Clinical and Preclinical Targeting of Oncogenic Pathways in PDAC: Targeted Therapeutic Approaches for the Deadliest Cancer
Abstract
1. Introduction
1.1. Background and Significance of Pancreatic Cancer
1.2. Rationale for Targeted Therapies in Pancreatic Cancer
2. Molecular Basis of Pancreatic Cancer
2.1. Genetic Mutations and Alterations in Pancreatic Cancer
2.2. Signaling Pathways Implicated in Pancreatic Cancer Progression
2.2.1. KRAS Signaling Pathway
2.2.2. TGF-β Pathway
2.2.3. Hedgehog
2.2.4. WNT
2.2.5. NOTCH
2.3. Role of Tumor Microenvironment in Pancreatic Cancer Development
3. Targeted Therapeutic Approaches
3.1. Inhibition of RAF/MEK/ERK
3.2. Inhibition of Other Main Signaling Pathways
3.2.1. PI3K/AKT/mTOR Pathway Inhibitors
| Drug | Target | References |
|---|---|---|
| Everolimus | mTOR | [53] |
| Sonolisib (PX-866) | PI3K | [54] |
| Alpelisib | PI3Ka | [55] |
| Buparlisib | AKT | [58] |
| Copanlisib | PI3K (p110a) | [61] |
| Pictilisib | PI3K | [62] |
| Perifosine | AKT | [65] |
| Uprosertib | AKT | [69] |
| Oleandrin | AKT | [70] |
| Afuresertib | AKT | [73] |
| Archexin | AKT | [74] |
| MK-2206 | AKT | [75] |
| TCN | AKT | [31] |
| Sirolimus | mTOR | [78] |
| Ridaforolimus | mTOR | [85] |
| Vistusertib | mTORC1/C2 | [86] |
| Temsirolimus | mTOR | [87] |
| Dactolisib | PI3K/mTOR | [89] |
| Voxtalisib | PI3K/mTOR | [90] |
| Omipalisib | PI3K/mTOR | [93] |
| PF4691502 | PI3K/mTOR | [95] |
| Gedatolisib | PI3K/mTOR | [95] |
3.2.2. Wnt/β-Catenin Pathway Inhibitors
3.2.3. Hedgehog Pathway Inhibitors
3.3. Angiogenesis and Vascular-Targeted Therapies
3.3.1. VEGF and VEGFR Inhibitors
| Drug | Target | References |
|---|---|---|
| Brivanib | FGF/VEGF | [131] |
| Aflibercept | VEGF-A, B | [133] |
| Bevacizumab | VEGF | [135,136] |
| Sorafenib | tyrosine kinase | [137] |
| Vatalanib | tyrosine kinases | [139] |
| Axitinib | VEGR1,2,3 | [140] |
| Regorafenib | tyrosine kinases | [143] |
| Foretinib | VEGFR-2 | [147] |
| Cediranib | Pan VEGFR | [148] |
| PF4691502 | PI3K/mTOR | [95] |
| Gedatolisib | PI3K/mTOR | [95] |
3.3.2. PDGF Inhibitors
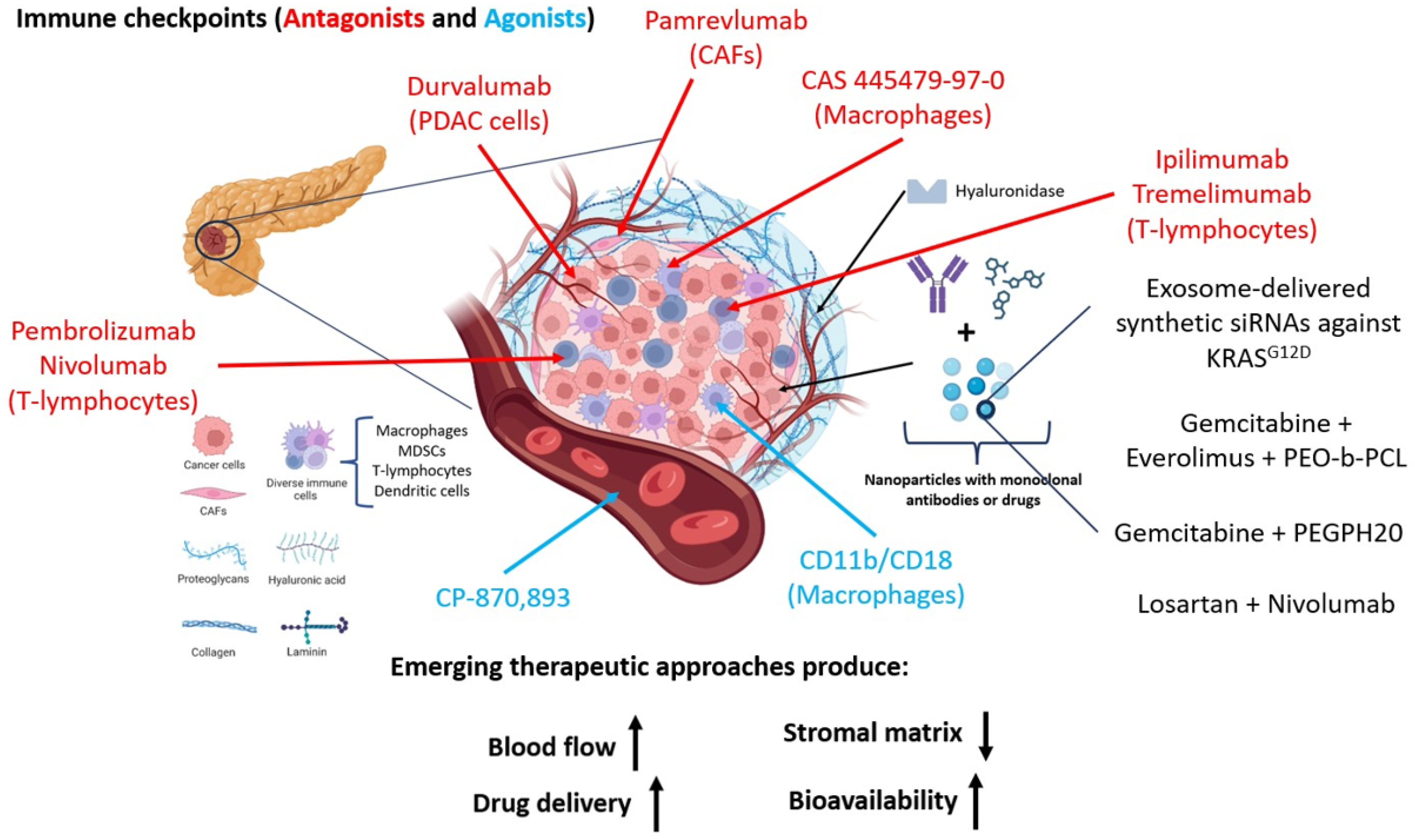
3.4. Immune Checkpoint Inhibitors in Pancreatic Cancer
3.4.1. PD-1/PDL-1 Inhibitors
3.4.2. CTLA-4 Inhibitors
4. Clinical Trials and Current Treatment Landscape
4.1. Overview of Recent and Ongoing Clinical Trials
4.2. Targeted Therapies in Combination with Standard of Care
4.3. Challenges and Limitations in Clinical Implementation
5. Emerging Trends and Future Directions
5.1. Novel Targeted Agents in Preclinical Development
| Drug or Intervention | Target | Phase of Trial | Preclinical References | Clinical References |
|---|---|---|---|---|
| adjuvant chemotherapy FOLFIRINOX | folate reductase, thymidylate synthetase, topoisomerase I, DNA | NA | NA | [41] |
| Gemcitabine | topoisomerase I | NA | NA | [41] |
| Gemcitabine + capecitabine | thymidylate synthetase | NA | NA | [41] |
| Gemcitabine/nab-paclitaxel + Pembrolizumab | PD-1 | NA | NA | [173] |
| Sotorasib | KRASG12C | I/II | NA | [24] |
| Irinotecan + 5-FU | topoisomerase I, thymidylate synthetase | NA | NA | [173] |
| Adoptive T cell | HLA-restricted mutant KRAS neoantigen | NA | NA | [173] |
| Exosome-delivered synthetic siRNAs | KRASG12D | NA | NA | [173] |
| Autologous CAR-T cells | B7-H3 antigen | I | Clinical trials | NA |
| Vismodegib + chemotherapy | SMO | NA | NA | [173] |
| TAMs (novel agonists) + pembrolizumab | CD11b/CD18 + PD-1 | NA | [173] | NA |
| Gemcitabine + CD40 agonists | CD40 | NA | [173] | NA |
| Gemcitabine + erlotinib | EGFR | NA | NA | [3] |
| Gemcitabine + TH-302 | DNA | NA | NA | [3] |
| MM-398 + 5-FU + folinic acid | NA | NA | [3] | |
| Deltarasin | KRAS | NA | NA | [3] |
| Chloroquine or hidroxychloroquine | Lysosomes | NA | [3] | NA |
| GVAX pancreas | pancreatic tumor cells | NA | [3] | NA |
| Gemcitabine/or nab-paclitaxel + Ipilimumab | CTLA-4 | NA | NA | [3] |
| Durvalumab + tremelimumab | PD-L1 + CTLA-4 | II | NA | [11] |
| FOLFIRINOX + CCR2 inhibitors | CCR2 | NA | [175] | NA |
| CCR2 inhibitors + Nivolumab + chemotherapy | PD-1 | NA | NA | [11] |
| CD40 agonist + FLT3L | CD40 + Tyrosine kinase 3 | NA | NA | [11] |
| CD40 agonist + FLT3L | CD40 + Tyrosine kinase 3 | NA | NA | [11] |
| LCL-161 | ABCB1-ATPase/ABCB1 | NA | [175] | NA |
| DRI-C21045 + anti-PD1 + GnP | CD40 | II | NA | [11] |
| Gemcitabine + everolimus + thermosensitive hydrogels | mTOR | NA | [31,144] | NA |
| GSH + UNC0638 | GSS + EHMT2 | NA | [37,75] | NA |
| Gemcitabine-nab-paclitaxel + ATRA | RARs (RAR-α, RAR-β, RAR-γ) | II | NA | [176] |
| Losartan + chemotherapy + nivolumab | AT1R | II | NA | [176] |
| FG-3019+ gemcitabine-nab-paclitaxel or FOLFIRINOX | CTGF (connective tissue growth factor) | NA | [176] | [176] |
| Gemcitabine + Napabucasin | STAT3 | NA | [176] | NA |
| 64Cu-DOTA-ECLli | CCR2 | I | NA | Clinical trials |
| 1ºAzacitidine and/or Romidepsin + nab-paclitaxel/Gemcitabine | DNMT/HDAC | I/II | NA | Clinical trials |
| 2ºDurvalumab + low-dose Lenalidomide | PD-L1/TNF-α, IL-1β, IL-6 and GM-SCF | I/II | NA | Clinical trials |
| nab-Paclitaxel/Gemcitabine + Camrelizumab/Radiotherapy | PD-1 | Observational | NA | Clinical trials |
| Zimberelimab/SBRT + quemliclustat and/or etrumadenant | PD-1/CD73/A2a or A2b | II | NA | Clinical trials |
5.2. Combination Strategies for Enhanced Efficacy
5.3. Advancements in Drug Delivery for Targeted Therapies
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Klatte, D.C.F.; Boekestijn, B.; Onnekink, A.M.; Dekker, F.W.; van der Geest, L.G.; Wasser, M.N.J.M.; Feshtali, S.; Mieog, J.S.D.; Luelmo, S.A.C.; Morreau, H.; et al. Surveillance for Pancreatic Cancer in High-Risk Individuals Leads to Improved Outcomes: A Propensity Score-Matched Analysis. Gastroenterology 2023, 164, 1223–1231. [Google Scholar] [CrossRef]
- Grant, T.J.; Hua, K.; Singh, A. Molecular Pathogenesis of Pancreatic Cancer. Prog. Mol. Biol. Transl. Sci. 2016, 144, 241–275. [Google Scholar] [CrossRef]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Laethem, J.L.V.; Conroy, T.; et al. Cancer of the Pancreas: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up †. Ann. Oncol. 2015, 26, v56–v68. [Google Scholar] [CrossRef] [PubMed]
- Sohal, D.P.S.; Kennedy, E.B.; Cinar, P.; Conroy, T.; Copur, M.S.; Crane, C.H.; Garrido-Laguna, I.; Lau, M.W.; Johnson, T.; Krishnamurthi, S.; et al. Metastatic Pancreatic Cancer: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3217–3230. [Google Scholar] [CrossRef]
- Schneider, M.; Hackert, T.; Strobel, O.; Büchler, M.W. Technical Advances in Surgery for Pancreatic Cancer. Br. J. Surg. 2021, 108, 777–785. [Google Scholar] [CrossRef]
- Strobel, O.; Neoptolemos, J.; Jäger, D.; Büchler, M.W. Optimizing the Outcomes of Pancreatic Cancer Surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26. [Google Scholar] [CrossRef]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic Cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Hosein, A.N.; Dougan, S.K.; Aguirre, A.J.; Maitra, A. Translational Advances in Pancreatic Ductal Adenocarcinoma Therapy. Nat. Cancer 2022, 3, 272–286. [Google Scholar] [CrossRef]
- Barceló, C.; Etchin, J.; Mansour, M.R.; Sanda, T.; Ginesta, M.M.; Lobo, V.J.S.-A.; Real, F.X.; Capellà, G.; Estanyol, J.M.; Jaumot, M.; et al. Ribonucleoprotein HNRNPA2B1 Interacts with and Regulates Oncogenic KRAS in Pancreatic Ductal Adenocarcinoma Cells. Gastroenterology 2014, 147, 882–892.e8. [Google Scholar] [CrossRef]
- Barceló, C.; Paco, N.; Morell, M.; Alvarez-Moya, B.; Bota-Rabassedas, N.; Jaumot, M.; Vilardell, F.; Capella, G.; Agell, N. Phosphorylation at Ser-181 of Oncogenic KRAS Is Required for Tumor Growth. Cancer Res. 2014, 74, 1190–1199. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Fang, Y.T.; Yang, W.W.; Niu, Y.R.; Sun, Y.K. Recent advances in targeted therapy for pancreatic adenocarcinoma. World J Gastrointest Oncol. 2023, 4, 571–595. [Google Scholar] [CrossRef]
- Maitra, A.; Hruban, R.H. Pancreatic Cancer. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 157–188. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Zhang, X.; Parsons, D.W.; Lin, J.C.-H.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Kamiyama, H.; Jimeno, A.; et al. Core Signaling Pathways in Human Pancreatic Cancers Revealed by Global Genomic Analyses. Science 2008, 321, 1801–1806. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.-M.; Gingras, M.-C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.C.; Quinn, M.C.; et al. Genomic Analyses Identify Molecular Subtypes of Pancreatic Cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef]
- Regel, I.; Mayerle, J.; Mukund, M.U. Current Strategies and Future Perspectives for Precision Medicine in Pancreatic Cancer. Cancers 2020, 12, 1024. [Google Scholar] [CrossRef] [PubMed]
- Pagliarini, R.; Shao, W.; Sellers, W.R. Oncogene Addiction: Pathways of Therapeutic Response, Resistance, and Road Maps toward a Cure. EMBO Rep. 2015, 16, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Solimini, N.L.; Elledge, S.J. Principles of Cancer Therapy: Oncogene and Non-Oncogene Addiction. Cell 2009, 136, 823–837. [Google Scholar] [CrossRef]
- Waddell, N.; Pajic, M.; Patch, A.-M.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; Quek, K.; et al. Whole Genomes Redefine the Mutational Landscape of Pancreatic Cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef]
- Regad, T. Targeting RTK Signaling Pathways in Cancer. Cancers 2015, 7, 1758–1784. [Google Scholar] [CrossRef]
- Huang, L.; Guo, Z.; Wang, F.; Fu, L. KRAS Mutation: From Undruggable to Druggable in Cancer. Signal Transduct. Target. Ther. 2021, 6, 386. [Google Scholar] [CrossRef]
- David, C.J.; Huang, Y.-H.; Chen, M.; Su, J.; Zou, Y.; Bardeesy, N.; Iacobuzio-Donahue, C.A.; Massagué, J. TGF-β Tumor Suppression through a Lethal EMT. Cell 2016, 164, 1015–1030. [Google Scholar] [CrossRef]
- Wakefield, L.M.; Hill, C.S. Beyond TGFβ: Roles of Other TGFβ Superfamily Members in Cancer. Nat. Rev. Cancer 2013, 13, 328–341. [Google Scholar] [CrossRef]
- Siegel, P.M.; Massagué, J. Cytostatic and Apoptotic Actions of TGF-β in Homeostasis and Cancer. Nat. Rev. Cancer 2003, 3, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.A.; Freitas, J.P.; Hussain, S.M.; Glazer, E.S. TGF-β Inhibitors in Metastatic. J. Gastrointest. Cancer 2019, 50, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Padua, D.; Massagué, J. Roles of TGFβ in Metastasis. Cell Res. 2009, 19, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.P.; Wang, S.C.; Hebrok, M. KRAS, Hedgehog, Wnt and the Twisted Developmental Biology of Pancreatic Ductal Adenocarcinoma. Nat. Rev. Cancer 2010, 10, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Yamauchi, T.; Husain, K.; Sebti, S.; Malafa, M. Triciribine Phosphate Monohydrate, an AKT Inhibitor, Enhances Gemcitabine Activity in Pancreatic Cancer Cells. Anticancer Res. 2015, 35, 4599–4604. [Google Scholar] [PubMed]
- Onishi, H.; Katano, M. Hedgehog Signaling Pathway as a New Therapeutic Target in Pancreatic Cancer. World J. Gastroenterol. 2014, 20, 2335–2342. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Cho, J. Hedgehog Pathway Inhibitors as Targeted Cancer Therapy and Strategies to Overcome Drug Resistance. Int. J. Mol. Sci. 2022, 23, 1733. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, K.Y.; Dawson, D.W. WNT Ligand Dependencies in Pancreatic Cancer. Front. Cell Dev. Biol. 2021, 9, 671022. [Google Scholar] [CrossRef]
- Tai, D.; Wells, K.; Arcaroli, J.; Vanderbilt, C.; Aisner, D.L.; Messersmith, W.A.; Lieu, C.H. Targeting the WNT Signaling Pathway in Cancer Therapeutics. Oncology 2015, 20, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Acebron, S.P.; Niehrs, C. β-Catenin-Independent Roles of Wnt/LRP6 Signaling. Trends Cell Biol. 2016, 26, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ahmad, A.; Li, Y.; Azmi, A.S.; Miele, L.; Sarkar, F.H. Targeting Notch to Eradicate Pancreatic Cancer Stem Cells for Cancer Therapy. Anticancer. Res. 2011, 31, 1105–1113. [Google Scholar]
- Harbuzariu, A.; Oprea-Ilies, G.M.; Gonzalez-Perez, R.R. The Role of Notch Signaling and Leptin-Notch Crosstalk in Pancreatic Cancer. Medicines 2018, 5, 68. [Google Scholar] [CrossRef]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The Tumour Microenvironment in Pancreatic Cancer—Clinical Challenges and Opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef]
- Chia, S.K.; Ellard, S.L.; Mates, M.; Welch, S.; Mihalcioiu, C.; Miller, W.H.; Gelmon, K.; Lohrisch, C.; Kumar, V.; Taylor, S.; et al. A Phase-I Study of Lapatinib in Combination with Foretinib, a c-MET, AXL and Vascular Endothelial Growth Factor Receptor Inhibitor, in Human Epidermal Growth Factor Receptor 2 (HER-2)-Positive Metastatic Breast Cancer. Breast Cancer Res. 2017, 19, 54. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Springfeld, C.; Hackert, T. A Review of Pancreatic Cancer. JAMA 2021, 23, 2436. [Google Scholar] [CrossRef] [PubMed]
- Jacobetz, M.A.; Chan, D.S.; Neesse, A.; Bapiro, T.E.; Cook, N.; Frese, K.K.; Feig, C.; Nakagawa, T.; Caldwell, M.E.; Zecchini, H.I.; et al. Hyaluronan Impairs Vascular Function and Drug Delivery in a Mouse Model of Pancreatic Cancer. Gut 2013, 62, 112. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Cuevas, C.; Chang, A.E.; Goel, V.K.; Von Hoff, D.D.; Hingorani, S.R. Enzymatic Targeting of the Stroma Ablates Physical Barriers to Treatment of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2012, 21, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Eibl, G.; Guha, S.; Duffy, J.P.; Reber, H.A.; Hines, O.J. Nerve Growth Factor Stimulates MMP-2 Expression and Activity and Increases Invasion by Human Pancreatic Cancer Cells. Clin. Exp. Metastasis 2004, 21, 285. [Google Scholar] [CrossRef]
- Fukuda, A.; Wang, S.C.; Morris, J.P.; Folias, A.E.; Liou, A.; Kim, G.E.; Akira, S.; Boucher, K.M.; Firpo, M.A.; Mulvihill, S.J.; et al. Stat3 and MMP7 Contribute to Pancreatic Ductal Adenocarcinoma Initiation and Progression. Cancer Cell 2011, 19, 441–455. [Google Scholar] [CrossRef]
- Waters, A.M.; Der, C.J. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a031435. [Google Scholar] [CrossRef]
- Macaya, I.; Roman, M.; Welch, C.; Entrialgo-Cadierno, R.; Salmon, M.; Santos, A.; Feliu, I.; Kovalski, J.; Lopez, I.; Rodriguez-Remirez, M.; et al. Signature-Driven Repurposing of Midostaurin for Combination with MEK1/2 and KRASG12C Inhibitors in Lung Cancer. Nat. Commun. 2023, 14, 6332. [Google Scholar] [CrossRef]
- Qian, Y.; Gong, Y.; Fan, Z.; Luo, G.; Huang, Q.; Deng, S.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. Molecular Alterations and Targeted Therapy in Pancreatic Ductal Adenocarcinoma. J. Hematol. Oncol. 2020, 13, 130. [Google Scholar] [CrossRef]
- Conway, J.R.; Herrmann, D.; Evans, T.J.; Morton, J.P.; Timpson, P. Combating Pancreatic Cancer with PI3K Pathway Inhibitors in the Era of Personalised Medicine. Gut 2019, 68, 742–758. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wei, J.; Liu, P. Attacking the PI3K/Akt/MTOR Signaling Pathway for Targeted Therapeutic Treatment in Human Cancer. Semin. Cancer Biol. 2022, 85, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Mehra, S.; Deshpande, N.; Nagathihalli, N. Targeting Pi3k Pathway in Pancreatic Ductal Adenocarcinoma: Rationale and Progress. Cancers 2021, 13, 4434. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, M.; Moosavi, F.; Martini, M.; Giovannetti, E.; Firuzi, O. Prospects of Targeting PI3K/AKT/MTOR Pathway in Pancreatic Cancer. Crit. Rev. Oncol. Hematol. 2022, 176, 103749. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, D.R.; Karim, S.A.; Sano, M.; Gay, D.M.; Jacob, W.; Yu, J.; Mizukami, Y.; Gopinathan, A.; Jodrell, D.I.; Evans, T.R.J.; et al. MTORC2 Signaling Drives the Development and Progression of Pancreatic Cancer. Cancer Res. 2016, 76, 6911–6923. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Henary, H.; Falchook, G.S.; Naing, A.; Fu, S.; Moulder, S.; Wheler, J.J.; Tsimberidou, A.; Durand, J.B.; Khan, R.; et al. First-in-Human Study of Pbi-05204, an Oleander-Derived Inhibitor of Akt, Fgf-2, Nf-$\kappa$Β and P70s6k, in Patients with Advanced Solid Tumors. Investig. New Drugs 2014, 32, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Patel, H.; Alanazi, S.; Kilroy, M.K.; Garrett, J.T. PI3K Inhibitors in Cancer: Clinical Implications and Adverse Effects. Int. J. Mol. Sci. 2021, 22, 3464. [Google Scholar] [CrossRef] [PubMed]
- Thibault, B.; Ramos-Delgado, F.; Pons-Tostivint, E.; Therville, N.; Cintas, C.; Arcucci, S.; Cassant-Sourdy, S.; Reyes-Castellanos, G.; Tosolini, M.; Villard, A.V.; et al. Pancreatic Cancer Intrinsic PI3K$\alpha$ Activity Accelerates Metastasis and Rewires Macrophage Component. EMBO Mol. Med. 2021, 13, e13502. [Google Scholar] [CrossRef] [PubMed]
- Soares, H.P.; Ming, M.; Mellon, M.; Young, S.H.; Han, L.; Sinnet-Smith, J.; Rozengurt, E. Dual PI3K/MTOR Inhibitors Induce Rapid Overactivation of the MEK/ERK Pathway in Human Pancreatic Cancer Cells through Suppression of MTORC2. Mol. Cancer Ther. 2015, 14, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Bedard, P.L.; Tabernero, J.; Janku, F.; Awainberg, Z.; Paz-Ares, L.; Vansteenkiste, J.; Cutsem, E.V.; Pérez-García, J.; Stathis, A.; Britten, C.D.; et al. A Phase Ib Dose-Escalation Study of the Oral Pan-PI3K Inhibitor Buparlisib (BKM120) in Combination with the Oral MEK1/2 Inhibitor Trametinib (GSK1120212) in Patients with Selected Advanced Solid Tumors. Clin. Cancer Res. 2015, 21, 730–738. [Google Scholar] [CrossRef]
- Ma, Y.; Sender, S.; Sekora, A.; Kong, W.; Bauer, P.; Ameziane, N.; Al-Ali, R.; Krake, S.; Radefeldt, M.; Weiss, F.U.; et al. The Inhibitory Response to PI3K/AKT Pathway Inhibitors MK-2206 and Buparlisib Is Related to Genetic Differences in Pancreatic Ductal Adenocarcinoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 4295. [Google Scholar] [CrossRef]
- McRee, A.J.; Sanoff, H.K.; Carlson, C.; Ivanova, A.; O’Neil, B.H. A Phase I Trial of MFOLFOX6 Combined with the Oral PI3K Inhibitor BKM120 in Patients with Advanced Refractory Solid Tumors. Investig. New Drugs 2015, 33, 1225–1231. [Google Scholar] [CrossRef]
- Patnaik, A.; Appleman, L.J.; Tolcher, A.W.; Papadopoulos, K.P.; Beeram, M.; Rasco, D.W.; Weiss, G.J.; Sachdev, J.C.; Chadha, M.; Fulk, M.; et al. First-in-Human Phase I Study of Copanlisib (BAY 80-6946), an Intravenous Pan-Class I Phosphatidylinositol 3-Kinase Inhibitor, in Patients with Advanced Solid Tumors and Non-Hodgkin’s Lymphomas. Ann. Oncol. 2016, 27, 1928–1940. [Google Scholar] [CrossRef]
- Sarker, D.; Ang, J.E.; Baird, R.; Kristeleit, R.; Shah, K.; Moreno, V.; Clarke, P.A.; Raynaud, F.I.; Levy, G.; Ware, J.A.; et al. First-in-Human Phase I Study of Pictilisib (GDC-0941), a Potent Pan-Class I Phosphatidylinositol-3-Kinase (PI3K) Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2015, 21, 77–86. [Google Scholar] [CrossRef]
- Stewart, A.; Coker, E.A.; Pölsterl, S.; Georgiou, A.; Minchom, A.R.; Carreira, S.; Cunningham, D.; O’Brien, M.E.R.; Raynaud, F.I.; Bono, J.S.D.; et al. Differences in Signaling Patterns on PI3K Inhibition Reveal Context Specificity in KRAS Mutant Cancers. Mol. Cancer Ther. 2019, 18, 1396–1404. [Google Scholar] [CrossRef]
- Shapiro, G.I.; LoRusso, P.; Kwak, E.; Pandya, S.; Rudin, C.M.; Kurkjian, C.; Cleary, J.M.; Pilat, M.J.; Jones, S.; de Crespigny, A.; et al. Phase Ib Study of the MEK Inhibitor Cobimetinib (GDC-0973) in Combination with the PI3K Inhibitor Pictilisib (GDC-0941) in Patients with Advanced Solid Tumors. Investig. New Drugs 2019, 38, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Hedley, D.; Moore, M.J.; Hirte, H.; Siu, L.; Vincent, M.; Jonker, D.; Mwang, H.; Nagai, J.; Dancey, J. A Phase II Trial of Perifosine as Second Line Therapy for Advanced Pancreatic Cancer. A Study of the Princess Margaret Hospital [PMH] Phase II Consortium. J. Clin. Oncol. 2005, 23, 4166. [Google Scholar] [CrossRef]
- Marsh, R.D.W.; Lima, C.M.R.; Levy, D.E.; Mitchell, E.P.; Rowland, K.M.; Benson, A.B. A Phase II Trial of Perifosine in Locally Advanced, Unresectable, or Metastatic Pancreatic Adenocarcinoma. Am. J. Clin. Oncol. Cancer Clin. Trials 2007, 30, 26–31. [Google Scholar] [CrossRef]
- Xin, H.; Zhong, C.; Nudleman, E.; Ferrara, N. Evidence for Pro-Angiogenic Functions of VEGF-Ax. Cell 2016, 167, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Shen, X.D.; Cheng, L.; Hong, D.F.; Chen, B. Perifosine Inhibits S6K1-Gli1 Signaling and Enhances Gemcitabine-Induced Anti-Pancreatic Cancer Efficiency. Cancer Chemother. Pharmacol. 2014, 73, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Tolcher, A.W.; Kurzrock, R.; Valero, V.; Gonzalez, R.; Heist, R.S.; Tan, A.R.; Means-Powell, J.; Werner, T.L.; Becerra, C.; Wang, C.; et al. Phase I Dose-Escalation Trial of the Oral AKT Inhibitor Uprosertib in Combination with the Oral MEK1/MEK2 Inhibitor Trametinib in Patients with Solid Tumors. Cancer Chemother. Pharmacol. 2020, 85, 673–683. [Google Scholar] [CrossRef]
- Yang, P.; Menter, D.G.; Cartwright, C.; Chan, D.; Dixon, S.; Suraokar, M.; Mendoza, G.; Llansa, N.; Newman, R.A. Oleandrin-Mediated Inhibition of Human Tumor Cell Proliferation: Importance of Na,K-ATPase $\alpha$ Subunits as Drug Targets. Mol. Cancer Ther. 2009, 8, 2319–2328. [Google Scholar] [CrossRef]
- Hong, D.S.; Bowles, D.W.; Falchook, G.S.; Messersmith, W.A.; George, G.C.; O’Bryant, C.L.; Vo, A.C.H.; Klucher, K.; Herbst, R.S.; Eckhardt, S.G.; et al. A Multicenter Phase I Trial of PX-866, an Oral Irreversible Phosphatidylinositol 3-Kinase Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2012, 18, 4173–4182. [Google Scholar] [CrossRef]
- Roth, M.T.; Cardin, D.B.; Borazanci, E.H.; Steinbach, M.; Picozzi, V.J.; Rosemury, A.; Wadlow, R.C.; Newman, R.A.; Berlin, J. A Phase II, Single-Arm, Open-Label, Bayesian Adaptive Efficacy and Safety Study of PBI-05204 in Patients with Stage IV Metastatic Pancreatic Adenocarcinoma. Oncol. 2020, 25, e1446–e1450. [Google Scholar] [CrossRef]
- Elhariri, A.; Alhaj, A.; Ahn, D.; Sonbol, M.B.; Bekaii-Saab, T.; Wu, C.; Rutenberg, M.S.; Stauffer, J.; Starr, J.; Majeed, U.; et al. Targeting KRAS in Pancreatic Adenocarcinoma: Progress in Demystifying the Holy Grail. World J. Clin. Oncol. 2023, 14, 285–296. [Google Scholar] [CrossRef]
- Stanciu, S.; Ionita-Radu, F.; Stefani, C.; Miricescu, D.; Stanescu-Spinu, I.I.; Greabu, M.; Totan, A.R.; Jinga, M. Targeting PI3K/AKT/MTOR Signaling Pathway in Pancreatic Cancer: From Molecular to Clinical Aspects. Int. J. Mol. Sci. 2022, 23, 10132. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, G.; Qiu, Z. Akt Inhibitor MK–2206 Reduces Pancreatic Cancer Cell Viability and Increases the Efficacy of Gemcitabine. Oncol. Lett. 2020, 19, 1999–2004. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Dadon, T.; Chenna, V.; Yabuuchi, S.; Bannerji, R.; Booher, R.; Strack, P.; Azad, N.; Nelkin, B.D.; Maitra, A. Combined Inhibition of Cyclin-Dependent Kinases (Dinaciclib) and AKT(MK-2206) Blocks Pancreatic Tumor Growth and Metastases in Patient-Derived Xenograft Models. Mol. Cancer Ther. 2015, 14, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.G.; Zahurak, M.; Shah, M.; Weekes, C.D.; Hansen, A.; Siu, L.L.; Spreafico, A.; LoConte, N.; Anders, N.M.; Miles, T.; et al. A Phase I Study of Dinaciclib in Combination with MK-2206 in Patients with Advanced Pancreatic Cancer. Clin. Transl. Sci. 2020, 13, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Gangadhar, T.C.; Cohen, E.E.W.; Wu, K.; Janisch, L.; Geary, D.; Kocherginsky, M.; House, L.K.; Ramirez, J.; Undevia, S.D.; Maitland, M.L.; et al. Two Drug Interaction Studies of Sirolimus in Combination with Sorafenib or Sunitinib in Patients with Advanced Malignancies. Clin. Cancer Res. 2011, 17, 1956–1963. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Laguna, I.; Tan, A.C.; Uson, M.; Angenendt, M.; Ma, W.W.; Villaroel, M.C.; Zhao, M.; Rajeshkumar, N.V.; Jimeno, A.; Donehower, R.; et al. Integrated Preclinical and Clinical Development of MTOR Inhibitors in Pancreatic Cancer. Br. J. Cancer 2010, 103, 649–655. [Google Scholar] [CrossRef]
- Garrido-Laguna, I.; Hidalgo, M. Pancreatic Cancer: From State-of-the-Art Treatments to Promising Novel Therapies. Nat. Rev. Clin. Oncol. 2015, 12, 319–334. [Google Scholar] [CrossRef]
- Wolpin, B.M.; Hezel, A.F.; Abrams, T.; Blaszkowsky, L.S.; Meyerhardt, J.A.; Chan, J.A.; Enzinger, P.C.; Allen, B.; Clark, J.W.; Ryan, D.P.; et al. Oral MTOR Inhibitor Everolimus in Patients with Gemcitabine-Refractory Metastatic Pancreatic Cancer. J. Clin. Oncol. 2009, 27, 193–198. [Google Scholar] [CrossRef]
- Yao, J.C.; Lombard-Bohas, C.; Baudin, E.; Kvols, L.K.; Rougier, P.; Ruszniewski, P.; Hoosen, S.; Peter, J.S.; Haas, T.; Lebwohl, D.; et al. Daily Oral Everolimus Activity in Patients with Metastatic Pancreatic Neuroendocrine Tumors after Failure of Cytotoxic Chemotherapy: A Phase II Trial. J. Clin. Oncol. 2010, 28, 69–76. [Google Scholar] [CrossRef]
- Kordes, S.; Pollak, M.N.; Zwinderman, A.H.; Math^ot, R.A.; Weterman, M.J.; Beeker, A.; Punt, C.J.; Richel, D.J.; Wilmink, J.W. Metformin in Patients with Advanced Pancreatic Cancer: A Double-Blind, Randomised, Placebo-Controlled Phase 2 Trial. Lancet Oncol. 2015, 16, 839–847. [Google Scholar] [CrossRef]
- Bever, K.M.; Borazanci, E.H.; Thompson, E.A.; Durham, J.N.; Pinero, K.; Jameson, G.S.; Vrana, A.; Liu, M.; Wilt, C.; Wu, A.A.; et al. An Exploratory Study of Metformin with or without Rapamycin as Maintenance Therapy after Induction Chemotherapy in Patients with Metastatic Pancreatic Adenocarcinoma. Oncotarget 2020, 11, 1929–1941. [Google Scholar] [CrossRef] [PubMed]
- Nemunaitis, J.; Hochster, H.S.; Lustgarten, S.; Rhodes, R.; Ebbinghaus, S.; Turner, C.D.; Dodion, P.F.; Mita, M.M. A Phase I Trial of Oral Ridaforolimus (AP23573; MK-8669) in Combination with Bevacizumab for Patients with Advanced Cancers. Clin. Oncol. 2013, 25, 336–342. [Google Scholar] [CrossRef]
- Basu, B.; Dean, E.; Puglisi, M.; Greystoke, A.; Ong, M.; Burke, W.; Cavallin, M.; Bigley, G.; Womack, C.; Harrington, E.A.; et al. First-in-Human Pharmacokinetic and Pharmacodynamic Study of the Dual m-TORC 1/2 Inhibitor AZD2014. Clin. Cancer Res. 2015, 21, 3412–3419. [Google Scholar] [CrossRef]
- Javle, M.M.; Shroff, R.T.; Xiong, H.; Varadhachary, G.A.; Fogelman, D.; Reddy, S.A.; Davis, D.; Zhang, Y.; Wolff, R.A.; Abbruzzese, J.L. Inhibition of the Mammalian Target of Rapamycin (MTOR) in Advanced Pancreatic Cancer: Results of Two Phase II Studies. BMC Cancer 2010, 10, 368. [Google Scholar] [CrossRef]
- Karavasilis, V.; Samantas, E.; Koliou, G.A.; Kalogera-Fountzila, A.; Pentheroudakis, G.; Varthalitis, I.; Linardou, H.; Rallis, G.; Skondra, M.; Papadopoulos, G.; et al. Gemcitabine Combined with the MTOR Inhibitor Temsirolimus in Patients with Locally Advanced or Metastatic Pancreatic Cancer. A Hellenic Cooperative Oncology Group Phase I/II Study. Target. Oncol. 2018, 13, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, N.; Yen, P.L.; Schwarz, M.A.; Schwarz, R.E. The Efficacy of a Novel, Dual PI3K/MTOR Inhibitor NVP-BEZ235 to Enhance Chemotherapy and Antiangiogenic Response in Pancreatic Cancer. J. Cell. Biochem. 2012, 113, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, D.; Chiorean, E.G.; Harris, W.B.; Hoff, D.D.V.; Stejskal-Barnett, A.; Qi, W.; Anthony, S.P.; Younger, A.E.; Rensvold, D.M.; Cordova, F.; et al. Phase i Pharmacokinetic and Pharmacodynamic Study of the Pan-PI3K/MTORC Vascular Targeted pro-Drug SF1126 in Patients with Advanced Solid Tumours and B-Cell Malignancies. Eur. J. Cancer 2012, 48, 3319–3327. [Google Scholar] [CrossRef]
- Schram, A.M.; Gandhi, L.; Mita, M.M.; Damstrup, L.; Campana, F.; Hidalgo, M.; Grande, E.; Hyman, D.M.; Heist, R.S. A Phase Ib Dose-Escalation and Expansion Study of the Oral MEK Inhibitor Pimasertib and PI3K/MTOR Inhibitor Voxtalisib in Patients with Advanced Solid Tumours. Br. J. Cancer 2018, 119, 1471–1476. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Tabernero, J.; Markman, B.; Patnaik, A.; Tolcher, A.W.; Baselga, J.; Shi, W.; Egile, C.; Ruiz-Soto, R.; Laird, A.D.; et al. Phase i Safety, Pharmacokinetic, and Pharmacodynamic Study of SAR245409 (XL765), a Novel, Orally Administered PI3K/MTOR Inhibitor in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2014, 20, 2445–2456. [Google Scholar] [CrossRef]
- Jack, J.; Pierce, A.; Bye, B.; Walsh, M.; Chalise, P.; VanSaun, M.N. Abstract 4027: Dual MEK and AKT Inhibition Suppresses Pancreatic Cancer Growth and Migration. Cancer Res. 2022, 82, 4027. [Google Scholar] [CrossRef]
- Munster, P.; Aggarwal, R.; Hong, D.; Schellens, J.H.M.; Noll, R.V.D.; Specht, J.; Witteveen, P.O.; Werner, T.L.; Dees, E.C.; Bergsland, E.; et al. First-in-Human Phase i Study of GSK2126458, an Oral Pan-Class i Phosphatidylinositol-3-Kinase Inhibitor, in Patients with Advanced Solid Tumor Malignancies. Clin. Cancer Res. 2016, 22, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Wainberg, Z.A.; Alsina, M.; Soares, H.P.; Braña, I.; Britten, C.D.; Conte, G.D.; Ezeh, P.; Houk, B.; Kern, K.A.; Leong, S.; et al. A Multi-Arm Phase I Study of the PI3K/MTOR Inhibitors PF-04691502 and Gedatolisib (PF-05212384) plus Irinotecan or the MEK Inhibitor PD-0325901 in Advanced Cancer. Target. Oncol. 2017, 12, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.; Hosseini, M.; Shahidsales, S.; Maftouh, M.; Ferns, G.A.; Ghayour-Mobarhan, M.; Hassanian, S.M.; Avan, A. Targeting the Akt/PI3K Signaling Pathway as a Potential Therapeutic Strategy for the Treatment of Pancreatic Cancer. Curr. Med. Chem. 2017, 24, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Makena, M.R.; Gatla, H.; Verlekar, D.; Sukhavasi, S.; Pandey, M.K.; Pramanik, K.C. Wnt/beta-Catenin Signaling: The Culprit in Pancreatic Carcinogenesis and Therapeutic Resistance. Int. J. Mol. Sci. 2019, 20, 4242. [Google Scholar] [CrossRef] [PubMed]
- Turpin, A.; Neuzillet, C.; Colle, E.; Dusetti, N.; Nicolle, R.; Cros, J.; de Mestier, L.; Bachet, J.B.; Hammel, P. Therapeutic Advances in Metastatic Pancreatic Cancer: A Focus on Targeted Therapies. Ther. Adv. Med. Oncol. 2022, 14, 17588359221118019. [Google Scholar] [CrossRef]
- Rodon, J.; Argilés, G.; Connolly, R.M.; Vaishampayan, U.; de Jonge, M.; Garralda, E.; Giannakis, M.; Smith, D.C.; Dobson, J.R.; McLaughlin, M.E.; et al. Phase 1 Study of Single-Agent WNT974, a First-in-Class Porcupine Inhibitor, in Patients with Advanced Solid Tumours. Br. J. Cancer 2021, 125, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Madan, B.; Harmston, N.; Nallan, G.; Montoya, A.; Faull, P.; Petretto, E.; Virshup, D.M. Temporal Dynamics of Wnt-Dependent Transcriptome Reveal an Oncogenic Wnt/MYC/Ribosome Axis. J. Clin. Investig. 2018, 128, 5620–5633. [Google Scholar] [CrossRef]
- Messersmith, W.; Cohen, S.; Shahda, S.; Lenz, H.-J.; Weekes, C.; Dotan, E.; Denlinger, C.; O’Neil, B.; Kapoun, A.; Zhang, C.; et al. Phase 1b Study of WNT Inhibitor Vantictumab (VAN, Human Monoclonal Antibody) with Nab-Paclitaxel (Nab-P) and Gemcitabine (G) in Patients (Pts) with Previously Untreated Stage IV Pancreatic Cancer (PC). Ann. Oncol. 2016, 27, vi228. [Google Scholar] [CrossRef]
- Le, P.N.; McDermott, J.D.; Jimeno, A. Targeting the Wnt Pathway in Human Cancers: Therapeutic Targeting with a Focus on OMP-54F28. Pharmacol. Ther. 2015, 146, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.M.; Cancilla, B.; Yeung, V.P.; Cattaruzza, F.; Chartier, C.; Murriel, C.L.; Cain, J.; Tam, R.; Cheng, C.Y.; Evans, J.W.; et al. WNT Antagonists Exhibit Unique Combinatorial Antitumor Activity with Taxanes by Potentiating Mitotic Cell Death. Sci. Adv. 2017, 3, e1700090. [Google Scholar] [CrossRef] [PubMed]
- Dotan, E.; Cardin, D.B.; Lenz, H.-J.; Messersmith, W.A.; O’Neil, B.; Cohen, S.J.; Denlinger, C.S.; Shahda, S.; Kapoun, A.M.; Brachmann, R.K.; et al. Phase Ib Study of WNT Inhibitor Ipafricept (IPA) with Nab-Paclitaxel (Nab-P) and Gemcitabine (G) in Patients (Pts) with Previously Untreated Stage IV Pancreatic Cancer (MPC). J. Clin. Oncol. 2019, 37, 369. [Google Scholar] [CrossRef]
- Fang, Y.T.; Yang, W.W.; Niu, Y.R.; Sun, Y.K. Recent Advances in Targeted Therapy for Pancreatic Adenocarcinoma. J. Hematol. Oncol. 2023, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.J.; Kahn, M. Safely Targeting Cancer Stem Cells via Selective Catenin Coactivator Antagonism. Cancer Sci. 2014, 105, 1087–1092. [Google Scholar] [CrossRef]
- Ko, A.H.; Chiorean, E.G.; Kwak, E.L.; Lenz, H.-J.; Nadler, P.I.; Wood, D.L.; Fujimori, M.; Inada, T.; Kouji, H.; McWilliams, R.R. Final Results of a Phase Ib Dose-Escalation Study of PRI-724, a CBP/Beta-Catenin Modulator, plus Gemcitabine (GEM) in Patients with Advanced Pancreatic Adenocarcinoma (APC) as Second-Line Therapy after FOLFIRINOX or FOLFOX. J. Clin. Oncol. 2016, 34, e15721. [Google Scholar] [CrossRef]
- Manegold, P.; Lai, K.K.Y.; Wu, Y.; Teo, J.L.; Lenz, H.J.; Genyk, Y.S.; Pandol, S.J.; Wu, K.; Lin, D.P.; Chen, Y.; et al. Differentiation Therapy Targeting the $\beta$-Catenin/CBP Interaction in Pancreatic Cancer. Cancers 2018, 10, 95. [Google Scholar] [CrossRef]
- Quatannens, D.; Verhoeven, Y.; Dam, P.V.; Lardon, F.; Prenen, H.; Roeyen, G.; Peeters, M.; Smits, E.L.J.; Audenaerde, J.V. Targeting Hedgehog Signaling in Pancreatic Ductal Adenocarcinoma. Pharmacol. Ther. 2022, 236, 108107. [Google Scholar] [CrossRef]
- Carr, R.M.; Duma, N.; McCleary-Wheeler, A.L.; Almada, L.L.; Marks, D.L.; Graham, R.P.; Smyrk, T.C.; Lowe, V.; Borad, M.J.; Kim, G.; et al. Targeting of the Hedgehog/GLI and MTOR Pathways in Advanced Pancreatic Cancer, a Phase 1 Trial of Vismodegib and Sirolimus Combination. Pancreatology 2020, 20, 1115–1122. [Google Scholar] [CrossRef]
- Jesus-Acosta, A.D.; Sugar, E.A.; O’Dwyer, P.J.; Ramanathan, R.K.; Hoff, D.D.V.; Rasheed, Z.; Zheng, L.; Begum, A.; Anders, R.; Maitra, A.; et al. Phase 2 Study of Vismodegib, a Hedgehog Inhibitor, Combined with Gemcitabine and Nab-Paclitaxel in Patients with Untreated Metastatic Pancreatic Adenocarcinoma. Br. J. Cancer 2020, 122, 498–505. [Google Scholar] [CrossRef]
- Pijnappel, E.N.; Wassenaar, N.P.M.; Gurney-Champion, O.J.; Klaassen, R.; van der Lee, K.S.; Pleunis-van Empel, M.; Richel, D.J.; Legdeur, M.-C.J.C.; Nederveen, A.J.; Laarhoven, H.W.M.V.; et al. Phase I/II Study of LDE225 in Combination with Gemcitabine and Nab-Paclitaxel in Patients with Metastatic Pancreatic Cancer. J. Clin. Oncol. 2021, 39, e16239. [Google Scholar] [CrossRef]
- Ko, A.H.; LoConte, N.; Tempero, M.A.; Walker, E.J.; Kelley, R.K.; Lewis, S.; Chang, W.C.; Kantoff, E.; Vannier, M.W.; Catenacci, D.V.; et al. A Phase I Study of FOLFIRINOX Plus IPI-926, a Hedgehog Pathway Inhibitor, for Advanced Pancreatic Adenocarcinoma. Pancreas 2016, 45, 370–375. [Google Scholar] [CrossRef]
- Richards, D.A.; Stephenson, J.; Wolpin, B.M.; Becerra, C.; Hamm, J.T.; Messersmith, W.A.; Devens, S.; Cushing, J.; Schmalbach, T.; Fuchs, C.S. A Phase Ib Trial of IPI-926, a Hedgehog Pathway Inhibitor, plus Gemcitabine in Patients with Metastatic Pancreatic Cancer. J. Clin. Oncol. 2012, 30, 213. [Google Scholar] [CrossRef]
- Ueno, H.; Kondo, S.; Yoshikawa, S.; Inoue, K.; Andre, V.; Tajimi, M.; Murakami, H. A Phase I and Pharmacokinetic Study of Taladegib, a Smoothened Inhibitor, in Japanese Patients with Advanced Solid Tumors. Investig. New Drugs 2018, 36, 647–656. [Google Scholar] [CrossRef]
- Michaud, N.R.; Wang, Y.; McEachern, K.A.; Jordan, J.J.; Mazzola, A.M.; Hernandez, A.; Jalla, S.; Chesebrough, J.W.; Hynes, M.J.; Belmonte, M.A.; et al. Novel Neutralizing Hedgehog Antibody Medi-5304 Exhibits Antitumor Activity by Inhibiting Paracrine Hedgehog Signaling. Mol. Cancer Ther. 2014, 13, 386–398. [Google Scholar] [CrossRef]
- Rodgers, U.R.; Lanyon-Hogg, T.; Masumoto, N.; Ritzefeld, M.; Burke, R.; Blagg, J.; Magee, A.I.; Tate, E.W. Characterization of Hedgehog Acyltransferase Inhibitors Identifies a Small Molecule Probe for Hedgehog Signaling by Cancer Cells. ACS Chem. Biol. 2016, 11, 3256–3262. [Google Scholar] [CrossRef]
- Cortes, J.E.; Gutzmer, R.; Kieran, M.W.; Solomon, J.A. Hedgehog Signaling Inhibitors in Solid and Hematological Cancers. Cancer Treat. Rev. 2019, 76, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Brunetti, O.; Gnoni, A.; Cascinu, S.; Gasparini, G.; Lorusso, V.; Ribatti, D.; Silvestris, N. Angiogenesis in Pancreatic Ductal Adenocarcinoma: A Controversial Issue. Oncotarget. 2016, 7, 58649–58658. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Kang, G.; Wang, T.; Huang, H. Tumor Angiogenesis and Anti-Angiogenic Gene Therapy for Cancer (Review). Oncol. Lett. 2018, 16, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Wiszniak, S.; Schwarz, Q. Exploring the Intracrine Functions of VEGF-A. Biomolecules 2021, 11, 128. [Google Scholar] [CrossRef]
- Lupo, G.; Caporarello, N.; Olivieri, M.; Cristaldi, M.; Motta, C.; Bramanti, V.; Avola, R.; Salmeri, M.; Nicoletti, F.; Anfuso, C.D. Anti-Angiogenic Therapy in Cancer: Downsides and New Pivots for Precision Medicine. Front. Pharmacol. 2017, 7, 519. [Google Scholar] [CrossRef]
- Bry, M.; Kivelä, R.; Leppänen, V.M.; Alitalo, K. Vascular endothelial growth factor-B in physiology and disease. Physiol. Rev. 2014, 94, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Chen, R.; Sun, G.; Liu, X.; Lin, X.; He, C.; Xing, L.; Liu, L.; Jensen, L.D.; Kumar, A.; et al. VEGF-B Prevents Excessive Angiogenesis by Inhibiting FGF2/FGFR1 Pathway. Signal Transduct. Target. Ther. 2023, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- McMahon, G. VEGF receptor signaling in tumor angiogenesis. Oncologist 2000, 5 (Suppl. S1), 3–10. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sampedro, A.; Gaggia, G.; Ney, A.; Mahamed, I.; Acedo, P. The State-of-the-Art of Phase II/III Clinical Trials for Targeted Pancreatic Cancer Therapies. J. Clin. Med. 2021, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Annese, T.; Tamma, R.; Ruggieri, S.; Ribatti, D. Angiogenesis in Pancreatic Cancer: Pre-Clinical and Clinical Studies. Cancers 2019, 11, 381. [Google Scholar] [CrossRef]
- Lopes-Coelho, F.; Martins, F.; Pereira, S.A.; Serpa, J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 3765. [Google Scholar] [CrossRef]
- Pàez-Ribes, M.; Allen, E.; Hudock, J.; Takeda, T.; Okuyama, H.; Viñals, F.; Inoue, M.; Bergers, G.; Hanahan, D.; Casanovas, O. Antiangiogenic Therapy Elicits Malignant Progression of Tumors to Increased Local Invasion and Distant Metastasis. Cancer Cell 2009, 15, 220–231. [Google Scholar] [CrossRef]
- Lu, Z.; Weniger, M.; Jiang, K.; Boeck, S.; Zhang, K.; Bazhin, A.; Miao, Y.; Werner, J.; D’Haese, J.G. Therapies Targeting the Tumor Stroma and the VEGF/VEGFR Axis in Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis. Target. Oncol. 2018, 13, 447–459. [Google Scholar] [CrossRef]
- Allen, E.; Walters, I.B.; Hanahan, D. Brivanib, a Dual FGF/VEGF Inhibitor, Is Active Both First and Second Line against Mouse Pancreatic Neuroendocrine Tumors Developing Adaptive/Evasive Resistance to VEGF Inhibition. Clin. Cancer Res. 2011, 17, 5299–5310. [Google Scholar] [CrossRef]
- Cai, Z.W.; Zhang, Y.; Borzilleri, R.M.; Qian, L.; Barbosa, S.; Wei, D.; Zheng, X.; Wu, L.; Fan, J.; Shi, Z.; et al. Discovery of Brivanib Alaninate ((S)-((R)-1-(4-(4-Fluoro-2-Methyl-1H-Indol-5-Yloxy)-5-Methylpyrrolo [2,1-f][1,2,4]Triazin-6-Yloxy)Propan-2-Yl) 2-Aminopropanoate), a Novel Prodrug of Dual Vascular Endothelial Growth Factor Receptor-2 and Fibroblast Growth. J. Med. Chem. 2008, 51, 1976–1980. [Google Scholar] [CrossRef]
- Gaya, A.; Tse, V. A preclinical and clinical review of aflibercept for the management of cancer. Cancer Treat Rev. 2012, 38, 484–493. [Google Scholar] [CrossRef]
- Rougier, P.; Riess, H.; Manges, R.; Karasek, P.; Humblet, Y.; Barone, C.; Santoro, A.; Assadourian, S.; Hatteville, L.; Philip, P.A. Randomised, Placebo-Controlled, Double-Blind, Parallel-Group Phase III Study Evaluating Aflibercept in Patients Receiving First-Line Treatment with Gemcitabine for Metastatic Pancreatic Cancer. Eur. J. Cancer 2013, 49, 2633–2642. [Google Scholar] [CrossRef]
- Kindler, H.L.; Niedzwiecki, D.; Hollis, D.; Sutherland, S.; Schrag, D.; Hurwitz, H.; Innocenti, F.; Mulcahy, M.F.; O’Reilly, E.; Wozniak, T.F.; et al. Gemcitabine plus Bevacizumab Compared with Gemcitabine plus Placebo in Patients with Advanced Pancreatic Cancer: Phase III Trial of the Cancer and Leukemia Group B (CALGB 80303). J. Clin. Oncol. 2010, 28, 3617–3622. [Google Scholar] [CrossRef]
- Astsaturov, I.A.; Meropol, N.J.; Alpaugh, R.K.; Burtness, B.A.; Cheng, J.D.; McLaughlin, S.; Rogatko, A.; Xu, Z.; Watson, J.C.; Weiner, L.M.; et al. Phase II and Coagulation Cascade Biomarker Study of Bevacizumab with or without Docetaxel in Patients with Previously Treated Metastatic Pancreatic Adenocarcinoma. Am. J. Clin. Oncol. Cancer Clin. Trials 2011, 34, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, S.; Mey, V.; Nannizzi, S.; Pasqualetti, G.; Crea, F.; Tacca, M.D.; Danesi, R. Synergistic Cytotoxicity and Molecular Interaction on Drug Targets of Sorafenib and Gemcitabine in Human Pancreas Cancer Cells. Chemotherapy 2010, 56, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, N.; Zhang, C.; Hinz, S.; Schwarz, M.A.; Schwarz, R.E. Enhancing Sorafenib-Mediated Sensitization to Gemcitabine in Experimental Pancreatic Cancer through EMAP II. J. Exp. Clin. Cancer Res. 2013, 32, 12. [Google Scholar] [CrossRef]
- Dragovich, T.; Laheru, D.; Dayyani, F.; Bolejack, V.; Smith, L.; Seng, J.; Burris, H.; Rosen, P.; Hidalgo, M.; Ritch, P.; et al. Phase II Trial of Vatalanib in Patients with Advanced or Metastatic Pancreatic Adenocarcinoma after First-Line Gemcitabine Therapy (PCRT O4-001). Cancer Chemother. Pharmacol. 2014, 74, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Rixe, O. Axitinib—A selective inhibitor of the vascular endothelial growth factor (VEGF) receptor. Target Oncol. 2009, 4, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kindler, H.L.; Ioka, T.; Richel, D.J.; Bennouna, J.; Létourneau, R.; Okusaka, T.; Funakoshi, A.; Furuse, J.; Park, Y.S.; Ohkawa, S.; et al. Axitinib plus Gemcitabine versus Placebo plus Gemcitabine in Patients with Advanced Pancreatic Adenocarcinoma: A Double-Blind Randomised Phase 3 Study. Lancet Oncol. 2011, 12, 256–262. [Google Scholar] [CrossRef]
- Ioka, T.; Okusaka, T.; Ohkawa, S.; Boku, N.; Sawaki, A.; Fujii, Y.; Kamei, Y.; Takahashi, S.; Namazu, K.; Umeyama, Y.; et al. Efficacy and Safety of Axitinib in Combination with Gemcitabine in Advanced Pancreatic Cancer: Subgroup Analyses by Region, Including Japan, from the Global Randomized Phase III Trial. Jpn. J. Clin. Oncol. 2015, 45, 439–448. [Google Scholar] [CrossRef]
- Mayer, B.; Karakhanova, S.; Bauer, N.; Liu, L.; Zhu, Y.; Philippov, P.P.; Werner, J.; Bazhin, A.V. A Marginal Anticancer Effect of Regorafenib on Pancreatic Carcinoma Cells In Vitro, Ex Vivo, and In Vivo. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 1125–1134. [Google Scholar] [CrossRef]
- Salmon, J.S.; Hwang, J.J.; Robinson, M.M.; Symanowski, J.T.; Dillon, L.M.; Roy, L.D.; Beldner, M.A.; Preston, K.; Buige, S.; Nazemzadeh, R.; et al. Phase II Study of Regorafenib (Reg) in Patients with Previously Treated Advanced Pancreatic Cancer (APC). J. Clin. Oncol. 2017, 35, e15751. [Google Scholar] [CrossRef]
- Barati Bagherabad, M.; Afzaljavan, F.; ShahidSales, S.; Hassanian, S.M.; Avan, A. Targeted therapies in pancreatic cancer: Promises and failures. J. Cell Biochem. 2019, 120, 2726–2741. [Google Scholar] [CrossRef]
- Breuer, S.; Maimon, O.; Appelbaum, L.; Peretz, T.; Hubert, A. TL-118—Anti-Angiogenic Treatment in Pancreatic Cancer: A Case Report. Med. Oncol. 2013, 30, 585. [Google Scholar] [CrossRef]
- Chen, H.M.; Tsai, C.H.; Hung, W.C. Foretinib Inhibits Angiogenesis, Lymphangiogenesis and Tumor Growth of Pancreatic Cancer in Vivo by Decreasing VEGFR-2/3 and TIE-2 Signaling. Oncotarget 2015, 6, 14940–14952. [Google Scholar] [CrossRef] [PubMed]
- Momeny, M.; Alishahi, Z.; Eyvani, H.; Esmaeili, F.; Zaghal, A.; Ghaffari, P.; Tavakkoly-Bazzaz, J.; Alimoghaddam, K.; Ghavamzadeh, A.; Ghaffari, S.H. Anti-Tumor Activity of Cediranib, a Pan-Vascular Endothelial Growth Factor Receptor Inhibitor, in Pancreatic Ductal Adenocarcinoma Cells. Cell. Oncol. 2020, 43, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Weissmueller, S.; Manchado, E.; Saborowski, M.; John, P.M.I.; Wagenblast, E.; Davis, C.A.; Moon, S.H.; Pfister, N.T.; Tschaharganeh, D.F.; Kitzing, T.; et al. Mutant P53 Drives Pancreatic Cancer Metastasis through Cell-Autonomous PDGF Receptor $\beta$ Signaling. Cell 2014, 157, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Reni, M.; Cereda, S.; Milella, M.; Novarino, A.; Passardi, A.; Mambrini, A.; Lucca, G.D.; Aprile, G.; Belli, C.; Danova, M.; et al. Maintenance Sunitinib or Observation in Metastatic Pancreatic Adenocarcinoma: A Phase II Randomised Trial. Eur. J. Cancer 2013, 49, 3609–3615. [Google Scholar] [CrossRef] [PubMed]
- Kavian, N.; Servettaz, A.; Marut, W.; Nicco, C.; Chéreau, C.; Weill, B.; Batteux, F. Sunitinib Inhibits the Phosphorylation of Platelet-Derived Growth Factor Receptor $\beta$ in the Skin of Mice with Scleroderma-like Features and Prevents the Development of the Disease. Arthritis Rheum. 2012, 64, 1990–2000. [Google Scholar] [CrossRef]
- Awasthi, N.; Hinz, S.; Brekken, R.A.; Schwarz, M.A.; Schwarz, R.E. Nintedanib, a Triple Angiokinase Inhibitor, Enhances Cytotoxic Therapy Response in Pancreatic Cancer. Cancer Lett. 2015, 358, 59–66. [Google Scholar] [CrossRef]
- Hilberg, F.; Roth, G.J.; Krssak, M.; Kautschitsch, S.; Sommergruber, W.; Tontsch-Grunt, U.; Garin-Chesa, P.; Bader, G.; Zoephel, A.; Quant, J.; et al. BIBF 1120: Triple Angiokinase Inhibitor with Sustained Receptor Blockade and Good Antitumor Efficacy. Cancer Res. 2008, 68, 4774–4782. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Xu, Q.C.; Zhu, Y.Q.; Liu, Z.D.; Zhao, G.Y.; Liu, Q.; Wang, X.Y.; Wang, J.Q.; Xu, X.; Su, Q.; et al. Imatinib Facilitates Gemcitabine Sensitivity by Targeting Epigenetically Activated PDGFC Signaling in Pancreatic Cancer. Mol. Ther. 2023, 31, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Y.; Wang, Y.; Mukhopadhyay, D.; Bi, Y.; Ji, B. Aurora Kinase a Inhibitor MLN8237 Suppresses Pancreatic Cancer Growth. Pancreatology 2022, 22, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Marech, I.; Patruno, R.; Zizzo, N.; Gadaleta, C.; Introna, M.; Zito, A.F.; Gadaleta, C.D.; Ranieri, G. Masitinib (AB1010), from canine tumor model to human clinical development: Where we are? Crit. Rev. Oncol. Hematol. 2014, 91, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Purvey, S.; Saif, M.W. Masitinib in Treatment of Pancreatic Cancer. Expert Opin. Pharmacother. 2018, 19, 759–764. [Google Scholar] [CrossRef]
- Taeger, J.; Moser, C.; Hellerbrand, C.; Mycielska, M.E.; Glockzin, G.; Schlitt, H.J.; Geissler, E.K.; Stoeltzing, O.; Lang, S.A. Targeting FGFR/PDGFR/VEGFR Impairs Tumor Growth, Angiogenesis, and Metastasis by Effects on Tumor Cells, Endothelial Cells, and Pericytes in Pancreatic Cancer. Mol. Cancer Ther. 2011, 10, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Siddiqui, B.A.; Anandhan, S.; Yadav, S.S.; Subudhi, S.K.; Gao, J.; Goswami, S.; Allison, J.P. The Next Decade of Immune Checkpoint Therapy. Cancer Discov. 2021, 11, 838–857. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Eso, Y.; Okada, H.; Takai, A.; Takahashi, K.; Seno, H. Recent Advances in Immunotherapy for Hepatocellular Carcinoma. Cancers 2020, 12, 775. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Qureshi, O.S.; Zheng, Y.; Nakamura, K.; Attridge, K.; Manzotti, C.; Schmidt, E.M.; Baker, J.; Jeffery, L.E.; Kaur, S.; Briggs, Z.; et al. Trans-Endocytosis of CD80 and CD86: A Molecular Basis for the Cell-Extrinsic Function of CTLA-4. Science 2011, 332, 600–603. [Google Scholar] [CrossRef]
- Eso, Y.; Seno, H. Current status of treatment with immune checkpoint inhibitors for gastrointestinal, hepatobiliary, and pancreatic cancers. Therap. Adv. Gastroenterol. 2020, 13, 1756284820948773. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Smithy, J.W.; O’Reilly, E.M. Pancreas cancer: Therapeutic trials in metastatic disease. J. Surg. Oncol. 2021, 123, 1475–1488. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Beatty, G.L.; Dougan, S.K. Broadening the Impact of Immunotherapy to Pancreatic Cancer: Challenges and Opportunities. Gastroenterology 2019, 156, 2056–2072. [Google Scholar] [CrossRef] [PubMed]
- Yazdanifar, M.; Zhou, R.; Grover, P.; Williams, C.; Bose, M.; Moore, L.J.; Wu, S.T.; Maher, J.; Dreau, D.; Mukherjee, P. Overcoming Immunological Resistance Enhances the Efficacy of a Novel Anti-TMUC1-CAR T Cell Treatment against Pancreatic Ductal Adenocarcinoma. Cells 2019, 8, 1070. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Lutz, E.; Uram, J.N.; Sugar, E.A.; Onners, B.; Solt, S.; Zheng, L.; Diaz, L.A.; Donehower, R.C.; Jaffee, E.M.; et al. Evaluation of Ipilimumab in Combination with Allogeneic Pancreatic Tumor Cells Transfected with a GM-CSF Gene in Previously Treated Pancreatic Cancer. J. Immunother. 2013, 36, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Royal, R.E.; Levy, C.; Turner, K.; Mathur, A.; Hughes, M.; Kammula, U.S.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Lowy, I.; et al. Phase 2 Trial of Single Agent Ipilimumab (Anti-CTLA-4) for Locally Advanced or Metastatic Pancreatic Adenocarcinoma. J. Immunother. 2010, 33, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. New Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Zheng, W.; Dong, J.; Wang, Y.; Lai, J.; Liu, X.; Yin, F. Tumor microenvironment in pancreatic ductal adenocarcinoma: Implications in immunotherapy. World J. Gastroenterol. 2022, 28, 3297–3313. [Google Scholar] [CrossRef] [PubMed]
- Katayama, E.S.; Hue, J.J.; Bajor, D.L.; Ocuin, L.M.; Ammori, J.B.; Hardacre, J.M.; Winter, J.M. A Comprehensive Analysis of Clinical Trials in Pancreatic Cancer: What Is Coming down the Pike? Oncotarget 2020, 11, 3489–3501. [Google Scholar] [CrossRef] [PubMed]
- Halbrook, C.J.; Lyssiotis, C.A.; Magliano, M.P.D.; Maitra, A. Pancreatic Cancer: Advances and Challenges. Cell 2023, 186, 1729–1754. [Google Scholar] [CrossRef] [PubMed]
- Gabitova-Cornell, L.; Surumbayeva, A.; Peri, S.; Franco-Barraza, J.; Restifo, D.; Weitz, N.; Ogier, C.; Goldman, A.R.; Hartman, T.R.; Francescone, R.; et al. Cholesterol Pathway Inhibition Induces TGF-β Signaling to Promote Basal Differentiation in Pancreatic Cancer. Cancer Cell 2020, 38, 567–583.e11. [Google Scholar] [CrossRef]
- Shaya, J.; Kato, S.; Adashek, J.J.; Patel, H.; Fanta, P.T.; Botta, G.P.; Sicklick, J.K.; Kurzrock, R. Personalized matched targeted therapy in advanced pancreatic cancer: A pilot cohort analysis. npj Genom. Med. 2023, 8, 1. [Google Scholar] [CrossRef]
- Min, K.K.M.; Ffrench, C.B.; Jessup, C.F.; Shepherdson, M.; Barreto, S.G.; Bonder, C.S. Overcoming the Fibrotic Fortress in Pancreatic Ductal Adenocarcinoma: Challenges and Opportunities. Cancers 2023, 15, 2354. [Google Scholar] [CrossRef]
- Peschke, K.; Jakubowsky, H.; Schäfer, A.; Maurer, C.; Lange, S.; Orben, F.; Bernad, R.; Harder, F.N.; Eiber, M.; Öllinger, R.; et al. Identification of Treatment-induced Vulnerabilities in Pancreatic Cancer Patients Using Functional Model Systems. EMBO Mol. Med. 2022, 14, e14876. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-G.; Malek, E.; Choi, S.H.; Ignatz-Hoover, J.J.; Driscoll, J.J. Novel Therapies Emerging in Oncology to Target the TGF-β Pathway. J. Hematol. Oncol. 2021, 14, 55. [Google Scholar] [CrossRef] [PubMed]

| Functions | Incidence | Genes |
|---|---|---|
| Epigenetic Regulators | 35% | ARID1A, KMT2C, KMT2D, KDM6A, SMARCA4, SETD2, ARID2, PBRM1, HDAC1, CREBBP, SETDB1, SETD1B, EP300, JARID2, KMT2A, SMARCA1, SMARCA2, KDM5C, SETBP1, KDM2B, ARID3C, DNMT3B, DNMT1, ARID4A, KDM5A, SMARCB1, SMARCD1, SETD1A, KAT6B |
| DNA Damage Response | 9% | BRCA1, BRCA2, PALB2, ATM, ATR, MLH1, MSH2, MSH6, RPA1, STK11, FANCA, FANCC |
| Drug | Target | References |
|---|---|---|
| Sunitinib | PDGFRβ) | [150,151] |
| Nintedanib | PDGFRα/β | [152,153] |
| Imatinib | PDGFR | [154] |
| Masitinib | PDGFR-3 | [156] |
| TK1258 (Dovitinib) | PDGFB | [158] |
| Clinical Trials Identifier | Targeted Therapy | Mechanism of Action |
|---|---|---|
| NCT03351296 | Bevacizumab | Vascular endothelial growth factor (VEGF) inhibitor |
| NCT02259725 | Regorafenib | Multi kinase inhibitor |
| NCT05934331 | Toripalimab | Monoclonal antibody against programmed cell death protein 1 (PD-1) |
| NCT05580445 | ||
| NCT06111274 | ||
| NCT05862324 | Activated T-lymphocyte cell therapy | Anti-tumoural immunity activation |
| NCT04137536 | ||
| NCT03269526 | ||
| NCT02311361 | Tremelimumab | Monoclonal antibody against cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) |
| NCT02305810 | Everolimus | |
| NCT01088815 | Hedgehog inhibitor | Inhibitor of the Hedgehog pathway |
| NCT02501902 | Palbociclib | CDK4/6 inhibitor |
| NCT03558945 | neoantigen peptide-based vaccines | Vaccines |
| NCT04161755 | mRNA vaccines |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez, D.J.; Javed, A.; Rubio-Tomás, T.; Seye-Loum, N.; Barceló, C. Clinical and Preclinical Targeting of Oncogenic Pathways in PDAC: Targeted Therapeutic Approaches for the Deadliest Cancer. Int. J. Mol. Sci. 2024, 25, 2860. https://doi.org/10.3390/ijms25052860
Jiménez DJ, Javed A, Rubio-Tomás T, Seye-Loum N, Barceló C. Clinical and Preclinical Targeting of Oncogenic Pathways in PDAC: Targeted Therapeutic Approaches for the Deadliest Cancer. International Journal of Molecular Sciences. 2024; 25(5):2860. https://doi.org/10.3390/ijms25052860
Chicago/Turabian StyleJiménez, Diego J., Aadil Javed, Teresa Rubio-Tomás, Ndioba Seye-Loum, and Carles Barceló. 2024. "Clinical and Preclinical Targeting of Oncogenic Pathways in PDAC: Targeted Therapeutic Approaches for the Deadliest Cancer" International Journal of Molecular Sciences 25, no. 5: 2860. https://doi.org/10.3390/ijms25052860
APA StyleJiménez, D. J., Javed, A., Rubio-Tomás, T., Seye-Loum, N., & Barceló, C. (2024). Clinical and Preclinical Targeting of Oncogenic Pathways in PDAC: Targeted Therapeutic Approaches for the Deadliest Cancer. International Journal of Molecular Sciences, 25(5), 2860. https://doi.org/10.3390/ijms25052860







