Comparative Analysis of the Mitochondrial Genomes of Chloropidae and Their Implications for the Phylogeny of the Family
Abstract
1. Introduction
2. Results
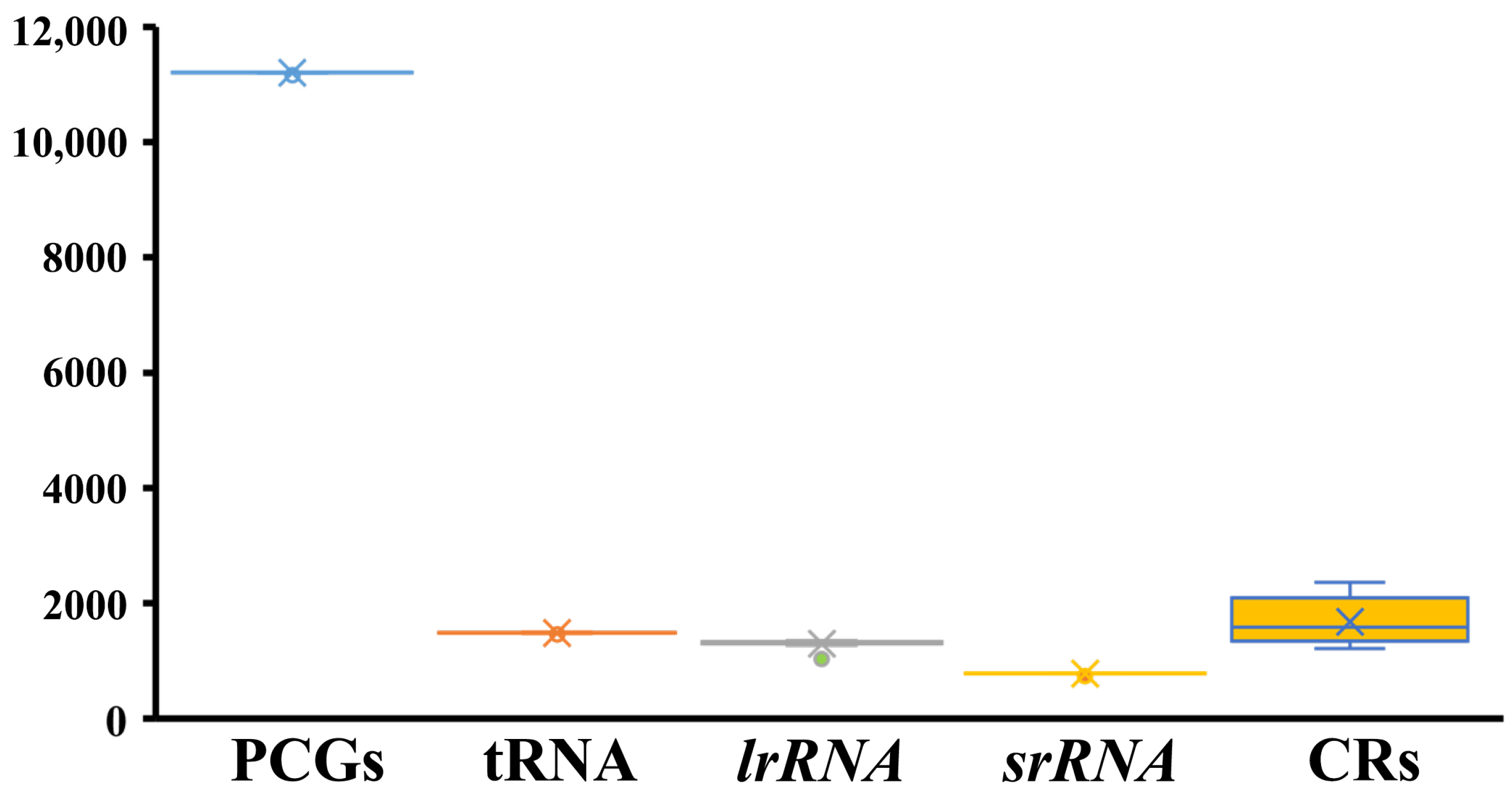
2.1. General Features and Genome Organization
2.2. Base Composition
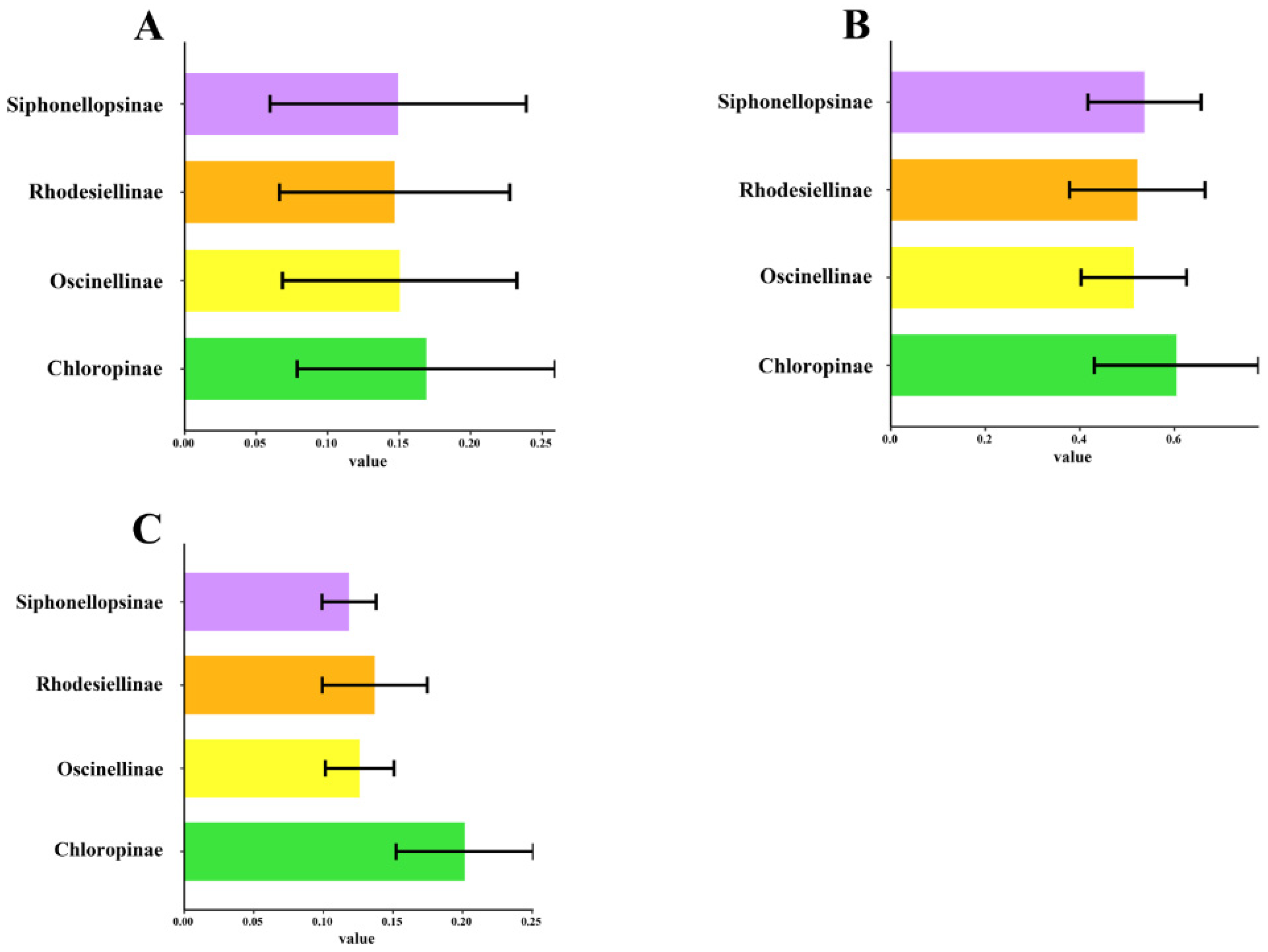
2.3. Protein-Coding Genes, Codon Usage, and Evolutionary Rate Analysis
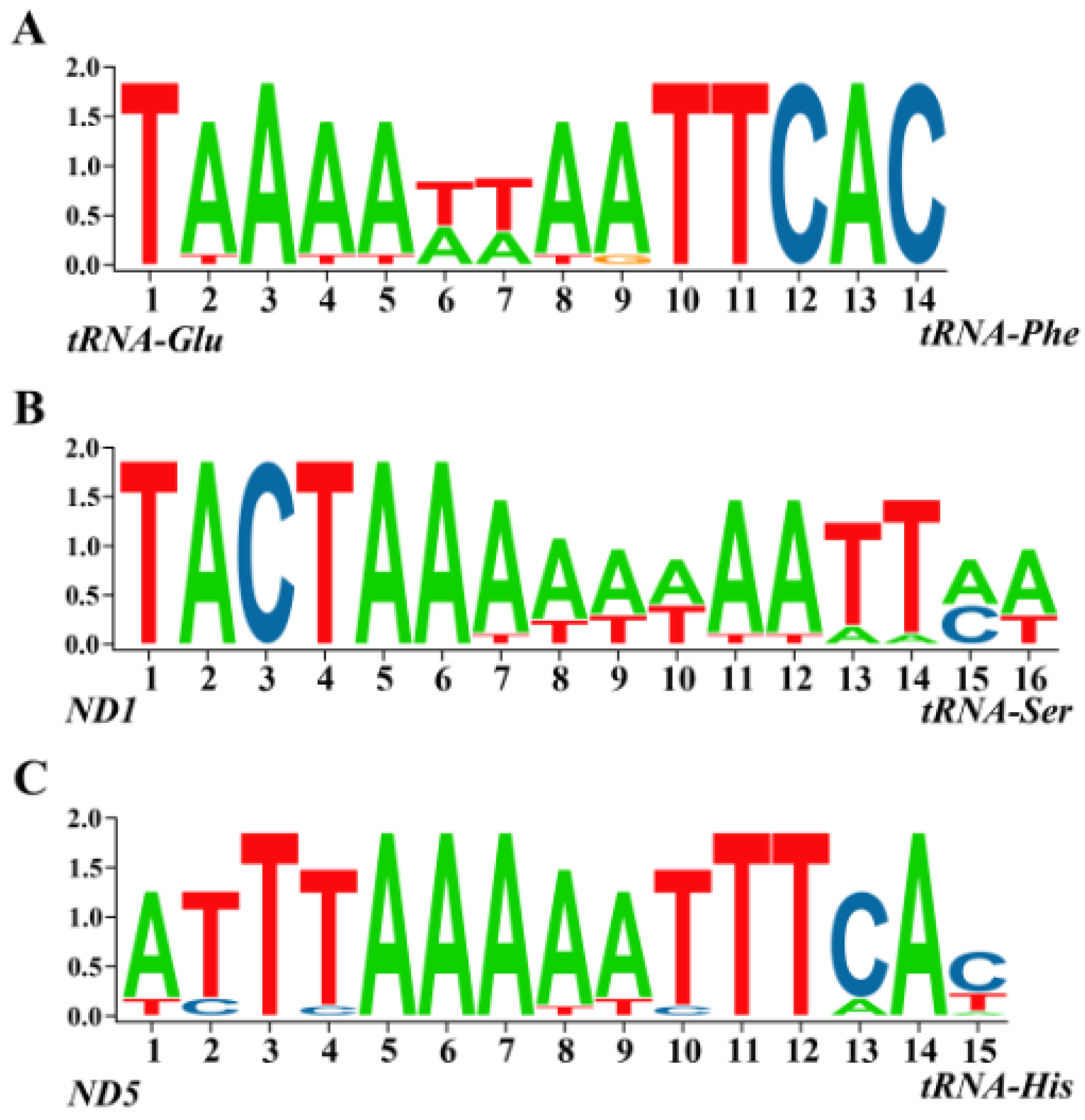
2.4. Intergenic Sequences
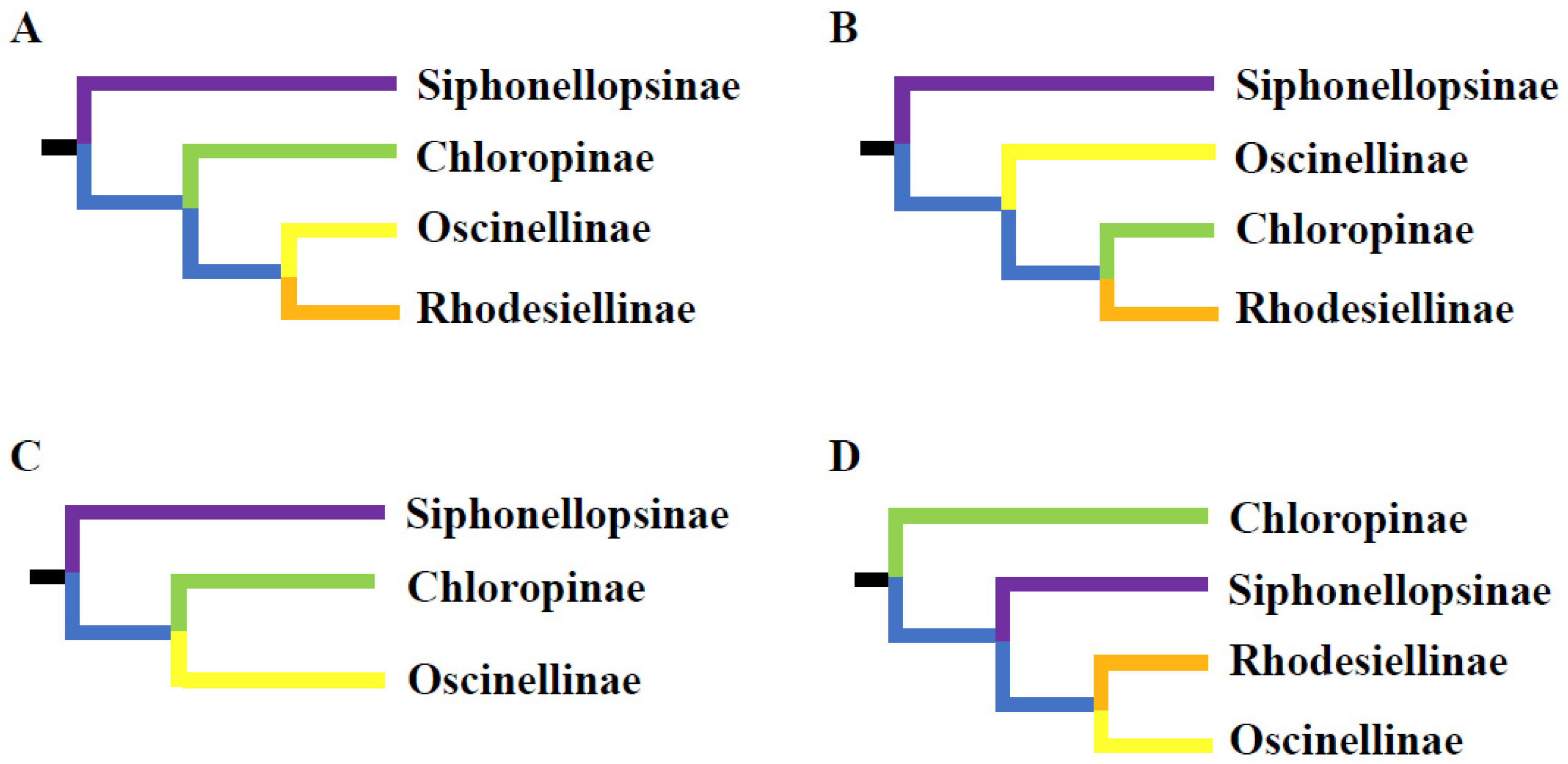
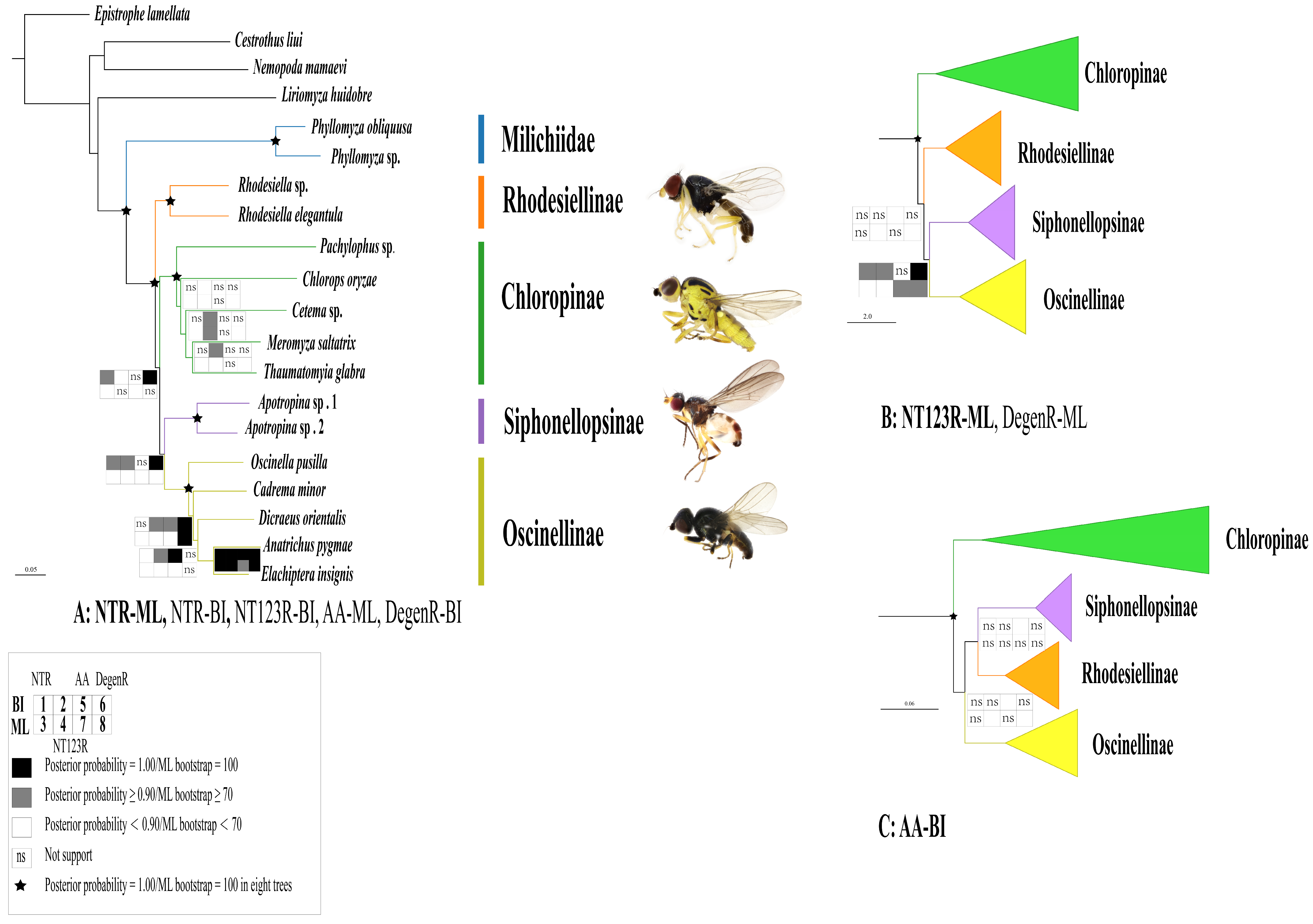
2.5. Phylogenetic Analyses
3. Discussion
4. Methods and Materials
4.1. Taxon Sampling and DNA Extraction
4.2. Mitochondrial Genome Sequencing and Assembly
4.3. Bioinformatic Analysis
| Family | Subfamily | Species | Accession Number | Reference |
|---|---|---|---|---|
| Outgroup | ||||
| Syrphidae | Syrphinae | Epistrophe lamellata | MZ398236 | [51] |
| Sepsidae | Sciomyzoidea | Nemopoda mamaevi | KM605250 | [31] |
| Lauxaniidae | Cestrotus liui | KX372559 | [32] | |
| Agromyzidae | Phytomyzinae | Liriomyza huidobrensis | JN570505 | [52] |
| Milichiidae | Phyllomyza sp. | OP612805 | this study | |
| Phyllomyza obliquusa | MT462165 | [16] | ||
| Ingroup | ||||
| Siphonellopsinae | Apotropina sp.1 | OP612811 | this study | |
| Apotropina sp.2 | OP612809 | this study | ||
| Chloropidae | Chloropinae | Cetema sp. | OR522694 | this study |
| Chlorops oryzae | NC_059894 | [34] | ||
| Meromyza saltatrix | NC_072204 | this study | ||
| Pachylophus sp. | MT462163 | [16] | ||
| Thaumatomyia glabra | NC_072209 | this study | ||
| Rhodesiellinae | Rhodesiella sp. | OP612804 | this study | |
| Rhodesiella elegantula | NC_072206 | this study | ||
| Oscinellinae | Anatrichus pygmaeus | NC_063616 | [33] | |
| Cadrema minor | NC_072207 | this study | ||
| Dicraeus orientalis | NC_057210 | [35] | ||
| Elachiptera insignis | NC_072208 | this study | ||
| Osciella pusilla | NC_072205 | this study |
4.4. Phylogenetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nartshuk, E.P. A check list of the world genera of the family Chloropidae (Diptera, Cyclorrhapha, Muscomorpha). Zootaxa 2012, 3267, 1–43. [Google Scholar] [CrossRef]
- Riccardi, P.R.; Amorim, D.D.S. Phylogenetic relationships and classification of the Chloropinae of the world (Diptera: Chloropidae). Zool. J. Linn. Soc. 2020, 190, 889–941. [Google Scholar] [CrossRef]
- Kanmiya, K. A systematic study of the Japanese Chloropidae (Diptera). Mem. Ent. Soc. Wash. 1983, 11, 1–370. [Google Scholar]
- Ismay, J.W.; Ismay, B.; Deeming, J.C. 96. Chloropidae. In Manual of Afrotropical Diptera. Volume 3. Brachycera-Cyclorrhapha, Excluding Calyptratae; Kirk-Spriggs, A.H., Sinclair, B.J., Eds.; Suricata 8; South African National Biodiversity Institute: Pretoria, South Africa, 2021; pp. 2035–2114. [Google Scholar]
- Wiegmann, B.M.; Trautwein, M.D.; Winkler, I.S.; Barr, N.B.; Kim, J.W.; Lambkin, C.; Bertone, M.A.; Cassel, B.K.; Bayless, K.M.; Heimberg, A.M.; et al. Episodic radiations in the fly tree of life. Proc. Natl. Acad. Sci. USA 2011, 108, 5690–5695. [Google Scholar] [CrossRef] [PubMed]
- Evenhuis, N.L. Catalog of the Fossil Flies of the World (Insecta: Diptera); Backhuis Publishers: Leiden, The Netherlands, 1994; pp. 1–600. [Google Scholar]
- Paganelli, C.H.M.; Sabrosky, C.W. Hippelates flies (Diptera: Chloropidae) possibly associated with Brazilian purpuric fever. P. Entomol. Soc. Wash. 1993, 95, 165–174. [Google Scholar]
- Nartshuk, E.P. Grass-fly larvae (Diptera, Chloropidae): Diversity, habitats, and feeding specializations. Entomol. Rev. 2014, 94, 514–525. [Google Scholar] [CrossRef]
- Bower, C.; Towle, B.; Bickel, D. Reproductive success and pollination of the Tuncurry midge orchid (Genoplesium littorale) (Orchidaceae) by chloropid flies. Telopea. 2015, 18, 43–55. [Google Scholar] [CrossRef]
- Oelschlägel, B.; Nuss, M.; von Tschirnhaus, M.; Pätzold, C.; Neinhuis, C.; Dötterl, S.; Wanke, S. The betrayed thief–the extraordinary strategy of Aristolochia rotunda to deceive its pollinators. New Phytol. 2015, 206, 342–351. [Google Scholar] [CrossRef]
- Wiesenborn, W.D. Conspecific pollen on insects visiting female flowers on the oak parasite Phoradendron coryae (Viscaceae). West. N. Am. Nat. 2016, 76, 265–274. [Google Scholar] [CrossRef][Green Version]
- Kidyoo, A.; Kidyoo, M.; McKey, D.; Proffit, M.; Deconninck, G.; Wattana, P.; Uamjan, N.; Ekkaphan, P. Pollinator and floral odor specificity among four synchronopatric species of Ceropegia (Apocynaceae) suggests ethological isolation that prevents reproductive, Interference. Sci. Rep. 2022, 12, 13788. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, M.; Oikawa, S.; Kanmiya, K. Siphunculina quinquangula (Loew) (Diptera, Chloropidae) new to Japan: Emergence from the remains stage of pig carcass, with the implications for forensic entomology. Med. Entomol. Zool. 2013, 64, 103–106. [Google Scholar] [CrossRef]
- Buck, M. A new family and genus of acalypterate flies from the Neotropical region, with a phylogenetic analysis of Carnoidea family relationships (Diptera, Schizophora). Syst. Entomol. 2006, 31, 377–404. [Google Scholar] [CrossRef]
- Nartshuk, E.P.; Andersson, H. The frit flies (Chloropidae, Diptera) of Fennoscandia and Denmark. Fauna. Ent. Scand. 2013, 43, 1–282. [Google Scholar]
- Song, N.; Xi, Y.Q.; Yin, X.M. Phylogenetic relationships of Brachycera (Insecta: Diptera) inferred from mitochondrial genome sequences. Zool. J. Linn. Soc. 2022, 196, 720–739. [Google Scholar] [CrossRef]
- Andersson, H. Taxonomic and phylogenetic studies on Chloropidae (Diptera) with special reference to Old World genera. Ent. Scand. Suppl. 1997, 8, 1–200. [Google Scholar]
- Sabrosky, C.W. An Annotated List of Genotypes of the Chloropidae of the World (Diptera). Ann. Entomol. Soc. Am. 1941, 34, 735–765. [Google Scholar] [CrossRef]
- Wheeler, T.A. 92. Chloropidae (frit flies, grass flies, eye gnats). In Manual of Central American Diptera; Brown, B.V., Borkent, A., Cumming, J.M., Wood, D.M., Woodley, N.E., Zumbadoeds, M.A., Eds.; NRC Research Press: Ottawa, Canada, 2010; Volume 2, pp. 1137–1153. [Google Scholar]
- Ismay, J.; Nartshuk, E.P. Family Chloropidae. In Contributions to a Manual of Palaearctic Diptera (with Special Reference to Flies of Economic Importance). Appendix volume; Papp, L., Darvas, B., Eds.; Science Herald: Budapest, Hungary, 2000; pp. 387–429. [Google Scholar]
- Mlynarek, J.J.; Wheeler, T.A. Phylogeny and revised classification of the tribe Elachipterini (Diptera: Chloropidae). Zootaxa 2018, 4471, 1–36. [Google Scholar] [CrossRef]
- Nartshuk, E.P. Classification of the superfamily Chloropoidea (Diptera, Cyclorrhapha). Entomol. Rev. 1984, 62, 180–193. [Google Scholar]
- Nartshuk, E.P. Chloropid flies (Diptera: Chloropoidea): Their system, evolution and association with plants. Trudy. Zool. Inst. 1987, 136, 1–268. (In Russian) [Google Scholar]
- Cherian, P.T. Chloropidae (Part 1). Siphonellopsinae and Rhodesiellinae. The Fauna of India and Adjacent Countries. Diptera Volume IX; Zoological Survey of India: Kolkata, India, 2002; pp. 1–368. [Google Scholar]
- Andersson, H. Problem vid kladistik analys av flugfamiljen Chloropidae. Ent. Tidskr. 1979, 100, 180–187. [Google Scholar]
- Brake, I. Phylogenetic systematics of the Milichiidae (Diptera, Schizophora). Entomol. Scand. 2000, 57, 1–120. [Google Scholar]
- Bazyar, Z.A. Comparative Morphology of Oscinellinae Genera (Diptera: Chloropidae). Ph.D. Thesis, Universidade de Sao Paulo, Sao Paulo, Brazil, 2019. [Google Scholar]
- Ramakodi, M.P.; Singh, B.; Wells, J.D.; Guerrero, F.; Ray, D.A. A 454 sequencing approach to dipteran mitochondrial genome research. Genomics 2015, 105, 53–60. [Google Scholar] [CrossRef]
- Holland, B.R.; Jermiin, L.S.; Moulton, V. Improved consensus network techniques for genome-scale phylogeny. Mol. Biol. Evol. 2005, 23, 848–855. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Kang, Z.H.; Ding, S.M.; Wang, Y.Y.; Borkent, C.; Saigusa, T.; Yang, D. Mitochondrial Genomes Provide Insights into the Phylogeny of Culicomorpha (Insecta: Diptera). Int. J. Mol. Sci. 2019, 20, 747. [Google Scholar] [CrossRef] [PubMed]
- Li, X.K.; Ding, S.M.; Cameron, S.L.; Kang, Z.H.; Wang, Y.Y.; Yang, D. The first mitochondrial genome of the sepsid fly Nemopoda mamaevi Ozerov, 1997 (Diptera: Sciomyzoidea: Sepsidae), with mitochondrial genome phylogeny of cyclorrhapha. PLoS ONE 2012, 10, e0123594. [Google Scholar] [CrossRef] [PubMed]
- Li, X.K.; Li, W.L.; Ding, S.M.; Cameron, S.L.; Mao, M.; Shi, L.; Yang, D. Mitochondrial Genomes Provide Insights into the Phylogeny of Lauxanioidea (Diptera: Cyclorrhapha). Int. J. Mol. Sci. 2017, 18, 773. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.D.; Yang, D.; Liu, X.Y. The complete mitochondrial genome of Anatrichus pygmaeus Lamb, 1918 (Diptera, Chloropidae). Mitochondrial DNA B 2022, 7, 1285–1287. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.Y.; Du, R.B.; Liu, Y.H. The complete mitogenome of Chlorops oryzae Matsumura (Diptera: Chloropidae). Mitochondrial DNA B 2021, 6, 1844–1846. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; Li, X.; Cai, X.D.; Du, B.T.; Liu, X.Y.; Yang, D. The complete mitochondrial genome of Dicraeus orientalis Becker, 1911 (Diptera: Chloropidae). Mitochondrial DNA B 2021, 6, 951–952. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.P.; Xu, W.T.; Zhang, D.; Li, J.Q. Comparative analysis of the mitochondrial genomes of flesh flies and their evolutionary implication. Int. J. Biol. Macromol. 2021, 174, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Roberti, M.; Polosa, P.L.; Bruni, F.; Musicco, C.; Gadaleta, M.N.; Cantatore, P. DmTTF, a novel mitochondrial transcription termination factor that recognizes two sequences of Drosophila melanogaster mitochondrial DNA. Nucleic Acids Res. 2003, 31, 1597–1604. [Google Scholar] [CrossRef]
- Cameron, S.L.; Whiting, M.F. Mitochondrial genomic comparisons of the subterranean termites from the Genus Reticulitermes (Insecta: Isoptera: Rhinotermitidae). Genome 2007, 50, 188–202. [Google Scholar] [CrossRef]
- Taanman, J.W. The mitochondrial genome: Structure, transcription, translation and replication. Biochim. Biophys. Acta Bioenerg. 1999, 1410, 103–123. [Google Scholar] [CrossRef]
- Lee, C.; Wen, J. Phylogeny of Panax using chloroplast trnC-trnD intergenic region and the utility of trnC-trnD in interspecific studies of plants. Mol. Phylogenet. Evol. 2004, 31, 894–903. [Google Scholar] [CrossRef]
- Yang, D.; Yang, C.K. Chloropidae of China (Diptera). In Flies of China 1; Xue, W.Q., Chao, C.M., Eds.; Liaoning Science and Technology Press: Shenyang, China, 1998; pp. 547–573. [Google Scholar]
- Gillett, C.P.; Crampton-Platt, A.; Timmermans, M.J.; Jordal, B.H.; Emerson, B.C.; Vogler, A.P. Bulk de novo mitogenome assembly from pooled total DNA elucidates the phylogeny of weevils (Coleoptera: Curculionoidea). Mol. Biol. Evol. 2014, 31, 2223–2237. [Google Scholar] [CrossRef]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef]
- Peng, Y.; Leung, H.C.M.; Yiu, S.M.; Chin, F.Y.L. IDBA-UD: A de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics 2012, 28, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Hajibabaei, M.; Janzen, D.H.; Burns, J.M.; Hallwachs, W.; Hebert, P.D. DNA barcodes distinguish species of tropical Lepidoptera. Proc. Natl. Acad. Sci. USA 2006, 103, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Alzohairy, A.M. Bioedit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Donath, A.; Jühling, F.; Al-Arab, M.; Bernhart, S.H.; Reinhardt, F.; Stadler, P.F.; Middendorf, M.; Bernt, M. Improved annotation of protein-coding genes boundaries in metazoan mitochondrial genomes. Nucleic Acids Res. 2019, 47, 10543–10552. [Google Scholar] [CrossRef] [PubMed]
- Laslett, D.; Canback, B. ARWEN: A program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics 2008, 24, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, F.L.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A toolkit incorporating gamma-series methods and sliding window strategies. Genom. Proteom. Bioinf. 2010, 8, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, Y.; Li, J. Eighteen mitochondrial genomes of Syrphidae (Insecta: Diptera: Brachycera) with a phylogenetic analysis of Muscomorpha. PLoS One 2023, 18, e0278032. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Lei, Z.R.; Wen, J.Z.; Wang, H.H.; Li, X.; Dong, B.X.; Ren, B.Z. The complete mitochondrial genome of Liriomyza huidobrensis and comparison with L. trifolii and L.sativae (Diptera: Agromyzidae). Mitochondrial DNA 2014, 25, 104–105. [Google Scholar] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Xiang, C.Y.; Gao, F.L.; Jakovlić, I.; Lei, H.P.; Hu, Y.; Zhang, H.; Zou, H. Using PhyloSuite for molecular phylogeny and tree-based analyses. iMeta 2023, 2, e87. [Google Scholar] [CrossRef]
- Regier, J.C.; Shultz, J.W.; Zwick, A.; Hussey, A.; Ball, B.; Wetzer, R.; Martin, J.W.; Cunningham, W.C. Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature 2010, 463, 1079–1083. [Google Scholar] [CrossRef]
- Zwick, A.; Regier, J.C.; Zwickl, D.J. Resolving discrepancy between nucleotides and amino acids in deep-level arthropod phylogenomics: Differentiating serine codons in 21-amino-acid models. PLoS ONE 2012, 7, e47450. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New Methods for Selecting Partitioned Models of Evolution for Molecular and Morphological Phylogenetic Analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Chen, J.; Cai, X.; Yang, D.; Li, X.; Liu, X. Comparative Analysis of the Mitochondrial Genomes of Chloropidae and Their Implications for the Phylogeny of the Family. Int. J. Mol. Sci. 2024, 25, 2920. https://doi.org/10.3390/ijms25052920
Liu J, Chen J, Cai X, Yang D, Li X, Liu X. Comparative Analysis of the Mitochondrial Genomes of Chloropidae and Their Implications for the Phylogeny of the Family. International Journal of Molecular Sciences. 2024; 25(5):2920. https://doi.org/10.3390/ijms25052920
Chicago/Turabian StyleLiu, Jiuzhou, Jiajia Chen, Xiaodong Cai, Ding Yang, Xuankun Li, and Xiaoyan Liu. 2024. "Comparative Analysis of the Mitochondrial Genomes of Chloropidae and Their Implications for the Phylogeny of the Family" International Journal of Molecular Sciences 25, no. 5: 2920. https://doi.org/10.3390/ijms25052920
APA StyleLiu, J., Chen, J., Cai, X., Yang, D., Li, X., & Liu, X. (2024). Comparative Analysis of the Mitochondrial Genomes of Chloropidae and Their Implications for the Phylogeny of the Family. International Journal of Molecular Sciences, 25(5), 2920. https://doi.org/10.3390/ijms25052920






