Plasma-Activated Polydimethylsiloxane Microstructured Pattern with Collagen for Improved Myoblast Cell Guidance
Abstract
1. Introduction
2. Results and Discussion
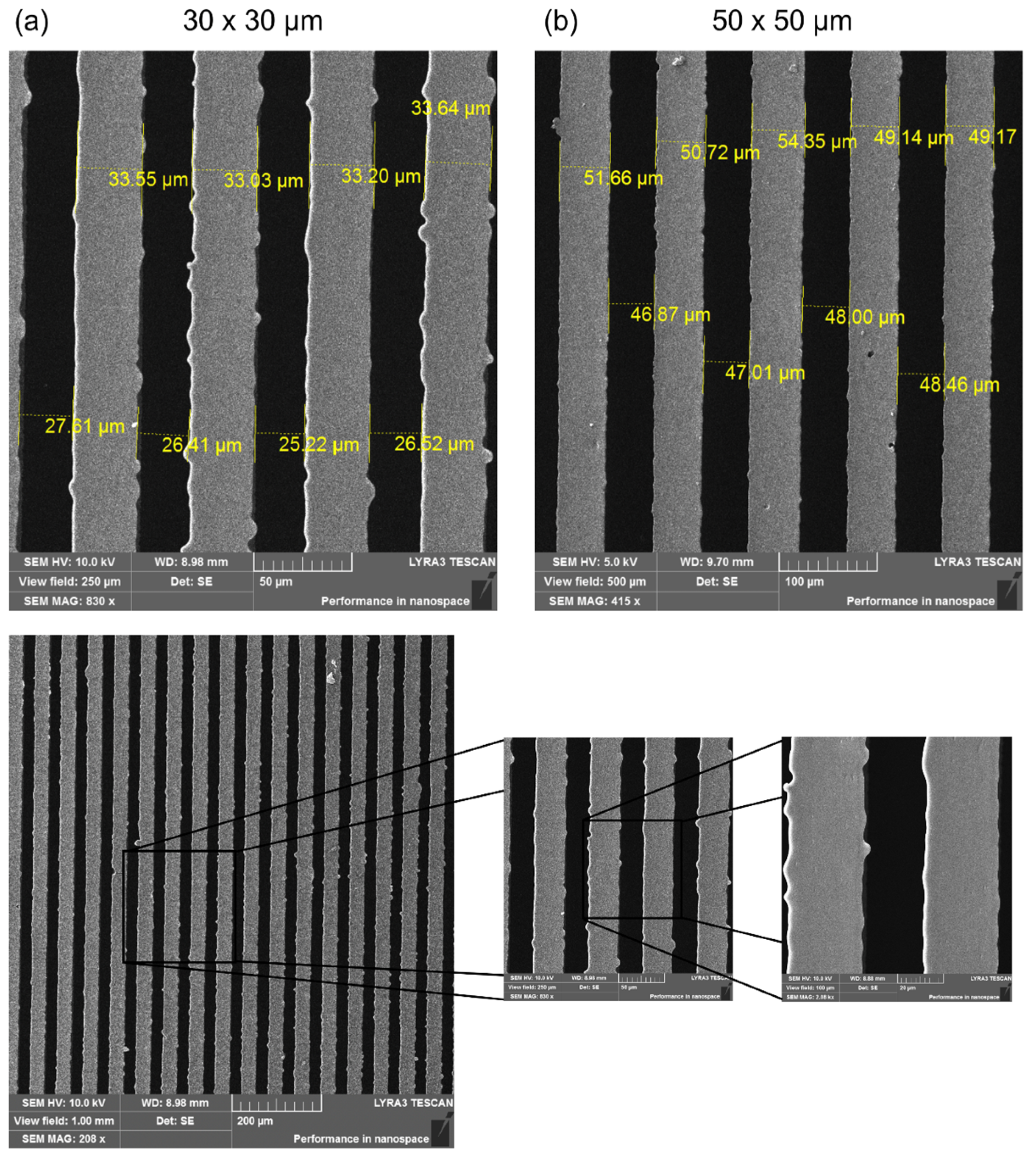
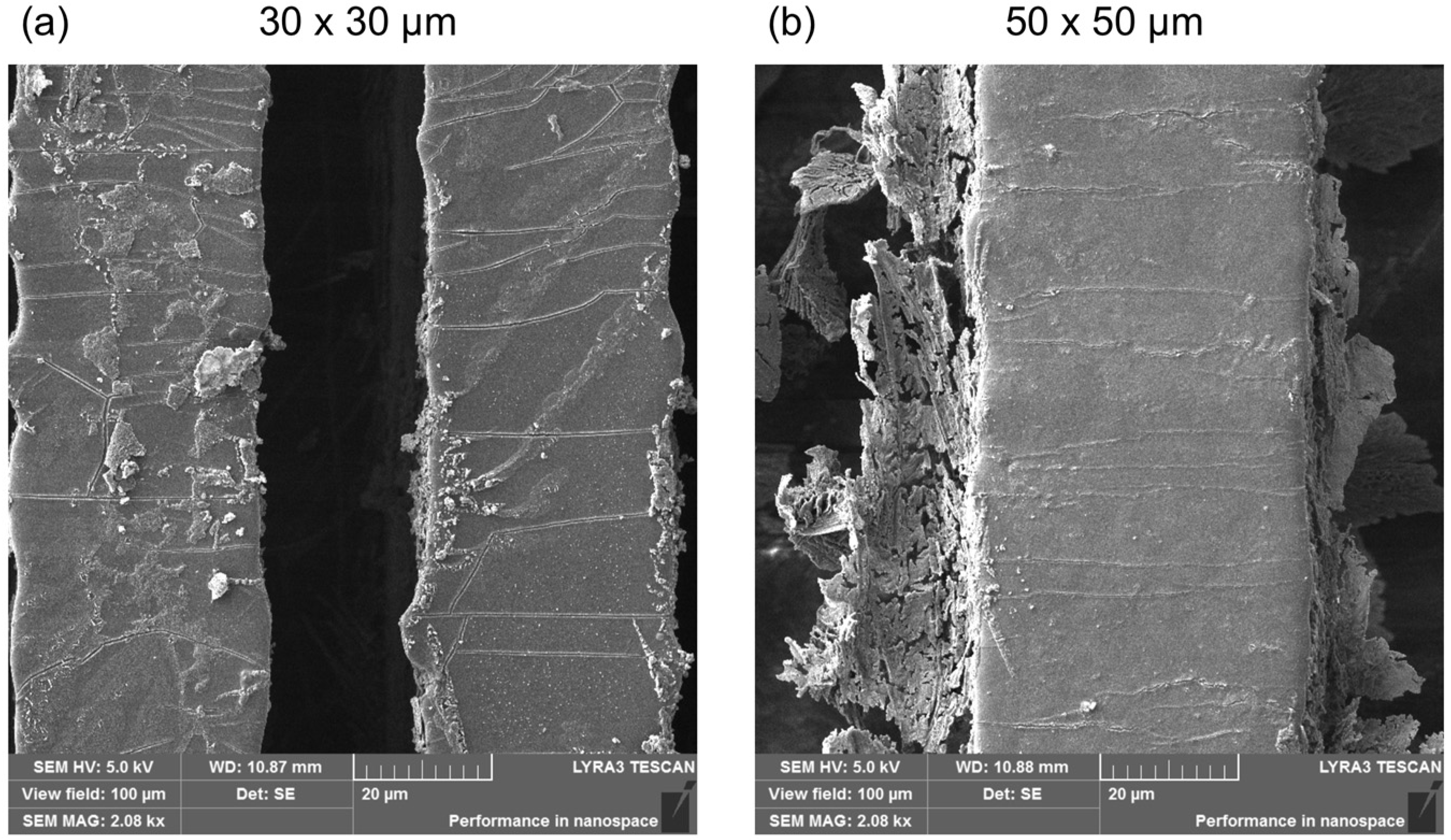
2.1. Surface Morphology of the Replicas
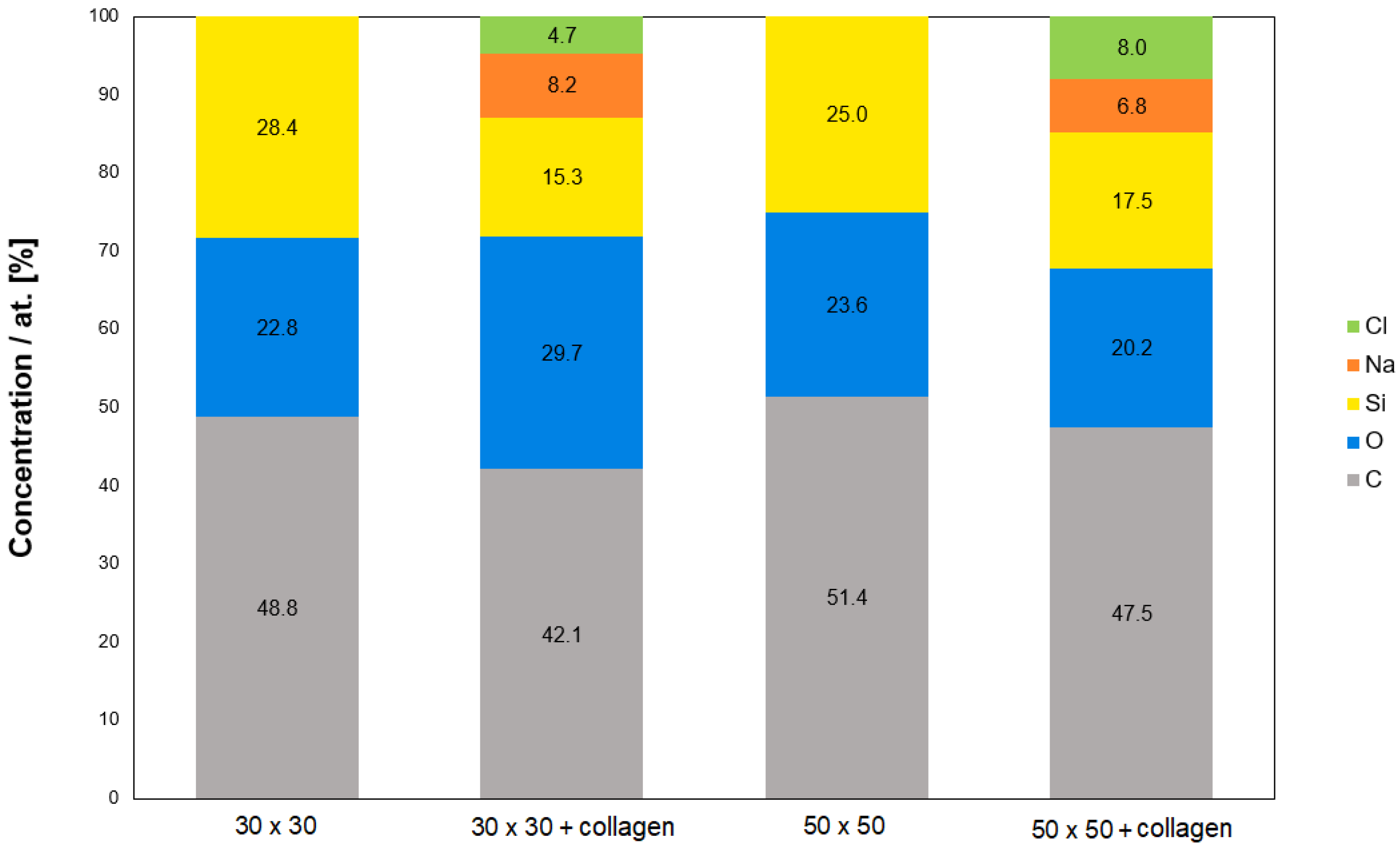
2.2. Study of Surface Chemistry
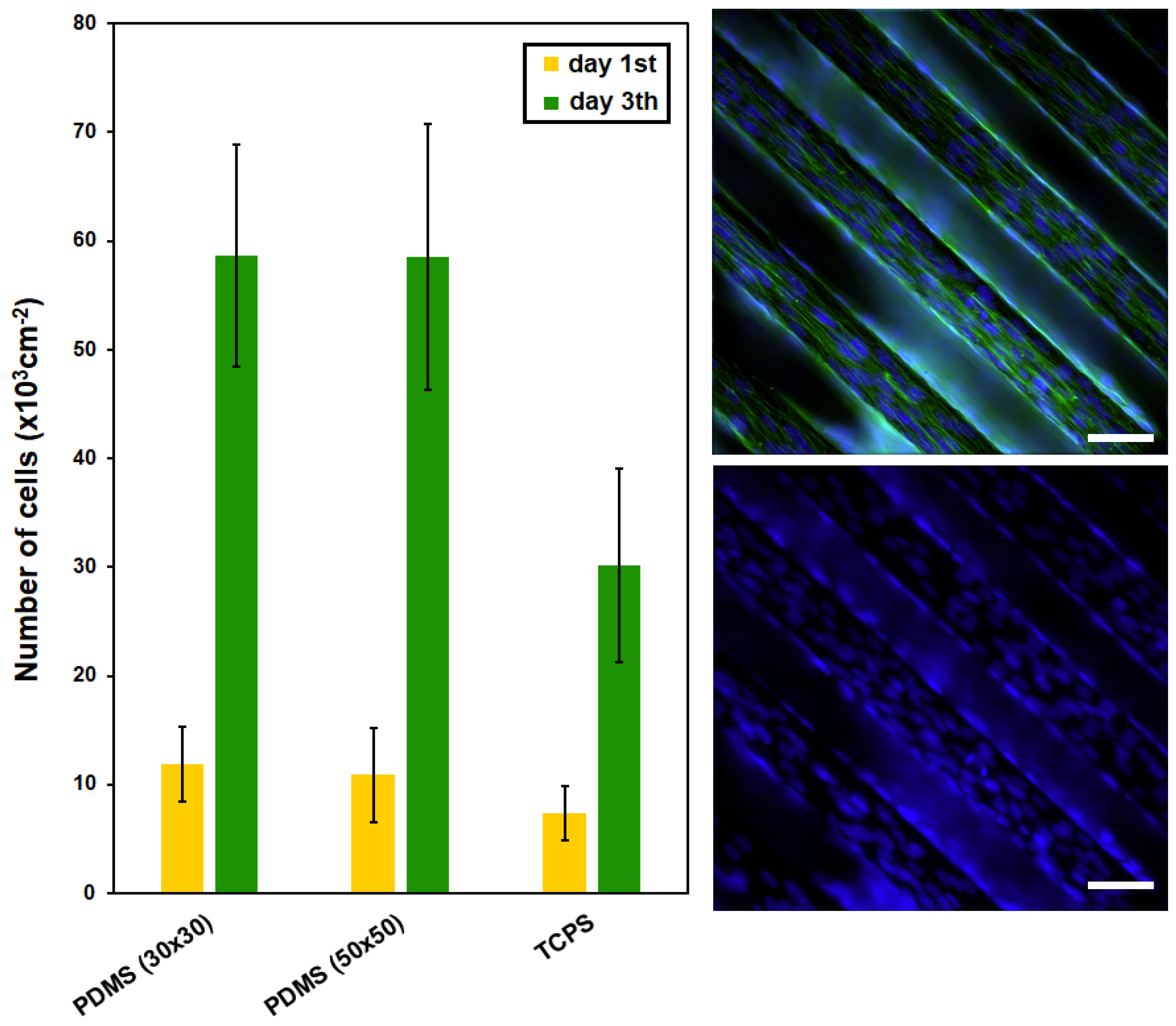
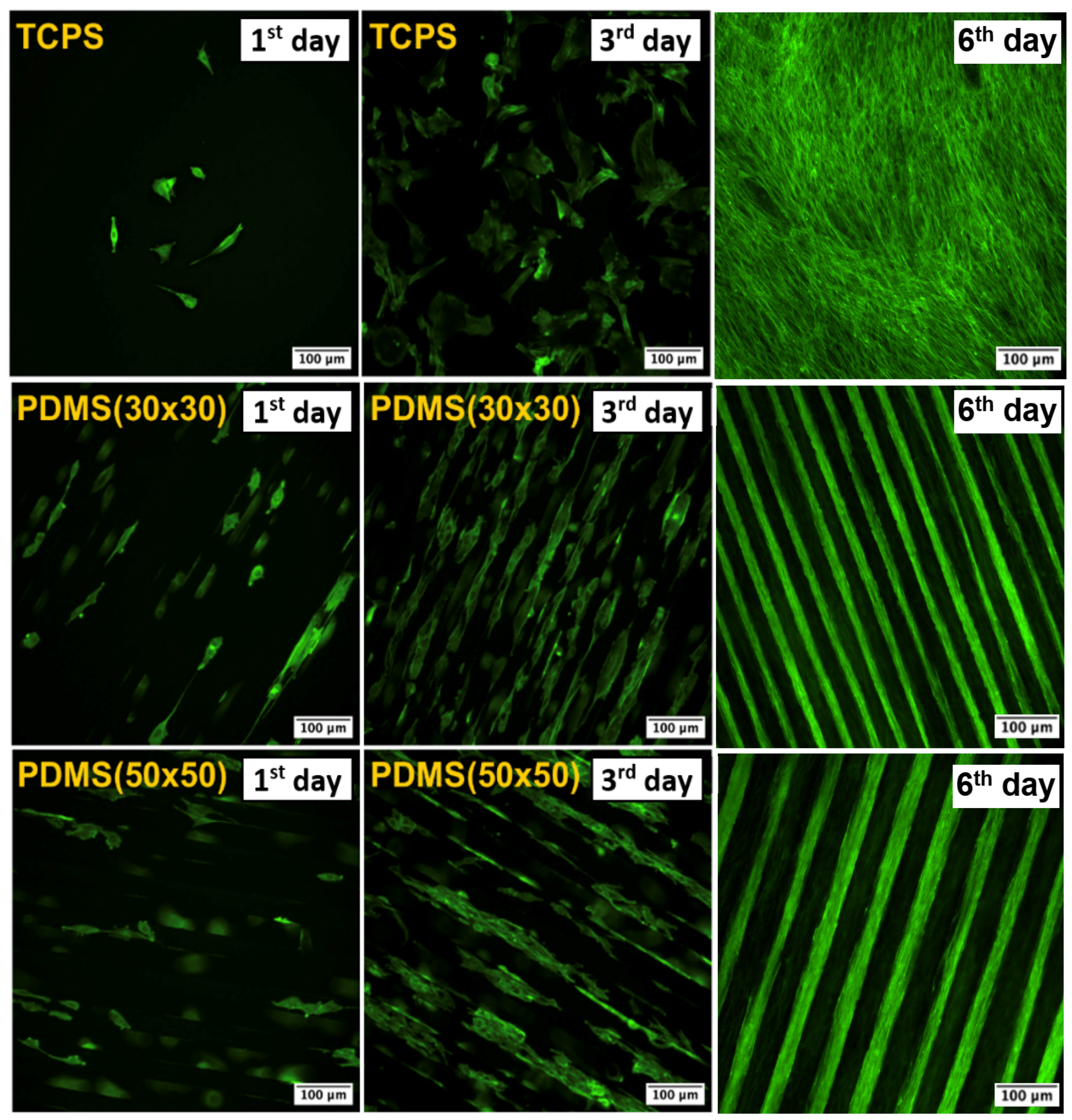
2.3. Study of PDMS Cytocompatibility
3. Materials and Methods
3.1. Materials Used in Photolithography and Replication
3.1.1. PDMS Polymer
3.1.2. Phosphor Bronze Wafer
3.1.3. Photoresist (SU-8 3035)
3.2. Preparation of Microstructured Substrates
3.3. Preparation of Photoresist Pattern Replicas
3.4. Plasma Modification of Replicas
3.5. Coating of Functionalized Replicas
3.6. Analytical Methods
3.7. Cytocompatibility Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajendran, A.K.; Kim, H.D.; Kim, J.-W.; Bae, J.W.; Hwang, N.S. Nanotechnology in tissue engineering and regenerative medicine. Korean J. Chem. Eng. 2023, 40, 286–301. [Google Scholar] [CrossRef]
- Kirkpatrick, C.J.; Mittermayer, C. Theoretical and practical aspects of testing potential biomaterials in vitro. J. Mater. Sci. Mater. Med. 1990, 1, 9–13. [Google Scholar] [CrossRef]
- De Jong, W.H.; Carraway, J.W.; Geertsma, R.E. Chapter 6—In vivo and in vitro testing for the biological safety evaluation of biomaterials and medical devices. In Biocompatibility and Performance of Medical Devices, 2nd ed.; Boutrand, J.P., Ed.; Woodhead Publishing: Sawston, UK, 2020; pp. 123–166. [Google Scholar]
- Hunckler, M.D.; Levine, A.D. Navigating ethical challenges in the development and translation of biomaterials research. Front. Bioeng. Biotechnol. 2022, 10, 949280. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, C.A. The history of tissue engineering. J. Cell. Mol. Med. 2006, 10, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354, S32–S34. [Google Scholar] [CrossRef] [PubMed]
- Duval, C.; Cohen, C.; Chagnoleau, C.; Flouret, V.; Bourreau, E.; Bernerd, F. Key Regulatory Role of Dermal Fibroblasts in Pigmentation as Demonstrated Using a Reconstructed Skin Model: Impact of Photo-Aging. PLoS ONE 2014, 9, e114182. [Google Scholar] [CrossRef] [PubMed]
- El Ghalbzouri, A.; Jonkman, M.F.; Dijkman, R.; Ponec, M. Basement Membrane Reconstruction in Human Skin Equivalents Is Regulated by Fibroblasts and/or Exogenously Activated Keratinocytes. J. Investig. Dermatol. 2005, 124, 79–86. [Google Scholar] [CrossRef]
- van Osch, G.J.; van der Veen, S.W.; Verwoerd-Verhoef, H.L. In Vitro Redifferentiation of Culture-Expanded Rabbit and Human Auricular Chondrocytes for Cartilage Reconstruction. Plast. Reconstr. Surg. 2001, 107, 433–440. [Google Scholar] [CrossRef]
- Yamanouchi, K.; Satomura, K.; Gotoh, Y.; Kitaoka, E.; Tobiume, S.; Kume, K.; Nagayama, M. Bone Formation by Transplanted Human Osteoblasts Cultured Within Collagen Sponge with Dexamethasone In Vitro. J. Bone Miner. Res. 2001, 16, 857–867. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Wang, R.; Ji, C.; Wei, Y.; Shi, M.; Wang, Y.; Du, Y.; Zhang, Y.; Yuan, Q.; et al. Bioactive Core–Shell CaF2 Upconversion Nanostructure for Promotion and Visualization of Engineered Bone Reconstruction. ACS Nano 2020, 14, 16085–16095. [Google Scholar] [CrossRef]
- Demoor, M.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Fabre, H.; Lafont, J.; Denoix, J.-M.; Audigié, F.; Mallein-Gerin, F.; et al. Cartilage tissue engineering: Molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim. Et. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 2414–2440. [Google Scholar] [CrossRef]
- Smetana, K. Kmenové buňky. Kontakt 2010, 12, 251–254. [Google Scholar]
- Dahlberg, A.; Delaney, C.; Bernstein, I.D. Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood 2011, 117, 6083–6090. [Google Scholar] [CrossRef]
- Quintarelli, C.; Dotti, G.; Hasan, S.T.; De Angelis, B.; Hoyos, V.; Errichiello, S.; Mims, M.; Luciano, L.; Shafer, J.; Leen, A.M.; et al. High-avidity cytotoxic T lymphocytes specific for a new PRAME-derived peptide can target leukemic and leukemic-precursor cells. Blood 2011, 117, 3353–3362. [Google Scholar] [CrossRef][Green Version]
- Griffith, L.; Naughton, G. Tissue Engineering—Current Challenges and Expanding Opportunities. Science 2002, 295, 1009–1014. [Google Scholar] [CrossRef]
- Cook, J.L.; Hung, C.T.; Kuroki, K.; Stoker, A.M.; Cook, C.R.; Pfeiffer, F.M.; Sherman, S.L.; Stannard, J.P. Animal models of cartilage repair. Bone Jt. Res. 2014, 3, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Loskill, P.; Shao, K.; Huebsch, N.; Hong, S.; Marcus, S.G.; Marks, N.; Mandegar, M.; Conklin, B.R.; Lee, L.P.; et al. Human iPSC-based cardiac microphysiological system for drug screening applications. Sci. Rep. 2015, 5, 8883. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.M.; Solari, R.; Holgate, S.T. Animal models of asthma: Value, limitations and opportunities for alternative approaches. Drug Discov. Today 2011, 16, 659–670. [Google Scholar] [CrossRef]
- Park, K. Facing the truth about nanotechnology in drug delivery. ACS Nano 2013, 7, 7442–7447. [Google Scholar] [CrossRef]
- Däullary, T.; Fey, C.; Berger, C.; Metzger, M.; Zdzieblo, D. Bioartificial gut—Current state of small intestinal tissue engineering. In Biomaterials for Organ and Tissue Regeneration; Elsevier: Amsterdam, The Netherlands, 2020; pp. 273–297. [Google Scholar]
- Dai, C.; Shih, S.; Khachemoune, A. Skin substitutes for acute and chronic wound healing: An updated review. J. Dermatol. Treat. 2020, 31, 639–648. [Google Scholar] [CrossRef]
- Dvořánková, B.; Holíková, Z.; Vacík, J.; Königová, R.; Kapounková, Z.; Michálek, J.; Přádn, M.; Smetana, K. Reconstruction of epidermis by grafting of keratinocytes cultured on polymer support—Clinical study. Int. J. Dermatol. 2003, 42, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandal, B.B. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials 2019, 216, 119267. [Google Scholar] [CrossRef] [PubMed]
- Tiruvannamalai-Annamalai, R.; Armant, D.R.; Matthew, H.W. A glycosaminoglycan based, modular tissue scaffold system for rapid assembly of perfusable, high cell density, engineered tissues. PLoS ONE 2014, 9, e84287. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yoshinari, M.; Takemoto, S.; Hattori, M.; Kawada, E.; Liu, B.; Oda, Y. Adhesion of mouse fibroblasts on hexamethyldisiloxane surfaces with wide range of wettability. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 81B, 66–75. [Google Scholar] [CrossRef]
- Ikada, Y. Surface modification of polymers for medical applications. Biomaterials 1994, 15, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.; Ashokkumar, M.; Ajayan, P.M. Chapter 3—Micro- and nanopatterning of biomaterial surfaces. In Fundamental Biomaterials: Metals; Brodoceanu, D., Kraus, T., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 67–78. [Google Scholar]
- Michaljaničová, I.; Slepička, P.; Heitz, J.; Barb, R.; Sajdl, P.; Švorčík, V. Comparison of KrF and ArF excimer laser treatment of biopolymer surface. Appl. Surf. Sci. 2015, 339, 144–150. [Google Scholar] [CrossRef]
- Slepička, P.; Siegel, J.; Lyutakov, O.; Slepičková Kasálková, N.; Kolská, Z.; Bačáková, L.; Švorčík, V. Polymer nanostructures for bioapplications induced by laser treatment. Biotechnol. Adv. 2018, 36, 839–855. [Google Scholar] [CrossRef]
- Neděla, O.; Slepička, P.; Sajdl, P.; Veselý, M.; Švorčík, V. Surface analysis of ripple pattern on PS and PEN induced with ring-shaped mask due to KrF laser treatment. Surf. Interface Anal. 2017, 49, 25–33. [Google Scholar] [CrossRef]
- Fajstavr, D.; Slepička, P.; Švorčík, V. LIPSS with gold nanoclusters prepared by combination of heat treatment and KrF exposure. Appl. Surf. Sci. 2019, 465, 919–928. [Google Scholar] [CrossRef]
- Fajstavr, D.; Michaljaničová, I.; Slepička, P.; Neděla, O.; Sajdl, P.; Kolská, Z.; Švorčík, V. Surface instability on polyethersulfone induced by dual laser treatment for husk nanostructure construction. React. Funct. Polym. 2018, 125, 20–28. [Google Scholar] [CrossRef]
- Krajcar, R.; Siegel, J.; Slepička, P.; Fitl, P.; Švorčík, V. Silver nanowires prepared on PET structured by laser irradiation. Mater. Lett. 2014, 117, 184–187. [Google Scholar] [CrossRef]
- Michaljaničová, I.; Slepička, P.; Kasálková, N.S.; Sajdl, P.; Švorčík, V. Plasma and laser treatment of PMP for biocompatibility improvement. Vacuum 2014, 107, 184–190. [Google Scholar] [CrossRef]
- Slepicka, P.; Slepickova Kasalkova, N.; Siegel, J.; Kolska, Z.; Bacakova, L.; Svorcik, V. Nano-structured and functionalized surfaces for cytocompatibility improvement and bactericidal action. Biotechnol. Adv. 2015, 33, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Michaljaničová, I.; Slepička, P.; Rimpelová, S.; Slepičková Kasálková, N.; Švorčík, V. Regular pattern formation on surface of aromatic polymers and its cytocompatibility. Appl. Surf. Sci. 2016, 370, 131–141. [Google Scholar] [CrossRef]
- Slepička, P.; Michaljaničová, I.; Rimpelová, S.; Švorčík, V. Surface roughness in action–Cells in opposition. Mater. Sci. Eng. C 2017, 76, 818–826. [Google Scholar] [CrossRef]
- Ehrhardt, M.; Lai, S.; Lorenz, P.; Zimmer, K. Guiding of LIPSS formation by excimer laser irradiation of pre-patterned polymer films for tailored hierarchical structures. Appl. Surf. Sci. 2020, 506, 144785. [Google Scholar] [CrossRef]
- Rahmawan, Y.; Xu, L.; Yang, S. Self-assembly of nanostructures towards transparent, superhydrophobic surfaces. J. Mater. Chem. A 2013, 1, 2955–2969. [Google Scholar] [CrossRef]
- Arole, V.; Munde, S. Fabrication of nanomaterials by top-down and bottom-up approaches–an overview. J. Mater. Sci 2014, 1, 89–93. [Google Scholar]
- Cheng, X. Nanostructures: Fabrication and applications. In Nanolithography; Elsevier: Amsterdam, The Netherlands, 2014; pp. 348–375. [Google Scholar]
- Ifkovits, J.L.; Burdick, J.A. Review: Photopolymerizable and Degradable Biomaterials for Tissue Engineering Applications. Tissue Eng. 2007, 13, 2369–2385. [Google Scholar] [CrossRef] [PubMed]
- Kaihara, S.; Borenstein, J.; Koka, R.; Lalan, S.; Ochoa, E.R.; Ravens, M.; Pien, H.; Cunningham, B.; Vacanti, J.P. Silicon micromachining to tissue engineer branched vascular channels for liver fabrication. Tissue Eng. 2000, 6, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.; Piraino, F.; Khademhosseini, A. Chapter 9—Microfabrication Technology in Tissue Engineering. In Tissue Engineering, 2nd ed.; Blitterswijk, C.A.V., De Boer, J., Eds.; Academic Press: Oxford, UK, 2014; pp. 283–310. [Google Scholar]
- Kim, H.N.; Jiao, A.; Hwang, N.S.; Kim, M.S.; Kang, D.H.; Kim, D.-H.; Suh, K.-Y. Nanotopography-guided tissue engineering and regenerative medicine. Adv. Drug Deliv. Rev. 2013, 65, 536–558. [Google Scholar] [CrossRef]
- Kelleher, C.M.; Vacanti, J.P. Engineering extracellular matrix through nanotechnology. J. R. Soc. Interface 2010, 7 (Suppl. S6), S717–S729. [Google Scholar] [CrossRef]
- Marczak, J.; Kusinski, J.; Major, R.; Rycyk, A.; Sarzynski, A.; Strzelec, M.; Czyz, K. Laser interference patterning of diamond-like carbon layers for directed migration and growth of smooth muscle cell depositions. Opt. Appl. 2014, 44, 575–586. [Google Scholar]
- Molnar, P.; Wang, W.; Natarajan, A.; Rumsey, J.W.; Hickman, J.J. Photolithographic patterning of C2C12 myotubes using vitronectin as growth substrate in serum-free medium. Biotechnol. Prog. 2007, 23, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Fujita, H.; Nagamori, E. Micropatterning of single myotubes on a thermoresponsive culture surface using elastic stencil membranes for single-cell analysis. J. Biosci. Bioeng. 2010, 109, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.F.; Lee, R.J.; Li, S. Engineering of aligned skeletal muscle by micropatterning. Am. J. Transl. Res. 2010, 2, 43. [Google Scholar] [PubMed]
- Ostrovidov, S.; Hosseini, V.; Ahadian, S.; Fujie, T.; Parthiban, S.P.; Ramalingam, M.; Bae, H.; Kaji, H.; Khademhosseini, A. Skeletal muscle tissue engineering: Methods to form skeletal myotubes and their applications. Tissue Eng. Part B Rev. 2014, 20, 403–436. [Google Scholar] [CrossRef] [PubMed]
- Skoog, S.A.; Kumar, G.; Narayan, R.J.; Goering, P.L. Biological responses to immobilized microscale and nanoscale surface topographies. Pharmacol. Ther. 2018, 182, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, B.; Wang, X.; Zhou, G.; Zhang, W.; Yi, B.; Wang, W.; Liu, W. Synergistic effects of mechanical stimulation and crimped topography to stimulate natural collagen development for tendon engineering. Acta Biomater. 2022, 145, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Rosella, E.; Jia, N.; Mantovani, D.; Greenera, J. A microfluidic approach for development of hybrid collagen-chitosan extracellular matrix-like membranes for on-chip cell cultures. J. Mater. Sci. Technol. 2021, 63, 54–61. [Google Scholar] [CrossRef]
- Oztürk-Oncel, M.O.; Erkoc-Biradli, F.Z.; Rasier, R.; Marcali, M.; Elbuken, C.; Garipcan, B. Rose petal topography mimicked poly(dimethylsiloxane) substrates for enhanced corneal endothelial cell behavior. Mater. Sci. Eng. C 2021, 126, 112147. [Google Scholar] [CrossRef] [PubMed]
- Comelles, J.; Fernandez-Majada, V.; Acevedo, V.; Rebollo-Calderon, B.; Martínez, E. Soft topographical patterns trigger a stiffness-dependent cellular response to contact guidance. Mater. Today Bio. 2023, 19, 100593. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Kulwatno, J.; Mills, K.L. Rapid fabrication of collagen bundles mimicking tumor-associated collagen architectures. Acta Biomater. 2020, 108, 128–141. [Google Scholar] [CrossRef]
- Nuhn, J.A.M.; Perez, A.M.; Schneider, I.C. Contact guidance diversity in rotationally aligned collagen matrices. Acta Biomater. 2018, 66, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.; David, G.; Jacobs, D.; Kuczwara, P.; Woessner, A.E.; Kim, J.W.; Quinn, K.P.; Song, Y. Neuro-regenerative behavior of adipose-derived stem cells in aligned collagen I hydrogels. Mater. Today Bio 2023, 22, 100762. [Google Scholar] [CrossRef] [PubMed]
- Muzzio, N.; Martinez-Cartagena, M.E.; Romero, G. Soft nano and microstructures for the photomodulation of cellular signaling and behavior. Adv. Drug Deliv. Rev. 2022, 190, 114554. [Google Scholar] [CrossRef]
- Almonacid Suarez, A.M.; van der Hama, I.; Brinker, M.G.L.; van Rijn, P.; Harmsen, M.C. Topography-driven alterations in endothelial cell phenotype and contact guidance. Heliyon 2020, 6, e04329. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Neyer, A.; Kuckuk, R.; Heise, H. Optical absorption in transparent PDMS materials applied for multimode waveguides fabrication. Opt. Mater. 2008, 30, 1157–1161. [Google Scholar] [CrossRef]
- Colas, A.; Curtis, J. Handbook of Polymer Applications in Medicine and Medical Devices: 7. Silicones; Elsevier Inc.: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Dahrouch, M.; Schmidt, A.; Leemans, L.; Linssen, H.; Götz, H. Synthesis and properties of poly(butylene terephthalate)-poly(ethylene oxide)-poly(dimethylsiloxane) block copolymers. Macromol. Symp. 2003, 199, 147–162. [Google Scholar] [CrossRef]
- Ariati, R.; Sales, F.; Souza, A.; Lima, R.A.; Ribeiro, J. Polydimethylsiloxane Composites Characterization and Its Applications: A Review. Polymers 2021, 13, 4258. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities—Properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Zhou, J.; Ellis, A.V.; Voelcker, N.H. Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis 2010, 31, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Yang, S. Surface modification of PDMS by atmospheric-pressure plasma-enhanced chemical vapor deposition and analysis of long-lasting surface hydrophilicity. Sens. Actuators B Chem. 2012, 162, 425–434. [Google Scholar] [CrossRef]
- Wu, M.H. Simple poly (dimethylsiloxane) surface modification to control cell adhesion. Sens. Actuators B Chem. 2009, 41, 11–16. [Google Scholar] [CrossRef]
- Sugiura, S.; Edahiro, J.-I.; Sumaru, K.; Kanamori, T. Surface modification of polydimethylsiloxane with photo-grafted poly (ethylene glycol) for micropatterned protein adsorption and cell adhesion. Colloids Surf. B Biointerfaces 2008, 63, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Kidambi, S.; Udpa, N.; Schroeder, S.A.; Findlan, R.; Lee, I.; Chan, C. Cell adhesion on polyelectrolyte multilayer coated polydimethylsiloxane surfaces with varying topographies. Tissue Eng. 2007, 13, 2105–2117. [Google Scholar] [CrossRef]
- Armugam, A.; Teong, S.P.; Lim, D.S.W.; Chan, S.P.; Yi, G.; Yew, D.S.; Beh, C.W.; Zhang, Y. Broad spectrum antimicrobial PDMS-based biomaterial for catheter fabrication. Biomater. Res. 2021, 25, 33. [Google Scholar] [CrossRef]
- Tohfafarosh, M.; Sevit, A.; Patel, J.; Kiel, J.W.; Greenspon, A.; Prutkin, J.M.; Kurtz, S.M. Characterization of outer insulation in long-term-implanted leads. J. Long-Term Eff. Med. Implant. 2016, 26, 225–232. [Google Scholar] [CrossRef]
- Holländer, J.; Hakala, R.; Suominen, J.; Moritz, N.; Yliruusi, J.; Sandler, N. 3D printed UV light cured polydimethylsiloxane devices for drug delivery. Int. J. Pharm. 2018, 544, 433–442. [Google Scholar] [CrossRef]
- Spiller, D.; Losi, P.; Briganti, E.; Sbrana, S.; Kull, S.; Martinelli, I.; Soldani, G. PDMS content affects in vitro hemocompatibility of synthetic vascular grafts. J. Mater. Sci. Mater. Med. 2007, 18, 1097–1104. [Google Scholar] [CrossRef]
- Daniels, A. Silicone breast implant materials. Swiss Med. Wkly. 2012, 142, w13614. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Mirzadeh, H.; Simjoo, M. Hydrophilic interpenetrating polymer networks of poly (dimethyl siloxane)(PDMS) as biomaterial for cochlear implants. J. Biomater. Sci. Polym. Ed. 2006, 17, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Fiddes, L.K.; Raz, N.; Srigunapalan, S.; Tumarkan, E.; Simmons, C.A.; Wheeler, A.R.; Kumacheva, E. A circular cross-section PDMS microfluidics system for replication of cardiovascular flow conditions. Biomaterials 2010, 31, 3459–3464. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-H.; Moya, M.L.; Hughes, C.C.W.; George, S.C.; Lee, A.P. A microfluidic platform for generating large-scale nearly identical human microphysiological vascularized tissue arrays. Lab A Chip 2013, 13, 2990–2998. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T. PDMS-based microfluidic devices for biomedical applications. Microelectron. Eng. 2002, 61, 907–914. [Google Scholar] [CrossRef]
- Bosc, D.; Maalouf, A.; Haesaert, S.; Henrio, F. Investigation into defects occurring on the polymer surface, during the photolithography process. Appl. Surf. Sci. 2007, 253, 6162–6164. [Google Scholar] [CrossRef]
- Juárez-Moreno, A.; Brito-Argáez, L.G.; Ávila-Ortega, A.; Oliva, A.I.; Avilés, F.; Cauich-Rodríguez, J.V. Effect of the type of plasma on the polydimethylsiloxane/collagen,composites adhesive properties. Int. J. Adhes. Adhes. 2017, 77, 85–95. [Google Scholar] [CrossRef]
- Chuah, Y.J.; Heng, Z.T.; Tan, J.S.; Tay, L.M.; Lim, C.S.; Kang, Y.; Wang, D.A. Surface modifications to polydimethylsiloxane substrate for stabilizing prolonged bone marrow stromal cell culture. Colloids Surf. B Biointerfaces 2020, 191, 110995. [Google Scholar] [CrossRef]
- Jandura, D.; Pudis, D.; Berezina, S. Photonic devices prepared by embossing in PDMS. Appl. Surf. Sci. 2017, 395, 145–149. [Google Scholar] [CrossRef]
- Potrich, C.; Lunelli, L.; Cocuzza, M.; Marasso, S.L.; Pirri, C.F.; Pederzolli, C. Simple PDMS microdevice for biomedical applications. Talanta 2019, 193, 44–50. [Google Scholar] [CrossRef]
- Aggarwal, R.T.; Lai, L.; Li, H. Microarray fabrication techniques for multiplexed bioassay applications. Anal. Biochem. 2023, 683, 115369. [Google Scholar] [CrossRef]
- Roughton, B.C.; Iyer, L.K.; Bertelsen, E.; Topp, E.M.; Camarda, K.V. Protein aggregation and lyophilization: Protein structural descriptors as predictors of aggregation propensity. Comput. Chem. Eng. 2013, 58, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-L.; Kim, D.-E. Self-healing Characteristics of Collagen Coatings with Respect to Surface Abrasion. Sci. Rep. 2016, 6, 20563. [Google Scholar] [CrossRef]
- Kefallinou, D.; Grigoriou, M.; Constantoudis, V.; Raptis, I.; Xenogiannopoulou, E.; Dimoulas, A.; Boumpas, D.T.; Tserepi, A. Enhanced and stabilized mesenchymal stem cell growth inside plasma pre-treated and collagen-coated PDMS-based microfluidic chambers. Surf. Coat. Technol. 2023, 466, 129658. [Google Scholar] [CrossRef]
- Wu, S.T.; Huang, C.Y.; Weng, C.C.; Chang, C.C.; Li, B.R.; Hsu, C.S. Rapid Prototyping of an Open-Surface Microfluidic Platform Using Wettability-Patterned Surfaces Prepared by an Atmospheric-Pressure Plasma Jet. ACS Omega 2019, 4, 16292–16299. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Ma, Z.; Zhao, Q.; Li, Y.; Zhu, K.; Zhang, H.; Chen, X. Arrow-shaped patterned microchannel for enhancing droplet coalescence and demulsification of oil-in-water emulsions with high oil content. Colloids Surf. A Physicochem. Eng. Asp. 2024, 685, 133177. [Google Scholar] [CrossRef]
- Wang, W.; Bai, X.; Sun, S.; Gao, Y.; Li, F.; Hu, S. Polysiloxane-Based Polyurethanes with High Strength and Recyclability. Int. J. Mol. Sci. 2022, 23, 12613. [Google Scholar] [CrossRef] [PubMed]
- Somaiah, C.; Kumar, A.; Mawrie, D.; Sharma, A.; Patil, S.D.; Bhattacharyya, J.; Swaminathan, R.; Jaganathan, B.G. Collagen Promotes Higher Adhesion, Survival and Proliferation of Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0145068. [Google Scholar] [CrossRef]
- Chua, P.F.; Lim, W.K. The strategic uses of collagen in adherent cell cultures. Cell Biol. Int. 2023, 47, 367–373. [Google Scholar] [CrossRef]
- Chuah, Y.J.; Koh, Y.T.; Lim, K.; Menon, N.V.; Wu, Y.; Kang, Y. Simple surface engineering of polydimethylsiloxane with polydopamine for stabilized mesenchymal stem cell adhesion and multipotency. Sci. Rep. 2015, 5, 18162. [Google Scholar] [CrossRef]
- Lam, M.T.; Sim, S.; Zhu, X.; Takayama, S. The effect of continuous wavy micropatterns on silicone substrates on the alignment of skeletal muscle myoblasts and myotubes. Biomaterials 2006, 27, 4340–4347. [Google Scholar] [CrossRef] [PubMed]
- Akther, F.; Yakob, S.B.; Nguyen, N.-T.; Ta, H.T. Surface Modification Techniques for Endothelial Cell Seeding in PDMS Microfluidic Devices. Biosensors 2020, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Palchesko, R.N.; Zhang, L.; Sun, Y.; Feinberg, A.W. Development of polydimethylsiloxane substrates with tunable elastic modulus to study cell mechanobiology in muscle and nerve. PLoS ONE 2012, 7, e51499. [Google Scholar] [CrossRef] [PubMed]
- Borók, A.; Laboda, K.; Bonyár, A. PDMS Bonding Technologies for Microfluidic Applications: A Review. Biosensors 2021, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Parizek, M.; Slepickova Kasalkova, N.; Bacakova, L.; Slepicka, P.; Lisa, V.; Blazkova, M.; Svorcik, V. Improved Adhesion, Growth and Maturation of Vascular Smooth Muscle Cells on Polyethylene Grafted with Bioactive Molecules and Carbon Particles. Int. J. Mol. Sci. 2009, 10, 4352–4374. [Google Scholar] [CrossRef]
- Yao, L.; Wang, S.; Cui, W.; Sherlock, R.; O’connell, C.; Damodaran, G.; Gorman, A.; Windebank, A.; Pandit, A. Effect of functionalized micropatterned PLGA on guided neurite growth. Acta Biomater. 2009, 5, 580–588. [Google Scholar] [CrossRef]
- Bielajew, B.J.; Hu, J.C.; Athanasiou, K.A. Collagen: Quantification, biomechanics, and role of minor subtypes in cartilage. Nat. Rev. Mater. 2020, 5, 730–747. [Google Scholar] [CrossRef]


| Type of Modification | Application | Ref. |
|---|---|---|
| Plasma treatment | Microfluidic applications | [67,68] |
| PE CVD | Applications that require stable hydrophilic surface property in PDMS | [69] |
| Photografting of PEG | Protein adsorption and cell adhesion | [71] |
| Imidazolium antimicrobial compound | Catheter fabrication | [73] |
| UV-light curing | Drug delivery | [75] |
| Soft lithography | Replication of cardiovascular flow conditions | [80] |
| Bulk oxygen, nitrogen, or argon + collagen | Adhesive free skin substitutes | [82] |
| Bulk plasma treatment and collagen | Bone marrow-derived stromal cells | [83] |
| Hot embossing | Photonic devices | [85,86,87] |
| Different bulk/ surface treatments | Biomedical applications | [88,89] |
| Surface plasma treatment and collagen | Mesenchymal stem cells | [90] |
| Different bulk/surface treatments | Wettability and recyclability applications | [91,92,93] |
| Plasma/collagen treatment | Tissue engineering—different cell types | [94,95,96,97,98] |
| Bulk modification | Cell mechanobiology in muscle and nerve | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slepičková Kasálková, N.; Juřicová, V.; Fajstavr, D.; Frýdlová, B.; Rimpelová, S.; Švorčík, V.; Slepička, P. Plasma-Activated Polydimethylsiloxane Microstructured Pattern with Collagen for Improved Myoblast Cell Guidance. Int. J. Mol. Sci. 2024, 25, 2779. https://doi.org/10.3390/ijms25052779
Slepičková Kasálková N, Juřicová V, Fajstavr D, Frýdlová B, Rimpelová S, Švorčík V, Slepička P. Plasma-Activated Polydimethylsiloxane Microstructured Pattern with Collagen for Improved Myoblast Cell Guidance. International Journal of Molecular Sciences. 2024; 25(5):2779. https://doi.org/10.3390/ijms25052779
Chicago/Turabian StyleSlepičková Kasálková, Nikola, Veronika Juřicová, Dominik Fajstavr, Bára Frýdlová, Silvie Rimpelová, Václav Švorčík, and Petr Slepička. 2024. "Plasma-Activated Polydimethylsiloxane Microstructured Pattern with Collagen for Improved Myoblast Cell Guidance" International Journal of Molecular Sciences 25, no. 5: 2779. https://doi.org/10.3390/ijms25052779
APA StyleSlepičková Kasálková, N., Juřicová, V., Fajstavr, D., Frýdlová, B., Rimpelová, S., Švorčík, V., & Slepička, P. (2024). Plasma-Activated Polydimethylsiloxane Microstructured Pattern with Collagen for Improved Myoblast Cell Guidance. International Journal of Molecular Sciences, 25(5), 2779. https://doi.org/10.3390/ijms25052779







