Identification of Endothelial Cell Protein C Receptor by Urinary Proteomics as Novel Prognostic Marker in Non-Recovery Kidney Injury
Abstract
1. Introduction
1.1. Clinical Needs for Novel AKI Biomarkers in Heart Failure
1.2. The Rationale for Discovering an AKI Biomarker from the Urine Proteome
1.3. Biomarker Discovery by Shotgun Proteomics
2. Results
2.1. AKI-Associated Changes of the Urine Proteome
2.2. Pathway Analysis of Dysregulated Proteins
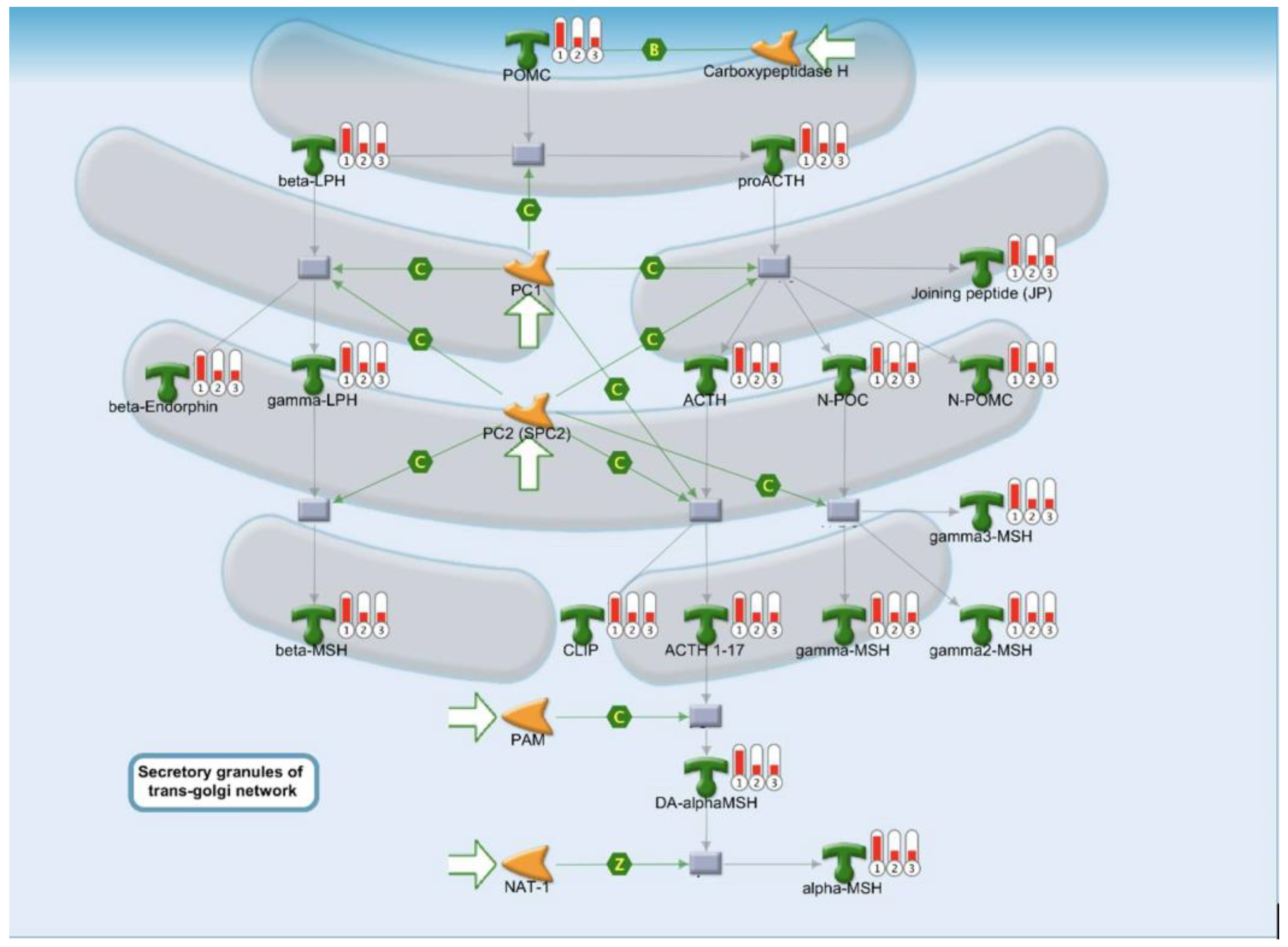
2.3. Protein Folding, Maturation, and POMC Processing Pathways
2.4. Immune Responses Via the Alternative Complement Pathway and Lectin-Induced Pathway
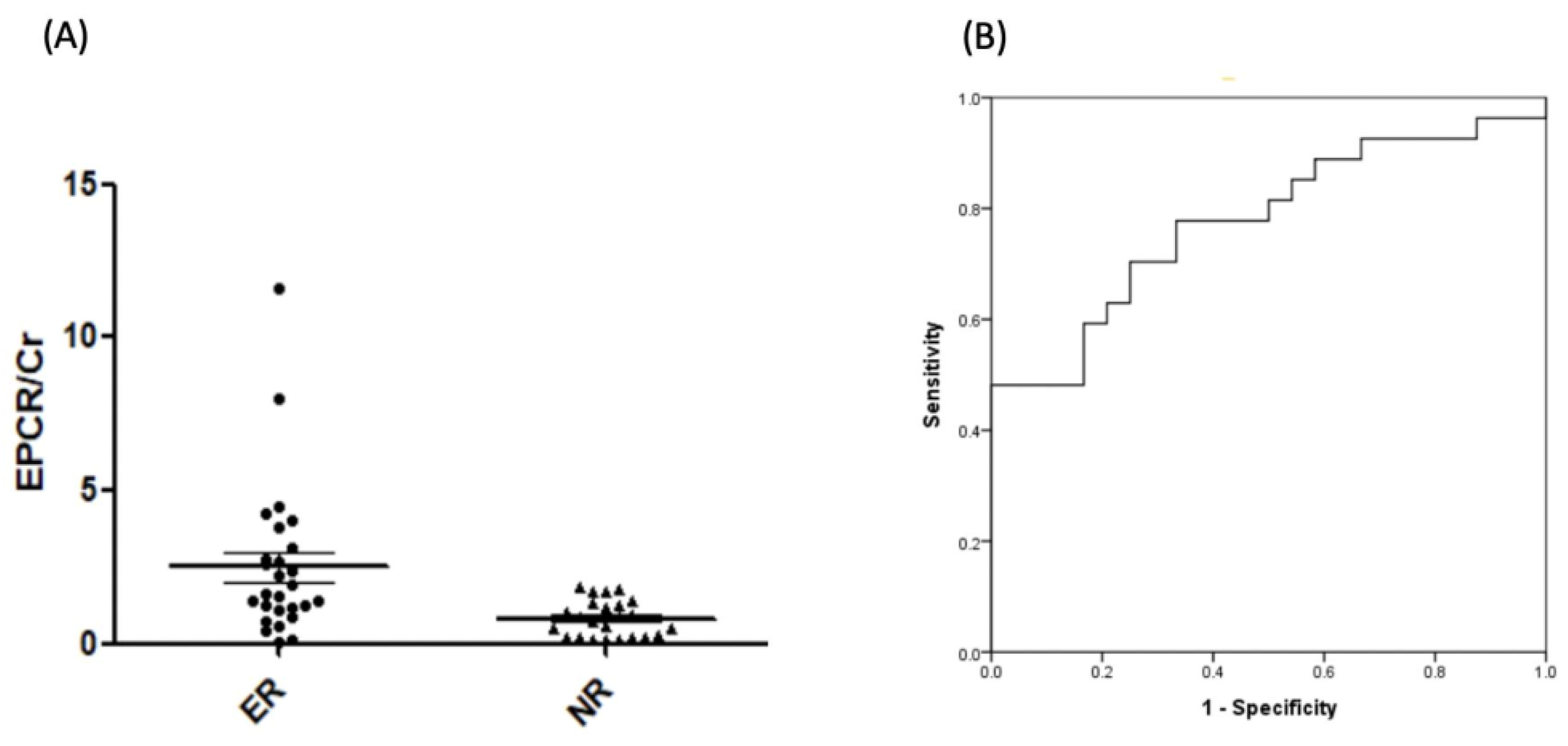
2.5. Verification of EPCR by ELISA
3. Discussion
3.1. Advances in Urinary Proteome Identification
3.2. Difficulties in Urinary Biomarker Verification
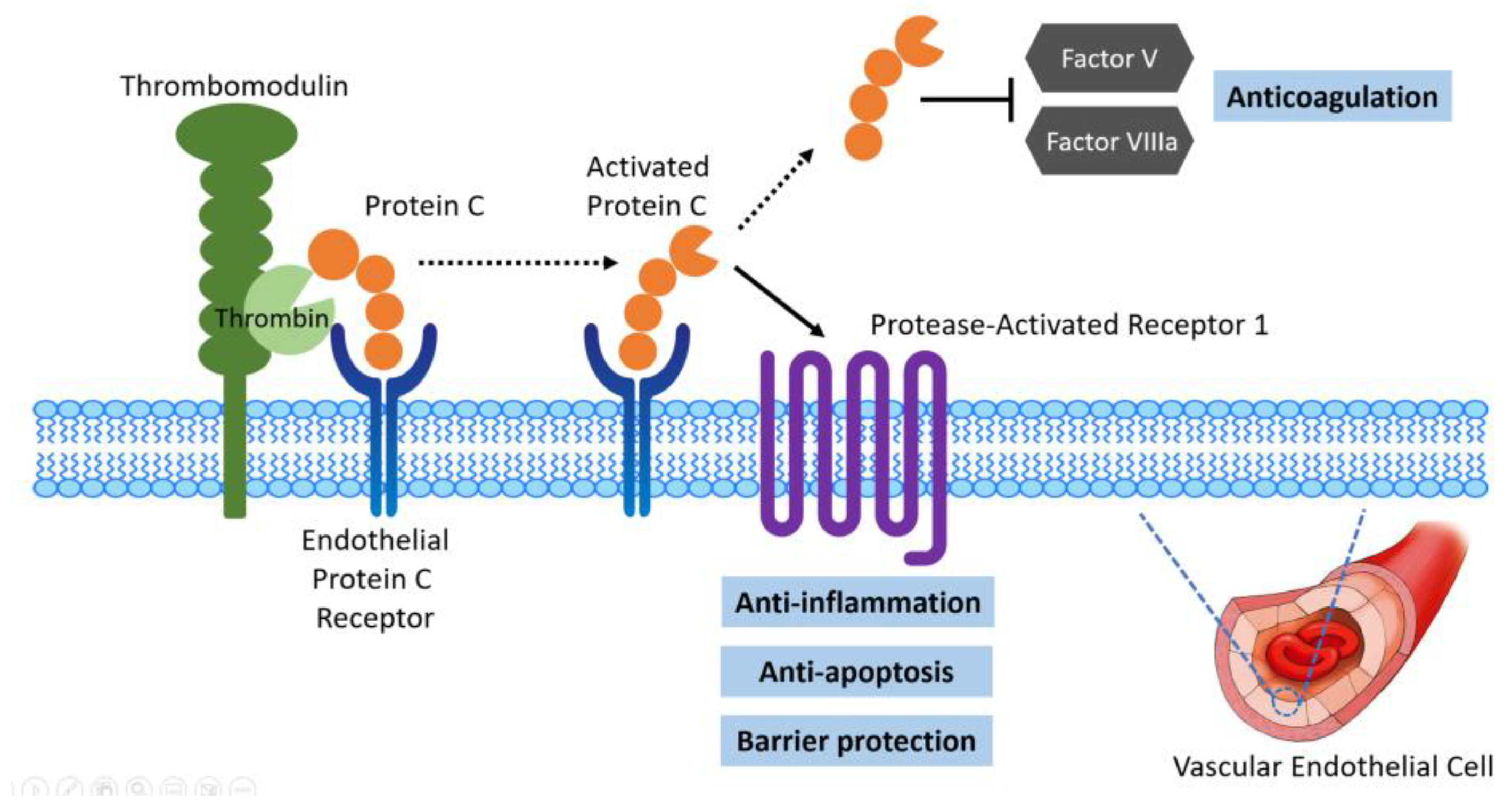
3.3. Protein Function of EPCR and Relationship with Non-Recovery of AKI
3.4. Limitations
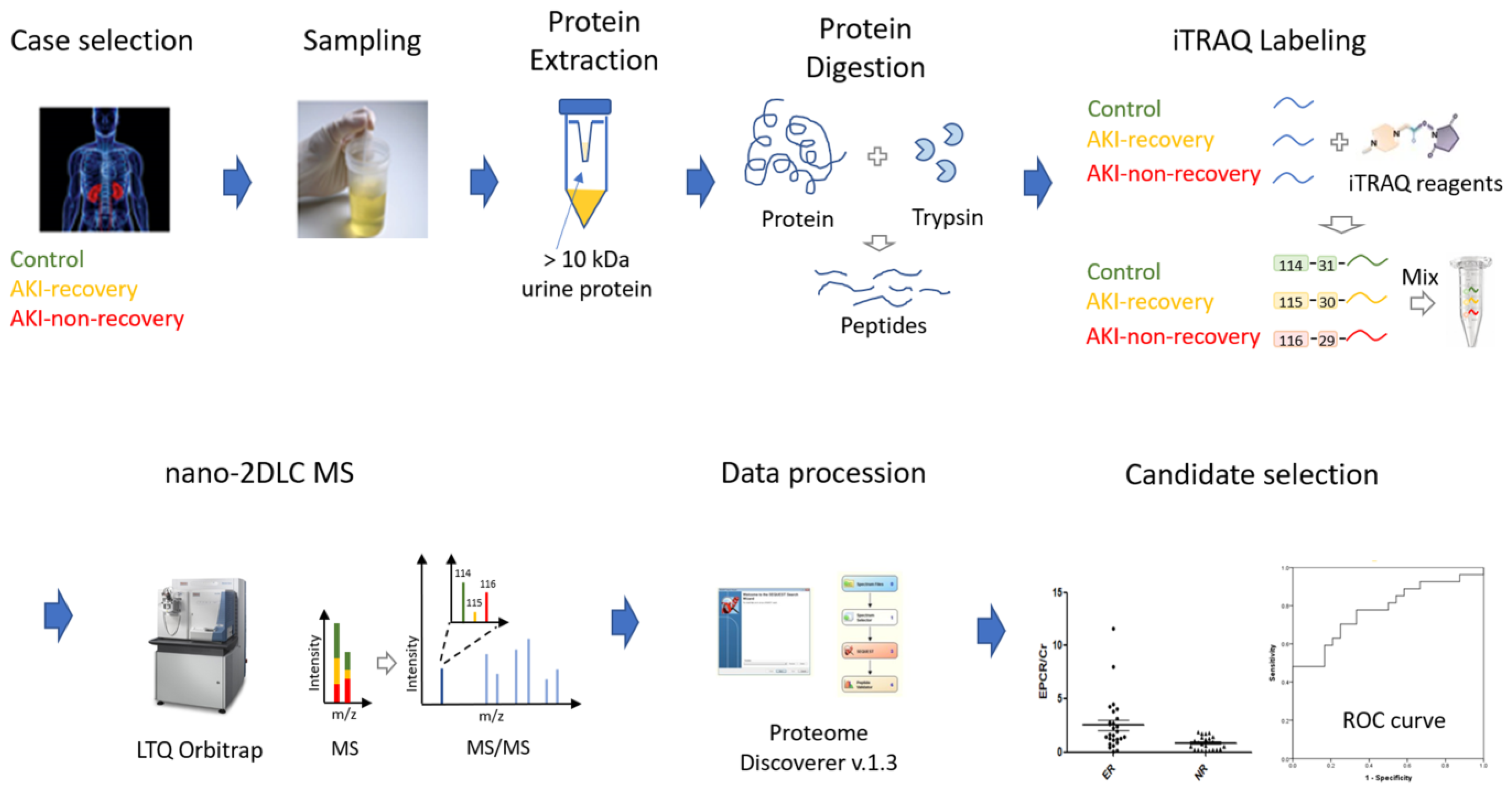
4. Methods and Materials
4.1. Patient Enrollment
4.2. Collection and Pretreatment of Urine Samples
4.3. iTRAQ Labeling and Fractionation of Labeled Peptides
4.4. LC-ESI MS/MS Analysis by Orbitrap-Elite MS
4.5. MS Data Processing, Database Searching for Protein Identification, Protein Quantification, and Target Protein Selection
4.6. ELISA for Quantification of Urinary EPCR Levels
4.7. Statistical Analysis
4.8. Network Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADHF | acute decompensated heart failure |
| AKI | acute kidney injury |
| AMI | acute myocardial infraction |
| AUROC | area under the receiver operating characteristic curve |
| BCA | bicinchoninic acid |
| BNP | B-type natriuretic peptide |
| Cr | creatinine |
| CCU | coronary care unit |
| CHF | congestive heart failure |
| CKD | chronic kidney disease |
| CRP | C-reactive protein |
| DM | diabetes mellitus |
| EPCR | endothelial cell protein C receptor |
| ER | endoplasmic reticulum |
| ICU | intensive care unit |
| IL-18 | interleukin-18 |
| IGFBP7 | insulin-like growth factor binding protein 7 |
| i-TRAQ | isobaric tag for relative and absolute quantitation |
| IRE-1 | inositol-requiring enzyme 1 |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| KIM-1 | kidney injury molecule one |
| LC | liquid chromatography |
| L-FABP | liver type fatty acid-binding protein |
| MS | mass spectrometry |
| NGAL | neutrophil gelatinase-associated lipocalin |
| TIMP-2 | tissue inhibitor of metalloproteinases 2 |
| URR | unfolded protein response |
| WSF | worsening renal function |
References
- Lee, C.C.; Kuo, G.; Chan, M.J.; Fan, P.C.; Chen, J.J.; Yen, C.L.; Tsai, T.Y.; Chen, Y.C.; Tian, Y.C.; Chang, C.H. Characteristics of and Outcomes After Dialysis-Treated Acute Kidney Injury, 2009–2018: A Taiwanese Multicenter Study. Am. J. Kidney Dis. 2023, 81, 665–674.e1. [Google Scholar] [CrossRef]
- Tsai, T.Y.; Chien, H.; Tsai, F.C.; Pan, H.C.; Yang, H.Y.; Lee, S.Y.; Hsu, H.H.; Fang, J.T.; Yang, C.W.; Chen, Y.C. Comparison of Rifle, Akin, and Kdigo Classifications for Assessing Prognosis of Patients on Extracorporeal Membrane Oxygenation. J. Formos. Med. Assoc. 2017, 116, 844–851. [Google Scholar] [CrossRef]
- Chang, C.H.; Lin, C.Y.; Tian, Y.C.; Jenq, C.C.; Chang, M.Y.; Chen, Y.C.; Fang, J.T.; Yang, C.W. Acute Kidney Injury Classification: Comparison of Akin and Rifle Criteria. Shock 2010, 33, 247–252. [Google Scholar] [CrossRef]
- McIlroy, D.R.; Wagener, G.; Lee, H.T. Biomarkers of Acute Kidney Injury: An Evolving Domain. Anesthesiology 2010, 112, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Jackson, K.; Rao, V.S.; Tang, W.H.W.; Brisco-Bacik, M.A.; Chen, H.H.; Felker, G.M.; Hernandez, A.F.; O’Connor, C.M.; Sabbisetti, V.S.; et al. Worsening Renal Function in Patients with Acute Heart Failure Undergoing Aggressive Diuresis Is Not Associated With Tubular Injury. Circulation 2018, 137, 2016–2028. [Google Scholar] [CrossRef] [PubMed]
- van Duijl, T.T.; Soonawala, D.; de Fijter, J.W.; Ruhaak, L.R.; Cobbaert, C.M. Rational Selection of a Biomarker Panel Targeting Unmet Clinical Needs in Kidney Injury. Clin. Proteom. 2021, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Liao, W.R.; Wang, H.T.; Chen, H.W.; Chen, S.F. Targeted Protein Quantitation in Human Body Fluids by Mass Spectrometry. Mass Spectrom. Rev. 2022, 42, e21788. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Gong, J.; Zhao, L.; Li, Y.; Wang, Y.; He, Q. iTRAQ-Based Comparative Proteomics Analysis Reveals Specific Urinary Biomarkers for Various Kidney Diseases. Biomark. Med. 2020, 14, 839–854. [Google Scholar] [CrossRef]
- Rauniyar, N.; Yates III, J.R. Isobaric Labeling-Based Relative Quantification in Shotgun Proteomics. J. Proteome Res. 2014, 13, 5293–5309. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Chen, C.L.; Chen, H.W.; Chung, T.; Wu, C.C.; Chen, C.D.; Hsu, C.W.; Chen, M.C.; Tsui, K.H.; Chang, P.L.; et al. Discovery of Novel Bladder Cancer Biomarkers by Comparative Urine Proteomics Using iTRAQ Technology. J. Proteome Res. 2010, 9, 5803–5815. [Google Scholar] [CrossRef]
- Chen, C.L.; Lai, Y.F.; Tang, P.; Chien, K.Y.; Yu, J.S.; Tsai, C.H.; Chen, H.W.; Wu, C.C.; Chung, T.; Hsu, C.W.; et al. Comparative and Targeted Proteomic Analyses of Urinary Microparticles from Bladder Cancer and Hernia Patients. J. Proteome Res. 2012, 11, 5611–5629. [Google Scholar] [CrossRef] [PubMed]
- Inagi, R.; Ishimoto, Y.; Nangaku, M. Proteostasis in Endoplasmic Reticulum—New Mechanisms in Kidney Disease. Nat. Rev. Nephrol. 2014, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Dara, L.; Ji, C.; Kaplowitz, N. The Contribution of Endoplasmic Reticulum Stress to Liver Diseases. Hepatology 2011, 53, 1752–1763. [Google Scholar] [CrossRef]
- Credle, J.J.; Finer-Moore, J.S.; Papa, F.R.; Stroud, R.M.; Walter, P. On the Mechanism of Sensing Unfolded Protein in the Endoplasmic Reticulum. Proc. Natl. Acad. Sci. USA 2005, 102, 18773–18784. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Shu, S.; Guo, C.; Tang, C.; Dong, Z. Endoplasmic Reticulum Stress in Ischemic and Nephrotoxic acute Kidney injury. Ann. Med. 2018, 50, 381–390. [Google Scholar] [CrossRef]
- Chang, M.; Chen, B.; Shaffner, J.; Dworkin, L.D.; Gong, R. Melanocortin System in Kidney Homeostasis and Disease: Novel Therapeutic Opportunities. Front. Physiol. 2021, 12, 651236. [Google Scholar] [CrossRef]
- Si, J.; Ge, Y.; Zhuang, S.; Wang, L.J.; Chen, S.; Gong, R. Adrenocorticotropic Hormone Ameliorates Acute Kidney Injury by Steroidogenic-Dependent and-Independent Mechanisms. Kidney Int. 2013, 83, 635–646. [Google Scholar] [CrossRef]
- Endo, M.; Ohi, H.; Ohsawa, I.; Fujita, T.; Matsushita, M.; Fujita, T. Glomerular Deposition of Mannose-Binding Lectin (MBL) Indicates a Novel Mechanism of Complement Activation in IgA Nephropathy. Nephrol. Dial. Transplant. 1998, 13, 1984–1990. [Google Scholar] [CrossRef]
- Roos, A.; Rastaldi, M.P.; Calvaresi, N.; Oortwijn, B.D.; Schlagwein, N.; van Gijlswijk-Janssen, D.J.; Stahl, G.L.; Matsushita, M.; Fujita, T.; van Kooten, C.; et al. Glomerular Activation of the Lectin Pathway of Complement in IgA Nephropathy Is Associated with More Severe Renal Disease. J. Am. Soc. Nephrol. 2006, 17, 1724–1734. [Google Scholar] [CrossRef]
- Endo, M.; Ohi, H.; Ohsawa, I.; Fujita, T.; Matsushita, M. Complement Activation through the Lectin Pathway in Patients with Henoch-Schonlein Purpura Nephritis. Am. J. Kidney Dis. 2000, 35, 401–407. [Google Scholar] [CrossRef]
- Hovind, P.; Hansen, T.K.; Tarnow, L.; Thiel, S.; Steffensen, R.; Flyvbjerg, A.; Parving, H.H. Mannose-Binding Lectin as a Predictor of Microalbuminuria in Type 1 Diabetes: An Inception Cohort Study. Diabetes 2005, 54, 1523–1527. [Google Scholar] [CrossRef]
- Asgari, E.; Farrar, C.A.; Lynch, N.; Ali, Y.M.; Roscher, S.; Stover, C.; Zhou, W.; Schwaeble, W.J.; Sacks, S.H. Mannan-Binding Lectin-Associated Serine Protease 2 Is Critical for the Development of Renal Ischemia Reperfusion Injury and Mediates Tissue Injury in the Absence of Complement C4. FASEB J. 2014, 28, 3996–4003. [Google Scholar] [CrossRef]
- Hansen, S.W.K.; Aagaard, J.B.; Bjerrum, K.B.; Hejbol, E.K.; Nielsen, O.; Schroder, H.D.; Skjoedt, K.; Sorensen, A.L.; Graversen, J.H.; Henriksen, M.L. CL-L1 and CL-K1 Exhibit Widespread Tissue Distribution with High and Co-Localized Expression in Secretory Epithelia and Mucosa. Front. Immunol. 2018, 9, 1757. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, M.H.; Catarino, S.J.; Goeldner, I.; Boldt, A.B.; de Messias-Reason, I.J. The Lectin Pathway of Complement and Rheumatic Heart Disease. Front. Pediatr. 2014, 2, 148. [Google Scholar] [CrossRef] [PubMed]
- Fliser, D.; Novak, J.; Thongboonkerd, V.; Argiles, A.; Jankowski, V.; Girolami, M.A.; Jankowski, J.; Mischak, H. Advances in Urinary Proteome Analysis and Biomarker Discovery. J. Am. Soc. Nephrol. 2007, 18, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Hao, L.; Ricke, W.A.; Li, L. Biomarker Discovery in Mass Spectrometry-Based Urinary Proteomics. Proteom. Clin. Appl. 2016, 10, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Zangar, R.C.; Daly, D.S.; White, A.M. ELISA Microarray Technology as a High-Throughput System for Cancer Biomarker Validation. Expert. Rev. Proteom. 2006, 3, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Makawita, S.; Diamandis, E.P. The Bottleneck in the Cancer Biomarker Pipeline and Protein Quantification through Mass Spectrometry-Based Approaches: Current Strategies for Candidate Verification. Clin. Chem. 2010, 56, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Addona, T.A.; Shi, X.; Keshishian, H.; Mani, D.R.; Burgess, M.; Gillette, M.A.; Clauser, K.R.; Shen, D.; Lewis, G.D.; Farrell, L.A.; et al. A Pipeline that Integrates the Discovery and Verification of Plasma Protein Biomarkers Reveals Candidate Markers for Cardiovascular Disease. Nat. Biotechnol. 2011, 29, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.A.; Murphy, R.P.; Cummins, P.M. Thrombomodulin and the Vascular Endothelium: Insights into Functional, Regulatory, and Therapeutic Aspects. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1585–H1597. [Google Scholar] [CrossRef]
- Sharfuddin, A.A.; Sandoval, R.M.; Berg, D.T.; McDougal, G.E.; Campos, S.B.; Phillips, C.L.; Jones, B.E.; Gupta, A.; Grinnell, B.W.; Molitoris, B.A. Soluble Thrombomodulin Protects Ischemic Kidneys. J. Am. Soc. Nephrol. 2009, 20, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.L.; Lee, K.T.; Chang, C.H.; Chen, Y.C.; Lin, S.M.; Chu, P.H. Elevated Plasma Thrombomodulin and Angiopoietin-2 Predict the Development of Acute Kidney Injury in Patients with Acute Myocardial Infarction. Crit. Care 2014, 18, R100. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gerlitz, B.; Richardson, M.A.; Bull, C.; Berg, D.T.; Syed, S.; Galbreath, E.J.; Swanson, B.A.; Jones, B.E.; Grinnell, B.W. Distinct Functions of Activated Protein C Differentially Attenuate Acute Kidney Injury. J. Am. Soc. Nephrol. 2009, 20, 267–277. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chen, H.W.; Domanski, D.; Smith, D.S.; Liang, K.H.; Wu, C.C.; Chen, C.L.; Chung, T.; Chen, M.C.; Chang, Y.S.; et al. Multiplexed Quantification of 63 Proteins in Human Urine by Multiple Reaction Monitoring-Based Mass Spectrometry for Discovery of Potential Bladder Cancer Biomarkers. J. Proteom. 2012, 75, 3529–3545. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Lin, T.S.; Tsai, C.H.; Wu, C.C.; Chung, T.; Chien, K.Y.; Wu, M.; Chang, Y.S.; Yu, J.S.; Chen, Y.T. Identification of Potential Bladder Cancer Markers in Urine by Abundant-Protein Depletion Coupled with Quantitative Proteomics. J. Proteom. 2013, 85, 28–43. [Google Scholar] [CrossRef]


| (A) | ||||
| iTRAQ Tags | Diagnosis | Patient Size | Average Age | iTRAQ Labeling Tag |
| 114 | Control | 15 | 73.4 ± 7.4 | 114 |
| 115 | AKI with recovery | 9 | 74.4 ± 8.5 | 115 |
| 116 | AKI with Non-recovery | 6 | 71.8 ± 3.8 | 116 |
| (B) | ||||
| With 1 Unique Peptide | ||||
| With Quantitative Results | 2384 | 2308 | ||
| Without Quantitative Results | 76 | |||
| Protein (Peptide) of False Discovery Rate | 0.01 | |||
| Both ratios of 115/114 and 116/114 tags >2 | 189 | |||
| AKI > control | 589 | |||
| Both ratios of 115/114 and 116/114 tags >2 | 189 | |||
| Ether one ratio of 115/114 or 116/114 tags >2 | 400 | |||
| AKI < control | 537 | |||
| Both ratios of 115/114 and 116/114 tags >2 | 109 | |||
| Ether one ratio of 115/114 or 116/114 tags <2 | 428 | |||
| No significant changes in concentrations | 177 | |||
| Loss of identification in one ratio; and not consistence in two ratios | 5 | |||
| AKI Patients | |||
|---|---|---|---|
| Variable | Non-Recovery (n = 24) | Recovery (n = 27) | p |
| Baseline | |||
| Age (years) | 67.1 ± 13.8 | 70.1 ± 14.0 | 0.442 |
| Gender, Male (%) | 17 (70.8) | 20 (74.1) | 1.000 |
| Diabetes mellitus, n (%) | 16 (66.7) | 10 (37.0) | 0.051 |
| Serum creatinine baseline (mg/dL) | 1.91 ± 0.97 | 1.72 ± 0.83 | 0.455 |
| Serum creatinine sample day (mg/dL) | 3.32 ± 1.52 | 3.00 ± 1.11 | 0.388 |
| APACHE III score | 51.7 ± 22.9 | 63.7 ± 33.8 | 0.150 |
| SOFA score | 5.1 ± 3.3 | 6.2 ± 4.0 | 0.295 |
| Shock, n (%) | 5 (20.8) | 6 (22.2) | 1.000 |
| Functional class | 0/20/4 | 4/19/4 | 0.145 |
| EPCR/Cr (ng/dL) | 0.82 ± 0.59 | 2.49 ± 2.49 | 0.002 |
| Outcomes | |||
| RRT, n (%) | 7 (29.2) | 0 (0.0) | 0.003 |
| LOS days | 22.2 ± 21.7 | 14.4 ± 12.3 | 0.122 |
| Mortality, n (%) | 4 (16.7) | 5 (18.5) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-H.; Lee, C.-C.; Chen, Y.-C.; Fan, P.-C.; Chu, P.-H.; Chu, L.J.; Yu, J.-S.; Chen, H.-W.; Yang, C.-W.; Chen, Y.-T. Identification of Endothelial Cell Protein C Receptor by Urinary Proteomics as Novel Prognostic Marker in Non-Recovery Kidney Injury. Int. J. Mol. Sci. 2024, 25, 2783. https://doi.org/10.3390/ijms25052783
Chang C-H, Lee C-C, Chen Y-C, Fan P-C, Chu P-H, Chu LJ, Yu J-S, Chen H-W, Yang C-W, Chen Y-T. Identification of Endothelial Cell Protein C Receptor by Urinary Proteomics as Novel Prognostic Marker in Non-Recovery Kidney Injury. International Journal of Molecular Sciences. 2024; 25(5):2783. https://doi.org/10.3390/ijms25052783
Chicago/Turabian StyleChang, Chih-Hsiang, Cheng-Chia Lee, Yung-Chang Chen, Pei-Chun Fan, Pao-Hsien Chu, Lichieh Julie Chu, Jau-Song Yu, Hsiao-Wei Chen, Chih-Wei Yang, and Yi-Ting Chen. 2024. "Identification of Endothelial Cell Protein C Receptor by Urinary Proteomics as Novel Prognostic Marker in Non-Recovery Kidney Injury" International Journal of Molecular Sciences 25, no. 5: 2783. https://doi.org/10.3390/ijms25052783
APA StyleChang, C.-H., Lee, C.-C., Chen, Y.-C., Fan, P.-C., Chu, P.-H., Chu, L. J., Yu, J.-S., Chen, H.-W., Yang, C.-W., & Chen, Y.-T. (2024). Identification of Endothelial Cell Protein C Receptor by Urinary Proteomics as Novel Prognostic Marker in Non-Recovery Kidney Injury. International Journal of Molecular Sciences, 25(5), 2783. https://doi.org/10.3390/ijms25052783








