TRP Ion Channels in Immune Cells and Their Implications for Inflammation
Abstract
1. Introduction
2. Distribution of TRP Ion Channels in Immune Cells
2.1. TRPs in Macrophages
2.2. TRPs in Mast Cells
2.3. TRPs in Neutrophils
2.4. TRPs in Specific Immunocytes
3. Involvement of TRPs in Inflammation
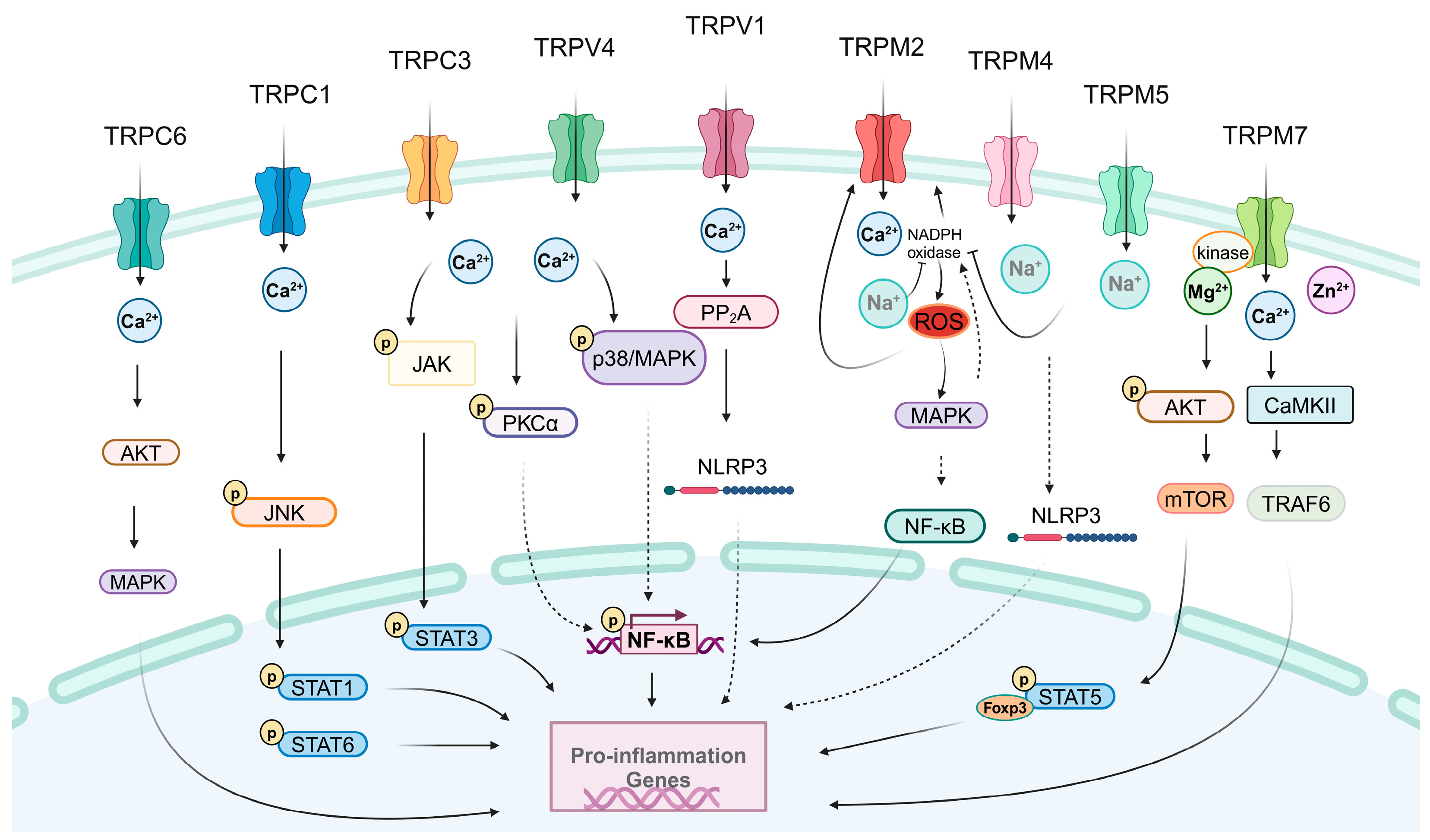
3.1. Pro-inflammatory Effects of TRPs
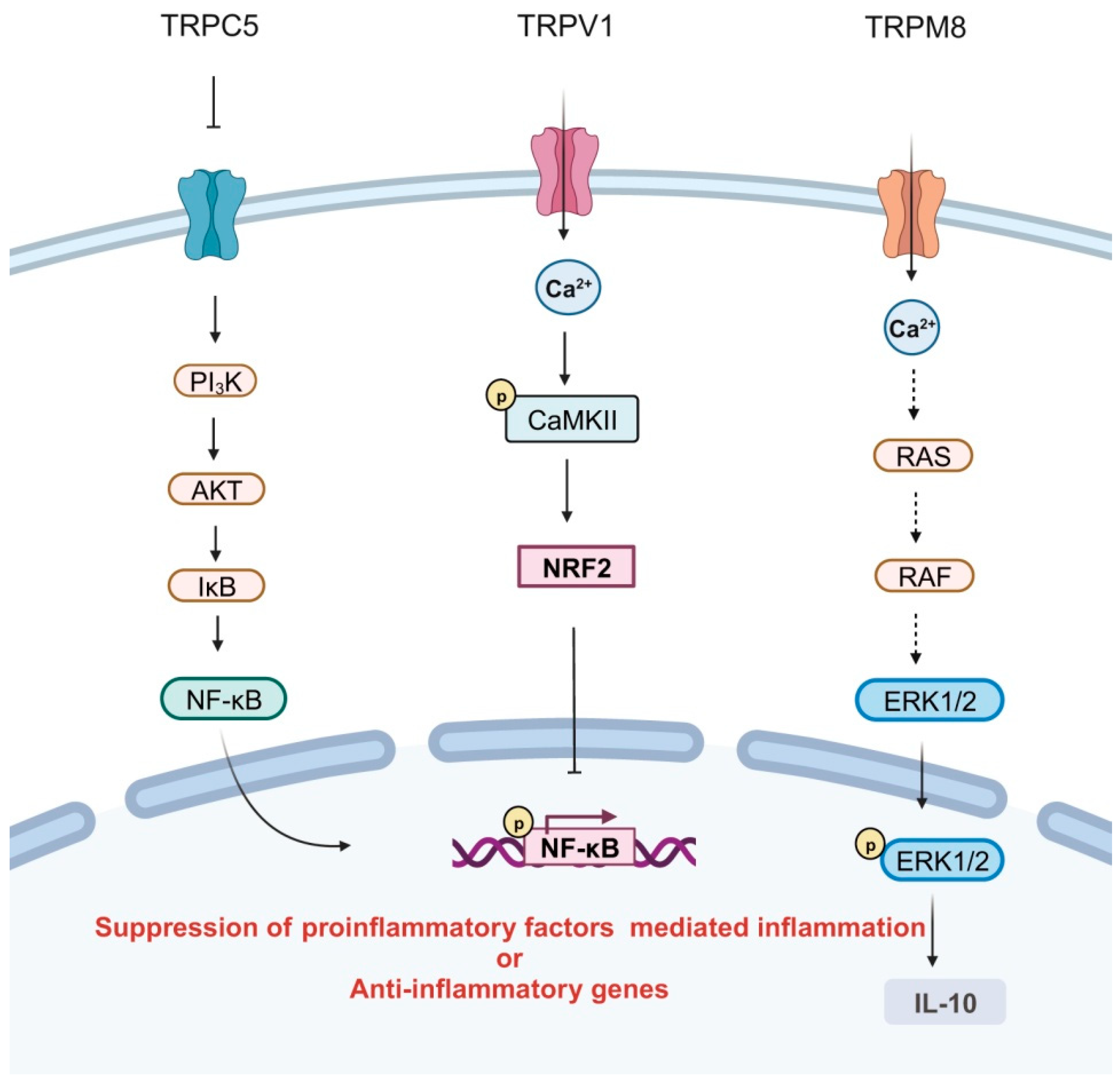
3.2. Anti-Inflammatory Effects of TRPs
3.3. The Paradoxical Roles of TRPs in Inflammation
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Leigh, T.; Scalia, R.G.; Autieri, M.V. Resolution of inflammation in immune and nonimmune cells by interleukin-19. Am. J. Physiol. Cell Physiol. 2020, 319, C457–C464. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Sanchez, N.; Alonso-Alonso, S.; Nagy, L. Regenerative inflammation: When immune cells help to re-build tissues. FEBS J. 2022. [Google Scholar] [CrossRef]
- Asano, H.; Hasegawa-Ishii, S.; Arae, K.; Obara, A.; Laumet, G.; Dantzer, R.; Shimada, A. Infiltration of peripheral immune cells into the olfactory bulb in a mouse model of acute nasal inflammation. J. Neuroimmunol. 2022, 368, 577897. [Google Scholar] [CrossRef]
- Pillon, N.J.; Smith, J.A.B.; Alm, P.S.; Chibalin, A.V.; Alhusen, J.; Arner, E.; Carninci, P.; Fritz, T.; Otten, J.; Olsson, T.; et al. Distinctive exercise-induced inflammatory response and exerkine induction in skeletal muscle of people with type 2 diabetes. Sci. Adv. 2022, 8, eabo3192. [Google Scholar] [CrossRef]
- Chang, Z.; Li, R.; Zhang, J.; An, L.; Zhou, G.; Lei, M.; Deng, J.; Yang, R.; Song, Z.; Zhong, W.; et al. Distinct immune and inflammatory response patterns contribute to the identification of poor prognosis and advanced clinical characters in bladder cancer patients. Front. Immunol. 2022, 13, 1008865. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gao, Q.; Jiang, H.; Liu, C.; Du, Y.; Li, L. Changes of macrophage and CD4+ T cell in inflammatory response in type 1 diabetic mice. Sci. Rep. 2022, 12, 14929. [Google Scholar] [CrossRef] [PubMed]
- Shaker, T.; Chattopadhyaya, B.; Amilhon, B.; Cristo, G.D.; Weil, A.G. Transduction of inflammation from peripheral immune cells to the hippocampus induces neuronal hyperexcitability mediated by Caspase-1 activation. Neurobiol. Dis. 2021, 160, 105535. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Kipnis, J. Smelling Danger: Olfactory Stem Cells Control Immune Defense during Chronic Inflammation. Cell Stem Cell 2019, 25, 449–451. [Google Scholar] [CrossRef]
- Chen, X.; Jaiswal, A.; Costliow, Z.; Herbst, P.; Creasey, E.A.; Oshiro-Rapley, N.; Daly, M.J.; Carey, K.L.; Graham, D.B.; Xavier, R.J. pH sensing controls tissue inflammation by modulating cellular metabolism and endo-lysosomal function of immune cells. Nat. Immunol. 2022, 23, 1063–1075. [Google Scholar] [CrossRef]
- Cheng, W.; Zheng, J. Distribution and Assembly of TRP Ion Channels. Adv. Exp. Med. Biol. 2021, 1349, 111–138. [Google Scholar] [CrossRef]
- Nilius, B.; Flockerzi, V. Mammalian transient receptor potential (TRP) cation channels. Preface. Handb. Exp. Pharmacol. 2014, 223, v–vi. [Google Scholar] [PubMed]
- Zhao, J.; Lin King, J.V.; Paulsen, C.E.; Cheng, Y.; Julius, D. Irritant-evoked activation and calcium modulation of the TRPA1 receptor. Nature 2020, 585, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Earley, S.; Santana, L.F.; Lederer, W.J. The physiological sensor channels TRP and piezo: Nobel Prize in Physiology or Medicine 2021. Physiol. Rev. 2022, 102, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Franulic, I.; Raddatz, N.; Castillo, K.; Gonzalez-Nilo, F.D.; Latorre, R. A folding reaction at the C-terminal domain drives temperature sensing in TRPM8 channels. Proc. Natl. Acad. Sci. USA 2020, 117, 20298–20304. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Wang, H.; Kamm, G.B.; Pohle, J.; Reis, F.C.; Heppenstall, P.; Wende, H.; Siemens, J. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 2016, 353, 1393–1398. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, M.; Zubcevic, L.; Borschel, W.F.; Lander, G.C.; Lee, S.Y. Structure of the cold- and menthol-sensing ion channel TRPM8. Science 2018, 359, 237–241. [Google Scholar] [CrossRef]
- Nam, J.H.; Kim, W.K. The Role of TRP Channels in Allergic Inflammation and its Clinical Relevance. Curr. Med. Chem. 2020, 27, 1446–1468. [Google Scholar] [CrossRef]
- Khalil, M.; Alliger, K.; Weidinger, C.; Yerinde, C.; Wirtz, S.; Becker, C.; Engel, M.A. Functional Role of Transient Receptor Potential Channels in Immune Cells and Epithelia. Front. Immunol. 2018, 9, 174. [Google Scholar] [CrossRef]
- Nascimento Da Conceicao, V.; Sun, Y.; Ramachandran, K.; Chauhan, A.; Raveendran, A.; Venkatesan, M.; DeKumar, B.; Maity, S.; Vishnu, N.; Kotsakis, G.A.; et al. Resolving macrophage polarization through distinct Ca2+ entry channel that maintains intracellular signaling and mitochondrial bioenergetics. iScience 2021, 24, 103339. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.; Meana, C.; López-López, J.R.; Balsinde, J.; Balboa, M.A. Lipin-1-derived diacylglycerol activates intracellular TRPC3 which is critical for inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 8243–8260. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Guo, G.; Qi, Y.; Xiong, Y.; Ma, X.; Wu, N.; Dong, C.; Yang, C. Inhibition of Canonical Transient Receptor Potential 5 Channels Polarizes Macrophages to an M1 Phenotype. Pharmacology 2020, 105, 202–208. [Google Scholar] [CrossRef]
- Lv, Z.; Xu, X.; Sun, Z.; Yang, Y.X.; Guo, H.; Li, J.; Sun, K.; Wu, R.; Xu, J.; Jiang, Q.; et al. TRPV1 alleviates osteoarthritis by inhibiting M1 macrophage polarization via Ca2+/CaMKII/Nrf2 signaling pathway. Cell Death Dis. 2021, 12, 504. [Google Scholar] [CrossRef]
- Sanjai Kumar, P.; Nayak, T.K.; Mahish, C.; Sahoo, S.S.; Radhakrishnan, A.; De, S.; Datey, A.; Sahu, R.P.; Goswami, C.; Chattopadhyay, S.; et al. Inhibition of transient receptor potential vanilloid 1 (TRPV1) channel regulates chikungunya virus infection in macrophages. Arch. Virol. 2021, 166, 139–155. [Google Scholar] [CrossRef]
- Xu, J.; Yang, Y.; Hou, Z.; Jia, H.; Wang, Y. TRPV2-spike protein interaction mediates the entry of SARS-CoV-2 into macrophages in febrile conditions. Theranostics 2021, 11, 7379–7390. [Google Scholar] [CrossRef]
- Raudszus, R.; Paulig, A.; Urban, N.; Deckers, A.; Grassle, S.; Vanderheiden, S.; Jung, N.; Brase, S.; Schaefer, M.; Hill, K. Pharmacological inhibition of TRPV2 attenuates phagocytosis and lipopolysaccharide-induced migration of primary macrophages. Br. J. Pharmacol. 2023, 180, 2736–2749. [Google Scholar] [CrossRef]
- Rayees, S.; Joshi, J.C.; Tauseef, M.; Anwar, M.; Baweja, S.; Rochford, I.; Joshi, B.; Hollenberg, M.D.; Reddy, S.P.; Mehta, D. PAR2-Mediated cAMP Generation Suppresses TRPV4-Dependent Ca2+ Signaling in Alveolar Macrophages to Resolve TLR4-Induced Inflammation. Cell Rep. 2019, 27, 793–805.e794. [Google Scholar] [CrossRef]
- Zong, P.; Feng, J.; Yue, Z.; Yu, A.S.; Vacher, J.; Jellison, E.R.; Miller, B.; Mori, Y.; Yue, L. TRPM2 deficiency in mice protects against atherosclerosis by inhibiting TRPM2-CD36 inflammatory axis in macrophages. Nat. Cardiovasc. Res. 2022, 1, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ying, F.; Tian, X.; Lei, Z.; Li, X.; Lo, C.Y.; Li, J.; Jiang, L.; Yao, X. TRPM2 Promotes Atherosclerotic Progression in a Mouse Model of Atherosclerosis. Cells 2022, 11, 1423. [Google Scholar] [CrossRef] [PubMed]
- Beceiro, S.; Radin, J.N.; Chatuvedi, R.; Piazuelo, M.B.; Horvarth, D.J.; Cortado, H.; Gu, Y.; Dixon, B.; Gu, C.; Lange, I.; et al. TRPM2 ion channels regulate macrophage polarization and gastric inflammation during Helicobacter pylori infection. Mucosal Immunol. 2017, 10, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Serafini, N.; Dahdah, A.; Barbet, G.; Demion, M.; Attout, T.; Gautier, G.; Arcos-Fajardo, M.; Souchet, H.; Jouvin, M.H.; Vrtovsnik, F.; et al. The TRPM4 channel controls monocyte and macrophage, but not neutrophil, function for survival in sepsis. J. Immunol. 2012, 189, 3689–3699. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, A.; Vettore, V.; Rezzonico-Jost, T.; Hampe, S.; Rottoli, E.; Nadolni, W.; Perotti, M.; Meier, M.A.; Hermanns, C.; Geiger, S.; et al. TRPM7 kinase activity is essential for T cell colonization and alloreactivity in the gut. Nat. Commun. 2017, 8, 1917. [Google Scholar] [CrossRef]
- Schappe, M.S.; Stremska, M.E.; Busey, G.W.; Downs, T.K.; Seegren, P.V.; Mendu, S.K.; Flegal, Z.; Doyle, C.A.; Stipes, E.J.; Desai, B.N. Efferocytosis requires periphagosomal Ca2+-signaling and TRPM7-mediated electrical activity. Nat. Commun. 2022, 13, 3230. [Google Scholar] [CrossRef]
- Qiao, W.; Wong, K.H.M.; Shen, J.; Wang, W.; Wu, J.; Li, J.; Lin, Z.; Chen, Z.; Matinlinna, J.P.; Zheng, Y.; et al. TRPM7 kinase-mediated immunomodulation in macrophage plays a central role in magnesium ion-induced bone regeneration. Nat. Commun. 2021, 12, 2885. [Google Scholar] [CrossRef]
- Khalil, M.; Babes, A.; Lakra, R.; Försch, S.; Reeh, P.W.; Wirtz, S.; Becker, C.; Neurath, M.F.; Engel, M.A. Transient receptor potential melastatin 8 ion channel in macrophages modulates colitis through a balance-shift in TNF-alpha and interleukin-10 production. Mucosal Immunol. 2016, 9, 1500–1513. [Google Scholar] [CrossRef]
- Plesch, E.; Chen, C.C.; Butz, E.; Scotto Rosato, A.; Krogsaeter, E.K.; Yinan, H.; Bartel, K.; Keller, M.; Robaa, D.; Teupser, D.; et al. Selective agonist of TRPML2 reveals direct role in chemokine release from innate immune cells. eLife 2018, 7, e39720. [Google Scholar] [CrossRef]
- Maione, S.; Piscitelli, F.; Gatta, L.; Vita, D.; De Petrocellis, L.; Palazzo, E.; de Novellis, V.; Di Marzo, V. Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. Br. J. Pharmacol. 2011, 162, 584–596. [Google Scholar] [CrossRef]
- Romano, B.; Borrelli, F.; Fasolino, I.; Capasso, R.; Piscitelli, F.; Cascio, M.; Pertwee, R.; Coppola, D.; Vassallo, L.; Orlando, P.; et al. The cannabinoid TRPA1 agonist cannabichromene inhibits nitric oxide production in macrophages and ameliorates murine colitis. Br. J. Pharmacol. 2013, 169, 213–229. [Google Scholar] [CrossRef]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and non-immune functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, Y.; Monji, A. TRPC Channels and Brain Inflammation. Adv. Exp. Med. Biol. 2017, 976, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hou, B.; Liang, P.; Lu, X.; Wu, Y.; Zhang, X.; Fan, Y.; Liu, Y.; Chen, T.; Liu, W.; et al. TRPV1 channel mediates NLRP3 inflammasome-dependent neuroinflammation in microglia. Cell Death Dis. 2021, 12, 1159. [Google Scholar] [CrossRef] [PubMed]
- Akyuva, Y.; Nazıroğlu, M.; Yıldızhan, K. Selenium prevents interferon-gamma induced activation of TRPM2 channel and inhibits inflammation, mitochondrial oxidative stress, and apoptosis in microglia. Metab. Brain Dis. 2021, 36, 285–298. [Google Scholar] [CrossRef]
- He, Y.; Chang, Y.; Peng, Y.; Zhu, J.; Liu, K.; Chen, J.; Wu, Y.; Ji, Z.; Lin, Z.; Wang, S.; et al. Glibenclamide Directly Prevents Neuroinflammation by Targeting SUR1-TRPM4-Mediated NLRP3 Inflammasome Activation In Microglia. Mol. Neurobiol. 2022, 59, 6590–6607. [Google Scholar] [CrossRef]
- Conti, P.; Lauritano, D.; Caraffa, A.; Gallenga, C.E.; Kritas, S.K.; Ronconi, G.; Pandolfi, F. New insight into systemic mastocytosis mediated by cytokines IL-1beta and IL-33: Potential inhibitory effect of IL-37. Eur. J. Pharmacol. 2019, 858, 172473. [Google Scholar] [CrossRef]
- Wu, C.; Boey, D.; Bril, O.; Grootens, J.; Vijayabaskar, M.S.; Sorini, C.; Ekoff, M.; Wilson, N.K.; Ungerstedt, J.S.; Nilsson, G.; et al. Single-cell transcriptomics reveals the identity and regulators of human mast cell progenitors. Blood Adv. 2022, 6, 4439–4449. [Google Scholar] [CrossRef]
- Vennekens, R.; Olausson, J.; Meissner, M.; Bloch, W.; Mathar, I.; Philipp, S.E.; Schmitz, F.; Weissgerber, P.; Nilius, B.; Flockerzi, V.; et al. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat. Immunol. 2007, 8, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Wykes, R.C.; Lee, M.; Duffy, S.M.; Yang, W.; Seward, E.P.; Bradding, P. Functional transient receptor potential melastatin 7 channels are critical for human mast cell survival. J. Immunol. 2007, 179, 4045–4052. [Google Scholar] [CrossRef]
- Castanheira, F.V.S.; Kubes, P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 2019, 133, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, O.; Strodthoff, C.; Horstmann, M.; Nielsen, N.; Jung, F.; Schimmelpfennig, S.; Heitzmann, M.; Schwab, A. TRPC1 regulates fMLP-stimulated migration and chemotaxis of neutrophil granulocytes. Biochim. Biophys. Acta 2015, 1853, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, O.; Umlauf, D.; Frank, S.; Schimmelpfennig, S.; Bertrand, J.; Pap, T.; Hanley, P.J.; Fabian, A.; Dietrich, A.; Schwab, A. TRPC6 regulates CXCR2-mediated chemotaxis of murine neutrophils. J. Immunol. 2013, 190, 5496–5505. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lu, K.; Lu, Y.; Liao, J.; Zhang, S.; Yang, S.; Zhao, N.; Dong, Q.; Chen, L.; Wu, Q.; et al. Transient receptor potential vanilloid 4 (TRPV4) in neutrophils enhances myocardial ischemia/reperfusion injury. J. Leukoc. Biol. 2023, 114, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Nepal, S.; Tsukasaki, Y.; Hecquet, C.M.; Soni, D.; Rehman, J.; Tiruppathi, C.; Malik, A.B. Neutrophil Activation of Endothelial Cell-Expressed TRPM2 Mediates Transendothelial Neutrophil Migration and Vascular Injury. Circ. Res. 2017, 121, 1081–1091. [Google Scholar] [CrossRef]
- Nadolni, W.; Immler, R.; Hoelting, K.; Fraticelli, M.; Ripphahn, M.; Rothmiller, S.; Matsushita, M.; Boekhoff, I.; Gudermann, T.; Sperandio, M.; et al. TRPM7 Kinase Is Essential for Neutrophil Recruitment and Function via Regulation of Akt/mTOR Signaling. Front. Immunol. 2020, 11, 606893. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Roche, P.A. Macropinocytosis in phagocytes: Regulation of MHC class-II-restricted antigen presentation in dendritic cells. Front. Physiol. 2015, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Sumoza-Toledo, A.; Lange, I.; Cortado, H.; Bhagat, H.; Mori, Y.; Fleig, A.; Penner, R.; Partida-Sánchez, S. Dendritic cell maturation and chemotaxis is regulated by TRPM2-mediated lysosomal Ca2+ release. FASEB J. 2011, 25, 3529–3542. [Google Scholar] [CrossRef] [PubMed]
- Assas, M.B.; Wakid, M.H.; Zakai, H.A.; Miyan, J.A.; Pennock, J.L. Transient receptor potential vanilloid 1 expression and function in splenic dendritic cells: A potential role in immune homeostasis. Immunology 2016, 147, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Yao, E.; Zhang, G.; Huang, J.; Yang, X.; Peng, L.; Huang, X.; Luo, X.; Ren, J.; Huang, R.; Yang, L.; et al. Immunomodulatory effect of oleoylethanolamide in dendritic cells via TRPV1/AMPK activation. J. Cell. Physiol. 2019, 234, 18392–18407. [Google Scholar] [CrossRef]
- Bretou, M.; Sáez, P.J.; Sanséau, D.; Maurin, M.; Lankar, D.; Chabaud, M.; Spampanato, C.; Malbec, O.; Barbier, L.; Muallem, S.; et al. Lysosome signaling controls the migration of dendritic cells. Sci. Immunol. 2017, 2, eaak9573. [Google Scholar] [CrossRef]
- MartIn-Fontecha, A.; Sebastiani, S.; Höpken, U.E.; Uguccioni, M.; Lipp, M.; Lanzavecchia, A.; Sallusto, F. Regulation of dendritic cell migration to the draining lymph node: Impact on T lymphocyte traffic and priming. J. Exp. Med. 2003, 198, 615–621. [Google Scholar] [CrossRef]
- Weber, M.; Hauschild, R.; Schwarz, J.; Moussion, C.; de Vries, I.; Legler, D.F.; Luther, S.A.; Bollenbach, T.; Sixt, M. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science 2013, 339, 328–332. [Google Scholar] [CrossRef]
- Saravia, J.; Chapman, N.M.; Chi, H. Helper T cell differentiation. Cell. Mol. Immunol. 2019, 16, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Sel, S.; Rost, B.R.; Yildirim, A.O.; Sel, B.; Kalwa, H.; Fehrenbach, H.; Renz, H.; Gudermann, T.; Dietrich, A. Loss of classical transient receptor potential 6 channel reduces allergic airway response. Clin. Exp. Allergy 2008, 38, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Mendu, S.K.; Stremska, M.E.; Schappe, M.S.; Moser, E.K.; Krupa, J.K.; Rogers, J.S.; Stipes, E.J.; Parker, C.A.; Braciale, T.J.; Perry, J.S.A.; et al. Targeting the ion channel TRPM7 promotes the thymic development of regulatory T cells by promoting IL-2 signaling. Sci. Signal. 2020, 13, eabb0619. [Google Scholar] [CrossRef]
- Reina-Campos, M.; Scharping, N.E.; Goldrath, A.W. CD8+ T cell metabolism in infection and cancer. Nat. Rev. Immunol. 2021, 21, 718–738. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Dongming, Z.; Hong, C.; Quan, N.; Sishi, L.; Caixia, L. TRPC3 Overexpression Promotes the Progression of Inflammation-Induced Preterm Labor and Inhibits T Cell Activation. Cell. Physiol. Biochem. 2018, 45, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Acharya, T.K.; Kumar, S.; Rokade, T.P.; Chang, Y.T.; Goswami, C. TRPV4 regulates mitochondrial Ca2+-status and physiology in primary murine T cells based on their immunological state. Life Sci. 2023, 318, 121493. [Google Scholar] [CrossRef]
- Seifert, M.; Küppers, R. Human memory B cells. Leukemia 2016, 30, 2283–2292. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Okumura, R.; Ono, C.; Okuzaki, D.; Kawai, T.; Okochi, Y.; Tanimura, N.; Murakami, M.; Kayama, H.; Umemoto, E.; et al. TRPM5 Negatively Regulates Calcium-Dependent Responses in Lipopolysaccharide-Stimulated B Lymphocytes. Cell Rep. 2020, 31, 107755. [Google Scholar] [CrossRef]
- Goodridge, J.P.; Jacobs, B.; Saetersmoen, M.L.; Clement, D.; Hammer, Q.; Clancy, T.; Skarpen, E.; Brech, A.; Landskron, J.; Grimm, C.; et al. Remodeling of secretory lysosomes during education tunes functional potential in NK cells. Nat. Commun. 2019, 10, 514. [Google Scholar] [CrossRef]
- Jarczak, D.; Nierhaus, A. Cytokine Storm-Definition, Causes, and Implications. Int. J. Mol. Sci. 2022, 23, 11740. [Google Scholar] [CrossRef] [PubMed]
- Roe, K. An inflammation classification system using cytokine parameters. Scand. J. Immunol. 2021, 93, e12970. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. The spectrum of inflammatory responses. Science 2021, 374, 1070–1075. [Google Scholar] [CrossRef]
- Bhat, A.A.; Nisar, S.; Singh, M.; Ashraf, B.; Masoodi, T.; Prasad, C.P.; Sharma, A.; Maacha, S.; Karedath, T.; Hashem, S.; et al. Cytokine- and chemokine-induced inflammatory colorectal tumor microenvironment: Emerging avenue for targeted therapy. Cancer Commun. 2022, 42, 689–715. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, D.H.; Park, M.S.; Jung, Y.S.; Hong, J.T. C-C chemokine receptor type 5 deficiency exacerbates alcoholic fatty liver disease through pro-inflammatory cytokines and chemokines-induced hepatic inflammation. J. Gastroenterol. Hepatol. 2017, 32, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, Y.; Zhou, L.; Fu, Z.; Yang, C.; Zhao, L.; Li, S.; Chen, Y.; Wu, Y.; Ling, Z.; et al. TRPC6-dependent Ca2+ signaling mediates airway inflammation in response to oxidative stress via ERK pathway. Cell Death Dis. 2020, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.I.; Lee, H.T.; Lin, H.C.; Tsay, H.J.; Tsai, F.C.; Shyue, S.K.; Lee, T.S. Role of transient receptor potential ankyrin 1 channels in Alzheimer’s disease. J. Neuroinflamm. 2016, 13, 92. [Google Scholar] [CrossRef]
- Yap, J.M.G.; Ueda, T.; Takeda, N.; Fukumitsu, K.; Fukuda, S.; Uemura, T.; Tajiri, T.; Ohkubo, H.; Maeno, K.; Ito, Y.; et al. An inflammatory stimulus sensitizes TRPA1 channel to increase cytokine release in human lung fibroblasts. Cytokine 2020, 129, 155027. [Google Scholar] [CrossRef]
- Ninomiya, Y.; Tanuma, S.I.; Tsukimoto, M. Differences in the effects of four TRPV1 channel antagonists on lipopolysaccharide-induced cytokine production and COX-2 expression in murine macrophages. Biochem Biophys Res Commun 2017, 484, 668–674. [Google Scholar] [CrossRef]
- Yamashiro, K.; Sasano, T.; Tojo, K.; Namekata, I.; Kurokawa, J.; Sawada, N.; Suganami, T.; Kamei, Y.; Tanaka, H.; Tajima, N.; et al. Role of transient receptor potential vanilloid 2 in LPS-induced cytokine production in macrophages. Biochem. Biophys. Res. Commun. 2010, 398, 284–289. [Google Scholar] [CrossRef]
- Dutta, B.; Goswami, R.; Rahaman, S.O. TRPV4 Plays a Role in Matrix Stiffness-Induced Macrophage Polarization. Front. Immunol. 2020, 11, 570195. [Google Scholar] [CrossRef]
- Yin, J.; Michalick, L.; Tang, C.; Tabuchi, A.; Goldenberg, N.; Dan, Q.; Awwad, K.; Wang, L.; Erfinanda, L.; Nouailles, G.; et al. Role of Transient Receptor Potential Vanilloid 4 in Neutrophil Activation and Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 2016, 54, 370–383. [Google Scholar] [CrossRef]
- Miyake, T.; Shirakawa, H.; Kusano, A.; Sakimoto, S.; Konno, M.; Nakagawa, T.; Mori, Y.; Kaneko, S. TRPM2 contributes to LPS/IFNγ-induced production of nitric oxide via the p38/JNK pathway in microglia. Biochem. Biophys. Res. Commun. 2014, 444, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Hollenhorst, M.I.; Nandigama, R.; Evers, S.B.; Gamayun, I.; Abdel Wadood, N.; Salah, A.; Pieper, M.; Wyatt, A.; Stukalov, A.; Gebhardt, A.; et al. Bitter taste signaling in tracheal epithelial brush cells elicits innate immune responses to bacterial infection. J. Clin. Investig. 2022, 132, e150951. [Google Scholar] [CrossRef]
- Zhong, W.; Ma, M.; Xie, J.; Huang, C.; Li, X.; Gao, M. Adipose-specific deletion of the cation channel TRPM7 inhibits TAK1 kinase-dependent inflammation and obesity in male mice. Nat. Commun. 2023, 14, 491. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, B.; Nadolni, W.; Hampe, S.; Hoelting, K.; Fraticelli, M.; Zaborsky, N.; Madlmayr, A.; Sperrer, V.; Fraticelli, L.; Addington, L.; et al. Inactivation of TRPM7 Kinase Targets AKT Signaling and Cyclooxygenase-2 Expression in Human CML Cells. Function 2023, 4, zqad053. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hua, Y.; Vergarajauregui, S.; Diab, H.I.; Puertollano, R. Novel Role of TRPML2 in the Regulation of the Innate Immune Response. J. Immunol. 2015, 195, 4922–4932. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, D.H. Knockout of Trpa1 Exacerbates Renal Ischemia-Reperfusion Injury with Classical Activation of Macrophages. Am. J. Hypertens. 2021, 34, 110–116. [Google Scholar] [CrossRef]
- Bertin, S.; Aoki-Nonaka, Y.; Lee, J.; de Jong, P.R.; Kim, P.; Han, T.; Yu, T.; To, K.; Takahashi, N.; Boland, B.S.; et al. The TRPA1 ion channel is expressed in CD4+ T cells and restrains T-cell-mediated colitis through inhibition of TRPV1. Gut 2017, 66, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.S.; Majhi, R.K.; Tiwari, A.; Acharya, T.; Kumar, P.S.; Saha, S.; Kumar, A.; Goswami, C.; Chattopadhyay, S. Transient receptor potential ankyrin1 channel is endogenously expressed in T cells and is involved in immune functions. Biosci. Rep. 2019, 39, BSR20191437. [Google Scholar] [CrossRef]
- Feng, J.; Yang, P.; Mack, M.R.; Dryn, D.; Luo, J.; Gong, X.; Liu, S.; Oetjen, L.K.; Zholos, A.V.; Mei, Z.; et al. Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nat. Commun. 2017, 8, 980. [Google Scholar] [CrossRef]
- Ueno, K.; Saika, S.; Okada, Y.; Iwanishi, H.; Suzuki, K.; Yamada, G.; Asamura, S. Impaired healing of cutaneous wound in a Trpv1 deficient mouse. Exp. Anim. 2023, 72, 224–232. [Google Scholar] [CrossRef]
- Di, A.; Gao, X.P.; Qian, F.; Kawamura, T.; Han, J.; Hecquet, C.; Ye, R.D.; Vogel, S.M.; Malik, A.B. The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nat. Immunol. 2011, 13, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Boukenna, M.; Rougier, J.S.; Aghagolzadeh, P.; Pradervand, S.; Guichard, S.; Hämmerli, A.F.; Pedrazzini, T.; Abriel, H. Multiomics uncover the proinflammatory role of Trpm4 deletion after myocardial infarction in mice. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H504–H518. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.S.; Hildner, K.; Murphy, K.M.; Allen, P.M. Trpm4 differentially regulates Th1 and Th2 function by altering calcium signaling and NFAT localization. J. Immunol. 2010, 185, 2836–2846. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.; Miralles, F.; Eder, C. TRPM7 regulates proliferation and polarisation of macrophages. J. Cell Sci. 2014, 127, 4561–4566. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.Y.; Sun, M.R.; Wu, C.L.; Li, Y.; Du, J.J.; Zeng, J.Y.; Bi, H.L.; Sun, Y.H. Activation of calcium-sensing receptor increases TRPC3/6 expression in T lymphocyte in sepsis. Mol. Immunol. 2015, 64, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Reese, R.M.; Dourado, M.; Anderson, K.; Warming, S.; Stark, K.L.; Balestrini, A.; Suto, E.; Lee, W.; Riol-Blanco, L.; Shields, S.D.; et al. Behavioral characterization of a CRISPR-generated TRPA1 knockout rat in models of pain, itch, and asthma. Sci. Rep. 2020, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Nevius, E.; Srivastava, P.K.; Basu, S. Oral ingestion of Capsaicin, the pungent component of chili pepper, enhances a discreet population of macrophages and confers protection from autoimmune diabetes. Mucosal Immunol. 2012, 5, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Tominaga, M. The role of TRPM2 in pancreatic beta-cells and the development of diabetes. Cell Calcium 2014, 56, 332–339. [Google Scholar] [CrossRef]
- Bousquet, J.; Czarlewski, W.; Zuberbier, T.; Mullol, J.; Blain, H.; Cristol, J.P.; De La Torre, R.; Pizarro Lozano, N.; Le Moing, V.; Bedbrook, A.; et al. Potential Interplay between Nrf2, TRPA1, and TRPV1 in Nutrients for the Control of COVID-19. Int. Arch. Allergy Immunol. 2021, 182, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Diep, P.T. TRPV1, Nrf2, and COVID-19: Could Oxytocin Have a Beneficial Role to Play? Int. Arch. Allergy Immunol. 2022, 183, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Kuebler, W.M.; Jordt, S.E.; Liedtke, W.B. Urgent reconsideration of lung edema as a preventable outcome in COVID-19: Inhibition of TRPV4 represents a promising and feasible approach. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L1239–L1243. [Google Scholar] [CrossRef] [PubMed]
| Immune Cells | TRP Channels | Reference |
|---|---|---|
| Macrophages | TRPA1 TRPML2 TRPC1, TRPC3, TRPC5 TRPV1, TRPV2, TRPV4 TRPM2, TRPM4, TRPM7, TRPM8 | [37] [35] [19,20,21] [22,23,24,25,26] [27,28,29,30,31,32,33,34] |
| Microglia | TRPC3 TRPV1 TRPM2, TRPM4 | [39] [40] [41,42] |
| Neutrophils | TRPV4 TRPC1, TRPC6 TRPM2, TRPM7 | [50] [48,49] [51,52] |
| Dendritic cells | TRPV1 TRPM2 TRPML1 | [55,56] [54] [57] |
| Mast cells | TRPM4, TRPM7 | [45,46] |
| B lymphocytes | TRPM5 | [68] |
| T lymphocytes | TRPA1,TRPV1 TRPV4 TRPM7 TRPC3, TRPC6 | [61] [66] [31,63] [62,65] |
| Natural killer cells | TRPML1 | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.; Gao, C.; Li, M.; Lan, R.; Wei, S.; Fan, R.; Cheng, W. TRP Ion Channels in Immune Cells and Their Implications for Inflammation. Int. J. Mol. Sci. 2024, 25, 2719. https://doi.org/10.3390/ijms25052719
Yan Q, Gao C, Li M, Lan R, Wei S, Fan R, Cheng W. TRP Ion Channels in Immune Cells and Their Implications for Inflammation. International Journal of Molecular Sciences. 2024; 25(5):2719. https://doi.org/10.3390/ijms25052719
Chicago/Turabian StyleYan, Qiyue, Chuanzhou Gao, Mei Li, Rui Lan, Shaohan Wei, Runsong Fan, and Wei Cheng. 2024. "TRP Ion Channels in Immune Cells and Their Implications for Inflammation" International Journal of Molecular Sciences 25, no. 5: 2719. https://doi.org/10.3390/ijms25052719
APA StyleYan, Q., Gao, C., Li, M., Lan, R., Wei, S., Fan, R., & Cheng, W. (2024). TRP Ion Channels in Immune Cells and Their Implications for Inflammation. International Journal of Molecular Sciences, 25(5), 2719. https://doi.org/10.3390/ijms25052719






