The Auditory Pathway in Congenitally Cytomegalovirus-Infected Human Fetuses
Abstract
1. Introduction
2. Results
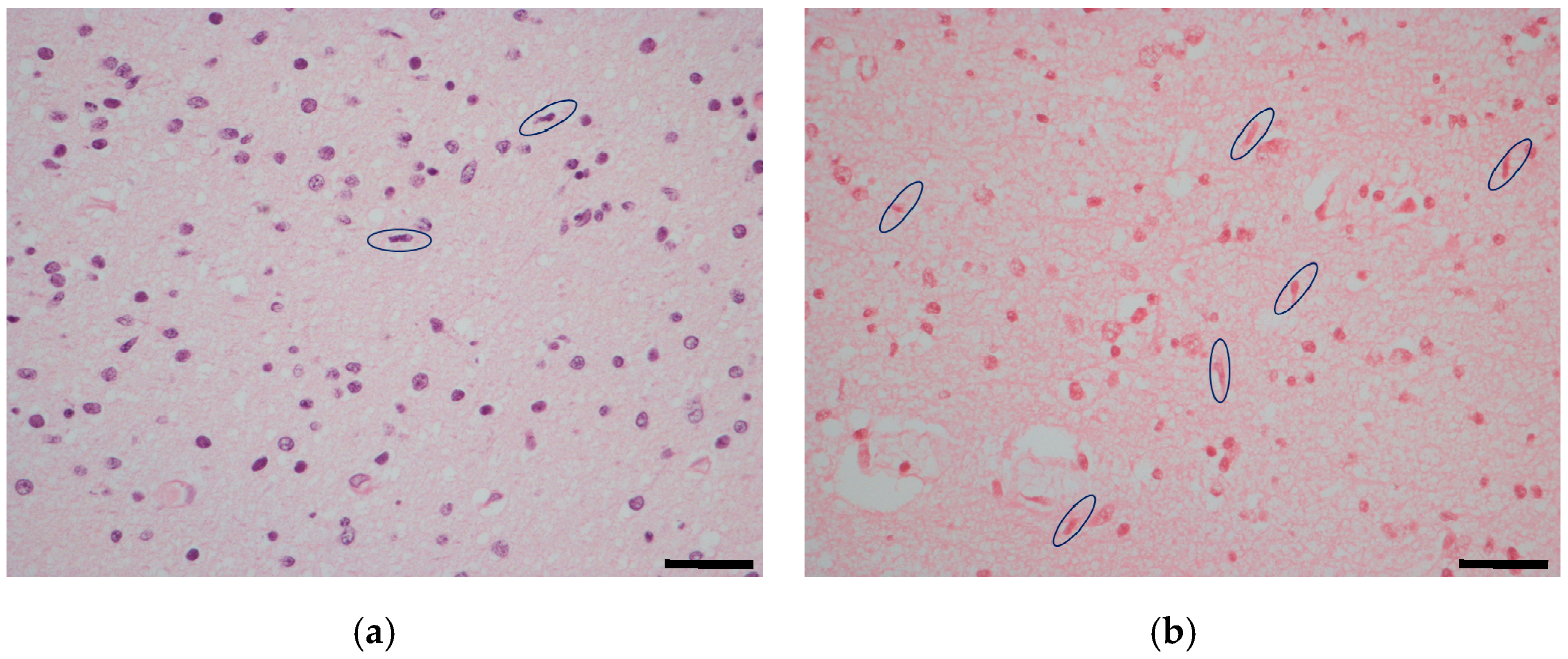
2.1. Temporal Lobe
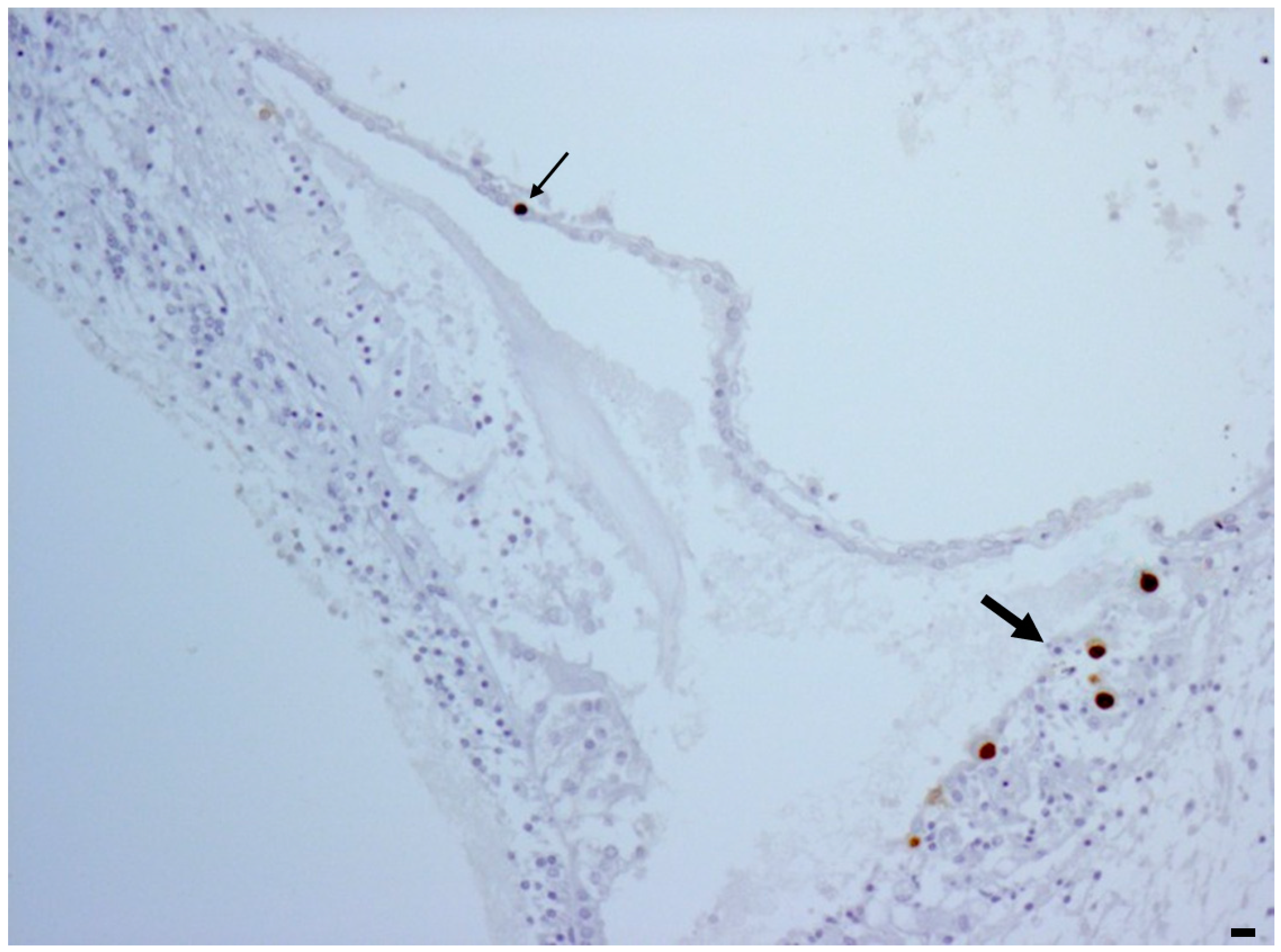
2.2. Inner Ear
3. Discussion
4. Materials and Methods
- Tissue necrosis: 0 = absent; 1 = focal; 2 = diffuse;
- Microglial Nodules: 0 = absent; 1 = occasional; 2 = scattered; 3 = multiple.
- Microglial activation: 0 = absent; 1 = focal; 2 = diffuse;
- Astrocytosis:0 = absent; 1 = focal with ramified astrocytes; 2 = diffuse with ramified astrocytes; 3 = 1 or 2 plus astrocyte nuclear clearing—Azheimer’s type II astrocytes—and/or apoptosis;
- Vascular changes: 0 = no changes; 1 = focal increased number of vessels; 2 = diffuse increased number of vessels, 3 = 1 or 2 plus plump endothelial cells.
Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rivera, L.B.; Boppana, S.B.; Fowler, K.B.; Britt, W.J.; Stagno, S.; Pass, R.F. Predictors of hearing loss in children with symptomatic congenital cytomegalovirus infection. Pediatrics 2002, 110, 762–767. [Google Scholar] [CrossRef]
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.J. Congenital cytomegalovirus (CMV) epidemiology and awareness. J. Clin. Virol. Off. Publ. Pan. Am. Soc. Clin. Virol. 2009, 46 (Suppl. 4), S6–S10. [Google Scholar] [CrossRef]
- Ross, S.A.; Kimberlin, D. Clinical outcome and the role of antivirals in congenital cytomegalovirus infection. Antiviral. Res. 2021, 191, 105083. [Google Scholar] [CrossRef]
- Korver, A.M.H.; de Vries, J.J.C.; Konings, S.; de Jong, J.W.; Dekker, F.W.; Vossen, A.C.T.M.; Frijns, J.H.M.; Oudesluys-Murphy, A.M.; the DECIBEL Collaborative Study Group. DECIBEL study: Congenital cytomegalovirus infection in young children with permanent bilateral hearing impairment in The Netherlands. J. Clin. Virol. 2009, 46 (Suppl. 4), S27–S31. [Google Scholar] [CrossRef]
- Goderis, J.; De Leenheer, E.; Smets, K.; Van Hoecke, H.; Keymeulen, A.; Dhooge, I. Hearing loss and congenital CMV infection: A systematic review. Pediatrics 2014, 134, 972–982. [Google Scholar] [CrossRef]
- Avettand-Fenoël, V.; Marlin, S.; Vauloup-Fellous, C.; Loundon, N.; François, M.; Couloigner, V.; Rouillon, I.; Drouin-Garraud, V.; Laccourreye, L.; Denoyelle, F.; et al. Congenital cytomegalovirus is the second most frequent cause of bilateral hearing loss in young French children. J. Pediatr. 2013, 162, 593–599. [Google Scholar] [CrossRef]
- Nance, W.E.; Lim, B.G.; Dodson, K.M. Importance of congenital cytomegalovirus infections as a cause for pre-lingual hearing loss. J. Clin. Virol. 2006, 35, 221–225. [Google Scholar] [CrossRef]
- Leruez-Ville, M.; Foulon, I.; Pass, R.; Ville, Y. Cytomegalovirus infection during pregnancy: State of the science. Am. J. Obstet. Gynecol. 2020, 223, 330–349. [Google Scholar] [CrossRef]
- Fowler, K.B.; Boppana, S.B. Congenital cytomegalovirus infection. Semin. Perinatol. 2018, 42, 149–154. [Google Scholar] [CrossRef]
- Cushing, S.L.; Purcell, P.L.; Papaiaonnou, V.; Neghandi, J.; Daien, M.; Blaser, S.I.; Ertl-Wagner, B.; Wagner, M.; Sheng, M.; James, A.L.; et al. Hearing Instability in Children with Congenital Cytomegalovirus: Evidence and Neural Consequences. Laryngoscope 2022, 132 (Suppl. 11), S1–S24. [Google Scholar] [CrossRef]
- Diogo, M.C.; Glatter, S.; Binder, J.; Kiss, H.; Prayer, D. The MRI spectrum of con-genital cyto-megalovirus infection. Prenat. Diagn. 2020, 40, 110–124. [Google Scholar] [CrossRef]
- Gabrielli, L.; Bonasoni, M.P.; Lazzarotto, T.; Lega, S.; Santini, D.; Foschini, M.P.; Guerra, B.; Baccolini, F.; Piccirilli, G.; Chiereghin, A.; et al. Histological findings in foetuses congenitally infected by cytomegalovirus. J. Clin. Virol. 2009, 46 (Suppl. 4), S16–S21. [Google Scholar] [CrossRef]
- Gabrielli, L.; Bonasoni, M.P.; Santini, D.; Piccirilli, G.; Chiereghin, A.; Petrisli, E.; Dolcetti, R.; Guerra, B.; Piccioli, M.; Lanari, M.; et al. Congenital cytomegalovirus infection: Patterns of fetal brain damage. Clin. Microbiol. Infect. 2012, 18, E419–E427. [Google Scholar] [CrossRef]
- Gabrielli, L.; Bonasoni, M.P.; Santini, D.; Piccirilli, G.; Chiereghin, A.; Guerra, B.; Landini, M.P.; Capretti, M.G.; Lanari, M.; Lazzarotto, T. Human fetal inner ear involvement in congenital cytomegalovirus infection. Acta Neuropathol. Commun. 2013, 1, 63. [Google Scholar] [CrossRef]
- Piccirilli, G.; Gabrielli, L.; Bonasoni, M.P.; Chiereghin, A.; Turello, G.; Borgatti, E.C.; Simonazzi, G.; Felici, S.; Leone, M.; Salfi, N.C.M.; et al. Fetal Brain Damage in Human Fetuses with Congenital Cytomegalovirus Infection: Histological Features and Viral Tropism. Cell Mol. Neurobiol. 2023, 43, 1385–1399. [Google Scholar] [CrossRef]
- Davis, L.E.; Johnsson, L.G.; Kornfeld, M. Cytomegalovirus labyrinthitis in an infant: Morphological, virological, and immunofluorescent studies. J. Neuropathol. Exp. Neurol. 1981, 40, 9–19. [Google Scholar] [CrossRef]
- Peña-Alonso, R.; Navarrete-Navarro, S.; Ramon-Garcia, G.; Hernandez-Mote, R.; Rodriguez-Jurado, R. Cytomegalovirus infection in children: Frequency, anatomopathologic characteristics and underlying risk factors in 1618 autopsies. Arch. Med. Res. 1996, 27, 25–30. [Google Scholar]
- Teissier, N.; Fallet-Bianco, C.; Delezoide, A.L.; Laquerrière, A.; Marcorelles, P.; Khung-Savatovsky, S.; Nardelli, J.; Cipriani, S.; Csaba, Z.; Picone, O.; et al. Cytomegalo-virus-induced brain malformations in fetuses. J. Neuropathol. Exp. Neurol. 2014, 73, 143–158. [Google Scholar] [CrossRef]
- Teissier, N.; Delezoide, A.L.; Mas, A.E.; Khung-Savatovsky, S.; Bessières, B.; Nardelli, J.; Vauloup-Fellous, C.; Picone, O.; Houhou, N.; Oury, J.F.; et al. Inner ear lesions in congenital cytomegalovirus infection of human fetuses. Acta Neuropathol. 2011, 122, 763–774. [Google Scholar] [CrossRef]
- Christov, F.; Nelson, E.G.; Gluth, M.B. Human Superior Olivary Nucleus Neuron Populations in Subjects With Normal Hearing and Presbycusis. Ann. Otol. Rhinol. Laryngol. 2018, 127, 527–535. [Google Scholar] [CrossRef]
- Pickles, J.O. Auditory pathways: Anatomy and physiology. Handb. Clin. Neurol. 2015, 129, 3–25. [Google Scholar] [CrossRef]
- Zachlod, D.; Kedo, O.; Amunts, K. Anatomy of the temporal lobe: From macro to micro. Handb. Clin. Neurol. 2022, 187, 17–51. [Google Scholar] [CrossRef]
- Pillion, J.P.; Shiffler, D.E.; Hoon, A.H.; Lin, D.D. Severe auditory processing disorder secondary to viral meningoencephalitis. Int. J. Audiol. 2014, 53, 427–431. [Google Scholar] [CrossRef]
- Kaga, K.; Kaga, M.; Tamai, F.; Shindo, M. Auditory agnosia in children after herpes encephalitis. Acta Otolaryngol. 2003, 123, 232–235. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflammation 2021, 18, 258. [Google Scholar] [CrossRef]
- Vidal-Itriago, A.; Radford, R.A.W.; Aramideh, J.A.; Maurel, C.; Scherer, N.M.; Don, E.K.; Lee, A.; Chung, R.S.; Graeber, M.B.; Morsch, M. Microglia morphophysiological diversity and its implications for the CNS. Front. Immunol. 2022, 13, 997786. [Google Scholar] [CrossRef]
- Lio, C.W.; McDonald, B.; Takahashi, M.; Dhanwani, R.; Sharma, N.; Huang, J.; Pham, E.; Benedict, C.A.; Sharma, S. cGAS-STING Signaling Regulates Initial Innate Control of Cytomegalovirus Infection. J. Virol. 2016, 90, 7789–7797. [Google Scholar] [CrossRef]
- Cheeran, M.C.; Hu, S.; Yager, S.L.; Gekker, G.; Peterson, P.K.; Lokensgard, J.R. Cytomegalovirus induces cytokine and chemokine production differentially in microglia and astrocytes: Antiviral implications. J. Neuro-Oncol. 2001, 7, 135–147. [Google Scholar]
- Rauf, A.; Badoni, H.; Abu-Izneid, T.; Olatunde, A.; Rahman, M.M.; Painuli, S.; Semwal, P.; Wilairatana, P.; Mubarak, M.S. Neuroinflammatory Markers: Key Indicators in the Pathology of Neurodegenerative Diseases. Molecules 2022, 27, 3194. [Google Scholar] [CrossRef]
- Swaroop, S.; Mahadevan, A.; Shankar, S.K.; Adlakha, Y.K.; Basu, A. HSP60 critically regulates endogenous IL-1β production in activated microglia by stimulating NLRP3 inflammasome pathway. J. Neuroinflammation 2018, 15, 177. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, D.; Li, G. The role of microglia in viral encephalitis: A review. J. Neuroinflammation 2019, 16, 76. [Google Scholar] [CrossRef]
- Cheeran, M.C.; Hu, S.; Sheng, W.S.; Peterson, P.K.; Lokensgard, J.R. CXCL10 production from cytomegalovirus-stimulated microglia is regulated by both human and viral interleukin-10. J. Virol. 2003, 77, 4502–4515. [Google Scholar] [CrossRef]
- Vasek, M.J.; Garber, C.; Dorsey, D.; Durrant, D.M.; Bollman, B.; Soung, A.; Yu, J.; Perez-Torres, C.; Frouin, A.; Wilton, D.K.; et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature 2016, 534, 538–543. [Google Scholar] [CrossRef]
- Holloway, O.G.; Canty, A.J.; King, A.E.; Ziebell, J.M. Rod microglia and their role in neurological diseases. Semin. Cell. Dev. Biol. 2019, 94, 96–103. [Google Scholar] [CrossRef]
- Rocamonde, B.; Hasan, U.; Mathieu, C.; Dutartre, H. Viral-induced neuroinflammation: Different mechanisms converging to similar exacerbated glial responses. Front. Neurosci. 2023, 17, 1108212. [Google Scholar] [CrossRef] [PubMed]
- Masliah, E.; Achim, C.L.; Ge, N.; DeTeresa, R.; Terry, R.D.; Wiley, C.A. Spectrum of human immunodeficiency virus-associated neocortical damage. Ann. Neurol. 1992, 32, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, L.; Bonasoni, M.P.; Foschini, M.P.; Silini, E.M.; Spinillo, A.; Revello, M.G.; Chiereghin, A.; Piccirilli, G.; Petrisli, E.; Turello, G.; et al. Histological Analysis of Term Placentas from Hyperimmune Globulin-Treated and Untreated Mothers with Primary Cytomegalovirus Infection. Fetal. Diagn. Ther. 2019, 45, 111–117. [Google Scholar] [CrossRef] [PubMed]
| CMV Load (Copies/5 ng DNA) | Rod-Shaped Microglial Cells (n°/10 Fields 40 HPF) | |||
|---|---|---|---|---|
| N° cases | Groups | Temporal lobe | Inner ear | Auditory cortex |
| 5 | Control | 0 | 0 | 10–30 |
| 4 | Mild CD | 14–33 | 0 | 33–35 |
| 7 | Moderate CD | 80–238 | 0–900 | 44–190 |
| 3 | Severe CD | 158–395 | 1000–1675 | 90–208 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabrielli, L.; Bonasoni, M.P.; Piccirilli, G.; Petrisli, E.; Venturoli, S.; Cantiani, A.; Pavoni, M.; Marsico, C.; Capretti, M.G.; Simonazzi, G.; et al. The Auditory Pathway in Congenitally Cytomegalovirus-Infected Human Fetuses. Int. J. Mol. Sci. 2024, 25, 2636. https://doi.org/10.3390/ijms25052636
Gabrielli L, Bonasoni MP, Piccirilli G, Petrisli E, Venturoli S, Cantiani A, Pavoni M, Marsico C, Capretti MG, Simonazzi G, et al. The Auditory Pathway in Congenitally Cytomegalovirus-Infected Human Fetuses. International Journal of Molecular Sciences. 2024; 25(5):2636. https://doi.org/10.3390/ijms25052636
Chicago/Turabian StyleGabrielli, Liliana, Maria Paola Bonasoni, Giulia Piccirilli, Evangelia Petrisli, Simona Venturoli, Alessia Cantiani, Matteo Pavoni, Concetta Marsico, Maria Grazia Capretti, Giuliana Simonazzi, and et al. 2024. "The Auditory Pathway in Congenitally Cytomegalovirus-Infected Human Fetuses" International Journal of Molecular Sciences 25, no. 5: 2636. https://doi.org/10.3390/ijms25052636
APA StyleGabrielli, L., Bonasoni, M. P., Piccirilli, G., Petrisli, E., Venturoli, S., Cantiani, A., Pavoni, M., Marsico, C., Capretti, M. G., Simonazzi, G., & Lazzarotto, T. (2024). The Auditory Pathway in Congenitally Cytomegalovirus-Infected Human Fetuses. International Journal of Molecular Sciences, 25(5), 2636. https://doi.org/10.3390/ijms25052636







