Solution Structures of Two Different FRP-OCP Complexes as Revealed via SEC-SANS
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. SANS Experiment
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vialet-Chabrand, S.; Matthews, J.S.; Simkin, A.J.; Raines, C.A.; Lawson, T. Importance of Fluctuations in Light on Plant Photosynthetic Acclimation. Plant Physiol. 2017, 173, 2163–2179. [Google Scholar] [CrossRef] [PubMed]
- Lokstein, H.; Renger, G.; Gotze, J.P. Photosynthetic Light-Harvesting (Antenna) Complexes—Structures and Functions. Molecules 2021, 26, 3378. [Google Scholar] [CrossRef] [PubMed]
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from Cyanobacteria: Biotechnological Potential and Optimization Strategies. Biomolecules 2021, 11, 735. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Muzzopappa, F.; Kirilovsky, D. Elucidation of the essential amino acids involved in the binding of the cyanobacterial Orange Carotenoid Protein to the phycobilisome. Biochim. Biophys. Acta (BBA)—Bioenerg. 2022, 1863, 148504. [Google Scholar] [CrossRef]
- Khorobrykh, S.; Havurinne, V.; Mattila, H.; Tyystjarvi, E. Oxygen and ROS in Photosynthesis. Plants 2020, 9, 91. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, Y.; Zhang, A.; Lu, C. Non-Photochemical Quenching: From Light Perception to Photoprotective Gene Expression. Int. J. Mol. Sci. 2022, 23, 687. [Google Scholar] [CrossRef]
- Niyogi, K.K.; Truong, T.B. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr. Opin. Plant Biol. 2013, 16, 307–314. [Google Scholar] [CrossRef]
- Hussain, S.; Ulhassan, Z.; Brestic, M.; Zivcak, M.; Weijun, Z.; Allakhverdiev, S.I.; Yang, X.; Safdar, M.E.; Yang, W.; Liu, W. Photosynthesis research under climate change. Photosynth. Res. 2021, 150, 5–19. [Google Scholar] [CrossRef]
- Melnicki, M.R.; Leverenz, R.L.; Sutter, M.; Lopez-Igual, R.; Wilson, A.; Pawlowski, E.G.; Perreau, F.; Kirilovsky, D.; Kerfeld, C.A. Structure, Diversity, and Evolution of a New Family of Soluble Carotenoid-Binding Proteins in Cyanobacteria. Mol. Plant 2016, 9, 1379–1394. [Google Scholar] [CrossRef]
- Kerfeld, C.A.; Sawaya, M.R.; Brahmandam, V.; Cascio, D.; Ho, K.K.; Trevithick-Sutton, C.C.; Krogmann, D.W.; Yeates, T.O. The Crystal Structure of a Cyanobacterial Water-Soluble Carotenoid Binding Protein. Structure 2003, 11, 55–65. [Google Scholar] [CrossRef]
- Wilson, A.; Ajlani, G.; Verbavatz, J.M.; Vass, I.; Kerfeld, C.A.; Kirilovsky, D. A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria. Plant Cell 2006, 18, 992–1007. [Google Scholar] [CrossRef]
- Wilson, A.; Kinney, J.N.; Zwart, P.H.; Punginelli, C.; d’Haene, S.; Perreau, F.; Klein, M.G.; Kirilovsky, D.; Kerfeld, C.A. Structural determinants underlying photoprotection in the photoactive orange carotenoid protein of cyanobacteria. J. Biol. Chem. 2010, 285, 18364–18375. [Google Scholar] [CrossRef]
- Thurotte, A.; Lopez-Igual, R.; Wilson, A.; Comolet, L.; Bourcier de Carbon, C.; Xiao, F.; Kirilovsky, D. Regulation of Orange Carotenoid Protein Activity in Cyanobacterial Photoprotection. Plant Physiol. 2015, 169, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Konold, P.E.; Van Stokkum, I.H.M.; Muzzopappa, F.; Wilson, A.; Groot, M.L.; Kirilovsky, D.; Kennis, J.T.M. Photoactivation Mechanism, Timing of Protein Secondary Structure Dynamics and Carotenoid Translocation in the Orange Carotenoid Protein. J. Am. Chem. Soc. 2019, 141, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Orf, G.S.; Lu, Y.; Jiang, J.; King, J.D.; Wolf, N.R.; Gross, M.L.; Blankenship, R.E. Dramatic Domain Rearrangements of the Cyanobacterial Orange Carotenoid Protein upon Photoactivation. Biochemistry 2016, 55, 1003–1009. [Google Scholar] [CrossRef]
- Sutter, M.; Wilson, A.; Leverenz, R.L.; Lopez-Igual, R.; Thurotte, A.; Salmeen, A.E.; Kirilovsky, D.; Kerfeld, C.A. Crystal structure of the FRP and identification of the active site for modulation of OCP-mediated photoprotection in cyanobacteria. Proc. Natl. Acad. Sci. USA 2013, 110, 10022–10027. [Google Scholar] [CrossRef]
- Kirilovsky, D.; Kerfeld, C.A. The Orange Carotenoid Protein: A blue-green light photoactive protein. Photochem. Photobiol. Sci. 2013, 12, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Cogdell, R.J.; Gardiner, A.T. Activated OCP unlocks nonphotochemical quenching in cyanobacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 12547–12548. [Google Scholar] [CrossRef]
- Niziński, S.; Schlichting, I.; Colletier, J.-P.; Kirilovsky, D.; Burdzinski, G.; Sliwa, M. Is orange carotenoid protein photoactivation a single-photon process? Biophys. Rep. 2022, 2, 100072. [Google Scholar] [CrossRef]
- Golub, M.; Moldenhauer, M.; Schmitt, F.J.; Feoktystov, A.; Mandar, H.; Maksimov, E.; Friedrich, T.; Pieper, J. Solution Structure and Conformational Flexibility in the Active State of the Orange Carotenoid Protein: Part I. Small-Angle Scattering. J. Phys. Chem. B 2019, 123, 9525–9535. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, H.; Niedzwiedzki, D.M.; Prado, M.; Jiang, J.; Gross, M.L.; Blankenship, R.E. Molecular mechanism of photoactivation and structural location of the cyanobacterial orange carotenoid protein. Biochemistry 2014, 53, 13–19. [Google Scholar] [CrossRef]
- Chukhutsina, V.U.; van Thor, J.J. Molecular activation mechanism and structural dynamics of orange carotenoid protein. Physchem 2022, 2, 235–252. [Google Scholar] [CrossRef]
- Leverenz, R.L.; Sutter, M.; Wilson, A.; Gupta, S.; Thurotte, A.; Bourcier De Carbon, C.; Petzold, C.J.; Ralston, C.; Perreau, F.; Kirilovsky, D.; et al. A 12 Å carotenoid translocation in a photoswitch associated with cyanobacterial photoprotection. Science 2015, 348, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; King, J.D.; Wolf, N.R.; Prado, M.; Gross, M.L.; Blankenship, R.E. Mass spectrometry footprinting reveals the structural rearrangements of cyanobacterial orange carotenoid protein upon light activation. Biochim. Biophys. Acta (BBA)—Bioenerg. 2014, 1837, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Guttman, M.; Leverenz, R.L.; Zhumadilova, K.; Pawlowski, E.G.; Petzold, C.J.; Lee, K.K.; Ralston, C.Y.; Kerfeld, C.A. Local and global structural drivers for the photoactivation of the orange carotenoid protein. Proc. Natl. Acad. Sci. USA 2015, 112, E5567–E5574. [Google Scholar] [CrossRef] [PubMed]
- Bandara, S.; Ren, Z.; Lu, L.; Zeng, X.; Shin, H.; Zhao, K.H.; Yang, X. Photoactivation mechanism of a carotenoid-based photoreceptor. Proc. Natl. Acad. Sci. USA 2017, 114, 6286–6291. [Google Scholar] [CrossRef]
- Kirilovsky, D. Modulating energy transfer from Phycobilisomes to photosystems: State transitions and OCP-related non-photochemical quenching. In Photosynthesis in Algae: Biochemical and Physiological Mechanisms; Springer: Cham, Swizerland, 2020; pp. 367–396. [Google Scholar]
- Sluchanko, N.N.; Slonimskiy, Y.B.; Moldenhauer, M.; Friedrich, T.; Maksimov, E.G. Deletion of the short N-terminal extension in OCP reveals the main site for FRP binding. FEBS Lett. 2017, 591, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Martin, M.A.; Sauer, P.V.; Kirst, H.; Sutter, M.; Bina, D.; Greber, B.J.; Nogales, E.; Polivka, T.; Kerfeld, C.A. Structures of a phycobilisome in light-harvesting and photoprotected states. Nature 2022, 609, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Golub, M.; Moldenhauer, M.; Schmitt, F.J.; Lohstroh, W.; Maksimov, E.G.; Friedrich, T.; Pieper, J. Solution Structure and Conformational Flexibility in the Active State of the Orange Carotenoid Protein. Part II: Quasielastic Neutron Scattering. J. Phys. Chem. B 2019, 123, 9536–9545. [Google Scholar] [CrossRef]
- Golub, M.; Moldenhauer, M.; Schmitt, F.J.; Lohstroh, W.; Friedrich, T.; Pieper, J. Light-Induced Conformational Flexibility of the Orange Carotenoid Protein Studied by Quasielastic Neutron Scattering with In Situ Illumination. J. Phys. Chem. Lett. 2023, 14, 295–301. [Google Scholar] [CrossRef]
- Sluchanko, N.N.; Slonimskiy, Y.B.; Shirshin, E.A.; Moldenhauer, M.; Friedrich, T.; Maksimov, E.G. OCP-FRP protein complex topologies suggest a mechanism for controlling high light tolerance in cyanobacteria. Nat. Commun. 2018, 9, 3869. [Google Scholar] [CrossRef] [PubMed]
- Golub, M.; Moldenhauer, M.; Matsarskaia, O.; Martel, A.; Grudinin, S.; Soloviov, D.; Kuklin, A.; Maksimov, E.G.; Friedrich, T.; Pieper, J. Stages of OCP-FRP Interactions in the Regulation of Photoprotection in Cyanobacteria, Part 2: Small-Angle Neutron Scattering with Partial Deuteration. J. Phys. Chem. B 2023, 127, 1901–1913. [Google Scholar] [CrossRef]
- Tsoraev, G.V.; Bukhanko, A.; Budylin, G.S.; Shirshin, E.A.; Slonimskiy, Y.B.; Sluchanko, N.N.; Kloz, M.; Cherepanov, D.A.; Shakina, Y.V.; Ge, B. Stages of OCP-FRP Interactions in the Regulation of Photoprotection in Cyanobacteria, Part 1: Time-Resolved Spectroscopy. J. Phys. Chem. B 2023, 127, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Bloustine, J.; Berejnov, V.; Fraden, S. Measurements of protein-protein interactions by size exclusion chromatography. Biophys. J. 2003, 85, 2619–2623. [Google Scholar] [CrossRef] [PubMed]
- Krueger, S. Small-angle neutron scattering contrast variation studies of biological complexes: Challenges and triumphs. Curr. Opin. Struct. Biol. 2022, 74, 102375. [Google Scholar] [CrossRef]
- Whitten, A.E.; Jeffries, C.M. Chapter Three—Data analysis and modeling of small-angle neutron scattering data with contrast variation from bio-macromolecular complexes. Methods Enzymol. 2023, 678, 55–96. [Google Scholar] [CrossRef]
- Mahieu, E.; Gabel, F. Biological small-angle neutron scattering: Recent results and development. Acta Crystallogr. Sect. D: Struct. Biol. 2018, 74, 715–726. [Google Scholar] [CrossRef]
- Receveur, V.; Durand, D.; Desmadril, M.; Calmettes, P. Repulsive interparticle interactions in a denatured protein solution revealed by small angle neutron scattering. Methods Enzymol. 1998, 426, 57–61. [Google Scholar] [CrossRef]
- Poklar Ulrih, N. Analytical techniques for the study of polyphenol–protein interactions. Crit. Rev. Food Sci. Nutr. 2017, 57, 2144–2161. [Google Scholar] [CrossRef]
- Di Cola, E.; Grillo, I.; Ristori, S. Small angle X-ray and neutron scattering: Powerful tools for studying the structure of drug-loaded liposomes. Pharmaceutics 2016, 8, 10. [Google Scholar] [CrossRef]
- Pynn, R. Neutron scattering—A non-destructive microscope for seeing inside matter. In Neutron Applications in Earth, Energy and Environmental Sciences; Springer: Boston, MA, USA, 2009; pp. 15–36. [Google Scholar]
- Cardoso, M.B.; Smolensky, D.; Heller, W.T.; O’Neill, H. Insight into the structure of light-harvesting complex II and its stabilization in detergent solution. J. Phys. Chem. B 2009, 113, 16377–16383. [Google Scholar] [CrossRef]
- Golub, M.; Lokstein, H.; Soloviov, D.; Kuklin, A.; Wieland, D.C.F.; Pieper, J. Light-Harvesting Complex II Adopts Different Quaternary Structures in Solution as Observed Using Small-Angle Scattering. J. Phys. Chem. Lett. 2022, 13, 1258–1265. [Google Scholar] [CrossRef]
- Tang, K.-H.; Blankenship, R.E. Neutron and light scattering studies of light-harvesting photosynthetic antenna complexes. Photosynth. Res. 2012, 111, 205–217. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, H.; Heller, W.T.; Helton, K.E.; Urban, V.S.; Greenbaum, E. Small-angle X-ray scattering study of photosystem I− detergent complexes: Implications for membrane protein crystallization. J. Phys. Chem. B 2007, 111, 4211–4219. [Google Scholar] [CrossRef] [PubMed]
- Le, R.K.; Harris, B.J.; Iwuchukwu, I.J.; Bruce, B.D.; Cheng, X.; Qian, S.; Heller, W.T.; O’Neill, H.; Frymier, P.D. Analysis of the solution structure of Thermosynechococcus elongatus photosystem I in n-dodecyl-β-d-maltoside using small-angle neutron scattering and molecular dynamics simulation. Arch. Biochem. Biophys. 2014, 550–551, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Golub, M.; Kölsch, A.; Feoktystov, A.; Zouni, A.; Pieper, J. Insights into Solution Structures of Photosynthetic Protein Complexes from Small-Angle Scattering Methods. Crystals 2021, 1, 203. [Google Scholar] [CrossRef]
- Golub, M.; Hussein, R.; Ibrahim, M.; Hecht, M.; Wieland, D.C.F.; Martel, A.; Machado, B.; Zouni, A.; Pieper, J. Solution Structure of the Detergent-Photosystem II Core Complex Investigated by Small-Angle Scattering Techniques. J. Phys. Chem. B 2020, 124, 8583–8592. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, E.A.; Nizinski, S.; Wilson, A.; Levantino, M.; De Zitter, E.; Munro, R.; Muzzopappa, F.; Thureau, A.; Zala, N.; Burdzinski, G.; et al. Oligomerization processes limit photoactivation and recovery of the orange carotenoid protein. Biophys. J. 2022, 121, 2849–2872. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A. Phycocyanin aggregation: A small angle neutron scattering and size exclusion chromatographic study. J. Mol. Biol. 1988, 200, 579–591. [Google Scholar] [CrossRef]
- Rubinson, K.A.; Stanley, C.; Krueger, S. Small-angle neutron scattering and the errors in protein structures that arise from uncorrected background and intermolecular interactions. J. Appl. Crystallogr. 2008, 41, 456–465. [Google Scholar] [CrossRef]
- Thurotte, A.; Bourcier de Carbon, C.; Wilson, A.; Talbot, L.; Cot, S.; Lopez-Igual, R.; Kirilovsky, D. The cyanobacterial Fluorescence Recovery Protein has two distinct activities: Orange Carotenoid Protein amino acids involved in FRP interaction. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 308–317. [Google Scholar] [CrossRef]
- Martel, A.; Cocho, C.; Caporaletti, F.; Jacques, M.; El Aazzouzi, A.; Lapeyre, F.; Porcar, L. Upgraded D22 SEC–SANS setup dedicated to the biology community. J. Appl. Crystallogr. 2023, 56, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst, C. GRASANSP, Graphical Reduction and Analysis SANS Program for MatlabTM. 2001. Available online: https://www.ill.eu/sites/saxs-sans/Manuals/grasp_manual.pdf (accessed on 27 March 2023).
- Doucet, M.; Cho, J.H.; Alina, G.; Attala, Z.; Bakker, J.; Bouwman, W.; Butler, P.; Campbell, K.; Cooper-Benun, T.; Durniak, C.; et al. SasView Version 5.0.4. 2021. Available online: https://zenodo.org/records/4467703 (accessed on 4 February 2023).
- Liu, H.; Zwart, P.H. Determining pair distance distribution function from SAXS data using parametric functionals. J. Struct. Biol. 2012, 180, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Debye, P. Scattering from non-crystalline substances. Ann. Physik 1915, 46, 809–823. [Google Scholar] [CrossRef]
- Svergun, D. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 1992, 25, 495–503. [Google Scholar] [CrossRef]
- Feigin, L.A.; Svergun, D. Structure Analysis by Small-Angle X-ray and Neutron Scattering, 1st ed.; Plenum Press: New York, NY, USA, 1987. [Google Scholar]
- Franke, D.; Svergun, D. DAMMIF, a program for rapid ab-initio shape determination in small-angle scattering. J. Appl. Crystallogr. 2009, 42, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Stuhrmann, H.B. Interpretation of small-angle scattering functions of dilute solutions and gases. A representation of the structures related to a one-particle scattering function. Acta Crystallogr. Sect. A 1970, 26, 297–306. [Google Scholar] [CrossRef]
- Grudinin, S.; Garkavenko, M.; Kazennov, A. Pepsi-SAXS: An adaptive method for rapid and accurate computation of small-angle X-ray scattering profiles. Acta Crystallogr. Sect. D Struct. Biol. 2017, 73, 449–464. [Google Scholar] [CrossRef]
- Svergun, D.; Richard, S.; Koch, M.; Sayers, Z.; Kuprin, S.; Zaccai, G. Protein hydration in solution: Experimental observation by x-ray and neutron scattering. Proc. Natl. Acad. Sci. USA 1998, 95, 2267–2272. [Google Scholar] [CrossRef]

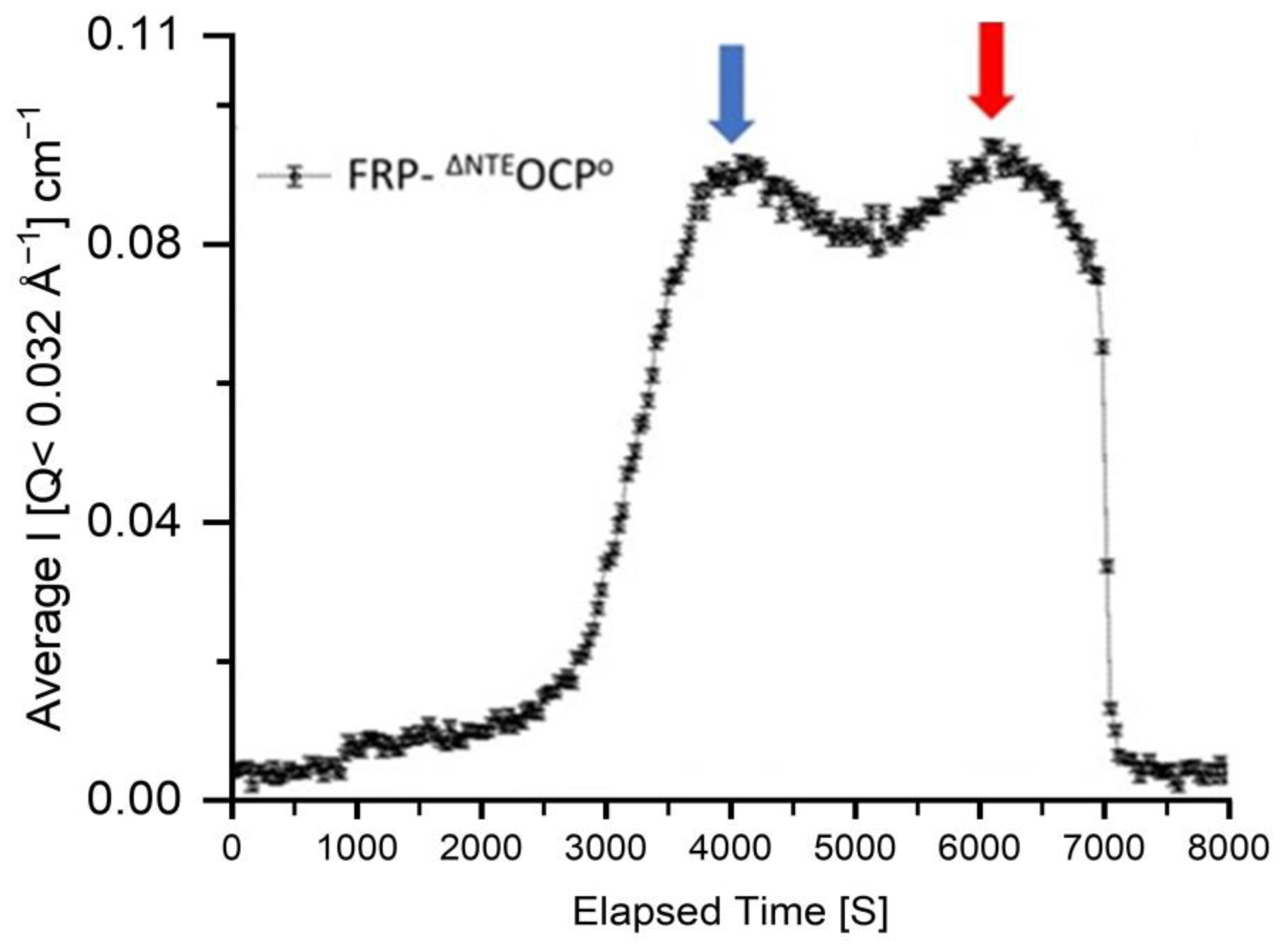



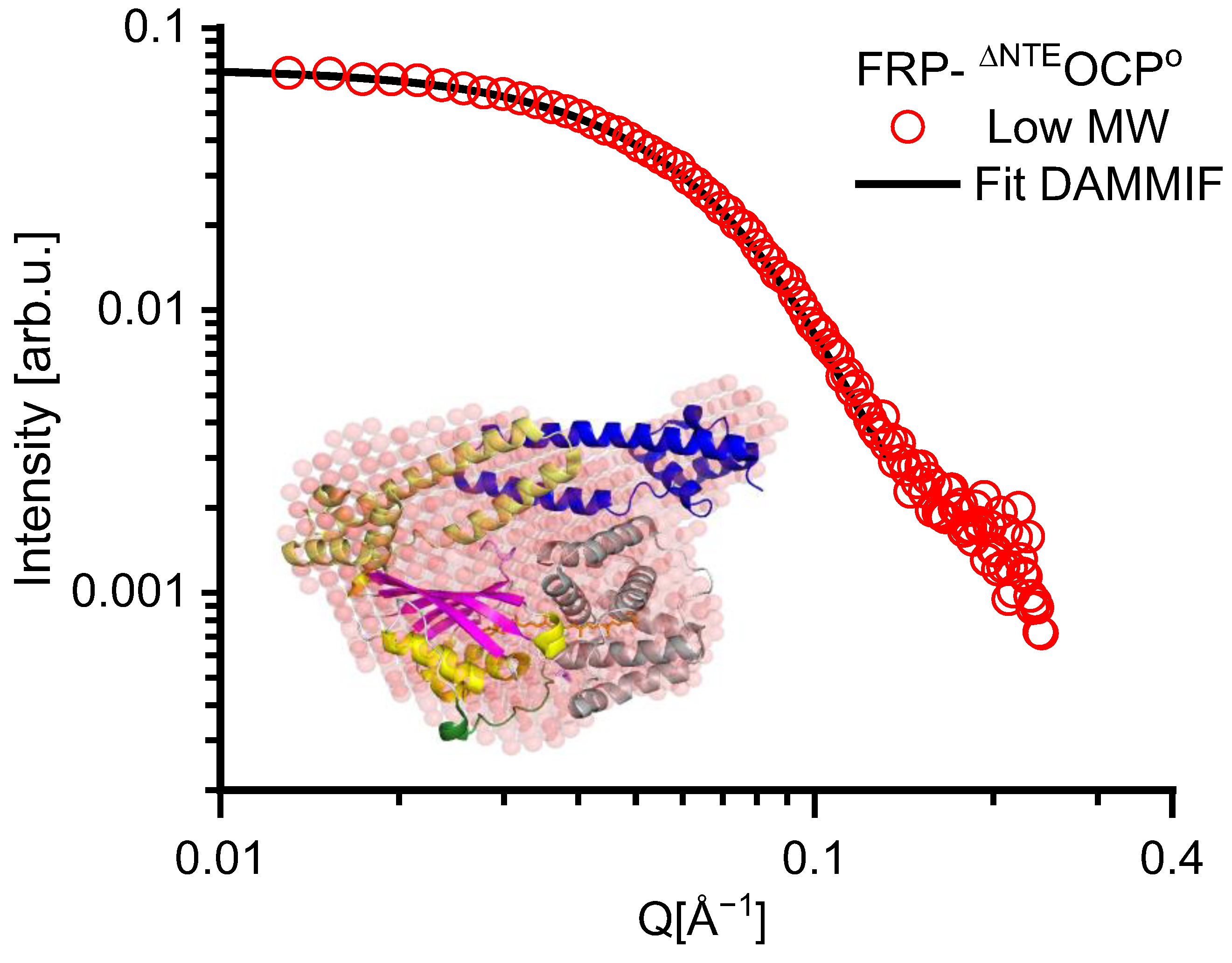
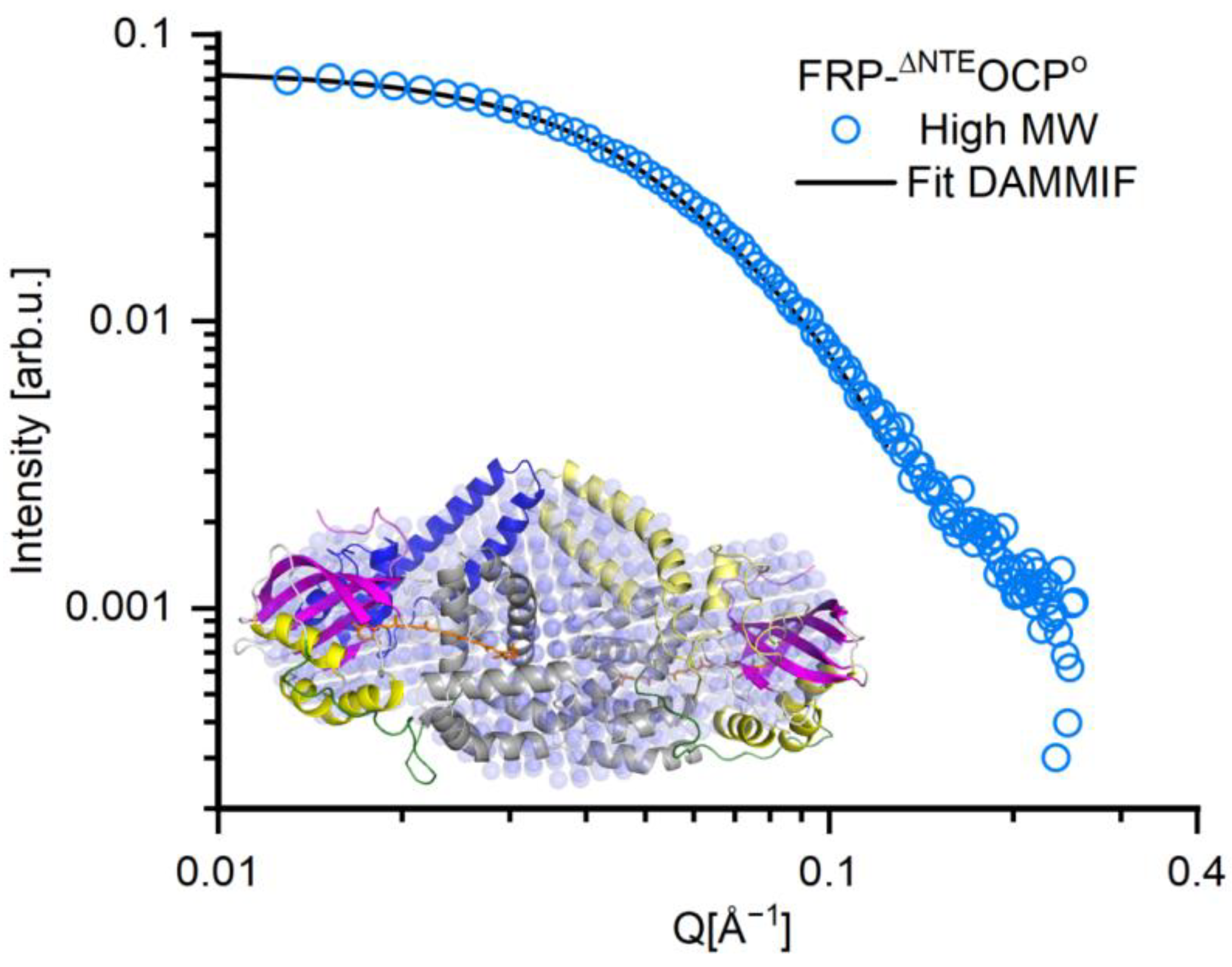

| FRP-∆NTEOCPO High MW | FRP-∆NTEOCPO Low MW | |
|---|---|---|
| Guinier Analysis | ||
| I(0) | 0.075 ± 0.00044 | 0.072 ± 0.00043 |
| Rg(Å) | 32.08 ± 0.033 | 27.04 ± 0.027 |
| QRg Range | 0.56 < QRg < 1.29 | 0.35 < QRg < 1.26 |
| P(r) Analysis | ||
| I(0) | 0.075 | 0.072 |
| Rg(Å) | 34.04 ± 0.08 | 27.51 ± 0.07 |
| DMAX(Å) | 140 | 102 |
| Ranges of data points taken for the fit | 3–113 | 1–115 |
| Kratky plot | folded | folded |
| Parameters of elliptical cylinder model | ||
| Minor radius (Å) | 13.49 ± 6.29 | 14.64 ± 0.35 |
| Length (Å) | 100 ± 10.44 | 73 ± 1.32 |
| Axis ratio | 2.06 ± 1.48 | 2.07 ± 0.98 |
| Scaling factor | 0.001 ± 0.0003 | 0.001 ± 0.00001 |
| X2 | 0.74 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajizadeh, M.; Golub, M.; Moldenhauer, M.; Matsarskaia, O.; Martel, A.; Porcar, L.; Maksimov, E.; Friedrich, T.; Pieper, J. Solution Structures of Two Different FRP-OCP Complexes as Revealed via SEC-SANS. Int. J. Mol. Sci. 2024, 25, 2781. https://doi.org/10.3390/ijms25052781
Hajizadeh M, Golub M, Moldenhauer M, Matsarskaia O, Martel A, Porcar L, Maksimov E, Friedrich T, Pieper J. Solution Structures of Two Different FRP-OCP Complexes as Revealed via SEC-SANS. International Journal of Molecular Sciences. 2024; 25(5):2781. https://doi.org/10.3390/ijms25052781
Chicago/Turabian StyleHajizadeh, Mina, Maksym Golub, Marcus Moldenhauer, Olga Matsarskaia, Anne Martel, Lionel Porcar, Eugene Maksimov, Thomas Friedrich, and Jörg Pieper. 2024. "Solution Structures of Two Different FRP-OCP Complexes as Revealed via SEC-SANS" International Journal of Molecular Sciences 25, no. 5: 2781. https://doi.org/10.3390/ijms25052781
APA StyleHajizadeh, M., Golub, M., Moldenhauer, M., Matsarskaia, O., Martel, A., Porcar, L., Maksimov, E., Friedrich, T., & Pieper, J. (2024). Solution Structures of Two Different FRP-OCP Complexes as Revealed via SEC-SANS. International Journal of Molecular Sciences, 25(5), 2781. https://doi.org/10.3390/ijms25052781






