Oxytocin Modifies the Excitability and the Action Potential Shape of the Hippocampal CA1 GABAergic Interneurons
Abstract
1. Introduction
2. Results
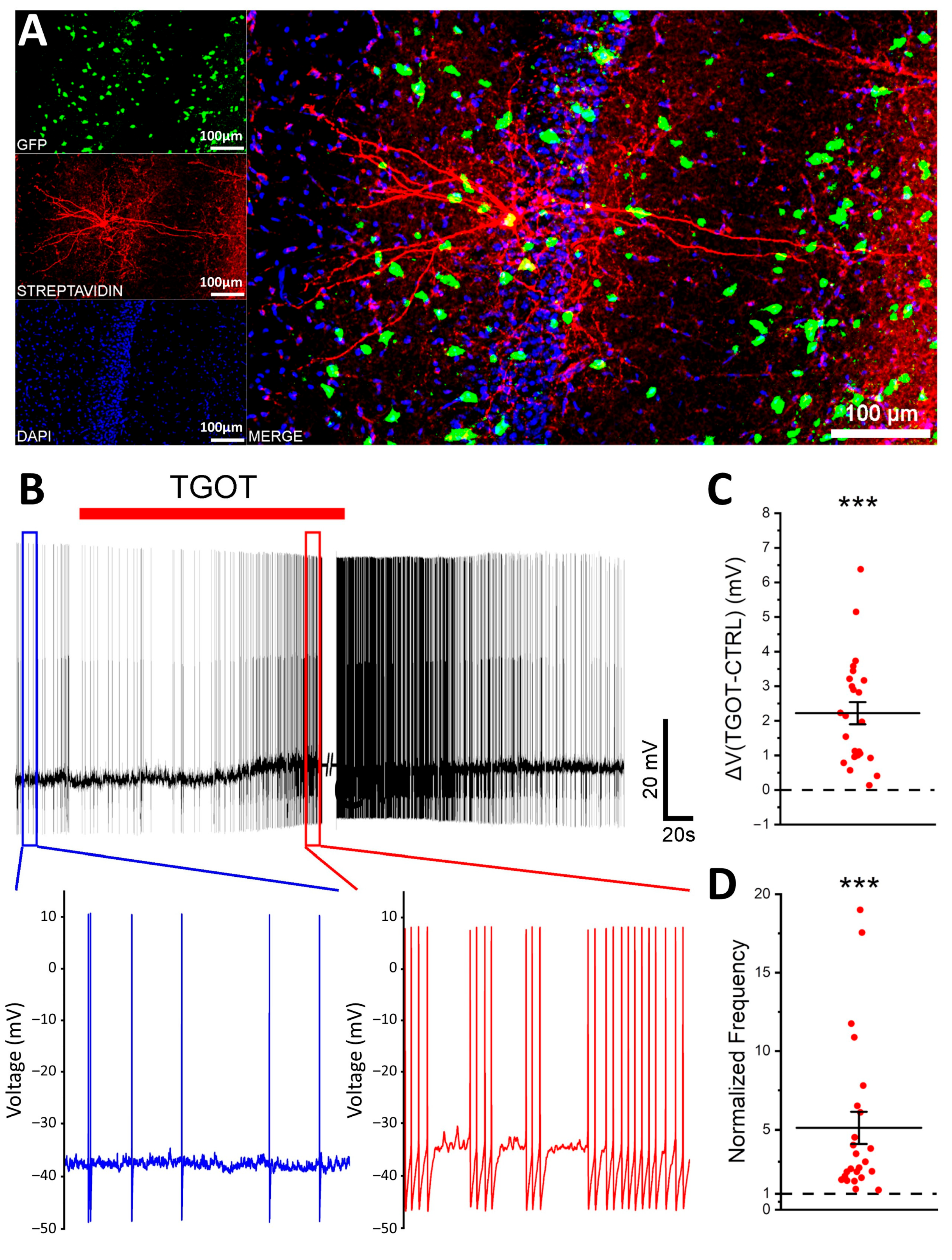
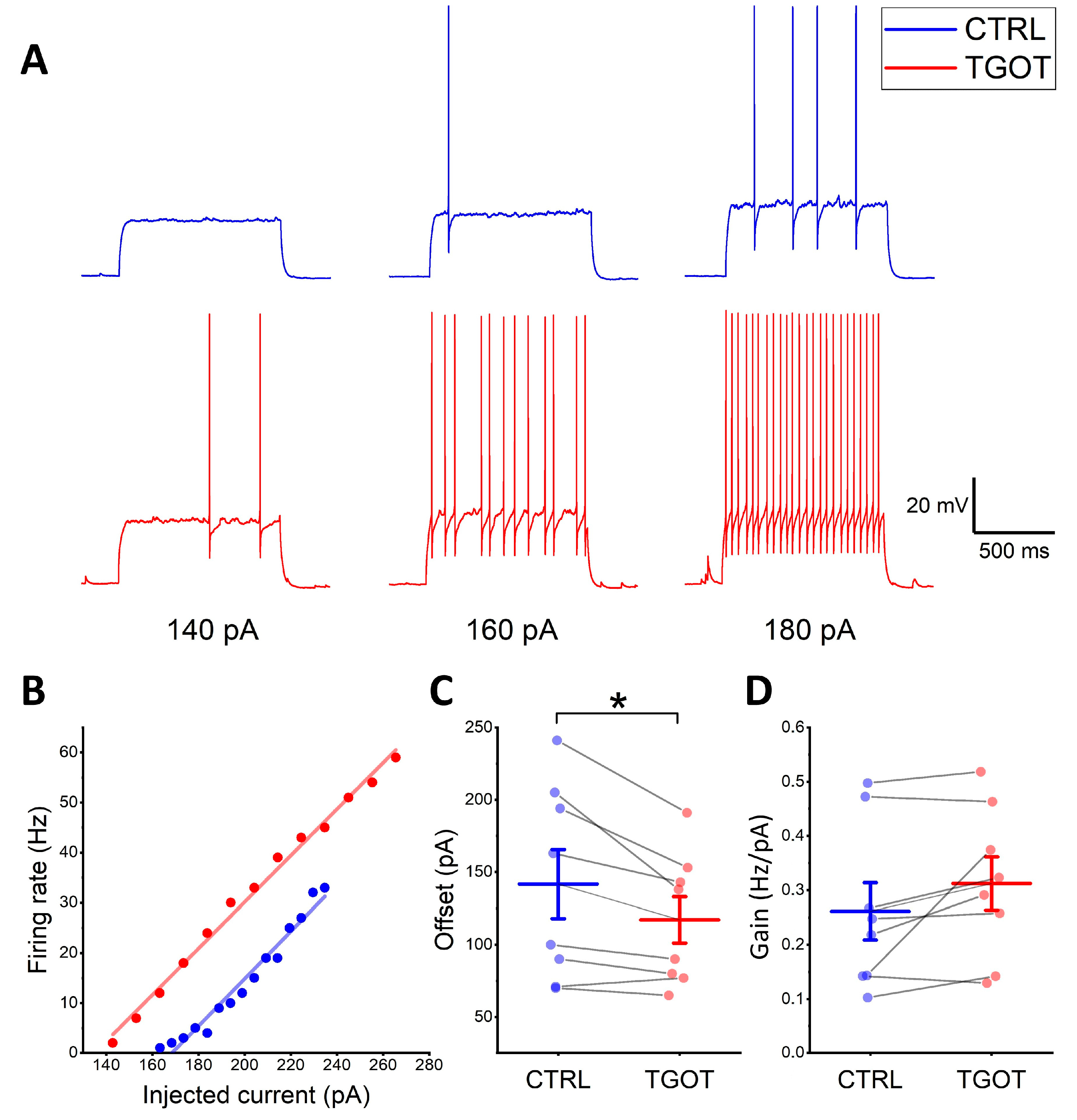
2.1. OTR Activation Not Only Depolarizes and Increases the Firing Frequency of Specific GABAergic Interneurons in CA1 but Also Enhances Their Excitability
2.2. OTR Activation Alters the Shape of the Action Potentials of the OTR-Expressing CA1 GABAergic Interneurons
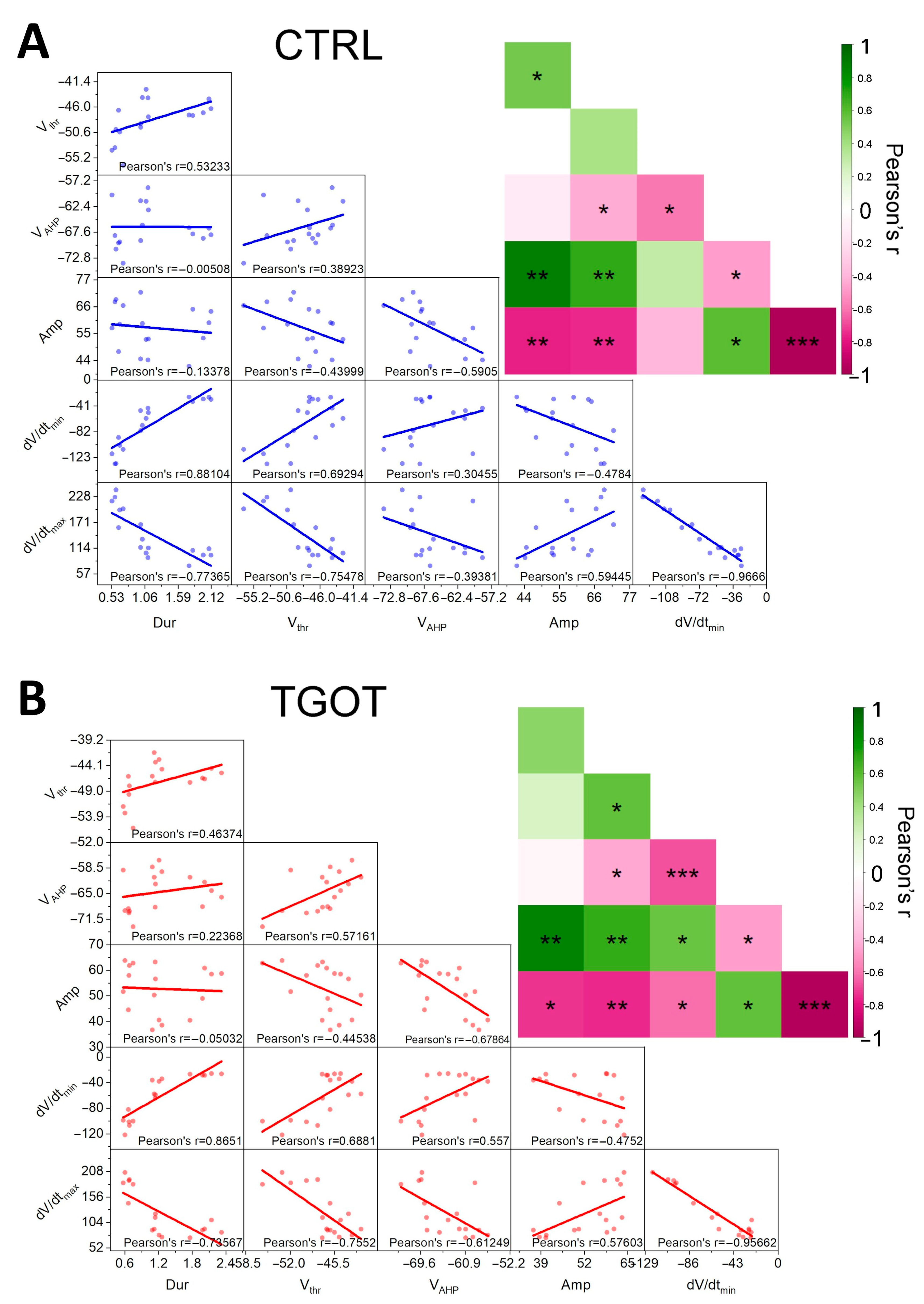
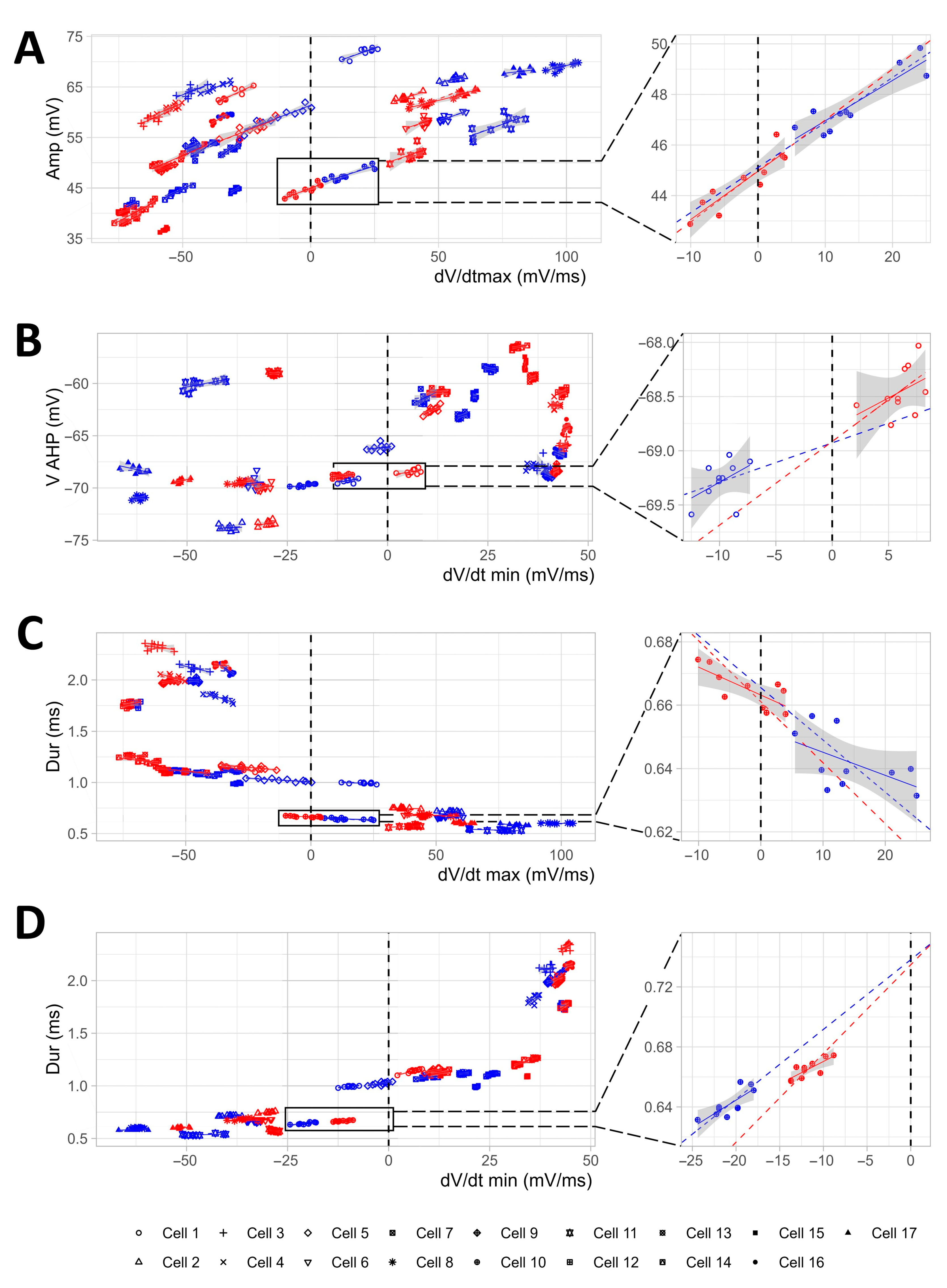
2.3. The Linear Mixed-Effects Models Corroborate the Results and Highlight the Presence/Absence of Interactions and Causal Dependencies among the Effects Induced by TGOT
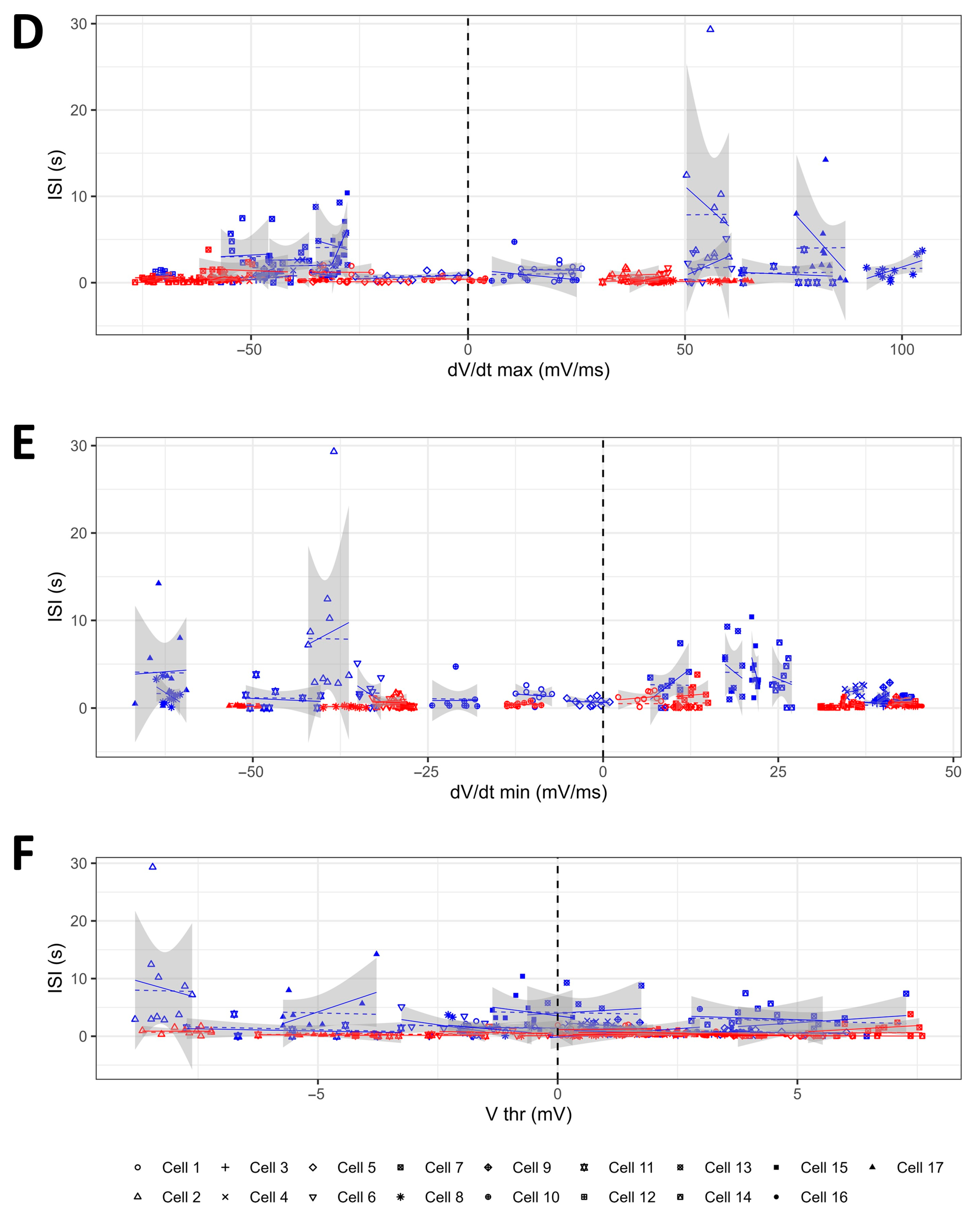
2.4. The Modifications of the AP Shape Induced by OTR Activation in the OTR-Expressing CA1 GABAergic Interneurons Do Not Result in the Increase in the Firing Frequency of These Neurons
3. Discussion
4. Materials and Methods
4.1. Animals and Brain Slices Preparation
4.2. Patch-Clamp Recordings
4.3. Immunostaining of the Recorded Neurons
4.4. Analysis of the Electrophysiological Recordings
4.5. Statistics and Linear Mixed-Effects Models
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swanson, L.W.; Sawchenko, P.E. Hypothalamic integration: Organization of the paraventricular and supraoptic nuclei. Annu. Rev. Neurosci. 1983, 6, 269–324. [Google Scholar] [CrossRef]
- Brown, C.H.; Ludwig, M.; Tasker, J.G.; Stern, J.E. Somato-dendritic vasopressin and oxytocin secretion in endocrine and autonomic regulation. J. Neuroendocr. 2020, 32, e12856. [Google Scholar] [CrossRef] [PubMed]
- Stoop, R. Neuromodulation by oxytocin and vasopressin. Neuron 2012, 76, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Mitre, M.; Minder, J.; Morina, E.X.; Chao, M.V.; Froemke, R.C. Oxytocin Modulation of Neural Circuits. Curr. Top. Behav. Neurosci. 2018, 35, 31–53. [Google Scholar] [PubMed]
- Son, S.; Manjila, S.B.; Newmaster, K.T.; Wu, Y.-T.; Vanselow, D.J.; Ciarletta, M.; Anthony, T.E.; Cheng, K.C.; Kim, Y. Whole-Brain Wiring Diagram of Oxytocin System in Adult Mice. J. Neurosci. 2022, 42, 5021–5033. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, Z.R.; Young, L.J. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 2008, 322, 900–904. [Google Scholar] [CrossRef]
- Heinrichs, M.; von Dawans, B.; Domes, G. Oxytocin, vasopressin, and human social behavior. Front. Neuroendocrinol. 2009, 30, 548–557. [Google Scholar] [CrossRef]
- Olivera-Pasilio, V.; Dabrowska, J. Oxytocin Promotes Accurate Fear Discrimination and Adaptive Defensive Behaviors. Front. Neurosci. 2020, 14, 583878. [Google Scholar] [CrossRef]
- Iovino, M.; Messana, T.; De Pergola, G.; Iovino, E.; Dicuonzo, F.; Guastamacchia, E.; Giagulli, V.A.; Triggiani, V. The Role of Neurohypophyseal Hormones Vasopressin and Oxytocin in Neuropsychiatric Disorders. Endocrine Metab. Immune Disord. Drug Targets 2018, 18, 341–347. [Google Scholar] [CrossRef]
- Abramova, O.; Zorkina, Y.; Ushakova, V.; Zubkov, E.; Morozova, A.; Chekhonin, V. The role of oxytocin and vasopressin dysfunction in cognitive impairment and mental disorders. Neuropeptides 2020, 83, 102079. [Google Scholar] [CrossRef]
- Sweatt, J.D. Hippocampal function in cognition. Psychopharmacol. 2004, 174, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Immordino-Yang, M.H.; Singh, V. Hippocampal contributions to the processing of social emotions. Hum. Brain Mapp. 2013, 34, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.D.; Watson, P.D.; Duff, M.C.; Cohen, N.J. The role of the hippocampus in flexible cognition and social behavior. Front. Hum. Neurosci. 2014, 8, 742. [Google Scholar] [CrossRef] [PubMed]
- Talpo, F.; Spaiardi, P.; Castagno, A.N.; Maniezzi, C.; Raffin, F.; Terribile, G.; Sancini, G.; Pisani, A.; Biella, G.R. Neuromodulatory functions exerted by oxytocin on different populations of hippocampal neurons in rodents. Front. Cell. Neurosci. 2023, 17, 1082010. [Google Scholar] [CrossRef] [PubMed]
- Mitre, M.; Marlin, B.J.; Schiavo, J.K.; Morina, E.; Norden, S.E.; Hackett, T.A.; Aoki, C.J.; Chao, M.V.; Froemke, R.C. A Distributed Network for Social Cognition Enriched for Oxytocin Receptors. J. Neurosci. 2016, 36, 2517–2535. [Google Scholar] [CrossRef] [PubMed]
- Raam, T.; McAvoy, K.M.; Besnard, A.; Veenema, A.H.; Sahay, A. Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat. Commun. 2017, 8, 2001. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Hsu, K.-S. Oxytocin receptor signaling in the hippocampus: Role in regulating neuronal excitability, network oscillatory activity, synaptic plasticity and social memory. Prog. Neurobiol. 2018, 171, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cilz, N.I.; Cymerblit-Sabba, A.; Young, W.S. Oxytocin and vasopressin in the rodent hippocampus. Genes Brain Behav. 2019, 18, e12535. [Google Scholar] [CrossRef]
- Maniezzi, C.; Talpo, F.; Spaiardi, P.; Toselli, M.; Biella, G. Oxytocin Increases Phasic and Tonic GABAergic Transmission in CA1 Region of Mouse Hippocampus. Front. Cell. Neurosci. 2019, 13, 178. [Google Scholar] [CrossRef]
- Owen, S.F.; Tuncdemir, S.N.; Bader, P.L.; Tirko, N.N.; Fishell, G.; Tsien, R.W. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature 2013, 500, 458–462. [Google Scholar] [CrossRef]
- Zaninetti, M.; Raggenbass, M. Oxytocin receptor agonists enhance inhibitory synaptic transmission in the rat hippocampus by activating interneurons in stratum pyramidale. Eur. J. Neurosci. 2000, 12, 3975–3984. [Google Scholar] [CrossRef] [PubMed]
- Tirko, N.N.; Eyring, K.W.; Carcea, I.; Mitre, M.; Chao, M.V.; Froemke, R.C.; Tsien, R.W. Oxytocin Transforms Firing Mode of CA2 Hippocampal Neurons. Neuron 2018, 100, 593–608.e3. [Google Scholar] [CrossRef] [PubMed]
- Busnelli; Chini, B. Molecular Basis of Oxytocin Receptor Signalling in the Brain: What We Know and What We Need to Know. Curr. Top. Behav. Neurosci. 2018, 35, 3–29. [Google Scholar] [PubMed]
- Yu, Z.; Guindani, M.; Grieco, S.F.; Chen, L.; Holmes, T.C.; Xu, X. Beyond t test and ANOVA: Applications of mixed-effects models for more rigorous statistical analysis in neuroscience research. Neuron 2022, 110, 21–35. [Google Scholar] [CrossRef]
- Koerner, T.K.; Zhang, Y. Application of Linear Mixed-Effects Models in Human Neuroscience Research: A Comparison with Pearson Correlation in Two Auditory Electrophysiology Studies. Brain Sci. 2017, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Jenerick, H. Phase plane trajectories of the muscle spike potential. Biophys. J. 1963, 3, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Bean, B.P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007, 8, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, A.L.; Huxley, A.F. The components of membrane conductance in the giant axon of Loligo. J. Physiol. 1952, 116, 473–496. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistics notes: Calculating correlation coefficients with repeated observations: Part 1--correlation within subjects. BMJ 1995, 310, 446. [Google Scholar] [CrossRef]
- Shan, G.; Zhang, H.; Jiang, T. Correlation Coefficients for a Study with Repeated Measures. Comput. Math. Methods Med. 2020, 2020, 7398324. [Google Scholar] [CrossRef]
- Lazic, S.E.; Clarke-Williams, C.J.; Munafò, M.R. What exactly is ‘N’ in cell culture and animal experiments? PLoS Biol. 2018, 16, e2005282. [Google Scholar] [CrossRef]
- Fourcaud-Trocmé, N.; Zbili, M.; Duchamp-Viret, P.; Kuczewski, N. Afterhyperpolarization Promotes the Firing of Mitral Cells through a Voltage-Dependent Modification of Action Potential Threshold. Eneuro 2022, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Azouz, R.; Gray, C.M. Dynamic spike threshold reveals a mechanism for synaptic coincidence detection in cortical neurons in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 8110–8115. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, R.S.; Johnson, E.C.; Jones, D.L.; Ratnam, R. A dynamic spike threshold with correlated noise predicts observed patterns of negative interval correlations in neuronal spike trains. Biol. Cybern. 2022, 116, 611–633. [Google Scholar] [CrossRef] [PubMed]
- Tateno, T.; Harsch, A.; Robinson, H.P. Threshold firing frequency-current relationships of neurons in rat somatosensory cortex: Type 1 and type 2 dynamics. J. Neurophysiol. 2004, 92, 2283–2294. [Google Scholar] [CrossRef]
- Raggenbass, M. Vasopressin- and oxytocin-induced activity in the central nervous system: Electrophysiological studies using in-vitro systems. Prog. Neurobiol. 2001, 64, 307–326. [Google Scholar] [CrossRef]
- Zbili, M.; Debanne, D. Past and Future of Analog-Digital Modulation of Synaptic Transmission. Front. Cell. Neurosci. 2019, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, S.; Tasaki, I. A study on the mechanism of impulse transmission across the giant synapse of the squid. J. Physiol. 1958, 143, 114–137. [Google Scholar] [CrossRef]
- Shapiro, E.; Castellucci, V.F.; Kandel, E.R. Presynaptic membrane potential affects transmitter release in an identified neuron in Aplysia by modulating the Ca2+ and K+ currents. Proc. Natl. Acad. Sci. USA 1980, 77, 629–633. [Google Scholar] [CrossRef]
- Clark, B.; Häusser, M. neural coding: Hybrid analog and digital signalling in axons. Curr. Biol. 2006, 16, R585–R588. [Google Scholar] [CrossRef]
- Alle, H.; Geiger, J.R. Analog signalling in mammalian cortical axons. Curr. Opin. Neurobiol. 2008, 18, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Eyring, K.W.; König, G.M.; Kostenis, E.; Tsien, R.W. Oxytocin-Modulated Ion Channel Ensemble Controls Depolarization, Integration and Burst Firing in CA2 Pyramidal Neurons. J. Neurosci. 2022, 42, 7707–7720. [Google Scholar] [CrossRef]
- Gravati, M.; Busnelli, M.; Bulgheroni, E.; Reversi, A.; Spaiardi, P.; Parenti, M.; Toselli, M.; Chini, B. Dual modulation of inward rectifier potassium currents in olfactory neuronal cells by promiscuous G protein coupling of the oxytocin receptor. J. Neurochem. 2010, 114, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Mitterdorfer, J.; Bean, B.P. Potassium currents during the action potential of hippocampal CA3 neurons. J. Neurosci. 2002, 22, 10106–10115. [Google Scholar] [CrossRef] [PubMed]
- Biella, G.R.; Spaiardi, P.; Jimenez-Moreno, R.; Magistretti, J.; Taglietti, V.; Toselli, M. A fast transient outward current in layer II/III neurons of rat perirhinal cortex. Pflügers Arch. Eur. J. Physiol. 2007, 455, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Breton, J.D.; Poisbeau, P.; Darbon, P. Antinociceptive action of oxytocin involves inhibition of potassium channel currents in lamina ii neurons of the rat spinal cord. Mol. Pain 2009, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Tamamaki, N.; Yanagawa, Y.; Tomioka, R.; Miyazaki, J.; Obata, K.; Kaneko, T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J. Comp. Neurol. 2003, 467, 60–79. [Google Scholar] [CrossRef] [PubMed]
- Stoop, R.; Pralong, E. Functional connections and epileptic spread between hippocampus, entorhinal cortex and amygdala in a modified horizontal slice preparation of the rat brain. Eur. J. Neurosci. 2000, 12, 3651–3663. [Google Scholar] [CrossRef]
- Magistretti, J.; Mantegazza, M.; Guatteo, E.; Wanke, E. Action potentials recorded with patch-clamp amplifiers: Are they genuine? Trends Neurosci. 1996, 19, 530–534. [Google Scholar] [CrossRef]
- Brickley, S.G.; Cull-Candy, S.G.; Farrant, M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J. Physiol. 1996, 497, 753–759. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Silver, R. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron 2003, 38, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Seay, M.J.; Buonomano, D.V. Creation of Neuronal Ensembles and Cell-Specific Homeostatic Plasticity through Chronic Sparse Optogenetic Stimulation. J. Neurosci. 2023, 43, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Sekerli, M.; DelNegro, C.A.; Lee, R.H.; Butera, R.J. Estimating Action potential thresholds from neuronal time-series: New metrics and evaluation of methodologies. IEEE Trans. Biomed. Eng. 2004, 51, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Binini, N.; Talpo, F.; Spaiardi, P.; Maniezzi, C.; Pedrazzoli, M.; Raffin, F.; Mattiello, N.; Castagno, A.N.; Masetto, S.; Yanagawa, Y.; et al. Membrane Resonance in Pyramidal and GABAergic Neurons of the Mouse Perirhinal Cortex. Front. Cell. Neurosci. 2021, 15, 703407. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Heisterkamp, S.; Van Willigen, B.; Maintainer, R. Package ‘nlme’. Linear Nonlinear Mix. Eff. Models. Version 2017, 3, 274. [Google Scholar]
- Revelle, W.; Revelle, M.W. Package ‘psych’. Compr. R Arch. Netw. 2015, 337–338. [Google Scholar]
- Fahrmeir, L.; Kneib, T.; Lang, S.; Marx, B. Regression: Models, Methods and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Lüdecke, D.; Lüdecke, M.D. Package ‘sjPlot’. R Package Version 2015, 1, 1–107. [Google Scholar]
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘ggplot2’. Creat. Elegant Data Vis. Using Gramm. Graph. Version 2016, 2, 1–189. [Google Scholar]
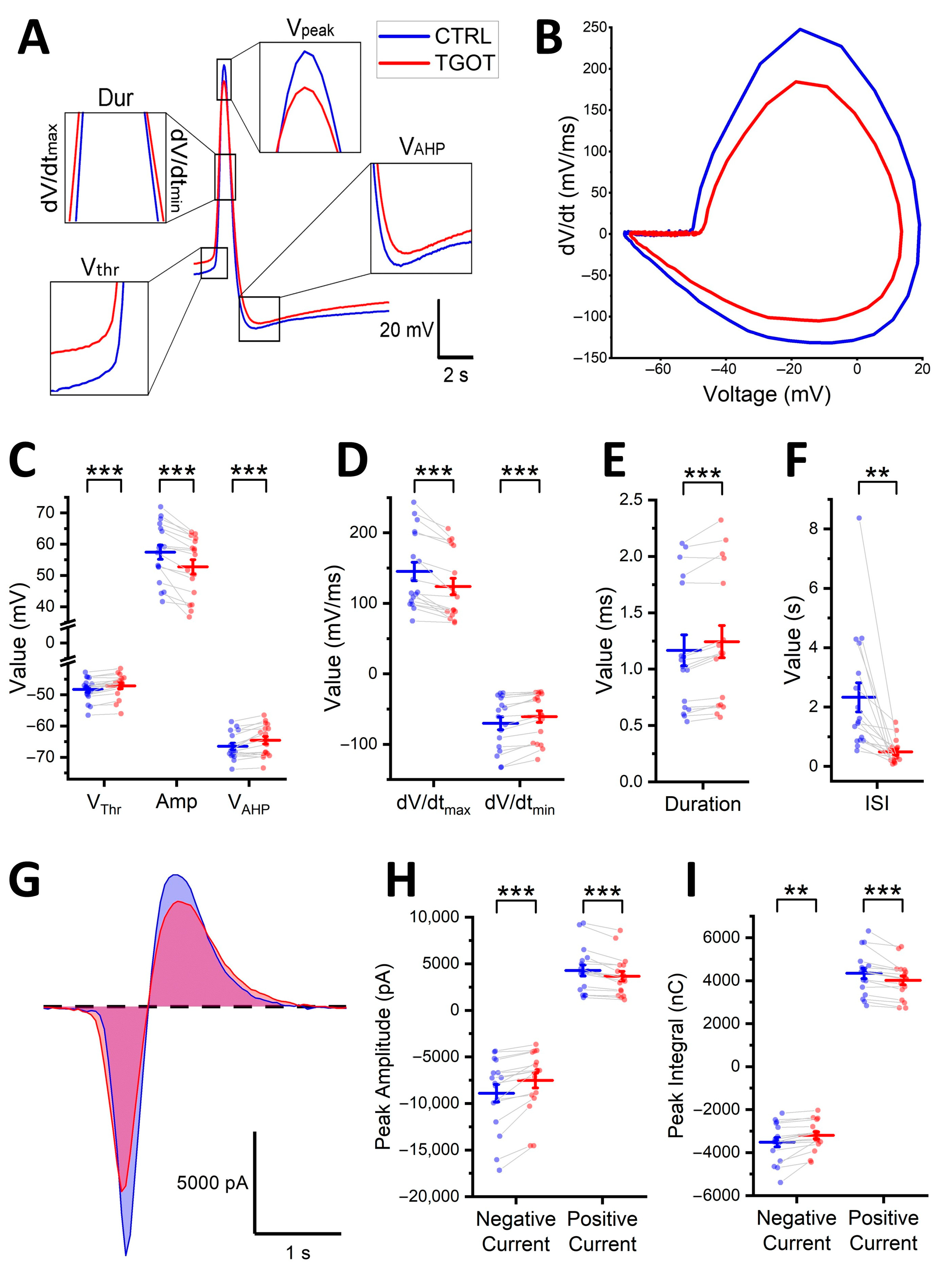

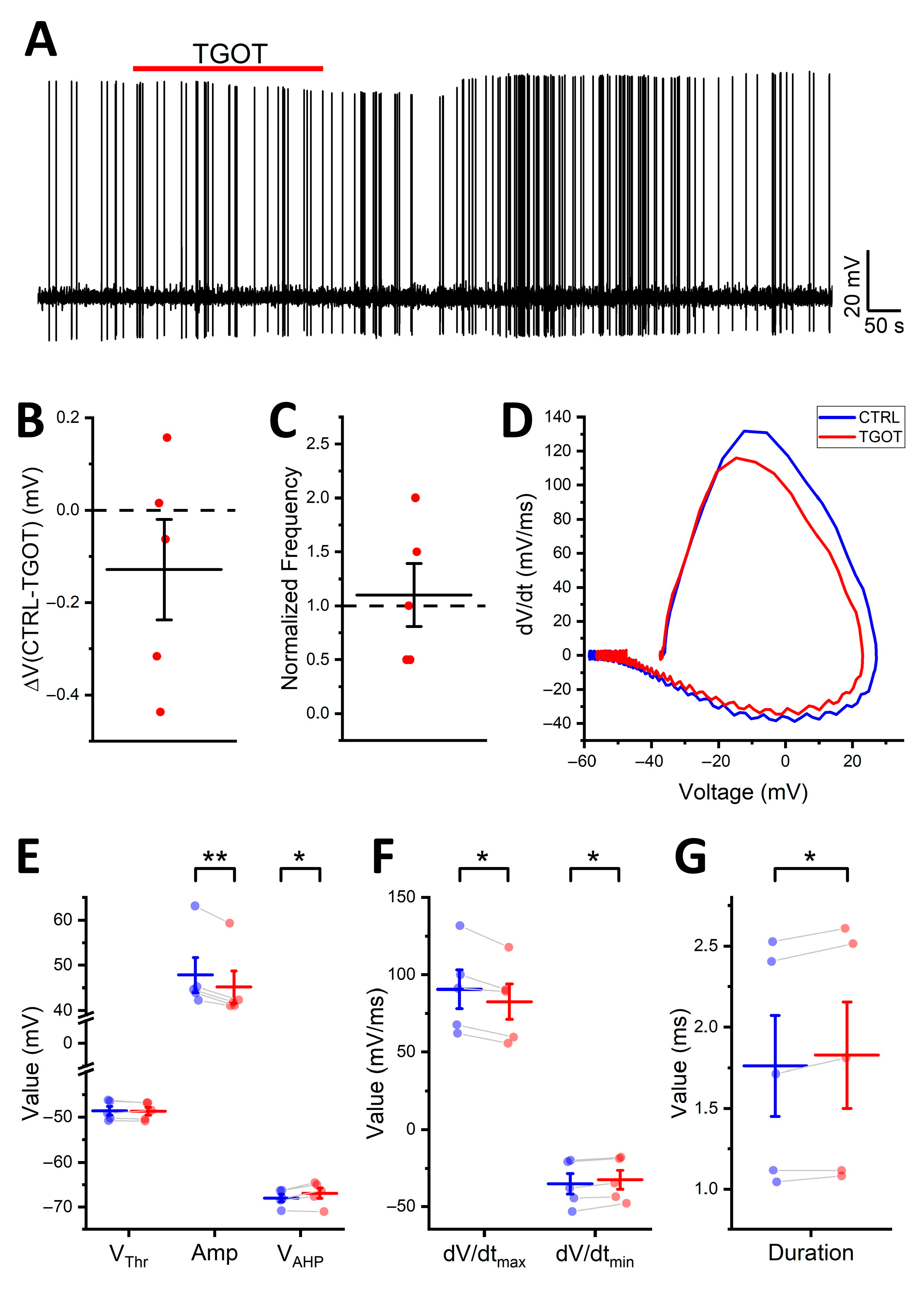
| CTRL | TGOT | ICC | p-Value from t-Test | p-Value from LMMs | |
|---|---|---|---|---|---|
| Vthr | −48.3 ± 0.9 mV | −47.1 ± 0.9 mV | 0.96 | <0.001 | <0.001 |
| Amp | 57.4 ± 2.3 mV | 52.7 ± 2.3 mV | 0.99 | <0.001 | <0.001 |
| VAHP | −66.5 ± 1.0 mV | −64.6 ± 1.2 mV | 1.00 | <0.001 | <0.001 |
| dV/dtmax | 145 ± 13 mV/ms | 124 ± 12 mV/ms | 0.99 | <0.001 | <0.001 |
| dV/dtmin | −70.2 ± 9.2 mV/ms | −60.8 ± 8.2 mV/ms | 1.00 | <0.001 | <0.001 |
| Dur | 1.17 ± 0.14 ms | 1.24 ± 0.14 ms | 1.00 | <0.001 | <0.001 |
| ISI | 2.33 ± 0.49 s | 0.49 ± 0.10 s | 0.35 | <0.01 | <0.001 |
| Effect of TGOT and dV/dtmax on Amp (mV) | Effect of TGOT and dV/dtmin on VAHP (mV) | Effect of TGOT and dV/dtmax on Dur (ms) | Effect of TGOT and dV/dtmin on Dur (ms) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | Estimates | Std. Error | p | Estimates | Std. Error | p | Estimates | Std. Error | p | Estimates | Std. Error | p |
| (Intercept) | 57.4349 *** | 2.0727 | <0.001 | −66.4859 *** | 0.9782 | <0.001 | 1.1668 *** | 0.1211 | <0.001 | 1.1668 *** | 0.1016 | <0.001 |
| Effect of dV/dtmax in CTRL | 0.1781 *** | 0.0077 | <0.001 | −0.0016 *** | 0.0002 | <0.001 | ||||||
| Effect of TGOT at x = 0 | −0.3996 | 0.4471 | 0.372 | 1.2087 ** | 0.3655 | 0.001 | 0.0363 * | 0.0151 | 0.017 | 0.0216 | 0.0144 | 0.134 |
| Interaction of dV/dtmax and TGOT | 0.0243 *** | 0.0073 | 0.001 | −0.0003 | 0.0002 | 0.209 | ||||||
| Effect of dV/dtmin in CTRL | 0.0364 ** | 0.0111 | 0.001 | 0.0047 *** | 0.0006 | <0.001 | ||||||
| Interaction of dV/dtmin and TGOT | 0.0407 *** | 0.0094 | <0.001 | 0.0013 *** | 0.0004 | 0.001 | ||||||
| Random Effects | ||||||||||||
| σ2 | 0.32 | 0.09 | 0.00 | 0.00 | ||||||||
| τ00 | 73.00 cell | 16.26 cell | 0.25 cell | 0.18 cell | ||||||||
| τ11 | 2.75 cell.cond(TGOT) | 1.99 cell.cond(TGOT) | 0.00 cell.cond(TGOT) | 0.00 cell.cond(TGOT) | ||||||||
| ρ01 | 0.10 cell | −0.06 cell | 0.37 cell | 0.09 cell | ||||||||
| ICC | 1.00 | 0.99 | 1.00 | 1.00 | ||||||||
| N | 17 cell | 17 cell | 17 cell | 17 cell | ||||||||
| Observations | 340 | 340 | 340 | 340 | ||||||||
| Marginal R2/Conditional R2 | 0.554/0.998 | 0.230/0.996 | 0.034/0.999 | 0.164/0.999 | ||||||||
| Effect of TGOT and Amp on ISI (s) | Effect of TGOT and VAHP on ISI (s) | Effect of TGOT and Dur on ISI (s) | Effect of TGOT and dV/dtmax on ISI (s) | Effect of TGOT and dV/dtmin on ISI (s) | Effect of TGOT and Dur on ISI (s) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | Est. ± SE | p | Est. ± SE | p | Est. ± SE | p | Est. ± SE | p | Est. ± SE | p | Est. ± SE | p |
| (Intercept) | 2.33 ± 0.50 *** | <0.001 | 2.33 ± 0.50 *** | <0.001 | 2.33 ± 0.50 *** | <0.001 | 2.33 ± 0.50 *** | <0.001 | 2.33 ± 0.50 *** | <0.001 | 2.33 ± 0.50 *** | <0.001 |
| Effect of Amp in CTRL | 0.01 ± 0.05 | 0.881 | ||||||||||
| Effect of TGOT at x = 0 | −1.83 ± 0.51 *** | <0.001 | −1.81 ± 0.51 *** | <0.001 | −1.83 ± 0.48 *** | <0.001 | −1.86 ± 0.50 *** | <0.001 | −1.84 ± 0.49 *** | <0.001 | −1.84 ± 0.44 *** | <0.001 |
| Interaction Amp and TGOT | −0.01 ± 0.05 | 0.919 | ||||||||||
| Effect of VAHP in CTRL | −0.06 ± 0.12 | 0.606 | ||||||||||
| Interaction of VAHP and TGOT | 0.05 ± 0.12 | 0.700 | ||||||||||
| Effect of Dur in CTRL | −1.24 ± 0.86 | 0.150 | ||||||||||
| Interaction of Dur and TGOT | 1.21 ± 0.87 | 0.165 | ||||||||||
| Effect of dV/dtmax in CTRL | 0.01 ± 0.01 | 0.480 | ||||||||||
| Interaction of dV/dtmax and TGOT | −0.01 ± 0.01 | 0.407 | ||||||||||
| Effect of dV/dtmin in CTRL | −0.01 ± 0.01 | 0.301 | ||||||||||
| Interaction of dV/dtmin and TGOT | 0.01 ± 0.01 | 0.303 | ||||||||||
| Effect of Vthr in CTRL | −0.19 ± 0.10 | 0.073 | ||||||||||
| Interaction of Vthr and TGOT | 0.19 ± 0.11 | 0.070 | ||||||||||
| Random Effects | ||||||||||||
| σ2 | 3.52 | 3.51 | 3.51 | 3.51 | 3.51 | 3.54 | ||||||
| τ00 | 3.95 cell | 3.95 cell | 3.47 cell | 3.80 cell | 3.70 cell | 2.88 cell | ||||||
| τ11 | 3.64 cell.cond(TGOT) | 3.68 cell.cond(TGOT) | 3.18 cell.cond(TGOT) | 3.45 cell.cond(TGOT) | 3.37 cell.cond(TGOT) | 2.55 cell.cond(TGOT) | ||||||
| ρ01 | −1.00 cell | −1.00 cell | −1.00 cell | −1.00 cell | −1.00 cell | −1.00 cell | ||||||
| ICC | 0.36 | 0.36 | 0.33 | 0.35 | 0.35 | 0.29 | ||||||
| N | 17 cell | 17 cell | 17 cell | 17 cell | 17 cell | 17 cell | ||||||
| Observations | 340 | 340 | 340 | 340 | 340 | 340 | ||||||
| Marginal R2/Conditional R2 | 0.134/0.447 | 0.138/0.450 | 0.170/0.446 | 0.143/0.445 | 0.153/0.447 | 0.179/0.417 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castagno, A.N.; Spaiardi, P.; Trucco, A.; Maniezzi, C.; Raffin, F.; Mancini, M.; Nicois, A.; Cazzola, J.; Pedrinazzi, M.; Del Papa, P.; et al. Oxytocin Modifies the Excitability and the Action Potential Shape of the Hippocampal CA1 GABAergic Interneurons. Int. J. Mol. Sci. 2024, 25, 2613. https://doi.org/10.3390/ijms25052613
Castagno AN, Spaiardi P, Trucco A, Maniezzi C, Raffin F, Mancini M, Nicois A, Cazzola J, Pedrinazzi M, Del Papa P, et al. Oxytocin Modifies the Excitability and the Action Potential Shape of the Hippocampal CA1 GABAergic Interneurons. International Journal of Molecular Sciences. 2024; 25(5):2613. https://doi.org/10.3390/ijms25052613
Chicago/Turabian StyleCastagno, Antonio Nicolas, Paolo Spaiardi, Arianna Trucco, Claudia Maniezzi, Francesca Raffin, Maria Mancini, Alessandro Nicois, Jessica Cazzola, Matilda Pedrinazzi, Paola Del Papa, and et al. 2024. "Oxytocin Modifies the Excitability and the Action Potential Shape of the Hippocampal CA1 GABAergic Interneurons" International Journal of Molecular Sciences 25, no. 5: 2613. https://doi.org/10.3390/ijms25052613
APA StyleCastagno, A. N., Spaiardi, P., Trucco, A., Maniezzi, C., Raffin, F., Mancini, M., Nicois, A., Cazzola, J., Pedrinazzi, M., Del Papa, P., Pisani, A., Talpo, F., & Biella, G. R. (2024). Oxytocin Modifies the Excitability and the Action Potential Shape of the Hippocampal CA1 GABAergic Interneurons. International Journal of Molecular Sciences, 25(5), 2613. https://doi.org/10.3390/ijms25052613







