Multi-Omics Analysis Reveals the IFI6 Gene as a Prognostic Indicator and Therapeutic Target in Esophageal Cancer
Abstract
1. Introduction
2. Results
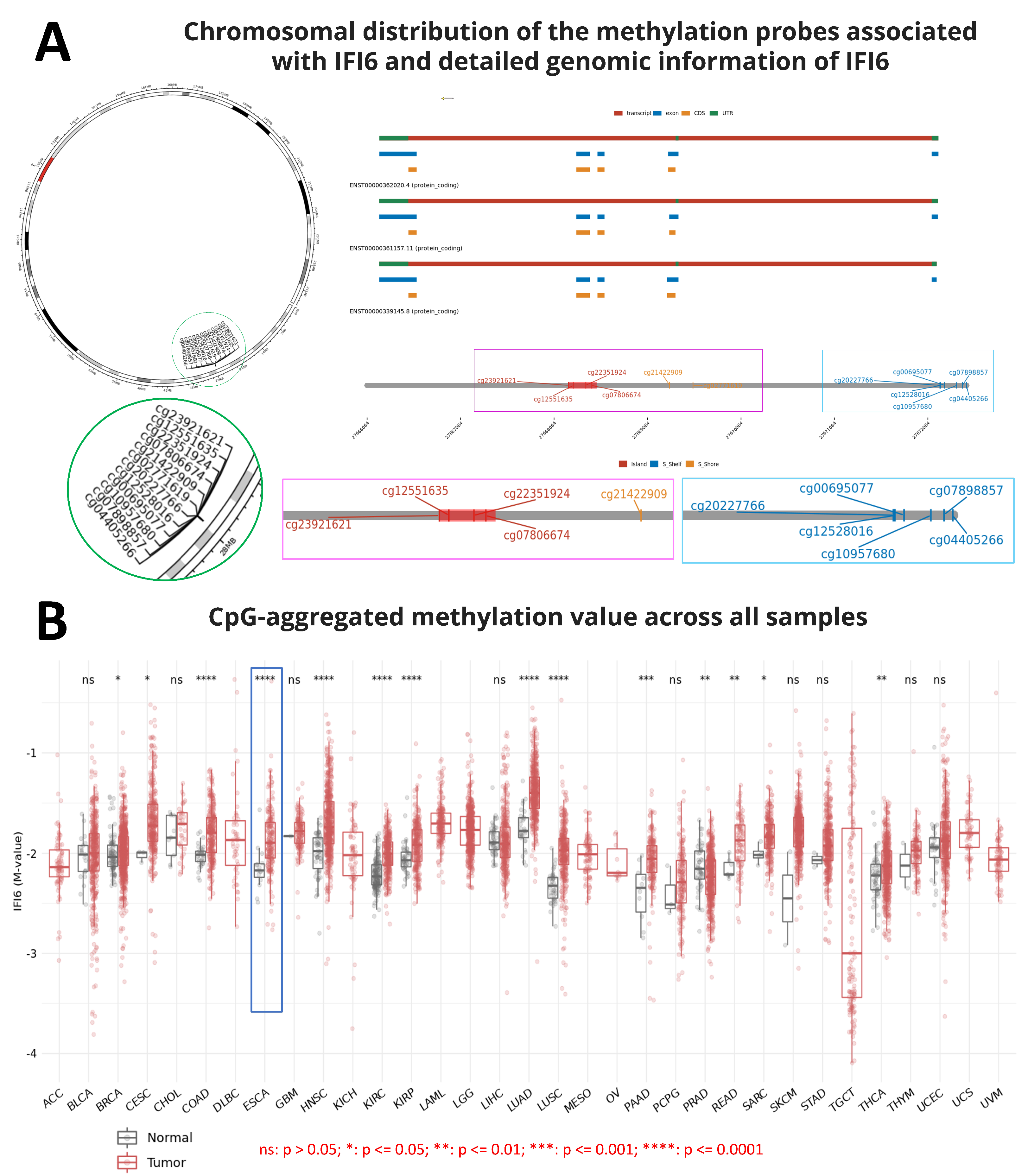
2.1. Screening of IFI6 Gene and Protein Information
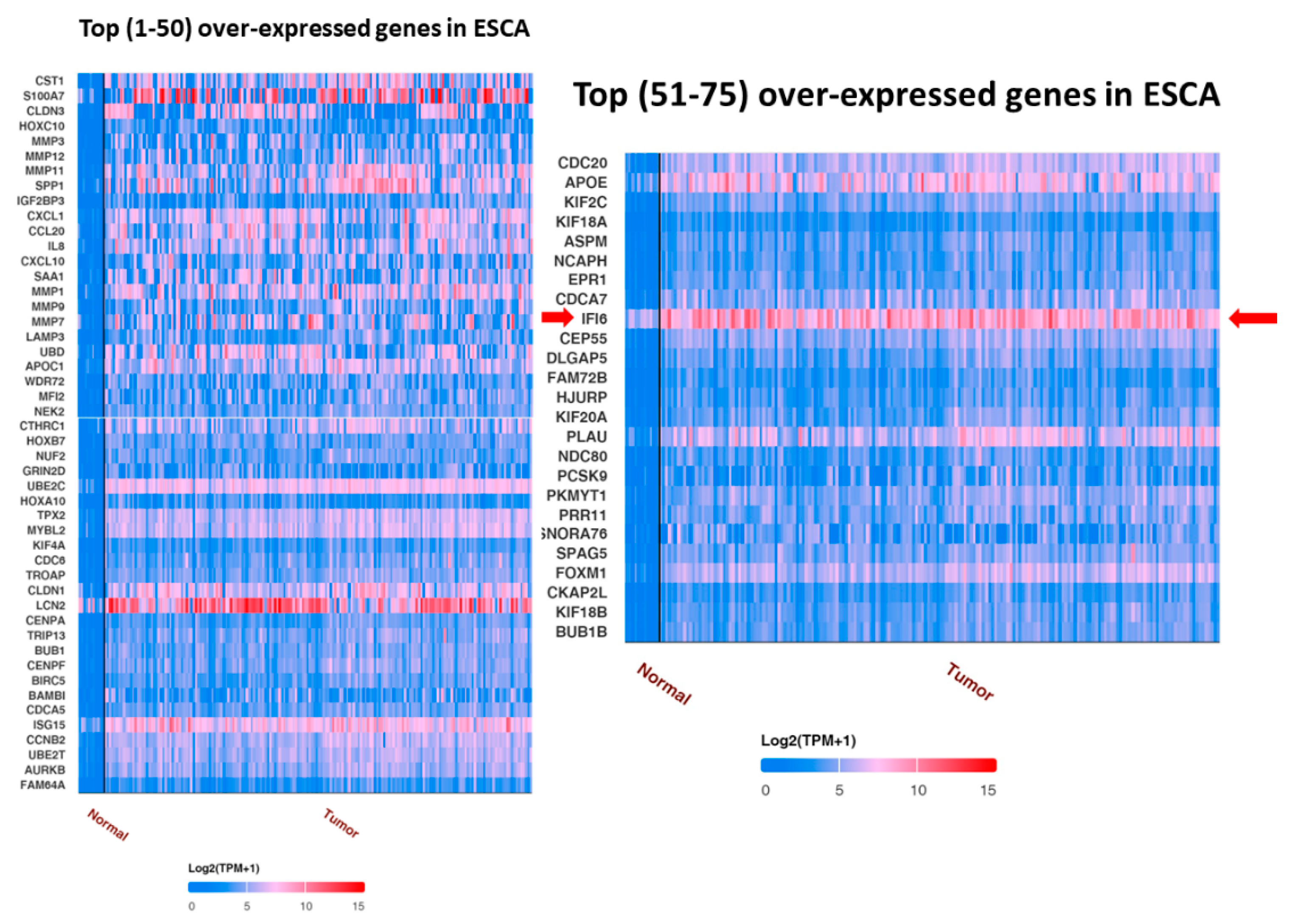
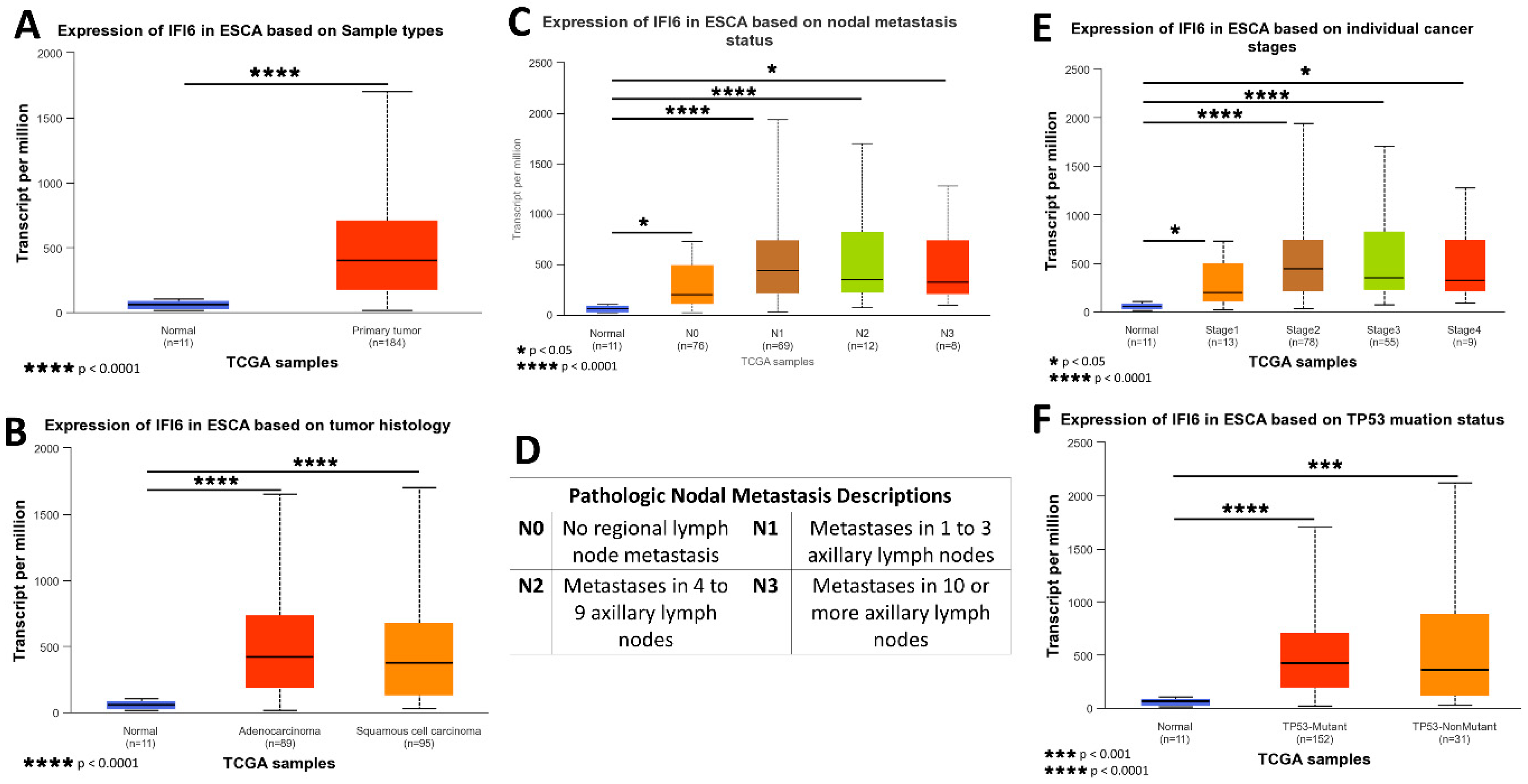
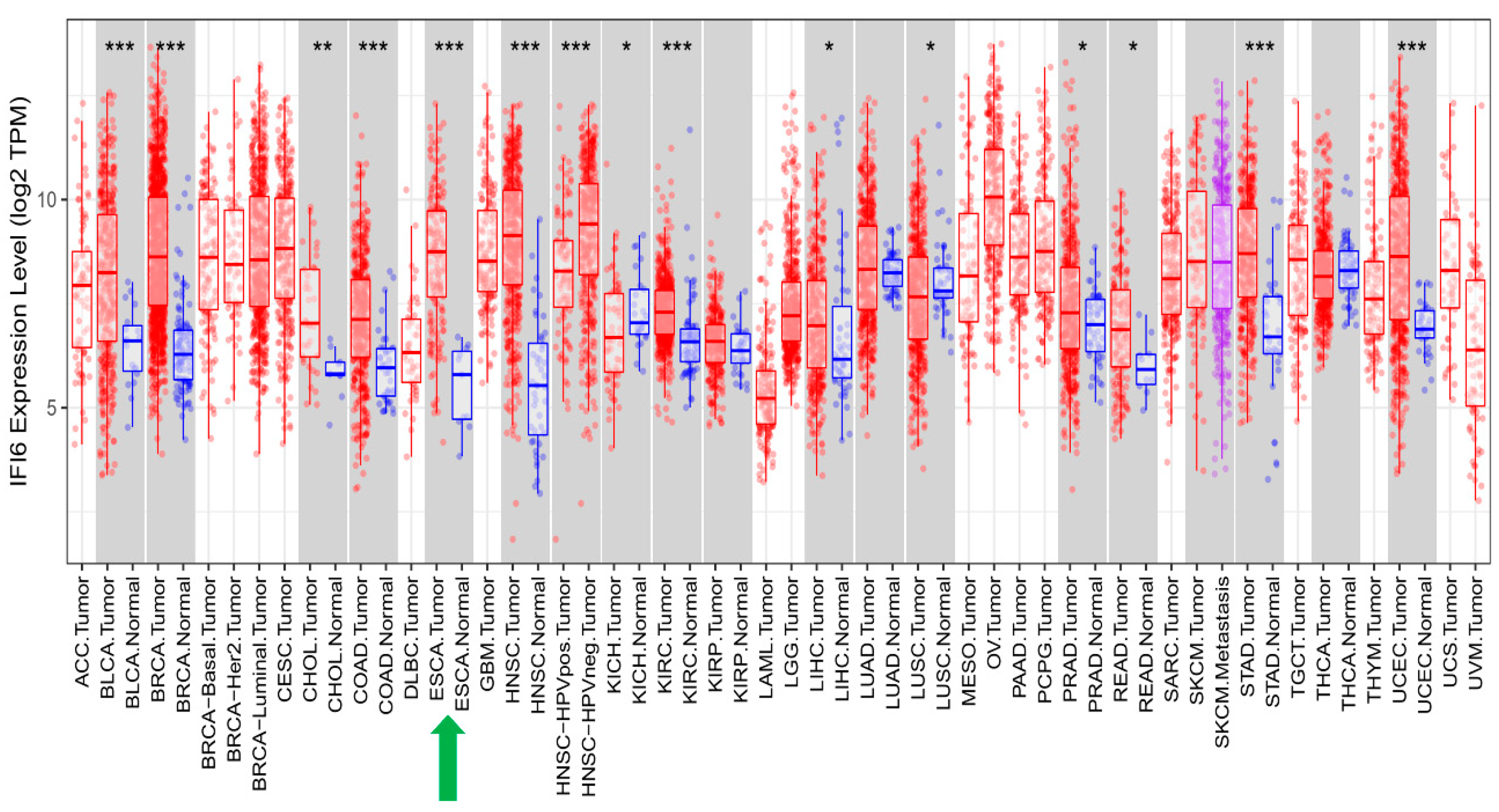
2.2. IFI6 Is an Over-Expressed Gene in ESCA
2.3. High Expression Levels of IFI6 in ESCA Show the Association with Poor Clinical Outcomes, including Advanced Disease Stage and Increased Risk of Metastasis
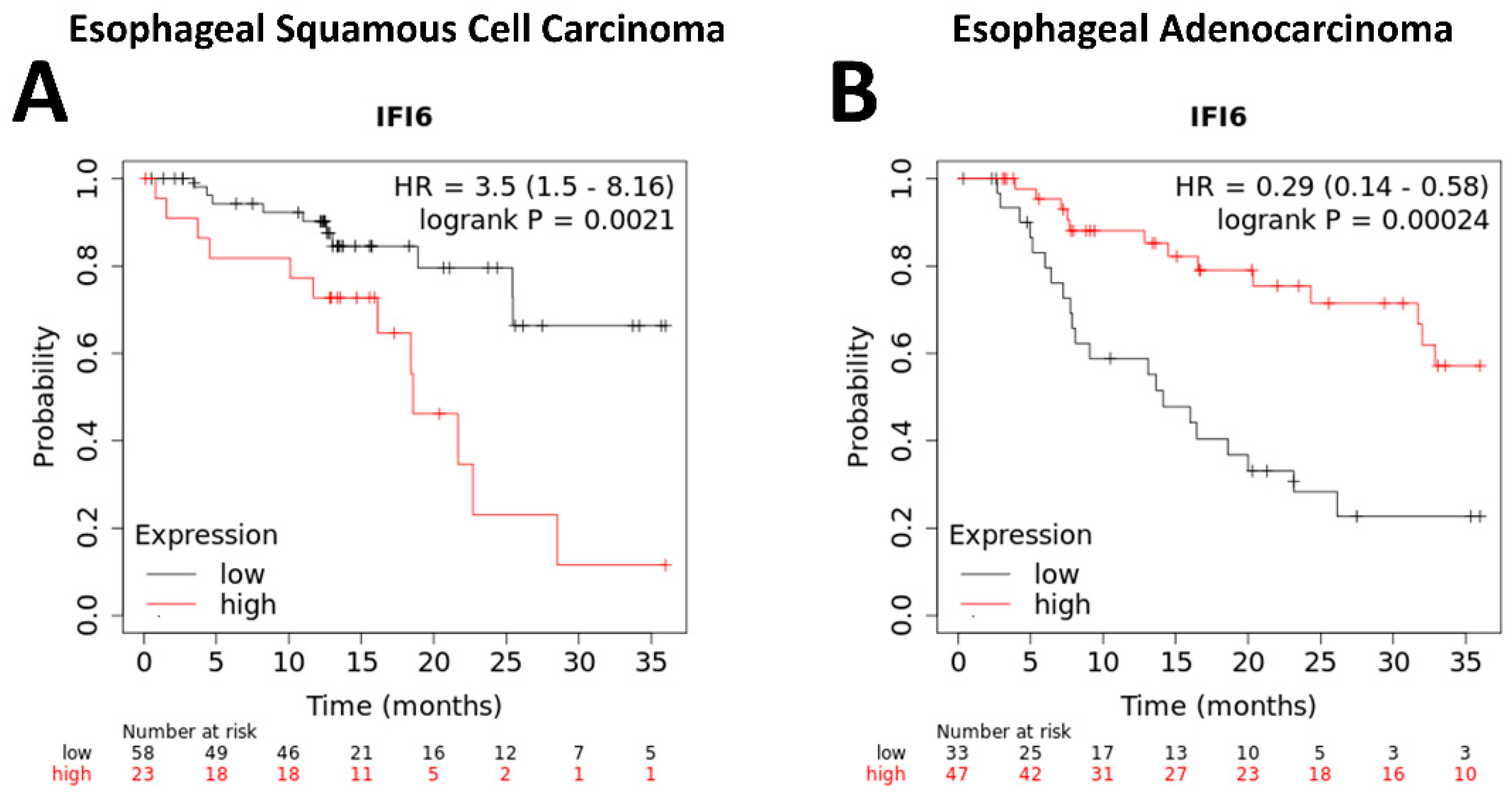
2.4. The Expression of IFI6 Is Associated with the Overall Survival of ESCA Patients
2.5. IFI6 Expression Correlates with Immune Infiltrates in ESCA
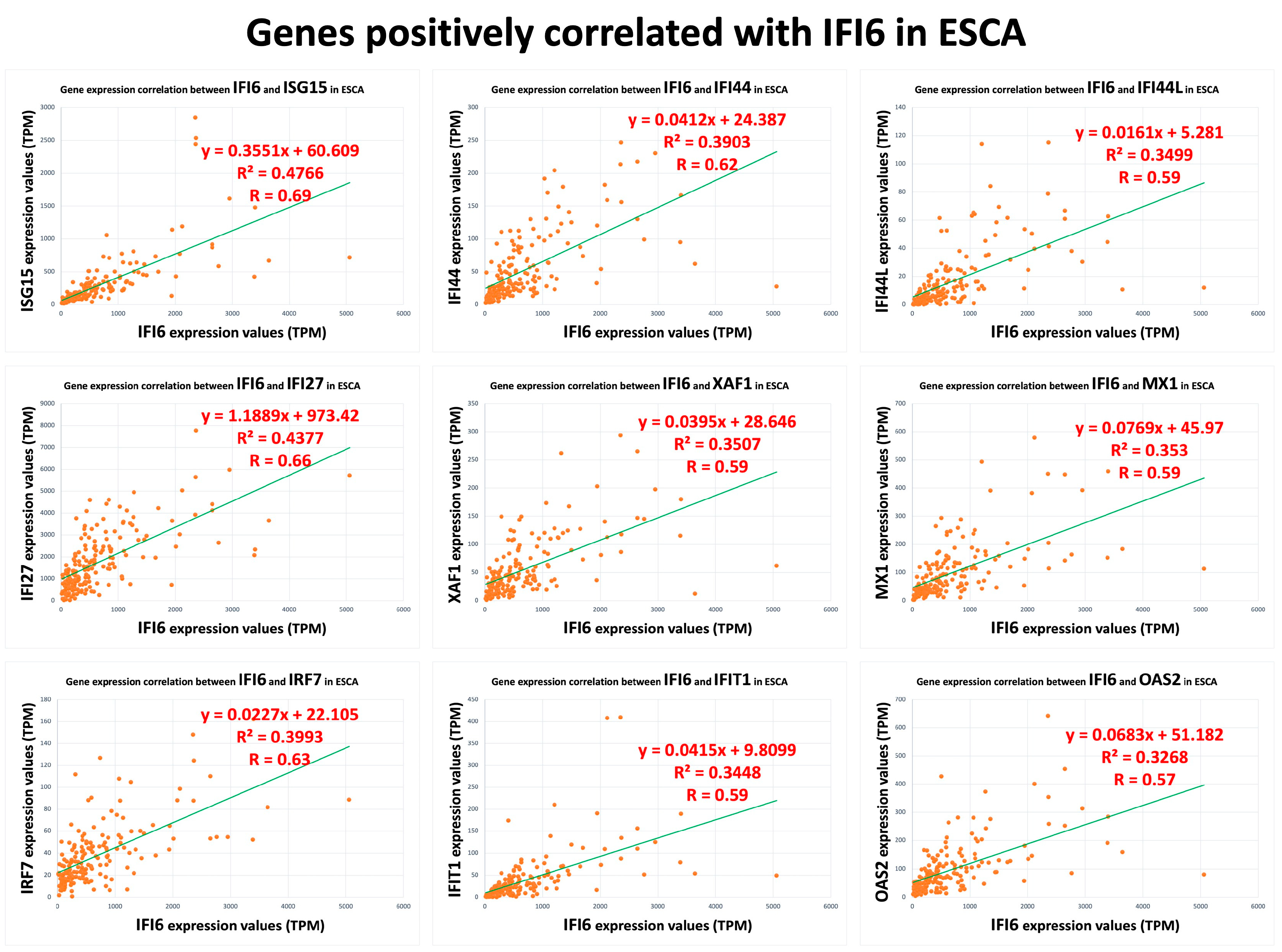
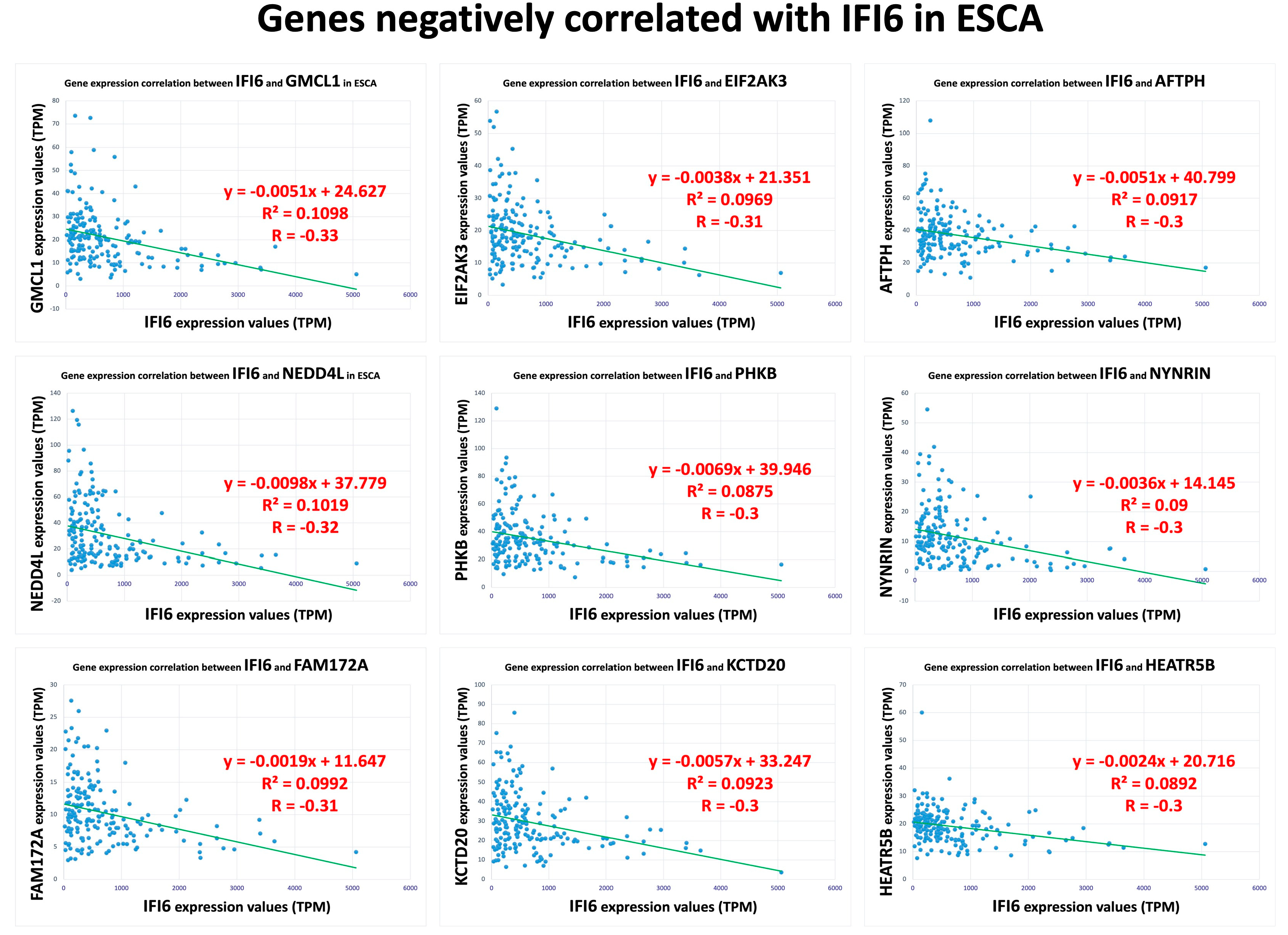
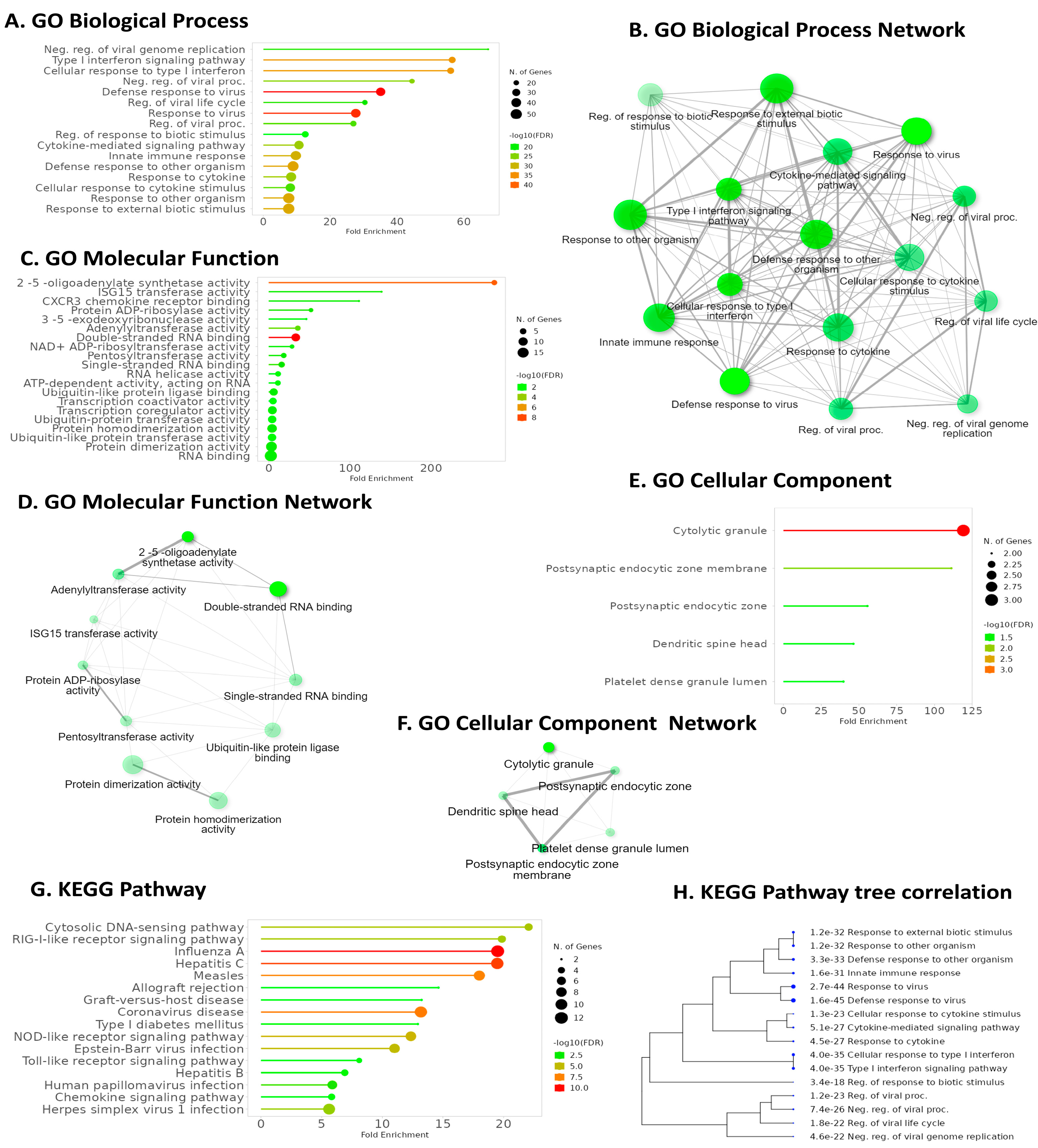
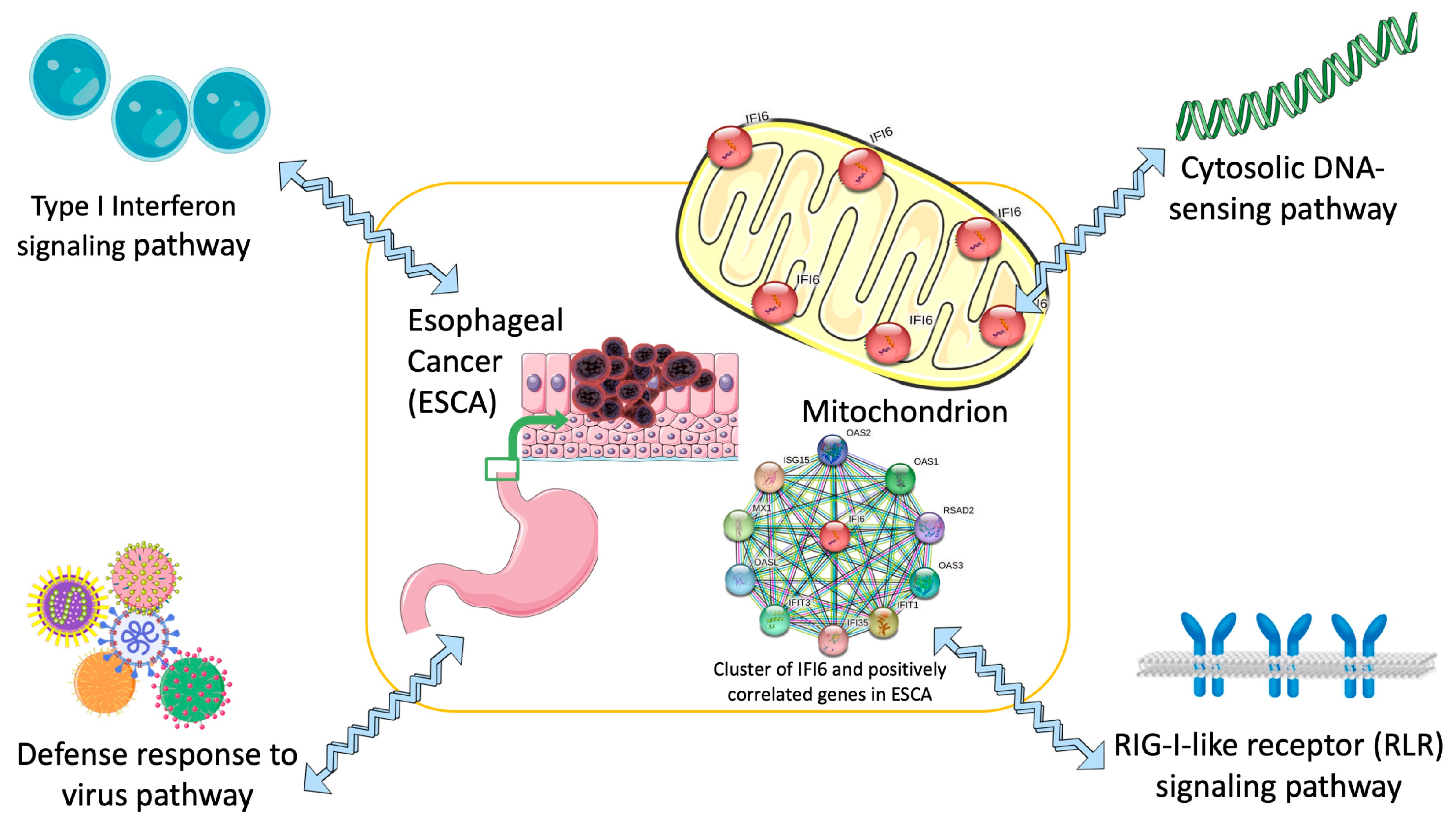
2.6. Pathway Analysis Reveals Common Pathways Associated with IFI6 and Its Positively Correlated Genes
3. Discussion
4. Materials and Methods
4.1. Gene and Protein Information
4.2. UALCAN Analysis
4.3. Survival Analysis
4.4. TIMER Analysis
4.5. GO and KEGG Functional Enrichment Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doghish, A.S.; El-Husseiny, A.A.; Abdelmaksoud, N.M.; El-Mahdy, H.A.; Elsakka, E.G.; Mageed, S.S.A.; Mahmoud, A.M.; Raouf, A.A.; Elballal, M.S.; El-Dakroury, W.A. The interplay of signaling pathways and miRNAs in the pathogenesis and targeted therapy of esophageal cancer. Pathol.-Res. Pract. 2023, 246, 154529. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Luan, S.; Zhou, J.; Xiao, X.; Yang, Y.; Mao, C.; Fang, P.; Chen, L.; Zeng, X. The development and progress of nanomedicine for esophageal cancer diagnosis and treatment. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Liu, C.Q.; Ma, Y.L.; Qin, Q.; Wang, P.H.; Luo, Y.; Xu, P.F.; Cui, Y. Epidemiology of esophageal cancer in 2020 and projections to 2030 and 2040. Thorac. Cancer 2023, 14, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, C.; Sun, N.; Xue, L.; Yang, Z.; Fang, L.; Zhang, Z.; Luo, Y.; Gao, S.; Xue, Q. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy for the treatment of esophageal squamous cell carcinoma: A propensity score-matched study from the National Cancer Center in China. J. Cancer Res. Clin. Oncol. 2022, 148, 943–954. [Google Scholar] [CrossRef]
- Han, J.; Wang, Z.; Liu, C. Survival and complications after neoadjuvant chemotherapy or chemoradiotherapy for esophageal cancer: A meta-analysis. Future Oncol. 2021, 17, 2257–2274. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Kato, K.; Shoji, H.; Iwasa, S.; Honma, Y.; Takashima, A.; Ushijima, T.; Ito, Y.; Itami, J.; Boku, N. Comparison of involved field radiotherapy and elective nodal irradiation in combination with concurrent chemotherapy for T1bN0M0 esophageal cancer. Int. J. Clin. Oncol. 2020, 25, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Simoni, N.; Pavarana, M.; Micera, R.; Weindelmayer, J.; Mengardo, V.; Rossi, G.; Cenzi, D.; Tomezzoli, A.; Del Bianco, P.; Giacopuzzi, S. Long-term outcomes of induction chemotherapy followed by chemo-radiotherapy as intensive neoadjuvant protocol in patients with esophageal cancer. Cancers 2020, 12, 3614. [Google Scholar] [CrossRef]
- Shang, X.; Zhao, G.; Liang, F.; Zhang, C.; Zhang, W.; Liu, L.; Li, R.; Duan, X.; Ma, Z.; Yue, J. Safety and effectiveness of pembrolizumab combined with paclitaxel and cisplatin as neoadjuvant therapy followed by surgery for locally advanced resectable (stage III) esophageal squamous cell carcinoma: A study protocol for a prospective, single-arm, single-center, open-label, phase-II trial (Keystone-001). Ann. Transl. Med. 2022, 10, 229. [Google Scholar]
- Liu, J.; Chen, H.; Qiao, G.; Zhang, J.-T.; Zhang, S.; Zhu, C.; Chen, Y.; Tang, J.; Li, W.; Wang, S. PLEK2 and IFI6, representing mesenchymal and immune-suppressive microenvironment, predicts resistance to neoadjuvant immunotherapy in esophageal squamous cell carcinoma. Cancer Immunol. Immunother. 2023, 72, 881–893. [Google Scholar] [CrossRef]
- Yang, W.; Xing, X.; Yeung, S.-C.J.; Wang, S.; Chen, W.; Bao, Y.; Wang, F.; Feng, S.; Peng, F.; Wang, X. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J. Immunother. Cancer 2022, 10, e003497. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Lin, W.; Shao, D.; Depypere, L.; Zhang, Z.; Li, Z.; Cui, F.; Du, Z.; Zeng, Y. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): A multicenter, phase 2 study. Int. J. Cancer 2022, 151, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Cheriyath, V.; Glaser, K.B.; Waring, J.F.; Baz, R.; Hussein, M.A.; Borden, E.C. G1P3, an IFN-induced survival factor, antagonizes TRAIL-induced apoptosis in human myeloma cells. J. Clin. Investig. 2007, 117, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Cheriyath, V.; Kaur, J.; Davenport, A.; Khalel, A.; Chowdhury, N.; Gaddipati, L. G1P3 (IFI6), a mitochondrial localised antiapoptotic protein, promotes metastatic potential of breast cancer cells through mtROS. Br. J. Cancer 2018, 119, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, L.; Jiang, M.; Chen, Q.; Jiang, Z.; Feng, H. AGR2-mediated lung adenocarcinoma metastasis novel mechanism network through repression with interferon coupling cytoskeleton to steroid metabolism-dependent humoral immune response. Cell. Immunol. 2014, 290, 102–106. [Google Scholar] [CrossRef]
- Lv, C.; Li, M. IFI6 predicts prognosis and promotes cell growth of human colorectal cancer. Trop. J. Pharm. Res. 2023, 22, 245–251. [Google Scholar] [CrossRef]
- Peng, Y.; Dong, S.; Yang, Z.; Song, Y.; Ding, J.; Hou, D.; Wang, L.; Zhang, Z.; Li, N.; Wang, H. Identification of docetaxel-related biomarkers for prostate cancer. Andrologia 2021, 53, e14079. [Google Scholar] [CrossRef]
- Tahara, E.; Tahara, H.; Kanno, M.; Naka, K.; Takeda, Y.; Matsuzaki, T.; Yamazaki, R.; Ishihara, H.; Yasui, W.; Barrett, J.C. G1P3, an interferon inducible gene 6-16, is expressed in gastric cancers and inhibits mitochondrial-mediated apoptosis in gastric cancer cell line TMK-1 cell. Cancer Immunol. Immunother. 2005, 54, 729–740. [Google Scholar] [CrossRef]
- Yin, X.; Yang, J.; Chen, J.; Ni, R.; Zhou, Y.; Song, H.; Jin, L.; Tang, T.; Pan, Y. LncRNA CTD-3252C9. 4 modulates pancreatic cancer cell survival and apoptosis through regulating IFI6 transcription. Cancer Cell Int. 2021, 21, 433. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Z.; Gao, Y.; Li, J.; Zhao, X.; Yue, W. Single-cell RNA-sequencing portraying functional diversity and clinical implications of IFI6 in ovarian cancer. Front. Cell Dev. Biol. 2021, 9, 677697. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gu, S.; Lu, T.; Wu, K.; Li, L.; Dong, C.; Zhou, Y. IFI6 depletion inhibits esophageal squamous cell carcinoma progression through reactive oxygen species accumulation via mitochondrial dysfunction and endoplasmic reticulum stress. J. Exp. Clin. Cancer Res. 2020, 39, 144. [Google Scholar] [CrossRef] [PubMed]
- Cheriyath, V.; Leaman, D.W.; Borden, E.C. Emerging roles of FAM14 family members (G1P3/ISG 6–16 and ISG12/IFI27) in innate immunity and cancer. J. Interferon Cytokine Res. 2011, 31, 173–181. [Google Scholar] [CrossRef]
- Villamayor, L.; Rivero, V.; López-García, D.; Topham, D.J.; Martínez-Sobrido, L.; Nogales, A.; DeDiego, M.L. Interferon alpha inducible protein 6 is a negative regulator of innate immune responses by modulating RIG-I activation. Front. Immunol. 2023, 14, 1105309. [Google Scholar] [CrossRef]
- Richardson, R.B.; Ohlson, M.B.; Eitson, J.L.; Kumar, A.; McDougal, M.B.; Boys, I.N.; Mar, K.B.; De La Cruz-Rivera, P.C.; Douglas, C.; Konopka, G. A CRISPR screen identifies IFI6 as an ER-resident interferon effector that blocks flavivirus replication. Nat. Microbiol. 2018, 3, 1214–1223. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T. The role of methylation in gene expression. Nat. Educ. 2008, 1, 116. [Google Scholar]
- Tran, T.-O.; Vo, T.H.; Lam, L.H.T.; Le, N.Q.K. ALDH2 as a potential stem cell-related biomarker in lung adenocarcinoma: Comprehensive multi-omics analysis. Comput. Struct. Biotechnol. J. 2023, 21, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.-J.; Kim, K.H.; Kim, J.-Y.; Jeong, S.; Kim, N. Identification of DNA-methylated CpG islands associated with gene silencing in the adult body tissues of the Ogye chicken using RNA-Seq and reduced representation bisulfite sequencing. Front. Genet. 2019, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Demers-Mathieu, V. Optimal Selection of IFN-α-Inducible Genes to Determine Type I Interferon Signature Improves the Diagnosis of Systemic Lupus Erythematosus. Biomedicines 2023, 11, 864. [Google Scholar] [CrossRef]
- Furie, R.; Khamashta, M.; Merrill, J.T.; Werth, V.P.; Kalunian, K.; Brohawn, P.; Illei, G.G.; Drappa, J.; Wang, L.; Yoo, S. Anifrolumab, an anti–interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol. 2017, 69, 376–386. [Google Scholar] [CrossRef]
- Kirou, K.A.; Lee, C.; George, S.; Louca, K.; Peterson, M.G.; Crow, M.K. Activation of the interferon-α pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005, 52, 1491–1503. [Google Scholar] [CrossRef]
- Zhang, H.-Z.; Jin, G.-F.; Shen, H.-B. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin. J. Cancer 2012, 31, 281. [Google Scholar] [CrossRef]
- Reed, P.; Johnston, B. The changing incidence of oesophageal cancer. Endoscopy 1993, 25, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Devesa, S.S. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg. Oncol. Clin. 2002, 11, 235–256. [Google Scholar] [CrossRef] [PubMed]
- Kervevan, J.; Chakrabarti, L.A. Role of CD4+ T cells in the control of viral infections: Recent advances and open questions. Int. J. Mol. Sci. 2021, 22, 523. [Google Scholar] [CrossRef]
- Wen, Y.; Jing, Y.; Yang, L.; Kang, D.; Jiang, P.; Li, N.; Cheng, J.; Li, J.; Li, X.; Peng, Z. The regulators of BCR signaling during B cell activation. Blood Sci. 2019, 1, 119–129. [Google Scholar] [CrossRef]
- Mak, T.W.; Saunders, M.E. The Immune Response: Basic and Clinical Principles; Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Shah, K.; Al-Haidari, A.; Sun, J.; Kazi, J.U. T cell receptor (TCR) signaling in health and disease. Signal Transduct. Target. Ther. 2021, 6, 412. [Google Scholar] [CrossRef] [PubMed]
- Shedlock, D.J.; Shen, H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 2003, 300, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Wiestner, A. Targeting B cell receptor signalling in cancer: Preclinical and clinical advances. Nat. Rev. Cancer 2018, 18, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.D.; Tong, X.; Moorman, A.C.; Ly, K.N.; Rupp, L.; Xu, F.; Gordon, S.C.; Holmberg, S.D.; Chronic Hepatitis Cohort Study (CHeCS) Investigators. Increased incidence of cancer and cancer-related mortality among persons with chronic hepatitis C infection, 2006–2010. J. Hepatol. 2015, 63, 822–828. [Google Scholar] [CrossRef]
- Huang, J.; Magnusson, M.; Törner, A.; Ye, W.; Duberg, A.-S. Risk of pancreatic cancer among individuals with hepatitis C or hepatitis B virus infection: A nationwide study in Sweden. Br. J. Cancer 2013, 109, 2917–2923. [Google Scholar] [CrossRef]
- Nyberg, A.H.; Sadikova, E.; Cheetham, C.; Chiang, K.M.; Shi, J.X.; Caparosa, S.; Younossi, Z.M.; Nyberg, L.M. Increased cancer rates in patients with chronic hepatitis C. Liver Int. 2020, 40, 685–693. [Google Scholar] [CrossRef]
- Ponvilawan, B.; Rittiphairoj, T.; Charoenngam, N.; Rujirachun, P.; Wattanachayakul, P.; Tornsatitkul, S.; Ungprasert, P. Association Between Chronic Hepatitis C Virus Infection and Esophageal Cancer: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2022, 56, 55–63. [Google Scholar] [CrossRef]
- Chu, Y.-Y.; Cheng, J.-S.; Wu, T.-S.; Chen, C.-W.; Chang, M.-Y.; Ku, H.-P.; Chien, R.-N.; Chang, M.-L. Association between hepatitis C virus infection and esophageal cancer: An Asian nationwide population-based cohort study. J. Clin. Med. 2021, 10, 2395. [Google Scholar] [CrossRef] [PubMed]
- Peri, S.; Navarro, J.D.; Kristiansen, T.Z.; Amanchy, R.; Surendranath, V.; Muthusamy, B.; Gandhi, T.; Chandrika, K.; Deshpande, N.; Suresh, S. Human protein reference database as a discovery resource for proteomics. Nucleic Acids Res. 2004, 32, D497–D501. [Google Scholar] [CrossRef]
- Li, Y.; Ge, D.; Lu, C. The SMART App: An interactive web application for comprehensive DNA methylation analysis and visualization. Epi genet. Chromatin 2019, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.; Varambally, S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef]
- Tran, T.-O.; Lam, L.H.T.; Le, N.Q.K. Hyper-methylation of ABCG1 as an epigenetics biomarker in non-small cell lung cancer. Funct. Integr. Genom. 2023, 23, 256. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.-H.; Ta, H.D.K.; Nguyen, T.T.T.; Wang, C.-Y.; Lee, K.-H.; Le, N.Q.K. Identification of a Novel Eight-Gene Risk Model for Predicting Survival in Glioblastoma: A Comprehensive Bioinformatic Analysis. Cancers 2023, 15, 3899. [Google Scholar] [CrossRef]

| General | |||
| HPRD ID: | 00959 | Molecular Weight (Da): | 13,298 |
| Gene Symbol: | IFI6 | Gene Map Locus: | 1p35 |
| Molecular Class: | Unclassified | Molecular Function: | Cytokine activity |
| Biological Process: | Anti-apoptosis; Immune response | ||
| Localization | |||
| Primary | Mitochondrion | ||
| Domains and Motifs | |||
| Domains | Motifs | ||
| Transmembrane Domain (TM) | 48–70 | Signal Peptide (SP) | 1–23 |
| Alternate Names | |||
| Alternate Names | G1P3; ISG16 | ||
| Interferon alpha inducible protein | |||
| Interferon induced protein IFI-6–16 | |||
| Isoform Specific Alternate Names | Interferon induced 6–16 protein isoform b | ||
| Gene | Coefficient | Hazard Ratio | 95% Confidence Interval | p-Value |
| B cell | −7.073 | 0.001 | 0.000–1.500300 × 101 | 0.156 |
| CD8+ T cell | 1.648 | 5.196 | 0.000–2.314727 × 105 | 0.763 |
| CD4+ T cell | −2.250 | 0.105 | 0.000–1.197976 × 106 | 0.786 |
| Macrophage | −13.143 | 0.000 | 0.000–2.780000 × 10−1 | 0.030 * |
| Neutrophil | −3.016 | 0.049 | 0.000–2.038430 × 108 | 0.790 |
| IFI6 | −0.128 | 0.880 | 0.759–1.019000 × 100 | 0.088 |
| CD4 | 0.617 | 1.853 | 1.211–2.834000 × 100 | 0.004 ** |
| BCR | −0.760 | 0.468 | 0.310–7.050000 × 10−1 | 0.000 *** |
| CD8A | 0.052 | 1.054 | 0.699–1.589000 × 100 | 0.803 |
| CD8B | −0.091 | 0.913 | 0.573–1.454000 × 100 | 0.702 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viet-Nhi, N.-K.; Minh Quan, T.; Cong Truc, V.; Anh Bich, T.; Hoang Nam, P.; Le, N.Q.K.; Chen, P.-Y.; Hung, S.-H. Multi-Omics Analysis Reveals the IFI6 Gene as a Prognostic Indicator and Therapeutic Target in Esophageal Cancer. Int. J. Mol. Sci. 2024, 25, 2691. https://doi.org/10.3390/ijms25052691
Viet-Nhi N-K, Minh Quan T, Cong Truc V, Anh Bich T, Hoang Nam P, Le NQK, Chen P-Y, Hung S-H. Multi-Omics Analysis Reveals the IFI6 Gene as a Prognostic Indicator and Therapeutic Target in Esophageal Cancer. International Journal of Molecular Sciences. 2024; 25(5):2691. https://doi.org/10.3390/ijms25052691
Chicago/Turabian StyleViet-Nhi, Nguyen-Kieu, Tran Minh Quan, Vu Cong Truc, Tran Anh Bich, Pham Hoang Nam, Nguyen Quoc Khanh Le, Po-Yueh Chen, and Shih-Han Hung. 2024. "Multi-Omics Analysis Reveals the IFI6 Gene as a Prognostic Indicator and Therapeutic Target in Esophageal Cancer" International Journal of Molecular Sciences 25, no. 5: 2691. https://doi.org/10.3390/ijms25052691
APA StyleViet-Nhi, N.-K., Minh Quan, T., Cong Truc, V., Anh Bich, T., Hoang Nam, P., Le, N. Q. K., Chen, P.-Y., & Hung, S.-H. (2024). Multi-Omics Analysis Reveals the IFI6 Gene as a Prognostic Indicator and Therapeutic Target in Esophageal Cancer. International Journal of Molecular Sciences, 25(5), 2691. https://doi.org/10.3390/ijms25052691









