Inulin-Coated ZnO Nanoparticles: A Correlation between Preparation and Properties for Biostimulation Purposes
Abstract
1. Introduction
2. Results and Discussion
2.1. XRD
2.2. IR
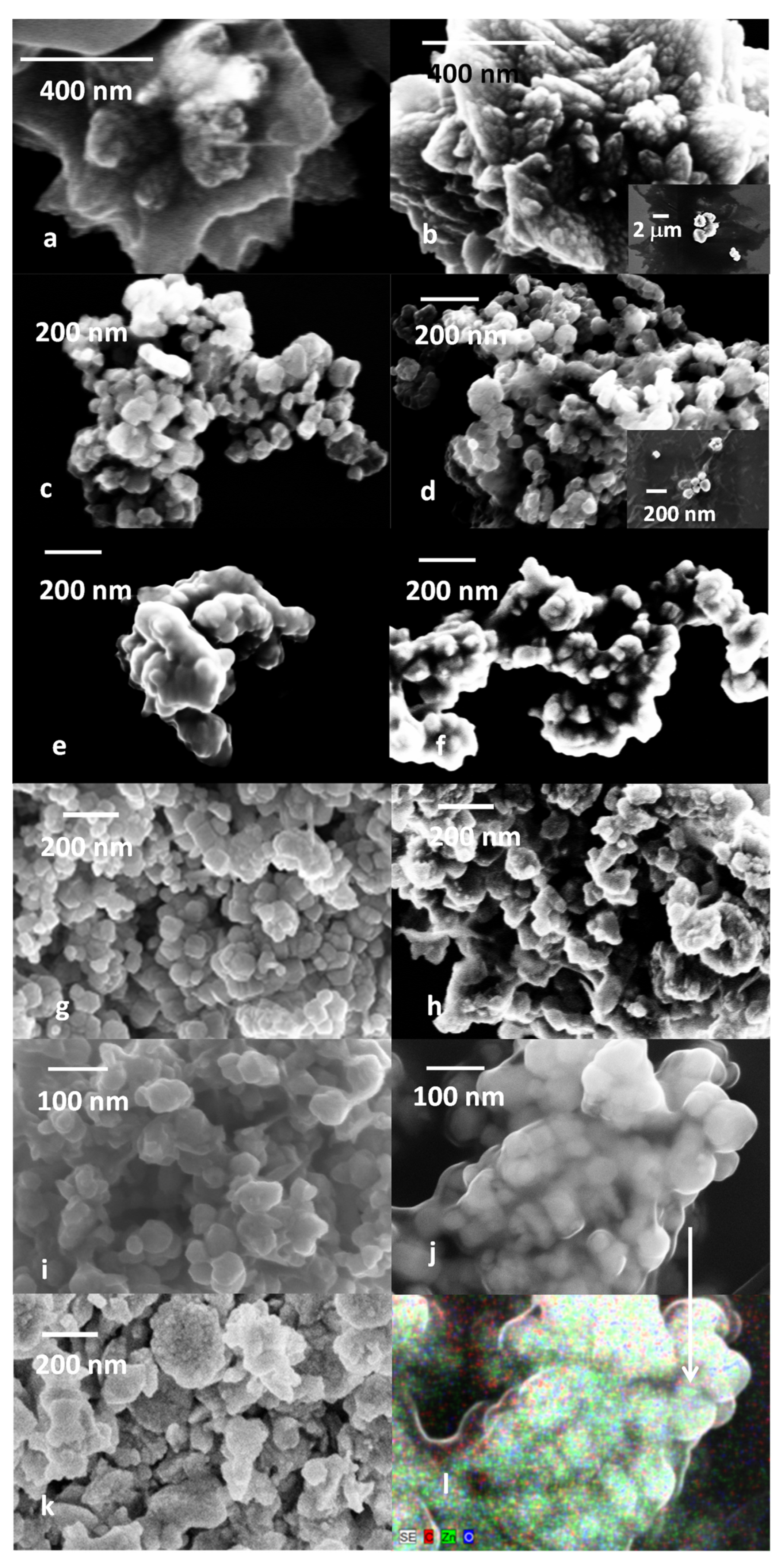
2.3. SEM
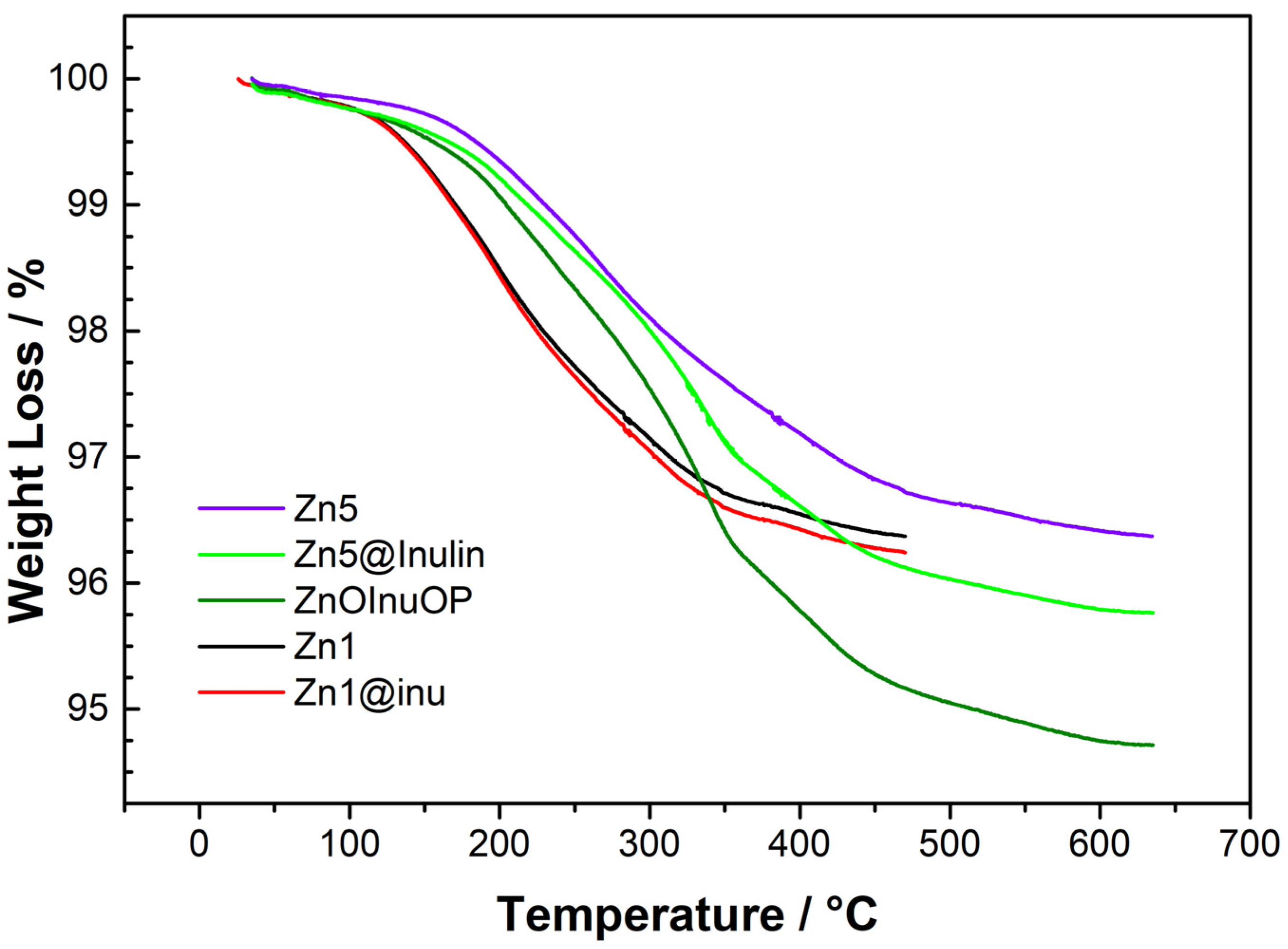
2.4. Thermogravimetric Analysis
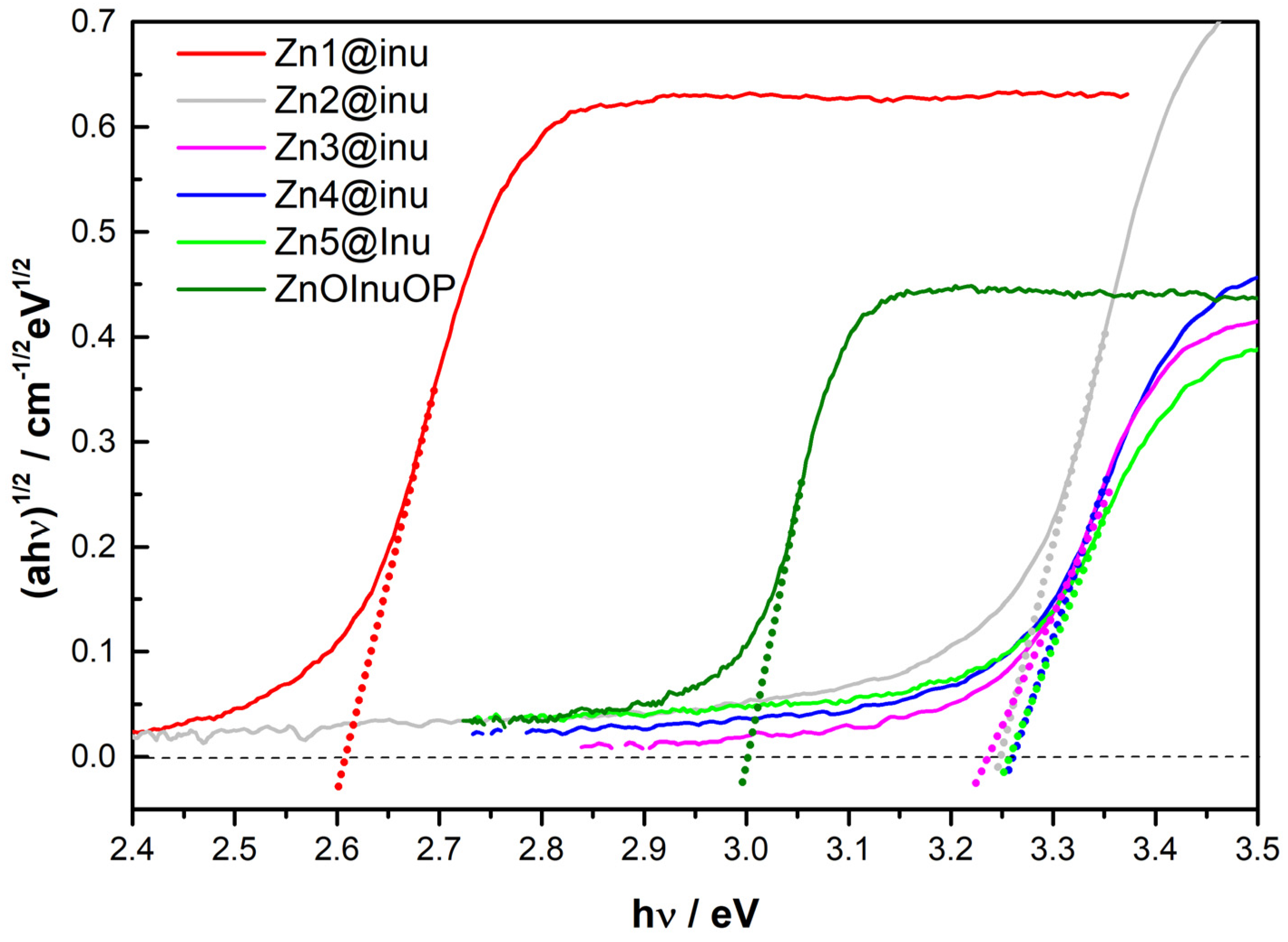
2.5. Reflectance Spectroscopy
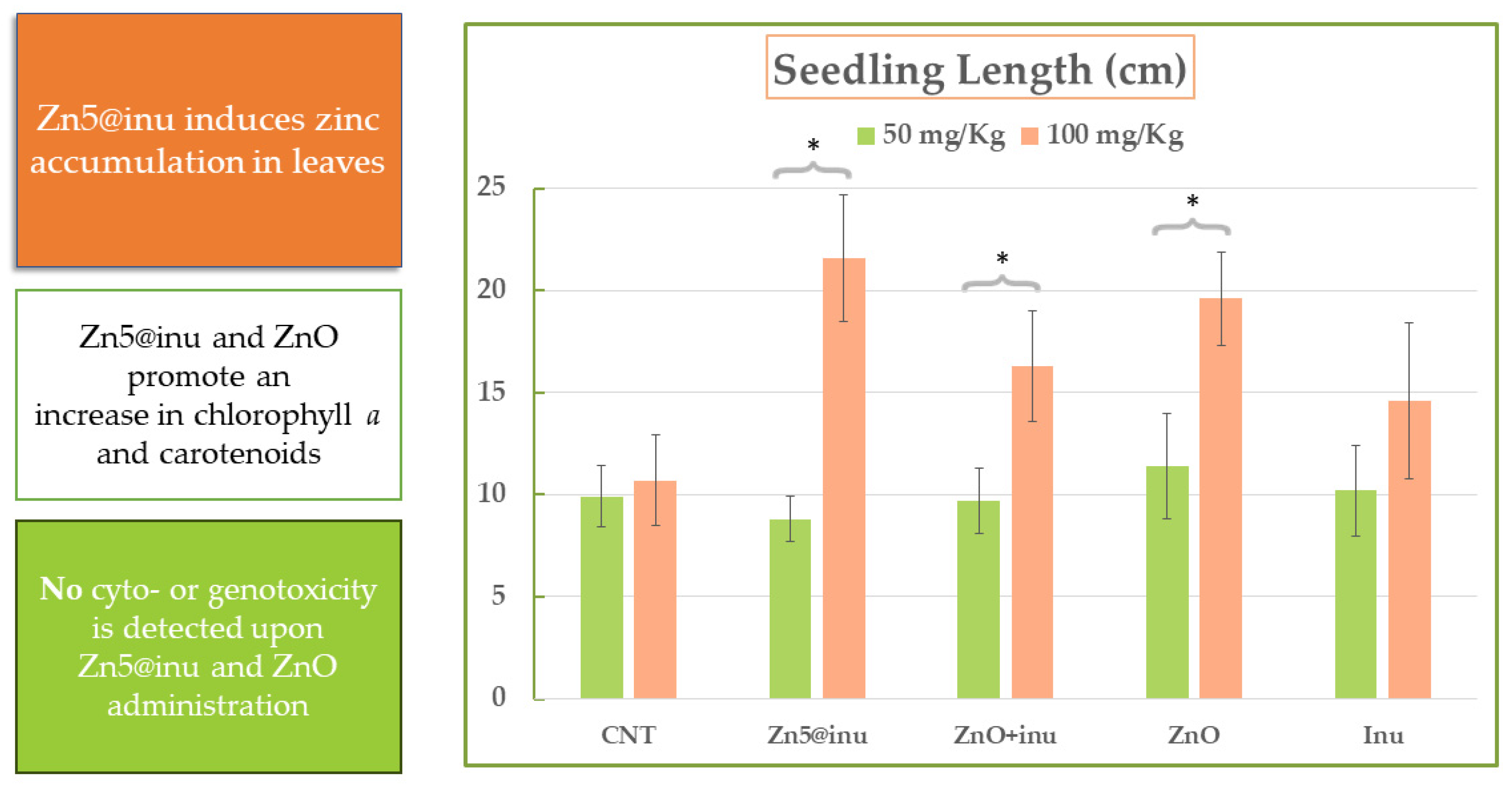
2.6. Plant Growth Assays—Proof of Principle
3. Materials and Methods
3.1. Chemicals and Equipment
3.2. Synthetic Procedures
3.2.1. Two-Step Synthesis
3.2.2. Gel-like Synthesis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, L.; Zhou, X.; Kang, Z.; Peralta-Videa, J.R.; Zhu, Y.G. Nano-enabled seed treatment: A new and sustainable approach to engineering climate-resilient crops. Sci. Total Environ. 2024, 910, 168640. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Afridi, M.S.; Javed, M.A.; Saleem, A.; Hafeez, A.; Khan, A.R.; Zeeshan, M.; Ali, B.; Azhar, W.; Sumaira; et al. Nano-Priming against Abiotic Stress: A Way Forward towards Sustainable Agriculture. Sustainability 2022, 14, 14880. [Google Scholar] [CrossRef]
- Nile, S.H.; Thiruvengadam, M.; Wang, Y.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Nile, A.; Sun, M.; Venkidasamy, B.; Xiao, J.; et al. Nano-priming as emerging seed priming technology for sustainable agriculture—Recent developments and future perspectives. J. Nanobiotechnol. 2022, 20, 254. [Google Scholar] [CrossRef]
- Khalaki, M.A.; Moameri, M.; Lajayer, B.A.; Tess Astatkie, T. Influence of nano-priming on seed germination and plant growth of forage and medicinal plants. Plant Growth Regul. 2021, 93, 13–28. [Google Scholar] [CrossRef]
- Nawaz, M.; Sun, J.; Shabbir, S.; Khattak, W.A.; Ren, G.; Nie, X.; Bo, Y.; Javed, Q.; Du, D.; Sonne, C. A review of plants strategies to resist biotic and abiotic environmental stressors. Sci. Total Environ. 2023, 900, 165832. [Google Scholar] [CrossRef]
- Limosani, F.; Bauer, E.M.; Cecchetti, D.; Biagioni, S.; Orlando, V.; Pizzoferrato, R.; Prosposito, P.; Carbone, M. Top-Down N-Doped Carbon Quantum Dots for Multiple Purposes: Heavy Metal Detection and Intracellular Fluorescence. Nanomaterials 2021, 11, 2249. [Google Scholar] [CrossRef]
- Valentini, F.; Ciambella, E.; Boaretto, A.; Rizzitelli, G.; Carbone, M.; Conte, V.; Cataldo, F.; Russo, V.; Casari, C.S.; Chillura-Martino, D.F.; et al. Sensor Properties of Pristine and Functionalized Carbon Nanohorns. Electroanalysis 2016, 28, 2489–2499. [Google Scholar] [CrossRef]
- Azzouz, A.; Hejji, L.; Kumar, V.; Kim, K.H. Nanomaterials-based aptasensors: An efficient detection tool for heavy-metal and metalloid ions in environmental and biological samples. Environ. Res. 2023, 238, 117170. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, Y. Nanomaterials for fluorescent detection of vitamin B2: A review. Anal. Biochem. 2023, 683, 115351. [Google Scholar] [CrossRef]
- Anusuyadevi, K.; Velmathi, S. Design strategies of carbon nanomaterials in fluorescent sensing of biomolecules and metal ions—A review. Results Chem. 2023, 5, 100918. [Google Scholar] [CrossRef]
- Geleta, G.S. Recent advances in electrochemical sensors based on MIPS and nanomaterials for detection of ascorbic dopamineand uric acid—A review. Sens. Bio-Sens. Res. 2024, 43, 100610. [Google Scholar] [CrossRef]
- Gowthaman, N.S.K.; Amalraj, M.; Kesavan, S.; Rajalakshmi, K.; Shankar, S.; Sinduja, B.; Arul, P.; Karthikeyan, R.; Loganathan, C.; Mangala Gowri, V.; et al. Zero-, one- and two-dimensional carbon nanomaterials as low-cost catalysts in optical and electrochemical sensing of biomolecules and environmental pollutants. Microchem. J. 2023, 194, 109291. [Google Scholar] [CrossRef]
- Jose, J.; Prakash, P.; Jeyaprabha, B.; Abraham, R.; Mathew, R.M.; Zacharia, E.S.; Thomas, V.; Thomas, J. Principle, design, strategies, and future perspectives of heavy metal ion detection using carbon nanomaterial-based electrochemical sensors: A review. J. Iran. Chem. Soc. 2023, 20, 775–791. [Google Scholar] [CrossRef]
- Kateshiya, M.R.; Desai, M.L.; Malek, N.I.; Kailasa, S.K. Advances in Ultra-small Fluorescence Nanoprobes for Detection of Metal Ions, Drugs, Pesticides and Biomarkers. J. Fluoresc. 2023, 33, 775–798. [Google Scholar] [CrossRef]
- Mahajan, M.R.; Patil, P.O. Design of zero-dimensional graphene quantum dots based nanostructures for the detection of organophosphorus pesticides in food and water: A review. Inorg. Chem. Commun. 2022, 144, 109883. [Google Scholar] [CrossRef]
- Malik, S.; Singh, J.; Goyat, R.; Saharan, Y.; Chaudhry, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Ameen, S.; Baskoutas, S. Nanomaterials-based biosensor and their applications: A review. Heliyon 2023, 9, e19929. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, B.; Xiao, Y.; Tian, X.; Wang, Y. Fluorescent Quantum Dots and Its Composites for Highly Sensitive Detection of Heavy Metal Ions and Pesticide Residues: A Review. Chemosensors 2023, 11, 405. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; Cao, P.; Li, P.; Xing, X.; Yu, Y.; Guo, R.; Yang, H. Recent Advances of Graphene Quantum Dots in Chemiresistive Gas Sensors. Nanomaterials 2023, 13, 2880. [Google Scholar] [CrossRef]
- Zúñiga, K.; Rebollar, G.; Avelar, M.; Campos-Terán, J.; Torres, E. Nanomaterial-Based Sensors for the Detection of Glyphosate. Water 2022, 14, 2436. [Google Scholar] [CrossRef]
- Carbone, M.; Aneggi, E.; Figueredo, F.; Susmel, S. NiO-Nanoflowers Decorating a Plastic Electrode for the Non-Enzymatic Amperometric Detection of H2O2 in Milk: Old Issue, New Challenge. Food Control 2022, 132, 108549. [Google Scholar] [CrossRef]
- Gontrani, L.; Donia, D.T.; Bauer, E.M.; Tagliatesta, P.; Carbone, M. Novel Synthesis of Zinc Oxide Nanoparticles from Type IV Deep Eutectic Solvents. Inorg. Chim. Acta 2023, 545, 121268. [Google Scholar] [CrossRef]
- Carbone, M. Cu Zn Co Nanosized Mixed Oxides Prepared from Hydroxycarbonate Precursors. J. Alloys Compd. 2016, 688, 202–209. [Google Scholar] [CrossRef]
- Carbone, M.; Piancastelli, M.N.; Casaletto, M.P.; Zanoni, R.; Besnard-Ramage, M.J.; Comtet, G.; Dujardin, G.; Hellner, L. Phenol adsorption on Si(111)7×7 studied by synchrotron radiation photoemission and photodesorption. Surf. Sci. 1999, 419, 114–119. [Google Scholar] [CrossRef]
- Donia, D.T.; Bauer, E.M.; Missori, M.; Roselli, L.; Cecchetti, D.; Tagliatesta, P.; Gontrani, L.; Carbone, M. Room Temperature Syntheses of ZnO and Their Structures. Symmetry 2021, 13, 733. [Google Scholar] [CrossRef]
- Aliannezhadi, M.; Mirsanai, S.Z.; Jamali, M.; Shariatmadar Tehrani, F. Optical and structural properties of bare MoO3 nanobelt, ZnO nanoflakes, and MoO3/ZnO nanocomposites: The effect of hydrothermal reaction times and molar ratios. Opt. Mater. 2024, 147, 114619. [Google Scholar] [CrossRef]
- Bharathi, P.; Harish, S.; Shimomura, M.; Mohan, M.K.; Archana, J.; Navaneethan, M. Ultrasensitive and reversible NO2 gas sensor based on SnS2/TiO2 heterostructures for room temperature applications. Chemosphere 2024, 346, 140486. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Chen, G.; Wang, Z.; Li, H.; Zhang, Z.; Liu, Y.; Lu, M. An ultra-sensitive electrochemical sensing platform based on nanoflower-like Au/ZnO array on carbon cloth for the rapid detection of the nitrite residues in food samples. Food Chem. 2024, 437, 137892. [Google Scholar] [CrossRef]
- Li, X.; Jia, F.; Luo, N.; Cai, H.; Chen, J.; Ren, W.; Cheng, J.; Xu, J. Self-assembly tourmaline@BiFeO3 composites with enhanced polarization for dual-selective C3H6O and H2S detection. Sens. Actuators B Chem. 2024, 399, 134806. [Google Scholar] [CrossRef]
- Lv, M.S.; Li, Y.N.; Chen, G.L.; Gao, R.; Zhang, X.F.; Deng, Z.P.; Xu, Y.M.; Huo, L.H.; Gao, S. Mesoporous In2O3/ZnO heterogeneous microtubes replicated from waste willow catkins for high response and rapid detection of NO2 gas at low temperature. Sens. Actuators B Chem. 2024, 400, 134880. [Google Scholar] [CrossRef]
- Malik, S.B.; Mejia-Centeno, K.V.; Martínez-Alanis, P.R.; Cabot, A.; Güell, F.; Annanouch, F.E.; Llobet, E. Synergistic effect of CeO2 nanoparticles and WO3 nanowires in gas sensing applications. Sens. Actuators B Chem. 2024, 400, 134879. [Google Scholar] [CrossRef]
- Murugesan, T.; Kumar, R.R.; Ranjan, A.; Lu, M.Y.; Lin, H.N. Fabrication of large-area Au nanoparticle decorated ZnO@ZIF-8 core-shell heterostructure nanorods for ppb-level NO2 gas sensing at room temperature. Sens. Actuators B Chem. 2024, 402, 135106. [Google Scholar] [CrossRef]
- Pandey, G.; Bhardwaj, M.; Kumar, S.; Lawaniya, S.D.; Kumar, M.; Dwivedi, P.K.; Awasthi, K. Synergistic effects of Pd-Ag decoration on SnO/SnO2 nanosheets for enhanced hydrogen sensing. Sens. Actuators B Chem. 2024, 402, 135062. [Google Scholar] [CrossRef]
- Proença, M.; Rodrigues, M.S.; Moura, C.; Machado, A.V.; Borges, J.; Vaz, F. Nanoplasmonic Au:CuO thin films functionalized with APTES to enhance the sensitivity of gas sensors. Sens. Actuators B Chem. 2024, 401, 134959. [Google Scholar] [CrossRef]
- Renganathan, B.; Gopakumar, C.K.; Priya, A.K.; Rao, S.K.; Sastikumar, D.; Silambarasan, M.; Kannapiran, N. Optimizing Gas Sensing Performance of CuO Nanoparticles via Sol-Gel Synthesis Approach for Efficient Detection of Ammonia Gas. Mater. Res. Bull. 2024, 170, 112556. [Google Scholar] [CrossRef]
- Carbone, M.; Missori, M.; Micheli, L.; Tagliatesta, P.; Bauer, E.M. NiO Pseudocapacitance and Optical Properties: Does the Shape Win? Materials 2020, 13, 1417. [Google Scholar] [CrossRef]
- Carbone, M. Zn Defective ZnCo2O4 Nanorods as High Capacity Anode for Lithium Ion Batteries. J. Electroanal. Chem. 2018, 815, 151–157. [Google Scholar] [CrossRef]
- Gontrani, L.; Bauer, E.M.; Talone, A.; Missori, M.; Imperatori, P.; Tagliatesta, P.; Carbone, M. CuO Nanoparticles and Microaggregates: An Experimental and Computational Study of Structure and Electronic Properties. Materials 2023, 16, 4800. [Google Scholar] [CrossRef]
- BhaskaraRao, B.V.; Pabba, D.P.; Aepuru, R.; Akbari-Fakhrabadi, A.; Lokhande, P.; Udayabhaskar, R.; Rosales-Vera, M.; Espinoza-González, R. Fe3O4 nanoparticles intercalated reduced graphene oxide nanosheets for supercapacitor and lithium-ion battery anode performance. J. Mater. Sci. Mater. Electron. 2023, 34, 1910. [Google Scholar] [CrossRef]
- Bhaviripudi, V.R.; Dwivedi, P.K.; Pabba, D.P.; Aepuru, R.; Nakate, U.T.; Espinoza-González, R.; Shelke, M.V. Evaluation of Fe3O4 incorporated functionalized carbon nanotube self-standing buckypaper as electrodes for solid-state symmetric supercapacitor. J. Energy Storage 2023, 73, 109101. [Google Scholar] [CrossRef]
- Jalilzadeh, H.; Outokesh, M.; Shafiekhani, A.; Hosseinpour, M.; Tayyebi, A. Magnetite nanoparticles embedded on reduced graphene oxide as an anode material for high capacity and long cycle-life Li-ion battery. J. Energy Storage 2023, 72, 108607. [Google Scholar] [CrossRef]
- Lv, H.; Xiao, Z.; Zhai, S.; Wang, X.; Hao, J.; An, Q. Designed formation of C/Fe3O4@Ni(OH)2 cathode with enhanced pseudocapacitance for asymmetric supercapacitors. J. Colloid Interface Sci. 2023, 635, 176–185. [Google Scholar] [CrossRef]
- Park, S.; Ji, S.; Kim, S.K.; Yoon, Y.; Yim, S.; Song, W.; Myung, S.; Lee, S.S.; An, K.S. Architectural engineering of vertically expanded graphene-CoMn2O4 compounds based interdigital electrode for in-plane micro-supercapacitor. J. Alloys Compd. 2023, 969, 172414. [Google Scholar] [CrossRef]
- Patil, S.L.; Pawar, O.Y.; Patil, H.S.; Sutar, S.S.; Kamble, G.U.; Kim, D.K.; Kim, J.H.; Kim, T.G.; Kamat, R.K.; Dongale, T.D.; et al. The g-C3N4-TiO2 nanocomposite for non-volatile memory and artificial synaptic device applications. J. Alloys Compd. 2023, 962, 171024. [Google Scholar] [CrossRef]
- Paunkumar, P.; Bhuvaneswari, C.; Priya, R.L.; Hariprasad, B.S.; Dhayanithi, C.A.; Babu, S.G. Functionalized Nanomaterials as Supercapacitor Devices: Current Trends and Beyond. In Materials Horizons: From Nature to Nanomaterials; Springer: Singapore, 2024; Volume F1329, pp. 93–127. [Google Scholar]
- Wang, K.; Cao, J.; Gao, J.; Zhao, J.; Jiang, W.; Ahmad, W.; Jiang, J.; Ling, M.; Liang, C.; Chen, J. Unveiling the structure-activity relationship of hollow spindle-like α-Fe2O3 nanoparticles via phosphorus doping engineering for enhanced lithium storage. SM&Ts 2023, 38, e00744. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Z.; Bai, S.; Wang, X.; Zhang, Y. Innovative BVO@CuO design: A high-performance vanadium-based anode material for Li-ion batteries. J. Alloys Compd. 2023, 958, 170485. [Google Scholar] [CrossRef]
- Wei, J.; Hu, F.; Shen, X.; Chen, B.; Chen, L.; Wang, Z.; Lv, C.; Ouyang, Q. Defective core–shell NiCo2S4/MnO2 nanocomposites for high performance solid-state hybrid supercapacitors. J. Colloid Interface Sci. 2023, 649, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Carbone, M.; Tallarida, M.; Dil, J.H.; Horn, K.; Casaletto, M.P.; Flammini, R.; Piancastelli, M.N. Adsorption of 2,3-butanediol on Si(100). Surf. Sci. 2004, 559, 179–185. [Google Scholar] [CrossRef]
- Carbone, M.; Zanoni, R.; Piancastelli, M.N.; Comtet, G.; Dujardin, G.; Hellner, L. Synchrotron radiation photoemission and photostimulated desorption of deuterated methanol on Si(111)7 × 7 and Si(100)2 × 1. Surf. Sci. 1996, 352–354, 391–395. [Google Scholar] [CrossRef]
- Bauer, E.M.; Talone, A.; Imperatori, P.; Briancesco, R.; Bonadonna, L.; Carbone, M. The Addition of Co into CuO–ZnO Oxides Triggers High Antibacterial Activity and Low Cytotoxicity. Nanomaterials 2023, 13, 2823. [Google Scholar] [CrossRef] [PubMed]
- Chioibas, R.; Borcan, F.; Mederle, O.; Stoian, D.; Soica, C.M. Comparative characterization of different samples containing nano-ZnO particles with applicability in topical therapies. Mater. Plast. 2019, 56, 652–656. [Google Scholar] [CrossRef]
- Carbone, M.; Sabbatella, G.; Antonaroli, S.; Remita, H.; Orlando, V.; Biagioni, S.; Nucara, A. Exogenous Control over Intracellular Acidification: Enhancement via Proton Caged Compounds Coupled to Gold Nanoparticles. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 2304–2307. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Delgadillo, R.; Velasco-Arias, D.; Martinez-Sanmiguel, J.J.; Diaz, D.; Zumeta-Dube, I.; Arevalo-Niño, K.; Cabral-Romero, C. Bismuth oxide aqueous colloidal nanoparticles inhibit Candida albicans growth and biofilm formation. Int. J. Nanomed. 2013, 8, 1645–1652. [Google Scholar] [CrossRef]
- Khan, M.F.; Hameedullah, M.; Ansari, A.H.; Ahmad, E.; Lohani, M.B.; Khan, R.H.; Alam, M.M.; Khan, W.; Husain, F.M.; Ahmad, I. Flower-shaped ZnO nanoparticles synthesized by a novel approach at near-room temperatures with antibacterial and antifungal properties. Int. J. Nanomed. 2014, 9, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Kyomuhimbo, H.D.; Michira, I.N.; Mwaura, F.B.; Derese, S.; Feleni, U.; Iwuoha, E.I. Silver–zinc oxide nanocomposite antiseptic from the extract of Bidens pilosa. SN Appl. Sci. 2019, 1, 681. [Google Scholar] [CrossRef]
- Liang, H.; Wang, H.; Sun, X.; Xu, W.; Meng, N.; Zhou, N. Development of ZnO/Ag nanoparticles supported polydopamine-modified montmorillonite nanocomposites with synergistic antibacterial performance. Appl. Clay Sci. 2023, 244, 107112. [Google Scholar] [CrossRef]
- Lin, W.; Fan, S.; Liao, K.; Huang, Y.; Cong, Y.; Zhang, J.; Jin, H.; Zhao, Y.; Ruan, Y.; Lu, H.; et al. Engineering zinc oxide hybrid selenium nanoparticles for synergetic anti-tuberculosis treatment by combining Mycobacterium tuberculosis killings and host cell immunological inhibition. Front. Cell. Infect. Microbiol. 2023, 12, 1074533. [Google Scholar] [CrossRef]
- Obeizi, Z.; Benbouzid, H.; Bouarroudj, T.; Bououdina, M. Excellent antimicrobial and anti-biofilm activities of Fe-SnO2 nanoparticles as promising antiseptics and disinfectants. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 015003. [Google Scholar] [CrossRef]
- Pomastowski, P.; Król-Górniak, A.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanocomposites—Extracellular synthesis, physicochemical characterization and antibacterial potential. Materials 2020, 13, 4347. [Google Scholar] [CrossRef]
- Sasani, M.; Fataei, E.; Safari, R.; Nasehi, F.; Mosayebi, M. Antibacterial effects of iron oxide and silver nanoparticles synthesized bbacillus subtilis: A comparative study. Desalination Water Treat. 2021, 231, 340–347. [Google Scholar] [CrossRef]
- Balu, S.K.; Andra, S.; Jeevanandam, J.; Kulabhusan, P.K.; Khamari, A.; Vedarathinam, V.; Hamimed, S.; Chan, Y.S.; Danquah, M.K. Exploring the potential of metal oxide nanoparticles as fungicides and plant nutrient boosters. Crop Prot. 2023, 174, 106398. [Google Scholar] [CrossRef]
- Deng, C.; Protter, C.R.; Wang, Y.; Borgatta, J.; Zhou, J.; Wang, P.; Goyal, V.; Brown, H.J.; Rodriguez-Otero, K.; Dimkpa, C.O.; et al. Nanoscale CuO charge and morphology control Fusarium suppression and nutrient biofortification in field-grown tomato and watermelon. Sci. Total Environ. 2023, 905, 167799. [Google Scholar] [CrossRef]
- Hassan, M.U.; Kareem, H.A.; Hussain, S.; Guo, Z.; Niu, J.; Roy, M.; Saleem, S.; Wang, Q. Enhanced salinity tolerance in Alfalfa through foliar nano-zinc oxide application: Mechanistic insights and potential agricultural applications. Rhizosphere 2023, 28, 100792. [Google Scholar] [CrossRef]
- Mandal, T.K. ZnO Nanoparticles: Sustainable Plant Production. In Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2024; Volume F1816, pp. 259–281. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Ullah, F.; Sayed, S.R.M.; Mahmoud, E.A. Seed priming with iron oxide nanoparticles improves yield and antioxidant status of garden pea (Pisum sativum L.) grown under drought stress. S. Afr. J. Bot. 2023, 162, 577–587. [Google Scholar] [CrossRef]
- Rabea, A.; Naeem, E.; Balabel, N.M.; Daigham, G.E. Management of potato brown rot disease using chemically synthesized CuO-NPs and MgO-NPs. Bot. Stud. 2023, 64, 20. [Google Scholar] [CrossRef] [PubMed]
- Filippou, P.; Tanou, G.; Molassiotis, A.; Fotopoulos, V. Plant Acclimation to Environmental Stress Using Priming Agents; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–27. [Google Scholar] [CrossRef]
- Méndez, A.A.; Pena, L.B.; Benavides, M.P.; Gallego, S.M. Priming with NO controls redox state and prevents cadmium-induced general up-regulation of methionine sulfoxide reductase gene family in Arabidopsis. Biochimie 2016, 131, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Nanoparticle-Mediated Seed Priming Improves Germination, Growth, Yield, and Quality of Watermelons (Citrullus lanatus) at multi-locations in Texas. Sci. Rep. 2020, 10, 5037. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, S.; Seisenbaeva, G.; Behers, L. Titania (TiO2) nanoparticles enhance the performance of growth-promoting rhizobacteria. Sci. Rep. 2018, 8, 617. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Atiq Hussain, S.; Casini, R.; Abd-ElGawad, A.M.; Elansary, H.O. Seed Nano-Priming with Calcium Oxide Maintains the Redox State by Boosting the Antioxidant Defense System in Water-Stressed Carom (Trachyspermum ammi L.) Plants to Confer Drought Tolerance. Nanomaterials 2023, 13, 1453. [Google Scholar] [CrossRef] [PubMed]
- Setty, J.; Samant, S.B.; Yadav, M.K.; Manjubala, M.; Pandurangam, V. Benefcial efects of bio-fabricated selenium nanoparticles as seed nanopriming agent on seed germination in rice (Oryza sativa L.). Sci. Rep. 2023, 13, 22349. [Google Scholar] [CrossRef]
- Yousaf, N.; Ishfaq, M.; Qureshi, H.A.; Saleem, A.; Yang, H.; Sardar, M.F.; Zou, C. Characterization of Root and Foliar-Applied Iron Oxide Nanoparticles (α-Fe2O3, γ-Fe2O3, Fe3O4, and Bulk Fe3O4) in Improving Maize (Zea mays L.) Performance. Nanomaterials 2023, 13, 3036. [Google Scholar] [CrossRef]
- Rai-Kalal, P.; Jajoo, A. Priming with zinc oxide nanoparticles improve germination and photosynthetic performance in wheat. Plant Physiol. Biochem. 2021, 160, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Donia, D.T.; Carbone, M. Seed Priming with Zinc Oxide Nanoparticles to Enhance Crop Tolerance to Environmental Stresses. Int. J. Mol. Sci. 2023, 24, 17612. [Google Scholar] [CrossRef]
- Zafar, S.; Perveen, S.; Khan, M.K.; Shaheen, M.R.; Hussain, R.; Sarwar, N.; Rashid, S.; Nafees, M.; Farid, G.; Alamri, S.; et al. Effect of zinc nanoparticles seed priming and foliar application on the growth and physiobiochemical indices of spinach (Spinacia oleracea L.) under salt stress. PLoS ONE 2022, 17, e0263194. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Zia ur Rehman, M.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Du, W.; Yang, J.; Peng, Q.; Liang, X.; Mao, H. Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: From toxicity and zinc biofortification. Chemosphere 2019, 227, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Durgude, S.A.; Ram, S.; Kumar, R.; Singh, S.V.; Singh, V.; Durgude, A.G.; Pramanick, B.; Maitra, S.; Gaber, A.; Hossain, A. Synthesis of Mesoporous Silica and Graphene-Based FeO and ZnO Nanocomposites for Nutritional Biofortification and Sustained the Productivity of Rice (Oryza sativa L.). J. Nanomater. 2022, 2022, 5120307. [Google Scholar] [CrossRef]
- Ghani, M.I.; Saleem, S.; Rather, S.A.; Rehmani, M.S.; Alamri, S.; Rajput, V.D.; Kalaji, H.M.; Saleem, N.; Sial, T.A.; Liu, M. Foliar application of zinc oxide nanoparticles: An effective strategy to mitigate drought stress in cucumber seedling by modulating antioxidant defense system and osmolytes accumulation. Chemosphere 2022, 289, 133202. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.M.; Behal, A.; Sushkova, S.N.; Mandzhieva, S.; Singh, R.; Gorovtsov, A.; Tsitsuashvili, V.S.; Purvis, W.O.; Ghazaryan, K.A.; et al. Effects of zinc-oxide nanoparticles on soil, plants, animals and soil organisms: A review. Environ. Nanotechnol. Monit. Manag. 2018, 9, 76–84. [Google Scholar] [CrossRef]
- Afsal, S.; Sharma, D.; Singh, N.K. Eco-friendly synthesis of phytochemical-capped iron oxide nanoparticles as nano-priming agent for boosting seed germination in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2021, 28, 40275–40287. [Google Scholar] [CrossRef]
- Mahakham, W.; Theerakulpisut, P.; Maensiri, S.; Phumying, S.; Sarmah, A.K. Environmentally benign synthesis of phytochemicals-capped gold nanoparticles as nanopriming agent for promoting maize seed germination. Sci. Total Environ. 2016, 573, 1089–1102. [Google Scholar] [CrossRef]
- Song, K.; Zhao, D.; Sun, H.; Gao, J.; Li, S.; Hu, T.; He, X. Green nanopriming: Responses of alfalfa (Medicago sativa L.) seedlings to alfalfa extract capped and light-induced silver nanoparticles. BMC Plant Biol. 2022, 22, 323. [Google Scholar] [CrossRef]
- Kalimuthu, R.; Sella, K.M.; Antony, D.; Rajaprakasam, S.; Chokkalingam, V.; Chidambaram, P.; Kanagarajam, S. Nanopriming Action of Microwave-Assisted Biofunctionalized ZnO Nanoparticles to Enhance the Growth under Moisture Stress in Vigna radiata. ACS Omega 2023, 8, 28143–28155. [Google Scholar] [CrossRef] [PubMed]
- Zungu, B.; Paumo, H.K.; Gaorongwe, J.L.; Tsuene, G.N.; Ruzvidzo, O.; Katata-Seru, L. Zn nutrients-loaded chitosan nanocomposites and their efficacy as nanopriming agents for maize (Zea mays) seeds. Front. Chem. 2023, 11, 1243884. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, D.; Luzi, F.; Tolisano, C.; Puglia, D.; Di Michele, A. Synthesis of a Lignin/Zinc Oxide Hybrid Nanoparticles System and Its Application by Nano-Priming in Maize. Nanomaterials 2022, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; De Rossi, S.; Donia, D.T.; Di Marco, G.; Gustavino, B.; Roselli, L.; Tagliatesta, P.; Canini, A.; Gismondi, A. Biostimulants promoting growth of Vicia faba L. seedlings: Inulin coated ZnO nanoparticles. Chem. Biol. Technol. Agric. 2023, 10, 134. [Google Scholar] [CrossRef]
- Redondo-Cuenca, A.; Herrera-Vàzquez, S.E.; Condezo-Hoyos, L.; Gómez-Ordóñez, E.; Rupérez, P. Inulin extraction from common inulin-containing plant sources. Ind. Crops Prod. 2021, 170, 113726. [Google Scholar] [CrossRef]
- Apolinário, A.C.; de Carvalho, E.M.; de Lima Damasceno, B.P.G.; da Silva, P.C.D.; Converti, A.; Pessoa, A., Jr.; da Silva, J.A. Extraction, isolation and characterization of inulin from Agave sisalana boles. Ind. Crops Prod. 2017, 108, 225–362. [Google Scholar] [CrossRef]
- Waqas Mazhar, M.; Ishtiaq, M.; Hussain, I.; Parveen, A.; Hayat Bhatti, K.; Azeem, M.; Thind, S.; Ajaib, M.; Maqbool, M.; Sardar, T.; et al. Seed nano-priming with Zinc Oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PLoS ONE 2022, 17, e0264967. [Google Scholar] [CrossRef]
- Abbas, S.F.; Bukhari, M.A.; Raza, M.A.S.; Abbasi, G.H.; Ahmad, Z.; Alqahtani, M.D.; Almutairi, K.F.; Abd Allah, E.F.; Iqbal, M.A. Enhancing Drought Tolerance in Wheat Cultivars through Nano-ZnO Priming by Improving Leaf Pigments and Antioxidant Activity. Sustainability 2023, 15, 5835. [Google Scholar] [CrossRef]
- El-Bassiouny, H.M.S.; Mahfouze, H.A.; Abdallah, M.M.S.; Bakry, B.A.; El-Enany, M.A.M. Physiological and Molecular Response of Wheat Cultivars to Titanium Dioxide or Zinc Oxide Nanoparticles under Water Stress Conditions. Int. J. Agron. 2022, 2022, 3806574. [Google Scholar] [CrossRef]
- Al-Salama, Y. Effect of Seed Priming with ZnO Nanoparticles and Saline Irrigation Water in Yield and Nutrients Uptake by Wheat. Plants Environ. Sci. Proc. 2022, 16, 37. [Google Scholar] [CrossRef]
- Salam, A.; Khan, A.R.; Liu, L.; Yang, S.; Azhar, W.; Ulhassan, Z.; Zeeshan, M.; Wu, J.; Fan, X.; Gan, Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J. Hazard. Mater. 2022, 423, 127021. [Google Scholar] [CrossRef]
- Wang, W.; Yamaji, N.; Ma, J.F. Molecular Mechanism of Cadmium Accumulation in Rice. In Cadmium Toxicity. Current Topics in Environmental Health and Preventive Medicine; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Kashyap, D.; Siddiqui, Z.A. Effect of different inocula of Meloidogyne incognita and Pseudomonas syringae pv. pisi with and without Rhizobium leguminosarum on growth, chlorophyll, carotenoid and proline contents of pea. Indian Phytopathol. 2020, 73, 499–506. [Google Scholar] [CrossRef]
- Li, M.; Ahammed, G.J.; Li, C.; Bao, X.; Yu, J.; Huang, C.; Yin, H.; Zhou, J. Brassinosteroid Ameliorates Zinc Oxide Nanoparticles-Induced Oxidative Stress by Improving Antioxidant Potential and Redox Homeostasis in Tomato Seedling. Front. Plant Sci. 2016, 7, 615. [Google Scholar] [CrossRef]
- Bauer, E.M.; Bogliardi, G.; Ricci, C.; Cecchetti, D.; De Caro, T.; Sennato, S.; Nucara, A.; Carbone, M. Syntheses of APTMS-Coated ZnO: An Investigation towards Penconazole Detection. Materials 2022, 15, 8050. [Google Scholar] [CrossRef]
- Aminoff, G. XXIV. Über Lauephotogramme und Struktur von Zinkit. Z. Krist.—Cryst. Mater. 1921, 56, 495–505. [Google Scholar] [CrossRef]
- Stahl, R.; Jung, C.; Lutz, H.D.; Kockelmann, W.; Jacobs, H. Kristallstrukturen und Wasserstoffbrückenbindungen bei β-Be(OH)2 und ϵ-Zn(OH)2. Z. Anorg. Allg. Chem. 1998, 624, 1130–1136. [Google Scholar] [CrossRef]
- Ghotbi, M.Y. Synthesis and characterization of nano-sized ɛ-Zn(OH)2 and its decomposed product, nano-zinc oxide. J. Alloys Compd. 2010, 491, 420–422. [Google Scholar] [CrossRef]
- Top, A.; Çetinkaya, H. Zinc oxide and zinc hydroxide formation via acquous precipitation: Effect of the preparation route and lysozyme addition. Mater. Chem. Phys. 2015, 167, 77–87. [Google Scholar] [CrossRef]
- Akram, W.; Garud, N. Optimization of inulin production process parameters using response surface methodology. Future J. Pharm. Sci. 2020, 6, 68. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Gholami-Daghian, M.; Esmaeili-Zare, M.; Sangsefidi, F.S. Solid State Synthesis and Characterization of Zinc Oxide (ZnO) Microflakes by [Bis(acetylacetonato)zinc(II)] and Sodium Hydroxide at Room Temperature. J. Clust. Sci. 2013, 24, 1093–1101. [Google Scholar] [CrossRef]
- Rauf, N.; Ilyas, S.; Heryanto, H.; Rahmat, R.; Fahri, A.N.; Rahmi, M.H.; Tahir, D. The Correlation between Structural and Optical Properties of Zinc Hydroxide Nanoparticle in Supports Photocatalytic Performance. Opt. Mater. 2021, 112, 110780. [Google Scholar] [CrossRef]
- Tian, H.; Ghorbanpour, M.; Kariman, K. Manganese oxide nanoparticle-induced changes in growth, redox reactions and elicitation of antioxidant metabolites in deadly nightshade (Atropa belladonna L.). Ind. Crops Prod. 2018, 126, 403–414. [Google Scholar] [CrossRef]
- Huang, D.; Dang, F.; Huang, Y.; Chen, N.; Zhou, D. Uptake, translocation, and transformation of silver nanoparticles in plants. Environ. Sci. Nano 2022, 9, 12–39. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. Ein Beitrag Zur Optik Der Farbanstriche. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Missori, M.; Pulci, O.; Teodonio, L.; Violante, C.; Kupchak, I.; Bagniuk, J.; Łojewska, J.; Mosca Conte, A. Optical response of strongly absorbing inhomogeneous materials: Application to paper degradation. Phys. Rev. B 2014, 89, 054201. [Google Scholar] [CrossRef]
- Missori, M. Optical spectroscopy of ancient paper and textiles. Riv. Nuovo Cimento 2016, 39, 293. [Google Scholar] [CrossRef]
- Smith, R.A. Semiconductors, 2nd ed.; Cambridge University Press: Cambridge, UK, 1978. [Google Scholar]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Islam, S.M.; Gayen, T.; Moussawi, A.; Shi, L.; Seredych, M.; Bandosz, T.J.; Alfano, R. Structural and optical characterization of Zn(OH)2 and its composites with graphite oxides. Opt. Lett. 2013, 38, 962–964. [Google Scholar] [CrossRef]
- Brus, L.E. Electron–electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef]
- Repp, S.; Erdem, E. Controlling the exciton energy of zinc oxide (ZnO) quantum dots by changing the confinement conditions. Spectrochim. Acta A 2016, 152, 637–644. [Google Scholar] [CrossRef]
- Xie, X.; Mao, C.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Yuan, X.; Zheng, Y.; et al. Tuning the Bandgap of Photo-Sensitive Polydopamine/Ag3PO4/Graphene Oxide Coating for Rapid, Noninvasive Disinfection of Implants. ACS Cent. Sci. 2018, 4, 724–738. [Google Scholar] [CrossRef]
- Guan, S.; Cheng, Y.; Hao, L.; Yoshida, H.; Chiaki, T.; Tianzhuo, Z.; Takaomi, I.; Tangbin, Q.; Lu, Y. Oxygen vacancies induced band gap narrowing for efficient visible-light response in carbon-doped TiO2. Sci. Rep. 2023, 13, 14105. [Google Scholar] [CrossRef]
- Ayoub, I.; Kumar, V.; Abolhassani, R.; Sehgal, R.; Sharma, V.; Sehgal, R.; Swart, H.C.; Mishra, Y.K. Advances in ZnO: Manipulation of defects for enhancing their technological potentials. Nanotechnol. Rev. 2022, 11, 575–619. [Google Scholar] [CrossRef]
- Cai, L.; Song, A.Y.; Li, W.; Hsu, P.C.; Lin, D.; Catrysse, P.B.; Liu, Y.; Peng, Y.; Chen, J.; Wang, H.; et al. Spectrally Selective Nanocomposite Textile for Outdoor Personal Cooling. Adv. Mater. 2018, 30, e1802152. [Google Scholar] [CrossRef]
- van der Leij, M. The possibility of black zinc oxide as spectrally selective coating for lower temperature solar collectors. J. Electrochem. Soc. 1978, 125, 1361. [Google Scholar] [CrossRef]
- Hu, P.; An, J.; Faulkner, M.M.; Wu, H.; Li, Z.; Tian, X.; Giraldo, J.P. Nanoparticle Charge and Size Control Foliar Delivery Efficiency to Plant Cells and Organelles. ACS Nano 2020, 14, 7970–7986. [Google Scholar] [CrossRef] [PubMed]
- Gustavino, B.; Carboni, G.; Petrillo, R.; Paoluzzi, G.; Santovetti, E.; Rizzoni, M. Exposure to 915 MHz radiation induces micronuclei in Vicia faba root tips. Mutagenesis 2016, 31, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhang, W.; Li, B.; Wang, Y.; Li, K.; Sodmergen; Han, C.; Zhang, Y.; Li, X. An endoplasmic reticulum response pathway mediates programmed cell death of root tip induced by water stress in Arabidopsis. New Phytol. 2010, 186, 681–695. [Google Scholar] [CrossRef] [PubMed]
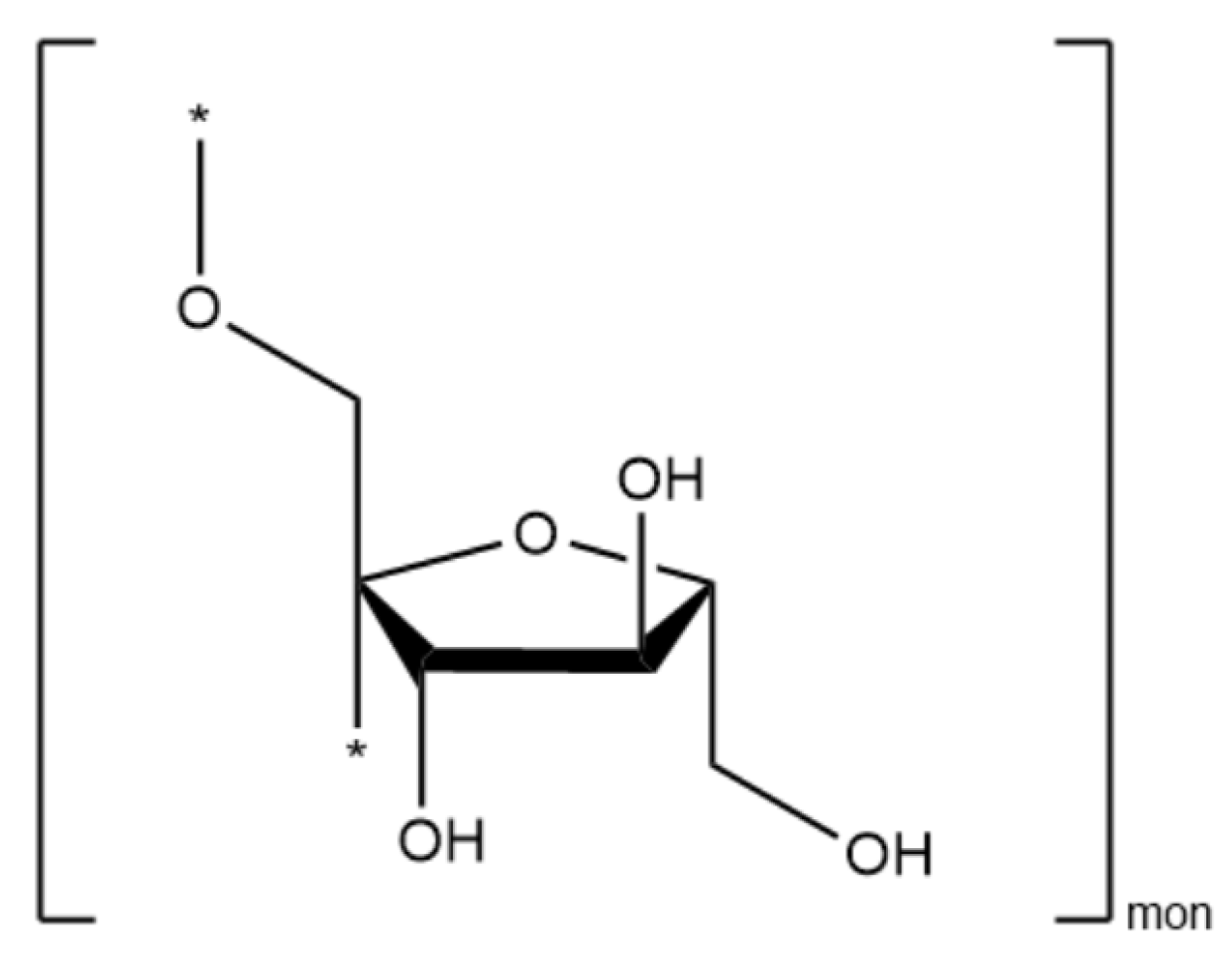

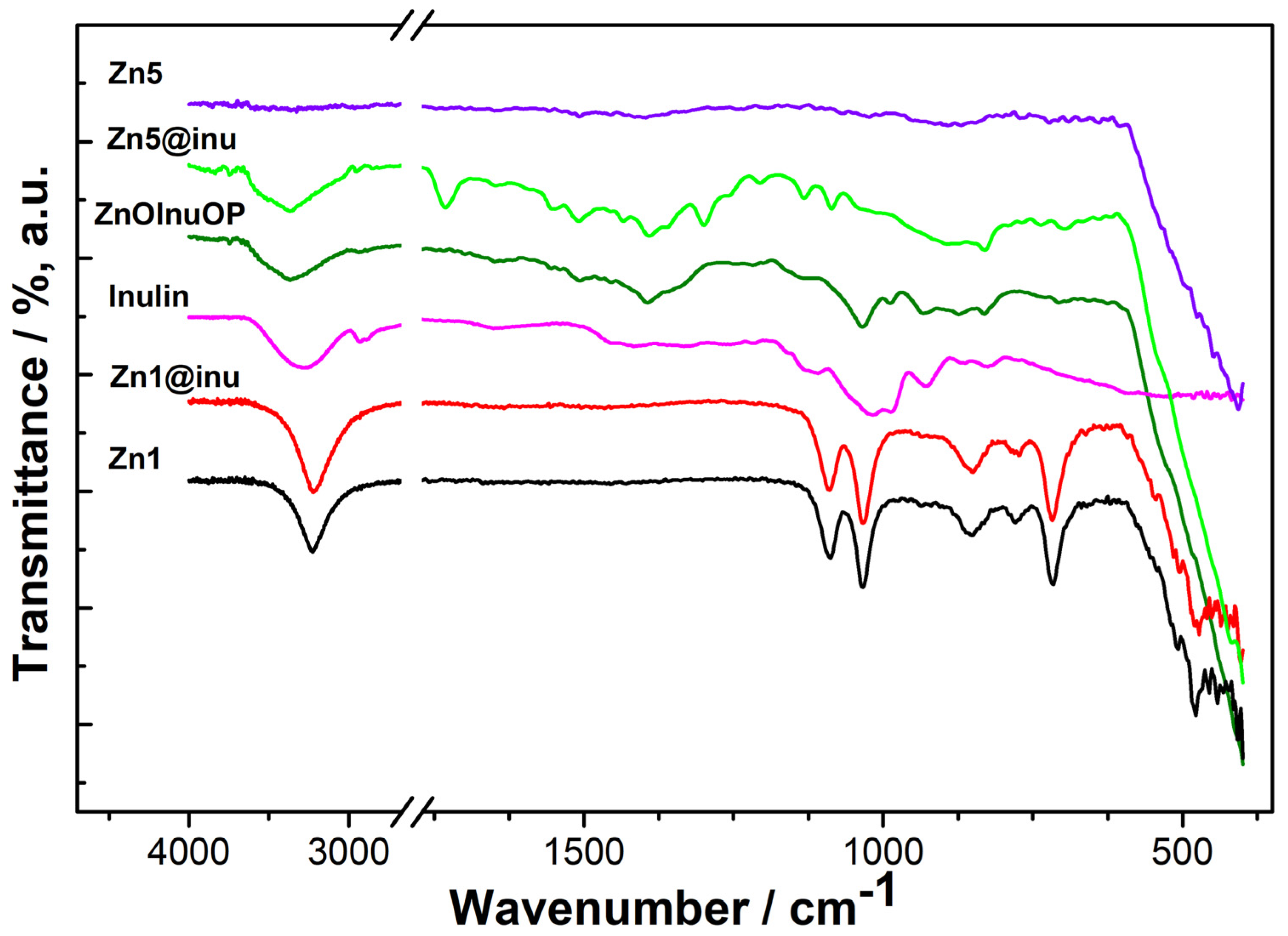
| Zn1 | Zn1@Inu | Inulin | ZnOInuOP | Zn5 | Zn5@Inu | Assignment |
|---|---|---|---|---|---|---|
| 401 | 401 | 400 | 400 | 400 | ν as Zn–O | |
| 482 | 482 | ν as Zn–O | ||||
| 507 | 507 | |||||
| 592 | 619 | ρ-CH2 | ||||
| 626 | 640 | ρ-CH2 | ||||
| 665 | ρ-CH2 | |||||
| 704 | 696 | ρ-CH2 | ||||
| 716 | 716 | δ-OH | ||||
| 731 | ||||||
| 768 | ρ-CH2 | |||||
| 779 | 779 | |||||
| 787 | ρ-CH2 | |||||
| 827 | 831 | 829 | 2-ketose | |||
| 851 | 851 | |||||
| 866 | 875 | 889 | Anomeric δ(C1–H), ring vibration (2-ketofuranose) | |||
| 1016 | ν as C–O–C | |||||
| 1034 | 1034 | 1034 | 1037 | ν as C–O–C, ν as Zn–O-Zn | ||
| 1088 | 1088 | 1086 | ν as C–O, ν as Zn–O-Zn | |||
| 1109 | ||||||
| 1134 | 1132 | ν as C–C | ||||
| 1219 | 1205 | βO-H | ||||
| 1269 | βO-H, δC–H | |||||
| 1300 | βO-H | |||||
| 1330 | δC–H, δH-C–H | |||||
| 1418 | 1394 | δC–H, δH-C–H | ||||
| 1454 | 1435 | δC–H | ||||
| 1508 | 1508 | ν C=O-Zn-O=C as | ||||
| 1556 | 1551 | ν C=O-Zn-O=C as | ||||
| 1653 | 1649 | 1649 | ν C=O-Zn-O=C as/δH2O | |||
| 1730 | ν C=O as | |||||
| 1980 | ||||||
| 2932 | 2924 | ν C–H | ||||
| 3210 | 3210 | 3304 | 3365 | 3370 | ν O–H intermolecular H-bonds |
| Sample | Average Size (nm) | Zn (at%) | O (at%) * | C (at%) | Si (at%) * |
|---|---|---|---|---|---|
| Zn1 | 1200 ± 50 | 49.9 | 50.1 | ||
| Zn1@inu | 1200 ± 50 | 45.0 | 50.7 | 5.0 | |
| Coating Zn1@inu | 50.2 | 48.8 | |||
| Zn2 | 62 ± 4 | 48.9 | 50.7 | 0.4 | |
| Zn2@inu | 63 ± 4 | 48.8 | 50.1 | 0.9 | |
| Coating Zn2@inu | 50.2 | 48.8 | 1.0 | ||
| Zn3 | 60 ± 10 | 49.6 | 50.4 | ||
| Zn3@inu | 60 ± 10 | 49.3 | 50.2 | 0.7 | |
| Zn4 | 52 ± 5 | 49.8 | 50.2 | ||
| Zn4@inu | 54 ± 4 | 49.0 | 50.1 | 0.9 | |
| Zn5 | 65 ± 4 | 50.1 | 49.9 | ||
| Zn5@inu | 58 ± 1 | 48.3 | 50.4 | 1.3 | |
| ZnOInuOP | 30 ± 10 | 48.7 | 50.3 | 1.0 |
| Sample | Weight Loss Difference | Degree of Coverage | Monomers |
|---|---|---|---|
| Zn1@inu | 0.15% | 53,867 | 1,616,000 |
| Zn2@inu | 0.41% | 431 | 12,917 |
| Zn3@inu | 0.42% | 381 | 11,430 |
| Zn4@inu | 0.60% | 354 | 10,629 |
| Zn5@inu | 0.72% | 590 | 17,700 |
| ZnInuOP | 1.67% | 192 | 5770 |
| Sample | Estimated Bandgap (eV) |
|---|---|
| Zn1@inu | 2.61 |
| Zn2@inu | 3.25 |
| Zn3@inu | 3.28 |
| Zn4@inu | 3.25 |
| Zn5@inu | 3.27 |
| ZnInuOP | 3.00 |
| Sample | TP (°C) | TD (°C) |
|---|---|---|
| Zn1 | 30 | |
| Zn1@inu | 30 | 30 |
| Zn2 | 60 | |
| Zn2@inu | 60 | 60 |
| Zn3 | 50 | |
| Zn3@inu | 50 | 40 |
| Zn4 | 40 | |
| Zn4@inu | 40 | 40 |
| Zn5 | 60 | |
| Zn5@inu | 60 | 40 |
| ZnInuOP | RT | 40 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gontrani, L.; Bauer, E.M.; Casoli, L.; Ricci, C.; Lembo, A.; Donia, D.T.; Quaranta, S.; Carbone, M. Inulin-Coated ZnO Nanoparticles: A Correlation between Preparation and Properties for Biostimulation Purposes. Int. J. Mol. Sci. 2024, 25, 2703. https://doi.org/10.3390/ijms25052703
Gontrani L, Bauer EM, Casoli L, Ricci C, Lembo A, Donia DT, Quaranta S, Carbone M. Inulin-Coated ZnO Nanoparticles: A Correlation between Preparation and Properties for Biostimulation Purposes. International Journal of Molecular Sciences. 2024; 25(5):2703. https://doi.org/10.3390/ijms25052703
Chicago/Turabian StyleGontrani, Lorenzo, Elvira Maria Bauer, Lorenzo Casoli, Cosimo Ricci, Angelo Lembo, Domenica Tommasa Donia, Simone Quaranta, and Marilena Carbone. 2024. "Inulin-Coated ZnO Nanoparticles: A Correlation between Preparation and Properties for Biostimulation Purposes" International Journal of Molecular Sciences 25, no. 5: 2703. https://doi.org/10.3390/ijms25052703
APA StyleGontrani, L., Bauer, E. M., Casoli, L., Ricci, C., Lembo, A., Donia, D. T., Quaranta, S., & Carbone, M. (2024). Inulin-Coated ZnO Nanoparticles: A Correlation between Preparation and Properties for Biostimulation Purposes. International Journal of Molecular Sciences, 25(5), 2703. https://doi.org/10.3390/ijms25052703









