Rapid and Convenient Single-Chain Variable Fragment-Employed Electrochemical C-Reactive Protein Detection System
Abstract
1. Introduction
2. Results
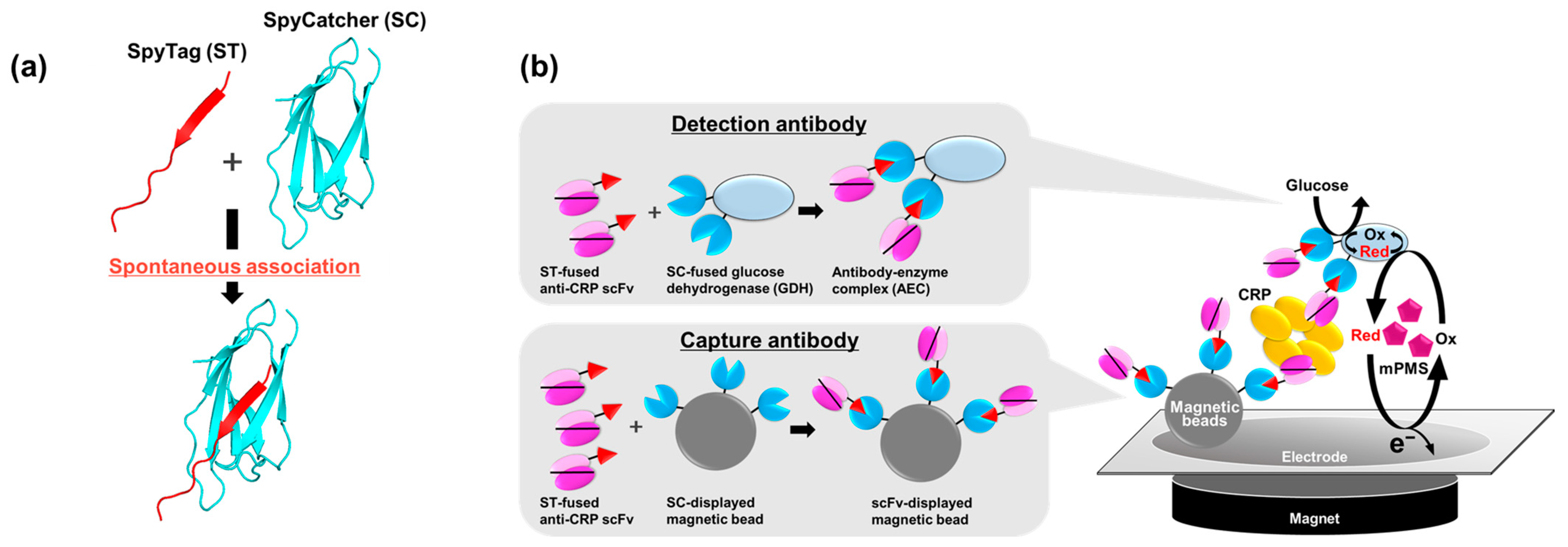
2.1. Preparation of Anti-CRP scFv and Anti-CRP scFv-SpyTag
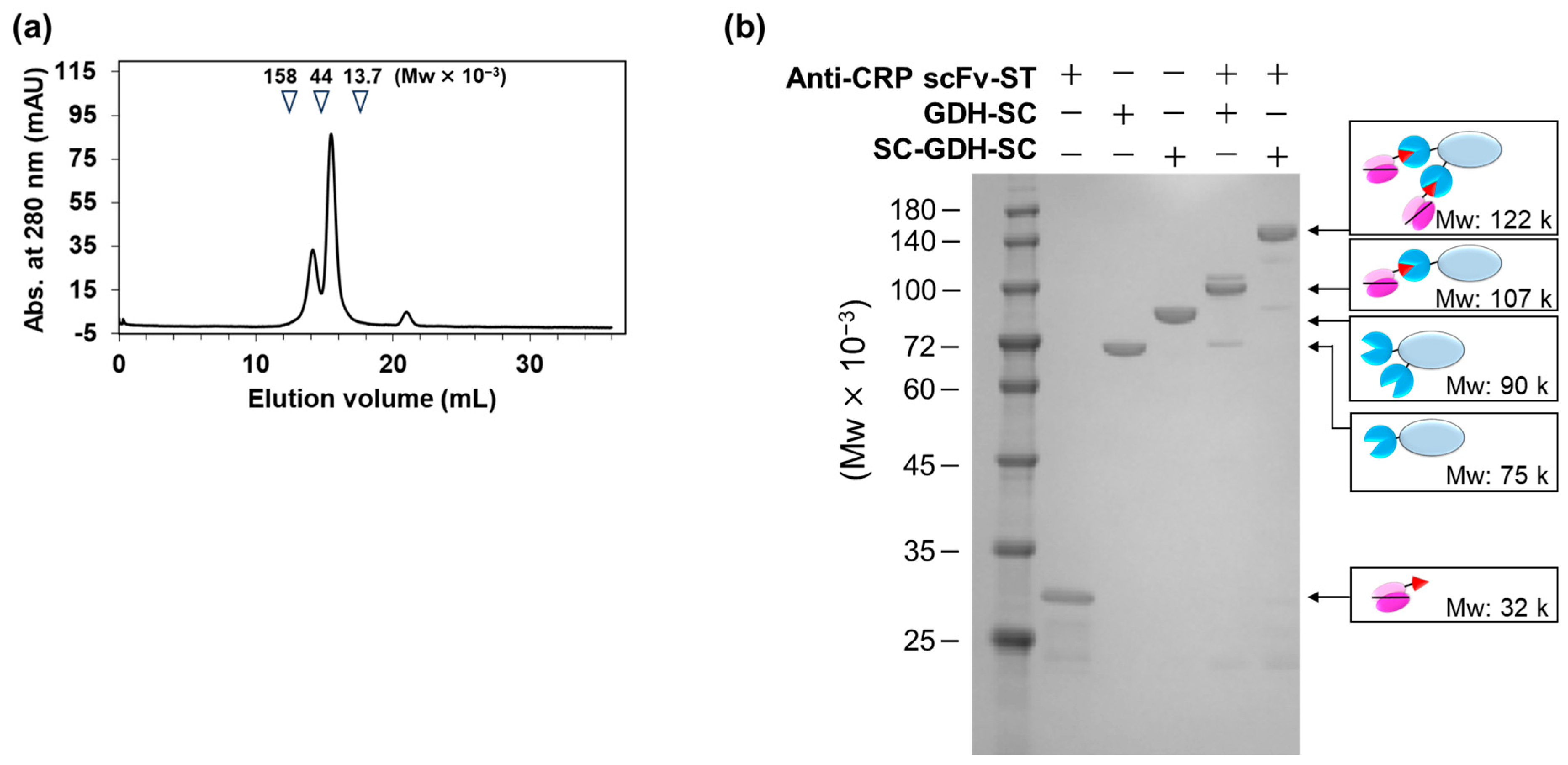
2.2. Fabrication of AECs
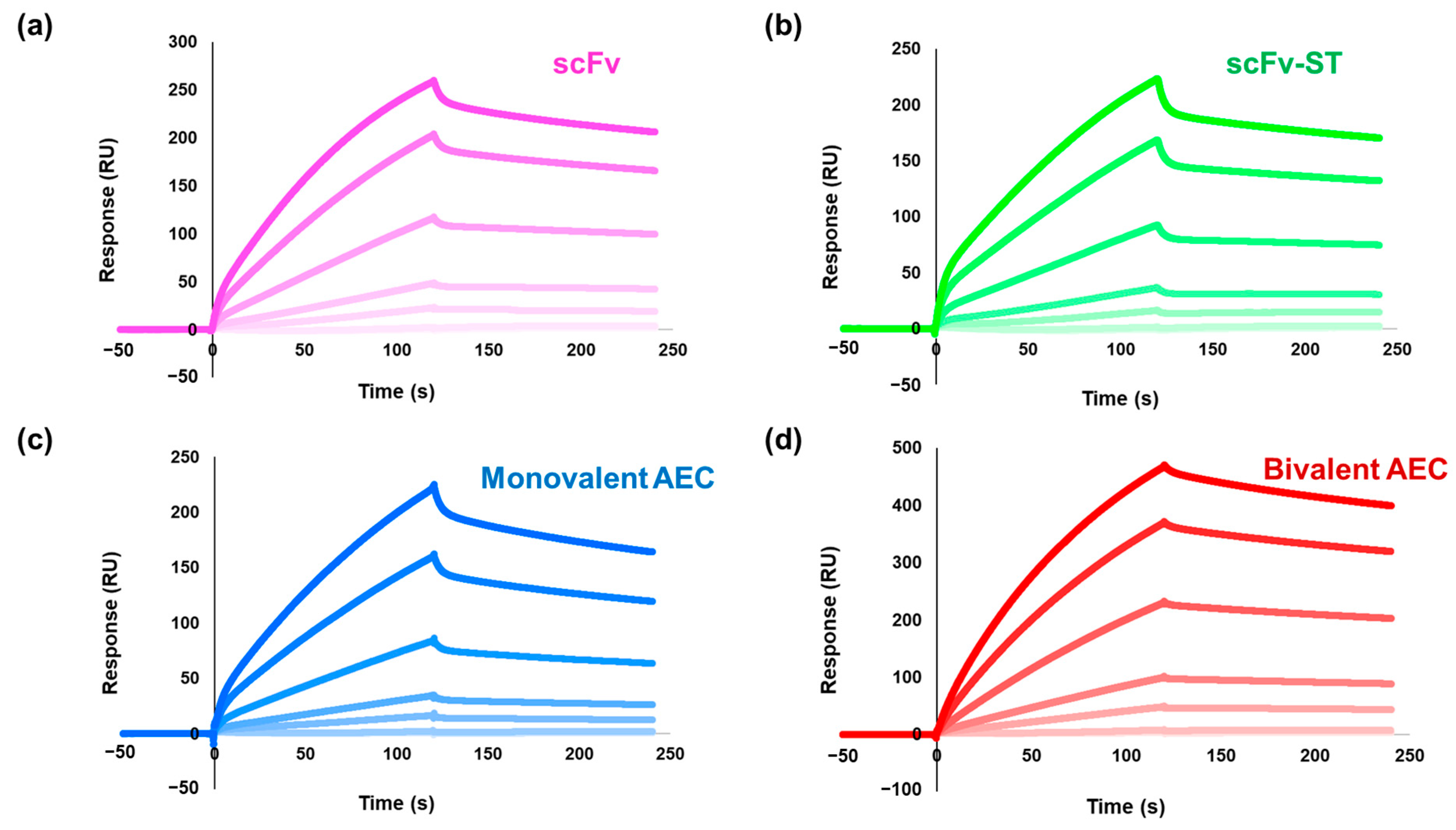
2.3. Affinity Analysis of the Antibodies by Surface Plasmon Resonance
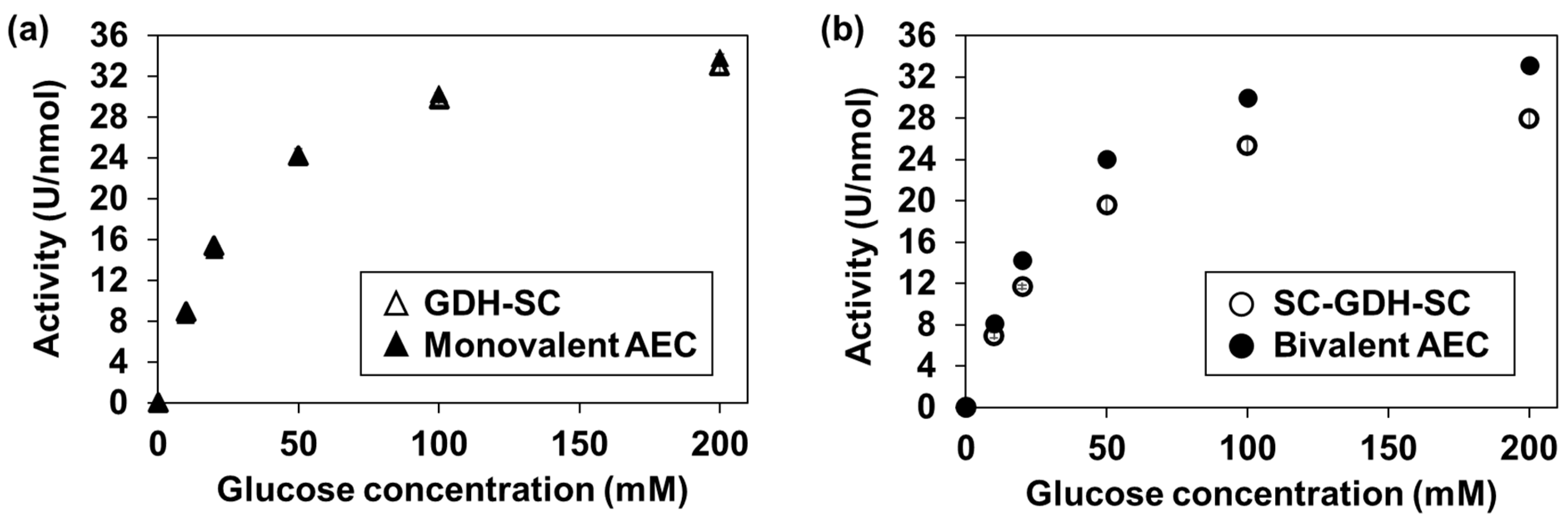
2.4. Glucose Dehydrogenase Activity Assay of the AECs
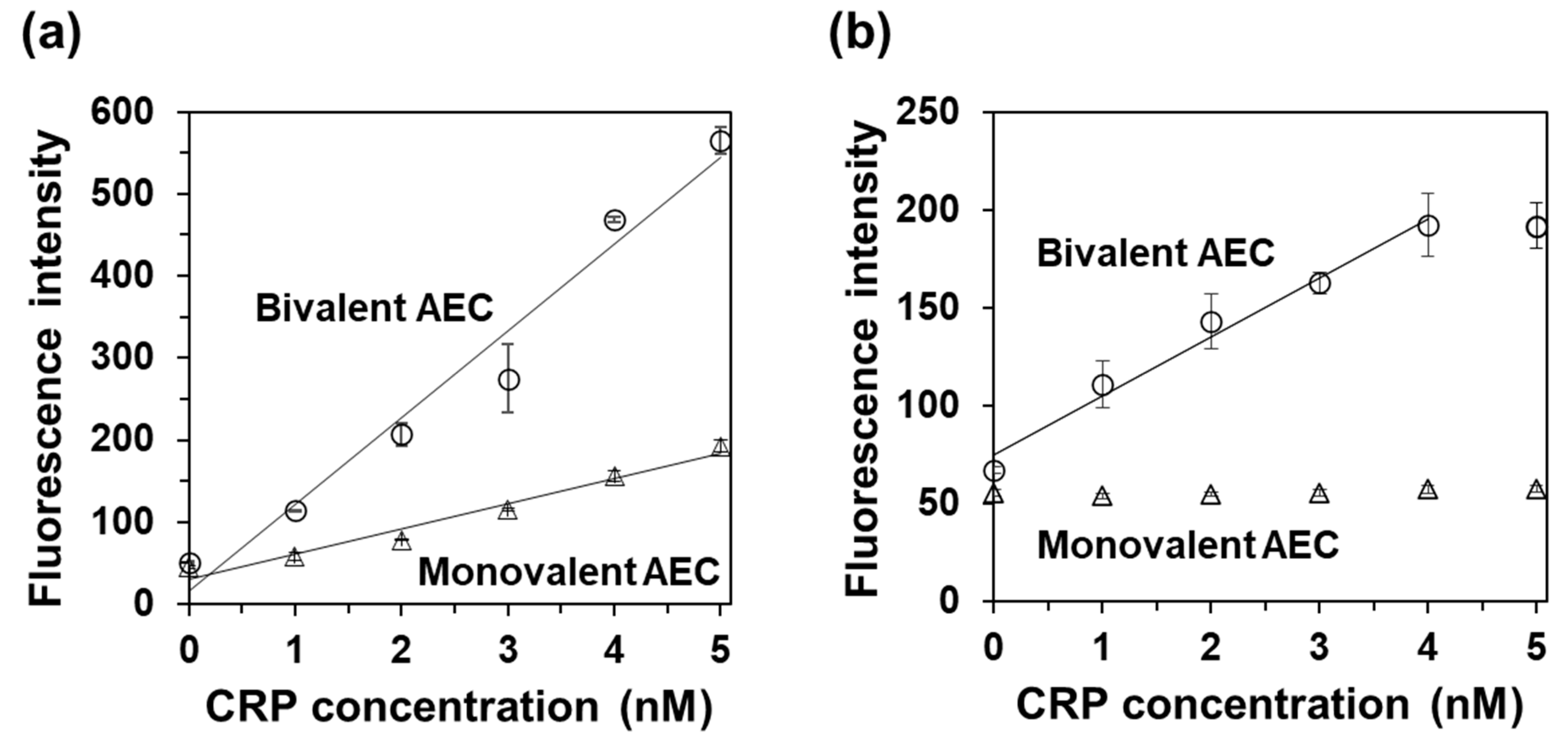
2.5. CRP Detection Using Bivalent AEC and Use of a Single scFv Clone as a Capture and Detection Antibody
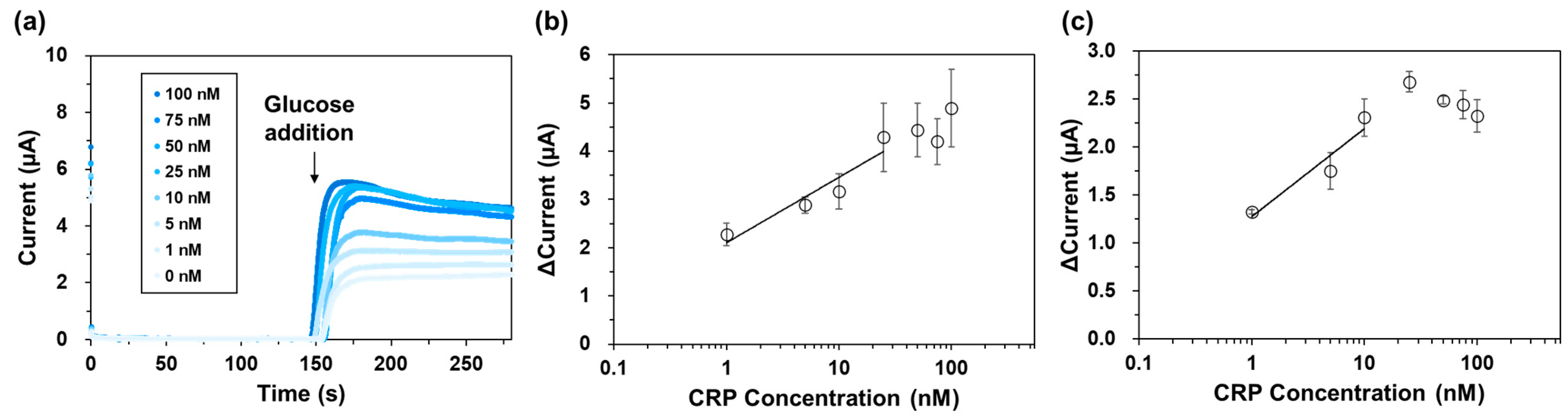
2.6. Electrochemical CRP Detection Using Magnetic Beads and the Bivalent AEC
3. Discussion
4. Materials and Methods
4.1. Construction of Expression Vectors
4.2. Recombinant Production of Proteins
4.3. Fabrication of AECs
4.4. Surface Plasmon Resonance (SPR) Analysis
4.5. Dehydrogenase Enzyme Activity Assay
4.6. Sandwich Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Electrochemical CRP Detection Using Magnetic Beads
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shulman, M.; Wilde, C.D.; Köhler, G. A better cell line for making hybridomas secreting specific antibodies. Nature 1978, 276, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.; Bradbury, A.R.M.; Knappik, A.; Plückthun, A.; Borrebaeck, C.A.K.; Dübel, S. Animal-free alternatives and the antibody iceberg. Nat. Biotechnol. 2020, 38, 1234–1239. [Google Scholar] [CrossRef]
- Kobayashi, N.; Iwakami, K.; Kotoshiba, S.; Niwa, T.; Kato, Y.; Mano, N.; Goto, J. Immunoenzymometric assay for a small molecule,11-deoxycortisol, with attomole-range sensitivity employing an scFv−enzyme fusion protein and anti-idiotype antibodies. Anal. Chem. 2006, 78, 2244–2253. [Google Scholar] [CrossRef] [PubMed]
- Koliasnikov, O.V.; Grigorenko, V.G.; Egorov, A.M.; Lange, S.; Schmid, R.D. Recombinant production of horseradish peroxidase conjugates with Fab antibodies in pichia pastoris for analytical applications. Acta Nat. 2011, 3, 85–92. [Google Scholar] [CrossRef][Green Version]
- Tian, B.; Wong, W.Y.; Hegmann, E.; Gaspar, K.; Kumar, P.; Chao, H. Production and characterization of a camelid single domain antibody-urease enzyme conjugate for the treatment of cancer. Bioconjug. Chem. 2015, 26, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Asano, R.; Tsukamoto, N.; Tsugawa, W.; Sode, K. Convenient and universal fabrication method for antibody-enzyme complexes as sensing elements using the SpyCatcher/SpyTag system. Anal. Chem. 2018, 90, 14500–14506. [Google Scholar] [CrossRef]
- Kimura, H.; Miura, D.; Tsugawa, W.; Ikebukuro, K.; Sode, K.; Asano, R. Rapid and homogeneous electrochemical detection by fabricating a high affinity bispecific antibody-enzyme complex using two Catcher/Tag systems. Biosens. Bioelectron. 2021, 175, 112885. [Google Scholar] [CrossRef]
- Miura, D.; Kimura, H.; Tsugawa, W.; Ikebukuro, K.; Sode, K.; Asano, R. Rapid, convenient, and highly sensitive detection of human hemoglobin in serum using a high-affinity bivalent antibody–enzyme complex. Talanta 2021, 234, 122638. [Google Scholar] [CrossRef]
- Kusnezow, W.; Hoheisel, J.D. Solid supports for microarray immunoassays. J. Mol. Recognit. 2003, 16, 165–176. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Mernaugh, R.L.; Zeng, X. Single chain fragment variable recombinant antibody functionalized gold nanoparticles for a highly sensitive colorimetric immunoassay. Biosens. Bioelectron. 2009, 24, 2853–2857. [Google Scholar] [CrossRef]
- Shen, Z.; Mernaugh, R.L.; Yan, H.; Yu, L.; Zhang, Y.; Zeng, X. Engineered recombinant single-chain fragment variable antibody for immunosensors. Anal. Chem. 2005, 77, 6834–6842. [Google Scholar] [CrossRef]
- Kumada, Y.; Hamasaki, K.; Shiritani, Y.; Nakagawa, A.; Kuroki, D.; Ohse, T.; Choi, D.H.; Katakura, Y.; Kishimoto, M. Direct immobilization of functional single-chain variable fragment antibodies (scFvs) onto a polystyrene plate by genetic fusion of a polystyrene-binding peptide (PS-tag). Anal. Bioanal. Chem. 2009, 395, 759–765. [Google Scholar] [CrossRef]
- Fatemi, F.; Amini, S.M.; Kharrazi, S.; Rasaee, M.J.; Mazlomi, M.A.; Asadi-Ghalehni, M.; Rajabibazl, M.; Sadroddiny, E. Construction of genetically engineered M13K07 helper phage for simultaneous phage display of gold binding peptide 1 and nuclear matrix protein 22 ScFv antibody. Colloids Surf. B Biointerfaces 2017, 159, 770–780. [Google Scholar] [CrossRef]
- Anderson, G.P.; Liu, J.L.; Shriver-Lake, L.C.; Zabetakis, D.; Sugiharto, V.A.; Chen, H.W.; Lee, C.R.; Defang, G.N.; Wu, S.L.; Venkateswaran, N.; et al. Oriented immobilization of single-domain antibodies using SpyTag/SpyCatcher yields improved limits of detection. Anal. Chem. 2019, 91, 9424–9429. [Google Scholar] [CrossRef]
- Saerens, D.; Huang, L.; Bonroy, K.; Muyldermans, S. Antibody fragments as probe in biosensor development. Sensors 2008, 8, 4669–4686. [Google Scholar] [CrossRef] [PubMed]
- Crivianu-Gaita, V.; Thompson, M. Aptamers, antibody scFv, and antibody Fab’ fragments: An overview and comparison of three of the most versatile biosensor biorecognition elements. Biosens. Bioelectron. 2016, 85, 32–45. [Google Scholar] [CrossRef]
- Syedmoradi, L.; Norton, M.L.; Omidfar, K. Point-of-care cancer diagnostic devices: From academic research to clinical translation. Talanta 2021, 225, 122002. [Google Scholar] [CrossRef]
- Clearfield, M.B. C-reactive protein: A new risk assessment tool for cardiovascular disease. J. Am. Osteopath. Assoc. 2005, 105, 409–416. [Google Scholar]
- Marshe, V.S.; Pira, S.; Mantere, O.; Bosche, B.; Looper, K.J.; Herrmann, N.; Müller, D.J.; Rej, S. C-reactive protein and cardiovascular risk in bipolar disorder patients: A systematic review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, N.A.; Keizer, E.; Plate, A.; Coenen, S.; Valeri, F.; Verbakel, J.Y.J.; Rosemann, T.; Neuner-Jehle, S.; Senn, O. Point-of-care C-reactive protein testing to reduce antibiotic prescribing for respiratory tract infections in primary care: Systematic review and meta-analysis of randomised controlled trials. Antibiotics 2020, 9, 610. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, E.J.; Conroy, P.J.; Hearty, S.; Keyes, T.E.; O’Kennedy, R.; Forster, R.J.; Dennany, L. Electrochemiluminescence platform for the detection of C-reactive proteins: Application of recombinant antibody technology to cardiac biomarker detection. RSC Adv. 2015, 5, 67874–67877. [Google Scholar] [CrossRef]
- Choi, D.H.; Katakura, Y.; Ninomiya, K.; Shioya, S. Rational screening of antibodies and design of sandwich enzyme linked immunosorbant assay on the basis of a kinetic model. J. Biosci. Bioeng. 2008, 105, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Hiraka, K.; Kojima, K.; Lin, C.E.; Tsugawa, W.; Asano, R.; La Belle, J.T.; Sode, K. Minimizing the effects of oxygen interference on L-lactate sensors by a single amino acid mutation in Aerococcus viridans L-lactate oxidase. Biosens. Bioelectron. 2018, 103, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, B.; Fierer, J.O.; Celik, E.; Chittock, E.C.; Schwarz-Linek, U.; Moy, V.T.; Howarth, M. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc. Nat. Acad. Sci. USA 2012, 109, E690–E697. [Google Scholar] [CrossRef] [PubMed]
- Sakai, G.; Kojima, K.; Mori, K.; Oonishi, Y.; Sode, K. Stabilization of fungi-derived recombinant FAD-dependent glucose dehydrogenase by introducing a disulfide bond. Biotechnol. Lett. 2015, 37, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose sensing for diabetes monitoring: Recent developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G. Analytical evaluation of sensor measurements. Anal. Bioanal. Chem. 2018, 410, 5–13. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, L.; Huo, W.; Lian, J.; Jesorka, A.; Shi, X.; Gao, Y. Dynamic range expansion of the C-reactive protein quantification with a tandem giant magnetoresistance biosensor. ACS Omega 2021, 6, 12923–12930. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Freitas, A.C.; Amaral, J.P.; Rocha-Santos, T.A.P.; Cardoso, S.; Duarte, A.C. Disposable immunosensors for C-reactive protein based on carbon nanotubes field effect transistors. Talanta 2013, 108, 165–170. [Google Scholar] [CrossRef]
- Siddiqui, M.F.; Khan, Z.A.; Park, S. Detection of C-reactive protein using histag-HRP functionalized nanoconjugate with signal amplified immunoassay. Nanomaterials 2020, 10, 1240. [Google Scholar] [CrossRef]
- Panraksa, Y.; Apilux, A.; Jampasa, S.; Puthong, S.; Henry, C.S.; Rengpipat, S.; Chailapakul, O. A facile one-step gold nanoparticles enhancement based on sequential patterned lateral flow immunoassay device for C-reactive protein detection. Sens. And Actuators B 2021, 329, 129241. [Google Scholar] [CrossRef]
- Hong, D.; Kim, K.; Jo, E.J.; Kim, M.G. Electrochemiluminescence-incorporated lateral flow immunosensors using Ru(bpy)32+-labeled gold nanoparticles for the full-range detection of physiological C-reactive protein levels. Anal. Chem. 2021, 93, 7925–7932. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.Y.; Shin, Y.B.; Li, T.; Park, J.H.; Kim, D.M.; Choi, D.H.; Kim, M.G. The use of an engineered single chain variable fragment in a localized surface plasmon resonance method for analysis of the C-reactive protein. Chem. Commun. (Camb) 2013, 49, 9497–9499. [Google Scholar] [CrossRef] [PubMed]
- Kumada, Y.; Tanibata, R.; Yamamoto, K.; Noguchi, H.; Angelini, A.; Horiuchi, J.I. Development and characterization of a latex turbidimetric immunoassay using rabbit anti-CRP single-chain Fv antibodies. J. Immunol. Methods 2023, 520, 113522. [Google Scholar] [CrossRef]
- Oloketuyi, S.; Bernedo, R.; Christmann, A.; Borkowska, J.; Cazzaniga, G.; Schuchmann, H.W.; Niedziółka-Jönsson, J.; Szot-Karpińska, K.; Kolmar, H.; de Marco, A. Native llama nanobody library panning performed by phage and yeast display provides binders suitable for C-reactive protein detection. Biosensors 2021, 11, 496. [Google Scholar] [CrossRef]
- Nishiyama, K.; Fukuyama, M.; Maeki, M.; Ishida, A.; Tani, H.; Hibara, A.; Tokeshi, M. One-step non-competitive fluorescence polarization immunoassay based on a Fab fragment for C-reactive protein quantification. Sens. Actuators B 2021, 326, 128982. [Google Scholar] [CrossRef]
| kon (×105, M−1s−1) | koff (×10−3, s−1) | KD (×10−9, M) | Chi2 | |
|---|---|---|---|---|
| scFv | 3.5 ± 0.024 | 1.6 ± 0.0070 | 4.8 ± 0.039 | 2.2 |
| scFv-ST | 3.4 ± 0.061 | 1.7 ± 0.016 | 5.0 ± 0.10 | 3.9 |
| Monovalent AEC | 0.67 ± 0.0060 | 1.9 ± 0.0099 | 28.7 ± 0.29 | 2.4 |
| Bivalent AEC | 1.8 ± 0.011 | 1.1 ± 0.0040 | 6.2 ± 0.045 | 6.2 |
| KM (mM) | Vmax (U nmol−1) | |
|---|---|---|
| GDH-SC | 36 | 33 |
| Monovalent AEC | 34 | 39 |
| SD-GDH-SC | 30 | 38 |
| Bivalent AEC | 33 | 40 |
| Used Antibody Format | Detection Technique | Linear Range (µg/L) | LOD (µg/L) | Required Time | POCT Application | Comment | Ref. |
|---|---|---|---|---|---|---|---|
| IgG | ELISA | 0–2 (in serum) | 0.032 (in serum) | 3 h | Difficult | It is the same configuration of a conventional ELISA. | [30] |
| IgG | Lateral flow immunoassay | 100–5000 (in buffer) | 1 (in buffer) | 15 min | Possible | Detection was performed by naked eyes. | [31] |
| IgG | Lateral flow immunoassay with electrochemiluminescence | 0.01–1000 (in serum) | 4.6 × 10−3 (in serum) | 15 min | Possible | Electrochemiluminescence image analysis is required on paper-based sensor strip. | [32] |
| scFv (Cys mutant) | Localized surface plasmon resonance | 1–10,000 (in serum) | N.A. | N.A. | Difficult | A large scale and sophisticated instrument is required. | [33] |
| scFv | Turbidimetry | 0–2960 (in buffer) | N.A. | 5 min | Difficult | It was detected by a clinical laboratory scale analyzer. | [34] |
| scFv and Ru+-complex labeled scFv | Electrochemiluminescence | 0.005–600 (in buffer) | 3.0 × 10−6 (in buffer) | 30 min | Difficult | A large-scale instrument is still required. | [21] |
| VHH | Electrochemical impedance spectroscopy | 250–1000 (in buffer) | 210 (in buffer) | N.A. | Difficult | Washing procedures must be required. | [35] |
| Fluorescence Labeled Fab | Fluorescence polarization | N.A. | 207 (in buffer) | 10 min | Difficult | A sophisticated optical instrument is required. | [36] |
| scFv and AEC composed of scFv and GDH | Amperometry | 115–2870 (in buffer) 115–1150 (in serum) | 276 (in buffer) 333 (in serum) | 20 min | Possible | An established glucose sensor-like strip can be used. | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, D.; Motohashi, S.; Goto, A.; Kimura, H.; Tsugawa, W.; Sode, K.; Ikebukuro, K.; Asano, R. Rapid and Convenient Single-Chain Variable Fragment-Employed Electrochemical C-Reactive Protein Detection System. Int. J. Mol. Sci. 2024, 25, 2859. https://doi.org/10.3390/ijms25052859
Miura D, Motohashi S, Goto A, Kimura H, Tsugawa W, Sode K, Ikebukuro K, Asano R. Rapid and Convenient Single-Chain Variable Fragment-Employed Electrochemical C-Reactive Protein Detection System. International Journal of Molecular Sciences. 2024; 25(5):2859. https://doi.org/10.3390/ijms25052859
Chicago/Turabian StyleMiura, Daimei, Saki Motohashi, Ayaka Goto, Hayato Kimura, Wakako Tsugawa, Koji Sode, Kazunori Ikebukuro, and Ryutaro Asano. 2024. "Rapid and Convenient Single-Chain Variable Fragment-Employed Electrochemical C-Reactive Protein Detection System" International Journal of Molecular Sciences 25, no. 5: 2859. https://doi.org/10.3390/ijms25052859
APA StyleMiura, D., Motohashi, S., Goto, A., Kimura, H., Tsugawa, W., Sode, K., Ikebukuro, K., & Asano, R. (2024). Rapid and Convenient Single-Chain Variable Fragment-Employed Electrochemical C-Reactive Protein Detection System. International Journal of Molecular Sciences, 25(5), 2859. https://doi.org/10.3390/ijms25052859









