inflammation. Crit. Care 2010, 14, R61. [Google Scholar] [CrossRef] [PubMed]
- Palojärvi, A.; Petäjä, J.; Siitonen, S.; Janér, C.; Andersson, S. Low monocyte HLA-DR expression as an indicator of immunodepression in very low birth weight infants. Pediatr. Res. 2012, 73, 469–475. [Google Scholar] [CrossRef]
- Frazier, W.J.; Hall, M.W. Immunoparalysis and Adverse Outcomes from Critical Illness. Pediatr. Clin. N. Am. 2008, 55, 647–668. [Google Scholar] [CrossRef] [PubMed]
- Girndt, M.; Sester, M.; Sester, U.; Kaul, H.; Köhler, H. Defective expression of B7-2 (CD86) on monocytes of dialysis patients correlates to the uremia-associated immune defect. Kidney Int. 2001, 59, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Kaewdoungtien, P.; Tachaboon, S.; Kangsumrith, N.; Srisawat, N. Immunomodulation Effect of Regional Citrate Anticoagulation in Acute Kidney Injury Requiring Renal Replacement Therapy. Blood Purif. 2023, 52, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Ashbrook, M.J.; Mcdonough, K.L.; Pituch, J.J.; Christopherson, P.L.; Cornell, T.T.; Selewski, D.T.; Shanley, T.P.; Blatt, N.B. Citrate modulates lipopolysaccharide-induced monocyte inflammatory responses. Clin. Exp. Immunol. 2015, 180, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Collier, S.; Davenport, A. Reducing the risk of infection in end-stage kidney failure patients treated by dialysis. Nephrol. Dial. Transpl. 2014, 29, 2158. [Google Scholar] [CrossRef]
- Brands, X.; Haak, B.W.; Klarenbeek, A.M.; Butler, J.; Uhel, F.; Qin, W.; Otto, N.A.; Jakobs, M.E.; Faber, D.R.; Lutter, R.; et al. An epigenetic and transcriptomic signature of immune tolerance in human monocytes through multi-omics integration. Genome Med. 2021, 13, 131. [Google Scholar] [CrossRef]
- Balomenos, D.; Martínez, A.C. Cell-cycle regulation in immunity, tolerance and autoimmunity. Immunol. Today 2000, 21, 551–555. [Google Scholar] [CrossRef]
- Nahrendorf, W.; Ivens, A.; Spence, P.J. Inducible mechanisms of disease tolerance provide an alternative strategy of acquired immunity to malaria. eLife 2021, 10, e63838. [Google Scholar] [CrossRef]
- Lowe, J.; Shatz, M.; Resnick, M.A.; Menendez, D. Modulation of immune responses by the tumor suppressor p53. BioDiscovery 2023, 8, e8947. [Google Scholar] [CrossRef]
- Łasut-Szyszka, B.; Rusin, M. The Wheel of p53 Helps to Drive the Immune System. Int. J. Mol. Sci. 2023, 24, 7645. [Google Scholar] [CrossRef]
- Kruiswijk, F.; Labuschagne, C.F.; Vousden, K.H. p53 in survival, death and metabolic health: A lifeguard with a licence to kill. Nat. Rev. Mol. Cell Biol. 2015, 16, 393–405. [Google Scholar] [CrossRef]
- Craddock, P.R.; Yawata, Y.; VanSanten, L.; Gilberstadt, S.; Silvis, S.; Jacob, H.S. Acquired Phagocyte Dysfunction. N. Engl. J. Med. 1974, 290, 1403–1407. [Google Scholar] [CrossRef]
- Tang, J.P.; Peters, C.W.; Quiros, C.; Wang, X.; Klomhaus, A.M.; Yamada, R.E.; Timmerman, J.M.; Moore, T.B.; Nowicki, T.S. Hypophosphatemia Due to Increased Effector Cell Metabolic Activity Is Associated with Neurotoxicity Symptoms in CD19-Targeted CAR T-cell Therapy. Cancer Immunol. Res. 2022, 10, 1433–1440. [Google Scholar] [CrossRef]
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front. Immunol. 2019, 10, 2759. [Google Scholar] [CrossRef]
- Rydzewska-Rosolowska, A.; Gozdzikiewicz-Lapinska, J.; Borawski, J.; Koc-Zorawska, E.; Mysliwiec, M.; Naumnik, B. Unexpected and striking effect of heparin-free dialysis on cytokine release. Int. Urol. Nephrol. 2017, 49, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Papayianni, A.; Alexopoulos, E.; Giamalis, P.; Gionanlis, L.; Belechri, A.-M.; Koukoudis, P.; Memmos, D. Circulating levels of ICAM-1, VCAM-1, and MCP-1 are increased in haemodialysis patients: Association with inflammation, dyslipidaemia, and vascular events. Nephrol. Dial. Transplant. 2002, 17, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Yanucil, C.; Kentrup, D.; Campos, I.; Czaya, B.; Heitman, K.; Westbrook, D.; Osis, G.; Grabner, A.; Wende, A.R.; Vallejo, J.; et al. Soluble α-klotho and heparin modulate the pathologic cardiac actions of fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2022, 102, 261–279. [Google Scholar] [CrossRef]
- Georgakis, M.K.; De Lemos, J.A.; Ayers, C.; Wnag, B.; Björkbacka, H.; Pana, T.A.; Thorand, B.; Sun, C.; Fani, L.; Malik, R.; et al. Association of Circulating Monocyte Chemoattractant Protein–1 Levels with Cardiovascular Mortality: A Meta-analysis of Population-Based Studies. JAMA Cardiol. 2021, 6, 587–592. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, Z.; Ouyang, N.; Ruan, X. Hyperphosphatemia and Cardiovascular Disease. Front. Cell Dev. Biol. 2021, 9, 644363. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef]
- Shute, J.K. Heparin, Low Molecular Weight Heparin, and Non-Anticoagulant Derivatives for the Treatment of Inflammatory Lung Disease. Pharmaceuticals 2023, 16, 584. [Google Scholar] [CrossRef] [PubMed]
- Mulloy, B. The non-anticoagulant promise of heparin and its mimetics. Curr. Opin. Pharmacol. 2019, 46, 50–54. [Google Scholar] [CrossRef]
- Tiranathanagul, K.; Jearnsujitwimol, O.; Susantitaphong, P.; Kijkriengkraikul, N.; Leelahavanichkul, A.; Srisawat, N.; Praditpornsilpa, K.; Eiam-Ong, S. Regional Citrate Anticoagulation Reduces Polymorphonuclear Cell Degranulation in Critically Ill Patients Treated with Continuous Venovenous Hemofiltration. Ther. Apher. Dial. 2011, 15, 556–564. [Google Scholar] [CrossRef]
- Döcke, W.D.; Höflich, C.; Davis, K.A.; Röttgers, K.; Meisel, C.; Kiefer, P.; Weber, S.U.; Hedwig-Geissing, M.; Kreuzfelder, E.; Tschentscher, P.; et al. Monitoring Temporary Immunodepression by Flow Cytometric Measurement of Monocytic HLA-DR Expression: A Multicenter Standardized Study. Clin. Chem. 2005, 51, 2341–2347. [Google Scholar] [CrossRef]
- Harms, H.; Prass, K.; Meisel, C.; Klehmet, J.; Rogge, W.; Drenckhahn, C.; Göhler, J.; Bereswill, S.; Göbel, U.; Wernecke, K.D.; et al. Preventive antibacterial therapy in acute ischemic stroke: A randomized controlled trial. PLoS ONE 2008, 3, e2158. [Google Scholar] [CrossRef] [PubMed]
- Cheron, A.; Floccard, B.; Allaouchiche, B.; Guignant, C.; Poitevin, F.; Malcus, C.; Crozon, J.; Faure, A.; Guillaume, C.; Marcotte, G.; et al. Lack of recovery in monocyte human leukocyte antigen-DR expression is independently associated with the development of sepsis after major trauma. Crit. Care 2010, 14, R208. [Google Scholar] [CrossRef] [PubMed]
- Joshi, I.; Carney, W.P.; Rock, E.P. Utility of monocyte HLA-DR and rationale for therapeutic GM-CSF in sepsis immunoparalysis. Front. Immunol. 2023, 14, 1130214. [Google Scholar] [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef] [PubMed]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 2016, 44, W90. [Google Scholar] [CrossRef] [PubMed]
- Supek, F.; Bošnjak, M.; Škunca, N.; Šmuc, T. REVIGO Summarizes and Visualizes Long Lists of Gene Ontology Terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523. [Google Scholar] [CrossRef]
- McKee, T.J.; Komarova, S.V. Is it time to reinvent basic cell culture medium? Am. J. Physiol. Cell Physiol. 2017, 312, C624–C626. [Google Scholar] [CrossRef]



| RCA | SHA | p-Value | |
|---|---|---|---|
| n = 6–12 * | n = 22–26 ** | ||
| Demographics | |||
| Age, in years | 60 ± 17 | 62 ± 15 | 0.82 |
| Sex, male | 8 (67) | 18 (69) | 0.87 |
| BMI | 28 ± 6 | 30 ± 5 | 0.10 |
| Blood analysis | |||
| Albumin, g/dL | 3.1 (3.0–3.5) | 3.2 (3.0–3.7) | 0.57 |
| BUN, mg/dL | 38 ± 16 | 41 ± 15 | 0.47 |
| Creatinine, mg/dL | 6.2 ± 3.1 | 6.3 ± 2.8 | 0.93 |
| CRP, mg/dL | 1.4 ± 0.9 | 2.4 ± 1.9 | 0.11 |
| Calcium, mmol/L | 2.2 (2.1–2.2) | 2.3 (2.1–2.4) | 0.08 |
| Ionized calcium, mmol/L | 1.13 ± 0.08 | 1.15 ± 0.08 | 0.392 |
| Phosphate, mg/dL | 4.3 ± 1.3 | 5.8 ± 1.8 | 0.02 |
| PTH, pmol/L | 91 (55–106) | 120 (52–242) | 0.43 |
| 1,25(OH)2D, pg/mL | 20 (10–28) | 15 (10–26) | 0.37 |
| Comorbidities and risk factors | |||
| CVD | 12 (46) | 8 (67) | 0.24 |
| Diabetes | 12 (46) | 4 (33) | 0.46 |
| Hypertension | 22 (85) | 12 (100) | 0.15 |
| Smoking | 8 (31) | 3 (25) | 0.72 |
| Dialysis | |||
| Weekly Kt/V | 1.4 ± 0.3 | 1.4 ± 0.3 | 0.64 |
| Session duration, hours | 4 | 4 | - |
| Membrane | polysulfone high flux | polysulfone high flux | - |
| RCA | SHA | Control | p-Value | |
|---|---|---|---|---|
| n = 9–12 * | n = 19–26 ** | n = 22–25 *** | ||
| Cytokines | ||||
| CX3CL1 | 610 (412–1086) | 663 (484–842) | 663 (479–811) | 0.96 |
| IFNγ | 9 (5–25) | 7 (2–24) | 12 (7–27) | 0.47 |
| IL-1β | 7 (3–16) | 3 (1–9) | 7 (2–10) | 0.22 |
| IL-4 | 3 (2–9) | 2 (2–6) | 4 (5–7) | 0.48 |
| IL-6 | 11 (8–21) # | 21 (7–40) # | 4 (3–8) | <0.001 |
| IL-10 | 313 (217–431) | 364 (319–571) | 396 (308–588) | 0.19 |
| MCP-1 | 260 (163–361) &,§ | 747 (557– 1077) # | 512 (353–590) | <0.0001 |
| Antimicrobial peptides | ||||
| BD1 | 1077 ± 134 # | 976 ± 163 # | 1441 ± 62 | <0.001 |
| BD2 | 299 ± 70 | 381 ± 137 | 303 ± 217 | 0.54 |
| BD3 | 48 (29–114) $ | 88 (65–142) # | 48 (35–86) | 0.004 |
| LL-37 | 4.5 (3.7–4.9) | 4.7 (4.0–6.0) | 4.7 (4.5–7.0) | 0.22 |
| CVD and endothelium-related biomarkers | ||||
| Angio-2 | 3605 (2168–6451) # | 4468 (2117–8116) # | 928 (649–1464) | <0.001 |
| FGF-23 | 3287 ± 2248 #,§ | 6418 ± 3573 # | 1207 ± 1031 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Marco, G.S.; Chasan, A.I.; Boeckel, G.R.; Beul, K.; Pavenstädt, H.; Roth, J.; Brand, M. Monocytes as Targets for Immunomodulation by Regional Citrate Anticoagulation. Int. J. Mol. Sci. 2024, 25, 2900. https://doi.org/10.3390/ijms25052900
Di Marco GS, Chasan AI, Boeckel GR, Beul K, Pavenstädt H, Roth J, Brand M. Monocytes as Targets for Immunomodulation by Regional Citrate Anticoagulation. International Journal of Molecular Sciences. 2024; 25(5):2900. https://doi.org/10.3390/ijms25052900
Chicago/Turabian StyleDi Marco, Giovana Seno, Achmet Imam Chasan, Göran Ramin Boeckel, Katrin Beul, Hermann Pavenstädt, Johannes Roth, and Marcus Brand. 2024. "Monocytes as Targets for Immunomodulation by Regional Citrate Anticoagulation" International Journal of Molecular Sciences 25, no. 5: 2900. https://doi.org/10.3390/ijms25052900
APA StyleDi Marco, G. S., Chasan, A. I., Boeckel, G. R., Beul, K., Pavenstädt, H., Roth, J., & Brand, M. (2024). Monocytes as Targets for Immunomodulation by Regional Citrate Anticoagulation. International Journal of Molecular Sciences, 25(5), 2900. https://doi.org/10.3390/ijms25052900





