Magnetic Hydroxyapatite Nanoparticles in Regenerative Medicine and Nanomedicine
Abstract
1. Introduction
2. Methods of Magnetic Hydroxyapatite Synthesis
2.1. Chemical Precipitation
2.1.1. HA-Based MNCs Obtained by Chemical Precipitation
2.1.2. HA Doped with Ions Giving Intrinsic Magnetic Properties Obtained by Chemical Precipitation
2.2. Mechanochemical Method
2.2.1. HA-Based MNCs Obtained by Mechanochemical Method
2.2.2. HA Doped with Ions Giving Intrinsic Magnetic Properties Obtained by Mechanochemical Method
2.3. Emulsion Synthesis Method
2.4. Hydrothermal Method
HA-Based MNCs Obtained by Hydrothermal Method
2.5. Template Method
HA-Based MNCs Obtained by Template Method
2.6. Sol–Gel Method
HA-Based MNCs Obtained by Sol–Gel Method
2.7. Synergistic Synthesis Method
HA-Based MNCs Obtained by Synergistic Synthesis Method
2.8. Microwave-Assisted Synthesis Method
2.8.1. HA-Based MNCs Obtained by Microwave-Assisted Synthesis Method
2.8.2. HA Doped with Ions Giving Intrinsic Magnetic Properties Obtained by Microwave-Assisted Synthesis Method
2.9. Biomimetic Fabrication Methods
HA-Based MNCs Obtained by Biomimetic Fabrication Methods
3. Magnetic Materials and Stimuli in Regenerative Medicine
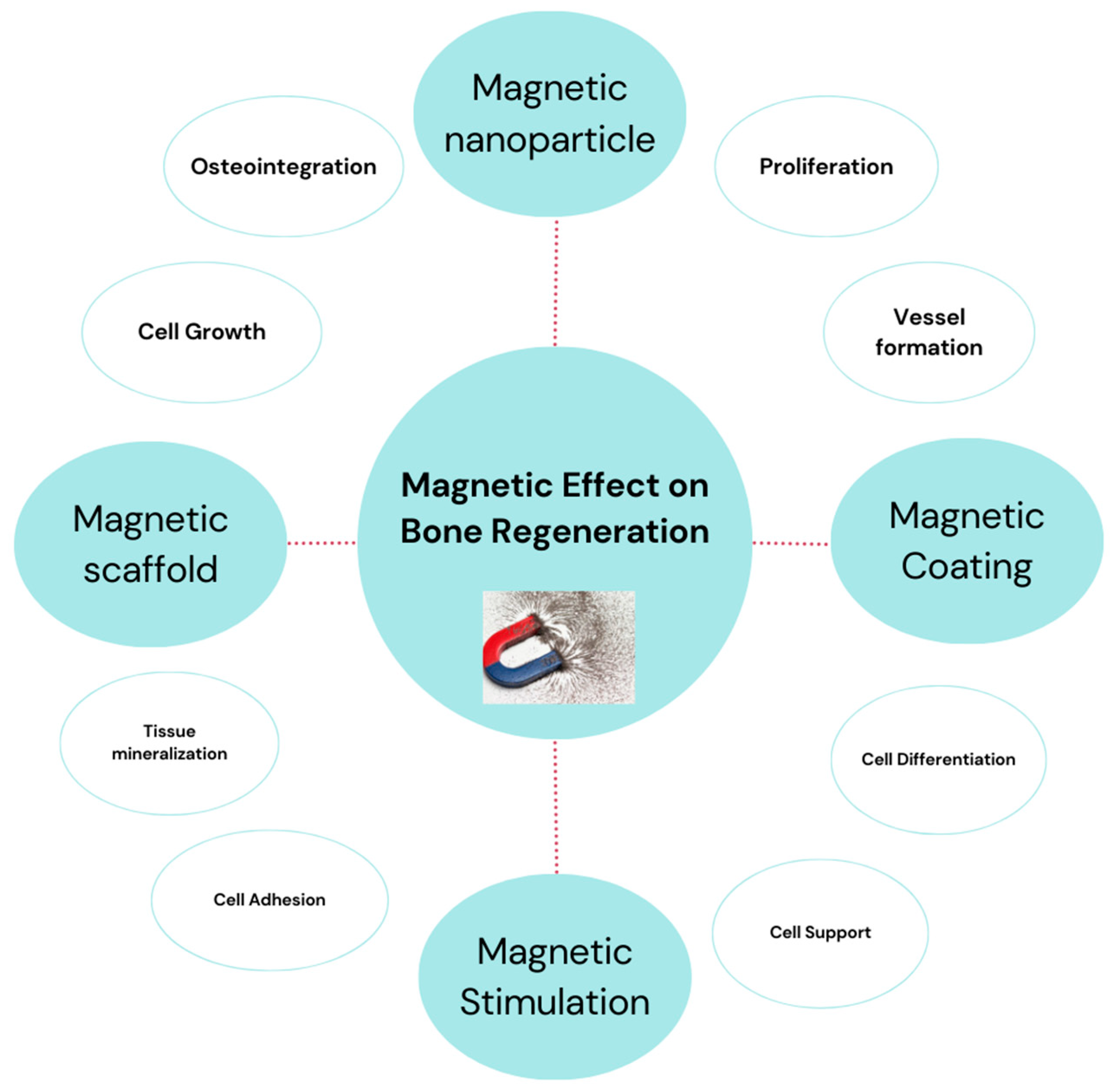
3.1. Magnetic Scaffolds
3.2. Magnetic Coatings
4. Magnetic Hydroxyapatite Nanoparticles in Nanomedicine
4.1. Magnetic Hydroxyapatite Nanoparticles as Drug Delivery Systems
4.2. Antimicrobial Agent
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abbreviation | Explanation |
| MNPs | Magnetic nanomaterials |
| MRI | Magnetic resonance imaging |
| MNCs | Magnetic nanomaterial composites |
| SPIONs | Superparamagnetic iron oxide nanoparticles |
| Fe-HA | Iron-doped hydroxyapatite |
| mHA | Magnetic hydroxyapatite |
| MF | Magnetic field |
| SMF | Static magnetic fields |
| AMF | Alternating magnetic fields |
References
- Tran, H.-V.; Ngo, N.M.; Medhi, R.; Srinoi, P.; Liu, T.; Rittikulsittichai, S.; Lee, T.R. Multifunctional iron oxide magnetic nanoparticles for biomedical applications: A review. Materials 2022, 15, 503. [Google Scholar] [CrossRef]
- Mittal, A.; Roy, I.; Gandhi, S. Magnetic nanoparticles: An overview for biomedical applications. Magnetochemistry 2022, 8, 107. [Google Scholar] [CrossRef]
- Chavan, N.; Dharmaraj, D.; Sarap, S.; Surve, C. Magnetic nanoparticles—A new era in nanotechnology. J. Drug Deliv. Sci. Technol. 2022, 77, 103899. [Google Scholar] [CrossRef]
- Schneider, M.G.M.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical applications of iron oxide nanoparticles: Current insights progress and perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef]
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic nanoparticles in biomedical applications: A review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Lavorato, G.C.; Das, R.; Masa, J.A.; Phan, M.-H.; Srikanth, H. Hybrid magnetic nanoparticles as efficient nanoheaters in biomedical applications. Nanoscale Adv. 2021, 3, 867–888. [Google Scholar] [CrossRef] [PubMed]
- Anik, M.I.; Hossain, M.K.; Hossain, I.; Mahfuz, A.M.U.B.; Rahman, M.T.; Ahmed, I. Recent progress of magnetic nanoparticles in biomedical applications: A review. Nano Sel. 2021, 2, 1146–1186. [Google Scholar] [CrossRef]
- Tran, N.; Webster, T.J. Magnetic nanoparticles: Biomedical applications and challenges. J. Mater. Chem. 2010, 20, 8760–8767. [Google Scholar] [CrossRef]
- Marouzi, S.; Sabouri, Z.; Darroudi, M. Greener synthesis and medical applications of metal oxide nanoparticles. Ceram. Int. 2021, 47, 19632–19650. [Google Scholar] [CrossRef]
- Shenoy, R.U.K.; Rama, A.; Govindan, I.; Naha, A. The purview of doped nanoparticles: Insights into their biomedical applications. OpenNano 2022, 8, 100070. [Google Scholar] [CrossRef]
- Tamboli, Q.Y.; Patange, S.M.; Mohanta, Y.K.; Sharma, R.; Zakde, K.R. Green synthesis of cobalt ferrite nanoparticles: An emerging material for environmental and biomedical applications. J. Nanomater. 2023, 2023, 9770212. [Google Scholar] [CrossRef]
- Laranjeira, M.S.; Ribeiro, T.P.; Magalhães, A.I.; Silva, P.C.; Santos, J.A.; Monteiro, F.J. Magnetic mesoporous silica nanoparticles as a theranostic approach for breast cancer: Loading and release of the poorly soluble drug exemestane. Int. J. Pharm. 2022, 619, 121711. [Google Scholar] [CrossRef] [PubMed]
- Comanescu, C. Magnetic Nanoparticles: Current Advances in Nanomedicine, Drug Delivery and MRI. Chemistry 2022, 4, 872–930. [Google Scholar] [CrossRef]
- Fopase, R.; Pandey, L.M. Iron oxide based magnetic nanomaterials for biomedical applications. Magnetochem. Mater. Appl. 2020, 66, 276. [Google Scholar]
- Iannotti, V.; Adamiano, A.; Ausanio, G.; Lanotte, L.; Aquilanti, G.; Coey, J.M.D.; Lantieri, M.; Spina, G.; Fittipaldi, M.; Margaris, G.; et al. Fe-Doping-Induced Magnetism in Nano-Hydroxyapatites. Inorg. Chem. 2017, 56, 4446–4458. [Google Scholar] [CrossRef]
- Panseri, S.; Cunha, C.; D’Alessandro, T.; Sandri, M.; Giavaresi, G.; Marcacci, M.; Hung, C.T.; Tampieri, A. Intrinsically superparamagnetic Fe-hydroxyapatite nanoparticles positively influence osteoblast-like cell behaviour. J. Nanobiotechnol. 2012, 10, 32. [Google Scholar] [CrossRef]
- Gobbo, O.L.; Sjaastad, K.; Radomski, M.W.; Volkov, Y.; Prina-Mello, A. Magnetic nanoparticles in cancer theranostics. Theranostics 2015, 5, 1249–1263. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Melnikov, M.Y. Magnetic nanoparticles for biomedical purposes: Modern trends and prospects. Magnetochemistry 2020, 6, 30. [Google Scholar] [CrossRef]
- Farzin, A.; Etesami, S.A.; Quint, J.; Memic, A.; Tamayol, A. Magnetic nanoparticles in cancer therapy and diagnosis. Adv. Healthc. Mater. 2020, 9, e1901058. [Google Scholar] [CrossRef]
- Dasari, A.; Xue, J.; Deb, S. Magnetic Nanoparticles in Bone Tissue Engineering. Nanomaterials 2022, 12, 757. [Google Scholar] [CrossRef]
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. [Google Scholar] [CrossRef]
- Yilmaz, A.; Bietenbeck, M.; Florian, A.; Faber, C.; Sechtem, U. Remote magnetic targeting of iron oxide nanoparticles for cardiovascular diagnosis and therapeutic drug delivery: Where are we now? Int. J. Nanomed. 2016, 11, 3191–3203. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, A.A.; Alorabi, A.Q.; Hassan, M.S.; Amna, T.; Azizi, M. Chitosan-Functionalized Hydroxyapatite-Cerium Oxide Heterostructure: An Efficient Adsorbent for Dyes Removal and Antimicrobial Agent. Nanomaterials 2022, 12, 2713. [Google Scholar] [CrossRef]
- Kalia, S.; Kango, S.; Kumar, A.; Haldorai, Y.; Kumari, B.; Kumar, R. Magnetic polymer nanocomposites for environmental and biomedical applications. Colloid Polym. Sci. 2014, 292, 2025–2052. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Guo, Z.; Poot, A.A.; Grijpma, D.W. Advanced polymer-based composites and structures for biomedical applications. Eur. Polym. J. 2021, 149, 110388. [Google Scholar] [CrossRef]
- Govindan, B.; Sabri, M.A.; Hai, A.; Banat, F.; Abu Haija, M. A Review of Advanced Multifunctional Magnetic Nanostructures for Cancer Diagnosis and Therapy Integrated into an Artificial Intelligence Approach. Pharmaceutics 2023, 15, 868. [Google Scholar] [CrossRef]
- Rocha-Santos, T.A. Sensors and biosensors based on magnetic nanoparticles. TrAC Trends Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Li, R.; Dai, Q.; Gao, R.; Liu, Q.; Zhang, M. Preparation of Fe3O4@C@ layered double hydroxide composite for magnetic separation of uranium. Ind. Eng. Chem. Res. 2013, 52, 10152–10159. [Google Scholar] [CrossRef]
- Nithya, R.; Thirunavukkarasu, A.; Sathya, A.B.; Sivashankar, R. Magnetic materials and magnetic separation of dyes from aqueous solutions: A review. Environ. Chem. Lett. 2021, 19, 1275–1294. [Google Scholar] [CrossRef]
- Kale, A.; Kale, S.; Yadav, P.; Gholap, H.; Pasricha, R.; Jog, J.P.; Lefez, B.; Hannoyer, B.; Shastry, P.; Ogale, S. Magnetite/CdTe magnetic–fluorescent composite nanosystem for magnetic separation and bio-imaging. Nanotechnology 2011, 22, 225101. [Google Scholar] [CrossRef]
- Ai, L.; Zhou, Y.; Jiang, J. Removal of methylene blue from aqueous solution by montmorillonite/CoFe2O4 composite with magnetic separation performance. Desalination 2011, 266, 72–77. [Google Scholar] [CrossRef]
- Minitha, C.R.; Martina Susan Arachy, M.; Rajendra Kumar, R.T. Influence of Fe3O4 nanoparticles decoration on dye adsorption and magnetic separation properties of Fe3O4/rGO nanocomposites. Sep. Sci. Technol. 2018, 53, 2159–2169. [Google Scholar] [CrossRef]
- Bao, Y.; Wen, T.; Samia, A.C.S.; Khandhar, A.; Krishnan, K.M. Magnetic nanoparticles: Material engineering and emerging applications in lithography and biomedicine. J. Mater. Sci. 2015, 51, 513–553. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.; Aifantis, K.E.; Fan, Y.; Feng, Q.; Cui, F.; Watari, F. Current investigations into magnetic nanoparticles for biomedical applications. J. Biomed. Mater. Res. Part A 2016, 104, 1285–1296. [Google Scholar] [CrossRef]
- Mody, V.V.; Cox, A.; Shah, S.; Singh, A.; Bevins, W.; Parihar, H. Magnetic nanoparticle drug delivery systems for targeting tumor. Appl. Nanosci. 2013, 4, 385–392. [Google Scholar] [CrossRef]
- Nordin, A.H.; Ahmad, Z.; Husna, S.M.N.; Ilyas, R.A.; Azemi, A.K.; Ismail, N.; Nordin, M.L.; Ngadi, N.; Siti, N.H.; Nabgan, W.; et al. The State of the Art of Natural Polymer Functionalized Fe3O4 Magnetic Nanoparticle Composites for Drug Delivery Applications: A Review. Gels 2023, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Rana, G.; Dhiman, P.; Sharma, A. Magnetic nanoferrite-based composites for pH sensitive drug delivery applications. In Magnetic Nanoferrites and Their Composites; Elsevier: Amsterdam, The Netherlands, 2023; pp. 165–191. [Google Scholar]
- Khizar, S.; Ahmad, N.M.; Zine, N.; Jaffrezic-Renault, N.; Errachid-El-Salhi, A.; Elaissari, A. magnetic nanoparticles: From synthesis to theranostic applications. ACS Appl. Nano Mater. 2021, 4, 4284–4306. [Google Scholar] [CrossRef]
- Mukherjee, P.; Kumar, A.; Bhamidipati, K.; Puvvada, N.; Sahu, S.K. Facile strategy to synthesize magnetic upconversion nanoscale metal–organic framework composites for theranostics application. ACS Appl. Bio Mater. 2019, 3, 869–880. [Google Scholar] [CrossRef]
- Kucharczyk, K.; Rybka, J.D.; Hilgendorff, M.; Krupinski, M.; Slachcinski, M.; Mackiewicz, A.; Giersig, M.; Dams-Kozlowska, H. Composite spheres made of bioengineered spider silk and iron oxide nanoparticles for theranostics applications. PLoS ONE 2019, 14, e0219790. [Google Scholar] [CrossRef] [PubMed]
- Masunga, N.; Mmelesi, O.K.; Kefeni, K.K.; Mamba, B.B. Recent advances in copper ferrite nanoparticles and nanocomposites synthesis, magnetic properties and application in water treatment: Review. J. Environ. Chem. Eng. 2019, 7, 103179. [Google Scholar] [CrossRef]
- Jadhav, J.; Biswas, S. Hybrid ZnO: Ag core-shell nanoparticles for wastewater treatment: Growth mechanism and plasmonically enhanced photocatalytic activity. Appl. Surf. Sci. 2018, 456, 49–58. [Google Scholar] [CrossRef]
- Khazeni, N. Synthesis and Characterization of Zirconium Tungstate-Zirconia Core-Shell Composite Particles. Master’s Thesis, Middle East Technical University, Ankara, Turkey, 2013. [Google Scholar]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle–hydrogel composites: Concept, design, and applications of these promising, multi-functional materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.K.; Richey, J.; Strand, M.; Leslie-Pelecky, D.L.; Flask, C.A.; Labhasetwar, V. Magnetic nanoparticles with dual functional properties: Drug delivery and magnetic resonance imaging. Biomaterials 2008, 29, 4012–4021. [Google Scholar] [CrossRef] [PubMed]
- Aghayi-Anaraki, M.; Safarifard, V. Fe3O4@MOF magnetic nanocomposites: Synthesis and applications. Eur. J. Inorg. Chem. 2020, 2020, 1916–1937. [Google Scholar] [CrossRef]
- Elkady, M.; Shokry, H.; Hamad, H. Microwave-assisted synthesis of magnetic hydroxyapatite for removal of heavy metals from groundwater. Chem. Eng. Technol. 2017, 41, 553–562. [Google Scholar] [CrossRef]
- Enriquez-Navas, P.M.; Garcia-Martin, M.L. Application of inorganic nanoparticles for diagnosis based on MRI. Front. Nanosci. 2012, 4, 233–245. [Google Scholar]
- Zhou, W.; Tang, K.; Zeng, S.; Qi, Y. Room temperature synthesis of rod-like FeC2O4·2H2O and its transition to maghemite, magnetite and hematite nanorods through controlled thermal decomposition. Nanotechnology 2008, 19, 065602. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Bagheri, S.; Abd Hamid, S.B. Progress in electrochemical synthesis of magnetic iron oxide nanoparticles. J. Magn. Magn. Mater. 2014, 368, 207–229. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Nwasike, C.; Yoo, E.; Purr, E.; Doiron, A.L. Activatable superparamagnetic iron oxide nanoparticles scavenge reactive oxygen species in macrophages and endothelial cells. RSC Adv. 2020, 10, 41305–41314. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, Q.; Huang, T.; Ling, D.; Gao, J. New insights into biocompatible iron oxide nanoparticles: A potential booster of gene delivery to stem cells. Small 2020, 16, e2001588. [Google Scholar] [CrossRef]
- Wang, N.; Xie, Y.; Xi, Z.; Mi, Z.; Deng, R.; Liu, X.; Kang, R.; Liu, X. Hope for bone regeneration: The versatility of iron oxide nanoparticles. Front. Bioeng. Biotechnol. 2022, 10, 937803. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhao, Y.; Zhang, F.; Chen, B.; Hu, X.; Weir, M.D.; Schneider, A.; Jia, L.; Gu, N.; Xu, H.H. Iron oxide nanoparticles in liquid or powder form enhanced osteogenesis via stem cells on injectable calcium phosphate scaffold. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102069. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Lu, J.; Wang, Q.; Yan, S.; Li, Y.; Zhang, Y.; Wang, P.; Jiang, Q.; Gu, N. Osteogenesis of iron oxide nanoparticles-labeled human precartilaginous stem cells in interpenetrating network printable hydrogel. Front. Bioeng. Biotechnol. 2022, 10, 872149. [Google Scholar] [CrossRef] [PubMed]
- Hachani, R.; Birchall, M.A.; Lowdell, M.W.; Kasparis, G.; Tung, L.D.; Manshian, B.B.; Soenen, S.J.; Gsell, W.; Himmelreich, U.; Gharagouzloo, C.A.; et al. Assessing cell-nanoparticle interactions by high content imaging of biocompatible iron oxide nanoparticles as potential contrast agents for magnetic resonance imaging. Sci. Rep. 2017, 7, 7850. [Google Scholar] [CrossRef] [PubMed]
- Demirer, G.S.; Okur, A.C.; Kizilel, S. Synthesis and design of biologically inspired biocompatible iron oxide nanoparticles for biomedical applications. J. Mater. Chem. B 2015, 3, 7831–7849. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.R.; Port, J.D.; Pandey, M.K. Use of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) via Multiple Imaging Modalities and Modifications to Reduce Cytotoxicity: An Educational Review. J. Nanotheranostics 2020, 1, 105–135. [Google Scholar] [CrossRef]
- Han, D.-W.; Hong, S.C.; Lee, J.H.; Lee, J.; Kim, H.Y.; Park, J.Y.; Cho, J.; Lee, J. Subtle cytotoxicity and genotoxicity differences in superparamagnetic iron oxide nanoparticles coated with various functional groups. Int. J. Nanomed. 2011, 6, 3219–3231. [Google Scholar] [CrossRef] [PubMed]
- Kefeni, K.K.; Msagati, T.A.; Nkambule, T.T.; Mamba, B.B. Spinel ferrite nanoparticles and nanocomposites for biomedical applications and their toxicity. Mater. Sci. Eng. C 2019, 107, 110314. [Google Scholar] [CrossRef]
- Gawande, M.B.; Monga, Y.; Zboril, R.; Sharma, R.K. Silica-decorated magnetic nanocomposites for catalytic applications. Coord. Chem. Rev. 2015, 288, 118–143. [Google Scholar] [CrossRef]
- Mariño, M.A.; Fulaz, S.; Tasic, L. Magnetic nanomaterials as biocatalyst carriers for biomass processing: Immobilization strategies, reusability, and applications. Magnetochemistry 2021, 7, 133. [Google Scholar] [CrossRef]
- Hojjati-Najafabadi, A.; Mansoorianfar, M.; Liang, T.; Shahin, K.; Wen, Y.; Bahrami, A.; Karaman, C.; Zare, N.; Karimi-Maleh, H.; Vasseghian, Y. Magnetic-MXene-based nanocomposites for water and wastewater treatment: A review. J. Water Process. Eng. 2022, 47, 102696. [Google Scholar] [CrossRef]
- Kandasamy, G.; Soni, S.; Sushmita, K.; Veerapu, N.S.; Bose, S.; Maity, D. One-step synthesis of hydrophilic functionalized and cytocompatible superparamagnetic iron oxide nanoparticles (SPIONs) based aqueous ferrofluids for biomedical applications. J. Mol. Liq. 2018, 274, 653–663. [Google Scholar] [CrossRef]
- Friedrich, R.P.; Janko, C.; Unterweger, H.; Lyer, S.; Alexiou, C. SPIONs and magnetic hybrid materials: Synthesis, toxicology and biomedical applications. Phys. Sci. Rev. 2021, 8, 1435–1464. [Google Scholar] [CrossRef]
- Cantarelli, I.X.; Pedroni, M.; Piccinelli, F.; Marzola, P.; Boschi, F.; Conti, G.; Sbarbati, A.; Bernardi, P.; Mosconi, E.; Perbellini, L.; et al. Multifunctional nanoprobes based on upconverting lanthanide doped CaF2: Towards biocompatible materials for biomedical imaging. Biomater. Sci. 2014, 2, 1158–1171. [Google Scholar] [CrossRef]
- Zilm, M.E.; Staruch, M.; Jain, M.; Wei, M. An intrinsically magnetic biomaterial with tunable magnetic properties. J. Mater. Chem. B 2014, 2, 7176–7185. [Google Scholar] [CrossRef]
- Mohammad, N.; Ahmad; Rosli, N.M.; Manan, M.A.; Marzuki, M.; Wahi, A. Sol gel deposited hydroxyapatite-based coating technique on porous titanium niobium for biomedical applications: A mini review. Mater. Today Proc. 2021, 41, 127–135. [Google Scholar] [CrossRef]
- Pokhrel, S. Hydroxyapatite: Preparation, properties and its biomedical applications. Adv. Chem. Eng. Sci. 2018, 8, 225. [Google Scholar] [CrossRef]
- Szcześ, A.; Hołysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef]
- Awasthi, S.; Pandey, S.K.; Arunan, E.; Srivastava, C. A review on hydroxyapatite coatings for the biomedical applications: Experimental and theoretical perspectives. J. Mater. Chem. B 2020, 9, 228–249. [Google Scholar] [CrossRef]
- Sobczak-Kupiec, A.; Drabczyk, A.; Florkiewicz, W.; Głąb, M.; Kudłacik-Kramarczyk, S.; Słota, D.; Tomala, A.; Tyliszczak, B. Review of the applications of biomedical compositions containing hydroxyapatite and collagen modified by bioactive components. Materials 2021, 14, 2096. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent advances in hydroxyapatite-based biocomposites for bone tissue regeneration in orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Han, S.S.; Kang, I.-K. Recent advances in the synthesis, functionalization and biomedical applications of hydroxyapatite: A review. RSC Adv. 2017, 7, 7442–7458. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite based materials for bone tissue engineering: A brief and comprehensive introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Halim, N.A.A.; Hussein, M.Z.; Kandar, M.K. Nanomaterials-upconverted hydroxyapatite for bone tissue engineering and a platform for drug delivery. Int. J. Nanomed. 2021, 16, 6477–6496. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Chen, J.; Liu, K.; Xing, H.; Song, C. Recent advances in biomedical engineering of nano-hydroxyapatite including dentistry, cancer treatment and bone repair. Compos. Part B Eng. 2021, 215, 108790. [Google Scholar] [CrossRef]
- Ren, B.; Chen, X.; Du, S.; Ma, Y.; Chen, H.; Yuan, G.; Li, J.; Xiong, D.; Tan, H.; Ling, Z.; et al. Injectable polysaccharide hydrogel embedded with hydroxyapatite and calcium carbonate for drug delivery and bone tissue engineering. Int. J. Biol. Macromol. 2018, 118, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Said, H.A.; Mabroum, H.; Lahcini, M.; Oudadesse, H.; Barroug, A.; Ben Youcef, H.; Noukrati, H. Manufacturing methods, properties, and potential applications in bone tissue regeneration of hydroxyapatite-chitosan biocomposites: A review. Int. J. Biol. Macromol. 2023, 243, 125150. [Google Scholar] [CrossRef]
- Mo, X.; Zhang, D.; Liu, K.; Zhao, X.; Li, X.; Wang, W. Nano-hydroxyapatite composite scaffolds loaded with bioactive factors and drugs for bone tissue engineering. Int. J. Mol. Sci. 2023, 24, 1291. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Song, G.; Huang, Y.; Yang, H.; Han, S.; Zhang, X.; Wang, Z.; Ma, J.; Bu, X.; Fu, L. Si, Sr, Ag co-doped hydroxyapatite/TiO2 coating: Enhancement of its antibacterial activity and osteoinductivity. RSC Adv. 2019, 9, 13348–13364. [Google Scholar] [CrossRef] [PubMed]
- Jebapriya, M.; Venkatesan, R.; Ansar, S.; Kim, S.-C. Enhancement of physicochemical characterization of nanocomposites on Ag+/Fe2+ codoped hydroxyapatite for antibacterial and anticancer properties. Colloids Surf. B Biointerfaces 2023, 229, 113463. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Nag, S.; Kottan, N.B.; Basu, B. In silico study on probing atomistic insights into structural stability and tensile properties of Fe-doped hydroxyapatite single crystals. Sci. Rep. 2022, 12, 20576. [Google Scholar] [CrossRef] [PubMed]
- Mollaei, M.; Varshosaz, J. Preparation and characterization of hydroxyapatite nanoparticles doped with nickel, tin, and molybdate ions for their antimicrobial effects. Drug Dev. Ind. Pharm. 2023, 49, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Surblyte, R.; Baltakys, K. Microwave synthesis of hydroxyapatite substituted with Zn2+ ions in the temperature range of 80–200 °C. In Proceedings of the Chemistry and Chemical Technology: International Conference CCT-2023, Vilnius, Lithuania, 10 March 2023. [Google Scholar]
- Syazwan, M.M.; Marliana, B.Y. The influence of simultaneous divalent cations (Mg2+, Co2+ and Sr2+) substitution on the physico-chemical properties of carbonated hydroxyapatite. Ceram. Int. 2019, 45, 14783–14788. [Google Scholar] [CrossRef]
- Pasandideh, Z.; Tajabadi, M.; Javadpour, J.; Mirkazemi, S.M. The effects of Fe3+ and Co2+ substitution in Ca10-x-yFexCoy(PO4)6(OH)2 hydroxyapatite nanoparticles: Magnetic, antibacterial, and improved drug release behavior. Ceram. Int. 2020, 46, 16104–16118. [Google Scholar] [CrossRef]
- Heshmatpour, F.; Lashteneshaee, S.H.; Samadipour, M. Study of in vitro bioactivity of nano hydroxyapatite composites doped by various cations. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2063–2068. [Google Scholar] [CrossRef]
- Huang, Y.; Qiao, H.; Nian, X.; Zhang, X.; Zhang, X.; Song, G.; Xu, Z.; Zhang, H.; Han, S. Improving the bioactivity and corrosion resistance properties of electrodeposited hydroxyapatite coating by dual doping of bivalent strontium and manganese ion. Surf. Coatings Technol. 2016, 291, 205–215. [Google Scholar] [CrossRef]
- Cacciotti, I. Multisubstituted hydroxyapatite powders and coatings: The influence of the codoping on the hydroxyapatite performances. Int. J. Appl. Ceram. Technol. 2019, 16, 1864–1884. [Google Scholar] [CrossRef]
- Ezekiel, I.; Kasim, S.R.; Ismail, Y.M.B.; Noor, A.-F.M. Nanoemulsion synthesis of carbonated hydroxyapatite nanopowders: Effect of variant CO32−/PO43− molar ratios on phase, morphology, and bioactivity. Ceram. Int. 2018, 44, 13082–13089. [Google Scholar] [CrossRef]
- Mushtaq, A.; Tang, Z.; Hou, Y.; Zhu, Z.; Tian, C.; Wu, Y.; Lu, Y.; Iqbal, M.Z.; Kong, X. Biocompatible magnetic hydroxyapatite Fe3O4-HAp nanocomposites for T1-magnetic resonance imaging guided photothermal therapy of breast cancer. Mater. Today Commun. 2022, 31, 103734. [Google Scholar] [CrossRef]
- Govindan, B.; Latha, B.S.; Nagamony, P.; Ahmed, F.; Alam Saifi, M.; Harrath, A.H.; Alwasel, S.; Mansour, L.; Alsharaeh, E.H. Designed synthesis of nanostructured magnetic hydroxyapatite based drug nanocarrier for anti-cancer drug delivery toward the treatment of human epidermoid carcinoma. Nanomaterials 2017, 7, 138. [Google Scholar] [CrossRef]
- Mondal, S.; Manivasagan, P.; Bharathiraja, S.; Moorthy, M.S.; Nguyen, V.T.; Kim, H.H.; Nam, S.Y.; Lee, K.D.; Oh, J. Hydroxyapatite coated iron oxide nanoparticles: A promising nanomaterial for magnetic hyperthermia cancer treatment. Nanomaterials 2017, 7, 426. [Google Scholar] [CrossRef]
- Tampieri, A.; D’alessandro, T.; Sandri, M.; Sprio, S.; Landi, E.; Bertinetti, L.; Panseri, S.; Pepponi, G.; Goettlicher, J.; Bañobre-López, M.; et al. Intrinsic magnetism and hyperthermia in bioactive Fe-doped hydroxyapatite. Acta Biomater. 2012, 8, 843–851. [Google Scholar] [CrossRef]
- Iafisco, M.; Sandri, M.; Panseri, S.; Delgado-López, J.M.; Gómez-Morales, J.; Tampieri, A. Magnetic bioactive and biodegradable hollow fe-doped hydroxyapatite coated poly(l-lactic) acid micro-nanospheres. Chem. Mater. 2013, 25, 2610–2617. [Google Scholar] [CrossRef]
- Adamiano, A.; Iafisco, M.; Sandri, M.; Basini, M.; Arosio, P.; Canu, T.; Tampieri, A. On the use of superparamagnetic hydroxyapatite nanoparticles as an agent for magnetic and nuclear in vivo imaging. Acta Biomater. 2018, 73, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, O.; Alaghmandfard, A.; Montazerian, M.; Baino, F. A critical review of bioceramics for magnetic hyperthermia. J. Am. Ceram. Soc. 2021, 105, 1723–1747. [Google Scholar] [CrossRef]
- Adamiano, A.; Wu, V.M.; Carella, F.; Lamura, G.; Canepa, F.; Tampieri, A.; Iafisco, M.; Uskoković, V. Magnetic calcium phosphates nanocomposites for the intracellular hyperthermia of cancers of bone and brain. Nanomedicine 2019, 14, 1267–1289. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in magnetic nanoparticles for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, Y.; Zuo, Y.; Li, Y.; Li, J. Fabrication of Magnetic Hydroxyapatite by a Homogeneous Precipitation Method. Mater. Sci. 2016, 6, 223–229. [Google Scholar]
- Scialla, S.; Palazzo, B.; Barca, A.; Carbone, L.; Fiore, A.; Monteduro, A.G.; Maruccio, G.; Sannino, A.; Gervaso, F. Simplified preparation and characterization of magnetic hydroxyapatite-based nanocomposites. Mater. Sci. Eng. C 2017, 76, 1166–1174. [Google Scholar] [CrossRef]
- Veerla, S.C.; Kim, D.R.; Kim, J.; Sohn, H.; Yang, S.Y. Controlled nanoparticle synthesis of Ag/Fe co-doped hydroxyapatite system for cancer cell treatment. Mater. Sci. Eng. C 2019, 98, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Karunamoorthi, R.; Kumar, G.S.; Prasad, A.I.; Vatsa, R.K.; Thamizhavel, A.; Girija, E.K. Fabrication of a novel biocompatible magnetic biomaterial with hyperthermia potential. J. Am. Ceram. Soc. 2013, 97, 1115–1122. [Google Scholar] [CrossRef]
- Abidi, S.S.A.; Murtaza, Q. Synthesis and characterization of nano-hydroxyapatite powder using wet chemical precipitation reaction. J. Mater. Sci. Technol. 2014, 30, 307–310. [Google Scholar] [CrossRef]
- Panneerselvam, R.; Anandhan, N.; Gopu, G.; Ganesan, K.P.; Marimuthu, T. Impact of different transition metal ions in the structural, mechanical, optical, chemico-physical and biological properties of nanohydroxyapatite. Appl. Surf. Sci. 2019, 506, 144802. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, Y.; Wang, C.; Sun, R.; Tang, Y. Effects of physico-chemical properties of ions-doped hydroxyapatite on adsorption and release performance of doxorubicin as a model anticancer drug. Mater. Chem. Phys. 2021, 276, 125440. [Google Scholar] [CrossRef]
- Sprio, S.; Dapporto, M.; Preti, L.; Mazzoni, E.; Iaquinta, M.R.; Martini, F.; Tognon, M.; Pugno, N.M.; Restivo, E.; Visai, L.; et al. Enhancement of the biological and mechanical performances of sintered hydroxyapatite by multiple ions doping. Front. Mater. 2020, 7, 224. [Google Scholar] [CrossRef]
- Yilmaz, B.; Alshemary, A.Z.; Evis, Z. Co-doped hydroxyapatites as potential materials for biomedical applications. Microchem. J. 2018, 144, 443–453. [Google Scholar] [CrossRef]
- Mansour, S.F.; El-Dek, S.I.; Dorozhkin, S.V.; Ahmed, M.K. Physico-mechanical properties of Mg and Ag doped hydroxyapatite/chitosan biocomposites. New J. Chem. 2017, 41, 13773–13783. [Google Scholar] [CrossRef]
- Tosan, F.; Rahnama, N.; Sakhaei, D.; Fathi, A.H.; Yari, A. Effects of doping metal nanoparticles in hydroxyapatite in Improving the physical and chemical properties of dental implants. Nanomed. Res. J. 2021, 6, 327–336. [Google Scholar]
- Aina, V.; Lusvardi, G.; Annaz, B.; Gibson, I.R.; Imrie, F.E.; Malavasi, G.; Menabue, L.; Cerrato, G.; Martra, G. Magnesium- and strontium-co-substituted hydroxyapatite: The effects of doped-ions on the structure and chemico-physical properties. J. Mater. Sci. Mater. Med. 2012, 23, 2867–2879. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh, M.; Ramesh, S.; Tan, C.; Chandran, H.; Noor, A.F.M.; Krishnasamy, S.; Alengaram, U.J. Effect of multi-ions doping on the properties of carbonated hydroxyapatite bioceramic. Ceram. Int. 2018, 45, 3473–3477. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Grumezescu, A.M. Magnetite nanoparticles: Synthesis methods—A comparative review. Methods 2022, 199, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Hachem, K.; Ansari, M.J.; Saleh, R.O.; Kzar, H.H.; Al-Gazally, M.E.; Altimari, U.S.; Hussein, S.A.; Mohammed, H.T.; Hammid, A.T.; Kianfar, E. Methods of chemical synthesis in the synthesis of nanomaterial and nanoparticles by the chemical deposition method: A review. BioNanoScience 2022, 12, 1032–1057. [Google Scholar] [CrossRef]
- Ma, G. Three common preparation methods of hydroxyapatite. IOP Conf. Ser. Mater. Sci. Eng. 2019, 688, 033057. [Google Scholar] [CrossRef]
- Ignatovich, Z.; Novik, K.; Abakshonok, A.; Koroleva, E.; Beklemisheva, A.; Panina, L.; Kaniukov, E.; Anisovich, M.; Shumskaya, A. One-Step Synthesis of Magnetic Nanocomposite with Embedded Biologically Active Substance. Molecules 2021, 26, 937. [Google Scholar] [CrossRef]
- Jakab, M.; Enisz-Bódogh, M.; Makó, É.; Kovács, K.; Orbán, S.; Horváth, B. Influence of wet chemical processing conditions on structure and properties of magnetic hydroxyapatite nanocomposites. Process. Appl. Ceram. 2020, 14, 321–328. [Google Scholar] [CrossRef]
- Fadli, A.; Iskandar, D.J.; Pane, K.B. The Effect of Addition of F3O4 and Sintering Temperature on Properties of the Magnetite/Hydroxyapatite Particles Produced through the Coprecipitation Technique. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 012018. [Google Scholar] [CrossRef]
- Ullah, I.; Zhang, W.; Yang, L.; Ullah, M.W.; Atta, O.M.; Khan, S.; Wu, B.; Wu, T.; Zhang, X. Impact of structural features of Sr/Fe co-doped HAp on the osteoblast proliferation and osteogenic differentiation for its application as a bone substitute. Mater. Sci. Eng. C 2020, 110, 110633. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Gloria, A.; Zhang, W.; Ullah, M.W.; Wu, B.; Li, W.; Domingos, M.; Zhang, X. Synthesis and characterization of sintered Sr/Fe-modified hydroxyapatite bioceramics for bone tissue engineering applications. ACS Biomater. Sci. Eng. 2019, 6, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, Q. Application of mechanochemical synthesis of advanced materials. J. Adv. Ceram. 2012, 1, 130–137. [Google Scholar] [CrossRef]
- Xu, C.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem. Commun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2011, 41, 413–447. [Google Scholar] [CrossRef]
- Godočíková, E.; Baláž, P.; Gock, E.; Choi, W.S.; Kim, B.S. Mechanochemical synthesis of the nanocrystalline semiconductors in an industrial mill. Powder Technol. 2006, 164, 147–152. [Google Scholar] [CrossRef]
- Sneha, M.; Sundaram, N.M. Preparation and characterization of an iron oxide-hydroxyapatite nanocomposite for potential bone cancer therapy. Int. J. Nanomed. 2015, 10, 99–106. [Google Scholar]
- Iwasaki, T.; Nakatsuka, R.; Murase, K.; Takata, H.; Nakamura, H.; Watano, S. Simple and rapid synthesis of magnetite/hydroxyapatite composites for hyperthermia treatments via a mechanochemical route. Int. J. Mol. Sci. 2013, 14, 9365–9378. [Google Scholar] [CrossRef]
- Isaev, D.D.; Kriventsov, V.V.; Petrov, S.A.; Bystrov, V.S.; Bulina, N.V. Substitution in the Structure of Hydroxyapatite Doped with Iron Cations upon Mechanochemical Synthesis. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2023, 17, 687–693. [Google Scholar] [CrossRef]
- Iwasaki, T. Mechanochemical synthesis of magnetite/hydroxyapatite nanocomposites for hyperthermia. In Materials Science-Advanced Topics; IntechOpen: London, UK, 2013; Volume 175. [Google Scholar]
- Kamitakahara, M.; Ohtoshi, N.; Kawashita, M.; Ioku, K. Spherical porous hydroxyapatite granules containing composites of magnetic and hydroxyapatite nanoparticles for the hyperthermia treatment of bone tumor. J. Mater. Sci. Mater. Med. 2016, 27, 1–7. [Google Scholar] [CrossRef]
- Makarova, S.V.; Bulina, N.V.; Vinokurova, O.B.; Ishchenko, A.V. Thermal Stability of Iron- and Silicon-Substituted Hydroxyapatite Prepared by Mechanochemical Method. Powders 2023, 2, 372–386. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Gafurov, M.R.; Murzakhanov, F.F.; Fomin, A.S.; Antonova, O.S.; Khairutdinova, D.R.; Komlev, V.S. Mesoporous iron (III)-doped hydroxyapatite nanopowders obtained via iron oxalate. Nanomaterials 2021, 11, 811. [Google Scholar] [CrossRef]
- Liu, X.; Okada, M.; Maeda, H.; Fujii, S.; Furuzono, T. Hydroxyapatite/biodegradable poly (l-lactide–co-ε-caprolactone) composite microparticles as injectable scaffolds by a Pickering emulsion route. Acta Biomater. 2011, 7, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Chesley, M.; Kennard, R.; Roozbahani, S.; Kim, S.M.; Kukk, K.; Mason, M. One-step hydrothermal synthesis with in situ milling of biologically relevant hydroxyapatite. Mater. Sci. Eng. C 2020, 113, 110962. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.F.; Alrowaili, Z.A.; El-Giar, E.M.; Ahmed, M.M.; El-Kady, A.M. Novel green synthesis of hydroxyapatite uniform nanorods via microwave-hydrothermal route using licorice root extract as template. Ceram. Int. 2020, 47, 3928–3937. [Google Scholar] [CrossRef]
- Murakami, S.; Hosono, T.; Jeyadevan, B.; Kamitakahara, M.; Ioku, K. Hydrothermal synthesis of magnetite/hydroxyapatite composite material for hyperthermia therapy for bone cancer. J. Ceram. Soc. Jpn. 2008, 116, 950–954. [Google Scholar] [CrossRef]
- Zheltova, V.; Vlasova, A.; Bobrysheva, N.; Abdullin, I.; Semenov, V.; Osmolowsky, M.; Voznesenskiy, M.; Osmolovskaya, O. Fe3O4@HAp core–shell nanoparticles as MRI contrast agent: Synthesis, characterization and theoretical and experimental study of shell impact on magnetic properties. Appl. Surf. Sci. 2020, 531, 147352. [Google Scholar] [CrossRef]
- Xie, Y.; Kocaefe, D.; Chen, C.; Kocaefe, Y. Review of research on template methods in preparation of nanomaterials. J. Nanomater. 2016, 2016, 2302595. [Google Scholar] [CrossRef]
- Liu, Y.; Goebl, J.; Yin, Y. Templated synthesis of nanostructured materials. Chem. Soc. Rev. 2012, 42, 2610–2653. [Google Scholar] [CrossRef]
- Sneha, M.; Sundaram, N.M.; Kandaswamy, A. Synthesis and characterization of magnetite/hydroxyapatite tubes using natural template for biomedical applications. Bull. Mater. Sci. 2016, 39, 509–517. [Google Scholar] [CrossRef]
- Mir, A.; Mallik, D.; Bhattacharyya, S.; Mahata, D.; Sinha, A.; Nayar, S. Aqueous ferrofluids as templates for magnetic hydroxyapatite nanocomposites. J. Mater. Sci. Mater. Med. 2010, 21, 2365–2369. [Google Scholar] [CrossRef]
- Singh, R.K.; El-Fiqi, A.M.; Patel, K.D.; Kim, H.-W. A novel preparation of magnetic hydroxyapatite nanotubes. Mater. Lett. 2012, 75, 130–133. [Google Scholar] [CrossRef]
- Cui, X.; Green, M.A.; Blower, P.J.; Zhou, D.; Yan, Y.; Zhang, W.; Djanashvili, K.; Mathe, D.; Veres, D.S.; Szigeti, K. Al(OH)3 facilitated synthesis of water-soluble, magnetic, radiolabelled and fluorescent hydroxyapatite nanoparticles. Chem. Commun. 2015, 51, 9332–9335. [Google Scholar] [CrossRef]
- Lett, J.A.; Sundareswari, M.; Ravichandran, K.; Latha, M.B.; Sagadevan, S.; Bin Johan, M.R. Tailoring the morphological features of sol–gel synthesized mesoporous hydroxyapatite using fatty acids as an organic modifier. RSC Adv. 2019, 9, 6228–6240. [Google Scholar] [CrossRef]
- Phatai, P.; Futalan, C.M.; Kamonwannasit, S.; Khemthong, P. Structural characterization and antibacterial activity of hydroxyapatite synthesized via sol-gel method using glutinous rice as a template. J. Sol-Gel Sci. Technol. 2019, 89, 764–775. [Google Scholar] [CrossRef]
- Yusoff, A.; Salimi, M.; Gopinath, S.; Abdullah, M.; Samsudin, E. Catechin adsorption on magnetic hydroxyapatite nanoparticles: A synergistic interaction with calcium ions. Mater. Chem. Phys. 2019, 241, 122337. [Google Scholar] [CrossRef]
- Yusoff, A.H.M.; Salimi, M.N.; Jamlos, M.F. Synthesis of superparamagnetic hydroxyapatite core-shell nanostructure by a rapid sol-gel route. e-J. Surf. Sci. Nanotechnol. 2017, 15, 121–126. [Google Scholar] [CrossRef]
- Mushtaq, A.; Ma, X.; Farheen, J.; Lin, X.; Tayyab, M.; Iqbal, M.Z.; Kong, X. Facile Synthesis of Metformin Loaded Mn3O4-HAp Magnetic Hydroxyapatite Nanocomposites for T1-Magnetic Resonance Imaging Guided Targeted Chemo-Phototherapy In Vitro. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 674, 131911. [Google Scholar] [CrossRef]
- Sangeetha, K.; Ashok, M.; Girija, E. Development of multifunctional cobalt ferrite/hydroxyapatite nanocomposites by microwave assisted wet precipitation method: A promising platform for synergistic chemo-hyperthermia therapy. Ceram. Int. 2019, 45, 12860–12869. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, M.; Zhao, Y.; Wang, J.; Wu, Y.; Li, K.; Liu, Y. Microwave-assisted hydrothermal synthesis of Fe-doped 1T/2H-MoS2 few-layer nanosheets for efficient electromagnetic wave absorbing. J. Alloys Compd. 2023, 947, 169544. [Google Scholar] [CrossRef]
- Hassan, M.N.; Mahmoud, M.M.; El-Fattah, A.A.; Kandil, S. Microwave-assisted preparation of Nano-hydroxyapatite for bone substitutes. Ceram. Int. 2016, 42, 3725–3744. [Google Scholar] [CrossRef]
- Chen, F.; Li, C.; Zhu, Y.-J.; Zhao, X.-Y.; Lu, B.-Q.; Wu, J. Magnetic nanocomposite of hydroxyapatite ultrathin nanosheets/Fe3O4 nanoparticles: Microwave-assisted rapid synthesis and application in pH-responsive drug release. Biomater. Sci. 2013, 1, 1074–1081. [Google Scholar] [CrossRef]
- Karunakaran, G.; Cho, E.-B.; Kumar, G.S.; Kolesnikov, E.; Karpenkov, D.Y.; Gopinathan, J.; Pillai, M.M.; Selvakumar, R.; Boobalan, S.; Gorshenkov, M.V. Sodium dodecyl sulfate mediated microwave synthesis of biocompatible superparamagnetic mesoporous hydroxyapatite nanoparticles using black Chlamys varia seashell as a calcium source for biomedical applications. Ceram. Int. 2019, 45, 15143–15155. [Google Scholar] [CrossRef]
- Agalya, P.; Saravanan, T.; Kumar, G.S.; Cholan, S.; Karunakaran, G.; Van Minh, N. Surfactant-assisted microwave synthesis of luminescent/magnetic bifunctional hydroxyapatite nanorods for dual-model imaging. Optik 2020, 225, 165564. [Google Scholar] [CrossRef]
- Chandra, V.S.; Elayaraja, K.; Arul, K.T.; Ferraris, S.; Spriano, S.; Ferraris, M.; Asokan, K.; Kalkura, S.N. Synthesis of magnetic hydroxyapatite by hydrothermal–microwave technique: Dielectric, protein adsorption, blood compatibility and drug release studies. Ceram. Int. 2015, 41, 13153–13163. [Google Scholar] [CrossRef]
- Iglesias, G.R.; Jabalera, Y.; Peigneux, A.; Fernández, B.L.C.; Delgado, V.; Jimenez-Lopez, C. Enhancement of magnetic hyperthermia by mixing synthetic inorganic and biomimetic magnetic nanoparticles. Pharmaceutics 2019, 11, 273. [Google Scholar] [CrossRef]
- Drozdov, A.S.; Komarova, K.S.; Mochalova, E.N.; Komedchikova, E.N.; Shipunova, V.O.; Nikitin, M.P. Fluorescent magnetic nanoparticles for bioimaging through biomimetic surface modification. Int. J. Mol. Sci. 2022, 24, 134. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kargozar, S.; Baino, F.; Han, S.S. Additive manufacturing methods for producing hydroxyapatite and hydroxyapatite-based composite scaffolds: A review. Front. Mater. 2019, 6, 313. [Google Scholar] [CrossRef]
- Gibson, I.R.; Bonfield, W. Novel synthesis and characterization of an AB-type carbonate-substituted hydroxyapatite. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2002, 59, 697–708. [Google Scholar] [CrossRef]
- Vijayalakshmi, U.; Rajeswari, S. Preparation and characterization of microcrystalline hydroxyapatite using sol gel method. Trends Biomater. Artif. Organs 2006, 19, 57–62. [Google Scholar]
- Luz, G.; Mano, J. Nanoscale design in biomineralization for developing new biomaterials for bone tissue engineering (BTE). In Tissue Engineering Using Ceramics and Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 153–195. [Google Scholar]
- Mann, S. Biomineralization and biomimetic materials chemistry. J. Mater. Chem. 1995, 5, 935–946. [Google Scholar] [CrossRef]
- Strbak, O.; Hnilicova, P.; Gombos, J.; Lokajova, A.; Kopcansky, P. Magnetotactic bacteria: From evolution to biomineralization and biomedical applications. Minerals 2022, 12, 1403. [Google Scholar] [CrossRef]
- Hakimi, F.; Abroon, M.; Sadighian, S.; Ramazani, A. Evaluation of bone-like apatite biomineralization on biomimetic graphene oxide/hydroxyapatite nanocomposite. Inorg. Chem. Commun. 2023, 149, 110450. [Google Scholar] [CrossRef]
- Tithito, T.; Sillapaprayoon, S.; Pimtong, W.; Thongbunchoo, J.; Charoenphandhu, N.; Krishnamra, N.; Lert-Itthiporn, A.; Maneeprakorn, W.; Pon-On, W. Development of Biomaterials Based on Biomimetic Trace Elements Co-Doped Hydroxyapatite: Physical, In Vitro Osteoblast-like Cell Growth and In Vivo Cytotoxicity in Zebrafish Studies. Nanomaterials 2023, 13, 255. [Google Scholar] [CrossRef]
- Bhatt, A.; Sakai, K.; Madhyastha, R.; Murayama, M.; Madhyastha, H.; Rath, S.N. Biosynthesis and characterization of nano magnetic hydroxyapatite (nMHAp): An accelerated approach using simulated body fluid for biomedical applications. Ceram. Int. 2020, 46, 27866–27876. [Google Scholar] [CrossRef]
- Labrag, J.; Abbadi, M.; Hnini, M.; El Bekkali, C.; Bouziani, A.; Robert, D.; Aurag, J.; Laghzizil, A.; Nunzi, J.-M. Antibiotic photocatalysis and antimicrobial activity of low-cost multifunctional Fe3O4@HAp nanocomposites. J. Environ. Health Sci. Eng. 2023, 21, 429–440. [Google Scholar] [CrossRef]
- Adamu, D.B.; Zereffa, E.; Segne, T.A.; Razali, M.H.; Lemu, B.R. Synthesis of iron-substituted hydroxyapatite nanomaterials by co-precipitation method for defluoridation. Mater. Res. Express 2023, 10, 045006. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, Q.; Yu, Y.; Ge, S. Spongy magnetic hydroxyapatite for the enhanced Pb2+ removal and its dynamic sorption mechanism. J. Environ. Chem. Eng. 2023, 11, 110213. [Google Scholar] [CrossRef]
- Wu, S.-C.; Hsu, H.-C.; Wang, H.-F.; Liou, S.-P.; Ho, W.-F. Synthesis and characterization of nano-hydroxyapatite obtained from eggshell via the hydrothermal process and the precipitation method. Molecules 2023, 28, 4926. [Google Scholar] [CrossRef]
- Gopi, D.; Ansari, M.T.; Shinyjoy, E.; Kavitha, L. Synthesis and spectroscopic characterization of magnetic hydroxyapatite nanocomposite using ultrasonic irradiation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 87, 245–250. [Google Scholar] [CrossRef]
- Kermanian, M.; Naghibi, M.; Sadighian, S. One-pot hydrothermal synthesis of a magnetic hydroxyapatite nanocomposite for MR imaging and pH-Sensitive drug delivery applications. Heliyon 2020, 6, e04928. [Google Scholar] [CrossRef]
- Phasuk, A.; Srisantitham, S.; Tuntulani, T.; Anutrasakda, W. Facile synthesis of magnetic hydroxyapatite-supported nickel oxide nanocomposite and its dye adsorption characteristics. Adsorption 2017, 24, 157–167. [Google Scholar] [CrossRef]
- Ravindranadh, K.; Babu, B.; Reddy, C.V.; Shim, J.; Rao, M.C.; Ravikumar, R.V.S.S.N. EPR and Optical Studies of Fe3+-Doped Ca–Li Hydroxyapatite Nanopowder: Mechanochemical Synthesis. Appl. Magn. Reson. 2014, 46, 1–15. [Google Scholar] [CrossRef]
- Al Jahoushi, K.A.; Ayesh, A.I.; El-Maghraby, H.F.; Alnoush, W.; Higgins, D.; Hassan, F.M.; Greish, Y.E. Tunable Hydroxyapatite/Magnetite Nanohybrids with Preserved Magnetic Properties. Adv. Mater. Interfaces 2022, 9, 2102120. [Google Scholar] [CrossRef]
- Vahdat, A.; Ghasemi, B.; Yousefpour, M. Mechanical properties of the hydroxyapatite and magnetic nanocomposite of hydroxyapatite adsorbents. S. Afr. J. Chem. Eng. 2020, 33, 90–94. [Google Scholar] [CrossRef]
- Vucinic-Vasic, M.; Antic, B.; Boskovic, M.; Antic, A.; Blanusa, J. Hydroxyapatite/iron oxide nanocomposite prepared by high energy ball milling. Process. Appl. Ceram. 2019, 13, 210–217. [Google Scholar] [CrossRef]
- Mendiratta, S.; Ali, A.A.A.; Hejazi, S.H.; Gates, I. Dual stimuli-responsive pickering emulsions from novel magnetic hydroxyapatite nanoparticles and their characterization using a microfluidic platform. Langmuir 2021, 37, 1353–1364. [Google Scholar] [CrossRef]
- Yan, B.; Wang, X.; Zhang, X.; Liu, S.; Lu, H.; Ran, R. One-step preparation of hydroxyapatite-loaded magnetic Polycaprolactone hollow microspheres for malachite green adsorption by Pickering emulsion template method. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 639, 128347. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, Y.; Chen, S.; Bai, Y.; Li, X.; Chen, W.; Wei, A. Synthesis of chitosan/hydroxyapatite/Fe3O4 microspheres with NIR-induced antibacterial property. Mater. Lett. 2023, 351, 135062. [Google Scholar] [CrossRef]
- Long, T.; Guo, Y.-P.; Tang, S.; Zhu, Z.-A. Emulsion fabrication of magnetic mesoporous carbonated hydroxyapatite microspheres for treatment of bone infection. RSC Adv. 2014, 4, 11816–11825. [Google Scholar] [CrossRef]
- Okada, M.; Takeda, S.; Furuzono, T. A Novel Approach to Prepare Hydroxyapatite-Coated Biodegradable Polymer Microspheres Loaded with Magnetic Fe3O4 via Nanoparticle-Stabilized Emulsions. Key Eng. Mater. 2012, 529–530, 223–228. [Google Scholar] [CrossRef]
- Foroughi, F.; Hassanzadeh-Tabrizi, S.; Amighian, J. Microemulsion synthesis and magnetic properties of hydroxyapatite-encapsulated nano CoFe2O4. J. Magn. Magn. Mater. 2015, 382, 182–187. [Google Scholar] [CrossRef]
- Shu, K.; Chuaicham, C.; Noguchi, Y.; Xu, L.; Sasaki, K. In-situ hydrothermal synthesis of Fe-doped hydroxyapatite photocatalyst derived from converter slag toward xanthate photodegradation and Cr(VI) reduction under visible-light irradiation. Chem. Eng. J. 2023, 459, 141474. [Google Scholar] [CrossRef]
- Chen, W.; Long, T.; Zhu, Z.-A.; Guo, Y.-P. Magnetic hydroxyapatite coatings with oriented nanorod arrays: Hydrothermal synthesis, structure and biocompatibility. J. Mater. Chem. B 2014, 2, 1653–1660. [Google Scholar] [CrossRef]
- Yazdani, N.; Javadpour, J.; Eftekhari, Y.; Hamrang, M. Hydrothermal synthesis of cobalt-doped hydroxyapatite nanoparticles: Structure, magnetic behaviour, bioactivity and antibacterial activity. Iran. J. Mater. Sci. Eng. 2019, 16, 39–48. [Google Scholar]
- Periyasamy, S.; Gopalakannan, V.; Viswanathan, N. Hydrothermal assisted magnetic nano-hydroxyapatite encapsulated alginate beads for efficient Cr(VI) uptake from water. J. Environ. Chem. Eng. 2018, 6, 1443–1454. [Google Scholar] [CrossRef]
- Mushtaq, A.; Zhang, H.; Cui, M.; Lin, X.; Huang, S.; Tang, Z.; Hou, Y.; Iqbal, M.Z.; Kong, X. ROS-responsive chlorin e6 and silk fibroin loaded ultrathin magnetic hydroxyapatite nanorods for T1-magnetic resonance imaging guided photodynamic therapy in vitro. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 656, 130513. [Google Scholar] [CrossRef]
- Gopalakannan, V.; Periyasamy, S.; Viswanathan, N. Fabrication of magnetic particles reinforced nano-hydroxyapatite/gelatin composite for selective Cr(vi) removal from water. Environ. Sci. Water Res. Technol. 2018, 4, 783–794. [Google Scholar] [CrossRef]
- Guo, Y.-P.; Long, T.; Tang, S.; Zhu, Z.-A. Hydrothermal fabrication of magnetic mesoporous carbonated hydroxyapatite microspheres: Biocompatibility, osteoinductivity, drug delivery property and bactericidal property. J. Mater. Chem. B 2014, 2, 2899–2909. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, X.; Kang, C.; Zhang, J.; Xu, Z.; Jiang, C.; Luo, P.; Fu, Z.; Ding, M.; Lv, Y. Synthesis of magnetic Fe-doped hydroxyapatite nanocages with highly efficient and selective adsorption for Cd2+. Mater. Lett. 2019, 253, 144–147. [Google Scholar] [CrossRef]
- Zuo, G.; Wan, Y.; Zhang, Y. Preparation and characterization of a novel laminated magnetic hydroxyapatite for application on gene delivery. Mater. Lett. 2011, 68, 225–227. [Google Scholar] [CrossRef]
- Sheikh, L.; Mahto, N.; Nayar, S. In situ synthesis of hydroxyapatite nanocomposites using iron oxide nanofluids at ambient conditions. J. Mater. Sci. Mater. Med. 2015, 26, 47. [Google Scholar] [CrossRef]
- Zeng, D.; Dai, Y.; Zhang, Z.; Wang, Y.; Cao, X.; Liu, Y. Magnetic solid-phase extraction of U(VI) in aqueous solution by Fe3O4@hydroxyapatite. J. Radioanal. Nucl. Chem. 2020, 324, 1329–1337. [Google Scholar] [CrossRef]
- Kayğili, Ö.; Dorozhkin, S.V.; Ates, T.; Al-Ghamdi, A.A.; Yakuphanoglu, F. Dielectric properties of Fe doped hydroxyapatite prepared by sol–gel method. Ceram. Int. 2014, 40, 9395–9402. [Google Scholar] [CrossRef]
- Xiao, X.; Yang, L.; Zhou, D.; Zhou, J.; Tian, Y.; Song, C.; Liu, C. Magnetic γ-Fe2O3/Fe-doped hydroxyapatite nanostructures as high-efficiency cadmium adsorbents. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 548–557. [Google Scholar] [CrossRef]
- Trinkunaite-Felsen, J.; Prichodko, A.; Semasko, M.; Skaudzius, R.; Beganskiene, A.; Kareiva, A. Synthesis and characterization of iron-doped/substituted calcium hydroxyapatite from seashells Macoma balthica (L.). Adv. Powder Technol. 2015, 26, 1287–1293. [Google Scholar] [CrossRef]
- Anjaneyulu, U.; Swaroop, V.; Vijayalakshmi, U. Preparation and characterization of novel Ag doped hydroxyapatite–Fe3O4–chitosan hybrid composites and in vitro biological evaluations for orthopaedic applications. RSC Adv. 2016, 6, 10997–11007. [Google Scholar] [CrossRef]
- Baskaran, P.; Udduttula, A.; Uthirapathy, V. Development and characterisation of novel Ce-doped hydroxyapatite–Fe3O4 nanocomposites and their in vitro biological evaluations for biomedical applications. IET Nanobiotechnol. 2018, 12, 138–146. [Google Scholar] [CrossRef]
- Anjaneyulu, U.; Priyadarshini, B.; Vijayalakshmi, U. Preparation of Ag doped hydroxyapatite-Fe3O4-Chitosan composites: In vitro biocompatibility study on MG-63 cells for orthopedic applications. Adv. Sci. Lett. 2018, 24, 5901–5906. [Google Scholar] [CrossRef]
- Dong, Y.-Y.; Liu, S.; Liu, Y.-J.; Meng, L.-Y.; Ma, M.-G. Ag@Fe3O4@cellulose nanocrystals nanocomposites: Microwave-assisted hydrothermal synthesis, antimicrobial properties, and good adsorption of dye solution. J. Mater. Sci. 2017, 52, 8219–8230. [Google Scholar] [CrossRef]
- Gu, L.; He, X.; Wu, Z. Mesoporous Fe3O4/hydroxyapatite composite for targeted drug delivery. Mater. Res. Bull. 2014, 59, 65–68. [Google Scholar] [CrossRef]
- Abbasi, Z.; Rezayati, S.; Bagheri, M.; Hajinasiri, R. Preparation of a novel, efficient, and recyclable magnetic catalyst, γ-Fe2O3@ HAp-Ag nanoparticles, and a solvent-and halogen-free protocol for the synthesis of coumarin derivatives. Chin. Chem. Lett. 2017, 28, 75–82. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Korkmaz, A.D.; Baykal, A.; Vakhitov, M.; Klygach, D.S.; Trukhanov, S.V.; Trukhanov, A.V. Influence of phase ratio on structural, magnetic, and microwave characteristics of hard/soft SrEr0.01Cr0.01Fe11.98O19/NiFe2O4 magnetic nanocomposites. Appl. Phys. A 2023, 129, 187. [Google Scholar] [CrossRef]
- Piri, F.; Mollahosseini, A.; Khadir, A.; Milani Hosseini, M. Synthesis of a novel magnetic zeolite–hydroxyapatite adsorbent via microwave-assisted method for protein adsorption via magnetic solid-phase extraction. J. Iran. Chem. Soc. 2019, 17, 1635–1648. [Google Scholar] [CrossRef]
- Saikiran, A.; Pavankumar, K.; Shishir, R.; Rameshbabu, N. Synthesis and characterization of Fe and Eu containing hydroxyapataite. Mater. Today Proc. 2021, 46, 1061–1065. [Google Scholar] [CrossRef]
- Karunakaran, G.; Cho, E.-B.; Kumar, G.S.; Kolesnikov, E.; Dmitry, A.; Ali, S. Microwave-assisted synthesis of superparamagnetic mesoporous Co-doped hydroxyapatite nanorods for various biomedical applications. Ceram. Int. 2020, 47, 8642–8652. [Google Scholar] [CrossRef]
- Piri, F.; Mollahosseini, A.; Khadir, A.; Hosseini, M.M. Enhanced adsorption of dyes on microwave-assisted synthesized magnetic zeolite-hydroxyapatite nanocomposite. J. Environ. Chem. Eng. 2019, 7, 103338. [Google Scholar] [CrossRef]
- Kheradmandfard, M.; Mahdavi, K.; Kharazi, A.Z.; Kashani-Bozorg, S.F.; Kim, D.E. In vitro study of a novel multi-substituted hydroxyapatite nanopowder synthesized by an ultra-fast, efficient and green microwave-assisted method. Mater. Sci. Eng. 2020, 117, 111310. [Google Scholar] [CrossRef]
- Ge, H.; Liu, X.; Yuan, H.; Zhang, G. Biomimetic one-pot preparation of surface biofunctionalized silica-coated magnetic composites for dual enzyme oriented immobilization without pre-purification. Enzym. Microb. Technol. 2023, 164, 110169. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yabutsuka, T.; Takai, S.; Yao, T. Biomimetic Method for Production of Magnetic Hydroxyapatite Microcapsules for Enzyme Immobilization. Trans. Mater. Res. Soc. Jpn. 2018, 43, 153–156. [Google Scholar] [CrossRef]
- Tampieri, A.; Iafisco, M.; Sandri, M.; Panseri, S.; Cunha, C.; Sprio, S.; Savini, E.; Uhlarz, M.; Herrmannsdörfer, T. Magnetic bioinspired hybrid nanostructured collagen–hydroxyapatite scaffolds supporting cell proliferation and tuning regenerative process. ACS Appl. Mater. Interfaces 2014, 6, 15697–15707. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Stanford, C.M.; Keller, J.C. Calcium and phosphate supplementation promotes bone cell mineralization: Implications for hydroxyapatite (HA)-enhanced bone formation. J. Biomed. Mater. Res. 2000, 52, 270–278. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J.; Zhao, L.; Zhang, F.; Liang, X.-J.; Guo, Y.; Weir, M.D.; Reynolds, M.A.; Gu, N.; Xu, H.H.K. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials 2018, 183, 151–170. [Google Scholar] [CrossRef]
- Yun, H.-M.; Ahn, S.-J.; Park, K.-R.; Kim, M.-J.; Kim, J.-J.; Jin, G.-Z.; Kim, H.-W.; Kim, E.-C. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials 2016, 85, 88–98. [Google Scholar] [CrossRef]
- Feng, S.-W.; Lo, Y.-J.; Chang, W.-J.; Lin, C.-T.; Lee, S.-Y.; Abiko, Y.; Huang, H.-M. Static magnetic field exposure promotes differentiation of osteoblastic cells grown on the surface of a poly-l-lactide substrate. Med. Biol. Eng. Comput. 2010, 48, 793–798. [Google Scholar] [CrossRef]
- Yang, J.; Feng, Y.; Li, Q.; Zeng, Y. Evidence of the static magnetic field effects on bone-related diseases and bone cells. Prog. Biophys. Mol. Biol. 2023, 177, 168–180. [Google Scholar] [CrossRef]
- Sengupta, S.; Balla, V.K. A review on the use of magnetic fields and ultrasound for non-invasive cancer treatment. J. Adv. Res. 2018, 14, 97–111. [Google Scholar] [CrossRef]
- Wu, D.; Kang, L.; Tian, J.; Wu, Y.; Liu, J.; Li, Z.; Wu, X.; Huang, Y.; Gao, B.; Wang, H.; et al. Exosomes derived from bone mesenchymal stem cells with the stimulation of Fe3O4 nanoparticles and static magnetic field enhance wound healing through upregulated miR-21-5p. Int. J. Nanomed. 2020, 15, 7979–7993. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2020, 19, 23–36. [Google Scholar] [CrossRef]
- Zhang, Q.-M.; Tokiwa, M.; Doi, T.; Nakahara, T.; Chang, P.-W.; Nakamura, N.; Hori, M.; Miyakoshi, J.; Yonei, S. Strong static magnetic field and the induction of mutations through elevated production of reactive oxygen species in Escherichia coli soxR. Int. J. Radiat. Biol. 2003, 79, 281–286. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Cui, X.; Wang, X.; Zhang, L.; Tang, P. Recent Advances on Magnetic Sensitive Hydrogels in Tissue Engineering. Front. Chem. 2020, 8, 124. [Google Scholar] [CrossRef]
- Zhang, G.; Zhen, C.; Yang, J.; Zhang, Z.; Wu, Y.; Che, J.; Shang, P. 1–2 T static magnetic field combined with Ferumoxytol prevent unloading-induced bone loss by regulating iron metabolism in osteoclastogenesis. J. Orthop. Transl. 2022, 38, 126–140. [Google Scholar] [CrossRef]
- Marędziak, M.; Śmieszek, A.; Tomaszewski, K.A.; Lewandowski, D.; Marycz, K. The effect of low static magnetic field on osteogenic and adipogenic differentiation potential of human adipose stromal/stem cells. J. Magn. Magn. Mater. 2016, 398, 235–245. [Google Scholar] [CrossRef]
- Cho, S.; Shon, M.J.; Son, B.; Eun, G.S.; Yoon, T.-Y.; Park, T.H. Tension exerted on cells by magnetic nanoparticles regulates differentiation of human mesenchymal stem cells. Mater. Sci. Eng. C 2022, 139, 213028. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Ding, C.; Dong, D.; Shang, P. Regulation of osteoblast differentiation and iron content in MC3T3-E1 cells by static magnetic field with different intensities. Biol. Trace Element Res. 2017, 184, 214–225. [Google Scholar] [CrossRef]
- Russo, A.; Bianchi, M.; Sartori, M.; Boi, M.; Giavaresi, G.; Salter, D.M.; Jelic, M.; Maltarello, M.C.; Ortolani, A.; Sprio, S.; et al. Bone regeneration in a rabbit critical femoral defect by means of magnetic hydroxyapatite macroporous scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 546–554. [Google Scholar] [CrossRef]
- De Santis, R.; Russo, A.; Gloria, A.; D’Amora, U.; Russo, T.; Panseri, S.; Sandri, M.; Tampieri, A.; Marcacci, M.; Dediu, V.A. Towards the design of 3D fiber-deposited poly (-caprolactone)/iron-doped hydroxyapatite nanocomposite magnetic scaffolds for bone regeneration. J. Biomed. Nanotechnol. 2015, 11, 1236–1246. [Google Scholar] [CrossRef]
- Biedrzycka, A.; Skwarek, E.; Hanna, U.M. Hydroxyapatite with magnetic core: Synthesis methods, properties, adsorption and medical applications. Adv. Colloid Interface Sci. 2021, 291, 102401. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, H.; Zhao, Y.; Zhang, F.; Li, X.; Wang, L.; Weir, M.D.; Ma, J.; Reynolds, M.A.; Gu, N.; et al. Novel magnetic calcium phosphate-stem cell construct with magnetic field enhances osteogenic differentiation and bone tissue engineering. Mater. Sci. Eng. C 2018, 98, 30–41. [Google Scholar] [CrossRef]
- Mostafa, S.I.; Ismail, M.S.; Mohammed, H.A.; Osman, M.F.; Elwassefy, N.A. Magnetic hydroxyapatite bisphosphonate-based composites: A bone-targeting nanosystem. Emergent Mater. 2022, 6, 1273–1284. [Google Scholar] [CrossRef]
- Li, Y.; Huang, L.; Tai, G.; Yan, F.; Cai, L.; Xin, C.; Al Islam, S. Graphene Oxide-loaded magnetic nanoparticles within 3D hydrogel form High-performance scaffolds for bone regeneration and tumour treatment. Compos. Part A Appl. Sci. Manuf. 2021, 152, 106672. [Google Scholar] [CrossRef]
- Shi, Z.; Jia, L.; Zhang, Q.; Sun, L.; Wang, X.; Qin, X.; Xia, Y. An altered oral microbiota induced by injections of superparamagnetic iron oxide nanoparticle-labeled periodontal ligament stem cells helps periodontal bone regeneration in rats. Bioeng. Transl. Med. 2023, 8, e10466. [Google Scholar] [CrossRef]
- Shuai, C.; Cheng, Y.; Yang, W.; Feng, P.; Yang, Y.; He, C.; Peng, S. Magnetically actuated bone scaffold: Microstructure, cell response and osteogenesis. Composit. Part B Eng. 2020, 192, 107986. [Google Scholar] [CrossRef]
- Chen, H.; Sun, J.; Wang, Z.; Zhou, Y.; Lou, Z.; Chen, B.; Wang, P.; Guo, Z.; Tang, H.; Ma, J.; et al. Magnetic Cell–Scaffold Interface Constructed by Superparamagnetic IONP Enhanced Osteogenesis of Adipose-Derived Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 44279–44289. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Yang, W.; He, C.; Peng, S.; Gao, C.; Yang, Y.; Qi, F.; Feng, P. A magnetic micro-environment in scaffolds for stimulating bone regeneration. Mater. Des. 2019, 185, 108275. [Google Scholar] [CrossRef]
- Bock, N.; Riminucci, A.; Dionigi, C.; Russo, A.; Tampieri, A.; Landi, E.; Goranov, V.; Marcacci, M.; Dediu, V. A novel route in bone tissue engineering: Magnetic biomimetic scaffolds. Acta Biomater. 2010, 6, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Mocanu-Dobranici, A.-E.; Costache, M.; Dinescu, S. Insights into the Molecular Mechanisms Regulating Cell Behavior in Response to Magnetic Materials and Magnetic Stimulation in Stem Cell (Neurogenic) Differentiation. Int. J. Mol. Sci. 2023, 24, 2028. [Google Scholar] [CrossRef]
- Bakhtiary, N.; Pezeshki-Modaress, M.; Najmoddin, N. Wet-electrospinning of nanofibrous magnetic composite 3-D scaffolds for enhanced stem cells neural differentiation. Chem. Eng. Sci. 2022, 264, 118144. [Google Scholar] [CrossRef]
- Mohd, N.; Razali, M.; Ghazali, M.J.; Abu Kasim, N.H. 3D-Printed Hydroxyapatite and Tricalcium Phosphates-Based Scaffolds for Alveolar Bone Regeneration in Animal Models: A Scoping Review. Materials 2022, 15, 2621. [Google Scholar] [CrossRef] [PubMed]
- Van Hede, D.; Liang, B.; Anania, S.; Barzegari, M.; Verlée, B.; Nolens, G.; Pirson, J.; Geris, L.; Lambert, F. 3D-printed synthetic hydroxyapatite scaffold with in silico optimized macrostructure enhances bone formation in vivo. Adv. Funct. Mater. 2021, 32, 2105002. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Van Hede, D.; Plougonven, E.; Nolens, G.; Verlée, B.; De Pauw, M.C.; Lambert, F. In vitro and in vivo biocompatibility of calcium-phosphate scaffolds three-dimensional printed by stereolithography for bone regeneration. J. Biomed. Mater. Res. Part A 2020, 108, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.; D’amora, U.; Gloria, A.; Tunesi, M.; Sandri, M.; Rodilossi, S.; Albani, D.; Forloni, G.; Giordano, C.; Cigada, A.; et al. Systematic Analysis of Injectable Materials and 3D Rapid Prototyped Magnetic Scaffolds: From CNS Applications to Soft and Hard Tissue Repair/Regeneration. Procedia Eng. 2013, 59, 233–239. [Google Scholar] [CrossRef]
- Panseri, S.; Cunha, C.; D’Alessandro, T.; Sandri, M.; Russo, A.; Giavaresi, G.; Marcacci, M.; Hung, C.T.; Tampieri, A. Magnetic hydroxyapatite bone substitutes to enhance tissue regeneration: Evaluation in vitro using osteoblast-like cells and in vivo in a bone defect. PLoS ONE 2012, 7, e38710. [Google Scholar] [CrossRef] [PubMed]
- Tavares, F.J.T.M.; Soares, P.I.P.; Silva, J.C.; Borges, J.P. Preparation and in vitro characterization of magnetic CS/PVA/HA/pSPIONs scaffolds for magnetic hyperthermia and bone regeneration. Int. J. Mol. Sci. 2023, 24, 1128. [Google Scholar] [CrossRef] [PubMed]
- Maglio, M.; Sartori, M.; Gambardella, A.; Shelyakova, T.; Dediu, V.A.; Santin, M.; Piñeiro, Y.; López, M.B.; Rivas, J.; Tampieri, A.; et al. Bone regeneration guided by a magnetized scaffold in an ovine defect model. Int. J. Mol. Sci. 2023, 24, 747. [Google Scholar] [CrossRef]
- Petretta, M.; Gambardella, A.; Desando, G.; Cavallo, C.; Bartolotti, I.; Shelyakova, T.; Goranov, V.; Brucale, M.; Dediu, V.A.; Fini, M.; et al. Multifunctional 3D-Printed Magnetic Polycaprolactone/Hydroxyapatite Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 3825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, H.S.; Liu, J.Q.; Yu, T.; Shen, Z.H.; Ye, J.D. Good hydration and cell-biological performances of superparamagnetic calcium phosphate cement with concentration-dependent osteogenesis and angiogenesis induced by ferric iron. J. Mater. Chem. B 2015, 3, 8782–8795. [Google Scholar] [CrossRef]
- Russo, T.; Peluso, V.; Gloria, A.; Oliviero, O.; Rinaldi, L.; Improta, G.; De Santis, R.; D’Antò, V. Combination design of time-dependent magnetic field and magnetic nanocomposites to guide cell behavior. Nanomaterials 2020, 10, 577. [Google Scholar] [CrossRef]
- Patrício, T.M.F.; Panseri, S.; Sandri, M.; Tampieri, A.; Sprio, S. New bioactive bone-like microspheres with intrinsic magnetic properties obtained by bio-inspired mineralisation process. Mater. Sci. Eng. C 2017, 77, 613–623. [Google Scholar] [CrossRef]
- Patrício, T.M.F.; Mumcuoglu, D.; Montesi, M.; Panseri, S.; Witte-Bouma, J.; Garcia, S.F.; Sandri, M.; Tampieri, A.; Farrell, E.; Sprio, S. Bio-inspired polymeric iron-doped hydroxyapatite microspheres as a tunable carrier of rhBMP-2. Mater. Sci. Eng. C 2020, 119, 111410. [Google Scholar] [CrossRef] [PubMed]
- Gloria, A.; Russo, T.; d’Amora, U.; Zeppetelli, S.; d’Alessandro, T.; Sandri, M.; Bañobre-López, M.; Piñeiro-Redondo, Y.; Uhlarz, M.; Tampieri, A. Magnetic poly (ε-caprolactone)/iron-doped hydroxyapatite nanocomposite substrates for advanced bone tissue engineering. J. R. Soc. Interface 2013, 10, 20120833. [Google Scholar] [CrossRef]
- De Santis, R.; Gloria, A.; Russo, T.; d’Amora, U.; Zeppetelli, S.; Dionigi, C.; Sytcheva, A.; Herrmannsdörfer, T.; Dediu, V.; Ambrosio, L. A basic approach toward the development of nanocomposite magnetic scaffolds for advanced bone tissue engineering. J. Appl. Polym. Sci. 2011, 122, 3599–3605. [Google Scholar] [CrossRef]
- Huang, Z.; He, Y.; Chang, X.; Liu, J.; Yu, L.; Wu, Y.; Li, Y.; Tian, J.; Kang, L.; Wu, D.; et al. A Magnetic Iron Oxide/Polydopamine Coating Can Improve Osteogenesis of 3D-Printed Porous Titanium Scaffolds with a Static Magnetic Field by Upregulating the TGFβ-Smads Pathway. Adv. Health Mater. 2020, 9, e2000318. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, Z.; Ma, B.; Yu, L.; He, Y.; Xu, D.; Wu, Y.; Wang, H.; Qiu, G. Enhanced in vitro biocompatibility and osteogenesis of titanium substrates immobilized with dopamine-assisted superparamagnetic Fe3O4 nanoparticles for hBMSCs. R. Soc. Open Sci. 2018, 5, 172033. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, S.; Zhou, J.; Zhu, K.; Cui, X.; Huang, W.; Rahaman, M.N.; Zhang, C.; Wang, D. Biocompatibility and osteogenic capacity of borosilicate bioactive glass scaffolds loaded with Fe3O4 magnetic nanoparticles. J. Mater. Chem. B 2015, 3, 4377–4387. [Google Scholar] [CrossRef]
- Dittler, M.L.; Zelís, P.M.; Beltrán, A.M.; Destch, R.; Grillo, C.A.; Gonzalez, M.C.; Boccaccini, A.R. Magnetic 3D scaffolds for tissue engineering applications: Bioactive glass (45S5) coated with iron-loaded hydroxyapatite nanoparticles. Biomed. Mater. 2021, 16, 055006. [Google Scholar] [CrossRef]
- Tang, B.; Shen, X.; Yang, Y.; Xu, Z.; Yi, J.; Yao, Y.; Cao, M.; Zhang, Y.; Xia, H. Enhanced cellular osteogenic differentiation on CoFe2O4/P (VDF-TrFE) nanocomposite coatings under static magnetic field. Colloids Surf. B Biointerfaces 2021, 198, 111473. [Google Scholar] [CrossRef]
- Lin, S.; Li, J.; Dong, L.; Cheng, K.; Lin, J.; Weng, W. Periodic-mechanical-stimulus enhanced osteogenic differentiation of mesenchymal stem cells on Fe3O4/mineralized collagen coatings. ACS Biomater. Sci. Eng. 2019, 5, 6446–6453. [Google Scholar] [CrossRef]
- Zhuang, J.; Lin, S.; Dong, L.; Cheng, K.; Weng, W. Magnetically actuated mechanical stimuli on Fe3O4/mineralized collagen coatings to enhance osteogenic differentiation of the MC3T3-E1 cells. Acta Biomater. 2018, 71, 49–60. [Google Scholar] [CrossRef]
- Dikina, A.D.; Lai, B.P.; Cao, M.; Zborowski, M.; Alsberg, E. Magnetic field application or mechanical stimulation via magnetic microparticles does not enhance chondrogenesis in mesenchymal stem cell sheets. Biomater. Sci. 2017, 5, 1241–1245. [Google Scholar] [CrossRef]
- Hao, L.; Li, L.; Wang, P.; Wang, Z.; Shi, X.; Guo, M.; Zhang, P. Synergistic osteogenesis promoted by magnetically actuated nano-mechanical stimuli. Nanoscale 2019, 11, 23423–23437. [Google Scholar] [CrossRef]
- Yu, C.; Yang, W.; Yang, L.; Ye, L.; Sun, R.; Gu, T.; Ying, X.; Wang, M.; Tang, R.; Fan, S.; et al. Synergistic Effect of Magneto-Mechanical Bioengineered Stem Cells and Magnetic Field to Alleviate Osteoporosis. ACS Appl. Mater. Interfaces 2023, 15, 19976–19988. [Google Scholar] [CrossRef]
- Hu, B.; El Haj, A.J.; Dobson, J. Receptor-Targeted, Magneto-Mechanical Stimulation of Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2013, 14, 19276–19293. [Google Scholar] [CrossRef]
- Deng, C.; Li, Z.; Lu, L.; Zhang, H.; Chen, R.; Liu, Y.; Cheng, Y. Sophisticated magneto-mechanical actuation promotes in situ stem cell assembly and chondrogenesis for treating osteoarthritis. ACS Nano 2023, 17, 21690–21707. [Google Scholar] [CrossRef]
- Son, B.; Kim, H.D.; Kim, M.; Kim, J.A.; Lee, J.; Shin, H.; Park, T.H. Physical Stimuli-Induced Chondrogenic Differentiation of Mesenchymal Stem Cells Using Magnetic Nanoparticles. Adv. Healthc. Mater. 2015, 4, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Kumar, R.; Harilal, S.; Mathew, G.E.; Parambi, D.G.T.; Prabhu, A.; Uddin, S.; Aleya, L.; Kim, H.; Mathew, B. Magnetic nanoparticles for hyperthermia in cancer treatment: An emerging tool. Environ. Sci. Pollut. Res. 2019, 27, 19214–19225. [Google Scholar] [CrossRef] [PubMed]
- Manjusha, V.; Rajeev, M.; Anirudhan, T. Magnetic nanoparticle embedded chitosan-based polymeric network for the hydrophobic drug delivery of paclitaxel. Int. J. Biol. Macromol. 2023, 235, 123900. [Google Scholar] [CrossRef]
- Paltanea, G.; Paltanea, V.M.; Antoniac, I.; Antoniac, A.; Nemoianu, I.V.; Robu, A.; Dura, H. A Review of Biomimetic and Biodegradable Magnetic Scaffolds for Bone Tissue Engineering and Oncology. Int. J. Mol. Sci. 2023, 24, 4312. [Google Scholar] [CrossRef]
- Silva, L.H.; Silva, S.M.; Lima, E.C.; Silva, R.C.; Weiss, D.J.; Morales, M.M.; Cruz, F.F.; Rocco, P.R. Effects of static magnetic fields on natural or magnetized mesenchymal stromal cells: Repercussions for magnetic targeting. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Panseri, S.; Montesi, M.; Sandri, M.; Iafisco, M.; Adamiano, A.; Ghetti, M.; Cenacchi, G.; Tampieri, A. Magnetic Labelling of Mesenchymal Stem Cells with Iron-Doped Hydroxyapatite Nanoparticles as Tool for Cell Therapy. J. Biomed. Nanotechnol. 2016, 12, 909–921. [Google Scholar] [CrossRef]
- Sadeghzadeh, H.; Dianat-Moghadam, H.; Del Bakhshayesh, A.R.; Mohammadnejad, D.; Mehdipour, A. A review on the effect of nanocomposite scaffolds reinforced with magnetic nanoparticles in osteogenesis and healing of bone injuries. Stem Cell Res. Ther. 2023, 14, 194. [Google Scholar] [CrossRef]
- Carvalho, T.S.S.; Torres, P.M.C.; Belo, J.H.; Mano, J.; Olhero, S.M. Bioactive Magnetic Materials in Bone Tissue Engineering: A Review of Recent Findings in CaP-Based Particles and 3D-Printed Scaffolds. Adv. NanoBiomed Res. 2023, 3, 2300035. [Google Scholar] [CrossRef]
- Tang, Y.-Q.; Wang, Q.-Y.; Ke, Q.-F.; Zhang, C.-Q.; Guan, J.-J.; Guo, Y.-P. Mineralization of ytterbium-doped hydroxyapatite nanorod arrays in magnetic chitosan scaffolds improves osteogenic and angiogenic abilities for bone defect healing. Chem. Eng. J. 2020, 387, 124166. [Google Scholar] [CrossRef]
- Cojocaru, F.-D.; Balan, V.; Verestiuc, L. Advanced 3D Magnetic Scaffolds for Tumor-Related Bone Defects. Int. J. Mol. Sci. 2022, 23, 16190. [Google Scholar] [CrossRef] [PubMed]
- Alturky, A.; Alatawi, A.; Abulyazied, D.; Abomostaf, H.; Elkomy, G.; Taha, M.; Youness, R. Magnetic and dielectric properties of hybrid nanocomposites of biologically extracted hydroxyapatite/hematite/silicon dioxide for potential use in bone replacement applications. ECS J. Solid State Sci. Technol. 2023, 12, 083001. [Google Scholar] [CrossRef]
- Zeyni, V.; Karimi, S.; Namazi, H. Surface PEGylation of ZIF-8 metal-organic framework based on magnetic hydroxyapatite as a pH/magnetic targeting responsive system for anticancer drug delivery. Microporous Mesoporous Mater. 2023, 354, 112544. [Google Scholar] [CrossRef]
- Naseri, K.; Khademi, E.; Mortazavi-Derazkola, S. Introducing a new pharmaceutical agent: Facile synthesis of CuFe12O19@HAp-APTES magnetic nanocomposites and its cytotoxic effect on HEK-293 cell as an efficient in vitro drug delivery system for atenolol. Arab. J. Chem. 2023, 16, 104404. [Google Scholar] [CrossRef]
- Tian, Y.; Han, W.; Yeung, K.L. Magnetic Microsphere Scaffold-Based Soft Microbots for Targeted Mesenchymal Stem Cell Delivery. Small 2023, 19, e2300430. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, H.; Lam, K.Y. Optimization of Deformable Magnetic-Sensitive Hydrogel-Based Targeting System in Suspension Fluid for Site-Specific Drug Delivery. Mol. Pharm. 2018, 15, 4632–4642. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Zhao, R.; Luo, D.; Dempsey, E.; Wang, X.; Iqbal, M.Z.; Kong, X. Magnetic hydroxyapatite nanocomposites: The advances from synthesis to biomedical applications. Mater. Des. 2021, 197, 109269. [Google Scholar] [CrossRef]
- Mondal, S.; Manivasagan, P.; Bharathiraja, S.; Moorthy, M.S.; Kim, H.H.; Seo, H.; Lee, K.D.; Oh, J. Magnetic hydroxyapatite: A promising multifunctional platform for nanomedicine application. Int. J. Nanomed. 2017, 12, 8389–8410. [Google Scholar] [CrossRef] [PubMed]
- Marrella, A.; Iafisco, M.; Adamiano, A.; Rossi, S.; Aiello, M.; Barandalla-Sobrados, M.; Carullo, P.; Miragoli, M.; Tampieri, A.; Scaglione, S.; et al. A combined low-frequency electromagnetic and fluidic stimulation for a controlled drug release from superparamagnetic calcium phosphate nanoparticles: Potential application for cardiovascular diseases. J. R. Soc. Interface 2018, 15, 20180236. [Google Scholar] [CrossRef] [PubMed]
- Davar, M. Cancer and concerns. Doctmedico J. 2023, 3, 258–263. [Google Scholar]
- Lara-Ochoa, S.; Ortega-Lara, W.; Guerrero-Beltrán, C.E. Hydroxyapatite Nanoparticles in Drug Delivery: Physicochemistry and Applications. Pharmaceutics 2021, 13, 1642. [Google Scholar] [CrossRef]
- Foroughi, F.; Hassanzadeh-Tabrizi, S.; Bigham, A. In situ microemulsion synthesis of hydroxyapatite-MgFe2O4 nanocomposite as a magnetic drug delivery system. Mater. Sci. Eng. C 2016, 68, 774–779. [Google Scholar] [CrossRef]
- Bharath, G.; Rambabu, K.; Hai, A.; Anwer, S.; Banat, F.; Ponpandian, N. Synthesis of one-dimensional magnetite hydroxyapatite nanorods on reduced graphene oxide sheets for selective separation and controlled delivery of hemoglobin. Appl. Surf. Sci. 2020, 501, 144215. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, Q.; Cui, D.; Han, L.; Chen, L.; Xu, J.; Niu, N. Metal-polyphenol network coated magnetic hydroxyapatite for pH-activated MR imaging and drug delivery. Colloids Surf. B Biointerfaces 2023, 222, 113076. [Google Scholar] [CrossRef]
- Iafisco, M.; Drouet, C.; Adamiano, A.; Pascaud, P.; Montesi, M.; Panseri, S.; Sarda, S.; Tampieri, A. Superparamagnetic iron-doped nanocrystalline apatite as a delivery system for doxorubicin. J. Mater. Chem. B 2015, 4, 57–70. [Google Scholar] [CrossRef]
- Pusta, A.; Tertis, M.; Crăciunescu, I.; Turcu, R.; Mirel, S.; Cristea, C. Recent Advances in the Development of Drug Delivery Applications of Magnetic Nanomaterials. Pharmaceutics 2023, 15, 1872. [Google Scholar] [CrossRef] [PubMed]
- Kuwahata, A.; Adachi, Y.; Yabukami, S. Ultra-short pulse magnetic fields on effective magnetic hyperthermia for cancer therapy. AIP Adv. 2023, 13, 025145. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Jahangirian, H.; Teow, S.-Y.; Afsah-Hejri, L.; Sukri, S.N.A.M.; Kuča, K. How Magnetic Composites are Effective Anticancer Therapeutics? A Comprehensive Review of the Literature. Int. J. Nanomed. 2023, 18, 3535–3575. [Google Scholar] [CrossRef]
- Fanti, A.; Lodi, M.B.; Vacca, G.; Mazzarella, G. Numerical investigation of bone tumor hyperthermia treatment using magnetic scaffolds. IEEE J. Electromagn. RF Microw. Med. Biol. 2018, 2, 294–301. [Google Scholar] [CrossRef]
- Munir, T.; Mahmood, A.; Rasul, A.; Imran, M.; Fakhar-E-Alam, M. Biocompatible polymer functionalized magnetic nanoparticles for antimicrobial and anticancer activities. Mater. Chem. Phys. 2023, 301, 127677. [Google Scholar] [CrossRef]
- Beiranvand, M.; Farhadi, S.; Mohammadi-Gholami, A. Ag NPs decorated on the magnetic rod-like hydroxyapatite/MIL-101(Fe) nanocomposite as an efficient catalyst for the reduction of some nitroaromatic compounds and as an effective antimicrobial agent. RSC Adv. 2023, 13, 13683–13697. [Google Scholar] [CrossRef]
- Martins, P.M.; Lima, A.C.; Ribeiro, S.; Lanceros-Mendez, S. Magnetic Nanoparticles for Biomedical Applications: From the Soul of the Earth to the Deep History of Ourselves. ACS Appl. Bio Mater. 2021, 4, 5839–5870. [Google Scholar] [CrossRef]
- Bealer, E.J.; Kavetsky, K.; Dutko, S.; Lofland, S.; Hu, X. Protein and Polysaccharide-Based Magnetic Composite Materials for Medical Applications. Int. J. Mol. Sci. 2019, 21, 186. [Google Scholar] [CrossRef]
- Izadi, A.; Meshkini, A.; Entezari, M.H. Mesoporous superparamagnetic hydroxyapatite nanocomposite: A multifunctional platform for synergistic targeted chemo-magnetotherapy. Mater. Sci. Eng. C 2019, 101, 27–41. [Google Scholar] [CrossRef]
- Katundi, D.; Bayraktar, E.; Gatamorta, F.; Miskioglu, I. Design of hydroxyapatite/magnetite (HAP/Fe3O4) based composites reinforced with ZnO and MgO for biomedical applications. Biomed. J. Sci. Tech. Res. 2019, 21, 16113. [Google Scholar] [CrossRef]
- Almutairi, L.A.; Yu, B.; Dyne, E.; Ojaym, A.A.; Kim, M.-H. Mild magnetic hyperthermia is synergistic with an antibiotic treatment against dual species biofilms consisting of S. aureus and P. aeruginosa by enhancing metabolic activity. Int. J. Hyperth. 2023, 40, 2226845. [Google Scholar] [CrossRef] [PubMed]

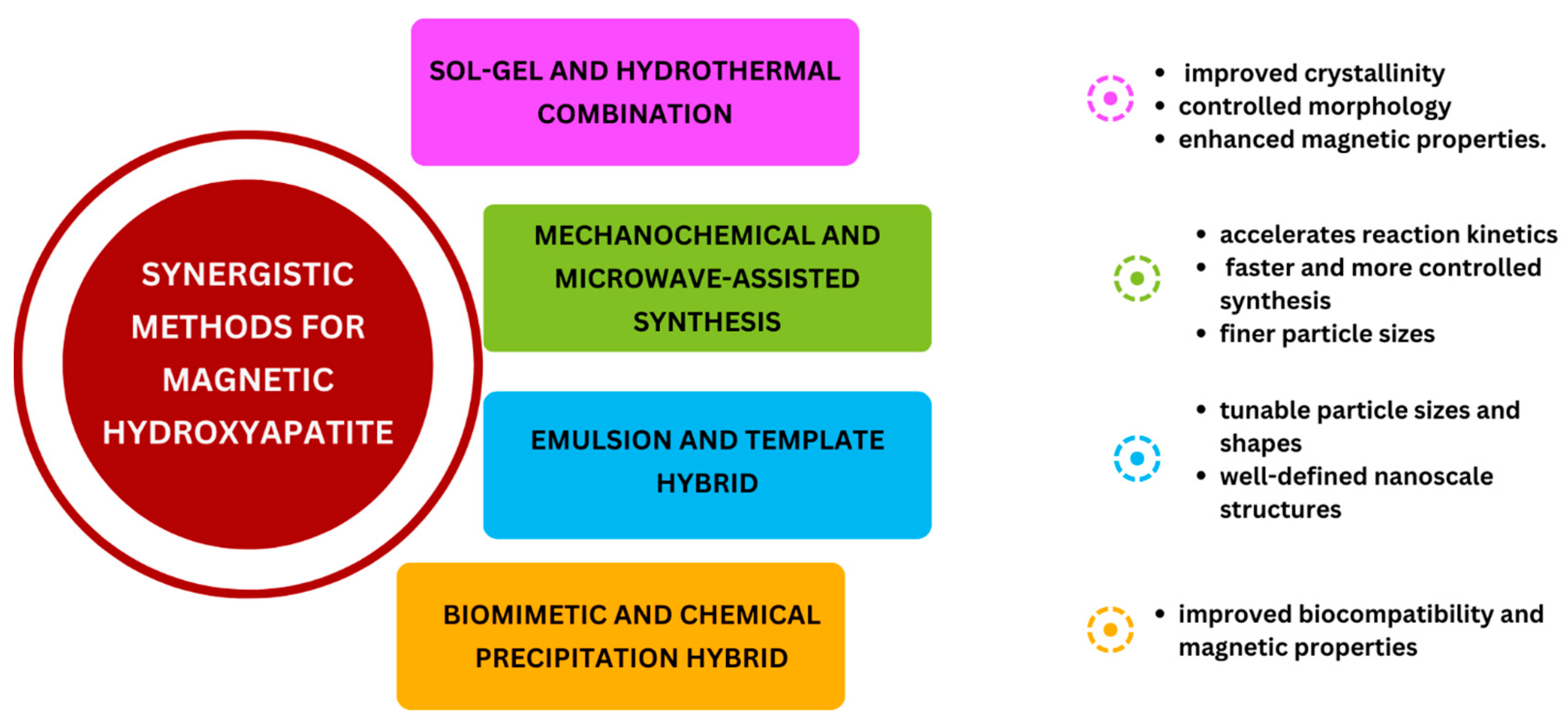
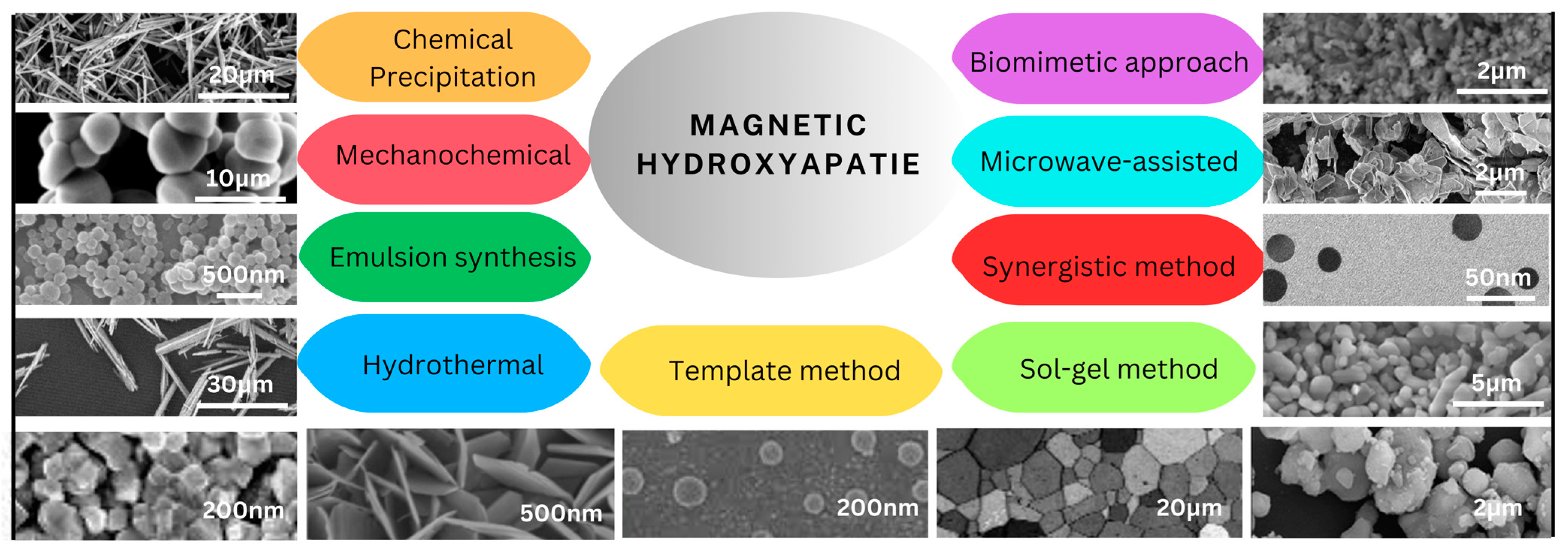
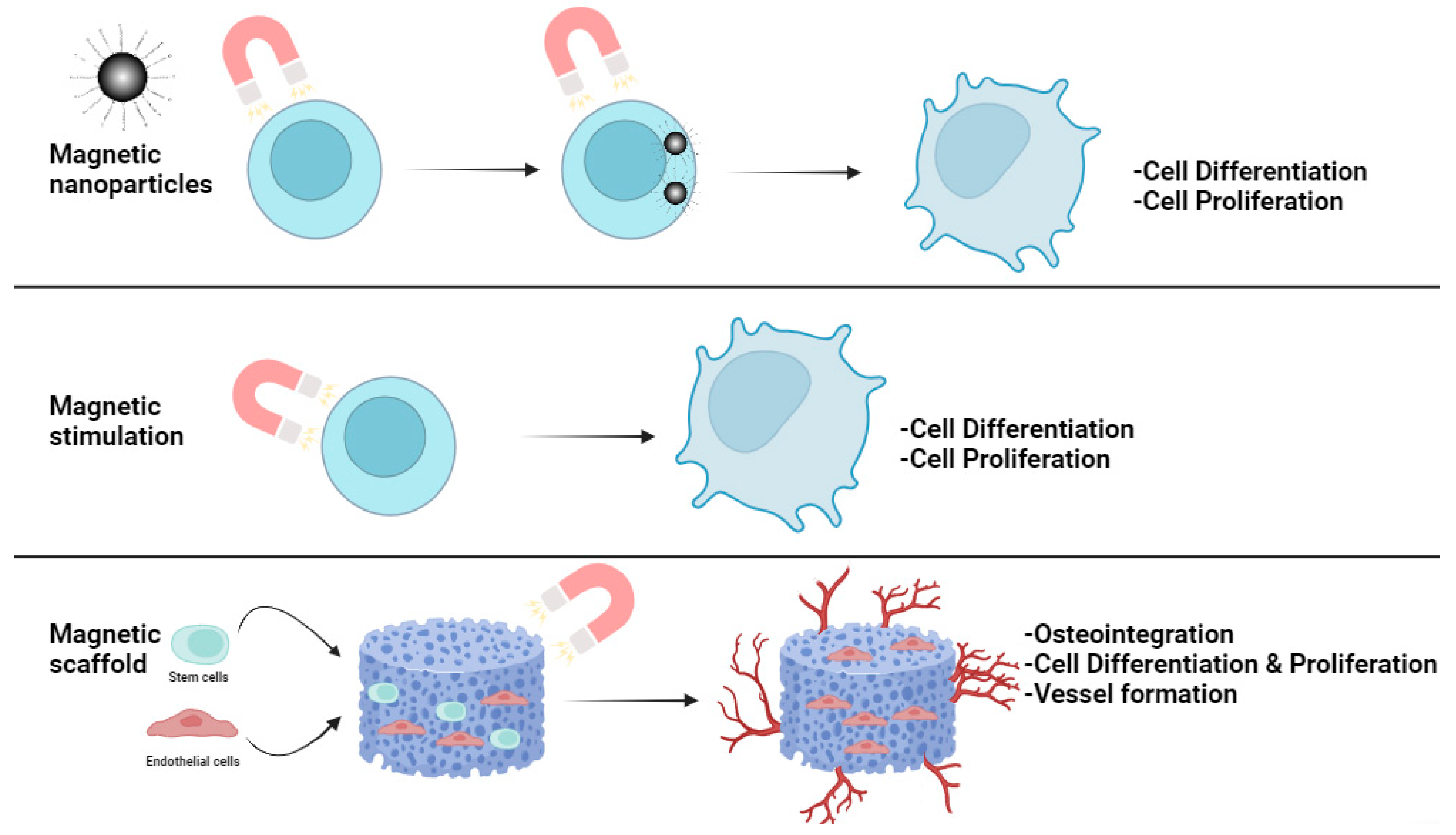
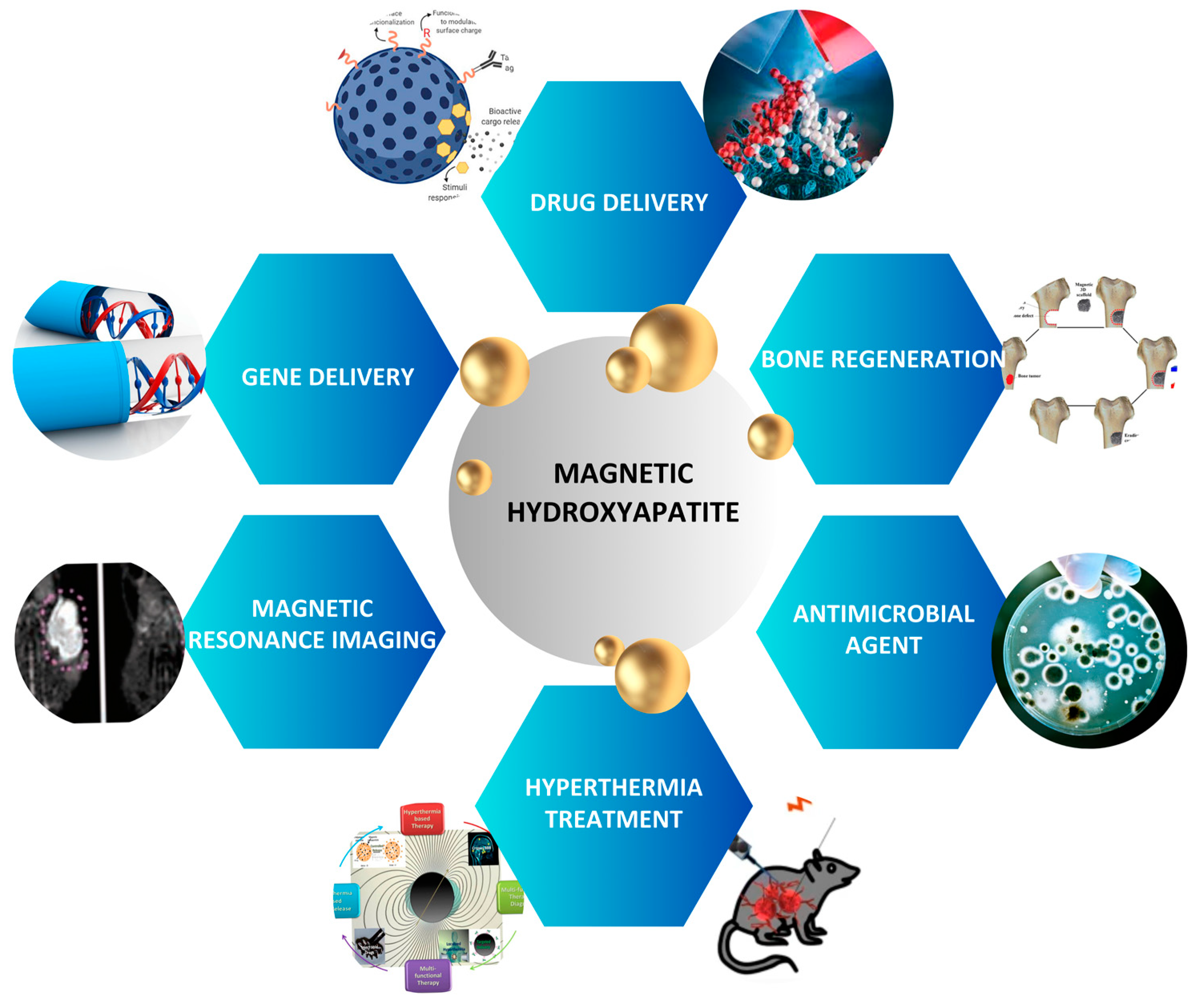
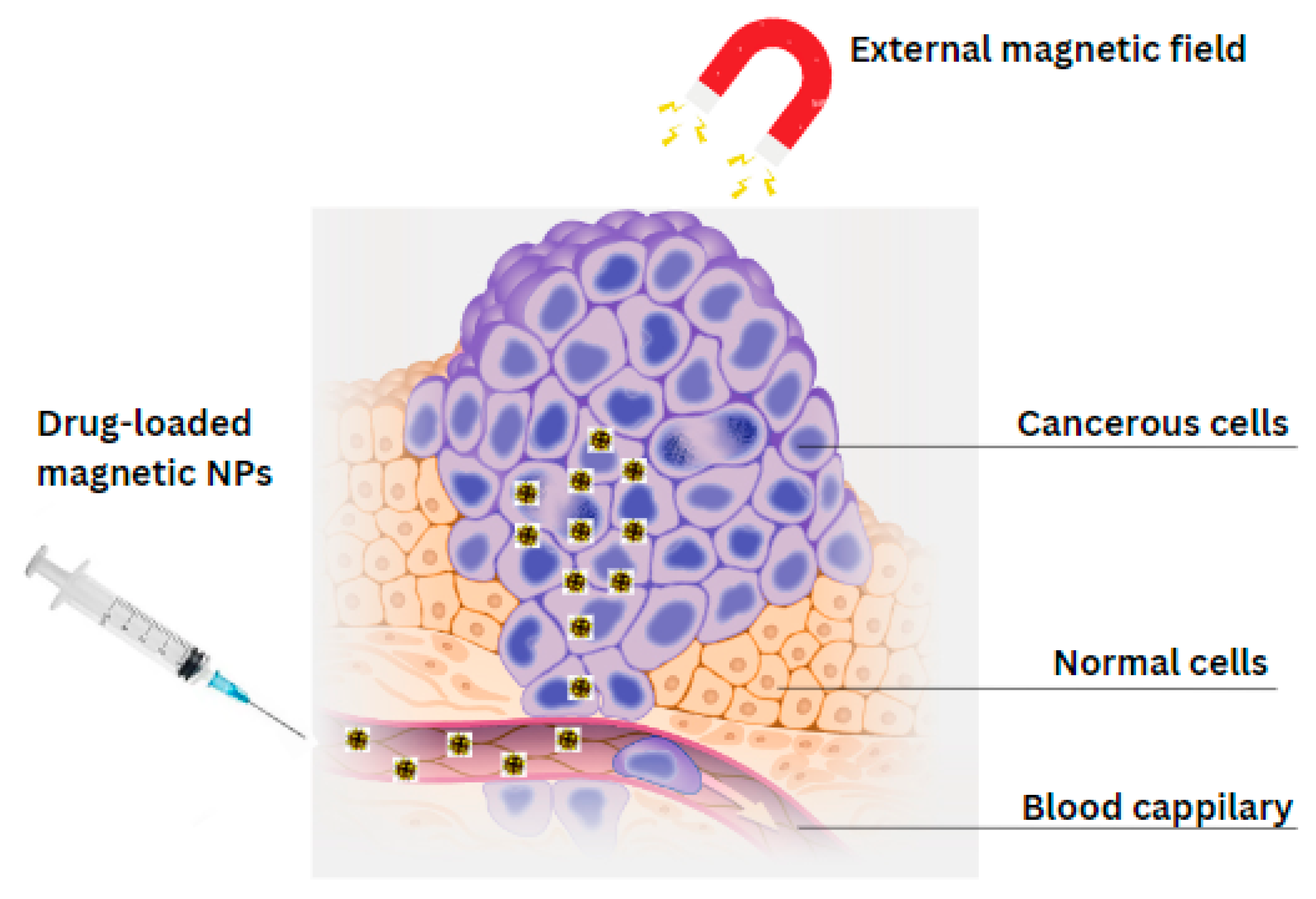
| Synthesis Method | Particles Morphology | Particles Size | Ca/P Molar Ratios | Biocompatibility | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|
| Chemical Precipitation method | Rod/Needle-like shape | 10–100 nm | Limited control (1.50–1.64) | High |
| Difficult to control particle morphology and size | [173,174,175,176,177,178] |
| Mechano-chemical method | Largely aggregated nanostructure | 5–100 nm | Generally good control (1.67–1.69) | High |
|
| [132,133,179,180,181,182] |
| Emulsion synthesis | Spherical shape | 10–400 nm | Moderate control depending on emulsification (1.50–1.67) | Moderate |
| May require the use of surfactants and stabilizers | [101,183,184,185,186,187,188] |
| Hydrothermal method | Rod-like shape | 10–200 nm | Moderate control (1.50–1.67) | High |
| Difficult to control particle size and morphology due to high temperature and pressure | [41,160,177,189,190,191,192,193,194,195] |
| Template method | Nano-spherical | 5–200 nm | Good control through template selection (1.50–1.67) | High |
|
| [184,186,196,197,198,199] |
| Sol-gel method | Fine-grained microstructure | 10–500 nm | Good control (1.35–1.68) | High |
|
| [200,201,202,203,204,205] |
| Synergistic synthesis | Spherical | 5–100 nm | Moderate control depending on synthesis methods (1.50–1.67) | High |
| Complex and time-consuming | [99,153,154,206,207,208] |
| Microwave-assisted synthesis | Highly agglomerated hexagonal platelets and small spherical particles | 10–150 nm | Moderate control depending on reaction conditions (1.50–1.67) | High |
| Non-uniform heating affect the quality and properties of synthesized nanoparticles | [178,209,210,211,212,213,214] |
| Biomimetic approach | Irregular, rough, and aggregated shapes | 10–50 nm | Moderate control depending on biomolecules used (1.50–1.67) | High |
|
| [170,172,215,216,217] |
| Type of Magnetic Field Applied | Intensity of Magnetic Field | Type of Cells | Results | Biological Effects | Advantages | Ref |
|---|---|---|---|---|---|---|
| HiMF of 16 T | 16 T | MC3T3-E1 | Iron accumulated into the cells |
|
| [231] |
| 1–2 T STF combined with Ferumoxytol | 1–2 T | Pre-osteoclast RAW264.7 cells |
| A potent tool for the translational therapy of orthopedic disorders |
| [228] |
| 0.5T SMF | 0.5T | Stem cells | MSCs’ osteogenic capacity is increased while their adipogenic differentiation potential is inhibited. |
|
| [229] |
| Static magnets | 200 mT | Stem cells | Increase in osteogenesis, gene and protein expression levels |
|
| [230] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inam, H.; Sprio, S.; Tavoni, M.; Abbas, Z.; Pupilli, F.; Tampieri, A. Magnetic Hydroxyapatite Nanoparticles in Regenerative Medicine and Nanomedicine. Int. J. Mol. Sci. 2024, 25, 2809. https://doi.org/10.3390/ijms25052809
Inam H, Sprio S, Tavoni M, Abbas Z, Pupilli F, Tampieri A. Magnetic Hydroxyapatite Nanoparticles in Regenerative Medicine and Nanomedicine. International Journal of Molecular Sciences. 2024; 25(5):2809. https://doi.org/10.3390/ijms25052809
Chicago/Turabian StyleInam, Hina, Simone Sprio, Marta Tavoni, Zahid Abbas, Federico Pupilli, and Anna Tampieri. 2024. "Magnetic Hydroxyapatite Nanoparticles in Regenerative Medicine and Nanomedicine" International Journal of Molecular Sciences 25, no. 5: 2809. https://doi.org/10.3390/ijms25052809
APA StyleInam, H., Sprio, S., Tavoni, M., Abbas, Z., Pupilli, F., & Tampieri, A. (2024). Magnetic Hydroxyapatite Nanoparticles in Regenerative Medicine and Nanomedicine. International Journal of Molecular Sciences, 25(5), 2809. https://doi.org/10.3390/ijms25052809






