Abstract
Microalgae have been reported to be excellent producers of bioactive molecules. Lutein is a pigment reported to have various beneficial effects for humans, and especially for eye well-being. In the current review, we summarize various methods that have been developed to optimize its extraction and bioactivities reported for human health. Several protective effects have been reported for lutein, including antioxidant, anticancer, anti-inflammatory, and cardioprotective activity. This review also reports attempts to increase lutein production by microalgae by changing culturing parameters or by using pilot-scale systems. Genetic engineering lutein production is also discussed. Considering the increasing aging of the worldwide population will create an increased need for lutein, a viable economic and eco-sustainable method to produce lutein is needed to face this market demand.
1. Introduction
Marine organisms are considered a great source of many essential nutrients for human health, and, among these, one of the most studied is the carotenoid lutein. It is a yellow-orange pigment with known protective activities and beneficial effects for humans, including decreasing the risk of cancer, improving cardiovascular health, and benefitting human eye well-being [1]. For instance, its presence in the retina of the human eye has been reported, where it plays a protective role against the damage caused by free radicals and harmful blue light. For this purpose, it is essential to take the right amount of lutein through one’s diet.
Carotenoids are molecules known to have antioxidant properties due to their rich composition in double bonds, which are able to react with reactive oxygen species with radical scavenging properties. Lutein is a compound with the formula C40H56O2, and its secondary structure is as reported in Figure 1. It is a xanthophyll, classified as a carotenoid. Xanthophylls are photosynthetic light-harvesting compounds known to be involved in photoprotection, in the presence of excessive light, by dissipating energy, but they are also structural entities within the light-harvesting complex (LHC).
Lutein is known to be synthesized by plants as a defensive molecule against ultraviolet light, pathogens, or predators [2]. It is not synthetized by animals but is accumulated after ingestion and gives rise to the yellow color, for instance, of egg yolk, animal fat, and human eye retinal macula [3]. Regarding its biosynthetic route, it is known to be synthetized from lycopene and α-carotene, as shown in the Kyoto Encyclopedia of Genes and Genomes (KEGG) PATHWAY Database (https://www.kegg.jp/pathway/map00906+C08601; accessed on 9 January 2024) and briefly shown in Figure 1.
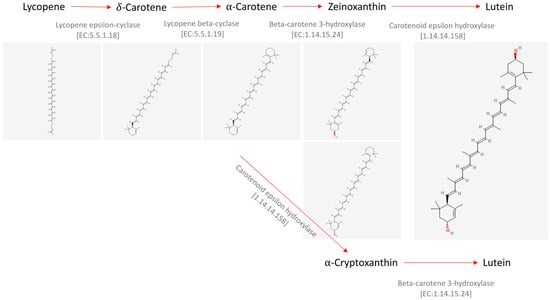

Figure 1.
Synthesis of the carotenoid lutein starting from lycopene. Chemical structures were retrieved from the public database PubChem [4,5,6,7,8,9].
Lutein, in its fatty acid ester form, is extensively found in flowers, yellow autumn leaves, fruits, and vegetables. Metabolites of lutein, including various geometrical isomers, dehydration, and oxidation products, have been identified in human plasma extracts. Lutein can exist in eight stereoisomeric forms due to the presence of three stereocenters at the 3, 3′, and 6′ carbon atoms [10].
Today, commercial production of lutein mainly relies on the bright yellow petals of the marigold flowers of the genus Tagetes. This source has several disadvantages depending on seasonality, and results are technically relatively complex [11]. Microalgae produce lutein at a rate three to six times higher compared to marigold flowers and require less water [12]. Studies have reported that chemical synthesis allows one to obtain only very low lutein yields [13], and it has been suggested to explore new lutein sources, such as microalgae. Lutein quantities reported for various microalgae will be discussed in the paragraphs below.
Marine organisms, such as microalgae and fish (e.g., salmons and sardines) are a rich source of lutein [14]. Saha et al. [11] reported that from some species of freshwater and marine microalgae, it is possible to obtain 5 g of free lutein per kg of algal biomass [15]. Fish feeding on microalgae accumulate it in their tissues and are therefore rich in lutein. A rich diet based on these fish can be an excellent source for lutein uptake, helping to maintain healthy vision even in old age and to prevent degenerative eye diseases and other pathologies, as explained in the paragraphs below. In fact, many studies confirm the importance of lutein for the prevention of human diseases [16,17,18].
The lutein obtained from these organisms can be used for the formulation of food supplements and also as food colorant (E161b) approved by the European Union [19]. In particular, microalgae are the principal source of lutein from the marine environment. Some freshwater species of microalgae are studied for their production of large amounts of lutein, such as Chlorella zofingiensis [20] and Auxenochlorella protothecoides [21], but other Chlorophyta, such as Muriellopsis sp. [22,23] and Scenedesmus sp. [24], have also been reported as great sources of lutein. In fact, these microalgae are used in the form of food supplements [19,25]. There are, for instance, various products based on Chlorella sp., which are available on the online shopping market in the form of tablets or powder and produced by companies based all over the world.
The lutein market is mainly related to dietary supplements and functional foods. Considering the increasing ageing of the worldwide population (and, hence, an increase in eye-related disorders), the consciousness of the beneficial roles of lutein in human health, and also its use in foods and beverages, lutein use has increased. While the global market for this compound was USD 371 million in 2023, according to MARKETSANDMARKETS [26], this is projected to reach USD 488 million by 2028, with a CAGR of 5.6%.
2. Extraction Procedures
Marine microalgae are organisms rich in lutein, and over the years, various methods have been developed to optimize its extraction (Figure 2) [11]. There are several lutein extraction methods for marine organisms, including solvent extractions, extraction with supercritical CO2, extraction with ionic solvents, microwave-assisted extraction (MAE), and extraction with ultrasounds [27]. These methods are explained in the following paragraphs.
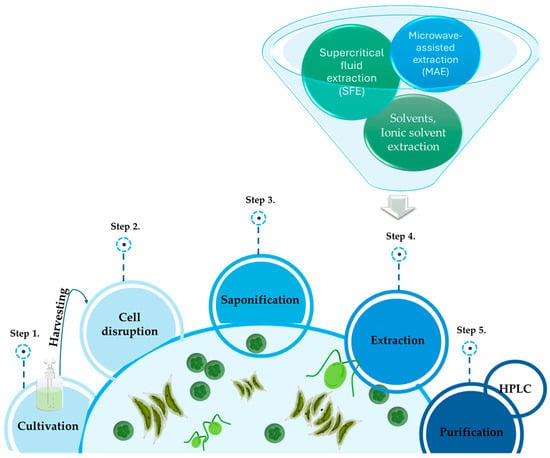
Figure 2.
The figure graphically summarizes the various steps commonly followed for lutein extraction.
2.1. Solvent Extraction
Extraction with organic solvents is the most commonly used method to obtain pigments from biomasses. Usually, non-polar solvents are used to extract carotenoids, given their high hydrophobicity, such as n-hexane, dichloromethane, dimethyl ether, and methanol, along with other solvents such as acetone and octane [28]. The biomass is macerated for a defined time in the chosen solvent and then homogenized to conduct a phase separation so as to separate the phase with the compound of interest. For lutein extraction, the most used solvents are acetone and ethanol, but also dichloromethane and methanol [29], although they have a much wider environmental impact, being highly toxic. The biomass mixture with the solvent is filtered, and the solvent is evaporated to obtain pure lutein. Following the extraction of biomass by the conventional method for obtaining pigments [30], the extract needs a further purification step to separate the carotenoids from the mixture and obtain high-purity lutein in order to meet marketing requirements [11,31,32]. To meet these needs, in 2002, Li and collaborators [31] set a simple and effective method to optimize the production of high-purity lutein following saponification and crude extraction with dichloromethane from the microalga Chlorella vulgaris. The raw extract obtained contained 30% of lutein. Since the raw extract contained water-soluble and liposoluble impurities, Li et al. measured the breakdown values of lutein in a two-phase ethanol–water-dichloromethane system in order to choose the best washing conditions to remove impurities. Dichloromethane extract was washed with 30% aqueous ethanol until the aqueous phase was almost colorless and the pH was almost neutral. The organic phase was then dried with rotary vaporization at 40 °C. Lutein obtained in this phase contained 70% lutein (w/w). Later, lutein was dissolved in 85% aqueous ethanol, and the fat-soluble impurities were extracted twice with hexane. The lutein obtained after filtration was dry-fed, and its purity determined with HPLC. The final purity was between 90 and 98%, and the yield was between 85 and 91%. In conclusion, the authors found that extraction with hexane was the best to remove the liposoluble impurities [31]. A few years later, Chan and collaborators [33] carried out a study to improve lutein’s commercial viability. They have chosen as their source of lutein the microalga Scenedesmus obliquus CNW-N as it has been reported to have a content of more than 0.25% of lutein. They compared the conventional method based on solvent extraction with their modified method to increase lutein yield from microalgal biomass. In the conventional method, the total extraction of pigments (chlorophyll and carotenoids) is obtained. The modified method instead allowed one to obtain only the carotenoids. The steps preceding the purification of lutein in the modified method are the cell-disrupting step [34], saponification, and solvent extraction. The cell-disrupting step can be achieved through several methods, such as by using an ultrasonicator or a bead-beater. For each method, 10–15 mg of algal dry cell weight was used. Among all methods, the disruption with bead-beater was the one that provided the best disruption results. The second step is saponification, which is essential to remove the ionizable lipids, completely disrupt the cells, and convert the esterified lutein contained in the microalgae into its free form. The microalgal biomass was mixed with potassium hydroxide KOH solution at a concentration of 0–80% (w/v). Chan et al. [33] modified the conventional saponification step to reduce the overall extraction time by 24 h (from 30 h to 6 h). The last step is solvent extraction. Chan et al. selected seven organic solvents: acetone, petroleum ether, n-hexane, diethyl ether, chloroform, dichloromethane, and methanol. The ratio of organic solvents to the sample mixture was 2:1 (v/v). The diethyl ether was found to be the best among solvents to extract lutein and was used to identify the optimal solvent-to-raffinate (S/R) ratio, leading to the highest lutein content extracted. The lutein content was measured by purging the organic solvent with an N2 flow and then dissolving the precipitate with acetone for HPLC measurements. Lutein obtained by the conventional method was 10–20% less than that obtained by the modified method. Recently, extraction methods have been developed with the use of green solvents, such as ethanol and biphasic water solvent mixtures [35]. It is interesting to note that lutein is marketed in the form of oil extracts ranging from 5 to 60%, since its crystalline form is unstable. Therefore, the algal biomass can be extracted with olio-resin in order to obtain 25% lutein extracts that are already marketable [36]. Various studies on methods for the extraction of lutein from microalgae were also included in the review by Saha et al. [11].
Kanda et al. yielded 0.30 mg g−1 dry lutein from wet microalga Monostroma nitidum using a method employing dimethyl ether (DME). DME resulted an ecofriendly and safe method to obtain a high yield of lutein with unheated drying of wet macroalgae in a single step. As the consumption of liquefied DME increased, the amount of water in the biomass was reduced. Almost all of the water was removed when 216 g DME was reached. In this way, there was no need for any other drying step. The liquefied DME was able to break the hydrogen bonds that were created between the water and the cells of M. nitidum. The water was immediately mixed with liquefied DME during the first stage of extraction. The water was extracted with a delay due to the resistance to transport in the latter phase of extraction. This transport resistance can be due both to spatial resistance, such as movement in a narrow space, and to physical-chemical resistance, such as the strong hydrogen bond interactions between cellulose, polysaccharides, and water [37].
Recently, in 2022, Patel et al. [29] evaluated what might be the best condition for extracting the maximum amount of lutein from microalgae. They cultivated Chlorella sorokiniana Kh12 under mixotrophy. The condition of mixotrophy 2X-(HT)-9k produced maximum biomass (3.46 g L−1) and lutein equal to 13.69 mg/g, which is among the highest yields reported so far. Seven minutes of bead beating was found to be the best disruption method to break the walls of the algae (compared to French press, mortar and pestle, and ultrasonic bath), obtaining maximum lutein (7.56 mg g−1) extraction, while methanol was found to be the best solvent to extract lutein from C. sorokiniana [29].
2.2. Supercritical Fluid Extraction (SFE)
SFE is a very efficient technique to extract natural compounds. With this method, lutein is effectively extracted using CO2 and ethanol as cosolvent. CO2, being chemically inert, non-flammable, non-toxic, retained from degradation [38], and especially economical, is the supercritical solvent preferred in SFE techniques [19,39]. The extraction process takes place in an extraction chamber, where the CO2 is brought to a supercritical state by applying pressure and temperature. In this state, CO2 has the properties of a gas and a liquid simultaneously, making it a very efficient solvent for the extraction of compounds such as lutein. During extraction, supercritical CO2 is passed through the plant material containing lutein. Supercritical CO2 acts as a solvent, dissolving lutein and other compounds. Then, the CO2 is separated from the solution containing lutein, allowing for purification. The advantage of using CO2 as a solvent is that it can be easily removed from the solution, leaving the purified lutein without solvent residues. This extraction method is widely used in the food and pharmaceutical industry to obtain lutein from natural sources. Supercritical CO2 extraction is a sustainable method because CO2 can be recovered and recycled, thus reducing environmental impacts. In addition, the use of low pressure during the extraction process can contribute to greater sustainability. Interest has been growing in this approach to recovering pharmaceutical and nutraceutical compounds precisely because it guarantees clean extracts and is not toxic. It would seem that, coupled with a co-solvent, the SFE method is among the most promising approaches for lutein extraction. Miguel et al., in their work in 2008, conducted a solvent extraction of carotenoids, following which they mixed the carotenoid-containing solvent with supercritical CO2 and optimized the pressure and temperature conditions to promote lutein precipitation [40]. According to Yen et al., the addition of polar co-solvents such as methanol or ethanol may increase lutein extraction efficiency [41]. They focused their research on the use of SFE methods for lutein extraction from the microalga Scenedesmus sp. Optimal parameters to increase the recovery yield of lutein have been tested, such as a temperature of 70 and pressure of 400 bar, whichh led to a recovery of 76.7% of lutein. There are still some criticisms in the development of techniques that use supercritical CO2, as shown by Ruen-Ngam et al., which assessed the impact of the CO2 flow rate for lutein extraction. They showed that the recovery of lutein (equal to 53%) was higher at a 0.4 mL min−1 flow rate, but with an increase of the flow rate to 0.5 mL min−1, the recovery of lutein was reduced. This phenomenon may be due to the prevalence of resistance to interparticulate diffusion [42].
A study by Gilbert-López et al. in 2017 [43] also aimed to develop a downstream platform for the exploitation of microalgal biomass. The basic approach was to extract and fractionate bioactive compounds from the microalgae Scenedesmus obliquus by applying a sequential process to produce increases in solvent polarity using non-toxic solvents. The phases used were supercritical CO2 extraction ScCO2, gas-expanded liquids (GXL) using 75% ethanol and 25% ScCO2 (v/v), and pressurized liquid extraction (PLE) using water. Lutein and β-carotene were the main pigments identified in the extracts by HPLC coupled to diode matrix detectors and mass spectrometry (HPLC-DAD-MS/MS), which were preferably extracted in the GXL phase [43].
2.3. Ionic Solvent Extraction
Another effective method to obtain lutein from marine organisms is the use of ionic solvents, ion-based liquids. Ionic solvents can be designed to be selective for lutein and can be used to extract lutein efficiently. These solvents are maintained in a liquid state at moderate temperatures from 0 °C to 140 °C [35,44]. Ionic solvent extraction draws attention because it combines both cellular disruption and extraction steps. Among the used solvents, there was the dipropylammonium dipropylcarbamate-methanol (DPCARB) [45,46]. The use of DPCARB has been reported to reduce the extraction time to 30 min compared to conventional methods with solvents by increasing the yield of lutein obtained to 0.96% DW (dry weight) [46]. Natural deep eutectic solvents (NADES), considered a subclass of ionic liquid solvents [47], are solvents that meet the criteria of green chemistry as they are biodegradable and economical [48]. These solvents were used for the recovery phase of lutein during purification, obtaining 4.41 mg g−1 against 3.91 mg g−1 lutein recovered with conventional solvents [49].
2.4. Microwave-Assisted Extraction (MAE)
Microwave-assisted extraction (MAE) offers a faster extraction process compared to conventional methods [50,51]. With this technique, microwaves are used to heat the samples in order to facilitate the release of bioactive compounds. It provides uniform heating throughout the sample, ensuring efficient extraction of the target compound. Another important aspect is that MAE requires less energy compared to traditional extraction methods, making it a more environmentally friendly option [48,50,52].
Low et al., in 2020 [53], described an optimization of a novel protocol based on the microwave-assisted binary phase solvent extraction method (MABS) for the extraction of lutein from Scenedesmus sp. With this protocol, 11.92 mg/g of lutein was recovered from the biomass. They found that the best binary phase solvent composition was a 60% potassium hydroxide solution with acetone in the ratio of 0.1 (mL mL−1). The conditions that allowed them to obtain the maximum content of lutein were 55 °C treatment temperature, 36 min extraction time, 0.7 (mg mL−1) biomass–solvent ratio, 250 W microwave power, and 250 rpm stirring speed [53].
Very recently, in 2022, Leema et al. [52] purified and characterized lutein from the microalga Chlorella sorokiniana using the optimized microwave-assisted extraction (MAE) method. The optimized conditions for microwave-assisted alkali pre-treatment resulted in a 3.26-fold increase in lutein yield compared to conventional extraction. The effectiveness of the MAE process was confirmed through visualization of the extracted algal biomass using scanning electron microscopy and X-ray diffraction analysis, which showed a significantly higher crystallinity index in the treated biomass. The lutein yield using the optimized MAE conditions was reported to be 20.69 ± 1.2 mg g−1. The conventional extraction method yielded lutein at a lower rate of 6.35 ± 0.44 mg g−1. X-ray diffraction analysis showed a significantly higher crystallinity index in the microwave-assisted alkali-treated biomass (83.85%) compared to the untreated control (17.28%) [52].
2.5. Ultrasonic Extraction
Ultrasonic extraction is an approach that uses high-frequency sound waves to break the cell walls of microalgae to release lutein. The organisms are immersed in a solvent and subjected to ultrasound for a certain period of time. In 2018, Gayathri et al. [54] focused their studies on the extraction of lutein from the marine microalga Chlorella salina by using an ultrasound-assisted microextraction (US-ME) technique. They found that the optimal conditions for lutein extraction from C. salina were a temperature of 40 °C, extraction time of 30 min, and a frequency of 35 kHz. Under these optimal conditions, the concentration of lutein obtained from C. salina was 2.92 ± 0.40 mg g−1 DW (dry weight). The results demonstrated that ultrasound-assisted microextraction (US-ME) is a very useful method for extracting lutein from microalgae, specifically from Chlorella salina. [54]. Ultrasonic extraction can be made more environmentally friendly by using low energy consumption and reducing extraction time. In addition, the use of ultrasounds can reduce the amount of solvent required for the extraction, contributing to greater sustainability.
2.6. Purification of Lutein
The essential step to obtain high-purity lutein is the purification that follows the extraction with the methods mentioned above. Among the mentioned techniques, the most promising method for extracting lutein on a large industrial scale is SFE, although other further studies are necessary to demonstrate the high yield of pure lutein. As mentioned above, the current methods of purification of carotenoids are based on the Willstätter method [19,48]. This name derives from the name of the Nobel Prize winner Richard Willstätter [55], who pioneered methods for the study and purification of secondary metabolites from natural sources, specifically pigments. In various laboratory studies following these extraction steps, the purification is conducted essentially with chromatography techniques, such as counter-current chromatography or solid support chromatography. High-performance counter-current chromatography has been shown to obtain a lutein purity equal to 92% [19,33].
3. Lutein: A Versatile Antioxidant in Human Health
Lutein has several effects on human health, depending mostly on its antioxidant capabilities. It has been shown that lutein is a strong antioxidant, with an effect that may vary depending on the cultivar of the plant from which it is extracted [56]. Notably, lutein contained in microalgal extracts presents interesting bioactive properties even without additional purification. For instance, lutein contained in an extract of the microalga Chlamydomonas reinhardtii demonstrated antioxidant effects on damaged DNA [57], while lutein contained in an extract of Tetraselmis sp. exhibited potent antioxidant, antiviral, and anti-inflammatory properties, suggesting its potential for sustainable industrial applications [58].
Mechanistically, in a murine model, lutein was proposed to scavenge reactive species and up-regulated genes associated with antioxidant responses, thereby enhancing oxygen transport [59]. Additionally, studies by Song et al. suggested that lutein, when combined with vitamin C, enhanced the total antioxidant defense system in rats [60]. Human trials further supported the antioxidant effects of lutein. In particular, Jȩdrzejczak-Pospiech et al. showed that the highest total antioxidant status increase was observed after the intake of the lowest lutein dose (8 mg d−1) [61]. Research involving lutein supplementation has also been conducted on infants. While administering lutein to pregnant women with gestational diabetes mellitus is linked to a reduction in oxidative stress in newborns at birth, these effects did not persist significantly after 48 h [62]. Similarly, administering 0.5 mg ± 0.02 mg/kg/d of lutein to newborns from 7 days of age was ineffective in enhancing their biological antioxidant capacity [63]. Conversely, recent findings demonstrated that neonatal supplementation with lutein in the early hours of life enhanced biological antioxidant potential and decreased total hydroperoxide levels in supplemented infants compared to untreated ones. This suggested a reduction in free-radical-induced damage at birth due to lutein supplementation [64].
In human health, lutein has two main roles: acting like a filter for high-energetic and damaging blue light at the eye level and acting as antioxidant [65]. The majority of lutein’s health benefits, along with its isomer zeaxanthin, depend on its antioxidant activity, which also contributes to its anti-inflammatory properties. Oxidative stress underlies the pathogenesis of various diseases, including diabetes, neurodegeneration, atherosclerosis, cancer, eye diseases, diabetic retinopathy, osteoporosis, cardiovascular diseases, skin diseases, liver injury, obesity, and colon diseases [66]. Reactive oxygen species (ROS) are produced both internally by mitochondrial respiration and externally by cytotoxic factors. Lutein, with its free-radical-scavenging activity, mitigates oxidative stress and inflammatory responses in different organs. It is notable that lutein’s efficacy in scavenging ROS may vary depending on the specific species. A study indicates that lutein effectively protects human cells against OH• radicals but shows less efficacy against NO2• and O2• [67].
Furthermore, lutein’s antioxidant effect remains largely consistent whether it is in a free or esterified form [68]. However, achieving a bioactive effect depends significantly on lutein’s bioavailability and transport [69], which are influenced by the formulation used. For instance, research by Evans et al. suggested that a starch-based formulation is to be preferred to an alginate-based product [70]. Additionally, the addition of excipients has been observed to enhance lutein’s bioavailability, as noted by Nemli et al. [71]. Moreover, the method of cooking food containing lutein also affects its bioavailability and effectiveness [72,73,74]. It has been shown that one of the effects of cooking may be the epimerization of the lutein, which is caused also by its natural metabolization by the human body. Products of this phenomenon may be 3-epilutein and 3-oxolutein, which also have a putative protective effect [75]. It is worth noting that lutein is subjected to blanching after the extraction, and this should be considered when formulating products that contain carotenoids as ingredients, for example by adding antioxidant agents. Cui et al. investigated the anti-oxidative and anti-inflammatory effects of lutein isomers on a Caco-2 cell model, revealing that also the type of isomer may significantly impact lutein’s health effects [76]. A remarkable human intervention study demonstrated that a single dose of marine Chlorella vulgaris increased the plasma concentrations of lutein and other carotenoids, suggesting potential health benefits associated with the use of Chlorella vulgaris as a source of carotenoids, polyunsaturated fatty acid (PUFA), and essential trace elements [77]. The effects of lutein on several human diseases are listed below, according to the most recent literature.
3.1. Lutein and Zeaxanthin: Guardians of Vision Health
Lutein and zeaxanthin have been reported to play pivotal roles in safeguarding vision by accumulating in two crucial tissues responsible for visual function: the macula lutea and the lens. This accumulation is tissue-specific; no others carotenoids are accumulated in the human body at the same concentrations [65]. The macula lutea, situated under the retina, owes its yellow color to the presence of these pigments. It contains the highest concentration of photoreceptors responsible for central vision. Here, lutein and zeaxanthin shield the tissue from high-energy blue light, which can cause light-induced retinal damage. Additionally, the macula is particularly susceptible to oxidative damage, where lutein also acts as an antioxidant [65]. The literature strongly supports these assertions. For example, there are several reports providing evidence for the effect of lutein on retinal neurodegeneration in diabetes [78].
In vitro studies have further elucidated the protective role of lutein and zeaxanthin. Murthy et al. investigated their dose-response effect on shielding retinal pigment epithelium from oxidative stress, finding that lutein demonstrated a cytoprotective effect at various concentrations, while zeaxanthin did not show similar efficacy [79]. Kamoshita et al. proposed a mechanism for lutein’s protective role, suggesting that it promotes tight junction repair and suppresses inflammation in a model of photo-stressed mice, thereby reducing local oxidative stress through direct scavenging and likely induction of endogenous antioxidant enzymes [80]. Leung et al. explored the impact of lutein, zeaxanthin, and docosahexaenoic acid (DHA) supplementation on retinal pigment epithelial (RPE) cells under oxidative stress, observing their regulation of inflammatory lipid mediators and reduction in non-enzymatic oxidation of omega-6 PUFA [81].
Oxidative-stress-induced cellular senescence contributes significantly to the pathogenesis of age-related macular degeneration (AMD). Liu et al. demonstrated lutein’s protective effect on acute retinal pigment epithelial 19 (ARPE-19) cells against oxidative stress damage induced by H2O2 [82]. Similarly, Chae et al. found that lutein effectively protected ARPE-19 cells from H2O2-induced effects [83]. Additionally, lutein mitigated UVB-mediated oxidative damage to retinal pigment epithelial cells [84].
Moving to animal models, lutein treatment over three months in ApoE-deficient mice, a model of genetic hypercholesterolemia with retinal alterations, significantly reduced vascular endothelial growth factor (VEGF) levels and matrix metalloproteinase-2 (MMP-2) activity, leading to improved retinal morphological alterations [85]. Padmanabha et al. investigated the effects of lutein and fatty acids in rats on oxidative stress and inflammation in cataracts, finding that lutein administration, particularly with EPA + DHA, reduced markers of oxidative stress [86]. Sahin et al. demonstrated the efficacy of lutein/zeaxanthin treatment on G-protein-coupled receptors in the retinas of rats exposed to intense LED illumination, significantly improving antioxidant capacity [87]. Additionally, lutein protected vestibulocochlear nerve tissue from acrolein-associated oxidative and proinflammatory damage [88]. Zhang et al. observed that lutein promoted the survival of photoreceptors in Pde6brd10 model mice, which are characterized by a degeneration of photoreceptors typical of retinitis pigmentosa, a retinal disease. They observed that lutein at the optimal protective dose of 200 mg kg−1 promoted the survival of photoreceptors compared with control, postponing photoreceptor degeneration [89]. However, intratympanic lutein application (1 mg mL−1) showed no protective effect against cisplatin-induced ototoxicity in Wistar rats, despite promising in vitro results [90]. Yu et al. showed the effects of lutein on light-induced retinopathy. They observed that lutein and zeaxanthin were effective in decreasing oxidative stress and preserving photoreceptors against light damage [91].
Human studies have yielded mixed results regarding lutein supplementation. While some studies found no statistically significant effects on age-related maculopathy (ARM) and atrophic age-related macular degeneration (AMD) in participants with 6 mg of lutein supplementation [92], others observed slight improvements in retinal function. In fact, a slight but encouraging improvement in retinal function, although not clinically significant, was observed by Berrow et al. [93]. However, supplementation with meso-zeaxanthin, lutein, and zeaxanthin showed benefits in patients with Alzheimer’s disease, resulting in improvements in visual function and macular pigment augmentation [94]. In patients with AMD, supplementation with lutein complex from marigold flower and wolfberry increased serum concentrations of lutein and zeaxanthin, along with antioxidant enzyme activities and macular pigment optical density. Other parameters were also improved, such as ocular comfort index (OCI) and macular pigment optical density (MPOD) [95].
In a separate investigation, a high-dose lutein/zeaxanthin supplement was examined for its impact on MPOD and skin carotenoid (SC) levels in healthy individuals. The supplementation of 20 mg/day of lutein, 4 mg/day of zeaxanthin, and other antioxidants (vitamin C, vitamin E, zinc, copper) for 16 weeks led to a notable increase in MPOD [96]. Moreover, a meta-analysis revealed that supplementation with xanthophyll carotenoids significantly raised macular pigment optical density in patients with AMD [97]. Furthermore, a study focusing on patients with chronic central serous chorioretinopathy investigated the effects of lutein supplementation. The group receiving supplementation exhibited significant improvements in mean visual acuity, along with a substantial reduction (28.6%) in mean subfoveal fluid height [98].
3.2. Protection of Skin
Similar to its function as a filter of high-energy blue light in plants and in human eyes, evidence suggests that lutein exerts a protective effect, whether ingested or applied topically to the skin, against UV-light exposure.
For instance, Sansone et al. [99] showed that an alcohol/water extract of the microalga Tetraselmis suecica, containing lutein, exhibited potent antioxidant and reparative activity in human lung cancer cells (A549). This extract also reduced prostaglandin E2 (PGE2) levels in cells damaged by H2O2 and demonstrated tissue-repairing effects on reconstructed human epidermal tissue cells (EpiDerm), suggesting a potential cosmeceutical application for this microalgal species [99]. In another randomized, double-blind, placebo-controlled intervention, researchers investigated the effects of dietary lutein supplementation on minimal erythema dose (MED) as an indicator of the skin’s photoprotective potential. Results indicated that MED significantly increased in the group receiving lutein supplementation, indicating enhanced resistance to erythema production following UV radiation. These findings suggest that dietary lutein supplementation can enhance the skin’s ability to protect against UV-induced damage [100].
3.3. Neurodegenerative Diseases
In the sphere of neurodegenerative diseases, inflammation plays a pivotal role, and lutein has shown promise in alleviating symptoms associated with various neurodegenerative conditions.
Liu et al. demonstrated the protective role of lutein against oxidative stress and apoptosis induced by the Aβ25–35 peptide in bEND.3 cells, which are cerebrovascular endothelial cells, a dysfunction that contributes significantly to neurodegeneration [101]. Furthermore, Hu et al. highlighted the protective effects of lutein and DHA on PC12 cells, a model for neurodegenerative disorders, against hydrogen-peroxide-induced oxidative stress, suggesting their potential as potent antioxidants with preventive or palliative effects on age-related neurodegenerative diseases [102]. Similarly, Chen et al. also found that lutein attenuated oxidative damage and apoptosis triggered by methylglyoxal in PC12 cells via the PI3K/Akt signaling pathway [103]. Pap et al. investigated the impact of lutein on antioxidant enzymes, cytokines, and iron metabolism in BV-2 microglia, the primary immune cells in the central nervous system, under stress conditions. They observed that lutein may have a preventive or suppressive effect on ROS-mediated microglia activation by reducing H2O2-induced ROS levels, modulating iron utilization, and altering the secretion of anti-inflammatory and pro-inflammatory cytokines in BV-2 cells [104]. In a subsequent study, Pap et al. demonstrated that lutein mitigates the effects of glutamate-induced ROS, inflammation, iron metabolism dysregulation, and lipid peroxidation in SH-SY5Y neuroblastoma cells, which serve as a model for neurodegenerative disorders [105]. In animal models, Fernandez et al. illustrated that lutein-loaded nanoparticles offer protection against locomotor damage and neurotoxicity induced by a Parkinson’s disease model in Drosophila melanogaster [106]. Oxidative stress is also implicated in the development of Alzheimer’s disease, characterized by memory impairment due to Aβ plaque formation in the brain. Nazari et al. demonstrated the memory-enhancing effects of lutein in a male rat model of Alzheimer’s disease [107]. Additionally, oxidative stress and inflammation are key contributors to diabetic cerebral and neurological dysfunction. Fatani et al. highlighted the role of lutein in combating oxidative injury and inflammation in the cerebral cortex of diabetic animals [108]. Furthermore, lutein has exhibited protective effects against monosodium-iodoacetate-induced (MIA) osteoarthritis in primary chondrocyte cells [109].
When considering research involving human subjects, it has been observed that phospholipid hydroperoxides (PLOOH) are abnormally elevated in the erythrocytes of individuals with dementia, including those diagnosed with Alzheimer’s disease. In a study involving six healthy subjects, the administration of a single capsule containing food-grade lutein (providing 9.67 mg of lutein) daily for four weeks resulted in the incorporation of lutein into human erythrocytes. Over the course of two and four weeks, there was a reduction in erythrocyte PLOOH levels following ingestion. The antioxidative effect of lutein was confirmed on erythrocyte membranes but not in plasma. These findings suggest that lutein may contribute to the prevention of dementia [110]. In another study, the effect of 2 months of Chlorella supplementation (8 g Chlorella/day/person; equivalent to 22.9 mg lutein/day/person) on PLOOH and carotenoid concentrations in erythrocytes, as well as plasma, was assessed in 12 normal senior subjects. Following one or two months of treatment, erythrocyte and plasma lutein concentrations increased in the Chlorella group but not in the placebo group. In the Chlorella-supplemented group, erythrocyte PLOOH concentrations after a total of two months of treatment were lower than the concentrations before supplementation. These results suggest that Chlorella ingestion improved erythrocyte antioxidant status and lowered PLOOH concentrations, potentially contributing to the maintenance of normal erythrocyte function and the prevention of senile dementia [111]. Furthermore, it has been shown that high serum levels of lycopene and lutein plus zeaxanthin are associated with a lower risk of Alzheimer’s disease mortality in adults [112]. In another study, phospholipid oxidation (particularly the phospholipid biomarker 1-palmitoyl-2- (5′-oxo-valeroyl)-sn-glycero-3-phosphocholine, POVPC) was analyzed as a marker of Alzheimer’s disease. The study revealed that serum POVPC levels are higher in Alzheimer’s disease patients compared to control subjects and are not reduced by carotenoid supplementation. Additionally, serum POVPC levels were found to correlate with cognitive function [113].
3.4. Lutein: A Potential Agent against Inflammation and Cancer
The involvement of acute inflammatory response in cancer development is well documented. Presently, there is a continual quest for novel compounds that could potentially prevent and treat both inflammatory diseases and cancer. Regarding chemotherapy drugs, which are known for their aggressive nature, research efforts have been boosted towards exploring alternative therapies, including natural plant-based substances like lutein. Lutein, a bioactive molecule, has been extensively studied for its potential anti-inflammatory and anticancer properties [114]. Notably, its antioxidant properties have been observed, with oxidized lutein showing increased cytotoxicity against human cervical carcinoma cells (HeLa) compared to normal lutein [115,116]. Various cancer types have been treated with lutein in different forms. A summary of lutein’s effects on various cancer types is reported in Table 1 and commented on below.
For instance, in a study on breast cancer cells MCF-7 and MDA-MB-231, lutein extracted from Spinacia oleracea was used at different concentrations (1 and 5 µM for MCF-7; 10 and 20 μM for MDA-MB-231) for a duration of 24 h. Lutein inhibited cell viability in a concentration-dependent manner and suppressed reactive oxygen species. It downregulated the expression of Nrf-2 protein and decreased SOD-2, HO-2 proteins, and XBP-1 transcription factor expression. Additionally, it inhibited phosphorylation of the AKT protein and expression of nuclear factor Nf-κB, while increasing caspase-3 activity, leading to apoptosis [117].
In a different in vivo study, lutein from Strobilanthes crispa was supplied to mice with doses of 50 mg/kg/day for 30 days. Lutein interacted with the X alpha receptor (RXRα), inhibiting breast cancer [118]. Another study on breast cancer cells MCF-7 and MDA-MB-468 showed that lutein increased the level of intracellular ROS and the pro-apoptotic/anti-apoptotic BAX/Bcl-2 ratio. It activated p53 protein signaling and up-regulated cellular HSP60, inhibiting tumor cell growth [119]. Despite inconsistencies in its molecular mechanisms, especially regarding its effects on reactive oxygen species and caspases, lutein shows promise in breast cancer treatment.
In non-small-cell lung cancer, lutein inhibited cell invasion and regulated signaling molecules involved in apoptosis. In particular, lutein, tested at concentrations of 25 and 50 μM on non-small-cell A549, caused a regulation of PI3K/AKT signaling molecules, inhibition of the anti-apoptotic signaling molecules Bcl2 and Bcl-XL, and an increase in the expression of the pro-apoptotic protein BAX, which activates the executioner caspase 3. This led to apoptosis of A549 cells [120]. No adverse effects were observed, suggesting its potential as an anti-tumor agent.
Another study showed that lutein reduced gene expression associated with cell proliferation in A549 at concentrations of 10, 30, and 50 μM. It modulated apoptotic proteins, indicating its role in regulating apoptosis-related genes. In particular, lutein was found to reduce the gene expression of CAS3, SOX2, NANOG, SLCA11, and ABCB1, while increasing the gene expression of CD44 and CD133. It also promotes the apoptosis induction in cells by decreasing the anti-apoptotic/pro-apoptotic BCL2/BAX protein ratio [121].
In both examined studies, it can be concluded that lutein acts on the gene expression of control proteins that regulate apoptosis, namely BAX, BCL2, and CAS3.
Studies on colon cancer in vivo demonstrated lutein’s chemoprotective effect by modulating proliferative proteins. Using a lutein extract from marigold flowers, Reynoso-Camacho et al. showed that lutein modulates the proliferative activity of K-ras, PKB, and β-catenin proteins, leading to a chemoprotective effect [122].
In various gastric cancer cell lines (AGS, MKN-74, MKN-1, and SNU-668), lutein was tested at different concentrations (5, 10, and 20 µM) for 24 h. Lutein increased ROS levels and activated NADPH oxidase, increasing the translocation of the p47 phox subunit of NADPH oxidase on the cell membrane. Furthermore, it increased NF-κB activation and apoptotic indices, such as Bax, caspase -3 cleavage, and DNA fragmentation, while reducing Bcl-2. It decreased cell viability in a dose-dependent manner [123].
Lutein extracted from carrot was tested on lymphoid leukemia cells (CCRF-CEM, Jurkat, MOLT-3) at concentrations from 0.5 µM to 100 µM for 24 and 48 h. No effects on apoptosis or cell proliferation were observed [124]. Similarly, in melanoma A375 tumor cells, the use of lutein at various concentrations (from 0.5 µM to 100 µM for 24 and 48 h) did not alter cell viability or membrane integrity [125].
With regard to prostate cancer, lutein extracted from marigold at a concentration of 10 µM for 18 h was tested on PC-3 cells. Lutein altered the expression of genes associated with growth and apoptosis [126].
In an in vivo study concerning hepatocellular carcinoma, lutein extracted from marigold reduced liver levels of enzymes such as alanine transaminase, aspartate transaminase, and alkaline phosphatase; reduced the activity of c-glutamyl transpeptidase; and increased glutathione [127].
In a study on cervical carcinoma, lutein from marigold petals used on HeLa cells at a concentration of 10 µm for different durations (24 h and 48 h) inhibited cell proliferation in a dose-dependent manner and increased ROS concentration. Furthermore, it promoted apoptosis by activating Bax and Caspase-3; there was also a significant downregulation of the expression of Bcl-2 (anti-apoptotic) and upregulation of the expression of Bax (pro-apoptotic) [128].
There are some studies in the literature assessing, in particular, the effect of lutein-rich microalgae extracts, which inhibited tumor growth across various cancer types by modulating apoptotic pathways. For example, an ethanolic extract from Chlorella was tested on lung cancer (A549 cells), cervical cancer (Hela cells), breast cancer (MCF7 cells), hepatocellular carcinoma (Huh7 cells), and cholangiocarcinoma (CCA cells; KKU213A cells), at a concentration of 400 μg mL−1. Several effects were observed: a reduction in procaspase-3, -8, and -9; an increase in the enzymatic activity of caspase and a reduction in the anti-apoptosis protein Bcl-2 (which induce cell death); and a reduction in the proteins phosphorylate-AKT and phosphorylate-mTOR, which led to inhibition of AKT/mTOR survival signaling. In all types of tumor cells considered, there was growth inhibition [129].
In another study, ethanolic extracts of Neochloris oleoabundans grown under different conditions were tested on three colon cancer cell lines, specifically HT-29, SW480, and HGUE-C-1, by using concentrations of 27, 83, or 250 μg mL−1 for 24 h. Results showed that most extracts showed dose-dependent anti-proliferative activity on cells, and that the action varied depending on the cell type. The anti-proliferative activity was linked to the concentration of lutein in the extract [130].
In another study, the effect of methanolic extracts of the microalgae Granulocystopsis sp. was assessed on various types of tumors, specifically breast cancer (HTB-22), colorectal cancer (HTB-38), prostate cancer (HBT-81), and skin melanoma (HTB-72) [131]. Each cell line reacts differently to the extract, with cell viability depending on the duration of the treatment. Apoptosis was favored in skin melanoma and prostate cancer cells. An increase in the activity of caspases 3 and 7 was observed in all tumor cells.
Numerous epidemiological studies [132,133,134,135,136,137,138,139] have investigated various tumor types including head and neck cancer, bladder cancer, liver cancer, breast cancer, lung cancer, prostate cancer, colon cancer, esophageal cancer, pancreatic cancer, and renal cell carcinoma. These studies are based on the dietary habits and lifestyle of the study participants, with carotenoids typically sourced from fruits and vegetables. In one specific study [134], it was noted that the risk of colorectal cancer is associated with an interactive effect between dietary intake of lutein/zeaxanthin and the DICER1 rs3742330 polymorphism. Across multiple studies, there is evidence of an inverse relationship between total carotenoid intake and cancer risk. Additionally, another study [140] examined the effects of lutein extracted from Medicago Sativa on various cancer types, including liver cancer (HepG2), breast cancer (MCF-7), lung cancer (A549), prostate cancer (PC3), and colon cancer (HCT116). Results indicated that while lutein exhibited activity against breast and liver cancer, it did not demonstrate efficacy against lung, prostate, or colon cancer.
3.5. Diverse Protective Effects across Various Diseases
Research has explored the impact of lutein on various other diseases. For instance, oxidative stress is also pivotal in cardiovascular disease, where hyperhomocysteinemia (HHcy)-mediated atherosclerosis (AS) is driven by oxidative stress and inflammation mechanisms. In a murine model, lutein has been shown to significantly intervene and inhibit Hcy-mediated oxidative stress activation while downregulating the expression of inflammation-associated molecules [141].
Li et al. assessed the role of lutein in protecting against osteoporosis in ovariectomized rats by suppressing inflammation and osteoclast-specific marker (NFATc1) expression through Nrf2 activation [142]. The efficacy of plant extracts rich in lutein for ulcerative colitis has also been shown: alcoholic plant extract of Tagetes erecta reduced colitis severity by attenuating inflammatory cytokine secretion and improved the endogenous antioxidant defense in 5% dextran sulfate sodium-induced ulcerative colitis in mice [143]. Mammadov demonstrated the effect of lutein on methotrexate-induced pulmonary toxicity in rats, revealing its ability to prevent biochemical and histopathological oxidative damage [144]. Additionally, lutein intake was significantly associated with lower lung function in current smokers [145]. Radhi and Al-Shawi revealed that lutein mitigated irinotecan-induced liver toxicity in rats [146]. In a rat model of polycystic ovary syndrome, Bandariyan et al. demonstrated that lutein improved antioxidant capacity and oocyte and embryo quality [147]. Zhang et al. highlighted lutein’s protective effects against reproductive injury induced by excessive alcohol in male rats through its antioxidant, anti-inflammatory, and anti-apoptotic properties [148]. Fatani et al. found protective effects against diabetes-induced oxidative stress in testicular cells of rats [149]. Moreover, lutein was shown to prevent cardiac and renal injury in streptozotocin-induced hyperglycemic rats, likely by ameliorating glucose intolerance [150]. Lutein was also effective against ischemia/reperfusion injury in rat skeletal muscle, downregulating oxidative stress and inflammatory mechanisms [151]. Furthermore, lutein exhibited beneficial effects on severe traumatic brain injury through anti-inflammatory and antioxidative mechanisms [152]. Human studies have indicated that the intake of β-carotene, lutein/zeaxanthin, and lycopene is associated with a lower risk of metabolic-dysfunction-associated fatty liver disease [153].


Table 1.
This table reports anticancer bioactivities identified for lutein, active concentrations, mechanisms of action when available, and relative references.
Table 1.
This table reports anticancer bioactivities identified for lutein, active concentrations, mechanisms of action when available, and relative references.
| Types of Cancer | Substance | Source | Model | Concentration | Mechanism of Action | Reference |
|---|---|---|---|---|---|---|
| Breast cancer | Lutein | Spinacia oleracea | In vitro (cells) MCF-7 and MDA-MB-231 cells | 1 and 5 µM for MCF-7 10 and 20 μM for MDA-MB-231 for 24 h | Inhibition of cell viability in a concentration-dependent manner Increase in cell death with higher concentrations of lutein Suppression of reactive oxygen species (ROS) levels Downregulation of Nrf-2 protein expression Decrease in expression of SOD-2, HO-2 proteins, and XBP-1 Inhibition of Akt phosphorylation and NF-κB expression Enhancement of caspase-3 activity, leading to apoptosis | [117] |
| Lutein | Strobilanthes crispus | In vivo (mouse) 4T1 breast cancer cell line | 50 mg/kg/day for 30 days | Activation of retinoid X receptor alpha (RXRa) by lutein Inhibition of breast cancer growth | [118] | |
| Lutein | - | In vitro MCF-7, MDA-MB-468 cells | 2.0 µM for 48 h | Inhibition of tumor cell growth, induction of cell cycle arrest, and cell death Increase in intracellular reactive oxygen species (ROS) levels Enhancement of the BAX/Bcl-2 ratio Activation of p53 signaling and upregulation of HSP60 | [119] | |
| Non-small-cell lung cancer (NSCLC) | Lutein | - | In vitro A549 cells | 25 and 50 μM (LC50 = 50 μM) | Inhibition of cell invasion and migration properties Suppression of PI3K/AKT signaling pathway Inhibition of intrinsic anti-apoptotic signaling molecules Bcl2 and Bcl-XL, leading to apoptosis Increase in expression of pro-apoptotic protein BAX, activating caspase 3 and inducing apoptosis | [120] |
| Lutein | - | In vitro A549 cells | 10, 30, 50 μM | Decrease in BCL2/BAX protein ratio, promoting apoptosis Reduction in gene expression of CAS3, SOX2, NANOG, SLCA11, and ABCB1 Increase in gene expression of CD44 and CD133 | [121] | |
| Colorectal Cancer | Lutein | Marigold flowers (Tagetes erecta) | In vivo | Chemoprotective effect against colon cancer Modulation of proliferative activity of K-ras, PKB, and β-catenin proteins | [122] | |
| Extract of microalgae Pressurized liquid extraction (EtOH) | Microalgae Neochloris oleoabundans | In vitro HT-29, SW480 HGUE-C-1 cells | 27, 83 or 250 μg mL−1 for 24 h | Different extracts exhibit dose-dependent anti-proliferative activities on cells | [130] | |
| Gastric Cancer | Lutein | Cayman Chemical n.10010811 | In vitro AGS, MKN-74, MKN-1, and SNU-668 cells | 5, 10, and 20 µM for 24 h | Induction of a dose-dependent decrease in cell viability Increase in ROS levels and induction of NADPH oxidase activation, promoting translocation of p47 phox subunit to the cell membrane Enhancement of NF-κB activation and induction of apoptotic indices, including Bax, caspase-3 cleavage, and DNA fragmentation Reduction in Bcl-2 levels | [123] |
| Lymphoid Leukemia | Lutein | Daucus carota L. | In vitro CCRF-CEM, Jurkat, MOLT-3 cells | 0.5 µM to100 µM for 24 and 48 h | No effect on apoptosis or cell proliferation | [124] |
| Melanoma | Lutein | - | In vitro A375 cells | 0.1, 0.3, 1, 3 and 10 µM of lutein for 24 h | No alteration in melanoma cell viability or membrane integrity by lutein | [125] |
| Prostate Cancer | Lutein | Marigold | In vitro PC-3 cells | 10 µM for 18 h | Alteration of expression of biomarker genes associated with growth and apoptosis by lutein | [126] |
| Hepatocellular carcinoma | Lutein | Marigold (Tagetes erecta L.) | In vivo | - | Reduction in levels of alanine transaminase, aspartate transaminase, and alkaline phosphatase Increase in levels of glutathione Reduction in activity of c-glutamyl transpeptidase | [127] |
| Lung cancer Cervical cancer Breast cancer Hepatocellular carcinoma Cholangiocarcinoma | Extracts of Chlorella (Gallic acid and lutein) (EtOH) | Chlorella sp. | In vitro A549, Hela, MCF7, Huh7, CCA, KKU213A cells | 400 μg mL−1 | Inhibition of growth in all types of tumor cells considered Reduction in procaspase-3, -8, and -9 levels, increase in caspase activity, and decrease in anti-apoptosis protein Bcl-2, leading to cell death Reduction in levels of phosphorylated-AKT and phosphorylated-mTOR proteins, inhibiting AKT/mTOR survival signaling | [129] |
| Breast cancer Colorectal cancer Prostate cancer Skin melanoma | Extract of Granulocystopsis (MeOH) | Granulocystopsis sp. | In vitro HTB-22, HTB-38, HTB-81 and HTB-72 cells | IC50 for 48 h: 16.70 µg/mL 17.20 µg/mL 13.74 µg/mL 17.44 µg/mL | Differential reactions of cell lines to the extract, but viability decreases in all depending on treatment duration Favorable induction of apoptosis in skin melanoma and prostate cancer cell lines Increase in caspase 3 and 7 activity in all treated tumor cells | [131] |
| Head and neck cancer (HNC): Oral and pharyngeal cancer Laryngeal cancer | Carotenoids: bcryptoxanthin, lycopene, and lutein plus zeaxanthin | Fruit and vegetables | Epidemiological studies | - | Carotenoids exhibit antioxidant, antimutagenic, and immunoregulatory actions Inverse association observed between carotenoid intake and risk of head and neck cancer (HNC) | [137] |
| Bladder cancer (BC) | Carotenoidis: lutein, αcarotene, βcarotene, lycopene, βcryptoxanthin, and zeaxanthin | Fruit and vegetables | Epidemiological studies | - | Inverse association between total carotenoid intake and risk of BC | [138] |
| Renal cell carcinoma (RCC) | Carotenoids: αcarotene, βcarotene, lutein, zeaxanthin, and lycopene | Fruit and vegetables | Epidemiological studies | - | Inverse associations between total carotenoid intake and risk of RCC | [139] |
4. Enhancement of Lutein Production in Microalgae
Although the lutein content in some terrestrial plants may be even higher than in microalgae, lutein production in microalgae can be enhanced by subjecting cell cultures to various types of stresses. The main conditions identified in the literature are summarized below and indicated in Table 2.
4.1. Metabolism
Microalgae exhibit diverse metabolic modes, including photoautotrophic, heterotrophic, mixotrophic, and photoheterotrophic modes according to their utilization of different sources of energy and carbon. Heterotrophic microalgae depend on organic carbon as the source of both energy and carbon, enabling them to proliferate in the absence of light. Photoautotrophic microalgae employ light as source of energy and inorganic carbon, such as CO2 or carbonate salts, as a source of carbon. Photoheterotrophic cultures utilize light energy and organic carbon, but they require light and cannot survive in darkness. Mixotrophic microalgae exhibit a versatile capacity to use both light energy and organic carbon as their energy sources, enabling the assimilation of both organic and inorganic carbon. Mixotrophic microalgae can conduct photosynthesis while also assimilating organic carbon. Importantly, the metabolic mode plays a pivotal role in influencing the ability of microalgae to accumulate lutein. For example, Yen et al. observed a preference for the autotrophic cultivation mode over mixotrophic cultivation mode in lutein production [154]. In a different study, Heo et al. demonstrated that, in Parachlorella sp. JD-076, autotrophic cultivation with 5% CO2 is more effective for lutein accumulation than mixotrophic culture, both in the presence of glucose and when glucose is present without CO2 [155]. Additionally, mixed strategies, involving an initial stage of mixotrophic cultivation followed by a second stage of autotrophic cultivation, were successfully implemented for lutein accumulation. This approach led to a lutein content of 11.22 ± 0.38 mg g−1 in Chlorella sorokiniana FZU60 [156].
4.2. Light
Light exerts a profound influence on lutein accumulation, with light quality, intensity, and supply strategies playing pivotal roles. The wavelength of light contributes to lutein accumulation, as evidenced by the fact that blue light enhances lutein content in Chlamydomonas sp. JSC4 [157]. In contrast, in Scenedesmus obliquus CWL-1, blue light does not increase lutein content but enhances lutein productivity [158].
Regarding light intensity, high lighting conditions generally lead to a decrease in lutein accumulation. For instance, Xie et al. demonstrated that increasing lighting conditions from 150 to 750 μmol m−2 s−1 resulted in a decrease in lutein content from 4.69 ± 0.08 to 3.30 ± 0.11 mg g−1 DW [159]. Similarly, other researchers observed that Chlorella sorokiniana Kh12 decreased lutein content when cultivated under stronger light intensities [160]. Indeed, exceptions to the general trends in light influence on lutein accumulation exist, as highlighted by Heo et al. They observed that Parachlorella sp. JD-076 increased lutein content up to 11.8 mg g−1 when exposed to an increased light intensity of 1000 μmol m−2 s−1, indicating a positive response to higher light levels [155]. Additionally, Ma et al. demonstrated a relationship between illumination intensity and lutein accumulation in Chlamydomonas sp., revealing an optimum intensity of 625 μmol m−2 s−1 for maximizing lutein accumulation before observing a decrease in content [161]. These exceptions highlight the complexity of the interplay between light conditions and lutein production in different microalgal species.
The method of light supply also plays a crucial role. Dineshkumar et al. observed that Chlorella minutissima accumulated 8.24 ± 0.12 mg g−1 and 7.96 ± 0.14 mg g−1 when linear and exponential light-feeding strategies were applied to the culture, outperforming the continuous lighting strategy with 6.37 ± 0.11 mg g−1 and leading to considerable energy savings [162].
4.3. Nitrogen
Nitrogen plays a crucial role in lutein accumulation, and the choice of nitrogen source significantly influences lutein concentration in microalgal cells. Shi et al. demonstrated that among nitrate, ammonium, and urea, urea proves to be the most effective nitrogen source for accumulating lutein in the heterotrophic culture of C. protothecoides [21]. In a different study, Ho et al. found that among Ca(NO3)2, (NH4)2SO4, and urea, a concentration of 8 mM Ca(NO3)2 was the most effective for lutein accumulation, resulting in 4.61 ± 0.11 mg g−1 in Scenedesmus obliquus FSP-3 grown in a photobioreactor [163]. In another case, the lutein production of Chlorella sorokiniana MB-1-M12 showed no significant differences for cultures grown with nitrate and ammonium, but a relatively lower level of lutein content was obtained when urea was used as a nitrogen source [164].
Regarding nitrogen concentration, maintaining nitrogen sufficiency is generally essential for achieving a high concentration of lutein. For instance, Tetraselmis sp. CTP4 accumulated up to 3.17 ± 0.18 mg g−1 DW when cultivated with a two-stage strategy in which, in the second stage, it was cultivated under nitrogen repletion (N-NO3), at 35 °C and 170 μmol m−2 s−1. [165]. Similarly, Ho et al. showed that in S. obliquus FSP-3, the content of lutein increased with the concentration of nitrogen in the medium, reaching 4.95 ± 0.22 mg g−1 with 24 mM of Ca(NO3)2 [163]. However, there seems to be an optimal concentration of nitrogen to optimize lutein content. Xie et al. demonstrated that the lutein content of Desmodesmus cultivated in a batch increased from 3.76 ± 0.23 mg g−1 DW to 4.97 ± 0.05 mg g−1 DW1 when nitrate concentration increased from 4.4 to 13.1 mM, but then decreased to 4.29 ± 0.13 mg g−1 DW when the nitrate concentration was 17.6 mM [159]. Furthermore, Xie et al. observed similar findings, with the content of lutein reaching a maximum of 5.56 mg g−1 in Desmodesmus sp. F51 when ammonium concentration was increased to 150 mg L−1, decreasing when nitrogen concentration further increased [166]. In their study on Chlorella sorokiniana FZU60, Xie et al. demonstrated that a higher concentration of NaNO3 (1 g L−1) resulted in the optimal lutein accumulation of 9.65 ± 0.25 mg g−1 [167]. Similar results were found by other authors [168]. In contrast, Shi et al. observed that nitrogen limitation enhanced lutein production by Chlorella protothecoides in heterotrophic fed-batch cultures of approx. 0.27 mg g−1 in the N-limited culture [169]. Despite reports indicating that nitrogen limitation can boost the synthesis of secondary carotenoids in certain algae species, lutein, typically considered a primary carotenoid, generally does not experience increased production under nitrogen deprivation. Nevertheless, in the case of heterotrophic growth, where algae are grown without relying on light, carotenoids might not serve the same function as they do in phototrophic mode. This distinction in function may explain the observed differences in regulation under nitrogen limitation in heterotrophic conditions.
4.4. Temperature
Temperature significantly influences lutein accumulation, generally with higher temperatures promoting increased lutein synthesis. For instance, Tetraselmis sp. CTP4 demonstrated enhanced lutein accumulation, reaching up to 2.15 ± 0.25 mg g−1 DW at a salinity of 35‰ and 30 °C, compared to 1.64 mg g−1 DW under standard conditions of 20 °C and a salinity of 35‰ [165]. Similarly, in Chlorella protothecoides, an increase in temperature from 24 to 32 °C led to a 25% rise in lutein content [169]. It was also reported that lutein concentration increased with temperature from 26 to 32 °C in Chlorella sorokiniana [170]. However, there is an optimal temperature range for lutein accumulation [156,160]. In contrast, Chlamydomonas sp. exhibited higher lutein accumulation when cultivated at 20–25 °C compared to higher temperatures [157]. The accumulation of lutein at low temperatures can be attributed to its role in regulating membrane fluidity. Carotenoids, including lutein, are proposed to play a crucial role as a biological adaptation to low temperatures. Specifically, they contribute to the regulation of intracellular thylakoid membrane fluidity, serving as a mechanism to counteract the negative effects associated with lower temperatures [157].
4.5. Organic Carbon Source
The choice of organic carbon source significantly impacts lutein production. For example, in the extremophile Chamydomonas acidophila, amongst several other carbon sources, in the presence of CO2, starch, urea, or glucose were the best carbon source, leading to an accumulation of 10 mg g−1 DW [171]. Another microalga, Chlorella sorokiniana MB-1-M12, showed a preference for acetate over glucose in lutein accumulation [164]. The concentration of the organic carbon source in the growth medium plays a role in lutein accumulation. In the growth of C. protothecoides, optimal concentrations of glucose were determined to be 10 g L−1, leading to a concentration of 4.38 mg g−1 DW after 92 h of cultivation, which increased to 4.44 mg g−1 DW after 238 h of cultivation [172]. Conversely, in the microalga C. sorokiniana Mb-1 grown in mixotrophic mode, lower acetate concentrations resulted in better lutein accumulation. When the acetate concentration was 1 g L−1, the lutein content reached 4.60 mg g−1 [168]. Likewise, Xie et al. found that Chlorella sorokiniana FZU60 accumulated more lutein when 1 g L−1 of sodium acetate was used; however, the lutein accumulation gradually decreased with an increase in acetate concentration [167].
4.6. Inorganic Carbon Source
The inorganic carbon source also plays a crucial role in lutein accumulation. In Desmodesmus sp. F51, the best lutein accumulation was achieved under a 2.5% CO2 supply, surpassing the addition of NaHCO3 or Na2CO3. When both CO2 supply and NaHCO3 were used, the concentration reached 3.98 ± 0.13 mg g−1 [166]. Additionally, CO2 concentration in autotrophic mode influences lutein accumulation, as demonstrated by Molino et al. in Scenedesmus almeriensis. This microalga accumulated the highest amount of lutein (5.71 mg g−1) under the highest tested CO2 concentration [173].
4.7. Abiotic Stress
Various abiotic stresses have been explored to understand their impact on lutein accumulation. Oxidative stress, for instance, demonstrated the ability to promote lutein formation and enhance lutein production in heterotrophic Chlorella protothecoides [174]. The cells likely increased lutein production to counteract ROS present in the growth medium. Specifically, the presence of 0.01 mmol L−1 H2O2 and 0.5 mmol L−1 NaClO resulted in an increase in lutein content from 1.75 to 1.98 mg g−1 DW. Salinity also influences lutein accumulation, generally leading to a decrease [160,170]. However, in the case of Coccomyxa onubensis, Bermejo et al. found that lutein content increased with salinity, reaching 7.80 mg g−1 with 500 mM of NaCl, although lutein productivity was optimized at 100 mM NaCl [175]. In the same research, they observed that illumination with photosynthetically active radiation (PAR)+UVA light increases lutein content to 7.07 mg g−1. Researchers have also evaluated pH effectiveness in accumulating lutein. In Chlorella sorokiniana MB-1-M12, it has been shown that the optimal pH for lutein accumulation was 7.5 [164].
4.8. Growth Strategy
Fed-batch conditions have proven to be effective in enhancing lutein content in microalgal cells. For instance, Xie et al. demonstrated that employing a fed-batch strategy with a continuous supply of 2.2 mM nitrate after the initial nitrate concentration was depleted maximized the lutein concentration in Desmodesmus sp. F51 to 5.05 ± 0.20 mg g−1 [159]. Other researchers observed similar findings when cultivating Chlorella protothecoides in fed-batch mode [169]. The effectiveness of the fed-batch strategy in inducing lutein accumulation was also confirmed by Ma et al. [176], by Xie et al. [167], and by Chen et al. [158]. In another case, a novel two-stage process integrating fed-batch and semi-batch modes was shown to be effective in promoting lutein accumulation [164].


Table 2.
Main culturing conditions used for lutein production optimization by microalgae.
Table 2.
Main culturing conditions used for lutein production optimization by microalgae.
| Microorganism | Culture Conditions | Metabolic Mode | Enhancing Condition Applied | Lutein Content | Comment | Ref. |
|---|---|---|---|---|---|---|
| Tetraselmis sp. CTP4 | Batch cultivation in 5-L reactors with modified algal medium at 100 μmol m−2 s−1 | Photoautotrophic | Temperature | 2.15 ± 0.25 mg g−1 | Lutein content demonstrates an increase with rising temperature, reaching optimal levels at 30 °C | [165] |
| Tetraselmis sp. CTP4 | First step: batch cultivation in 5 L reactors with modified algal medium | Photoautotrophic | Combination of several strategies | 3.17 ± 0.18 mg g−1 | Effective enhancement of lutein content achieved through a two-stage cultivation process involving nitrogen depletion, controlled temperature, and light optimization | [165] |
| Chlorella protothecoides | Batch cultivation in 3.7 L fermenter with modified basal medium | Heterotrophic | Glucose addition | 4.44 mg g−1 | Optimal glucose concentrations were found to be 10 g L−1 and 40 g L−1 | [172] |
| Chlorella protothecoides | Batch cultivation in 3.7 L fermenter with modified basal medium with 40 g L−1 of glucose | Heterotrophic | Nitrogen source | 4.58 mg g−1 | Urea identified as the most effective nitrogen source for enhancing lutein content | [21] |
| Chlorella protothecoides | Batch cultivation in modified basal medium in Erlenmeyer flasks (250-mL) | Heterotrophic | Oxidative stress | 1.98 mg g−1 | Increased lutein accumulation observed in the presence of 0.01 mmol L-1 H2O2 and 0.5 mmol L−1 NaClO | [174] |
| Chlamydomonas acidophila | Batch cultivation in 1 L reactor at 25 °C | Mixotrophic | Source of carbon | 10 mg g−1 | In the presence of CO2, starch, urea, or glucose were the best carbon source | [171] |
| Desmodesmus sp. F51 | Batch cultivation in photobioreactor at 150 μmol m−2 s−1, temperature at 35 °C | Photoautotrophic | Nitrogen | 4.97 ± 0.05 mg g−1 | Optimal lutein accumulation achieved at 13.2 mM of N-nitrate | [159] |
| Desmodesmus sp. F51 | Batch cultivation in photobioreactor (PBR) at 150 μmol m−2 s−1, temperature at 35 °C; nitrate concentration, 8.8 mM | Photoautotrophic | Light intensity | 4.69 ± 0.08 mg g−1 | Increasing light intensity leads to a decrease in lutein content | [159] |
| Desmodesmus sp. F51 | Fed-batch photobioreactor at 600 μmol m−2 s−1 at 35 °C temperature, initial nitrate concentration, 8.8 mM | Photoautotrophic | Growth strategy | 5.05 ± 0.20 mg g−1 | Increased lutein content observed in fed-batch cultivation with a 2.2 mM nitrate feeding concentration | [159] |
| Chlorella protothecoides | Fed-batch growth; batch condition with 40 g L−1 of glucose; fed-batch condition limited in nitrogen | Heterotrophic | Combination of several strategies | 5.35 mg g−1 | Effective enhancement of lutein content achieved through a three-step cultivation process involving growth strategy, nitrogen depletion, and temperature control | [169] |
| S. obliquus FSP-3 | Batch growth in photobioreactor, 24 mM Ca(NO3)2 | Photoautotrophic | Nitrogen concentration | 4.95 ± 0.22 mg g−1 | Lutein content shows a positive correlation with rising nitrogen concentration | [163] |
| Chlorella minutissima MCC-27 | Batch cultivation in 2 L airlift photobioreactor with BBM | Photoautotrophic | Light supply | 8.24 ± 0.12 mg g−1 | Light supply strategy has a role on lutein accumulation | [162] |
| Desmodesmus sp. F51 | Batch cultivation in 1 L glass photobioreactor with modified Bristol’s medium at 150 μmol m−2 s−1 | Photoautotrophic | Carbon and nitrogen sources | 5.56 mg g−1 | Optimal lutein accumulation achieved at 150 mg L−1 of N-ammonium | [166] |
| Chlorella sorokiniana Mb-1 | Batch cultivation in 1 L glass photobioreactor with modified Bristol’s medium at 150 μmol m−2 s−1 with 1.83 g L−1 of nitrate | Mixotrophic | Organic carbon concentration | 4.6 mg g−1 | Increased lutein content observed at the lowest tested concentrations of acetate (4.88 g L−1) | [168] |
| Chlamydomonas sp. JSC4 | Batch cultivation in photobioreactor with modified Bold 3 N medium, sea salt, and initial nitrate-N concentrations adjusted to 2% and 1000 mg L−1 | Photoautotrophic | Light quality | 3.10 mg g−1 | Blue light increases lutein content | [157] |
| Chlamydomonas sp. JSC4 | Batch cultivation in photobioreactor with modified Bold 3 N medium/two-stage cultivation | Photoautotrophic | Combination of several strategies | 4.24 ± 0.22 mg g−1 | Efficient enhancement of lutein content achieved through two-stage cultivation involving control of light quality and temperature | [157] |
| Chlorella sorokiniana C16 | Batch cultivation on Tris acetate phosphate (TAP) media | Mixotrophic | Temperature | 17.4 mg g−1 | Lutein content exhibits a positive trend, reaching its peak with increasing temperature up to 32 °C | [170] |
| Chlorella sorokiniana FZU60 | Batch cultivation in BG11 medium at 33 °C; light intensity, 150 µmol m−2 s−1, initial NaNO3 concentration of 1 g L−1 | Mixotrophic | Organic carbon concentration | 9.65 ± 0.25 mg g−1 | Optimal lutein accumulation achieved at 1 g L−1 of sodium acetate | [167] |
| Chlorella sorokiniana FZU60 | Batch cultivation in BG11 medium; light intensity, 150 µmol m−2 s−1, 0.75 g L−1 sodium nitrate, and 1 g L−1 sodium acetate | Mixotrophic | Temperature | 9.81 ± 0.49 mg g−1 | Peak lutein content achieved at an optimal temperature of 33 °C | [156] |
| Chlorella sorokiniana Kh12 | Batch cultivation on Tris acetate phosphate (TAP) media | Mixotrophic | Temperature | 17.25 mg g−1 | Peak lutein content achieved at an optimal temperature of 30 °C | [160] |
| Scenedesmus almeriensis | Batch cultivation in vertical bubble column photobioreactor in modified Mann & Myers medium | Autotrophic | CO2 concentration | 5.71 mg g−1 | Optimal lutein accumulation observed at the highest CO2 concentration tested (3% v/v) | [173] |
| Parachlorella sp. JD-076 | Batch cultivation in tubular-type photobioreactor in BG-11 medium at 35 °C | Autotrophic | Light intensity | 11.8 mg g−1 | Lutein content shows a positive correlation with rising light intensities | [155] |
| Coccomyxa onubensis | Batch cultivation in 2L Erlenmeyer flasks 140 µmol m−2 s−1 at 26 °C | Autotrophic | Salt | 7.80 mg g−1 | Enhanced lutein content observed under UVA + PAR illumination | [175] |
| Coccomyxa onubensis | Batch cultivation in 2L Erlenmeyer flasks 140 µmol m−2 s−1 at 26 °C | Autotrophic | UVA light | 7.07 mg g−1 | Lutein content demonstrated a positive correlation with rising salinity levels | [175] |
| Chlorella sorokiniana MB-1-M12 | Batch cultivation in 5L fermenter with BG-11 medium at 27 ± 1 °C | Heterotrophic | Two-stage process | 5.88 mg g−1 | The described two-stage process integrating fed-batch and semi-batch modes induced lutein accumulation | [164] |
| Scenedesmus obliquus CWL-1 | Batch cultivation in 7 L stirred tank reactor with 4.5 g L−1 of calcium nitrate | Mixotrophic | Combination of several strategies | 2.45 mg g−1 | Photoperiod and wavelength adjustment effective in inducing lutein | [158] |
5. Pilot-Scale Systems for Lutein Production
Various attempts have been made to produce lutein on a pilot scale. In an early effort, Moulton et al. cultivated Dunaliella viridis at pilot scale to produce various carotenoids. Laboratory experiments showed robust growth of D. viridis, achieving high levels of mixed carotenoids (approximately 13 mg L −1 carotenoid). However, outdoor pond experiments yielded less promising results [177]. Del Campo et al. [23] explored an alternative approach using a closed tubular reactor made of methyl polymethacrylate, equipped with an airlift system for cell culture recirculation and an external horizontal loop of tubes (90 m long, 2.4 cm inner diameter, and 2.2 m2 surface). They employed Muriellopsis sp. in semi-continuous mode, investigating the impact of dilution rate, mixing, and daily solar cycles on lutein and biomass productivity. Optimal productivity values, for both lutein (about 180 mg m−2 per day) and biomass (about 40 g m−2 per day), were achieved in May and July, with varying optimal dilution rates [23].
Jeon et al., after optimizing the medium recipe to enhance lutein productivity, validated the process on a larger scale of 25,000 L and 240,000 L. The lutein concentrations obtained at these scales (260.55 ± 3.23 mg L−1 and 263.13 ± 2.72 mg L−1, respectively) were comparable to the laboratory scale of 252.75 ± 12.92 mg L−1 [178].
McClure et al. identified optimal conditions for lutein production from Chlorella vulgaris in a 5 L photobioreactor, later validating the process at a 50 L scale. They achieved maximized lutein productivity (1.6 mg L−1 day−1) using specific parameters and demonstrated consistent system performance in semi-continuous operation for 32 days, maintaining high lutein concentrations (15–20 mg L−1) [179].
In a different study, Xie et al. evaluated the potential of Chlorella sorokiniana FZU60 for lutein production in a 50 L column photobioreactor using a two-stage strategy. Lutein content, production, and productivity reached 9.51 mg g−1, 33.55 mg L−1, and 4.67 mg L−1 d−1, respectively [180]. In another case, Coccomyxa onubensis, cultivated in an 800 L vertical tubular photobioreactor outdoors, demonstrated stable biomass production for at least one month, with a lutein content of 10 mg g−1 and a maximal lutein productivity of 1.42 mg L−1 d−1 [181]. The authors also cultivated the same microalga indoors, in two plastic bags of 400 L each. A maximal lutein concentration of 9.7 mg g−1 was obtained, leading to a maximal lutein productivity of 0.9 mg L−1 d−1.
More recently, Cavieres et al. integrated microalgal cultivation with wastewater treatment for carotenoid production. Muriellopsis sp. in an 800 L raceway removed 84% of nitrogen, 93% of phosphorus, and other chemical compounds within 4 days. Biomass productivity reached 104.25 mg·L–1·day–1, with 51% protein and a pigment content of 0.6% carotenoid, including 0.3% lutein [182]. Lastly, Chlorella sorokiniana TH01, cultivated in a 90 L flat-plate photobioreactor indoors, achieved the highest biomass and lutein productivity of 284–469 mg L−1 d−1 and 2.57–4.57 mg L−1 d−1, respectively [183].
The existing literature provides ample evidence supporting the technical feasibility of mass-producing lutein through microalgal cultivation. Over the years, various technical challenges have been addressed, indicating the viability of the process. To comprehensively assess the economic feasibility, additional evidence on technoeconomic aspects is essential. This will contribute to a more thorough understanding of the economic viability of the lutein production process.
6. Genetic Engineering Production
Many researchers have also tried to increase lutein production by metabolic engineering approaches. In the last few years, many attempts have been made to obtain encouraging yields. Chemical mutagenesis (such as with N-methyl-N′-nitro-N-nitrosoguanidine and ethyl methane sulfonate) as well as targeted genetic engineering of specific genes have been applied to the engineering of microalgal biosynthetic pathways for lutein production [184]. Huang et al., for instance, studied a mutant of Chlorella zofingiensis (CZ-bkt1) obtained by chemical mutagen (N-methyl-N’-nitro-N73 nitrosoguanidine) exposure. In addition, they cultivated it under different conditions and obtained the highest lutein production in high light irradiation and nitrogen deficiency (13.81 ± 1.23 mg g−1) and feeding with glucose culturing conditions (33.97 ± 2.61 mg L−1) [185]. Chlorella sorokiniana was also modified by random mutagenesis with N-methyl-N′-nitro-nitrosoguanidine (MNNG), obtaining a mutant MR-16 exhibiting 2.0-fold higher volumetric lutein content (42.0 mg L−1) and mutants DMR-5 and DMR-8 with lutein cellular content of 7.0 mg g−1 dry weight [186].
Regarding genetic engineering, transformation was generally performed by electroporation, shaking of cells with glass beads, and particle gun bombardment (biolistic method). The most studied genes were phytoene synthase (psy), phytoene desaturase (pds), and phytoene-β-carotene synthase (pbs). Cordero et al. studied the phytoene synthase gene, involved in the first step of carotenoid synthesis, from the green microalga Chlorella zofingiensis. The phytoene synthase gene was expressed in Chlamydomonas reinhardtii by nuclear transformation, resulting in gene overexpression and an increase of lutein production 2.2-fold higher with respect to untransformed cells [187]. Similarly, Liu et al. studied the gene phytoene desaturase from the green microalga Chlamydomonas reinhardtii. The authors applied a single amino acid substitution, L505F, and showed that this modification induced an increase of 29% of its activity and increased production of carotenoids [188]. In another similar study, Liu at al. nuclear-transformed Chlorella zofingiensis with a mutant version of phytoene desaturase gene (one amino acid substitution). This modification induced an increased production of carotenoids as well (total carotenoids 32%) compared to the control strain [189]. Recently, Rathod and co-workers inserted the gene coding for phytoene-β-carotene synthase from the red yeast Xanthophyllomyces dendrorhous in Chlamydomonas reinhardtii, by nuclear transformation. They obtained a species with the bifunctional enzyme with both phytoene synthase and lycopene cyclisation activities and obtained a 60% increase in lutein production under low light conditions [190].
Takemura et al. in 2021 applied heterologous expression by using as host Escherichia coli. In particular, they included, in the E. coli JM101 (DE3) genome, various genes involved in the lutein biosynthetic pathway, such as lycopene β-cyclase, lycopene ε-cyclase, and cytochrome P450 97C. They introduced in E. coli several plasmids containing some of the multiple genes and compared lutein productivity by obtaining 11 mg L−1 [191]. In the same year, Bian et al. tried to obtain good yields by heterologous expression in yeast and in particular in Saccharomyces cerevisae. During their experiments, they found as limiting step a competition of the cyclases for lycopene, forming β-carotene, and solved this problem by a temperature-responsive expression redirecting to α-carotene synthesis. At the end, they obtained 438 μg g−1 dry cells [192].
7. Conclusions
Overall, the information reported in this review shows that microalgae can be considered the main source of lutein, and carotenoids in general, in the marine environment [193]. Their use as lutein producers is advantageous compared to terrestrial plants because they are characterized by higher growth rates, combined with high photosynthetic efficiency and a great potential for carbon mitigation. Actually, the commercial supply of lutein is characterized by the bright yellow petals of the marigold flowers of the genus Tagetes, while chemical synthesis allows one to obtain very low yields [13]. Hence, novel eco-sustainable sources like microalgae can be a valid alternative, thanks also to the possibility of cultivating them in a controlled manner in photobioreactors.
In addition, nowadays, researchers are looking for new extraction methods that are environmentally sustainable. In fact, to overcome the high dosage of solvents and organic bases that are required in conventional methods of lutein extraction, researchers are focusing on the optimization of the extraction process, trying to limit environmental impacts [33]. Overall, it is always important to consider the environmental impact during the extraction process of natural substances, and therefore the suggestion is to try to use green solvents, such as vegetable oils, to perform extractions at low temperatures and/or with ultrasounds by using low energy consumption and reducing the extraction timing.
Author Contributions
Conceptualization, C.L.; writing—original draft preparation, E.M., S.L., A.M. and C.L.; writing—review and editing, E.M., S.L., A.M., F.S. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was realized with the cofounding of European Union FESR or FSE, PON Ricerca e Innovazione 2014–2020—DM 1062/2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.; Satriano, A.; Marchesini, G. The Effect of Lutein on Eye and Extra-Eye Health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Chemical Diversity and Defence Metabolism: How Plants Cope with Pathogens and Ozone Pollution. Int. J. Mol. Sci. 2009, 10, 3371–3399. [Google Scholar] [CrossRef] [PubMed]
- Kalariya, N.M.; Ramana, K.V.; VanKuijk, F.J.G.M. Focus on Molecules: Lutein. Exp. Eye Res. 2012, 102, 107–108. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 446925, Lycopene. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/446925#section=2D-Structure (accessed on 26 February 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5281230, Delta-Carotene. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5281230#section=2D-Structure (accessed on 26 February 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6419725, Alpha-Carotene. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6419725#section=2D-Structure (accessed on 26 February 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5281234, Zeinoxanthin. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5281234#section=2D-Structure (accessed on 26 February 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 23724629, Alpha-Cryptoxanthin. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/23724629#section=2D-Structure (accessed on 26 February 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5281243, Lutein. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5281243#section=2D-Structure (accessed on 26 February 2024).
- Nagy, V.; Agocs, A.; Deli, J. In Vitro and In Vivo Transformations of Lutein. Mini. Rev. Org. Chem. 2009, 6, 211–219. [Google Scholar] [CrossRef]
- Saha, S.K.; Ermis, H.; Murray, P. Marine Microalgae for Potential Lutein Production. Appl. Sci. 2020, 10, 6457. [Google Scholar] [CrossRef]
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein Production from Biomass: Marigold Flowers versus Microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, Y.; Li, S.; Nagarajan, D.; Varjani, S.; Lee, D.J.; Chang, J.S. Recent Advances in Lutein Production from Microalgae. Renew. Sustain. Energy Rev. 2022, 153, 111795. [Google Scholar] [CrossRef]
- Sun, Z.; Li, T.; Zhou, Z.; Jiang, Y. Microalgae as a Source of Lutein: Chemistry, Biosynthesis, and Carotenogenesis; Springer: Berlin/Heidelberg, Germany, 2015; pp. 37–58. [Google Scholar]
- Ochoa Becerra, M.; Mojica Contreras, L.; Hsieh Lo, M.; Mateos Díaz, J.; Castillo Herrera, G. Lutein as a Functional Food Ingredient: Stability and Bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- Sulich, A.; Hamułka, J.; Nogal, D. Dietary Sources of Lutein in Adults Suffering Eye Disease (AMD/Cataracts). Rocz. Panstw. Zakl. Hig. 2015, 66, 55–60. [Google Scholar]
- Raposo, M.; de Morais, A.; de Morais, R. Carotenoids from Marine Microalgae: A Valuable Natural Source for the Prevention of Chronic Diseases. Mar. Drugs 2015, 13, 5128–5155. [Google Scholar] [CrossRef]
- Li, L.H.; Lee, J.C.-Y.; Leung, H.H.; Lam, W.C.; Fu, Z.; Lo, A.C.Y. Lutein Supplementation for Eye Diseases. Nutrients 2020, 12, 1721. [Google Scholar] [CrossRef]
- Muhammad, G.; Butler, T.O.; Chen, B.; Lv, Y.; Xiong, W.; Zhao, X.; Solovchenko, A.E.; Zhao, A.; Mofijur, M.; Xu, J.; et al. Sustainable Production of Lutein—An Underexplored Commercially Relevant Pigment from Microalgae. Biomass Convers. Biorefin. 2022, 1–22. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Vargas, M.Á.; Rivas, J.; Guerrero, M.G. Accumulation of Astaxanthin and Lutein in Chlorella zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2004, 64, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.M.; Zhang, X.W.; Chen, F. Heterotrophic Production of Biomass and Lutein by Chlorella protothecoides on Various Nitrogen Sources. Enzyme Microb. Technol. 2000, 27, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J. Carotenoid Content of Chlorophycean Microalgae: Factors Determining Lutein Accumulation in Muriellopsis sp. (Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Vargas, M.Á.; Rivas, J.; Guerrero, M.G. Lutein Production by Muriellopsis sp. in an Outdoor Tubular Photobioreactor. J. Biotechnol. 2001, 85, 289–295. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Fernández, J.M.; Acién, F.G.; Rueda, A.; Pérez-Parra, J.; Molina, E. Influence of Culture Conditions on the Productivity and Lutein Content of the New Strain Scenedesmus almeriensis. Process Biochem. 2008, 43, 398–405. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Yi, L.; Liu, J.; Yang, S.; Liu, B.; Chen, F.; Sun, H. Lutein Production from Microalgae: A Review. Bioresour. Technol. 2023, 376, 128875. [Google Scholar] [CrossRef]
- Available online: https://www.marketsandmarkets.com/market-reports/lutein-market-69753879.html (accessed on 26 February 2024).
- Fatima, I.; Munir, M.; Qureshi, R.; Hanif, U.; Gulzar, N.; Sheikh, A.A. Advanced Methods of Algal Pigments Extraction: A Review. Crit. Rev. Food Sci. Nutr. 2023, 1–18. [Google Scholar] [CrossRef]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative Alternative Technologies to Extract Carotenoids from Microalgae and Seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- Patel, A.K.; Vadrale, A.P.; Tseng, Y.-S.; Chen, C.-W.; Dong, C.-D.; Singhania, R.R. Bioprospecting of Marine Microalgae from Kaohsiung Seacoast for Lutein and Lipid Production. Bioresour. Technol. 2022, 351, 126928. [Google Scholar] [CrossRef] [PubMed]
- Larsen, E.; Christensen, L.P. Simple Saponification Method for the Quantitative Determination of Carotenoids in Green Vegetables. J. Agric. Food Chem. 2005, 53, 6598–6602. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-B.; Jiang, Y.; Chen, F. Isolation and Purification of Lutein from the Microalga Chlorella vulgaris by Extraction after Saponification. J. Agric. Food Chem. 2002, 50, 1070–1072. [Google Scholar] [CrossRef]
- Kumar, R.; Yu, W.; Jiang, C.; Shi, C.; Zhao, Y. Improvement of The Isolation and Purification Of Lutein From Marigold Flower (Tagetes erecta L.) and Its Antioxidant Activity. J. Food Process Eng. 2010, 33, 1065–1078. [Google Scholar] [CrossRef]
- Chan, M.-C.; Ho, S.-H.; Lee, D.-J.; Chen, C.-Y.; Huang, C.-C.; Chang, J.-S. Characterization, Extraction and Purification of Lutein Produced by an Indigenous Microalga Scenedesmus obliquus CNW-N. Biochem. Eng. J. 2013, 78, 24–31. [Google Scholar] [CrossRef]
- Mendes-Pinto, M.M.; Raposo, M.F.J.; Bowen, J.; Young, A.J.; Morais, R. Evaluation of Different Cell Disruption Processes on Encysted Cells of Haematococcus pluvialis: Effects on Astaxanthin Recovery and Implications for Bio-Availability. J. Appl. Phycol. 2001, 13, 19–24. [Google Scholar] [CrossRef]
- Jeevan Kumar, S.P.; Vijay Kumar, G.; Dash, A.; Scholz, P.; Banerjee, R. Sustainable Green Solvents and Techniques for Lipid Extraction from Microalgae: A Review. Algal Res. 2017, 21, 138–147. [Google Scholar] [CrossRef]
- Maciassanchez, M.; Mantell, C.; Rodriguez, M.; Martinezdelaossa, E.; Lubian, L.; Montero, O. Comparison of Supercritical Fluid and Ultrasound-Assisted Extraction of Carotenoids and Chlorophyll a from Dunaliella salina. Talanta 2009, 77, 948–952. [Google Scholar] [CrossRef]
- Kanda, H.; Wahyudiono; Machmudah, S.; Goto, M. Direct Extraction of Lutein from Wet Macroalgae by Liquefied Dimethyl Ether without Any Pretreatment. ACS Omega 2020, 5, 24005–24010. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.; Romero-Castillo, K.; Parra-Arroyo, L.; Aguilar-Aguila-Isaías, M.; García-Reyes, I.; Ahmed, I.; Parra-Saldivar, R.; Bilal, M.; Iqbal, H. Mexican Microalgae Biodiversity and State-Of-The-Art Extraction Strategies to Meet Sustainable Circular Economy Challenges: High-Value Compounds and Their Applied Perspectives. Mar. Drugs 2019, 17, 174. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.P.; Marino, T.; Chianese, S.; Balducchi, R.; Musmarra, D. Recent Developments in Supercritical Fluid Extraction of Bioactive Compounds from Microalgae: Role of Key Parameters, Technological Achievements and Challenges. J. CO2 Util. 2020, 36, 196–209. [Google Scholar] [CrossRef]
- Miguel, F.; Martín, A.; Mattea, F.; Cocero, M.J. Precipitation of Lutein and Co-Precipitation of Lutein and Poly-Lactic Acid with the Supercritical Anti-Solvent Process. Chem. Eng. Process. Process Intensif. 2008, 47, 1594–1602. [Google Scholar] [CrossRef]
- Yen, H.-W.; Chiang, W.-C.; Sun, C.-H. Supercritical Fluid Extraction of Lutein from Scenedesmus Cultured in an Autotrophical Photobioreactor. J. Taiwan Inst. Chem. Eng. 2012, 43, 53–57. [Google Scholar] [CrossRef]
- Ruen-ngam, D.; Shotipruk, A.; Pavasant, P.; Machmudah, S.; Goto, M. Selective Extraction of Lutein from Alcohol Treated Chlorella vulgaris by Supercritical CO2. Chem. Eng. Technol. 2012, 35, 255–260. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Mendiola, J.A.; van den Broek, L.A.M.; Houweling-Tan, B.; Sijtsma, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Green Compressed Fluid Technologies for Downstream Processing of Scenedesmus obliquus in a Biorefinery Approach. Algal Res. 2017, 24, 111–121. [Google Scholar] [CrossRef]
- Ranjith Kumar, R.; Hanumantha Rao, P.; Arumugam, M. Lipid Extraction Methods from Microalgae: A Comprehensive Review. Front. Energy Res. 2015, 2, 61. [Google Scholar] [CrossRef]
- Pan, M.; Su, Y.; Zhu, X.; Pan, G.; Zhang, Y.; Angelidaki, I. Bioelectrochemically Assisted Sustainable Conversion of Industrial Organic Wastewater and Clean Production of Microalgal Protein. Resour. Conserv. Recycl. 2021, 168, 105441. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chong, Y.M.; Chang, W.S.; Yap, J.M.; Foo, S.C.; Khoiroh, I.; Lau, P.L.; Chew, K.W.; Ooi, C.W.; Show, P.L. Permeabilization of Chlorella sorokiniana and Extraction of Lutein by Distillable CO2-Based Alkyl Carbamate Ionic Liquids. Sep. Purif. Technol. 2021, 256, 117471. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (NADES): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- Paliwal, C.; Rehmanji, M.; Shaikh, K.M.; Zafar, S.U.; Jutur, P.P. Green Extraction Processing of Lutein from Chlorella saccharophila in Water-Based Ionic Liquids as a Sustainable Innovation in Algal Biorefineries. Algal Res. 2022, 66, 102809. [Google Scholar] [CrossRef]
- Fan, C.; Liu, Y.; Shan, Y.; Cao, X. A Priori Design of New Natural Deep Eutectic Solvent for Lutein Recovery from Microalgae. Food Chem. 2022, 376, 131930. [Google Scholar] [CrossRef] [PubMed]
- Nithya, S.; Krishnan, R.R.; Rao, N.R.; Naik, K.; Praveen, N.; Vasantha, V.L. Microwave-Assisted Extraction of Phytochemicals. In Drug Discovery and Design Using Natural Products; Springer Nature Switzerland: Cham, Switzerland, 2023; pp. 209–238. [Google Scholar]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. Acceleration of Microwave-Assisted Extraction Processes of Food Components by Integrating Technologies and Applying Emerging Solvents: A Review of Latest Developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Mary Leema, J.T.; Persia Jothy, T.; Dharani, G. Rapid Green Microwave Assisted Extraction of Lutein from Chlorella sorokiniana (NIOT-2)—Process Optimization. Food Chem. 2022, 372, 131151. [Google Scholar] [CrossRef]
- Low, K.L.; Idris, A.; Mohd Yusof, N. Novel Protocol Optimized for Microalgae Lutein Used as Food Additives. Food Chem. 2020, 307, 125631. [Google Scholar] [CrossRef]
- Gayathri, S.; Rajasree Radhika, S.R.; Suman, T.Y.; Aranganathan, L. Ultrasound-Assisted Microextraction of β, ε-Carotene-3, 3′-Diol (Lutein) from Marine Microalgae Chlorella salina: Effect of Different Extraction Parameters. Biomass Convers. Biorefinery 2018, 8, 791–797. [Google Scholar] [CrossRef]
- Available online: https://www.nobelprize.org/prizes/chemistry/1915/willstatter/biographical/ (accessed on 27 February 2024).
- Ingkasupart, P.; Manochai, B.; Song, W.T.; Hong, J.H. Antioxidant Activities and Lutein Content of 11 Marigold Cultivars (Tagetes spp.) Grown in Thailand. Food Sci. Technol. 2015, 35, 380–385. [Google Scholar] [CrossRef]
- Şahin, S.; Aybastıer, Ö.; Dawbaa, S.; Karkar, B.; Çakmak, T. Study of the Ability of Lutein and Neoxanthin as Standards and in the Extract of Chlamydomonas reinhardtii to Prevent Oxidatively Induced DNA Base Damage Using Ultrasensitive GC–MS/MS Analysis. Chromatographia 2020, 83, 919–926. [Google Scholar] [CrossRef]
- Kim, E.A.; Kang, N.; Heo, S.Y.; Oh, J.Y.; Lee, S.H.; Cha, S.H.; Kim, W.K.; Heo, S.J. Antioxidant, Antiviral, and Anti-Inflammatory Activities of Lutein-Enriched Extract of Tetraselmis Species. Mar. Drugs 2023, 21, 369. [Google Scholar] [CrossRef]
- Serpeloni, J.M.; de Cólus, I.M.S.; de Oliveira, F.S.; Aissa, A.F.; Mercadante, A.Z.; Bianchi, M.L.P.; Antunes, L.M.G. Diet Carotenoid Lutein Modulates the Expression of Genes Related to Oxygen Transporters and Decreases DNA Damage and Oxidative Stress in Mice. Food Chem. Toxicol. 2014, 70, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qian, S.; Na, X.; Zhao, A. Association between Dietary and Supplemental Antioxidants Intake and Lung Cancer Risk: Evidence from a Cancer Screening Trial. Antioxidants 2023, 12, 338. [Google Scholar] [CrossRef]
- Jȩdrzejczak-Pospiech, K.; Błaszczyk, J. The Effect of Lutein on the Total Antioxidant Status in Human Blood. Klin. Oczna 2017, 2017, 220–223. [Google Scholar]
- Lorenzoni, F.; Giampietri, M.; Ferri, G.; Lunardi, S.; Madrigali, V.; Battini, L.; Boldrini, A.; Ghirri, P. Lutein Administration to Pregnant Women with Gestational Diabetes Mellitus Is Associated to a Decrease of Oxidative Stress in Newborns. Gynecol. Endocrinol. 2013, 29, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Giannantonio, C.; Romagnoli, C.; Vento, G.; Gervasoni, J.; Persichilli, S.; Zuppi, C.; Cota, F. Effects of Lutein Supplementation on Biological Antioxidant Status in Preterm Infants: A Randomized Clinical Trial. J. Matern. Neonatal Med. 2013, 26, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Tei, M.; Longini, M.; Santacroce, A.; Turrisi, G.; Proietti, F.; Felici, C.; Picardi, A.; Bazzini, F.; Vasarri, P.; et al. Lipid and Protein Oxidation in Newborn Infants after Lutein Administration. Oxid. Med. Cell. Longev. 2014, 2014, 781454. [Google Scholar] [CrossRef] [PubMed]
- Alves-Rodrigues, A.; Shao, A. The Science behind Lutein. Toxicol. Lett. 2004, 150, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.J.; Kim, H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants 2021, 10, 1448. [Google Scholar] [CrossRef]
- Boehm, F.; Edge, R.; Truscott, T.G. Anti- and pro-Oxidative Mechanisms Comparing the Macular Carotenoids Zeaxanthin and Lutein with Other Dietary Carotenoids—A Singlet Oxygen, Free-Radica I in Vitro and Ex Vivo Study. Photochem. Photobiol. Sci. 2020, 19, 1001–1009. [Google Scholar] [CrossRef]
- Subagio, A.; Morita, N. No Effect of Esterification with Fatty Acid on Antioxidant Activity of Lutein. Food Res. Int. 2001, 34, 315–320. [Google Scholar] [CrossRef]
- Yang, C.; Fischer, M.; Kirby, C.; Liu, R.; Zhu, H.; Zhang, H.; Chen, Y.; Sun, Y.; Zhang, L.; Tsao, R. Bioaccessibility, Cellular Uptake and Transport of Luteins and Assessment of Their Antioxidant Activities. Food Chem. 2018, 249, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Beck, M.; Elliott, J.; Etheve, S.; Roberts, R.; Schalch, W. Effects of Formulation on the Bioavailability of Lutein and Zeaxanthin: A Randomized, Double-Blind, Cross-over, Comparative, Single-Dose Study in Healthy Subjects. Eur. J. Nutr. 2013, 52, 1381–1391. [Google Scholar] [CrossRef]
- Nemli, E.; Capanoglu, E.; McClements, D.J.; Tomas, M. Use of Excipient Emulsions for Improving the Bioaccessibility of Antioxidants in Tomato Sauce. Food Chem. 2023, 424, 136395. [Google Scholar] [CrossRef]
- Pugliese, A.; O’Callaghan, Y.; Tundis, R.; Galvin, K.; Menichini, F.; O’Brien, N.; Loizzo, M.R. In Vitro Investigation of the Bioaccessibility of Carotenoids from Raw, Frozen and Boiled Red Chili Peppers (Capsicum annuum). Eur. J. Nutr. 2014, 53, 501–510. [Google Scholar] [CrossRef]
- Zeb, A.; Haq, A.; Murkovic, M. Effects of Microwave Cooking on Carotenoids, Phenolic Compounds and Antioxidant Activity of Cichorium intybus L. (Chicory) Leaves. Eur. Food Res. Technol. 2019, 245, 365–374. [Google Scholar] [CrossRef]
- Mashiane, P.; Shoko, T.; Manhivi, V.; Slabbert, R.; Sultanbawa, Y.; Sivakumar, D. A Comparison of Bioactive Metabolites, Antinutrients, and Bioactivities of African Pumpkin Leaves (Momordica balsamina L.) Cooked by Different Culinary Techniques. Molecules 2022, 27, 1901. [Google Scholar] [CrossRef] [PubMed]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Fritz, F.R.; Nagy, T.; Agócs, A.; Deli, J. Protective Effects of 3′-Epilutein and 3′-Oxolutein against Glutamate-Induced Neuronal Damage. Int. J. Mol. Sci. 2023, 24, 12008. [Google Scholar] [CrossRef]
- Cui, N.; Yang, C.; Chen, X.; Zhang, J.; Zhang, L. Anti-Oxidative and Anti-Inflammatory Effects of Different Lutein Isomers in Caco-2 Cell Model. Food Biosci. 2023, 53, 102646. [Google Scholar] [CrossRef]
- Serra, A.T.; Silva, S.D.; Pleno de Gouveia, L.; Alexandre, A.M.R.C.; Pereira, C.V.; Pereira, A.B.; Partidário, A.C.; Silva, N.E.; Bohn, T.; Gonçalves, V.S.S.; et al. A Single Dose of Marine Chlorella vulgaris Increases Plasma Concentrations of Lutein, β-Carotene and Zeaxanthin in Healthy Male Volunteers. Antioxidants 2021, 10, 1164. [Google Scholar] [CrossRef]
- Ozawa, Y.; Sasaki, M. Lutein and Oxidative Stress-Mediated Retinal Neurodegeneration in Diabetes; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780124058859. [Google Scholar]
- Murthy, R.K.; Ravi, K.; Balaiya, S.; Brar, V.S.; Chalam, K.V. Lutein Protects Retinal Pigment Epithelium from Cytotoxic Oxidative Stress. Cutan. Ocul. Toxicol. 2014, 33, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Kamoshita, M.; Toda, E.; Osada, H.; Narimatsu, T.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Lutein Acts via Multiple Antioxidant Pathways in the Photo-Stressed Retina. Sci. Rep. 2016, 6, 30226. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.H.; Galano, J.M.; Crauste, C.; Durand, T.; Lee, J.C.Y. Combination of Lutein and Zeaxanthin, and DHA Regulated Polyunsaturated Fatty Acid Oxidation in H2O2-Stressed Retinal Cells. Neurochem. Res. 2020, 45, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, W.; Zhou, X.; Long, C.; Kuang, X.; Hu, J.; Tang, Y.; Liu, L.; He, J.; Huang, Z.; et al. Protective Effect of Lutein on ARPE-19 Cells upon H2O2-Induced G2/M Arrest. Mol. Med. Rep. 2017, 16, 2069–2074. [Google Scholar] [CrossRef]
- Chae, S.; Park, S.; Park, G. Lutein Protects Human Retinal Pigment Epithelial Cells from Oxidative Stress-induced Cellular Senescence. Mol. Med. Rep. 2018, 18, 5182–5190. [Google Scholar] [CrossRef]
- Aimjongjun, S.; Sutheerawattananonda, M.; Limpeanchob, N. Silk Lutein Extract and Its Combination with Vitamin E Reduce UVB-Mediated Oxidative Damage to Retinal Pigment Epithelial Cells. J. Photochem. Photobiol. B Biol. 2013, 124, 34–41. [Google Scholar] [CrossRef]
- Fernández-Robredo, P.; Sádaba, L.M.; Salinas-Alamán, A.; Recalde, S.; Rodríguez, J.A.; García-Layana, A. Effect of Lutein and Antioxidant Supplementation on VEGF Expression, MMP-2 Activity, and Ultrastructural Alterations in Apolipoprotein E-Deficient Mouse. Oxid. Med. Cell. Longev. 2013, 2013, 213505. [Google Scholar] [CrossRef] [PubMed]
- Padmanabha, S.; Vallikannan, B. Fatty Acids Modulate the Efficacy of Lutein in Cataract Prevention: Assessment of Oxidative and Inflammatory Parameters in Rats. Biochem. Biophys. Res. Commun. 2018, 500, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Gencoglu, H.; Akdemir, F.; Orhan, C.; Tuzcu, M.; Sahin, N.; Yilmaz, I.; Juturu, V. Lutein and Zeaxanthin Isomers May Attenuate Photo-Oxidative Retinal Damage via Modulation of G Protein-Coupled Receptors and Growth Factors in Rats. Biochem. Biophys. Res. Commun. 2019, 516, 163–170. [Google Scholar] [CrossRef]
- Erhan, E.; Salcan, I.; Bayram, R.; Suleyman, B.; Dilber, M.; Yazici, G.N.; Coban, T.A.; Altuner, D.; Suleyman, H. Protective Effect of Lutein against Acrolein-Induced Ototoxicity in Rats. Biomed. Pharmacother. 2021, 137, 111281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Liu, X.B.; Chen, X.M.; Kong, Q.H.; Liu, Y.S.; So, K.F.; Chen, J.S.; Xu, Y.; Mi, X.S.; Tang, S.B. Lutein Delays Photoreceptor Degeneration in a Mouse Model of Retinitis Pigmentosa. Neural Regen. Res. 2022, 17, 1596–1603. [Google Scholar] [CrossRef]
- Roldán-Fidalgo, A.; Martín Saldaña, S.; Trinidad, A.; Olmedilla-Alonso, B.; Rodríguez-Valiente, A.; García-Berrocal, J.R.; Ramírez-Camacho, R. In Vitro and in Vivo Effects of Lutein against Cisplatin-Induced Ototoxicity. Exp. Toxicol. Pathol. 2016, 68, 197–204. [Google Scholar] [CrossRef]
- Yu, M.; Yan, W.; Beight, C. Lutein and Zeaxanthin Isomers Protect against Light-Induced Retinopathy via Decreasing Oxidative and Endoplasmic Reticulum Stress in BALB/CJ Mice. Nutrients 2018, 10, 842. [Google Scholar] [CrossRef]
- Bartlett, H.E.; Eperjesi, F. Effect of Lutein and Antioxidant Dietary Supplementation on Contrast Sensitivity in Age-Related Macular Disease: A Randomized Controlled Trial. Eur. J. Clin. Nutr. 2007, 61, 1121–1127. [Google Scholar] [CrossRef]
- Berrow, E.J.; Bartlett, H.E.; Eperjesi, F.; Gibson, J.M. The Effects of a Lutein-Based Supplement on Objective and Subjective Measures of Retinal and Visual Function in Eyes with Age-Related Maculopathy—A Randomised Controlled Trial. Br. J. Nutr. 2013, 109, 2008–2014. [Google Scholar] [CrossRef]
- Nolan, J.M.; Loskutova, E.; Howard, A.; Mulcahy, R.; Moran, R.; Stack, J.; Bolger, M.; Coen, R.F.; Dennison, J.; Akuffo, K.O.; et al. The Impact of Supplemental Macular Carotenoids in Alzheimer’s Disease: A Randomized Clinical Trial. J. Alzheimer’s Dis. 2015, 44, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.-L.; Chiu, H.-F.; Chou, H.; Liao, H.-J.; Chen, S.-T.; Wong, Y.-C.; Shen, Y.-C.; Venkatakrishnan, K.; Wang, C.-K. Influence/Impact of Lutein Complex (Marigold Flower and Wolfberry) on Visual Function with Early Age-Related Macular Degeneration Subjects: A Randomized Clinical Trial. J. Funct. Foods 2016, 24, 122–130. [Google Scholar] [CrossRef]
- Obana, A.; Gohto, Y.; Nakazawa, R.; Moriyama, T.; Gellermann, W.; Bernstein, P.S. Effect of an Antioxidant Supplement Containing High Dose Lutein and Zeaxanthin on Macular Pigment and Skin Carotenoid Levels. Sci. Rep. 2020, 10, 10262. [Google Scholar] [CrossRef]
- Ma, L.; Liu, R.; Du, J.; Liu, T.; Wu, S.; Liu, X. Lutein, Zeaxanthin and Meso-Zeaxanthin Supplementation Associated with Macular Pigment Optical Density. Nutrients 2016, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Shinojima, A.; Sawa, M.; Sekiryu, T.; Oshima, Y.; Mori, R.; Hara, C.; Sugano, Y.; Kato, A.; Asato, H.; Yuzawa, M.; et al. A Multicenter Randomized Controlled Study of Antioxidant Supplementation with Lutein for Chronic Central Serous Chorioretinopathy. Ophthalmologica 2017, 237, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Sansone, C.; Galasso, C.; Orefice, I.; Nuzzo, G.; Luongo, E.; Cutignano, A.; Romano, G.; Brunet, C.; Fontana, A.; Esposito, F.; et al. The Green Microalga Tetraselmis suecica Reduces Oxidative Stress and Induces Repairing Mechanisms in Human Cells. Sci. Rep. 2017, 7, 41215. [Google Scholar] [CrossRef]
- Žmitek, K.; Žmitek, J.; Rogl Butina, M.; Hristov, H.; Pogačnik, T.; Pravst, I. Dietary Lutein Supplementation Protects against Ultraviolet-Radiation-Induced Erythema: Results of a Randomized Double-Blind Placebo-Controlled Study. J. Funct. Foods 2020, 75, 104265. [Google Scholar] [CrossRef]
- Liu, T.; Liu, W.; Zhao, J.; Meng, F.; Wang, H. Lutein Protects against β-Amyloid Peptide-Induced Oxidative Stress in Cerebrovascular Endothelial Cells through Modulation of Nrf-2 and NF-κb. Cell Biol. Toxicol. 2017, 33, 57–67. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, X.; Lian, F.; Yang, J.; Xu, X. Combination of Lutein and DHA Alleviate H2O2 Induced Cytotoxicity in PC12 Cells by Regulating the MAPK Pathway. J. Nutr. Sci. Vitaminol. 2021, 67, 234–242. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Liu, G.; Kang, J.; Wang, B.; Wang, J.; Li, J.; Wang, H. Lutein Attenuated Methylglyoxal-induced Oxidative Damage and Apoptosis in PC12 Cells via the PI3K Akt Signaling Pathway. J. Food Biochem. 2022, 46, e14382. [Google Scholar] [CrossRef]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Agócs, A.; Deli, J. Lutein Exerts Antioxidant and Anti-Inflammatory Effects and Influences Iron Utilization of BV-2 Microglia. Antioxidants 2021, 10, 363. [Google Scholar] [CrossRef]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Nagy, T.; Agócs, A.; Deli, J. Lutein Decreases Inflammation and Oxidative Stress and Prevents Iron Accumulation and Lipid Peroxidation at Glutamate-Induced Neurotoxicity. Antioxidants 2022, 11, 2269. [Google Scholar] [CrossRef]
- Fernandes, E.J.; Poetini, M.R.; Barrientos, M.S.; Bortolotto, V.C.; Araujo, S.M.; Santos Musachio, E.A.; De Carvalho, A.S.; Leimann, F.V.; Gonçalves, O.H.; Ramborger, B.P.; et al. Exposure to Lutein-Loaded Nanoparticles Attenuates Parkinson’s Model-Induced Damage in Drosophila melanogaster: Restoration of Dopaminergic and Cholinergic System and Oxidative Stress Indicators. Chem. Biol. Interact. 2021, 340, 109431. [Google Scholar] [CrossRef] [PubMed]
- Nazari, L.; Komaki, S.; Salehi, I.; Raoufi, S.; Golipoor, Z.; Kourosh-Arami, M.; Komaki, A. Investigation of the Protective Effects of Lutein on Memory and Learning Using Behavioral Methods in a Male Rat Model of Alzheimer’s Disease. J. Funct. Foods 2022, 99, 105319. [Google Scholar] [CrossRef]
- Fatani, A.J.; Parmar, M.Y.; Abuohashish, H.M.; Ahmed, M.M.; Al-Rejaie, S.S. Protective Effect of Lutein Supplementation on Oxidative Stress and Inflammatory Progression in Cerebral Cortex of Streptozotocin-Induced Diabetes in Rats. Neurochem. J. 2016, 10, 69–76. [Google Scholar] [CrossRef]
- Qiao, Y.-Q.; Jiang, P.-F.; Gao, Y.-Z. Lutein Prevents Osteoarthritis through Nrf2 Activation and Downregulation of Inflammation. Arch. Med. Sci. 2018, 14, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kiko, T.; Hatade, K.; Sookwong, P.; Arai, H.; Miyazawa, T. Antioxidant Effect of Lutein towards Phospholipid Hydroperoxidation in Human Erythrocytes. Br. J. Nutr. 2009, 102, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Nakagawa, K.; Takekoshi, H.; Higuchi, O.; Kato, S.; Kondo, M.; Kimura, F.; Miyazawa, T. Ingestion of Chlorella Reduced the Oxidation of Erythrocyte Membrane Lipids in Senior Japanese Subjects. J. Oleo Sci. 2013, 62, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Min, K. Serum Lycopene, Lutein and Zeaxanthin, and the Risk of Alzheimer’s Disease Mortality in Older Adults. Dement. Geriatr. Cogn. Disord. 2014, 37, 246–256. [Google Scholar] [CrossRef]
- Ademowo, O.S.; Dias, H.K.I.; Milic, I.; Devitt, A.; Moran, R.; Mulcahy, R.; Howard, A.N.; Nolan, J.M.; Griffiths, H.R. Phospholipid Oxidation and Carotenoid Supplementation in Alzheimer’s Disease Patients. Free Radic. Biol. Med. 2017, 108, 77–85. [Google Scholar] [CrossRef]
- Avila-Roman, J.; Garda-Gil, S.; Rodriguez-Luna, A.; Motilva, V.; Talero, E. Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids. Mar. Drugs 2021, 19, 531. [Google Scholar] [CrossRef]
- Lakshminarayana, R.; Sathish, U.V.; Dharmesh, S.M.; Baskaran, V. Antioxidant and Cytotoxic Effect of Oxidized Lutein in Human Cervical Carcinoma Cells (HeLa). Food Chem. Toxicol. 2010, 48, 1811–1816. [Google Scholar] [CrossRef]
- Lakshminarayana, R.; Aruna, G.; Sathisha, U.V.; Dharmesh, S.M.; Baskaran, V. Structural Elucidation of Possible Lutein Oxidation Products Mediated through Peroxyl Radical Inducer 2,2′-Azobis (2-Methylpropionamidine) Dihydrochloride: Antioxidant and Cytotoxic Influence of Oxidized Lutein in HeLa Cells. Chem. Biol. Interact. 2013, 203, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Kavalappa, Y.P.; Gopal, S.S.; Ponesakki, G. Lutein Inhibits Breast Cancer Cell Growth by Suppressing Antioxidant and Cell Survival Signals and Induces Apoptosis. J. Cell. Physiol. 2021, 236, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Baraya, Y.S.; Yankuzo, H.M.; Wong, K.K.; Yaacob, N.S. Strobilanthes Crispus Bioactive Subfraction Inhibits Tumor Progression and Improves Hematological and Morphological Parameters in Mouse Mammary Carcinoma Model. J. Ethnopharmacol. 2021, 267, 113522. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Smith, J.R.; Swanson, H.M.; Rubin, L.P. Carotenoid Lutein Selectively Inhibits Breast Cancer Cell Growth and Potentiates the Effect of Chemotherapeutic Agents through ROS-Mediated Mechanisms. Molecules 2018, 23, 905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Zhao, Y.N.; Shi, Z.Z.; Cong, D.; Bai, Y.S. Lutein Inhibits Cell Growth and Activates Apoptosis via the PI3K/AKT/MTOR Signaling Pathway in A549 Human Non-Small-Cell Lung Cancer Cells. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Fujiwara, A.; Onodera, D.; Motoki, T. Lutein Regulates the Expression of Apoptosis-Related Genes and Stem Cell Markers in A549 Human Lung Cancer Cells. Nat. Prod. Commun. 2017, 12, 897–900. [Google Scholar] [CrossRef]
- Reynoso-Camacho, R.; González-Jasso, E.; Ferriz-Martínez, R.; Villalón-Corona, B.; Loarca-Pina, G.F.; Salgado, L.M.; Ramos-Gomez, M. Dietary Supplementation of Lutein Reduces Colon Carcinogenesis in DMH-Treated Rats by Modulating K-Ras, PKB, and β-Catenin Proteins. Nutr. Cancer 2011, 63, 39–45. [Google Scholar] [CrossRef]
- Eom, J.W.; Lim, J.W.; Kim, H. Lutein Induces Reactive Oxygen Species-Mediated Apoptosis in Gastric Cancer AGS Cells via NADPH Oxidase Activation. Molecules 2023, 28, 1178. [Google Scholar] [CrossRef] [PubMed]
- Zaini, R.G.; Brandt, K.; Clench, M.R.; Le Maitre, C.L. Effects of Bioactive Compounds from Carrots (Daucus carota L.), Polyacetylenes, Beta-Carotene and Lutein on Human Lymphoid Leukaemia Cells. Anticancer. Agents Med. Chem. 2012, 12, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Keller, T.; Hendrix, C.; Hamilton, S.; Arena, R.; Tuason, M.; Gonzalez, S. Regulation of the Extracellular Matrix Remodeling by Lutein in Dermal Fibroblasts, Melanoma Cells, and Ultraviolet Radiation Exposed Fibroblasts. Arch. Dermatol. Res. 2007, 299, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Rafi, M.M.; Kanakasabai, S.; Gokarn, S.V.; Krueger, E.G.; Bright, J.J. Dietary Lutein Modulates Growth and Survival Genes in Prostate Cancer Cells. J. Med. Food 2015, 18, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, E.R.; Firdous, A.P.; Ramnath, V.; Kuttan, R. Effect of Carotenoid Lutein on N-Nitrosodiethylamine-Induced Hepatocellular Carcinoma and Its Mechanism of Action. Eur. J. Cancer Prev. 2013, 22, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Gansukh, E.; Mya, K.K.; Jung, M.; Keum, Y.-S.; Kim, D.H.; Saini, R.K. Lutein Derived from Marigold (Tagetes erecta) Petals Triggers ROS Generation and Activates Bax and Caspase-3 Mediated Apoptosis of Human Cervical Carcinoma (HeLa) Cells. Food Chem. Toxicol. 2019, 127, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Sawasdee, N.; Jantakee, K.; Wathikthinnakon, M.; Panwong, S.; Pekkoh, J.; Duangjan, K.; Yenchitsomanus, P.T.; Panya, A. Microalga Chlorella sp. Extract Induced Apoptotic Cell Death of Cholangiocarcinoma via AKT/MTOR Signaling Pathway. Biomed. Pharmacother. 2023, 160, 114306. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Pérez-Sánchez, A.; Valdés, A.; Ibrahim, O.H.M.; Suarez-Álvarez, S.; Ferragut, J.A.; Micol, V.; Cifuentes, A.; Ibáñez, E.; García-Cañas, V. Pressurized Liquid Extraction of Neochloris oleoabundans for the Recovery of Bioactive Carotenoids with Anti-Proliferative Activity against Human Colon Cancer Cells. Food Res. Int. 2017, 99, 1048–1055. [Google Scholar] [CrossRef]
- Tavares-Carreón, F.; De La Torre-Zavala, S.; Arocha-Garza, H.F.; Souza, V.; Galán-Wong, L.J.; Avilés-Arnaut, H. In Vitro Anticancer Activity of Methanolic Extract of Granulocystopsis sp., a Microalgae from an Oligotrophic Oasis in the Chihuahuan Desert. PeerJ 2020, 2020, e8686. [Google Scholar] [CrossRef]
- Yan, B.; Lu, M.-S.; Wang, L.; Mo, X.-F.; Luo, W.-P.; Du, Y.-F.; Zhang, C.-X. Specific Serum Carotenoids Are Inversely Associated with Breast Cancer Risk among Chinese Women: A Case–Control Study. Br. J. Nutr. 2016, 115, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.-X.; Xing, M.-Y.; Yu, L.-F.; Shen, P. Carotenoid Intake and Esophageal Cancer Risk: A Meta-Analysis. Asian Pacific J. Cancer Prev. 2013, 14, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Kwon, O.; Shin, A.; Kim, J. Dietary Lutein Plus Zeaxanthin Intake and DICER1 Rs3742330 A > G Polymorphism Relative to Colorectal Cancer Risk. Sci. Rep. 2019, 9, 3406. [Google Scholar] [CrossRef]
- Jansen, R.J.; Robinson, D.P.; Stolzenberg-Solomon, R.Z.; Bamlet, W.R.; de Andrade, M.; Oberg, A.L.; Rabe, K.G.; Anderson, K.E.; Olson, J.E.; Sinha, R.; et al. Nutrients from Fruit and Vegetable Consumption Reduce the Risk of Pancreatic Cancer. J. Gastrointest. Cancer 2013, 44, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Bosetti, C.; Filomeno, M.; Levi, F.; Garavello, W.; Galimberti, S.; Negri, E.; La Vecchia, C. Foods, Nutrients and the Risk of Oral and Pharyngeal Cancer. Br. J. Cancer 2013, 109, 2904–2910. [Google Scholar] [CrossRef] [PubMed]
- Leoncini, E.; Edefonti, V.; Hashibe, M.; Parpinel, M.; Cadoni, G.; Ferraroni, M.; Serraino, D.; Matsuo, K.; Olshan, A.F.; Zevallos, J.P.; et al. Carotenoid Intake and Head and Neck Cancer: A Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium. Eur. J. Epidemiol. 2016, 31, 369–383. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Michalek, J.E.; Mesa, R.A.; Parma, D.L.; Rodriguez, R.; Mansour, A.M.; Svatek, R.; Tucker, T.C.; Ramirez, A.G. Carotenoid Intake and Circulating Carotenoids Are Inversely Associated with the Risk of Bladder Cancer: A Dose-Response Meta-Analysis. Adv. Nutr. 2020, 11, 630–643. [Google Scholar] [CrossRef]
- Bock, C.H.; Ruterbusch, J.J.; Holowatyj, A.N.; Steck, S.E.; Van Dyke, A.L.; Ho, W.J.; Cote, M.L.; Hofmann, J.N.; Davis, F.; Graubard, B.I.; et al. Renal Cell Carcinoma Risk Associated with Lower Intake of Micronutrients. Cancer Med. 2018, 7, 4087–4097. [Google Scholar] [CrossRef]
- Omar, W.M.; Ahmed, A.E.; Raslan, M.; El-Nesr, K.; Ali, M.M.; De Abdelmaksoud, M.; Dahshan, D. El Effect of Lutein-Rich Extract on Human Cancer Cells. Middle East J. Cancer 2021, 12, 147–150. [Google Scholar] [CrossRef]
- Wang, S.; Wang, M.; Zhang, S.; Zhao, L. Oxidative Stress in Rats with Hyperhomo-Cysteinemia and Intervention Effect of Lutein. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 359–364. [Google Scholar]
- Li, H.; Huang, C.; Zhu, J.; Gao, K.; Fang, J.; Li, H. Lutein Suppresses Oxidative Stress and Inflammation by Nrf2 Activation in an Osteoporosis Rat Model. Med. Sci. Monit. 2018, 24, 5071–5075. [Google Scholar] [CrossRef]
- Meurer, M.C.; Mees, M.; Mariano, L.N.B.; Boeing, T.; Somensi, L.B.; Mariott, M.; da Silva, R.d.C.M.V.d.A.F.; dos Santos, A.C.; Longo, B.; Santos França, T.C.; et al. Hydroalcoholic Extract of Tagetes erecta L. Flowers, Rich in the Carotenoid Lutein, Attenuates Inflammatory Cytokine Secretion and Improves the Oxidative Stress in an Animal Model of Ulcerative Colitis. Nutr. Res. 2019, 66, 95–106. [Google Scholar] [CrossRef]
- Mammadov, R.; Suleyman, B.; Akturan, S.; Cimen, F.K.; Kurt, N.; Suleyman, Z.; Malkoc, İ. Effect of Lutein on Methotrexate-Induced Oxidative Lung Damage in Rats: A Biochemical and Histopathological Assessment. Korean J. Intern. Med. 2019, 34, 1279–1286. [Google Scholar] [CrossRef]
- Melo van Lent, D.; Leermakers, E.T.M.; Hofman, A.; Stricker, B.H.; Brusselle, G.G.; Franco, O.H.; Lahousse, L.; Kiefte-de Jong, J.C. Association between Lutein Intake and Lung Function in Adults: The Rotterdam Study. Br. J. Nutr. 2017, 117, 720–730. [Google Scholar] [CrossRef]
- Khudhai, A.R.; Al-Shawi, N.N. Possible Protective Effects of High- versus Low- Dose of Lutein in Combination with Irinotecan on Liver of Rats: Role of Oxidative Stress and Apoptosis. Indian J. Forensic Med. Toxicol. 2021, 15, 2439–2445. [Google Scholar] [CrossRef]
- Bandariyan, E.; Mogheiseh, A.; Ahmadi, A. The Effect of Lutein and Urtica dioica Extract on in Vitro Production of Embryo and Oxidative Status in Polycystic Ovary Syndrome in a Model of Mice. BMC Complement. Med. Ther. 2021, 21, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, H.; Xu, L.; Zhao, S.; Hu, S.; Ma, A.; Ma, Y. Lutein Can Alleviate Oxidative Stress, Inflammation, and Apoptosis Induced by Excessive Alcohol to Ameliorate Reproductive Damage in Male Rats. Nutrients 2022, 14, 2385. [Google Scholar] [CrossRef] [PubMed]
- Fatani, A.J.; Al-Rejaie, S.S.; Abuohashish, H.M.; Al-Assaf, A.; Parmar, M.Y.; Ahmed, M.M. Lutein Dietary Supplementation Attenuates Streptozotocin-Induced Testicular Damage and Oxidative Stress in Diabetic Rats. BMC Complement. Altern. Med. 2015, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- Sharavana, G.; Joseph, G.S.; Baskaran, V. Lutein Attenuates Oxidative Stress Markers and Ameliorates Glucose Homeostasis through Polyol Pathway in Heart and Kidney of STZ-Induced Hyperglycemic Rat Model. Eur. J. Nutr. 2017, 56, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Zhang, Q.; Yan, F.-F.; Wan, J.-F.; Lin, C.-S. Lutein Protects against Ischemia/Reperfusion Injury in Rat Skeletal Muscle by Modulating Oxidative Stress and Inflammation. Immunopharmacol. Immunotoxicol. 2015, 37, 329–334. [Google Scholar] [CrossRef]
- Tan, D.; Yu, X.; Chen, M.; Chen, J.; Xu, J. Lutein Protects against Severe Traumatic Brain Injury through Anti-Inflammation and Antioxidative Effects via ICAM-1/Nrf-2. Mol. Med. Rep. 2017, 16, 4235–4240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L.; Jia, L.; Liu, J. Association between Carotenoid Intake and Metabolic Dysfunction-Associated Fatty Liver Disease among US Adults: A Cross-Sectional Study. Medicine 2023, 102, e36658. [Google Scholar] [CrossRef]
- Yen, H.W.; Sun, C.H.; Ma, T.W. The Comparison of Lutein Production by Scenesdesmus sp. in the Autotrophic and the Mixotrophic Cultivation. Appl. Biochem. Biotechnol. 2011, 164, 353–361. [Google Scholar] [CrossRef]
- Heo, J.; Shin, D.S.; Cho, K.; Cho, D.H.; Lee, Y.J.; Kim, H.S. Indigenous Microalga Parachlorella sp. JD-076 as a Potential Source for Lutein Production: Optimization of Lutein Productivity via Regulation of Light Intensity and Carbon Source. Algal Res. 2018, 33, 1–7. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, Z.; Ho, S.H.; Ruan, C.; Li, J.; Xie, Y.; Shi, X.; Liu, L.; Chen, J. Two-Stage Bioprocess for Hyper-Production of Lutein from Microalga Chlorella sorokiniana FZU60: Effects of Temperature, Light Intensity, and Operation Strategies. Algal Res. 2020, 52, 102119. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, R.; Liu, X.; Ho, S.H.; Xie, Y.; Chen, J. Strategies Related to Light Quality and Temperature to Improve Lutein Production of Marine Microalga Chlamydomonas sp. Bioprocess Biosyst. Eng. 2019, 42, 435–443. [Google Scholar] [CrossRef]
- Chen, W.C.; Hsu, Y.C.; Chang, J.S.; Ho, S.H.; Wang, L.F.; Wei, Y.H. Enhancing Production of Lutein by a Mixotrophic Cultivation System Using Microalga Scenedesmus obliquus CWL-1. Bioresour. Technol. 2019, 291, 121891. [Google Scholar] [CrossRef]
- Xie, Y.; Ho, S.H.; Chen, C.N.N.; Chen, C.Y.; Ng, I.S.; Jing, K.J.; Chang, J.S.; Lu, Y. Phototrophic Cultivation of a Thermo-Tolerant Desmodesmus sp. for Lutein Production: Effects of Nitrate Concentration, Light Intensity and Fed-Batch Operation. Bioresour. Technol. 2013, 144, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Vadrale, A.P.; Dong, C.-D.; Haldar, D.; Wu, C.-H.; Chen, C.-W.; Singhania, R.R.; Patel, A.K. Bioprocess Development to Enhance Biomass and Lutein Production from Chlorella sorokiniana Kh12. Bioresour. Technol. 2023, 370, 128583. [Google Scholar] [CrossRef]
- Ma, R.; Zhao, X.; Xie, Y.; Ho, S.-H.; Chen, J. Enhancing Lutein Productivity of Chlamydomonas sp. via High-Intensity Light Exposure with Corresponding Carotenogenic Genes Expression Profiles. Bioresour. Technol. 2019, 275, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Dineshkumar, R.; Subramanian, G.; Dash, S.K.; Sen, R. Development of an Optimal Light-Feeding Strategy Coupled with Semi-Continuous Reactor Operation for Simultaneous Improvement of Microalgal Photosynthetic Efficiency, Lutein Production and CO2 Sequestration. Biochem. Eng. J. 2016, 113, 47–56. [Google Scholar] [CrossRef]
- Ho, S.H.; Xie, Y.; Chan, M.C.; Liu, C.C.; Chen, C.Y.; Lee, D.J.; Huang, C.C.; Chang, J.S. Effects of Nitrogen Source Availability and Bioreactor Operating Strategies on Lutein Production with Scenedesmus obliquus FSP-3. Bioresour. Technol. 2015, 184, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Lu, I.C.; Nagarajan, D.; Chang, C.H.; Ng, I.S.; Lee, D.J.; Chang, J.S. A Highly Efficient Two-Stage Cultivation Strategy for Lutein Production Using Heterotrophic Culture of Chlorella sorokiniana MB-1-M12. Bioresour. Technol. 2018, 253, 141–147. [Google Scholar] [CrossRef]
- Schüler, L.M.; Santos, T.; Pereira, H.; Duarte, P.; Katkam, N.G.; Florindo, C.; Schulze, P.S.C.; Barreira, L.; Varela, J.C.S. Improved Production of Lutein and β-Carotene by Thermal and Light Intensity Upshifts in the Marine Microalga Tetraselmis sp. CTP4. Algal Res. 2020, 45, 101732. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, X.; Chen, J.; Yang, X.; Ho, S.H.; Wang, B.; Chang, J.S.; Shen, Y. Enhancing Cell Growth and Lutein Productivity of Desmodesmus sp. F51 by Optimal Utilization of Inorganic Carbon Sources and Ammonium Salt. Bioresour. Technol. 2017, 244, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, J.; Ma, R.; Ho, S.H.; Shi, X.; Liu, L.; Chen, J. Bioprocess Operation Strategies with Mixotrophy/Photoinduction to Enhance Lutein Production of Microalga Chlorella sorokiniana FZU60. Bioresour. Technol. 2019, 290, 121798. [Google Scholar] [CrossRef]
- Chen, C.Y.; Ho, S.H.; Liu, C.C.; Chang, J.S. Enhancing Lutein Production with Chlorella sorokiniana Mb-1 by Optimizing Acetate and Nitrate Concentrations under Mixotrophic Growth. J. Taiwan Inst. Chem. Eng. 2017, 79, 88–96. [Google Scholar] [CrossRef]
- Shi, X.M.; Jiang, Y.; Chen, F. High-Yield Production of Lutein by the Green Microalga Chlorella protothecoides in Heterotrophic Fed-Batch Culture. Biotechnol. Prog. 2002, 18, 723–727. [Google Scholar] [CrossRef]
- Patel, A.K.; Vadrale, A.P.; Singhania, R.-R.; Chen, C.-W.; Chang, J.S.; Dong, C.-D. Enhanced Mixotrophic Production of Lutein and Lipid from Potential Microalgae Isolate Chlorella sorokiniana C16. Bioresour. Technol. 2023, 386, 129477. [Google Scholar] [CrossRef]
- Cuaresma, M.; Casal, C.; Forján, E.; Vílchez, C. Productivity and Selective Accumulation of Carotenoids of the Novel Extremophile Microalga Chlamydomonas acidophila Grown with Different Carbon Sources in Batch Systems. J. Ind. Microbiol. Biotechnol. 2011, 38, 167–177. [Google Scholar] [CrossRef]
- Shi, X.M.; Liu, H.J.; Zhang, X.W.; Chen, F. Production of Biomass and Lutein by Chlorella Protothecoides at Various Glucose Concentrations in Heterotrophic Cultures. Process Biochem. 1999, 34, 341–347. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Iovine, A.; Casella, P.; Marino, T.; Karatza, D.; Chianese, S.; Musmarra, D. Enhancing Biomass and Lutein Production from Scenedesmus almeriensis: Effect of Carbon Dioxide Concentration and Culture Medium Reuse. Front. Plant Sci. 2020, 11, 415. [Google Scholar] [CrossRef]
- Wei, D.; Chen, F.; Chen, G.; Zhang, X.W.; Liu, L.J.; Zhang, H. Enhanced Production of Lutein in Heterotrophic Chlorella protothecoides by Oxidative Stress. Sci. China Ser. C Life Sci. 2008, 51, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, E.; Ruiz-Domínguez, M.C.; Cuaresma, M.; Vaquero, I.; Ramos-Merchante, A.; Vega, J.M.; Vílchez, C.; Garbayo, I. Production of Lutein, and Polyunsaturated Fatty Acids by the Acidophilic Eukaryotic Microalga Coccomyxa onubensis under Abiotic Stress by Salt or Ultraviolet Light. J. Biosci. Bioeng. 2018, 125, 669–675. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, Z.; Tang, Z.; Ho, S.H.; Shi, X.; Liu, L.; Xie, Y.; Chen, J. Enhancement of Co-Production of Lutein and Protein in Chlorella sorokiniana FZU60 Using Different Bioprocess Operation Strategies. Bioresour. Bioprocess. 2021, 8, 82. [Google Scholar] [CrossRef]
- Moulton, T.P.; Burford, M.A. The Mass Culture of Dunaliella viridis (Volvocales, Chlorophyta) for Oxygenated Carotenoids: Laboratory and Pilot Plant Studies. Hydrobiologia 1990, 204–205, 401–408. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Kwon, J.S.; Kang, S.T.; Kim, B.R.; Jung, Y.; Han, J.G.; Park, J.H.; Hwang, J.K. Optimization of Culture Media for Large-Scale Lutein Production by Heterotrophic Chlorella vulgaris. Biotechnol. Prog. 2014, 30, 736–743. [Google Scholar] [CrossRef]
- McClure, D.D.; Nightingale, J.K.; Luiz, A.; Black, S.; Zhu, J.; Kavanagh, J.M. Pilot-Scale Production of Lutein Using Chlorella vulgaris. Algal Res. 2019, 44, 101707. [Google Scholar] [CrossRef]
- Xie, Y.; Li, J.; Ho, S.H.; Ma, R.; Shi, X.; Liu, L.; Chen, J. Pilot-Scale Cultivation of Chlorella sorokiniana FZU60 with a Mixotrophy/Photoautotrophy Two-Stage Strategy for Efficient Lutein Production. Bioresour. Technol. 2020, 314, 123767. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.; Montero, Z.; Cuaresma, M.; Ruiz-Domínguez, M.-C.; Mogedas, B.; Nores, I.G.; González del Valle, M.; Vílchez, C. Outdoor Large-Scale Cultivation of the Acidophilic Microalga Coccomyxa onubensis in a Vertical Close Photobioreactor for Lutein Production. Processes 2020, 8, 324. [Google Scholar] [CrossRef]
- Cavieres, L.; Bazaes, J.; Marticorena, P.; Riveros, K.; Medina, P.; Sepúlveda, C.; Riquelme, C. Pilot-Scale Phycoremediation Using Muriellopsis sp. For Wastewater Reclamation in the Atacama Desert: Microalgae Biomass Production and Pigment Recovery. Water Sci. Technol. 2021, 83, 331–343. [Google Scholar] [CrossRef]
- Van, T.; Do, C.; Dinh, C.T.; Dang, M.T.; Dang Tran, T.; Giang Le, T. A Novel Flat-Panel Photobioreactor for Simultaneous Production of Lutein and Carbon Sequestration by Chlorella sorokiniana TH01. Bioresour. Technol. 2022, 345, 126552. [Google Scholar] [CrossRef]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Microalgal Lutein Biosynthesis: Recent Trends and Challenges to Enhance the Lutein Content in Microalgal Cell Factories. Front. Mar. Sci. 2022, 9, 1015419. [Google Scholar] [CrossRef]
- Huang, W.; Lin, Y.; He, M.; Gong, Y.; Huang, J. Induced High-Yield Production of Zeaxanthin, Lutein, and β-Carotene by a Mutant of Chlorella zofingiensis. J. Agric. Food Chem. 2018, 66, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Cordero, B.F.; Obraztsova, I.; Couso, I.; Leon, R.; Vargas, M.A.; Rodriguez, H. Enhancement of Lutein Production in Chlorella sorokiniana (Chorophyta) by Improvement of Culture Conditions and Random Mutagenesis. Mar. Drugs 2011, 9, 1607–1624. [Google Scholar] [CrossRef] [PubMed]
- Cordero, B.F.; Couso, I.; León, R.; Rodríguez, H.; Vargas, M.Á. Enhancement of Carotenoids Biosynthesis in Chlamydomonas reinhardtii by Nuclear Transformation Using a Phytoene Synthase Gene Isolated from Chlorella zofingiensis. Appl. Microbiol. Biotechnol. 2011, 91, 341–351. [Google Scholar] [CrossRef]
- Liu, J.; Gerken, H.; Huang, J.; Chen, F. Engineering of an Endogenous Phytoene Desaturase Gene as a Dominant Selectable Marker for Chlamydomonas reinhardtii Transformation and Enhanced Biosynthesis of Carotenoids. Process Biochem. 2013, 48, 788–795. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Gerken, H.; Huang, J.; Jiang, Y.; Chen, F. Genetic Engineering of the Green Alga Chlorella zofingiensis: A Modified Norflurazon-Resistant Phytoene Desaturase Gene as a Dominant Selectable Marker. Appl. Microbiol. Biotechnol. 2014, 98, 5069–5079. [Google Scholar] [CrossRef] [PubMed]
- Rathod, J.P.; Vira, C.; Lali, A.M.; Prakash, G. Metabolic Engineering of Chlamydomonas reinhardtii for Enhanced β-Carotene and Lutein Production. Appl. Biochem. Biotechnol. 2020, 190, 1457–1469. [Google Scholar] [CrossRef]
- Takemura, M.; Kubo, A.; Watanabe, A.; Sakuno, H.; Minobe, Y.; Sahara, T.; Murata, M.; Araki, M.; Harada, H.; Terada, Y.; et al. Pathway Engineering for High-Yield Production of Lutein in Escherichia coli. Synth. Biol. 2021, 6, ysab012. [Google Scholar] [CrossRef]
- Bian, Q.; Zhou, P.; Yao, Z.; Li, M.; Yu, H.; Ye, L. Heterologous Biosynthesis of Lutein in S. cerevisiae Enabled by Temporospatial Pathway Control. Metab. Eng. 2021, 67, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Saide, A.; Martínez, K.A.; Ianora, A.; Lauritano, C. Unlocking the Health Potential of Microalgae as Sustainable Sources of Bioactive Compounds. Int. J. Mol. Sci. 2021, 22, 4383. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).