Piper sarmentosum Roxb. Inhibits Angiotensin-Converting Enzyme Activity in Phorbol 12-Myristate-13-Acetate-Induced Endothelial Cells
Abstract
1. Introduction
2. Results
2.1. LC-MS Analysis of AEPS
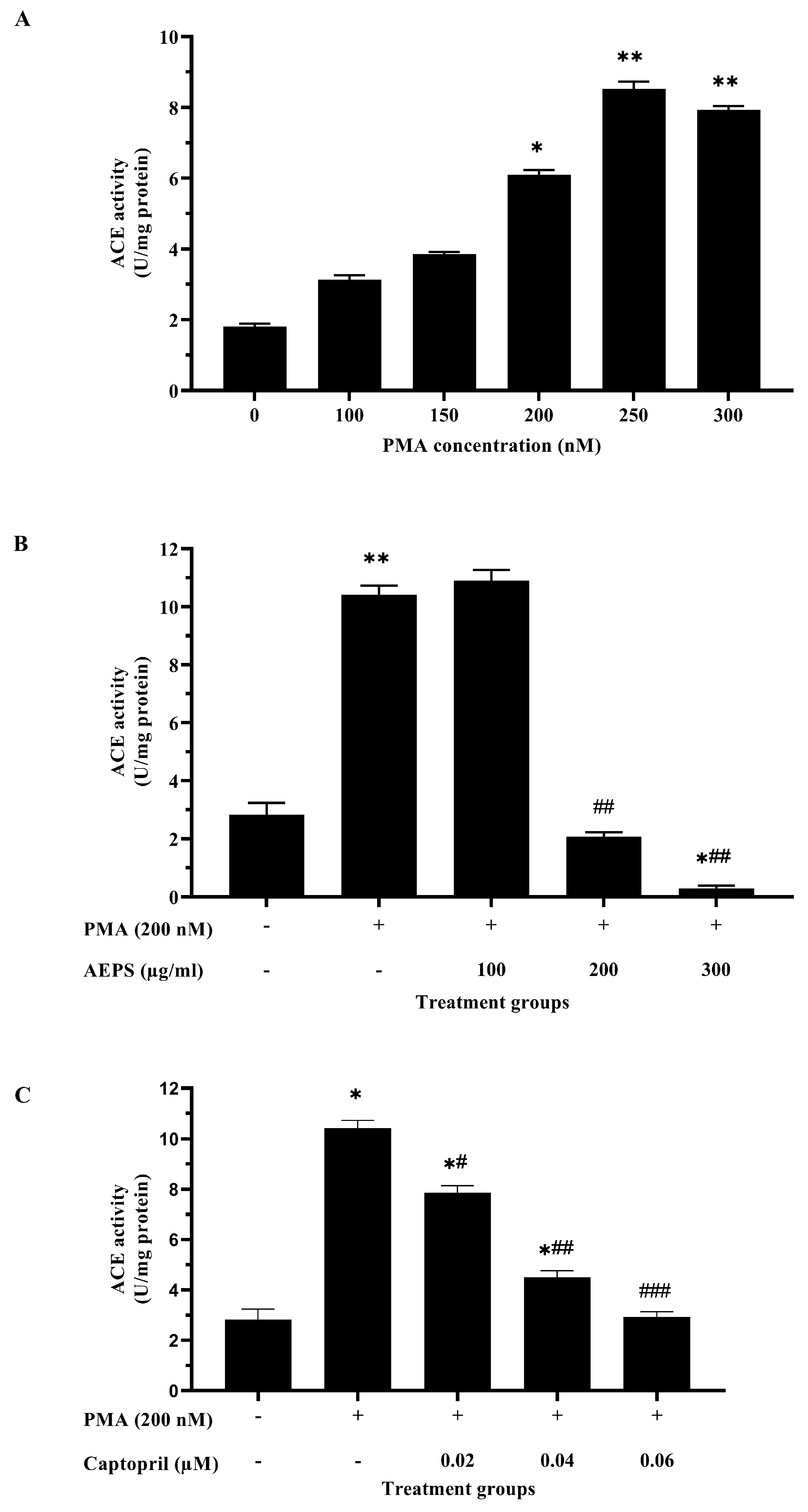
2.2. Determination of the Optimum Concentrations of PMA, AEPS, and Captopril for HUVEC Treatments
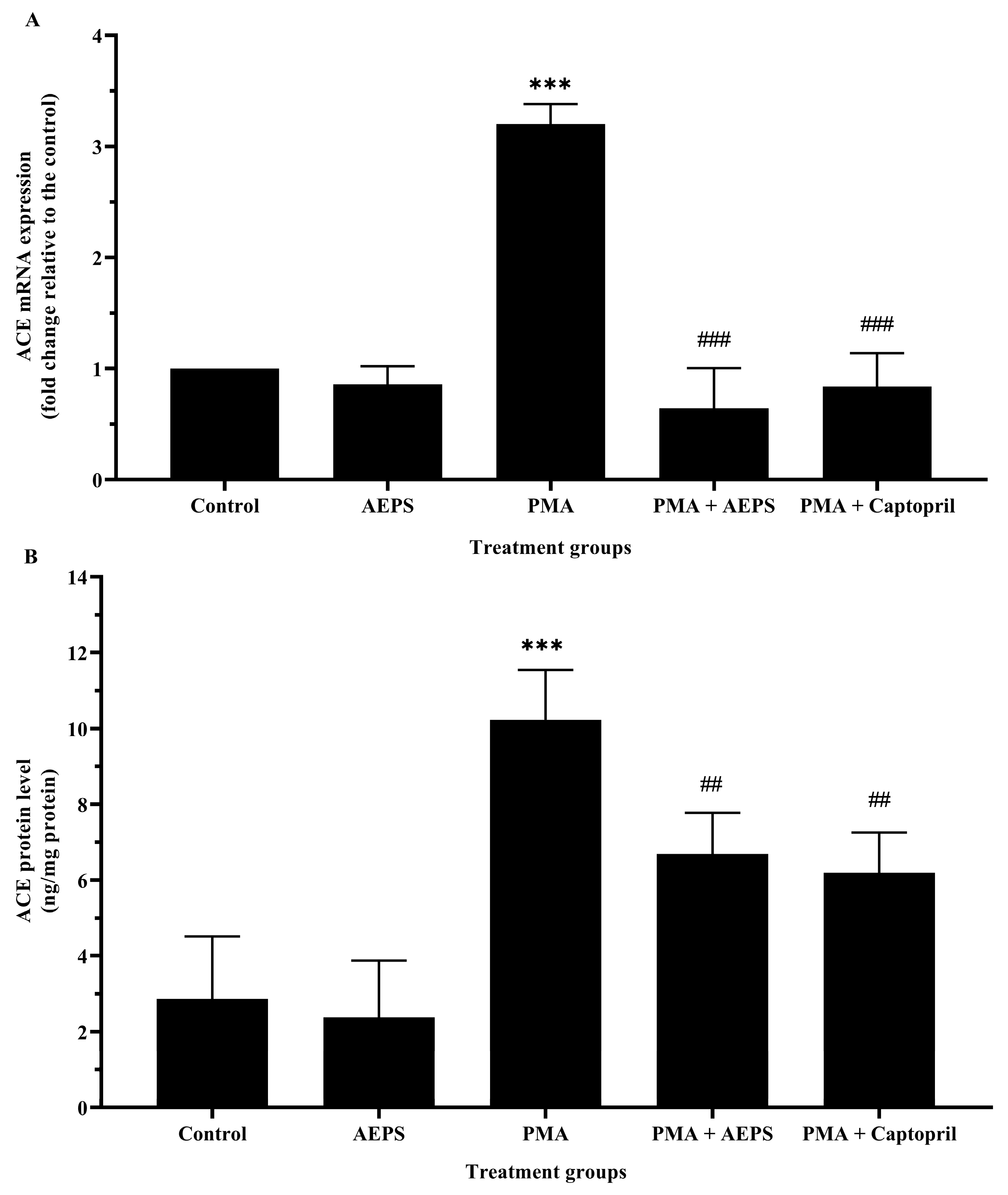
2.3. Effect of AEPS on ACE mRNA Expression and Protein Levels in PMA-Induced HUVEC
2.4. Effect of AEPS on ACE mRNA Expression and Protein Levels in PMA-Induced HUVEC
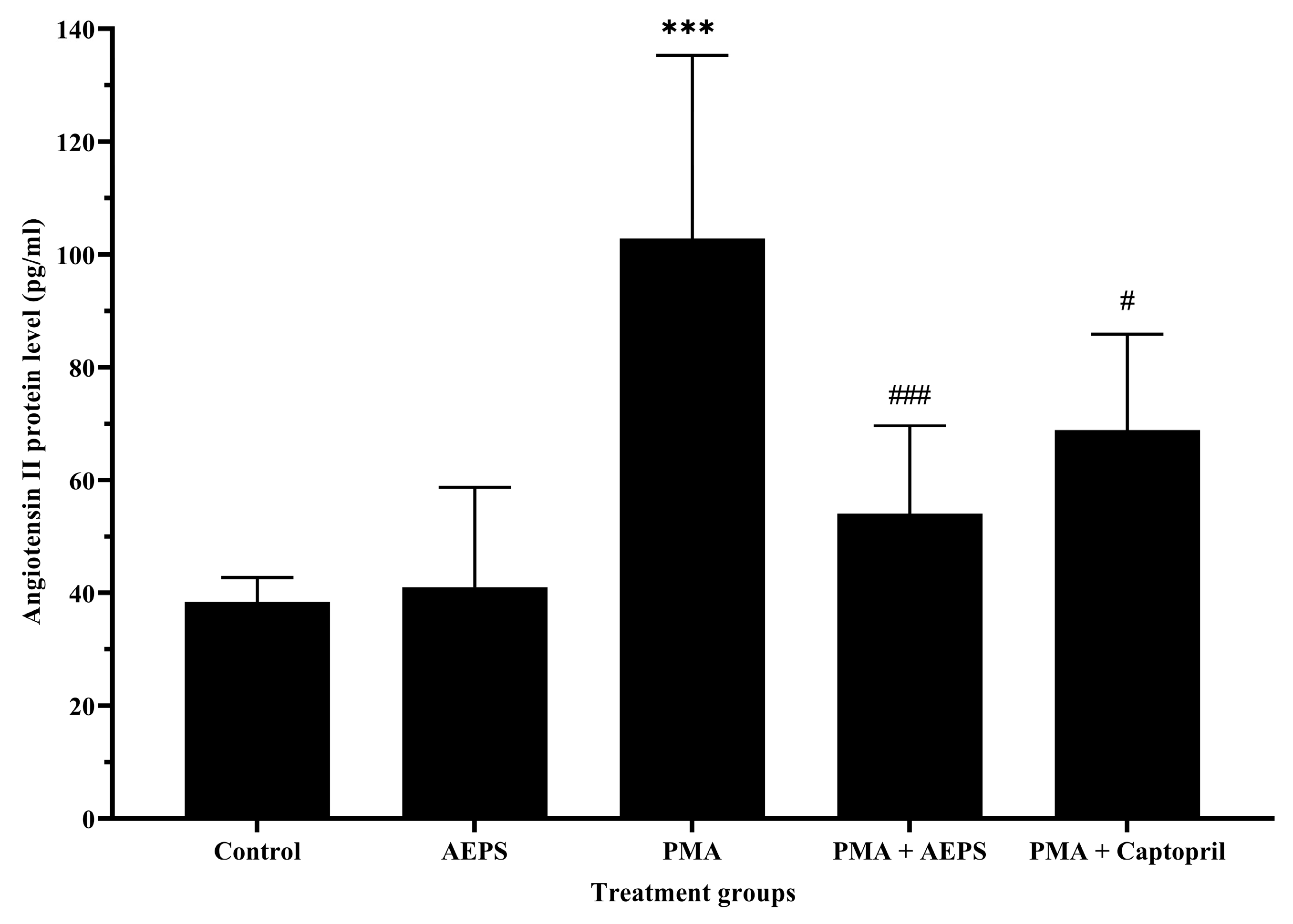
2.5. Effect of AEPS on Angiotensin II Protein Levels in PMA-Induced HUVEC
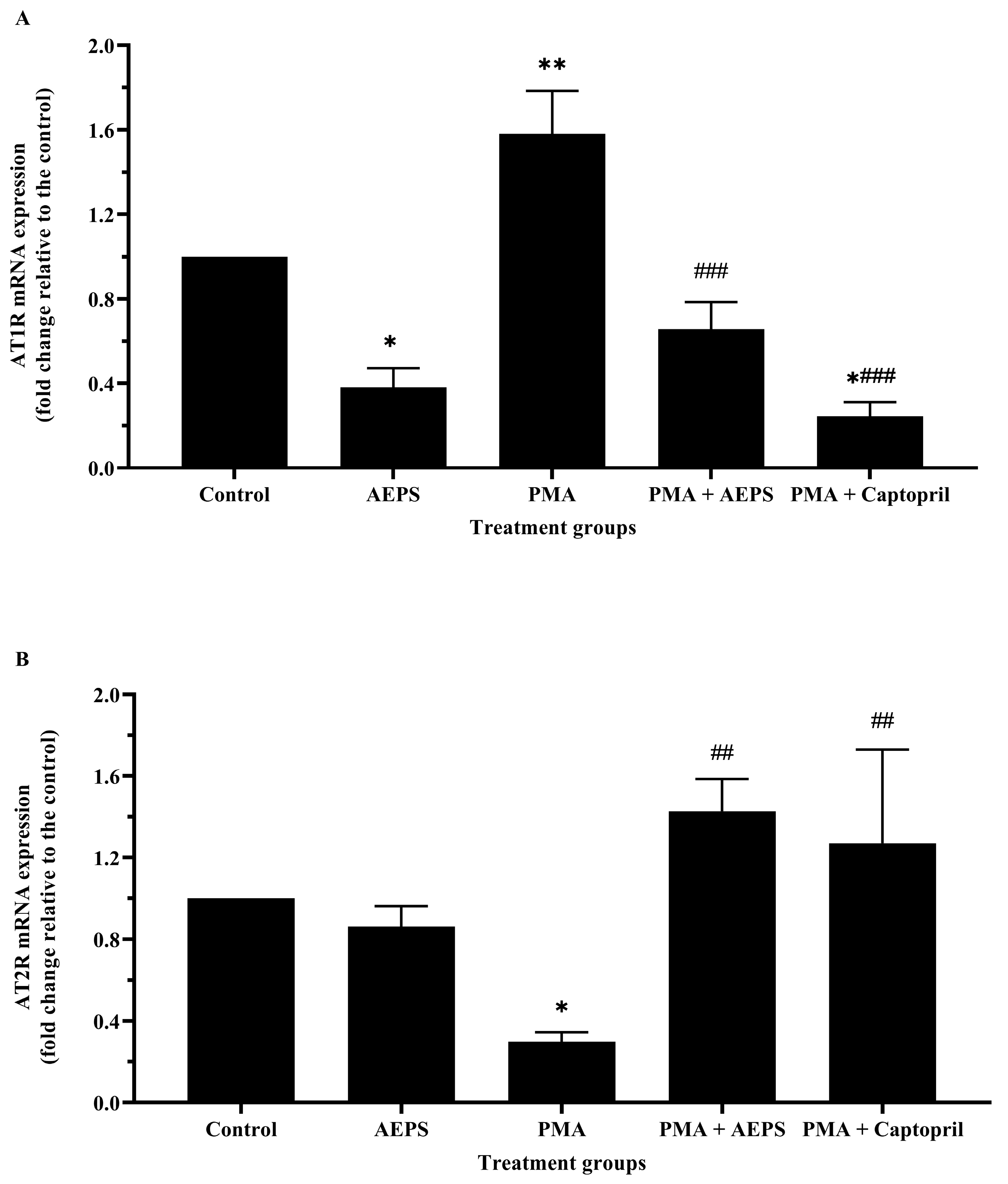
2.6. Effect of AEPS on AT1R and AT2R mRNA Expression in PMA-Induced HUVEC
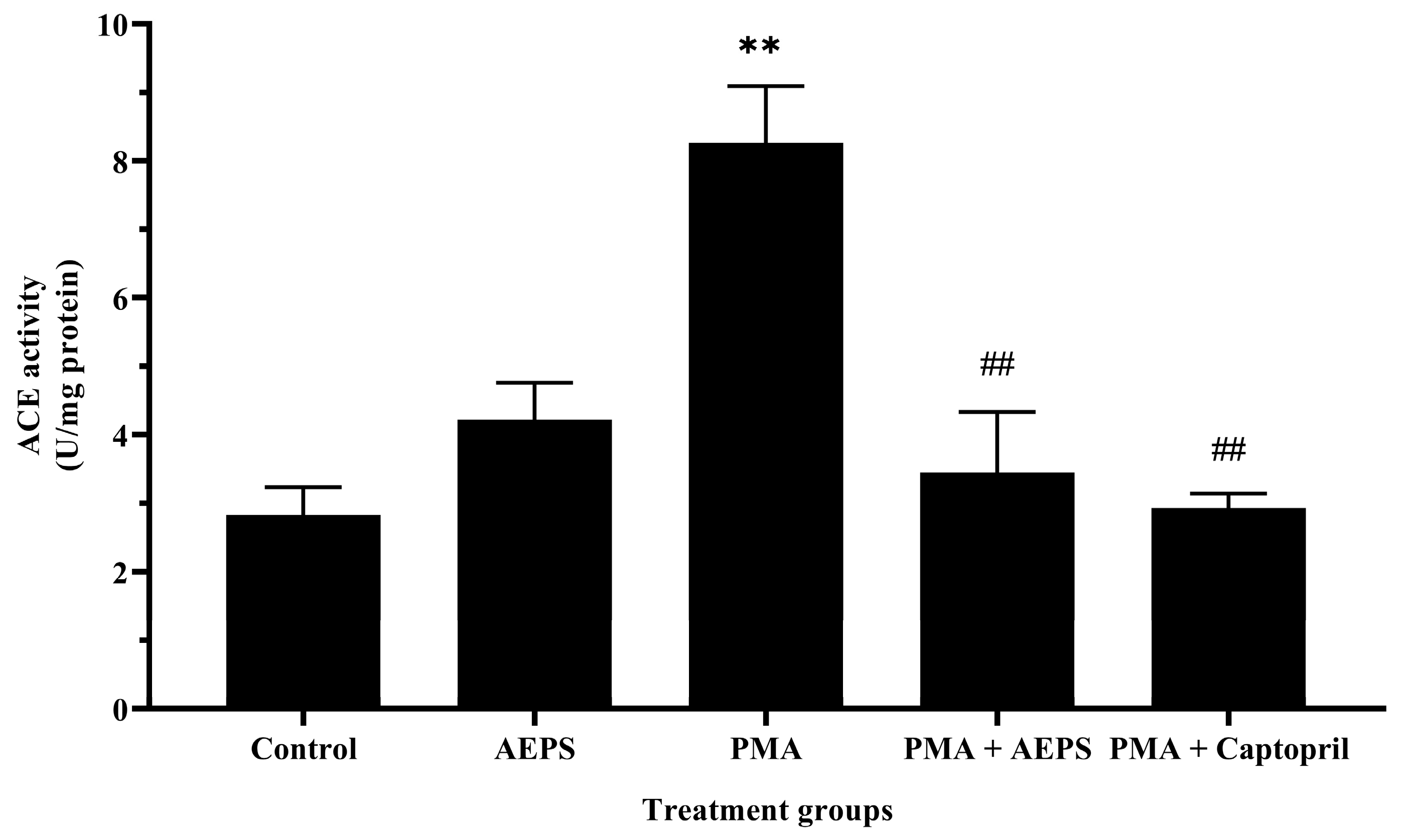
2.7. Effect of AEPS on Ex Vivo Aortic Contraction to PMA
3. Discussion
4. Materials and Methods
4.1. Aqueous Extract of P. sarmentosum Leaf Preparation
4.2. Liquid Chromatography (LC)-Mass Spectrometry (MS) Analysis of AEPS
4.3. HUVEC Isolation and Culture
4.4. Measurement of ACE Activity
4.5. Study Protocol
4.6. Measurement of ACE, AT1R, and AT2R mRNA Expression Using Quantitative Real Time Polymerase Chain Reaction (qPCR)
4.7. Determination of the ACE and Ang II Protein Levels
4.8. Ex Vivo Aortic Ring Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global Burden of Cardiovascular Diseases and Risk Factors. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155,722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef]
- Loperena, R.; Harrison, D.G. Oxidative Stress and Hypertensive Diseases. Med. Clin. N. Am. 2017, 101, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Đambić, V.; Pojatić, Đ.; Stažić, A.; Kibel, A. Significance of the Renin-Angiotensin System in Clinical Conditions; IntechOpen: London, UK, 2020. [Google Scholar]
- Liao, W.; Wu, J. The ACE2/Ang (1–7)/MasR axis as an emerging target for antihypertensive peptides. Crit. Rev. Food Sci. Nutr. 2021, 61, 2572–2586. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed]
- Gianzo, M.; Urizar-Arenaza, I.; Muñoa-Hoyos, I.; Larreategui, Z.; Garrido, N.; Casis, L.; Irazusta, J.; Subirán, N. Human sperm testicular angiotensin-converting enzyme helps determine human embryo quality. Asian J. Androl. 2018, 20, 498–504. [Google Scholar]
- Singh, K.D.; Karnik, S.S. Angiotensin Receptors: Structure, Function, Signaling and Clinical Applications. J. Cell Signal. 2016, 1, 111. [Google Scholar] [PubMed]
- Jukic, I.; Mihaljevic, Z.; Matic, A.; Mihalj, M.; Kozina, N.; Selthofer-Relatic, K.; Mihaljevic, D.; Koller, A.; Tartaro Bujak, I.; Drenjancevic, I. Angiotensin II type 1 receptor is involved in flow-induced vasomotor responses of isolated middle cerebral arteries: Role of oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1609–H1624. [Google Scholar] [CrossRef]
- Ranjit, A.; Khajehpour, S.; Aghazadeh-Habashi, A. Update on Angiotensin II Subtype 2 Receptor: Focus on Peptide and Nonpeptide Agonists. Mol. Pharmacol. 2021, 99, 469–487. [Google Scholar] [CrossRef]
- Colin, M.; Delaitre, C.; Foulquier, S.; Dupuis, F. The AT1/AT2 Receptor Equilibrium Is a Cornerstone of the Regulation of the Renin Angiotensin System beyond the Cardiovascular System. Molecules 2023, 28, 5481. [Google Scholar] [CrossRef]
- Ahmad, I.; Yanuar, A.; Mulia, K.; Mun’im, A. Review of Angiotensin-converting Enzyme Inhibitory Assay: Rapid Method in Drug Discovery of Herbal Plants. Pharmacogn. Rev. 2017, 11, 1–7. [Google Scholar]
- Khan, Z.; Shen, X.Z.; Bernstein, E.A.; Giani, J.F.; Eriguchi, M.; Zhao, T.V.; Gonzalez-Villalobos, R.A.; Fuchs, S.; Liu, G.Y.; Bernstein, K.E. Angiotensin-converting enzyme enhances the oxidative response and bactericidal activity of neutrophils. Blood 2017, 130, 328–339. [Google Scholar] [CrossRef]
- Damascena, H.L.; Silveira, W.A.A.; Castro, M.S.; Fontes, W. Neutrophil Activated by the Famous and Potent PMA (Phorbol Myristate Acetate). Cells 2022, 11, 2889. [Google Scholar] [CrossRef]
- Ringvold, H.C.; Khalil, R.A. Protein Kinase C as Regulator of Vascular Smooth Muscle Function and Potential Target in Vascular Disorders. Adv. Pharmacol. 2017, 78, 203–301. [Google Scholar]
- Chen, R.; Suchard, M.A.; Krumholz, H.M.; Schuemie, M.J.; Shea, S.; Duke, J.; Pratt, N.; Reich, C.G.; Madigan, D.; You, S.C.; et al. Comparative First-Line Effectiveness and Safety of ACE (Angiotensin Converting–Enzyme) Inhibitors and Angiotensin Receptor Blockers: A Multinational Cohort Study. Hypertension 2021, 78, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; Jahan, N.; Rahman, K.U.; Sultana, B.; Jamil, S. Identification of Hypotensive Biofunctional Compounds of Coriandrum sativum and Evaluation of Their Angiotensin-Converting Enzyme (ACE) Inhibition Potential. Oxid. Med. Cell. Longev. 2018, 2018, 4643736. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Zulkiflee, N.F.I.; Mohd Hasali, N.H. A Comprehensive Review on the Phytochemical Constituents, Antioxidant and Anticancer Properties of Piper sarmentosum. J. Pharm. 2022, 2, 107–121. [Google Scholar]
- Othman, N.S.; Che Roos, N.A.; Aminuddin, A.; Murthy, J.K.; Hamid, A.A.; Ugusman, A. Effects of Piper sarmentosum Roxb. on hypertension and diabetes mellitus: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 976247. [Google Scholar] [CrossRef]
- Mohd Zainudin, M.; Zakaria, Z.; Megat Mohd Nordin, N.A.; Othman, F. Does Oral Ingestion of Piper sarmentosum Cause Toxicity in Experimental Animals? Evid. Based Complement. Alternat. Med. 2013, 2013, 705950. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, W.; Dai, W.; Xin, H.; Rahmand, K.; Wang, Y.; Zhang, J.; Zhang, S.; Xu, L.; Han, T. Piper sarmentosum Roxb.: A review on its botany, traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol. 2020, 263, 112897. [Google Scholar] [CrossRef] [PubMed]
- Ware, I.; Franke, K.; Dube, M.; Ali El Enshasy, H.; Wessjohann, L.A. Characterization and Bioactive Potential of Secondary Metabolites Isolated from Piper sarmentosum Roxb. Int. J. Mol. Sci. 2023, 24, 1328. [Google Scholar] [CrossRef]
- Rahman, S.F.S.A.; Sijam, K.; Omar, D. Piper sarmentosum Roxb.: A Mini Review of Ethnobotany, Phytochemistry and Pharmacology. J. Anal. Pharm. Res. 2016, 2, 00031. [Google Scholar]
- Ugusman, A.; Zakaria, Z.; Hui, C.K.; Nordin, N.A.; Mahdy, Z.A. Flavonoids of Piper sarmentosum and its cytoprotective effects against oxidative stress. EXCLI J. 2012, 11, 705–714. [Google Scholar] [PubMed]
- Ismail, S.I.; Chua, K.H.; Aminuddin, A.; Ugusman, A. Piper sarmentosum as an Antioxidant: A Systematic Review. Sains Malays. 2018, 47, 2359–2368. [Google Scholar] [CrossRef]
- Ekeuku, S.O.; Nur Azlina, M.F.; Chin, K.Y. Effects of Piper sarmentosum on Metabolic Syndrome and Its Related Complications: A Review of Preclinical Evidence. Appl. Sci. 2021, 11, 9860. [Google Scholar] [CrossRef]
- Amran, A.A.; Zakaria, Z.; Othman, F.; Das, S.; Raj, S.; Nordin, N.A. Aqueous extract of Piper sarmentosum decreases atherosclerotic lesions in high cholesterolemic experimental rabbits. Lipids Health Dis. 2010, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zainudin, M.; Zakaria, Z.; Megat Mohd Nordin, N.A. The use of Piper sarmentosum leaves aqueous extract (KadukmyTM) as antihypertensive agent in spontaneous hypertensive rats. BMC Complement. Altern. Med. 2015, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zainudin, M.; Elshami, T.F.T.; Ismawi, H.R.; Hashim Fauzy, F.; Abdul Razak, T. Factors regulating nitric oxide production in spontaneously hypertensive rats treated with Piper sarmentosum aqueous extract. IIUM Med. J. Malays. 2019, 18, 1–7. [Google Scholar]
- Fauzy, F.H.; Zainudin, M.M.; Ismawi, H.R.; Elshami, T.F.T. Piper sarmentosum leaves aqueous extract attenuates vascular endothelial dysfunction in spontaneously hypertensive rats. Evid. Based Complement. Altern. Med. 2019, 2019, 7198592. [Google Scholar]
- Firdaus Azmi, M.; Aminuddin, A.; Azdina Jamal, J.; Hamid, A.A.; Ugusman, A. Quantified Piper sarmentosum Roxb. Leaves aqueous leaf extract and its antihypertensive effect in dexamethasone-induced hypertensive rats. Sains Malays. 2021, 50, 171–179. [Google Scholar] [CrossRef]
- Ugusman, A.; Md Fadze, N.; Hamid, A.A.; Asmawi, Z.; Aminuddin, A. Piper sarmentosum attenuates dexamethasone-induced hypertension by stimulating endothelial nitric oxide synthase. J. Res. Pharm. 2020, 24, 1–9. [Google Scholar] [CrossRef]
- Alwi, N.A.N.M.; Zakaria, Z.; Karim, A.A.H.; Nordin, N.A.M.M.; Ugusman, A. Antihypertensive effect of Piper sarmentosum in L-NAME-induced hypertensive rats. Sains Malays. 2018, 47, 2421–2428. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y.; Zhao, X.; Gong, W.; Wang, Y.; Cheng, Y. A novel aggregation-induced emission based fluorescent probe for an angiotensin converting enzyme (ACE) assay and inhibitor screening. Chem. Commun. 2014, 50, 15075–15078. [Google Scholar] [CrossRef] [PubMed]
- Villard, E.; Alonso, A.; Agrapart, M.; Challah, M.; Soubrier, F. Induction of angiotensin I-converting enzyme transcription by a protein kinase C-dependent mechanism in human endothelial cells. J. Biol. Chem. 1998, 273, 25191–25197. [Google Scholar] [CrossRef]
- Eyries, M.; Agrapart, M.; Alonso, A.; Soubrier, F. Phorbol ester induction of angiotensin-converting enzyme transcription is mediated by Egr-1 and AP-1 in human endothelial cells via ERK1/2 pathway. Circ. Res. 2002, 91, 899–906. [Google Scholar] [CrossRef]
- Vargas, R.A.V.; Varela Millán, J.M.; Fajardo Bonilla, E. Renin-angiotensin system: Basic and clinical aspects—A general perspective. Endocrinol. Diabetes Nutr. 2022, 69, 52–62. [Google Scholar] [CrossRef]
- Santos, R.A.S.; Oudit, G.Y.; Verano-Braga, T.; Canta, G.; Steckelings, U.M.; Bader, M. The renin-angiotensin system: Going beyond the classical paradigms. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H958–H970. [Google Scholar] [CrossRef]
- Bird, I.M.; Mason, J.I.; Rainey, W.E. Hormonal regulation of angiotensin II type 1 receptor expression and AT1-R mRNA levels in human adrenocortical cells. Endocr. Res. 1995, 21, 169–182. [Google Scholar] [CrossRef]
- Ugusman, A.; Zakaria, Z.; Hui, C.K.; Nordin, N.A. Piper sarmentosum increases nitric oxide production in oxidative stress: A study on human umbilical vein endothelial cells. Clinics 2010, 65, 709–714. [Google Scholar] [CrossRef]
- Messerli, F.H.; Bangalore, S.; Bavishi, C.; Rimoldi, S.F. Angiotensin-Converting Enzyme Inhibitors in Hypertension: To Use or Not to Use? J. Am. Coll. Cardiol. 2018, 71, 1474–1482. [Google Scholar] [CrossRef]
- Hettihewa, S.K.; Hemar, Y.; Rupasinghe, H.P.V. Flavonoid-Rich Extract of Actinidia macrosperma (A Wild Kiwifruit) Inhibits Angiotensin-Converting Enzyme In Vitro. Foods 2018, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.K.; Mukherjee, P.K.; Maiti, N.; Kumar, A.D.; Bhadra, S.; Saha, B.P. Evaluation of Angiotensin converting enzyme inhibition and anti-oxidant activity of Piper longum L. Indian J. Tradit. Knowl. 2013, 12, 478–482. [Google Scholar]
- Taqvi, S.I.; Shah, A.J.; Gilani, A.H. Blood pressure lowering and vasomodulator effects of piperine. J. Cardiovasc. Pharmacol. 2008, 52, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, R.; Cheng, X.; Yang, J.; Yang, Y.; Qu, H.; Li, S.; Lin, S.; Wei, D.; Bai, Y.; et al. Chemical constituents from the fruits of Piper longum L. and their vascular relaxation effect on rat mesenteric arteries. Nat. Prod. Res. 2022, 36, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Sundar, U.M.; Ugusman, A.; Chua, H.K.; Latip, J.; Aminuddin, A. Piper sarmentosum Promotes Endothelial Nitric Oxide Production by Reducing Asymmetric Dimethylarginine in Tumor Necrosis Factor-α-Induced Human Umbilical Vein Endothelial Cells. Front. Pharmacol. 2019, 10, 1033. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Kalisz, J.; Fornal, E. The Effects of Quercetin Supplementation on Blood Pressure–Meta-Analysis. Curr. Probl. Cardiol. 2022, 47, 101350. [Google Scholar] [CrossRef] [PubMed]
- Oyagbemi, A.A.; Bolaji-Alabi, F.B.; Ajibade, T.O.; Adejumobi, O.A.; Ajani, O.S.; Jarikre, T.A.; Omobowale, T.O.; Ola-Davies, O.E.; Soetan, K.O.; Aro, A.O.; et al. Novel antihypertensive action of rutin is mediated via inhibition of angiotensin converting enzyme/mineralocorticoid receptor/angiotensin 2 type 1 receptor (ATR1) signaling pathways in uninephrectomized hypertensive rats. J. Food Biochem. 2020, 44, e13534. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Fu, L.; Li, C.Y.; Xu, L.P.; Zhang, L.X.; Zhang, W.M. Quercetin, Hyperin, and Chlorogenic Acid Improve Endothelial Function by Antioxidant, Antiinflammatory, and ACE Inhibitory Effects. J. Food Sci. 2017, 82, 1239–1246. [Google Scholar] [CrossRef]
- Terao, J. Factors modulating bioavailability of quercetin-related flavonoids and the consequences of their vascular function. Biochem. Pharmacol. 2017, 139, 15–23. [Google Scholar] [CrossRef]
- Georgiou, N.; Kakava, M.G.; Routsi, E.A.; Petsas, E.; Stavridis, N.; Freris, C.; Zoupanou, N.; Moschovou, K.; Kiriakidi, S.; Mavromoustakos, T. Quercetin: A Potential Polydynamic Drug. Molecules 2023, 28, 8141. [Google Scholar] [CrossRef]
- Abd Rami, A.Z.; Aminuddin, A.; Hamid, A.A.; Mokhtar, M.H.; Ugusman, A. Nicotine Impairs the Anti-Contractile Function of Perivascular Adipose Tissue by Inhibiting the PPARγ–Adiponectin–AdipoR1 Axis. Int. J. Mol. Sci. 2023, 24, 15100. [Google Scholar] [CrossRef] [PubMed]
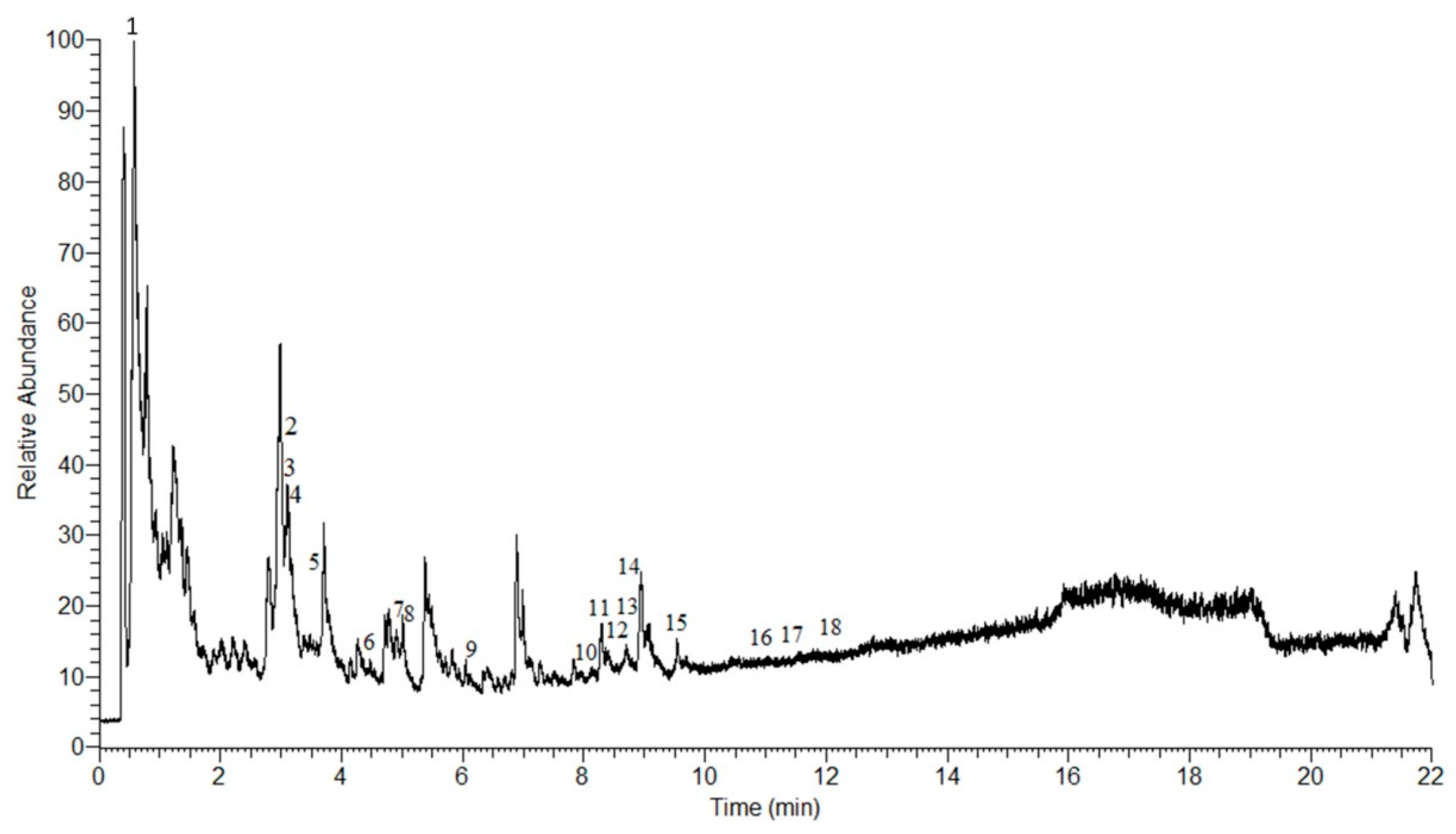

| No. | Retention Time (min) | m/z | Molecular Weight | Molecular Formula | Proposed Compound | Class |
|---|---|---|---|---|---|---|
| 1 | 0.70 | 755.20 | 756.21 | C33H40O20 | Quercetin 3-rhamninoside | Flavonoid |
| 2 | 3.14 | 167.03 | 166.02 | C8H6O4 | Piperonylic acid | Organic acid |
| 3 | 3.58 | 225.08 | 224.07 | C10H12N2O4 | 2,4,5-Trimethoxybenzaldehyde hydrazone N-oxide | Hydrazone |
| 4 | 3.65 | 304.11 | 303.11 | C16H17NO5 | Cenocladamide | Alkaloid |
| 5 | 3.81 | 288.12 | 287.11 | C16H17NO4 | Pipermethystine | Alkaloid |
| 6 | 4.54 | 200.10 | 199.09 | C13H13NO | N-(3-Phenylpropanoyl) pyrrole | Alkaloid |
| 7 | 5.05 | 300.15 | 299.15 | C18H21NO3 | 1-[7-(3,4-Methylenedioxyphenyl)-2,6-heptadienoyl] pyrrolidine | Alkaloid |
| 8 | 5.22 | 286.14 | 285.13 | C17H19NO3 | Piperine | Alkaloid |
| 9 | 6.27 | 216.10 | 215.09 | C13H13NO2 | Sarmentamide A | Amide |
| 10 | 8.16 | 318.13 | 317.12 | C17H19NO5 | Sarmentamide B | Amide |
| 11 | 8.43 | 274.14 | 273.13 | C16H19NO3 | Piperlonguminine | Alkaloid |
| 12 | 8.61 | 232.13 | 231.12 | C14H17NO2 | Awaine | Alkaloid |
| 13 | 8.71 | 318.13 | 317.12 | C17H19NO5 | Piplartine | Alkaloid |
| 14 | 8.78 | 306.13 | 305.12 | C16H19NO5 | Peepuloidine | Alkaloid |
| 15 | 9.500 | 306.07 | 305.06 | C18H11NO4 | Cepharadione A | Alkaloid |
| 16 | 11.53 | 222.18 | 221.17 | C14H23NO | Sarmentine | Alkaloid |
| 17 | 11.68 | 326.17 | 325.16 | C20H23NO3 | 1-[9-(3,4-Methylenedioxyphenyl)-2,4,8-nonatrienoyl] pyrrolidine | Alkaloid |
| 18 | 12.04 | 328.18 | 327.18 | C20H25NO3 | Retrofractamide | Amide |
| Gene | GenBank Accession No. | Type | Sequence |
|---|---|---|---|
| Angiotensin-converting enzyme (ACE) | NM_000789 | Forward Reverse | 5′-atg tag atg cag ggg act cg-3′ 5′-agg gca cca cca agt cat ag-3′ |
| Angiotensin II type 1 receptor (AT1R) | NM_032049 | Forward Reverse | 5′-gca caa tgc ttg tag cca aa-3′ 5′-ggg ttg aat ttt ggg act ca-3′ |
| Angiotensin II type 2 receptor (AT2R) | NM_000686 | Forward Reverse | 5′-ttc cct tcc atg ttc tga cc-3′ 5′-aaa cac act gcg gag ctt ct-3′ |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | NM_002046 | Forward Reverse | 5′-tcc ctg agc tga acg gga ag-3′ 5′-gga gga gtg ggt gtc gct gt-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugusman, A.; Ismail, S.M.; Nor Hisam, N.S.; Hui, C.K.; Saleh, M.S.M.; Abdul Karim, A.K.; Othman, N.S.; Hamid, A.A.; Aminuddin, A. Piper sarmentosum Roxb. Inhibits Angiotensin-Converting Enzyme Activity in Phorbol 12-Myristate-13-Acetate-Induced Endothelial Cells. Int. J. Mol. Sci. 2024, 25, 2806. https://doi.org/10.3390/ijms25052806
Ugusman A, Ismail SM, Nor Hisam NS, Hui CK, Saleh MSM, Abdul Karim AK, Othman NS, Hamid AA, Aminuddin A. Piper sarmentosum Roxb. Inhibits Angiotensin-Converting Enzyme Activity in Phorbol 12-Myristate-13-Acetate-Induced Endothelial Cells. International Journal of Molecular Sciences. 2024; 25(5):2806. https://doi.org/10.3390/ijms25052806
Chicago/Turabian StyleUgusman, Azizah, Siti Marjiana Ismail, Nur Syahidah Nor Hisam, Chua Kien Hui, Mohammed S. M. Saleh, Abdul Kadir Abdul Karim, Nur Syakirah Othman, Adila A. Hamid, and Amilia Aminuddin. 2024. "Piper sarmentosum Roxb. Inhibits Angiotensin-Converting Enzyme Activity in Phorbol 12-Myristate-13-Acetate-Induced Endothelial Cells" International Journal of Molecular Sciences 25, no. 5: 2806. https://doi.org/10.3390/ijms25052806
APA StyleUgusman, A., Ismail, S. M., Nor Hisam, N. S., Hui, C. K., Saleh, M. S. M., Abdul Karim, A. K., Othman, N. S., Hamid, A. A., & Aminuddin, A. (2024). Piper sarmentosum Roxb. Inhibits Angiotensin-Converting Enzyme Activity in Phorbol 12-Myristate-13-Acetate-Induced Endothelial Cells. International Journal of Molecular Sciences, 25(5), 2806. https://doi.org/10.3390/ijms25052806







