Abstract
As the burden of type 2 diabetes (T2D) continues to escalate globally, there is a growing need for novel, less-invasive biomarkers capable of early diabetes detection and monitoring of disease progression. Liquid biopsy, recognized for its minimally invasive nature, is increasingly being applied beyond oncology, and nevertheless shows its potential when the collection of the tissue biopsy is not possible. This diagnostic approach involves utilizing liquid biopsy markers such as cell-free nucleic acids, extracellular vesicles, and diverse metabolites for the molecular diagnosis of T2D and its related complications. In this context, we thoroughly examine recent developments in T2D liquid biopsy research. Additionally, we discuss the primary challenges and future prospects of employing liquid biopsy in the management of T2D. Prognosis, diagnosis and monitoring of T2D through liquid biopsy could be a game-changing technique for personalized diabetes management.
1. Introduction
The rapid increase in prevalence of type 2 diabetes mellitus (T2D) qualifies it as one of the fastest-growing global health emergencies of the 21st century [1]. T2D is a chronic condition necessitating continuous management and attention to avert its extensive health consequences, including myocardial infarction, cardiac failure, blindness, kidney failure, stroke, and amputation.
T2D is a multifactorial disease resulting from a combination of factors, including inadequate beta-pancreatic cell (β-cell) function, reduced insulin sensitivity, and chronic inflammation that precede the diabetes onset with up to 15 years. Currently, the diagnosis of T2D is made typically once hyperglycemia has become evident, a limitation that impedes early disease detection and precludes the possibility of timely interventions aimed at preserving β-cell mass and improving patient outcomes. Liquid biopsy can be a promising solution to this challenge, being useful in the diagnostic, management, and prognostic of the disease and providing a deeper understanding of the disease’s pathophysiology.
Early detection and intervention are crucial for preventing the progression of T2D and reducing the risk of complications such as nephropathy, cardiovascular disease, neuropathy, or retinopathy. Even though biomarkers such as glycemia or HbA1c are widely used for diagnosing T2D, presently, there is no biomarker that is able to predict the ongoing destruction of beta cells in the pancreas in the early stages of the disease as opposed to T1D, where specific autoantibodies against pancreatic islet cells or insulin are often present before the onset of symptoms and individuals at increased risk or in the early stages of T1D can be timely identified.
On the other hand, liquid biopsy shows its potential in the prognosis, diagnosis, and monitoring of cancer [2,3,4]. The use of a small amount of “body fluids” (usually blood), containing biomarkers that can provide relevant information for diagnostic and monitoring, present some relevant advantages such as reduced invasiveness, easy protocol for sample collection, lower costs, and real-time information [5,6,7].
This review study was conducted at the eBio-Hub Research Center affiliated to the National University of Science and Technology Politehnica Bucharest. PubMed, Scopus, Google Scholar, and Web of Science were searched using the following keywords: “type 2 diabetes”, “biomarker”, “microRNA”, “liquid biopsy”, and “cell free DNA.”
The main objectives of this review study are as follows: (i) to conduct a critical review of recent advancements in the field of T2D liquid biopsy and assess the current state of research and technology in T2D liquid biopsy; (ii) to provide an overview of the primary challenges associated with T2D liquid biopsy and explore the perspectives surrounding the potential of liquid biopsy to revolutionize personalized T2D management; (iii) to highlight the potential role of liquid biopsy as a game changer in advancing the field of personalized T2D management; and (iv) to address any gaps or limitations in current research on T2D liquid biopsy and to propose potential avenues for future research and development to enhance the applicability and effectiveness of liquid biopsy in T2D management.
2. Liquid Biopsy Markers in T2D
Biomarkers that allow for early diagnosis and effective monitoring have the potential to lead to better patient-centric therapeutic decisions. In T2D, identifying the precise timing of critical events such as β-cell stress, de-differentiation, or death is essential for developing effective treatments [8]. However, the inaccessibility of pancreatic tissue in humans for longitudinal, minimally invasive monitoring represents a significant obstacle. To address this shortcoming, liquid biopsy could be employed for the detection of β-cell markers. Liquid biopsies encompass a diverse range of clinically informative elements. Blood samples can undergo analysis for metabolites, exosomes, and cell-free nucleic acids, each offering valuable numerical and molecular insights. Employment of liquid biopsy in T2D patients can provide valuable insight regarding T2D complications, cardiovascular disease (CVD), and cancer risk. An overview of the key elements and the impact of the liquid biopsy markers in T2D is presented in Figure 1.
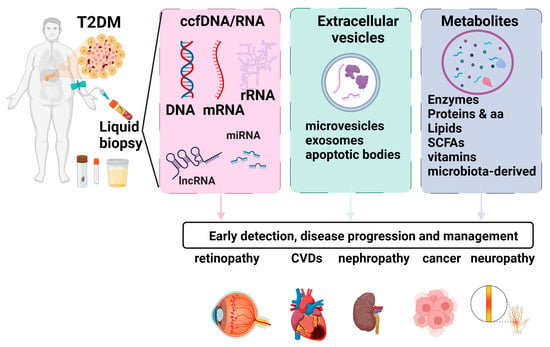
Figure 1.
Liquid biopsy markers in T2D. Liquid biopsies encompass a diverse range of clinically informative elements. Blood samples can undergo analysis for metabolites, exosomes, and cell-free nucleic acids, each offering valuable numerical and molecular insights. Employment of liquid biopsy in T2D patients can provide valuable insight regarding T2D complications, cardiovascular disease (CVD), and cancer risk. Figure created using biorender.com.
2.1. Cell-Free Nucleic Acids
2.1.1. Circulating Cell-Free DNA (ccfDNA)
Circulating cell-free DNA (ccfDNA), also known as cell-free DNA (cfDNA), refers to short (≈160 nt) double-stranded DNA fragments found in various biological fluids, including blood, urine, saliva, and cerebrospinal fluid. Generally, ccfDNA is actively released by living cells or can result after cell death (apoptosis, necrosis) [9].
Several methods have been utilized to assess cfDNA, including droplet digital polymerase chain reaction (ddPCR), beads, emulsion, amplification, and magnetics (BEAMing), tagged-amplicon deep sequencing (TAm-Seq), whole-genome bisulfite sequencing (WGBS-Seq), whole-exome sequencing (WES), and whole-genome sequencing (WGS).
BEAMing proves to be a cost-effective and highly sensitive screening method for identifying established mutations. The method of integrating PCR with flow cytometry has the capability to identify alterations at levels as minimal as 0.01%, demonstrating excellent concordance with tissue testing [10].
Droplet digital polymerase chain reaction (ddPCR) is capable of detecting genomic material at levels as low as 0.01–1.0%, making it a valuable tool for identifying potentially rare mutations and quantifying copy number variants [11]. Nevertheless, its applicability is limited to assessing the presence of well-characterized sequences.
In patients with cancer, whole-exome sequencing offers a comprehensive analysis of all existing tumor mutations, allowing for the identification of potential oncogenes and tumor suppressor genes. However, its sensitivity may be comparatively lower than other methods due to the inclusion of exomic alterations. Despite this, it stands out for its cost-effectiveness and high yield [12].
Tagged-amplicon deep sequencing (TAm-Seq) enables a highly specific and sensitive analysis, achieving an accuracy of approximately 97%. It has the capability to detect DNA levels as low as 2% through the utilization of primers to tag and identify genomic sequences. TAm-Seq boasts a high sequencing throughput, leading to reduced sequencing time and cost, allowing for the simultaneous sequencing of millions of DNA molecules. However, it is essential for the desired sequence to be pre-characterized for the methodology to be effective [13].
Whole-genome sequencing (WGS) examines the entirety of the sample genome to identify well-characterized and detrimental alterations, along with variants of unknown significance. It holds significant potential for a thorough assessment of genetic mutations and, in case of T2D, could be used to identify cancer risk; however, its application is constrained by challenges in quality assurance, ethical considerations, time, and cost. The interpretation of results can be challenging outside of specialized centers [12].
Whole-genome bisulfite sequencing (WGBS-Seq) stands as the benchmark in DNA methylation analysis. It provides a measurement for each cytosine with exceptional accuracy. While it has the capability to uncover partially methylated domains in cancer cells, the sensitivity of this method can be compromised due to potential variations in DNA degradation [12].
Cell-free DNA (cfDNA)-based biomarkers have shown promise as minimally invasive options for the early diagnosis and monitoring of diabetes in various studies [14,15].
CcfDNA fragments retain characteristics indicative of their tissue of origin. Determining the ccfDNA tissue of origin can be performed using different approaches: the detection of distinct SNPs and/or genetic mutations, genome-wide methylation or 5hmC profiling, and fragmentation profiling by nucleosome footprints [16].
Adipose tissue dysfunction, characterized by hypertrophy, inflammation, and cell death, is closely associated with the development of insulin resistance—a key feature of T2D. Hence, the detection of cfDNA fragments associated with adipose tissue degeneration can provide insights into the mechanisms underlying insulin resistance [17].
Combining cfDNA concentration with tissue-specific epigenetic signatures may offer a comprehensive approach to assessing metabolic risk and disease progression. For instance, Lehmann-Werman et al. used methylation profiling to identify circulating DNA linked to different pathological processes: pancreatic β-cell DNA from patients with type 1 diabetes and exocrine pancreatic DNA from patients with pancreatic cancer [18].
Karaglani et al. evaluated the methylation profile of a panel of specific genes related to β-cells, including INS (insulin), GCK (glucokinase), IAPP (islet amyloid polypeptide-amylin), KCNJ11 (potassium inwardly rectifying channel subfamily J member 11), and ABCC8 (ATP binding cassette subfamily C member 8) and, based on the data generated, they built classifying predictive models using automated machine learning. INS, IAPP, GCK, and KCNJ11 levels differed significantly between T2D patients and healthy controls. Based on ccfDNA parameters, methylation data and demographical information, automated machine learning analysis generated biosignatures, including GCK, IAPP, and KCNJ11 methylation, with a very high discriminating performance of T2D from healthy individuals [19].
While human leukocyte antigen (HLA) typing is primarily used for assessing the risk of developing type 1 diabetes, there is ongoing research exploring potential associations between specific HLA alleles and the risk of diabetes-related complications. These complications may include diabetic nephropathy, retinopathy, and neuropathy. HLA typing, combined with other genetic and clinical information, may contribute to individualized risk assessments for diabetes complications. Identifying individuals at higher risk may allow for targeted monitoring and early interventions to prevent or manage complications. Ongoing research is exploring the complex interplay between genetics, including HLA alleles, and the development of diabetes and its complications. Advances in genomic medicine and personalized medicine may lead to more refined risk assessments and tailored therapeutic approaches. The influence of variations in the HLA genotype on the development of type 2 diabetes complications is not clearly understood. Recently, Birinci et al. (2022) identified the HLA variant HLA-DR7 to be associated with diabetic rethynopathy development [20]. The presence of the HLA-DQB1*0501 allele demonstrated a protective association against diabetic nephropathy (DN) in individuals of Han ethnicity in China [21].
Cell-free mitochondrial DNA (ccf-mtDNA) is produced and discharged from cells into the systemic circulation in response to cellular damage or stress. This release may occur passively through various forms of cell death or actively from living cells through processes that are not fully comprehended at present [22]. Increased blood ccf-mtDNA levels have been associated with T2D associated with coronary heart disease or cognitive impairment [23,24].
2.1.2. Circulating Cell-Free RNA (ccfRNA)
Human blood is home to a wide range of cfRNA species, including microRNA (miRNA), messenger RNA (mRNA), long-noncoding RNA(lncRNA), Piwi-interactingRNA (piRNA), circular RNA (circRNA), transfer RNA (tRNA), and miscellaneous other noncoding RNA molecules [25].
Advances in high-throughput sequencing and bioinformatics have improved the ability to profile and analyze cfRNA. Emerging single-cell RNA sequencing (scRNA-seq) technologies enabled the study of cfRNA heterogeneity at the single-cell level [26].
2.1.3. miRNAs
Circulating miRNAs, small 17- to 23-bp-long noncoding RNAs have been proposed as potential biomarkers for T2D [27].
In streptozotocin-treated mice, miRNA-375, which is highly abundant in pancreatic islet cells, was reported to be an early indicator of T1D [28].
miRNA-375, miRNA-101, and miRNA-802, which have roles in pancreatic islet function and insulin secretion, have been identified as being significantly increased in T2D patients [29].
Elevated levels of miR-122 were shown to be strongly associated with an increased risk of developing T2D [30]. MicroRNA-122 levels were also found to be increased in patients with diabetic retinopathy [31].
Large-scale studies are necessary for clinical miRNA validation. Since miRNAs are involved in diverse regulatory processes, future studies are needed to unravel their roles in the overall pathogenesis of T2D. A shift from targeted analysis towards exploratory next generation sequencing (NGS) should be performed in order to potentially discover novel miRNA relevant for T2D pathogenesis [32].
Circulating long-noncoding RNAs (lncRNAs), which are RNA molecules longer than 200 nucleotides and that do not code for proteins, exhibit cell and tissue specificity and have been implicated in various cellular processes, including transcriptional regulation. In the context of T2D complications, circulating lncRNAs have been shown to play important roles as transcriptional activators and regulators. In a mouse model of diabetic nephropathy, Tug1 lncRNA was shown to modulate mitochondrial bioenergetics in glomerular podocytes [33].
Other lncRNAs such as GAS5, RNA-MEG3is, MALAT1, and CYP4B1-PS1-001 were reported to be involved in diabetes-induced microvascular dysfunction and inflammation [34,35,36,37].
The use of lncRNAs comes with several disadvantages, however, including a lack of conservation across species and technically challenging analysis. Also, the fact that lncRNAs harbor a multitude of roles at the cellular level makes their specific functional characterization difficult.
Even though cfRNA provides a snapshot of transcripts reflecting the health status of multiple RNAs, understanding the precise origins of cfRNA, including their cell types of origin, can be challenging due to the complex and dynamic nature of cfRNA in circulation [38].
2.2. Extracellular Vesicles
EVs play a significant role in intercellular communication and their shedding is influenced by intracellular elements such as calcium ions. EVs can interact with recipient cells through various mechanisms, including ligand–receptor binding, phagocytosis, direct fusion with plasma membranes, micropinocytosis, and lipid raft-mediated internalization or endocytosis [39].
EVs are classified based on their different sizes and biogenesis processes (Figure 2). The main types of EVs are microparticles (MPs), exosomes, and apoptotic bodies, each with distinct characteristics [40]. MPs that are also known as microvesicles or ectosomes originate from the outward budding or shedding of the plasma membrane of cells and have a diameter size range from 30 nm to approximately 1 μm. Exosomes originate from the inward budding of endocytic vesicles within the cell, forming early endosomes which mature into late endosomes, also known as multivesicular bodies (MVBs), where intraluminal vesicles (ILVs) are formed through the inward budding of the endosomal membrane. Exosomes are released when MVBs fuse with the cell’s plasma membrane, leading to the extracellular release of the ILVs as exosomes. Exosomes are generally smaller, typically ranging from 30 to 150 nm in diameter. Apoptotic bodies are distinct from MPs and exosomes in terms of their origin and are typically larger than MPs and exosomes [41].
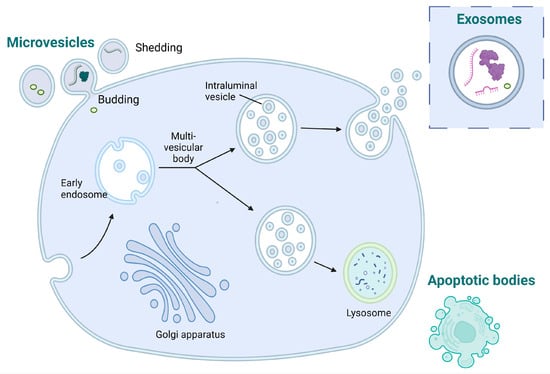
Figure 2.
Diagram illustrating the processes involved in the generation of microvesicles, exosomes, and apoptotic bodies.
2.2.1. Exosomes
Exosomes play a role in cell-to-cell communication by carrying proteins, lipids, RNA, and other molecules between cells. Exosomes have been implicated in various physiological and pathological processes, including those associated with diabetes [42]. The role of exosomes in diabetes-related pathophysiology, including vascular complications, inflammation, and coagulation changes, is an area of growing research interest.
While exosomes play a crucial role in the early detection and treatment of various conditions [43], their small size, low density (1.13–1.19 g/mL), and coexistence with similar components such as cell fragments and proteins in bodily fluids present significant challenges for their isolation [44]. Standardized methods for separation and quantification are crucial for both the research and clinical use of exosomes. In order to develop more efficient and rational exosome separation technologies, it is essential for us to comprehend the current separation technology landscape. Common separation techniques include ultracentrifugation, ultrafiltration, polymer precipitation, immunoaffinity, and size exclusion chromatography. However, these methods yield low isolation efficiency, sample loss, and compromised exosome recovery and purity. Ultracentrifugation, commonly regarded as the “gold standard” for exosome isolation, involves expensive instruments, large sample volumes, potential protein contamination, and complex isolation steps [45].
Proteins in exosomes obtained from the body fluids of individuals with T2D exhibit variations. For instance, dipeptidyl peptidase-IV (DPP IV), known for inactivating glucagon-like peptide-1 (GLP-1), is linked to T2D. Moreover, the microvesicle-bound form of DPP IV is the predominant type found in urine, and its levels are notably elevated in individuals with T2DM compared to control subjects [46]. Patients with diabetes exhibiting proteinuria show markedly elevated levels of Wilms tumor protein 1 in urinary exosomes, suggesting that exosomes can serve as early biomarkers for podocyte injury in individuals with diabetes [47].
Patients with T2D and associated microvascular complications exhibit markedly elevated levels of miR-7 in exosomes derived from serum compared to those without such complications. Consequently, alterations in these exosomal biomarkers precede changes at the organ level and offer greater specificity than examining whole urine or blood. This further strengthens the potential role of exosomes in the early diagnosis of diabetes and its complications [48].
Several studies have reported elevated levels of exosomes in the blood samples collected from individuals with diabetes, including both type 1 diabetes (T1D) and T2D. A comparative cross-sectional study performed on normoglycemic individuals and patients with prediabetes or diabetes mellitus reported that diabetics harbored elevated levels of plasma extracellular vesicles and that exosome concentration was positively associated with the insulin resistance index [49]. Meta-analyses have demonstrated a significant rise in circulating exosomes released by platelets, monocytes, and endothelial cells in individuals with diabetes. However, there is no discernible difference in exosomes from leukocytes between patients with diabetes and the control group [50].
The communication between different cell types via exosomes can promote the development of different T2D complications. For instance, endothelial cell-derived exosomes can interact with macrophages and smooth muscle cells altering their behavior and subsequently leading to atherosclerotic plaque formation [51].
Atherosclerotic lesions in the aortic endothelium can affect other normal cell types within the vascular wall via the transport of regulatory proteins carried by exosomes. Transfer of exosomes from the blood of db/db diabetic mice into db/m+ non-diabetic mice can severely impair the endothelial cell function in recipient mice [52].
Exosomes could serve as early biomarkers of podocyte injury in diabetic kidney disease (DKD) since significantly elevated levels of Wilms tumor protein 1 (WT1) were found in urinary exosomes of patients with diabetes and proteinuria [47].
It is known that vasa vasorum (VV) angiogenesis is increased for T2D patients and may promote atherosclerotic plaque rupture. Wang et al. [53] demonstrated the role of insulin resistance adipocyte-derived exosomes in modulating vasa vasorum angiogenesis tested on ApoE(−/−) mice.
Goetzl et al. [54] investigated the cargo proteins of human plasma endothelial cell-derived exosomes in patients with atherosclerotic cerebrovascular disease. Platelets exhibited significantly higher levels of platelet glycoprotein VI, integrin-linked kinase-1, high-mobility group box-1 protein, chemokine CXCL4, and thrombospondin-1.
2.2.2. Microbial Extracellular Vesicles
EVs are essential in various biological processes, including immune response regulation, tissue repair, and cell signaling. Microbiota-derived EVs (MEVs) have gained attention for their role in host cell–microbiota communication [55]. MEVs can transport molecules from bacteria, archaea, fungi, and other microorganisms to host cells, influencing host immune responses and other physiological processes.
EVs derived from gut microbiota have been linked to various host functions, including the regulation of the immune system and the suppression of cancer. There is also a suggestion that these EVs may play a role in modulating the gut–brain axis, as studies have reported their ability to reach the central nervous system and regulate cerebral function [56]. Apart from exhibiting beneficial effects and contributing to host homeostasis, changes in the profile of EVs derived from gut microbiota have been associated with the progression of several diseases, such as HIV, inflammatory bowel disease, or cancer therapy-induced intestinal mucositis.
It has been proposed that EVs derived from gut microbiota, possessing pro-inflammatory properties, might be sufficiently mild to be beneficial. They could contribute to maintaining gut barrier integrity and intestinal homeostasis by inducing host immune and defense responses. However, in cases of dysbiosis or host susceptibility to inflammatory disorders, an imbalance in the abundance of pro-inflammatory EVs from gut microbiota, initially considered beneficial to the host, may potentially result in exacerbated inflammatory responses that are ultimately harmful. Supporting this hypothesis, Hickey et al. observed that EVs derived from the commensal Bacteroides thetaiotaomicron could be taken up by intestinal macrophages, triggering an inflammatory response by inducing the production of pro-inflammatory cytokines (such as TNF-α and IL-1β) and contributing to the development of colitis in genetically susceptible mice [57].
There is growing evidence to suggest that the interplay between gut microbiota, their EVs, and the host’s immune system can have significant consequences for metabolic health, including the potential to improve obesity and diabetes. Indeed, some studies have indicated that interventions aimed at modulating the gut microbiota and their EVs, such as dietary changes or the use of probiotics or prebiotics, may lead to improvements in metabolic diseases.
A study by Chelakkot et al. found that oral administration of A. muciniphila EVs to mice that were fed a high-fat diet (HFD) resulted in a reduction in gut barrier permeability, reduced body weight gain, and improved glucose tolerance [58]. The beneficial effects of A. muciniphila EVs were confirmed by Ashrafian et al. who reported that A. muciniphila EVs had significant effects on alleviating weight gain and adiposity in HFD-induced obese mice [59]. Conversely, Choi et al. showed that that the stool EVs from HFD-fed mice had an adverse effect on glucose metabolism, promoting insulin resistance in both skeletal muscle and adipose tissue [60].
There are current constraints in characterizing EVs derived from gut microbiota, posing challenges in identifying the molecules responsible for their physiological effects. The isolation and characterization of the diverse profile of gut microbiota are considerably more intricate due to the absence of universal markers for bacterial EVs and their size similarity to mammalian EVs, thus complicating their differentiation from mammalian EVs in bodily fluids. Some authors have identified the bacterial origin of fecal EVs by amplifying DNA from the most prevalent phyla of gut microbiota through PCR [61]. However, the comprehensive identification of the origin of biofluids, fecal-derived EVs, and EV-associated molecules like miRNAs remains challenging due to the complex nature of the material.
2.2.3. Metabolites
While diagnosing diabetes or prediabetes currently relies on a straightforward blood glucose measurement, there are compelling reasons to advocate for concurrent metabolite assessments. Firstly, relying solely on a single measurement of plasma glucose for diagnosing diabetes or prediabetes can potentially yield false positive results, leading to misdiagnosis by healthcare professionals. In such scenarios, metabolite indices can serve as valuable complementary indicators. Secondly, these metabolites can offer a wealth of additional information about patients with diabetes or prediabetes.
In determining the progression to prediabetes and diabetes, there are several widely used and standardized biomarkers such as glycemia, HbA1c, glycated albumin, and fructosamine. The need to diagnose T2D even before β-cell function is lost determined the need to find other potential useful biomarkers, and a part of them are detailed in Table 1.

Table 1.
Biomarkers linked to detection of prediabetes and diabetes. Abbreviations: T2D—type 2 diabetes, LADA—latent autoimmune diabetes of the adult, CKD—chronic kidney disease, FPG—fasting plasma glucose, IFG—impaired fasting glucose, CVD—cardiovascular disease, SGLT2i—sodium-glucose inked transporter 2 inhibitors, IR—insulin resistance.
Further research is needed to pinpoint the most precise biomarkers, acknowledging that relying on a singular determinant is likely to have inherent constraints. Hence, combining several biomarkers could provide a more precise forecast of individuals with an increased likelihood of progressing from prediabetes to diabetes.
Several metabolite signatures linked to T2D and its progression have been recently described, and these include an alanine-to-glycine ratio, acylcarnitines, fetuin A, and branched chain amino acids [79,80,81,82,83]. However, these metabolites are still undergoing investigation and necessitate future validation in larger cohorts. Upon identification and validation in clinical studies, these biomarkers could potentially be integrated into point-of-care devices or wearable sensors, facilitating their rapid detection.
3. Salivary Markers
The conventional monitoring of serological parameters in diabetes typically involves invasive techniques, causing discomfort and distress. Collecting saliva is convenient, enhancing accessibility for both healthcare professionals and patients and is especially beneficial in scenarios where obtaining blood samples may pose challenges or discomfort. Saliva comprises a range of biomarkers, encompassing DNA, RNA, proteins (3000 proteins and 12,000 peptides), and metabolites [84]. An elegant review by Srinivasan et al. presented the wide array of salivary biomarkers linked to T2D, including glucose, glycosylated hemoglobin (HbA1c), amylase, alpha 2-macroglobulin, alpha defensins, carbonyls, dehydroprogesterone, ghrelin, high-density lipoprotein, C reactive protein, leptin, insulin, Il-1β, osteopontin, osteocalcin, transferrin receptor, urea, and uric acid [85,86].
In contrast to genetic T2D biomarkers, there is a limited body of research on mRNA biomarkers derived from saliva samples. A study examined differential gene expression in saliva from 13 T2D patients, revealing elevated levels of KRAS, SAT1, SLC13A2, and TMEM72, alongside reduced expressions of EGFR and PSMB2 genes [87]. Initially identified through microarray analysis, these six biomarkers were subsequently validated in a cohort of 13 T2D patients and 13 healthy controls. A logistic model incorporating four of these salivary biomarkers (KRAS, SAT1, EGFR, and PSMB2) successfully distinguished T2D patients from healthy controls, yielding an area under the curve (AUC) of 0.917 with a 95% confidence interval of 0.809–1.000. This suggests that salivary mRNAs hold promise as T2D biomarkers, although further investigations involving larger sample sizes are warranted. Subsequently, a validated panel comprising four salivary extracellular RNA biomarkers (IL1R2—interleukin 1 receptor type 2, VPS4B—vacuolar protein sorting 4 homolog B, CAP1—cyclase-associated actin cytoskeleton regulatory protein 1, LUZP6—leucine zipper protein 6) along with body mass index (BMI) has been identified. This panel demonstrates the capability to differentiate individuals with high and low insulin resistance, both in the general population and within subgroups of participants who are healthy and pre-diabetic [88].
There is still a need to identify reliable salivary biomarkers in order to diagnose prediabetes or to distinguish different T2D complications. For instance, differentiating between diabetic nephropathy (DN) and nondiabetic renal disease (NDRD) is crucial for tailoring more specific treatments. However, currently, there are no ideal biomarkers for making this distinction.
A recent study by Han et al. (2022) screened different salivary glycopatterns in DN and NDRD patients using lectin microarrays, with validation conducted through lectin blotting. Diagnostic models were then constructed using logistic regression and artificial neural network analyses, and their validity was confirmed in another cohort [89].
Oxidative stress occurs early in the development of T2D, often preceding the onset of clinical symptoms. Thus, monitoring oxidative stress markers allows for the detection of subtle changes at a molecular level before overt metabolic disturbances become evident [90]. Oxidative stress and inflammation can affect pancreatic beta cells, reducing their function and insulin secretion, further exacerbating glucose dysregulation. Prolonged exposure to oxidative stress, inflammation, and protein glycation contributes to the development of diabetic complications, including cardiovascular disease, neuropathy, nephropathy, and retinopathy. Understanding and targeting these interconnected pathways is essential for developing therapeutic interventions to manage T2D and prevent associated complications [91].
Numerous studies suggest that an imbalance in oxidant/antioxidant mediators plays a significant role in the development and advancement of metabolic syndrome, T2D, and cardiovascular disease. However, the majority of research has concentrated on the distribution of these indicators in tissues and blood, leaving their influence on saliva composition less explored. Products resulting from lipid peroxidation, protein oxidation, and DNA damage can be directly evaluated in saliva, potentially providing a means to diagnose systemic disorders associated with oxidative stress. The evaluation of salivary redox biomarkers appears to be applicable for both diagnosing and monitoring various health conditions, including obesity, diabetes, hypertensive disorders, and heart failure. In these conditions, there is a pathological depletion of molecules and enzymes with antioxidant properties in saliva, while oxidative and nitrosative by-products are favored. For instance, research has demonstrated elevated salivary oxidative biomarkers, including 4-hydroxynonenal (4-HNE), 8-isoprostanes (8-isoP), advanced oxidation protein products (AOPP), protein carbonyl groups (PC), and 8-hydroxy-D-guanosine (8-OHdG) [92].
The use of saliva samples for the molecular diagnostic of T2D comes with several caveats. For example, the local oral status and pathologies related to the oral cavity, such as periodontitis and dental caries may influence the redox balance of saliva, posing potential interference with its widespread routine clinical application [93]. Some biomarkers, such as pro-inflammatory cytokines, are common to many diseases, whereas others are more specific. The presence of other inflammatory comorbidities could also influence the reliability of salivary markers. Moreover, the oral microbiota, specific to each individual, might produce different metabolites and other signaling molecules which might hinder an accurate diagnostic.
The potential to identify a specific array of candidate molecules using saliva for distinguishing each stage of chronic disorder and creating panels of salivary mediators as appropriate molecular biomarkers, when integrated with the demographic, genetic, and anthropometric characteristics of patients, could offer an innovative diagnostic tool. Nevertheless, there is still a need to identify salivary markers specific for different T2D stages and complications. Once identified and validated in clinical studies, these biomarkers could further be incorporated into point-of-care devices or wearable sensors to enable their fast detection.
4. Challenges and Perspectives
The evident clinical validity of liquid biopsy is underscored by numerous ongoing or completed clinical trials seeking to demonstrate that decisions based on circulating components could improve diagnostic and enhance patient survival. Despite promising data from observational studies, albeit with limited patient numbers, the full clinical utility of liquid biopsies is yet to be conclusively established. Detecting and characterizing circulating biomarkers in the early disease stages pose challenges due to their low concentrations in patient biofluids. In oncology, advancements in circulating tumor DNA (ctDNA) analysis, utilizing ultra-sensitive state-of-the-art next-generation sequencing technologies and patient-specific panels, have facilitated tumor characterization, assessment of responses to neoadjuvant chemotherapies, and detection of minimal residual disease (MRD) post-surgery. While this methodological revolution has given rise to various NGS procedures with a shared goal of detecting ultra-low diluted cfDNA, important considerations for clinical extrapolation, particularly in terms of technical complexity, still remain. This complexity is especially pronounced at the bioinformatic level, where the development of non-specialized, user-friendly software is crucial for broader clinical application.
An important aspect to consider is the fact that genetic differences such as variations in allele frequencies, mutation patterns, or genomic rearrangements among racial and ethnic groups can impact the composition of cfDNA in liquid biopsies [94].
Hormonal factors, such as estrogen and testosterone levels, may influence the biology of certain cancers. Liquid biopsy results might reflect these hormonal influences, impacting the interpretation of biomarkers in male and female patients.
Discovering signatures for noncoding RNA in the blood is more demanding compared to other commonly used biomarkers. This is attributed to their low abundance in biofluids, the absence of suitable housekeeping noncoding RNA reference analytes, and elevated intra-patient variability.
Exosomes can carry bioactive molecules that contribute to inflammation and oxidative stress in blood vessels, ultimately leading to vascular damage. These elevated levels may be associated with diabetes-related complications and may serve as potential biomarkers for the disease. However, over the last few decades, a major limitation in clinical applications has been the drawbacks associated with conventional exosome isolation methods. This challenge has prompted efforts to create emerging separation platforms. Integration of emerging technologies such as microfluidics, electrical, centrifugal, and acoustical forces into exosome isolation technologies has advanced significantly and become more sophisticated. Microfluidic devices tailored for exosome isolation and purification are anticipated to be promising tools for early detection and biomedical applications [43].
Despite the notable progress, it is evident that current separation methods are not without their flaws. Understanding the role of exosomes in diabetes-related pathophysiology may open up new avenues for therapeutic interventions. Recent studies explore the use of exosomes for targeted drug delivery and regenerative medicine approaches. For example, mesenchymal stem cell exosomes contain growth factors and cytokines that can stimulate cell proliferation and tissue repair, promoting the regeneration of T2D damaged tissues [95].
Microfluidics-based techniques for the isolation of EVs [43] have gained significant attention due to their unique advantages, including the ability to combine EV isolation and disease detection on a single platform. Microfluidic-based systems harbor many advantages such as portability, small starting volume, fast isolation, and cost-efficiency. EVs can be separated from smaller cellular debris, proteins, and other particles using microfluidic nanowire and micropillar structures [96].
Future research should focus on understanding the biogenesis, cargo, and functions of EVs as it is crucial for unravelling their roles in cellular communication and their impact on health and disease. In addition to achieving high-purity exosome isolation, the development of more integrated, high-throughput, and high-recovery-rate devices will open up promising avenues for advances in exosome-based diagnostics and biomedical applications in the years to come. In addition, liquid biopsy combined with machine learning and AI will enable the practice of precision medicine by identifying the specific molecular characteristics of T2D patients to decipher the risk for complication development as well as helping in selecting targeted therapies that are most likely to be effective for a particular patient, minimizing unnecessary side effects.
In routine clinical practice, variations in individual responses to treatment may arise, and reliable indicators predicting glycemic response and the likelihood of adverse events in a given patient are currently lacking. The need to identify markers relevant for the patient drug response should be taken into account in the field of molecular diagnostic. For example, SGLT2 inhibitors, a novel category of antihyperglycemic medications, operate within the proximal tubules of the kidney. Various mutations within the SLC5A2 (solute carrier family 5 member 2) gene, which encode SGLT2, have been associated with familial renal glucosuria, characterized by unusually elevated urinary glucose excretion even in the presence of normal blood glucose levels. These mutations can affect SGLT2 expression, membrane localization, or transporter function. Alongside rare missense mutations, common genetic variants in the SLC5A2 gene have been documented, potentially influencing glucose homeostasis and playing a role in the risk of developing T2D as well as affecting the response to SGLT2 inhibitor treatments [97].
Another target for molecular detection in T2D patients could be represented by peroxisome proliferator-activated receptors (PPARs), which are fatty acid-activated transcription factors of nuclear hormone receptor superfamily that are well-established regulators of lipid metabolism, mitochondrial biogenesis, and energy homeostasis. Their activation has central implications in the function of oxidative tissues and organs such as cardiomyocytes, liver, and muscle. These receptors are important in the context of understanding the molecular mechanisms driving diabetic cardiomyopathy. Drugs such as pemafibrate, metformin, and GLP-1 agonists, classified as PPARα-related drugs, have exhibited effectiveness and safety in clinical studies by reducing lipid and glucose levels in patients with diabetes [98]. Hence, analyzing these receptors as liquid biopsy markers could aid in the personalized treatment of T2D patients at risk of developing cardiomyopathy.
There is an urgent need for more interventional clinical trials to facilitate the widespread adoption of liquid biopsy in clinical practice. For this, the standardization of preanalytical and analytical methods across multiple centers is crucial before liquid biopsy can be reliably incorporated into clinical settings. Policymakers and business leaders should actively engage in these clinical trials and discussions to inform national and international decisions. Existing international consortia (i.e., European Liquid Biopsy Society, BLOODPAC) are already spearheading significant initiatives to accomplish this mission, working towards the development and validation of a comprehensive set of standard operating procedures.
5. Conclusions
T2D has significant clinical implications, not only for glucose regulation, but also for overall metabolic and cardiovascular health. Understanding the underlying mechanisms of T2D is essential for developing effective treatments and interventions that address the causes of the disease, beyond managing symptoms.
Currently, there is an imperative need for the development of minimally invasive biomarkers, which aligns with patient-centered care, focusing on early detection, personalized treatment, and improved quality of life for individuals with T2D.
Collaboration across academia, the biotechnology and pharmaceutical industries, and various stakeholders is essential for advancing the field. We foresee that precision medicine strategies, which pinpoint high-risk patient populations and predict specific therapeutic benefits, will play a pivotal role in this progression.
Finally, the cost-effectiveness of traditional diagnostic tools versus innovative liquid biopsy biomarkers can vary depending on several factors, including the specific medical condition being diagnosed, the stage of disease, and the overall healthcare infrastructure. Adoption of innovative diagnostic tools can come with additional costs for the healthcare system. The regulatory landscape and the degree of adoption by healthcare professionals can influence the cost-effectiveness of diagnostic tools. Newly developed technologies may face higher initial costs due to research and development investments. The extent of insurance coverage for different diagnostic methods can play a crucial role in their cost-effectiveness for both healthcare providers and patients.
Hence, the cost-effectiveness of traditional diagnostic tools versus liquid biopsy biomarkers is a complex and dynamic issue. It often involves a trade-off between upfront costs, invasiveness, speed of results, and long-term outcomes. As technology advances and more evidence becomes available, the landscape may shift, and the choice between traditional and innovative diagnostic methods may become more nuanced.
Author Contributions
Conceptualization—G.G.P., M.M. and V.E., literature search, writing, review and editing—G.G.P., literature search, writing, review and data curation—V.E. and M.M., funding acquisition C.I. All authors have read and agreed to the published version of the manuscript.
Funding
G.G.P., M.M. and C.I. acknowledge the support from the European Union’s Horizon Europe framework program 2021–2027, under the Coordination and Support Actions, HORIZON-WIDERA-2022-TALENTS-01 (grant agreement—101087007—eBio-hub). Funded by the European Union. Views and opinions expressed are those of the authors only and do not necessarily reflect those of the European Union or European Research Executive Agency (REA). Neither the European Union nor the granting authority can be held responsible for them. G.G.P., M.M. and C.I. also acknowledge the support of project PN-IV-P8-8.1-PRE-HE-ORG-2023-0054.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid biopsy enters the clinic—Implementation issues and future challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef]
- Iliescu, F.S.; Poenar, D.P.; Yu, F.; Ni, M.; Chan, K.H.; Cima, I.; Taylor, H.K.; Cima, I.; Iliescu, C. Recent advances in microfluidic methods in cancer liquid biopsy. Biomicrofluidics 2019, 13, 041503. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi Naei, V.; Bordhan, P.; Mirakhorli, F.; Khorrami, M.; Shrestha, J.; Nazari, H.; Kulasinghe, A.; Ebrahimi Warkiani, M. Advances in novel strategies for isolation, characterization, and analysis of CTCs and ctDNA. Ther. Adv. Med. Oncol. 2023, 15, 17588359231192401. [Google Scholar] [CrossRef] [PubMed]
- Armakolas, A.; Kotsari, M.; Koskinas, J. Liquid Biopsies, Novel Approaches and Future Directions. Cancers 2023, 15, 1579. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, R.; Soon, R.H.; Zhang, P.; Jiang, K.; Lim, C.T. Cancer diagnosis: From tumor to liquid biopsy and beyond. Lab Chip 2019, 19, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Cima, I.; Wen Yee, C.; Iliescu, F.S.; Min Phyo, W.; Hon Lim, K.; Iliescu, C.; Han Tan, M. Label-free isolation of circulating tumor cells in microfluidic devices: Current research and perspectives. Biomicrofluidics 2013, 7, 011810. [Google Scholar] [CrossRef] [PubMed]
- Arosemena, M.; Meah, F.A.; Mather, K.J.; Tersey, S.A.; Mirmira, R.G. Cell-free DNA fragments as biomarkers of islet β-cell death in obesity and type 2 diabetes. Int. J. Mol. Sci. 2021, 22, 2151. [Google Scholar] [CrossRef] [PubMed]
- Jahr, S.; Hentze, H.; Englisch, S.; Hardt, D.; Fackelmayer, F.O.; Hesch, R.-D.; Knippers, R. DNA fragments in the blood plasma of cancer patients: Quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001, 61, 1659–1665. [Google Scholar]
- Khagi, Y.; Goodman, A.M.; Daniels, G.A.; Patel, S.P.; Sacco, A.G.; Randall, J.M.; Bazhenova, L.A.; Kurzrock, R. Hypermutated circulating tumor DNA: Correlation with response to checkpoint inhibitor–based immunotherapy. Clin. Cancer Res. 2017, 23, 5729–5736. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, C.W.; Shao, Y.; Wang, H.T.; Wu, Y.F.; Song, Y.Y.; Li, X.B.; Zhang, Z.; Wang, W.J.; Li, L.Q.; et al. Comparison of droplet digital PCR and conventional quantitative PCR for measuring EGFR gene mutation. Exp. Ther. Med. 2015, 9, 1383–1388. [Google Scholar] [CrossRef]
- Imperial, R.; Nazer, M.; Ahmed, Z.; Kam, A.E.; Pluard, T.J.; Bahaj, W.; Levy, M.; Kuzel, T.M.; Hayden, D.M.; Pappas, S.G.; et al. Matched whole-genome sequencing (WGS) and whole-exome sequencing (WES) of tumor tissue with circulating tumor DNA (ctDNA) analysis: Complementary modalities in clinical practice. Cancers 2019, 11, 1399. [Google Scholar] [CrossRef]
- Forshew, T.; Murtaza, M.; Parkinson, C.; Gale, D.; Tsui, D.W.; Kaper, F.; Dawson, S.-J.; Piskorz, A.M.; Jimenez-Linan, M.; Bentley, D.; et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci. Transl. Med. 2012, 4, 136ra168. [Google Scholar] [CrossRef]
- Akirav, E.M.; Lebastchi, J.; Galvan, E.M.; Henegariu, O.; Akirav, M.; Ablamunits, V.; Lizardi, P.M.; Herold, K.C. Detection of β cell death in diabetes using differentially methylated circulating DNA. Proc. Natl. Acad. Sci. USA 2011, 108, 19018–19023. [Google Scholar] [CrossRef]
- El Tarhouny, S.A.; Hadhoud, K.M.; Ebrahem, M.M.; Al Azizi, N.M. Assessment of cell-free DNA with microvascular complication of type II diabetes mellitus, using PCR and ELISA. Nucleosides Nucleotides Nucleic Acids 2010, 29, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, H.-D.; Wu, F.-X.; Wang, J. Identifying the tissues-of-origin of circulating cell-free DNAs is a promising way in noninvasive diagnostics. Brief. Bioinform. 2021, 22, bbaa060. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Fukuda, D.; Higashikuni, Y.; Tanaka, K.; Hirata, Y.; Murata, C.; Kim-Kaneyama, J.-R.; Sato, F.; Bando, M.; Yagi, S. Obesity-induced DNA released from adipocytes stimulates chronic adipose tissue inflammation and insulin resistance. Sci. Adv. 2016, 2, e1501332. [Google Scholar] [CrossRef] [PubMed]
- Lehmann-Werman, R.; Neiman, D.; Zemmour, H.; Moss, J.; Magenheim, J.; Vaknin-Dembinsky, A.; Rubertsson, S.; Nellgård, B.; Blennow, K.; Zetterberg, H. Identification of tissue-specific cell death using methylation patterns of circulating DNA. Proc. Natl. Acad. Sci. USA 2016, 113, E1826–E1834. [Google Scholar] [CrossRef]
- Karaglani, M.; Panagopoulou, M.; Cheimonidi, C.; Tsamardinos, I.; Maltezos, E.; Papanas, N.; Papazoglou, D.; Mastorakos, G.; Chatzaki, E. Liquid Biopsy in Type 2 Diabetes Mellitus Management: Building Specific Biosignatures via Machine Learning. J. Clin. Med. 2022, 11, 1045. [Google Scholar] [CrossRef]
- Birinci, A.; Birinci, H.; Abidinoglu, R.; Durupinar, B.; Oge, I. Diabetic retinopathy and HLA antigens in type 2 diabetes mellitus. Eur. J. Ophthalmol. 2002, 12, 89–93. [Google Scholar] [CrossRef]
- Ma, Z.J.; Sun, P.; Guo, G.; Zhang, R.; Chen, L.M. Association of the HLA-DQA1 and HLA-DQB1 Alleles in Type 2 Diabetes Mellitus and Diabetic Nephropathy in the Han Ethnicity of China. J. Diabetes Res. 2013, 2013, 452537. [Google Scholar] [CrossRef] [PubMed]
- Trumpff, C.; Michelson, J.; Lagranha, C.J.; Taleon, V.; Karan, K.R.; Sturm, G.; Lindqvist, D.; Fernström, J.; Moser, D.; Kaufman, B.A.; et al. Stress and circulating cell-free mitochondrial DNA: A systematic review of human studies, physiological considerations, and technical recommendations. Mitochondrion 2021, 59, 225–245. [Google Scholar] [CrossRef]
- Liu, J.; Zou, Y.; Tang, Y.; Xi, M.; Xie, L.; Zhang, Q.; Gong, J. Circulating cell-free mitochondrial deoxyribonucleic acid is increased in coronary heart disease patients with diabetes mellitus. J. Diabetes Investig. 2016, 7, 109–114. [Google Scholar] [CrossRef]
- Silzer, T.; Barber, R.; Sun, J.; Pathak, G.; Johnson, L.; O’Bryant, S.; Phillips, N. Circulating mitochondrial DNA: New indices of type 2 diabetes-related cognitive impairment in Mexican Americans. PLoS ONE 2019, 14, e0213527. [Google Scholar] [CrossRef] [PubMed]
- Umu, S.U.; Langseth, H.; Bucher-Johannessen, C.; Fromm, B.; Keller, A.; Meese, E.; Lauritzen, M.; Leithaug, M.; Lyle, R.; Rounge, T.B. A comprehensive profile of circulating RNAs in human serum. RNA Biol. 2018, 15, 242–250. [Google Scholar] [CrossRef]
- Guruprasad, P.; Lee, Y.G.; Kim, K.H.; Ruella, M. The current landscape of single-cell transcriptomics for cancer immunotherapy. J. Exp. Med. 2020, 218, e20201574. [Google Scholar] [CrossRef]
- Alvarez, M.L.; DiStefano, J.K. The role of non-coding RNAs in diabetic nephropathy: Potential applications as biomarkers for disease development and progression. Diabetes Res. Clin. Pract. 2013, 99, 1–11. [Google Scholar] [CrossRef]
- Erener, S.; Mojibian, M.; Fox, J.K.; Denroche, H.C.; Kieffer, T.J. Circulating miR-375 as a biomarker of β-cell death and diabetes in mice. Endocrinology 2013, 154, 603–608. [Google Scholar] [CrossRef]
- Higuchi, C.; Nakatsuka, A.; Eguchi, J.; Teshigawara, S.; Kanzaki, M.; Katayama, A.; Yamaguchi, S.; Takahashi, N.; Murakami, K.; Ogawa, D. Identification of circulating miR-101, miR-375 and miR-802 as biomarkers for type 2 diabetes. Metabolism 2015, 64, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Skroblin, P.; Moschen, A.R.; Yin, X.; Kaudewitz, D.; Zampetaki, A.; Barwari, T.; Whitehead, M.; Ramírez, C.M.; Goedeke, L. Circulating microRNA-122 is associated with the risk of new-onset metabolic syndrome and type 2 diabetes. Diabetes 2017, 66, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Pastukh, N.; Meerson, A.; Kalish, D.; Jabaly, H.; Blum, A. Serum miR-122 levels correlate with diabetic retinopathy. Clin. Exp. Med. 2019, 19, 255–260. [Google Scholar] [CrossRef]
- Turchinovich, A.; Baranova, A.; Drapkina, O.; Tonevitsky, A. Cell-free circulating nucleic acids as early biomarkers for NAFLD and NAFLD-associated disorders. Front. Physiol. 2018, 9, 1256. [Google Scholar] [CrossRef]
- Long, J.; Badal, S.S.; Ye, Z.; Wang, Y.; Ayanga, B.A.; Galvan, D.L.; Green, N.H.; Chang, B.H.; Overbeek, P.A.; Danesh, F.R. Long noncoding RNA Tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. J. Clin. Investig. 2016, 126, 4205–4218. [Google Scholar] [CrossRef]
- Carter, G.; Miladinovic, B.; Patel, A.A.; Deland, L.; Mastorides, S.; Patel, N.A. Circulating long noncoding RNA GAS5 levels are correlated to prevalence of type 2 diabetes mellitus. BBA Clin. 2015, 4, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.-Z.; Tian, W.; Fu, H.-T.; Li, C.-P.; Liu, B. Long noncoding RNA-MEG3 is involved in diabetes mellitus-related microvascular dysfunction. Biochem. Biophys. Res. Commun. 2016, 471, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, L.; Cao, C.; Lu, C.; Lian, W.; Han, J.; Zhang, X.; Zhang, J.; Tang, T.; Li, M. Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Exp. Cell Res. 2017, 350, 327–335. [Google Scholar] [CrossRef]
- Wang, M.; Wang, S.; Yao, D.; Yan, Q.; Lu, W. A novel long non-coding RNA CYP4B1-PS1-001 regulates proliferation and fibrosis in diabetic nephropathy. Mol. Cell. Endocrinol. 2016, 426, 136–145. [Google Scholar] [CrossRef]
- Chalasani, N.; Toden, S.; Sninsky, J.J.; Rava, R.P.; Braun, J.V.; Gawrieh, S.; Zhuang, J.; Nerenberg, M.; Quake, S.R.; Maddala, T. Noninvasive stratification of nonalcoholic fatty liver disease by whole transcriptome cell-free mRNA characterization. Am. J. Physiol.-Gastrointest. Liver Physiol. 2021, 320, G439–G449. [Google Scholar] [CrossRef]
- Fierabracci, A.; Del Fattore, A.; Muraca, M.; Vittorio Delfino, D.; Muraca, M. The use of mesenchymal stem cells for the treatment of autoimmunity: From animals models to human disease. Curr. Drug Targets 2016, 17, 229–238. [Google Scholar] [CrossRef]
- Rupert, D.L.; Claudio, V.; Lässer, C.; Bally, M. Methods for the physical characterization and quantification of extracellular vesicles in biological samples. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 3164–3179. [Google Scholar] [CrossRef] [PubMed]
- La Marca, V.; Fierabracci, A. Insights into the diagnostic potential of extracellular vesicles and their miRNA signature from liquid biopsy as early biomarkers of diabetic micro/macrovascular complications. Int. J. Mol. Sci. 2017, 18, 1974. [Google Scholar] [CrossRef] [PubMed]
- Akbar, N.; Azzimato, V.; Choudhury, R.P.; Aouadi, M. Extracellular vesicles in metabolic disease. Diabetologia 2019, 62, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, F.S.; Vrtačnik, D.; Neuzil, P.; Iliescu, C. Microfluidic technology for clinical applications of exosomes. Micromachines 2019, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yu, Z.; Chen, D.; Wang, Z.; Miao, J.; Li, Q.; Zhang, D.; Song, J.; Cui, D. Progress in microfluidics-based exosome separation and detection technologies for diagnostic applications. Small 2020, 16, 1903916. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, P.; Zhang, T.; Xu, Z.; Huang, X.; Wang, R.; Du, L. Review on strategies and technologies for exosome isolation and purification. Front. Bioeng. Biotechnol. 2022, 9, 811971. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.L.; Deng, J.T.; Guan, G.J.; Chen, S.H.; Liu, Y.T.; Cheng, J.; Li, Z.W.; Zhuang, X.H.; Sun, F.D.; Deng, H.P. Dipeptidyl peptidase-IV is a potential molecular biomarker in diabetic kidney disease. Diabetes Vasc. Dis. Res. 2012, 9, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Sakurai, A.; Ono, H.; Hayashi, S.; Yoshimoto, S.; Ochi, A.; Ueda, S.; Nishimura, K.; Shibata, E.; Tamaki, M. Urinary exosomal mRNA of WT1 as diagnostic and prognostic biomarker for diabetic nephropathy. J. Med. Investig. 2018, 65, 208–215. [Google Scholar] [CrossRef]
- Sun, Y.; Tao, Q.; Wu, X.; Zhang, L.; Liu, Q.; Wang, L. The utility of exosomes in diagnosis and therapy of diabetes mellitus and associated complications. Front. Endocrinol. 2021, 12, 756581. [Google Scholar] [CrossRef]
- Freeman, D.W.; Noren Hooten, N.; Eitan, E.; Green, J.; Mode, N.A.; Bodogai, M.; Zhang, Y.; Lehrmann, E.; Zonderman, A.B.; Biragyn, A. Altered extracellular vesicle concentration, cargo, and function in diabetes. Diabetes 2018, 67, 2377–2388. [Google Scholar] [CrossRef]
- Li, S.; Wei, J.; Zhang, C.; Li, X.; Meng, W.; Mo, X.; Zhang, Q.; Liu, Q.; Ren, K.; Du, R. Cell-derived microparticles in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Cell. Physiol. Biochem. 2016, 39, 2439–2450. [Google Scholar] [CrossRef]
- Kalucka, J.; Bierhansl, L.; Wielockx, B.; Carmeliet, P.; Eelen, G. Interaction of endothelial cells with macrophages—Linking molecular and metabolic signaling. Pflügers Arch.-Eur. J. Physiol. 2017, 469, 473–483. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Qu, D.; Wang, L.; Wong, C.M.; Lau, C.-W.; Huang, Y.; Wang, Y.F.; Huang, H.; Xia, Y. Serum exosomes mediate delivery of arginase 1 as a novel mechanism for endothelial dysfunction in diabetes. Proc. Natl. Acad. Sci. USA 2018, 115, E6927–E6936. [Google Scholar] [CrossRef]
- Wang, F.; Chen, F.-f.; Shang, Y.-y.; Li, Y.; Wang, Z.-h.; Han, L.; Li, Y.-h.; Zhang, L.; Ti, Y.; Zhang, W. Insulin resistance adipocyte-derived exosomes aggravate atherosclerosis by increasing vasa vasorum angiogenesis in diabetic ApoE−/− mice. Int. J. Cardiol. 2018, 265, 181–187. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Schwartz, J.B.; Mustapic, M.; Lobach, I.V.; Daneman, R.; Abner, E.L.; Jicha, G.A. Altered cargo proteins of human plasma endothelial cell–derived exosomes in atherosclerotic cerebrovascular disease. FASEB J. 2017, 31, 3689. [Google Scholar] [CrossRef]
- Shin, H.-S.; Gedi, V.; Kim, J.-K.; Lee, D.-K. Detection of Gram-negative bacterial outer membrane vesicles using DNA aptamers. Sci. Rep. 2019, 9, 13167. [Google Scholar] [CrossRef] [PubMed]
- Macia, L.; Nanan, R.; Hosseini-Beheshti, E.; Grau, G.E. Host-and microbiota-derived extracellular vesicles, immune function, and disease development. Int. J. Mol. Sci. 2019, 21, 107. [Google Scholar] [CrossRef] [PubMed]
- Hickey, C.A.; Kuhn, K.A.; Donermeyer, D.L.; Porter, N.T.; Jin, C.; Cameron, E.A.; Jung, H.; Kaiko, G.E.; Wegorzewska, M.; Malvin, N.P. Colitogenic Bacteroides thetaiotaomicron antigens access host immune cells in a sulfatase-dependent manner via outer membrane vesicles. Cell Host Microbe 2015, 17, 672–680. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.-K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, F.; Shahriary, A.; Behrouzi, A.; Moradi, H.R.; Keshavarz Azizi Raftar, S.; Lari, A.; Hadifar, S.; Yaghoubfar, R.; Ahmadi Badi, S.; Khatami, S. Akkermansia muciniphila-derived extracellular vesicles as a mucosal delivery vector for amelioration of obesity in mice. Front. Microbiol. 2019, 10, 2155. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kwon, Y.; Kim, D.-K.; Jeon, J.; Jang, S.C.; Wang, T.; Ban, M.; Kim, M.-H.; Jeon, S.G.; Kim, M.-S. Gut microbe-derived extracellular vesicles induce insulin resistance, thereby impairing glucose metabolism in skeletal muscle. Sci. Rep. 2015, 5, 15878. [Google Scholar] [CrossRef] [PubMed]
- Kameli, N.; Borman, R.; López-Iglesias, C.; Savelkoul, P.; Stassen, F.R. Characterization of feces-derived bacterial membrane vesicles and the impact of their origin on the inflammatory response. Front. Cell. Infect. Microbiol. 2021, 11, 667987. [Google Scholar] [CrossRef]
- Dorcely, B.; Katz, K.; Jagannathan, R.; Chiang, S.S.; Oluwadare, B.; Goldberg, I.J.; Bergman, M. Novel biomarkers for prediabetes, diabetes, and associated complications. Diabetes Metab. Syndr. Obes. 2017, 10, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Moellering, D.R.; Garvey, W.T. Use of HbA1c for diagnoses of diabetes and prediabetes: Comparison with diagnoses based on fasting and 2-hr glucose values and effects of gender, race, and age. Metab. Syndr. Relat. Disord. 2014, 12, 258–268. [Google Scholar] [CrossRef]
- Katulanda, G.W.; Katulanda, P.; Dematapitiya, C.; Dissanayake, H.A.; Wijeratne, S.; Sheriff, M.H.R.; Matthews, D.R. Plasma glucose in screening for diabetes and pre-diabetes: How much is too much? Analysis of fasting plasma glucose and oral glucose tolerance test in Sri Lankans. BMC Endocr Disord 2019, 19, 11. [Google Scholar] [CrossRef]
- Kaur, G.; Lakshmi, P.V.M.; Rastogi, A.; Bhansali, A.; Jain, S.; Teerawattananon, Y.; Bano, H.; Prinja, S. Diagnostic accuracy of tests for type 2 diabetes and prediabetes: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0242415. [Google Scholar] [CrossRef] [PubMed]
- Sumner, A.E.; Duong, M.T.; Aldana, P.C.; Ricks, M.; Tulloch-Reid, M.K.; Lozier, J.N.; Chung, S.T.; Sacks, D.B. A1C combined with glycated albumin improves detection of prediabetes in Africans: The Africans in America Study. Diabetes Care 2016, 39, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Furusyo, N.; Koga, T.; Ai, M.; Otokozawa, S.; Kohzuma, T.; Ikezaki, H.; Schaefer, E.J.; Hayashi, J. Utility of glycated albumin for the diagnosis of diabetes mellitus in a Japanese population study: Results from the Kyushu and Okinawa Population Study (KOPS). Diabetologia 2011, 54, 3028–3036. [Google Scholar] [CrossRef]
- Selvin, E.; Rawlings, A.M.; Grams, M.; Klein, R.; Sharrett, A.R.; Steffes, M.; Coresh, J. Prognostic utility of fructosamine and glycated albumin for incident diabetes and microvascular complications. Lancet Diabetes Endocrinol. 2014, 2, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Francis, L.M.; Ballantyne, C.M.; Hoogeveen, R.C.; Coresh, J.; Brancati, F.L.; Steffes, M.W. Nontraditional markers of glycemia: Associations with microvascular conditions. Diabetes Care 2011, 34, 960–967. [Google Scholar] [CrossRef]
- Malmström, H.; Walldius, G.; Grill, V.; Jungner, I.; Gudbjörnsdottir, S.; Hammar, N. Fructosamine is a useful indicator of hyperglycaemia and glucose control in clinical and epidemiological studies--cross-sectional and longitudinal experience from the AMORIS cohort. PLoS ONE 2014, 9, e111463. [Google Scholar] [CrossRef]
- Ying, L.; He, X.; Ma, X.; Shen, Y.; Su, H.; Peng, J.; Wang, Y.; Bao, Y.; Zhou, J.; Jia, W. Serum 1,5-anhydroglucitol when used with fasting plasma glucose improves the efficiency of diabetes screening in a Chinese population. Sci. Rep. 2017, 7, 11968. [Google Scholar] [CrossRef]
- Liang, M.; McEvoy, J.W.; Chen, Y.; Sharrett, A.R.; Selvin, E. Association of a Biomarker of Glucose Peaks, 1,5-Anhydroglucitol With Subclinical Cardiovascular Disease. Diabetes Care 2016, 39, 1752–1759. [Google Scholar] [CrossRef]
- Chan, C.L.; Pyle, L.; Kelsey, M.; Newnes, L.; Zeitler, P.S.; Nadeau, K.J. Screening for type 2 diabetes and prediabetes in obese youth: Evaluating alternate markers of glycemia-1,5-anhydroglucitol, fructosamine, and glycated albumin. Pediatr. Diabetes 2016, 17, 206–211. [Google Scholar] [CrossRef]
- Jiang, Y.; Owei, I.; Wan, J.; Ebenibo, S.; Dagogo-Jack, S. Adiponectin levels predict prediabetes risk: The Pathobiology of Prediabetes in A Biracial Cohort (POP-ABC) study. BMJ Open Diabetes Res. Care 2016, 4, e000194. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef]
- Festa, A.; D’Agostino, R., Jr.; Mykkänen, L.; Tracy, R.P.; Zaccaro, D.J.; Hales, C.N.; Haffner, S.M. Relative contribution of insulin and its precursors to fibrinogen and PAI-1 in a large population with different states of glucose tolerance. The Insulin Resistance Atherosclerosis Study (IRAS). Arterioscler. Thromb. Vasc. Biol. 1999, 19, 562–568. [Google Scholar] [CrossRef]
- Thorand, B.; Kolb, H.; Baumert, J.; Koenig, W.; Chambless, L.; Meisinger, C.; Illig, T.; Martin, S.; Herder, C. Elevated levels of interleukin-18 predict the development of type 2 diabetes: Results from the MONICA/KORA Augsburg Study, 1984–2002. Diabetes 2005, 54, 2932–2938. [Google Scholar] [CrossRef]
- Zaharieva, E.; Kamenov, Z.; Velikova, T.; Tsakova, A.; El-Darawish, Y.; Okamura, H. Interleukin-18 serum level is elevated in type 2 diabetes and latent autoimmune diabetes. Endocr. Connect. 2018, 7, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Watkins, S.M.; Lorenzo, C.; Wagenknecht, L.E.; Il’yasova, D.; Chen, Y.D.I.; Haffner, S.M.; Hanley, A.J. Branched-Chain Amino Acids and Insulin Metabolism: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2016, 39, 582–588. [Google Scholar] [CrossRef]
- Stefan, N.; Sun, Q.; Fritsche, A.; Machann, J.; Schick, F.; Gerst, F.; Jeppesen, C.; Joost, H.G.; Hu, F.B.; Boeing, H.; et al. Impact of the adipokine adiponectin and the hepatokine fetuin-A on the development of type 2 diabetes: Prospective cohort- and cross-sectional phenotyping studies. PLoS ONE 2014, 9, e92238. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Lee, Y.H.; Lee, S.G. Alanine to glycine ratio is a novel predictive biomarker for type 2 diabetes mellitus. Diabetes Obes. Metab. 2023, 26, 980–988. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, G.; Yang, C.; Zhou, K.; Shen, B.; Liang, H.; Jiang, X. The role of circulating microRNA-126 (miR-126): A novel biomarker for screening prediabetes and newly diagnosed type 2 diabetes mellitus. Int. J. Mol. Sci. 2014, 15, 10567–10577. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Liang, Y.; Hu, R.; Xu, W.; Liu, Y. Plasma Targeted Metabolomics Analysis for Amino Acids and Acylcarnitines in Patients with Prediabetes, Type 2 Diabetes Mellitus, and Diabetic Vascular Complications. Diabetes Metab. J. 2021, 45, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Loo, J.A.; Yan, W.; Ramachandran, P.; Wong, D.T. Comparative human salivary and plasma proteomes. J. Dent. Res. 2010, 89, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Blackburn, C.; Mohamed, M.; Sivagami, A.V.; Blum, J. Literature-based discovery of salivary biomarkers for type 2 diabetes mellitus. Biomark Insights 2015, 10, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.C.R.; da Silva, J.R.; Lima, C.P.V.; Stefani, C.M.; Damé-Teixeira, N. Salivary parameters of adults with diabetes mellitus: A systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Joshipura, K.; Vergara, J.L.; Wong, D.T. Detection of Type II Diabetes Mellitus Using Salivary Transcriptomic Biomarkers. Genom. Med. Biomark. Health Sci. 2012, 4, 7–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Li, F.; Grogan, T.R.; Vergara, J.L.; Luan, Q.; Park, M.S.; Chia, D.; Elashoff, D.; Joshipura, K.J.; et al. Salivary extracellular RNA biomarkers for insulin resistance detection in hispanics. Diabetes Res. Clin. Pract. 2017, 132, 85–94. [Google Scholar] [CrossRef]
- Han, Q.; Wang, X.; Ding, X.; Hao, J.; Li, Q.; Wang, J.; Yu, H.; Tang, Z.; Yang, F.; Cai, G.; et al. Salivary Glycopatterns as Potential Non-Invasive Biomarkers for Diagnosing and Reflecting Severity and Prognosis of Diabetic Nephropathy. Front. Endocrinol. 2022, 13, 790586. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, M.A.; Sadaf, A.; Younus, H. A structural study on the protection of glycation of superoxide dismutase by thymoquinone. Int. J. Biol. Macromol. 2014, 69, 476–481. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Anwar, S.; Raut, R.; Almatroudi, A.; Babiker, A.Y.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A. Therapeutic Potential of Myrrh, a Natural Resin, in Health Management through Modulation of Oxidative Stress, Inflammation, and Advanced Glycation End Products Formation Using In Vitro and In Silico Analysis. Appl. Sci. 2022, 12, 9175. [Google Scholar] [CrossRef]
- Fejfer, K.; Buczko, P.; Niczyporuk, M.; Ładny, J.R.; Hady, H.R.; Knaś, M.; Waszkiel, D.; Klimiuk, A.; Zalewska, A.; Maciejczyk, M. Oxidative modification of biomolecules in the nonstimulated and stimulated saliva of patients with morbid obesity treated with bariatric surgery. Biomed. Res. Int. 2017, 2017, 4923769. [Google Scholar] [CrossRef] [PubMed]
- Tóthová, L.U.; Kamodyova, N.; Červenka, T.; Celec, P. Salivary markers of oxidative stress in oral diseases. Front. Cell Infect. Microbiol. 2015, 5, 73. [Google Scholar] [CrossRef]
- Mather, K.A.; Thalamuthu, A. Unraveling the genetic contributions to complex traits across different ethnic groups. Nat. Med. 2020, 26, 467–469. [Google Scholar] [CrossRef]
- Xiong, J.; Hu, H.; Guo, R.; Wang, H.; Jiang, H. Mesenchymal stem cell exosomes as a new strategy for the treatment of diabetes complications. Front. Endocrinol. 2021, 12, 646233. [Google Scholar] [CrossRef]
- Yang, F.; Liao, X.; Tian, Y.; Li, G. Exosome separation using microfluidic systems: Size-based, immunoaffinity-based and dynamic methodologies. Biotechnol. J. 2017, 12, 1600699. [Google Scholar] [CrossRef]
- Klen, J.; Dolžan, V. Treatment Response to SGLT2 Inhibitors: From Clinical Characteristics to Genetic Variations. Int. J. Mol. Sci. 2021, 22, 9800. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cai, Y.; Jian, L.; Cheung, C.W.; Zhang, L.; Xia, Z. Impact of peroxisome proliferator-activated receptor-α on diabetic cardiomyopathy. Cardiovasc. Diabetol. 2021, 20, 2. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).