Insights on the Use of Transgenic Mice Models in Alzheimer’s Disease Research
Abstract
1. Introduction
1.1. Alzheimer’s Disease Pathology
1.2. Alzheimer’s Disease Research Models and Limitations
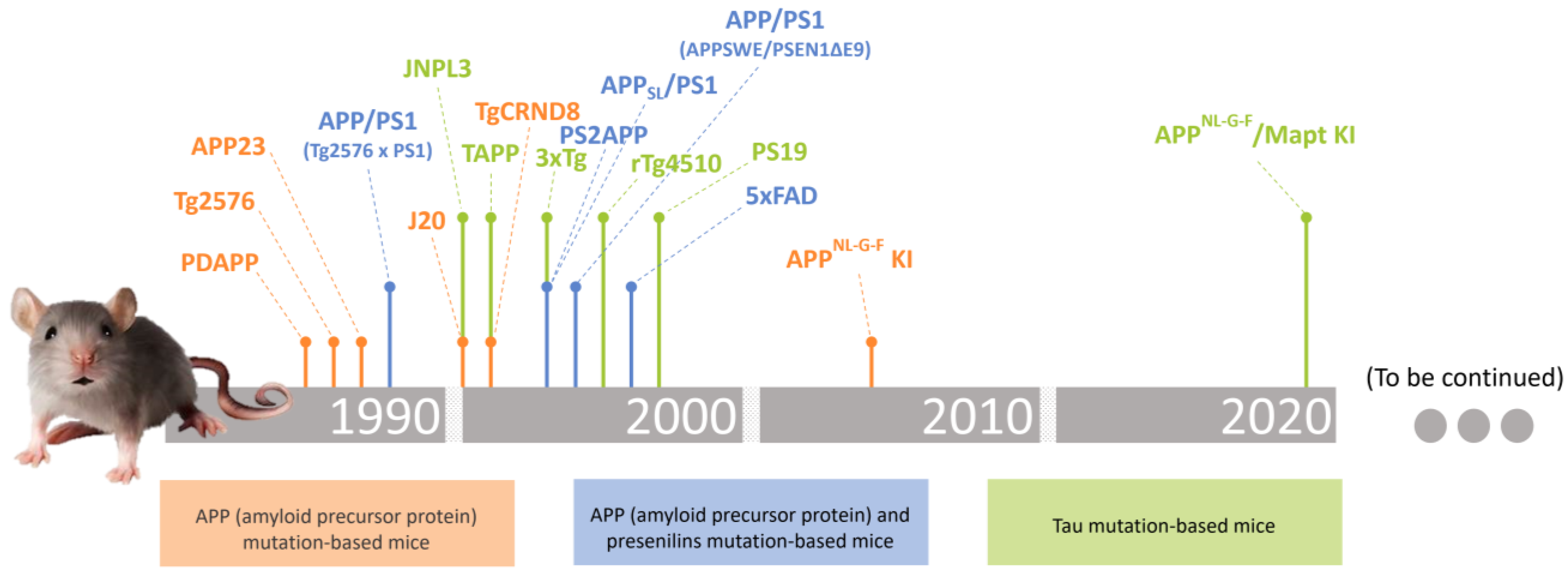
2. Transgenic Mice Models in Alzheimer’s Disease Research
2.1. APP (Amyloid Precursor Protein) Mutation-Based Mice
2.1.1. PDAPP Model
2.1.2. Tg2576 Model
2.1.3. APP23 Model
2.1.4. J20 Model
2.1.5. TgCRND8 Model
2.1.6. AppNL-G-F Knock-In Mice Model
| APP Mice Model | β-Amyloid Deposits (Onset) | Neurofibrillary Tangles (Onset) | Neuroinflammation | Neuronal Loss | Behavioural Impairments (Onset) | References |
|---|---|---|---|---|---|---|
| PDAPP |  (6–9 months) |  |  |  |  (3 months) | [46,47] |
| Tg2576 |  (11–13 months) |  |  |  |  (9 months) | [49,50] |
| APP23 |  (6 months) |  |  |  |  (3 months) | [51,52,53,54] |
| J20 |  (7–9 months) |  |  |  |  (4 months) | [55,56,57] |
| TgCRND8 |  (3 months) |  |  |  |  (3 months) | [58,59,60,61] |
| APPNL-G-F Knock-In |  (2 months) |  |  |  |  (6–9 months) | [43,64,65,66,67] |
 existing
existing  absent.
absent.2.2. APP (Amyloid Precursor Protein) and Presenilins Mutation-Based Mice
2.2.1. APP/PS1 (Tg2576 × PS1)
2.2.2. APP/PS1 (APPSWE/PSEN1ΔE9) Model
2.2.3. APPSL/PS1
2.2.4. PS2APP Model
2.2.5. APPSLPS1 Knock-In Mice Model
2.2.6. 5xFAD Model
| APP + PS Mice Model | β-Amyloid Deposits (Onset) | Neurofibrillary Tangles (Onset) | Neuroinflammation | Neuronal Loss | Behavioural Impairments (Onset) | References |
|---|---|---|---|---|---|---|
| APP/PS1 (Tg2576 × PS1) |  (6 months) |  |  |  |  (3–6 months) | [70,71,72,73] |
| APP/PS1 (APPswe/PSEN1ΔE9) |  (4 months) |  |  |  |  (8 months) | [75,76,77,78,79,80,81,82,83] |
| APPSL/PS1 |  (2.5 months) |  |  |  |  (9 months) | [85,86,87,88,89,90] |
| PS2APP |  (5–6 months) |  |  |  |  (7–8 months) | [92,93,94,95] |
| APPSLPS1 Knock-In |  (2–3 months) |  |  |  |  (6 months) | [98,99,100,101,102] |
| 5xFAD |  (2 months) |  |  |  |  (1–4 months) | [105,106,107,108,109,110,111,112,113] |
 existing
existing  absent.
absent.2.3. Tau Gene Mutation-Based Mice
2.3.1. JNPL3 Model
2.3.2. PS19 Model
2.3.3. rTg4510 Model
2.3.4. TAPP Model
2.3.5. 3xTg Model
2.3.6. APPNL-G-F/Mapt Knock-In Mice Model
| Tau Mice Model | β-Amyloid Deposits (Onset) | Neurofibrillary Tangles (Onset) | Neuroinflammation | Neuronal Loss | Behavioural Impairments (Onset) | References |
|---|---|---|---|---|---|---|
| JNPL3 |  |  (4.5 months) |  |  |  (7–10 months) | [118,119,120] |
| PS19 |  |  (5–6 months) |  |  |  (3–8 months) | [122,123,124,125] |
| rTg4510 |  |  (4 months) |  |  |  (2.5–4 months) | [126,127,128,129,130] |
| TAPP |  (6 months) |  (3 months) |  |  |  (7–10 months) | [131,132,133,134] |
| 3xTg |  (6 months) |  (12 months) |  |  |  (6 months) | [135,136,137,138,139] |
| APPNL-G-F/Mapt Knock-In |  (6 months) |  |  |  |  (9 months) | [140,141,142,143] |
 existing
existing  absent.
absent.3. Mice Modelling in Alzheimer’s Disease Research: Pros and Cons
4. Final Considerations and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s Dement. 2013, 9, 63–75. [Google Scholar] [CrossRef]
- WHO—World Health Organization. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 9 November 2023).
- Eurostat. Demography 2023 Edition. Available online: https://ec.europa.eu/eurostat/web/interactive-publications/demography-2023 (accessed on 10 November 2023).
- Harman, D. The aging process (free radicals/evolution/antioxidants/degenerative diseases/longevity). Proc. Natl. Acad. Sci. USA 1981, 78, 7124–7128. [Google Scholar] [CrossRef]
- Kritsilis, M.; Rizou, S.; Koutsoudaki, P.N.; Evangelou, K.; Gorgoulis, V.G.; Papadopoulos, D. Ageing, cellular senescence and neurodegenerative disease. Int. J. Mol. Sci. 2018, 19, 2937. [Google Scholar] [CrossRef]
- Bárrios, M.J.; Marques, R.; Fernandes, A.A. Aging with health: Aging in place strategies of a Portuguese population aged 65 years or older. Rev. Saúde Pública 2020, 54, 129. [Google Scholar] [CrossRef]
- WHO—World Health Organization. Dementia. Available online: https://www.who.int/news-room/facts-in-pictures/detail/dementia (accessed on 9 November 2023).
- Long, S.; Benoist, C.; Weidner, W. World Alzheimer Report 2023: Reducing Dementia Risk: Never too Early, Never too Late; Alzheimer’s Disease International: London, UK, 2023; 96p. [Google Scholar]
- Nunes, B.; Silva, R.D.; Cruz, V.T.; Roriz, J.M.; Pais, J.; Silva, M.C. Prevalence and pattern of cognitive impairment in rural and urban populations from Northern Portugal. BMC Neurol. 2010, 10, 42. [Google Scholar] [CrossRef]
- Gonçalves-Pereira, M.; Cardoso, A.; Verdelho, A.; Alves da Silva, J.; Caldas de Almeida, M.; Fernandes, A.; Raminhos, C.; Ferri, C.P.; Prina, A.M.; Price, M.; et al. The prevalence of dementia in a Portuguese community sample: A 10/66 dementia research group study. BMC Geriatr. 2017, 17, 261. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, R.L.; Ellis, C.E. Alzheimer’s disease and Parkinson’s disease. N. Engl. J. Med. 2003, 348, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Caito, S.W.; Newell-Caito, J.L. Simple in vivo models of Alzheimer’s Disease. In Drug Discovery Approaches for the Treatment of Neurodegenerative Disorders; Adejare, A., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2017; pp. 211–233. [Google Scholar]
- Armstrong, R.A. The molecular biology of senile plaques and neurofibrillary tangles in Alzheimer’s disease. Folia Neuropathol. 2009, 47, 289–299. [Google Scholar]
- Ittner, L.M.; Götz, J. Amyloid-β and tau—A toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosc. 2011, 12, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, I.B.; Wilhelmus, M.M.; Kox, M.; Veerhuis, R.; de Waal, R.M.; Verbeek, M.M. Apolipoprotein E protects cultured pericytes and astrocytes from D-Aβ1–40-mediated cell death. Brain Res. 2010, 1315, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Mawuenyega, K.G.; Sigurdson, W.; Ovod, V.; Munsell, L.; Kasten, T.; Morris, J.C.; Yarasheski, K.E.; Bateman, R.J. Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science 2010, 330, 1774. [Google Scholar] [CrossRef]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Ranjan, V.D.; Qiu, L.; Tan, E.K.; Zeng, L.; Zhang, Y. Modelling Alzheimer’s disease: Insights from in vivo to in vitro three-dimensional culture platforms. J. Tissue Eng. Regen. Med. 2018, 12, 1944–1958. [Google Scholar] [CrossRef]
- Paroni, G.; Bisceglia, P.; Seripa, D. Understanding the amyloid hypothesis in Alzheimer’s disease. J. Alzheimer’s Dis. 2019, 68, 493–510. [Google Scholar] [CrossRef]
- Guo, T.; Noble, W.; Hanger, D.P. Roles of tau protein in health and disease. Acta Neuropathol. 2017, 135, 665–704. [Google Scholar] [CrossRef] [PubMed]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments in Alzheimer disease: An update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.A.; Sosa, M.A.G.; De Gasperi, R. Transgenic mouse models of Alzheimer’s Disease. Mt. Sinai J. Med. 2010, 77, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.; Wisniewski, T. Alzheimer’s disease: Experimental models and reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Centeno, E.G.; Cimarosti, H.; Bithell, A. 2D versus 3D human induced pluripotent stem cell-derived cultures for neurodegenerative disease modelling. Mol. Neurodegener. 2018, 13, 27. [Google Scholar] [CrossRef]
- Sharma, N.S.; Karan, A.; Lee, D.; Yan, Z.; Xie, J. Advances in modeling Alzheimer’s disease in vitro. Adv. NanoBiomed Res. 2021, 1, 2100097. [Google Scholar] [CrossRef]
- Yokoyama, M.; Kobayashi, H.; Tatsumi, L.; Tomita, T. Mouse models of Alzheimer’s disease. Front. Mol. Neurosci. 2022, 15, 912995. [Google Scholar] [CrossRef]
- Sreenivasamurthy, S.; Laul, M.; Zhao, N.; Kim, T.; Zhu, D. Current progress of cerebral organoids for modeling Alzheimer’s disease origins and mechanisms. Bioeng. Transl. Med. 2023, 8, e10378. [Google Scholar] [CrossRef]
- Cenini, G.; Hebisch, M.; Iefremova, V.; Flitsch, L.J.; Breitkreuz, Y.; Tanzi, R.E.; Kim, D.Y.; Peitz, M.; Brüstle, O. Dissecting Alzheimer’s disease pathogenesis in human 2D and 3D models. Mol. Cell. Neurosc. 2021, 110, 103568. [Google Scholar] [CrossRef]
- Velasco, S.; Kedaigle, A.J.; Simmons, S.K.; Nash, A.; Rocha, M.; Quadrato, G.; Paulsen, B.; Nguyen, L.; Adiconis, X.; Regev, A.; et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 2019, 570, 523–527. [Google Scholar] [CrossRef]
- Liu, G.H.; Ding, Z.; Belmonte, J.C.I. iPSC technology to study human aging and aging-related disorders. Curr. Opin. Cell Biol. 2012, 24, 765–774. [Google Scholar] [CrossRef]
- Papaspyropoulos, A.; Tsolaki, M.; Foroglou, N.; Pantazaki, A.A. Modeling and targeting Alzheimer’s disease with organoids. Front. Pharmacol. 2020, 11, 396. [Google Scholar] [CrossRef]
- D’Avanzo, C.; Aronson, J.; Kim, Y.H.; Choi, S.H.; Tanzi, R.E.; Kim, D.Y. Alzheimer’s in 3D culture: Challenges and perspectives. Bioessays 2015, 37, 1139–1148. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, Y.; Quinti, L.; Tanzi, R.E.; Kim, D.Y. 3D culture models of Alzheimer’s disease: A road map to a “cure-in-a-dish”. Mol. Neurodegener. 2016, 11, 75. [Google Scholar] [CrossRef]
- Harvey, B.K.; Richie, C.T.; Hoffer, B.J.; Airavaara, M. Transgenic animal models of neurodegeneration based on human genetic studies. J. Neural Transm. 2011, 118, 27–45. [Google Scholar] [CrossRef][Green Version]
- Vandamme, T.F. Use of rodents as models of human diseases. J. Pharm. Bioallied Sci. 2014, 6, 2–9. [Google Scholar] [CrossRef]
- Esquerda-Canals, G.; Montoliu-Gaya, L.; Güell-Bosch, J.; Villegas, S. Mouse models of Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 57, 1171–1183. [Google Scholar] [CrossRef]
- Lecanu, L.; Papadopoulos, V. Modeling Alzheimer’s disease with non-transgenic rat models. Alz. Res. Ther. 2013, 5, 17. [Google Scholar] [CrossRef]
- Myers, A.; McGonigle, P. Overview of transgenic mouse models for Alzheimer’s disease. Curr. Protoc. Neurosci. 2019, 89, e81. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Varo, R.; Mejias-Ortega, M.; Fernandez-Valenzuela, J.J.; Nuñez-Diaz, C.; Caceres-Palomo, L.; Vegas-Gomez, L.; Sanchez-Mejias, E.; Trujillo-Estrada, L.; Garcia-Leon, J.A.; Moreno-Gonzalez, I.; et al. Transgenic mouse models of Alzheimer’s disease: An integrative analysis. Int. J. Mol. Sci. 2022, 23, 5404. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, M.; Medeiros, R.; LaFerla, F.M. Transgenic mouse models of Alzheimer disease: Developing a better model as a tool for therapeutic interventions. Curr. Pharm. Des. 2012, 18, 1131–1147. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.; Saito, T.; Saido, T.C. New mouse model of Alzheimer’s. ACS Chem. Neurosci. 2014, 5, 499–502. [Google Scholar] [CrossRef]
- Sasaguri, H.; Nilsson, P.; Hashimoto, S.; Nagata, K.; Saito, T.; De Strooper, B.; Hardy, J.; Vassar, R.; Winblad, B.; Saido, T.C. APP mouse models for Alzheimer’s disease preclinical studies. EMBO J. 2017, 36, 2473–2487. [Google Scholar] [CrossRef]
- Saito, T.; Matsuba, Y.; Mihira, N.; Takano, J.; Nilsson, P.; Itohara, S.; Iwata, N.; Saido, T.C. Single App knock-in mouse models of Alzheimer’s disease. Nat. Neurosci. 2014, 17, 661–663. [Google Scholar] [CrossRef]
- Sasaguri, H.; Hashimoto, S.; Watamura, N.; Sato, K.; Takamura, R.; Nagata, K.; Tsubuki, S.; Ohshima, T.; Yoshiki, A.; Sato, K.; et al. Recent advances in the modeling of Alzheimer’s disease. Front. Neurosc. 2022, 16, 807473. [Google Scholar] [CrossRef]
- Murrell, J.; Farlow, M.; Ghetti, B.; Benson, M.D. A mutation in the amyloid precursor protein associated with hereditary Alzheimer’s disease. Science 1991, 254, 97–99. [Google Scholar] [CrossRef]
- Games, D.; Adams, D.; Alessandrini, R.; Barbour, R.; Borthelette, P.; Blackwell, C.; Carr, T.; Clemens, J.; Donaldson, T.; Gillespie, F.; et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature 1995, 373, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Dodart, J.C.; Meziane, H.; Mathis, C.; Bales, K.R.; Paul, S.M.; Ungerer, A. Behavioral disturbances in transgenic mice overexpressing the V717F Β-amyloid precursor protein. Behav. Neurosc. 1999, 113, 982. [Google Scholar] [CrossRef] [PubMed]
- Mullan, M.; Crawford, F.; Axelman, K.; Houlden, H.; Lilius, L.; Winblad, B.; Lannfelt, L. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N–terminus of β–amyloid. Nat. Genet. 1992, 1, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, K.; Chapman, P.; Nilsen, S.; Eckman, C.; Harigaya, Y.; Younkin, S.; Yang, F.; Cole, G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 1996, 274, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Frautschy, S.A.; Yang, F.; Irrizarry, M.; Hyman, B.; Saido, T.C.; Hsiao, K.; Cole, G.M. Microglial response to amyloid plaques in APPsw transgenic mice. Am. J. Pathol. 1998, 152, 307–317. [Google Scholar] [PubMed]
- Sturchler-Pierrat, C.; Abramowski, D.; Duke, M.; Wiederhold, K.H.; Mistl, C.; Rothacher, S.; Ledermann, B.; Bürki, K.; Frey, P.; Paganetti, P.A.; et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. USA 1997, 94, 13287–13292. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, M.E.; Wiederhold, K.H.; Abramowski, D.; Phinney, A.L.; Probst, A.; Sturchler-Pierrat, C.; Staufenbiel, M.; Sommer, B.; Jucker, M. Neuron loss in APP transgenic mice. Nature 1998, 395, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.H.; Bondolfi, L.; Hunziker, D.; Schlecht, H.P.; Carver, K.; Maguire, E.; Abramowski, D.; Wiederhold, K.-H.; Sturchler-Pierrat, C.; Jucker, M.; et al. Progressive age-related impairment of cognitive behavior in APP23 transgenic mice. Neurobiol. Aging 2003, 24, 365–378. [Google Scholar] [CrossRef]
- Van Dam, D.; d’Hooge, R.; Staufenbiel, M.; Van Ginneken, C.; Van Meir, F.; De Deyn, P.P. Age-dependent cognitive decline in the APP23 model precedes amyloid deposition. Eur. J. Neurosc. 2003, 17, 388–396. [Google Scholar] [CrossRef]
- Mucke, L.; Masliah, E.; Yu, G.Q.; Mallory, M.; Rockenstein, E.M.; Tatsuno, G.; Hu, K.; Kholodenko, D.; Johnson-Wood, K.; McConlogue, L. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: Synaptotoxicity without plaque formation. J. Neurosci. 2000, 20, 4050–4058. [Google Scholar] [CrossRef]
- Wright, A.L.; Zinn, R.; Hohensinn, B.; Konen, L.M.; Beynon, S.B.; Tan, R.P.; Clark, I.A.; Abdipranoto, A.; Vissel, B. Neuroinflammation and neuronal loss precede Aβ plaque deposition in the hAPP-J20 mouse model of Alzheimer’s disease. PLoS ONE 2013, 8, e59586. [Google Scholar] [CrossRef]
- Karl, T.; Bhatia, S.; Cheng, D.; Kim, W.S.; Garner, B. Cognitive phenotyping of amyloid precursor protein transgenic J20 mice. Behav. Brain Res. 2012, 228, 392–397. [Google Scholar] [CrossRef]
- Chishti, M.A.; Yang, D.S.; Janus, C.; Phinney, A.L.; Horne, P.; Pearson, J.; Strome, R.; Zuker, N.; Loukides, J.; French, J.; et al. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J. Biol. Chem. 2001, 276, 21562–21570. [Google Scholar] [CrossRef]
- Dudal, S.; Krzywkowski, P.; Paquette, J.; Morissette, C.; Lacombe, D.; Tremblay, P.; Gervais, F. Inflammation occurs early during the Aβ deposition process in TgCRND8 mice. Neurobiol. Aging 2004, 25, 861–871. [Google Scholar] [CrossRef]
- Ugolini, F.; Lana, D.; Nardiello, P.; Nosi, D.; Pantano, D.; Casamenti, F.; Giovannini, M.G. Different patterns of neurodegeneration and glia activation in CA1 and CA3 hippocampal regions of TgCRND8 mice. Front. Aging Neurosci. 2018, 10, 372. [Google Scholar] [CrossRef]
- Romberg, C.; Horner, A.E.; Bussey, T.J.; Saksida, L.M. A touch screen-automated cognitive test battery reveals impaired attention, memory abnormalities, and increased response inhibition in the TgCRND8 mouse model of Alzheimer’s disease. Neurobiol. Aging 2013, 34, 731–744. [Google Scholar] [CrossRef]
- Nilsberth, C.; Westlind-Danielsson, A.; Eckman, C.B.; Condron, M.M.; Axelman, K.; Forsell, C.; Stenh, C.; Luthman, J.; Teplow, D.B.; Younkin, S.G.; et al. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Aβ protofibril formation. Nat. Neurosci. 2001, 4, 887–893. [Google Scholar] [CrossRef]
- Guardia-Laguarta, C.; Pera, M.; Clarimón, J.; Molinuevo, J.L.; Sánchez-Valle, R.; Lladó, A.; Coma, M.; Gómez-Isla, T.; Blesa, R.; Ferrer, I.; et al. Clinical, neuropathologic, and biochemical profile of the amyloid precursor protein I716F mutation. J. Neuropathol. Exp. Neurol. 2010, 69, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Mehla, J.; Lacoursiere, S.G.; Lapointe, V.; McNaughton, B.L.; Sutherland, R.J.; McDonald, R.J.; Mohajerani, M.H. Age-dependent behavioral and biochemical characterization of single APP knock-in mouse (APPNL-GF/NL-GF) model of Alzheimer’s disease. Neurobiol. Aging 2019, 75, 25–37. [Google Scholar] [CrossRef]
- Latif-Hernandez, A.; Sabanov, V.; Ahmed, T.; Craessaerts, K.; Saito, T.; Saido, T.; Balschun, D. The two faces of synaptic failure in AppNL-GF knock-in mice. Alz. Res. Ther. 2020, 12, 100. [Google Scholar] [CrossRef]
- Masuda, A.; Kobayashi, Y.; Kogo, N.; Saito, T.; Saido, T.C.; Itohara, S. Cognitive deficits in single App knock-in mouse models. Neurobiol. Learn. Mem. 2016, 135, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, Y.; Sekiya, M.; Saito, T.; Saido, T.C.; Iijima, K.M. Cognitive and emotional alterations in App knock-in mouse models of Aβ amyloidosis. BMC Neurosci. 2018, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Sapiéns, M.A.; Reza-Zaldívar, E.E.; Márquez-Aguirre, A.L.; Gómez-Pinedo, U.; Matias-Guiu, J.; Cevallos, R.R.; Mateos-Díaz, J.C.; Sánchez-González, V.J.; Canales-Aguirre, A.A. Presenilin mutations and their impact on neuronal differentiation in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Duff, K.; Eckman, C.; Zehr, C.; Yu, X.; Prada, C.-M.; Perez-Tur, J.; Hutton, M.; Buee, L.; Harigaya, Y.; Yager, D.; et al. Increased amyloid-β42 (43) in brains of mice expressing mutant presenilin 1. Nature 1996, 383, 710–713. [Google Scholar] [CrossRef]
- Holcomb, L.; Gordon, M.N.; McGowan, E.; Yu, X.; Benkovic, S.; Jantzen, P.; Wright, K.; Saad, I.; Mueller, R.; Morgan, D.; et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat. Med. 1998, 4, 97–100. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Picciano, M.; Malester, B.; LaFrancois, J.; Zehr, C.; Daeschner, J.M.; Olschowka, J.A.; Fonseca, M.I.; O’Banion, M.K.; Tenner, A.J.; et al. Inflammatory responses to amyloidosis in a transgenic mouse model of Alzheimer’s disease. Am. J. Pathol. 2001, 158, 1345–1354. [Google Scholar] [CrossRef]
- Takeuchi, A.; Irizarry, M.C.; Duff, K.; Saido, T.C.; Ashe, K.H.; Hasegawa, M.; Mann, D.M.A.; Hyman, B.T.; Iwatsubo, T. Age-related amyloid β deposition in transgenic mice overexpressing both Alzheimer mutant presenilin 1 and amyloid β precursor protein Swedish mutant is not associated with global neuronal loss. Am. J. Pathol. 2000, 157, 331–339. [Google Scholar] [CrossRef]
- Arendash, G.W.; King, D.L.; Gordon, M.N.; Morgan, D.; Hatcher, J.M.; Hope, C.E.; Diamond, D.M. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 2001, 891, 42–53. [Google Scholar] [CrossRef]
- Perez-Tur, J.; Froelich, S.; Prihar, G.; Crook, R.; Baker, M.; Duff, K.; Wragg, M.; Busfield, F.; Lendon, C.; Clark, R.F.; et al. A mutation in Alzheimer’s disease destroying a splice acceptor site in the presenilin-1 gene. Neuroreport 1995, 7, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Jankowsky, J.L.; Fadale, D.J.; Anderson, J.; Xu, G.M.; Gonzales, V.; Jenkins, N.A.; Copeland, N.G.; Lee, M.K.; Younkin, L.H.; Wagner, S.L.; et al. Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: Evidence for augmentation of a 42-specific γ secretase. Hum. Mol. Genet. 2004, 13, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alloza, M.; Robbins, E.M.; Zhang-Nunes, S.X.; Purcell, S.M.; Betensky, R.A.; Raju, S.; Prada, C.; Greenberg, S.M.; Bacskai, B.J.; Frosch, M.P. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol. Dis. 2006, 24, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Kang, Z.; Pei, G.; Le, Y. Amyloid deposition and inflammation in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Curr. Alzheimer Res. 2009, 6, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, W.; Orre, M.; Kooijman, L.; Dahmen, M.; Hol, E.M. Differential cell proliferation in the cortex of the APPswePS1dE9 Alzheimer’s disease mouse model. Glia 2012, 60, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Izco, M.; Martinez, P.; Corrales, A.; Fandos, N.; Garcia, S.; Insua, D.; Montañes, M.; Pérez-Grijalba, V.; Rueda, N.; Vidal, V.; et al. Changes in the brain and plasma Aβ peptide levels with age and its relationship with cognitive impairment in the APPswe/PS1dE9 mouse model of Alzheimer’s disease. Neuroscience 2014, 263, 269–279. [Google Scholar] [CrossRef]
- Toledo, E.M.; Inestrosa, N.C. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1ΔE9 mouse model of Alzheimer’s disease. Mol. Psychiatry 2010, 15, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Li, M.; Zhou, Y.; Ma, L.; Qiao, Q.; Hu, W.; Li, W.; Wills, Z.P.; Gan, W.B. Abnormal dendritic calcium activity and synaptic depotentiation occur early in a mouse model of Alzheimer’s disease. Mol. Neurodegener. 2017, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, R.; Kim, H.D.; Maxwell, J.A.; Fukuchi, K. Exploratory activity and spatial learning in 12-month-old APP695SWE/co+ PS1/ΔE9 mice with amyloid plaques. Neurosci. Lett. 2005, 390, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, L.A.; Frajmund, L.A.; van Nuijs, D.; van der Heijden, D.C.; Middeldorp, J.; Hol, E.M. Both male and female APPswe/PSEN1dE9 mice are impaired in spatial memory and cognitive flexibility at 9 months of age. Neurobiol. Aging 2022, 113, 28–38. [Google Scholar] [CrossRef]
- Goate, A.; Chartier-Harlin, M.-C.; Mullan, M.; Brown, J.; Crawford, F.; Fidani, L.; Giuffra, L.; Haynes, A.; Irving, N.; James, L.; et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 1991, 349, 704–706. [Google Scholar] [CrossRef]
- Blanchard, V.; Moussaoui, S.; Czech, C.; Touchet, N.; Bonici, B.; Planche, M.; Canton, T.; Jedidi, I.; Gohin, M.; Wirths, O.; et al. Time sequence of maturation of dystrophic neurites associated with Aβ deposits in APP/PS1 transgenic mice. Exp. Neurol. 2003, 184, 247–263. [Google Scholar] [CrossRef]
- Trujillo-Estrada, L.; Dávila, J.C.; Sánchez-Mejias, E.; Sánchez-Varo, R.; Gomez-Arboledas, A.; Vizuete, M.; Vitorica, J.; Gutiérrez, A. Early neuronal loss and axonal/presynaptic damage is associated with accelerated amyloid-β accumulation in AβPP/PS1 Alzheimer’s disease mice subiculum. J. Alzheimers Dis. 2014, 42, 521–541. [Google Scholar] [CrossRef]
- Jimenez, S.; Baglietto-Vargas, D.; Caballero, C.; Moreno-Gonzalez, I.; Torres, M.; Sanchez-Varo, R.; Ruano, D.; Vizuete, M.; Gutierrez, A.; Vitorica, J. Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer’s disease: Age-dependent switch in the microglial phenotype from alternative to classic. J. Neurosci. 2008, 28, 11650–11661. [Google Scholar] [CrossRef]
- Gomez-Arboledas, A.; Davila, J.C.; Sanchez-Mejias, E.; Navarro, V.; Nuñez-Diaz, C.; Sanchez-Varo, R.; Sanchez-Mico, M.V.; Trujillo-Estrada, L.; Fernandez-Valenzuela, J.J.; Vizuete, M.; et al. Phagocytic clearance of presynaptic dystrophies by reactive astrocytes in Alzheimer’s disease. Glia 2018, 66, 637–653. [Google Scholar] [CrossRef]
- Schmitz, C.; Rutten, B.P.F.; Pielen, A.; Schäfer, S.; Wirths, O.; Tremp, G.; Czech, C.; Blanchard, V.; Multhaup, G.; Rezaie, P.; et al. Hippocampal neuron loss exceeds amyloid plaque load in a transgenic mouse model of Alzheimer’s disease. Am. J. Pathol. 2004, 164, 1495–1502. [Google Scholar] [CrossRef]
- Trujillo-Estrada, L.; Jimenez, S.; De Castro, V.; Torres, M.; Baglietto-Vargas, D.; Moreno-Gonzalez, I.; Navarro, V.; Sanchez-Varo, R.; Sanchez-Mejias, E.; Davila, J.C.; et al. In vivo modification of Abeta plaque toxicity as a novel neuroprotective lithium-mediated therapy for Alzheimer’s disease pathology. Acta Neuropathol. Commun. 2013, 1, 73. [Google Scholar] [CrossRef]
- Levy-Lahad, E.; Wasco, W.; Poorkaj, P.; Romano, D.M.; Oshima, J.; Pettingell, W.H.; Yu, C.; Jondro, P.D.; Schmidt, S.D.; Wang, K.; et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science 1995, 269, 973–977. [Google Scholar] [CrossRef]
- Richards, J.G.; Higgins, G.A.; Ouagazzal, A.M.; Ozmen, L.; Kew, J.N.C.; Bohrmann, B.; Malherbe, P.; Brockhaus, M.; Loetscher, H.; Czech, C.; et al. PS2APP transgenic mice, coexpressing hPS2mut and hAPPswe, show age-related cognitive deficits associated with discrete brain amyloid deposition and inflammation. J. Neurosci. 2003, 23, 8989–9003. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, L.; Albientz, A.; Czech, C.; Jacobsen, H. Expression of transgenic APP mRNA is the key determinant for beta-amyloid deposition in PS2APP transgenic mice. Neurodegener. Dis. 2009, 6, 29–36. [Google Scholar] [CrossRef]
- Focke, C.; Blume, T.; Zott, B.; Shi, Y.; Deussing, M.; Peters, F.; Schmidt, C.; Kleinberger, G.; Lindner, S.; Gildehaus, F.-J.; et al. Early and longitudinal microglial activation but not amyloid accumulation predicts cognitive outcome in PS2APP mice. J. Nucl. Med. 2019, 60, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Kallop, D.Y.; Meilandt, W.J.; Gogineni, A.; Easley-Neal, C.; Wu, T.; Jubb, A.M.; Yaylaoglu, M.; Shamloo, M.; Tessier-Lavigne, M.; Scearce-Levie, K.; et al. A death receptor 6-amyloid precursor protein pathway regulates synapse density in the mature CNS but does not contribute to Alzheimer’s disease-related pathophysiology in murine models. J. Neurosci. 2014, 34, 6425–6437. [Google Scholar] [CrossRef] [PubMed]
- Campion, D.; Brice, A.; Dumanchin, C.; Puel, M.; Baulac, M.; De La Sayette, V.; Hannequin, D.; Duyckaerts, C.; Michon, A.; Martin, C.; et al. A novel presenilin 1 mutation resulting in familial Alzheimer’s disease with an onset age of 29 years. Neuroreport 1996, 7, 1582–1584. [Google Scholar] [CrossRef]
- Kwok, J.B.J.; Taddei, K.; Hallupp, M.; Fisher, C.; Brooks, W.S.; Broe, G.A.; Hardy, J.; Fulham, M.J.; Nicholson, G.A.; Stell, R.; et al. Two novel (M233T and ρ278T) presenilin-1 mutations in early-onset Alzheimer’s disease pedigrees and preliminary evidence for association of presenilin-1 mutations with a novel phenotype. Neuroreport 1997, 8, 1537–1542. [Google Scholar] [CrossRef]
- Casas, C.; Sergeant, N.; Itier, J.M.; Blanchard, V.; Wirths, O.; Van Der Kolk, N.; Vingtdeux, V.; Van De Steeg, E.; Ret, G.; Canton, T.; et al. Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Aβ42 accumulation in a novel Alzheimer transgenic model. Am. J. Pathol. 2004, 165, 1289–1300. [Google Scholar] [CrossRef]
- Wirths, O.; Weis, J.; Kayed, R.; Saido, T.C.; Bayer, T.A. Age-dependent axonal degeneration in an Alzheimer mouse model. Neurobiol. Aging 2007, 28, 1689–1699. [Google Scholar] [CrossRef]
- Barrier, L.; Ingrand, S.; Fauconneau, B.; Page, G. Gender-dependent accumulation of ceramides in the cerebral cortex of the APPSL/PS1Ki mouse model of Alzheimer’s disease. Neurobiol. Aging 2010, 31, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Page, G.; Bilan, A.R.; Ingrand, S.; Lafay-Chebassier, C.; Pain, S.; Pochat, M.C.P.; Bouras, C.; Bayer, T.; Hugon, J. Activated double-stranded RNA-dependent protein kinase and neuronal death in models of Alzheimer’s disease. Neuroscience 2006, 139, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Wirths, O.; Breyhan, H.; Schäfer, S.; Roth, C.; Bayer, T.A. Deficits in working memory and motor performance in the APP/PS1ki mouse model for Alzheimer’s disease. Neurobiol. Aging 2008, 29, 891–901. [Google Scholar] [CrossRef]
- Sherrington, R.; Rogaev, E.I.; Liang, Y.; Rogaeva, E.A.; Levesque, G.; Ikeda, M.; Chi, H.; Lin, C.; Li, G.; Holman, K.; et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 1995, 375, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Eckman, C.B.; Mehta, N.D.; Crook, R.; Perez-tur, J.; Prihar, G.; Pfeiffer, E.; Graff-Radford, N.; Hinder, P.; Yager, D.; Zenk, B.; et al. A new pathogenic mutation in the APP gene (I716V) increases the relative proportion of Aβ42 (43). Hum. Mol. Genet. 1997, 6, 2087–2089. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Eldik, L.V.; et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Eimer, W.A.; Vassar, R. Neuron loss in the 5XFAD mouse model of Alzheimer’s disease correlates with intraneuronal Aβ42 accumulation and caspase-3 activation. Mol. Neurodegener. 2013, 8, 2. [Google Scholar] [CrossRef]
- Girard, S.D.; Baranger, K.; Gauthier, C.; Jacquet, M.; Bernard, A.; Escoffier, G.; Marchetti, E.; Khrestchatisky, M.; Rivera, S.; Roman, F.S. Evidence for early cognitive impairment related to frontal cortex in the 5XFAD mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2013, 33, 781–796. [Google Scholar] [CrossRef]
- Boza-Serrano, A.; Yang, Y.; Paulus, A.; Deierborg, T. Innate immune alterations are elicited in microglial cells before plaque deposition in the Alzheimer’s disease mouse model 5xFAD. Sci. Rep. 2018, 8, 1550. [Google Scholar] [CrossRef] [PubMed]
- Forner, S.; Kawauchi, S.; Balderrama-Gutierrez, G.; Kramár, E.A.; Matheos, D.P.; Phan, J.; Javonillo, D.I.; Tran, K.M.; Hingco, E.; da Cunha, C.; et al. Systematic phenotyping and characterization of the 5xFAD mouse model of Alzheimer’s disease. Sci. Data 2021, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- Jawhar, S.; Trawicka, A.; Jenneckens, C.; Bayer, T.A.; Wirths, O. Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Aβ aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 2012, 33, 196.e29–196.e40. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wu, D.; Gu, L.-H.; Nie, B.-B.; Qi, X.-Y.; Wang, Y.-J.; Wu, F.-F.; Li, X.-L.; Bai, F.; Chen, X.-C.; et al. Spatial learning and memory impairments are associated with increased neuronal activity in 5XFAD mouse as measured by manganese-enhanced magnetic resonance imaging. Oncotarget 2016, 7, 57556–57570. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Wu, D.; Tang, X.; Qi, X.; Li, X.; Bai, F.; Chen, X.; Ren, Q.; Zhang, Z. Myelin changes at the early stage of 5XFAD mice. Brain Res. Bull. 2018, 137, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Chang, L.; Tseng, W.; Oakley, H.; Citron, M.; Klein, W.L.; Vassar, R.; Disterhoft, J.F. Temporal memory deficits in Alzheimer’s mouse models: Rescue by genetic deletion of BACE1. Eur. J. Neurosci. 2006, 23, 251–260. [Google Scholar] [CrossRef]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 22–35. [Google Scholar] [CrossRef]
- Noble, W.; Hanger, D.P.; Miller, C.C.; Lovestone, S. The importance of tau phosphorylation for neurodegenerative diseases. Front. Neurol. 2013, 4, 83. [Google Scholar] [CrossRef]
- Ghetti, B.; Oblak, A.L.; Boeve, B.F.; Johnson, K.A.; Dickerson, B.C.; Goedert, M. Invited review: Frontotemporal dementia caused by microtubule-associated protein tau gene (MAPT) mutations: A chameleon for neuropathology and neuroimaging. Neuropathol. Appl. Neurobiol. 2015, 41, 24–46. [Google Scholar] [CrossRef]
- Hutton, M.; Lendon, C.L.; Rizzu, P.; Baker, M.; Froelich, S.; Houlden, H.; Pickering-Brown, S.; Chakraverty, S.; Isaacs, A.; Grover, A.; et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998, 393, 702–705. [Google Scholar] [CrossRef]
- Lewis, J.; McGowan, E.; Rockwood, J.; Melrose, H.; Nacharaju, P.; Van Slegtenhorst, M.; Gwinn-Hardy, K.; Murphy, M.P.; Baker, M.; Yu, X.; et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 2000, 25, 402–405. [Google Scholar] [CrossRef]
- Lin, W.L.; Lewis, J.; Corral, A.R.; Dickson, D.W.; Hutton, M. Ultrastructural pathology of neurofibrillary tangles in transgenic mice carrying mutant (P301l) human Tau gene. Microsc. Microanal. 2000, 6, 584–585. [Google Scholar] [CrossRef]
- Morgan, D.; Munireddy, S.; Alamed, J.; DeLeon, J.; Diamond, D.M.; Bickford, P.; Hutton, M.; Lewis, J.; McGowan, E.; Gordon, M.N. Apparent behavioral benefits of tau overexpression in P301L tau transgenic mice. J. Alzheimer’s Dis. 2008, 15, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Bugiani, O.; Murrell, J.R.; Giaccone, G.; Hasegawa, M.; Ghigo, G.; Tabaton, M.; Morbin, M.; Primavera, A.; Carella, F.; Solaro, C.; et al. Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in tau. J. Neuropathol. Exp. Neurol. 1999, 58, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, Y.; Higuchi, M.; Zhang, B.; Huang, S.-M.; Iwata, N.; Saido, T.C.; Maeda, J.; Suhara, T.; Trojanowski, J.Q.; Lee, V.M.-Y. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 2007, 53, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Iba, M.; Inoue, H.; Higuchi, M.; Takao, K.; Tsukita, K.; Karatsu, Y.; Iwamoto, Y.; Miyakawa, T.; Suhara, T.; et al. P301S mutant human tau transgenic mice manifest early symptoms of human tauopathies with dementia and altered sensorimotor gating. PLoS ONE 2011, 6, e21050. [Google Scholar] [CrossRef] [PubMed]
- Lyons, C.E.; Graves, S.I.; Razzoli, M.; Jeganathan, K.; Mansk, R.P.; McGonigle, S.; Sabarinathan, N.; van Deursen, J.M.; Baker, D.J.; Bartolomucci, A. Chronic social and psychological stress impact select neuropathologies in the PS19 mouse model of tauopathy. Psychosom. Med. 2023. [Google Scholar] [CrossRef]
- Briggs, D.I.; Defensor, E.; Ardestani, P.M.; Yi, B.; Halpain, M.; Seabrook, G.; Shamloo, M. Role of endoplasmic reticulum stress in learning and memory impairment and Alzheimer’s disease-like neuropathology in the PS19 and APPSwe mouse models of tauopathy and amyloidosis. eNeuro 2017, 4, ENEURO.0025-17.2017. [Google Scholar] [CrossRef]
- Ramsden, M.; Kotilinek, L.; Forster, C.; Paulson, J.; McGowan, E.; SantaCruz, K.; Guimaraes, A.; Yue, M.; Lewis, J.; Carlson, G.; et al. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J. Neurosci. 2005, 25, 10637–10647. [Google Scholar] [CrossRef]
- Santacruz, K.; Lewis, J.; Spires, T.; Paulson, J.; Kotilinek, L.; Ingelsson, M.; Guimaraes, A.; DeTure, M.; Ramsden, M.; McGowan, E.; et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 2005, 309, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Spires, T.L.; Orne, J.D.; SantaCruz, K.; Pitstick, R.; Carlson, G.A.; Ashe, K.H.; Hyman, B.T. Region-specific dissociation of neuronal loss and neurofibrillary pathology in a mouse model of tauopathy. Am. J. Pathol. 2006, 168, 1598–1607. [Google Scholar] [CrossRef]
- Gamache, J.; Benzow, K.; Forster, C.; Kemper, L.; Hlynialuk, C.; Furrow, E.; Ashe, K.H.; Koob, M.D. Factors other than hTau overexpression that contribute to tauopathy-like phenotype in rTg4510 mice. Nat. Commun. 2019, 10, 2479. [Google Scholar] [CrossRef]
- Yue, M.; Hanna, A.; Wilson, J.; Roder, H.; Janus, C. Sex difference in pathology and memory decline in rTg4510 mouse model of tauopathy. Neurobiol. Aging 2011, 32, 590–603. [Google Scholar] [CrossRef]
- Lewis, J.; Dickson, D.W.; Lin, W.L.; Chisholm, L.; Corral, A.; Jones, G.; Yen, S.H.; Sahara, N.; Skipper, L.; Yager, D.; et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 2001, 293, 1487–1491. [Google Scholar] [CrossRef]
- Yuzwa, S.A.; Shan, X.; Jones, B.A.; Zhao, G.; Woodward, M.L.; Li, X.; Zhu, Y.; McEachern, E.J.; Silverman, M.A.; Watson, N.V.; et al. Pharmacological inhibition of O-GlcNAcase (OGA) prevents cognitive decline and amyloid plaque formation in bigenic tau/APP mutant mice. Mol. Neurodegener. 2014, 9, 42. [Google Scholar] [CrossRef]
- Hudák, A.; Letoha, A.; Vizler, C.; Letoha, T. Syndecan-3 as a novel biomarker in Alzheimer’s disease. Int. J. Mol. Sci. 2022, 23, 3407. [Google Scholar] [CrossRef] [PubMed]
- Saydoff, J.A.; Olariu, A.; Sheng, J.; Hu, Z.; Li, Q.; Garcia, R.; Pei, J.; Sun, G.Y.; von Borstel, R. Uridine prodrug improves memory in Tg2576 and TAPP mice and reduces pathological factors associated with Alzheimer’s disease in related models. J. Alzheimer’s Dis. 2013, 36, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Kitazawa, M.; Tseng, B.P.; LaFerla, F.M. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol. Aging 2003, 24, 1063–1070. [Google Scholar] [CrossRef]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Aβ and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Belfiore, R.; Rodin, A.; Ferreira, E.; Velazquez, R.; Branca, C.; Caccamo, A.; Oddo, S. Temporal and regional progression of Alzheimer’s disease-like pathology in 3xTg-AD mice. Aging Cell 2019, 18, e12873. [Google Scholar] [CrossRef]
- Filali, M.; Lalonde, R.; Theriault, P.; Julien, C.; Calon, F.; Planel, E. Cognitive and non-cognitive behaviors in the triple transgenic mouse model of Alzheimer’s disease expressing mutated APP, PS1, and Mapt (3xTg-AD). Behav. Brain Res. 2012, 234, 334–342. [Google Scholar] [CrossRef]
- Stover, K.R.; Campbell, M.A.; Van Winssen, C.M.; Brown, R.E. Early detection of cognitive deficits in the 3xTg-AD mouse model of Alzheimer’s disease. Behav. Brain Res. 2015, 289, 29–38. [Google Scholar] [CrossRef]
- Saito, T.; Mihira, N.; Matsuba, Y.; Sasaguri, H.; Hashimoto, S.; Narasimhan, S.; Zhang, B.; Murayama, S.; Higuchi, M.; Lee, V.M.Y.; et al. Humanization of the entire murine Mapt gene provides a murine model of pathological human tau propagation. J. Biol. Chem. 2019, 294, 12754–12765. [Google Scholar] [CrossRef]
- Hashimoto, S.; Matsuba, Y.; Kamano, N.; Mihira, N.; Sahara, N.; Takano, J.; Muramatsu, S.; Saido, T.C.; Saito, T. Tau binding protein CAPON induces tau aggregation and neurodegeneration. Nat. Commun. 2019, 10, 2394. [Google Scholar] [CrossRef] [PubMed]
- Spurrier, J.; Nicholson, L.; Fang, X.T.; Stoner, A.J.; Toyonaga, T.; Holden, D.; Siegert, T.R.; Laird, W.; Allnutt, M.A.; Chiasseu, M.; et al. Reversal of synapse loss in Alzheimer mouse models by targeting mGluR5 to prevent synaptic tagging by C1Q. Sci. Transl. Med. 2022, 14, eabi8593. [Google Scholar] [CrossRef]
- Stoner, A.; Fu, L.; Nicholson, L.; Zheng, C.; Toyonaga, T.; Spurrier, J.; Laird, W.; Cai, Z.; Strittmatter, S.M. Neuronal transcriptome, tau and synapse loss in Alzheimer’s knock-in mice require prion protein. Alzheimers Res. Ther. 2023, 15, 201. [Google Scholar] [CrossRef] [PubMed]
- Van Zutphen, L.F.M.; Baumans, V.; Beynen, A.C. Principles of Laboratory Animal Science: A Contribution to the Humane Use and Care of Animals and to the Quality of Experimental Results, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2001; 416p. [Google Scholar]
- Folkesson, R.; Malkiewicz, K.; Kloskowska, E.; Nilsson, T.; Popova, E.; Bogdanovic, N.; Ganten, U.; Ganten, D.; Bader, M.; Winblad, B.; et al. A transgenic rat expressing human APP with the Swedish Alzheimer’s disease mutation. Biochem. Biophys. Res. Commun. 2007, 358, 777–782. [Google Scholar] [CrossRef]
- Agca, C.; Fritz, J.J.; Walker, L.C.; Levey, A.I.; Chan, A.W.S.; Lah, J.J.; Agca, Y. Development of transgenic rats producing human beta-amyloid precursor protein as a model for Alzheimer’s disease: Transgene and endogenous APP genes are regulated tissue-specifically. BMC Neurosci. 2008, 9, 28. [Google Scholar] [CrossRef]
- Liu, L.; Orozco, I.J.; Planel, E.; Wen, Y.; Bretteville, A.; Krishnamurthy, P.; Wang, L.; Herman, M.; Figueroa, H.; Yu, W.H.; et al. A transgenic rat that develops Alzheimer’s disease-like amyloid pathology, deficits in synaptic plasticity and cognitive impairment. Neurobiol. Dis. 2008, 31, 46–57. [Google Scholar] [CrossRef]
- Flood, D.G.; Lin, Y.G.; Lang, D.M.; Trusko, S.P.; Hirsch, J.D.; Savage, M.J.; Scott, R.W.; Howland, D.S. A transgenic rat model of Alzheimer’s disease with extracellular Abeta deposition. Neurobiol. Aging 2009, 30, 1078–1090. [Google Scholar] [CrossRef]
- Games, D.; Buttini, M.; Kobayashi, D.; Schenk, D.; Seubert, P. Mice as models: Transgenic approaches and Alzheimer’s disease. J. Alzheimer’s Dis. 2006, 9 (Suppl. S3), 133–149. [Google Scholar] [CrossRef]
- Price, J.L.; Morris, J.C. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann. Neurol. 1999, 45, 358–368. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures; Alzheimer’s Association Report: Chicago, IL, USA, 2023; 128p. [Google Scholar]
- Wu, W.; Ji, Y.; Wang, Z.; Wu, X.; Li, J.; Gu, F.; Chen, Z.; Wang, Z. The FDA-approved anti-amyloid-β monoclonal antibodies for the treatment of Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Med. Res. 2023, 28, 544. [Google Scholar] [CrossRef]
- Gotz, J.; Ittner, L.M. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat. Rev. Neurosci. 2008, 9, 532–544. [Google Scholar] [CrossRef]
- Tiwari, V.; Solanki, V.; Tiwari, M. In-vivo and in-vitro techniques used to investigate Alzheimer’s disease. Front. Life Sci. 2015, 8, 332–347. [Google Scholar] [CrossRef]
- Sarasa, M.; Pesini, P. Natural non-transgenic animal models for research in Alzheimer’s disease. Curr. Alzheimer Res. 2009, 6, 171–178. [Google Scholar] [CrossRef]
- Klunk, W.E. Biological markers of Alzheimer’s disease. Neurobiol. Aging 1998, 19, 145–147. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef]
- LaFerla, F.M.; Green, K.N. Animal models of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006320. [Google Scholar] [CrossRef] [PubMed]
- Kokjohn, T.A.; Rohera, A.E. Amyloid precursor protein transgenic mouse models and Alzheimer’s disease: Understanding the paradigms, limitations, and contributions. Alzheimer’s Dement. 2009, 5, 340–347. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pádua, M.S.; Guil-Guerrero, J.L.; Prates, J.A.M.; Lopes, P.A. Insights on the Use of Transgenic Mice Models in Alzheimer’s Disease Research. Int. J. Mol. Sci. 2024, 25, 2805. https://doi.org/10.3390/ijms25052805
Pádua MS, Guil-Guerrero JL, Prates JAM, Lopes PA. Insights on the Use of Transgenic Mice Models in Alzheimer’s Disease Research. International Journal of Molecular Sciences. 2024; 25(5):2805. https://doi.org/10.3390/ijms25052805
Chicago/Turabian StylePádua, Mafalda Soares, José L. Guil-Guerrero, José A. M. Prates, and Paula Alexandra Lopes. 2024. "Insights on the Use of Transgenic Mice Models in Alzheimer’s Disease Research" International Journal of Molecular Sciences 25, no. 5: 2805. https://doi.org/10.3390/ijms25052805
APA StylePádua, M. S., Guil-Guerrero, J. L., Prates, J. A. M., & Lopes, P. A. (2024). Insights on the Use of Transgenic Mice Models in Alzheimer’s Disease Research. International Journal of Molecular Sciences, 25(5), 2805. https://doi.org/10.3390/ijms25052805








