(D-Ala2)GIP Inhibits Inflammatory Bone Resorption by Suppressing TNF-α and RANKL Expression and Directly Impeding Osteoclast Formation
Abstract
1. Introduction
2. Results
2.1. (D-Ala2)GIP Inhibited LPS-Induced Bone Resorption In Vivo
2.2. (D-Ala2)GIP Inhibited LPS-Induced Osteoclast Formation In Vivo
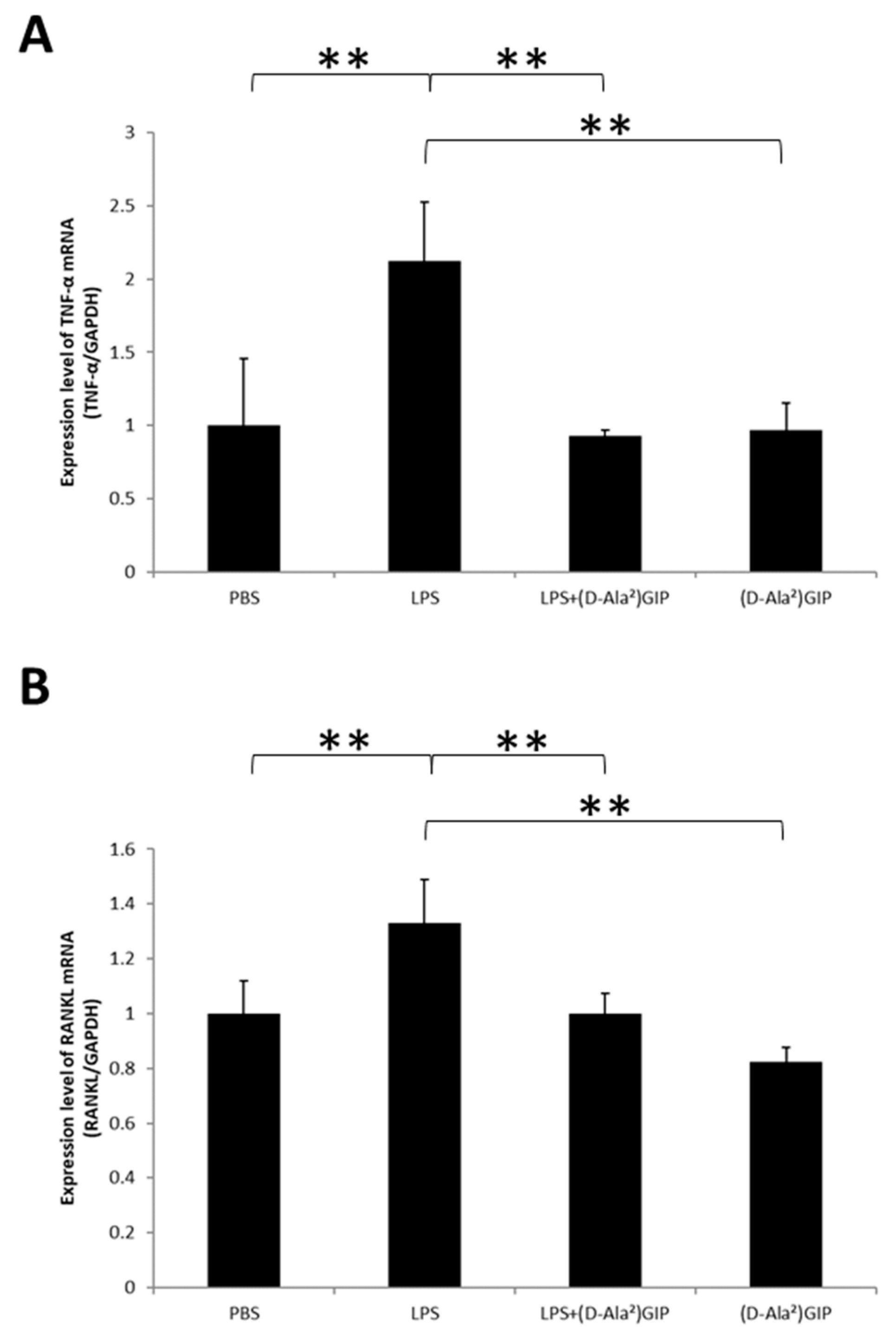
2.3. (D-Ala2)GIP Suppressed Expression of TNF-α and RANKL In Vivo
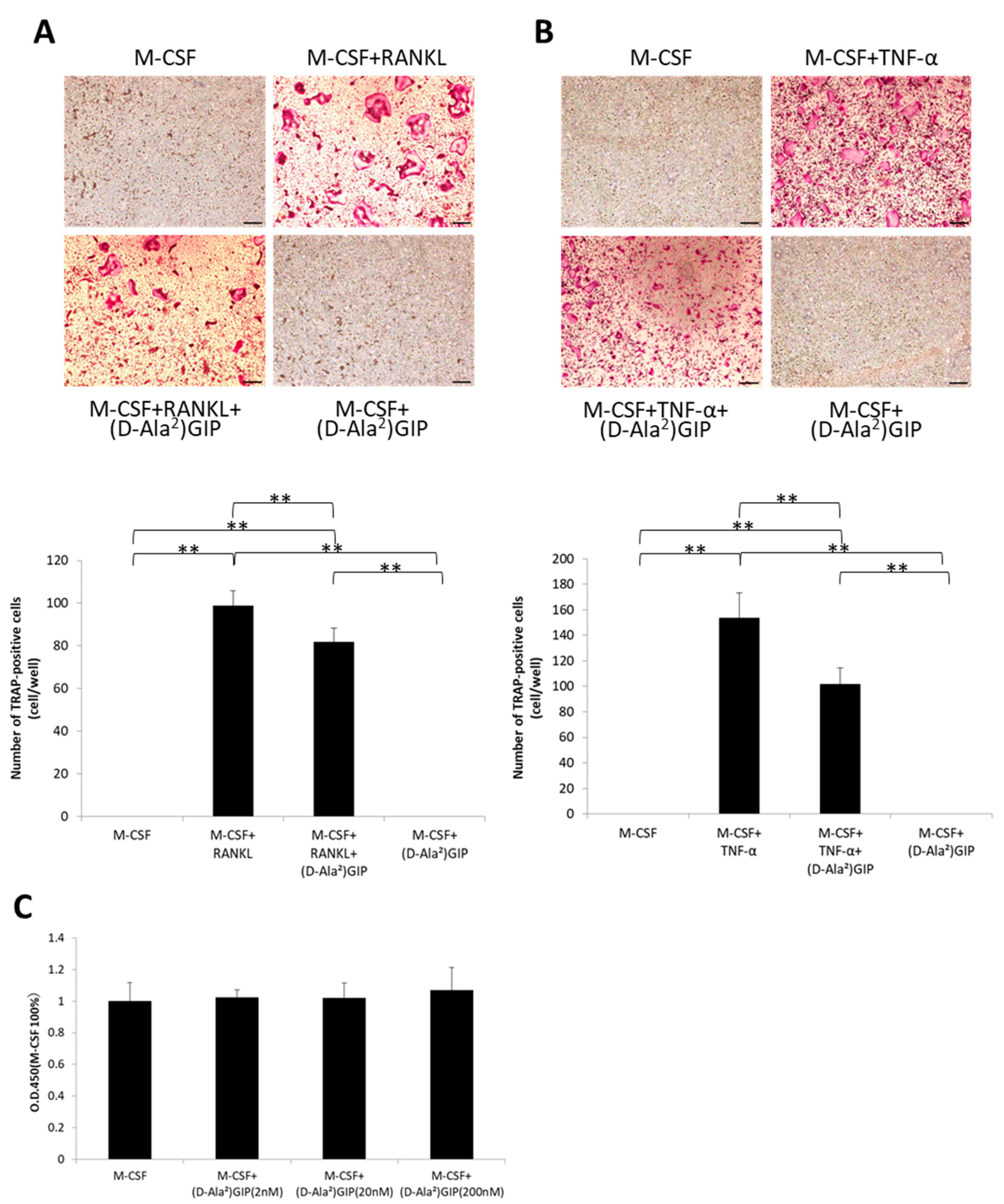
2.4. (D-Ala2)GIP Decreased Osteoclastogenesis Triggered by RANKL and TNF-α and Had No Effect on Cell Viability of Osteoclast Precursors In Vitro
2.5. (D-Ala2)GIP Downregulated LPS-Driven TNF-α Expression in Macrophages and RANKL Expression in Osteoblasts In Vitro
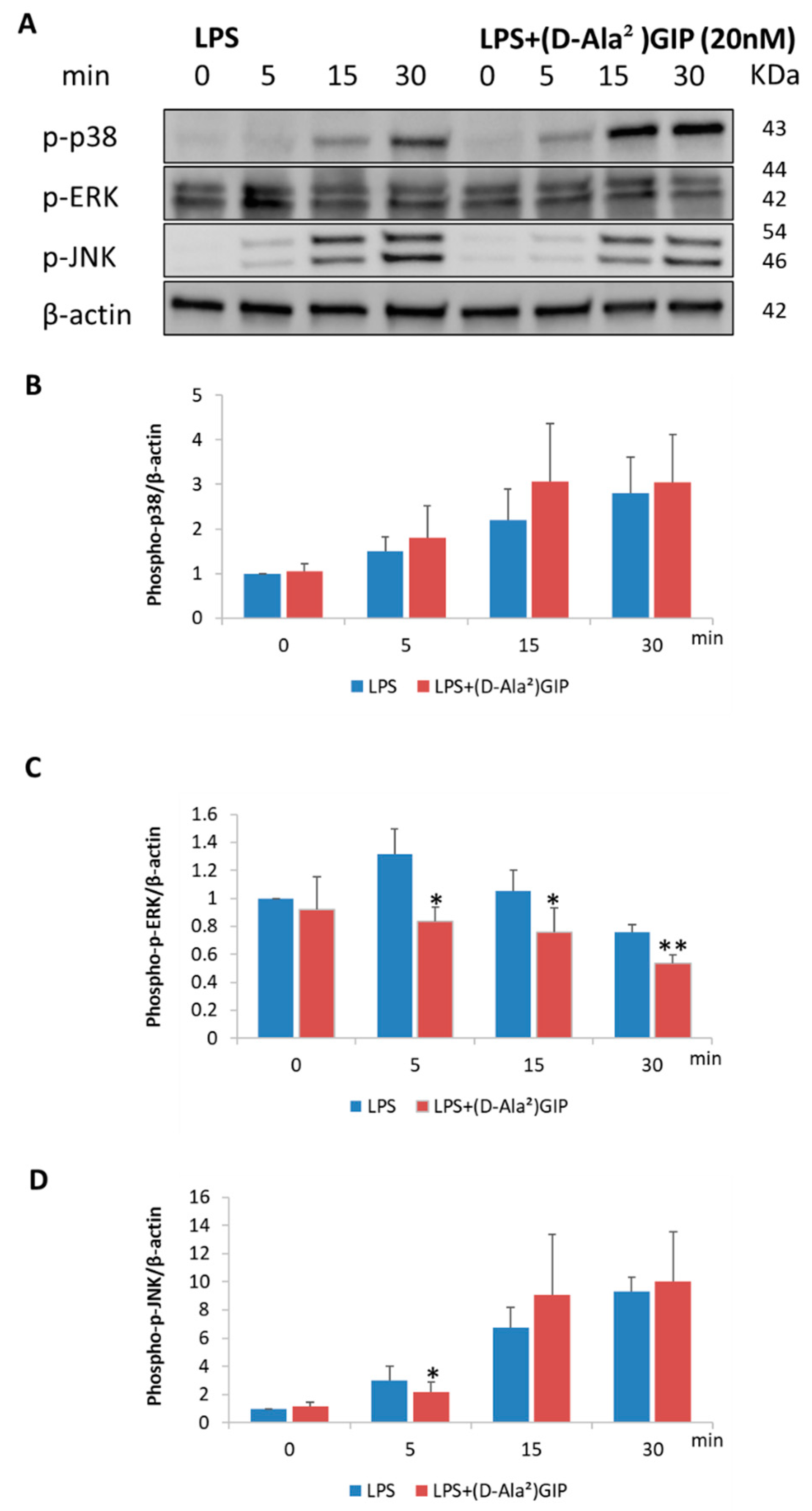
2.6. (D-Ala2)GIP Dampened LPS-Triggered Mitogen-Activated Protein Kinases (MAPKs) Signaling Pathway in Peritoneal Macrophages In Vitro
2.7. (D-Ala2)GIP Dampened LPS-Triggered MAPKs Signaling Pathway in Osteoblasts In Vitro
3. Discussion
4. Materials and Methods
4.1. Animal Model and Reagents
4.2. Histological Examination
4.3. Micro-CT Evaluation of Bone Destruction Area
4.4. Osteoclast Preparation
4.5. Cell Viability Assay of Osteoclast Precursors
4.6. Preparation of Peritoneal Macrophages
4.7. Preparation of Primary Osteoblasts
4.8. RNA Preparation and Real-Time RT-PCR Evaluation
4.9. Western Blotting Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Pop-Busui, R.; Januzzi, J.L.; Bruemmer, D.; Butalia, S.; Green, J.B.; Horton, W.B.; Knight, C.; Levi, M.; Rasouli, N.; Richardson, C.R. Heart failure: An underappreciated complication of diabetes. A consensus report of the American Diabetes Association. Diabetes Care 2022, 45, 1670–1690. [Google Scholar] [CrossRef]
- Wei, J.; Tian, J.; Tang, C.; Fang, X.; Miao, R.; Wu, H.; Wang, X.; Tong, X. The influence of different types of diabetes on vascular complications. J. Diabetes Res. 2022, 2022, 3448618. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Tran, T.; Everhart, J.E. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004, 126, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.G.; Huerta, M.; González-Usigli, H.A.; Torres-Sánchez, E.D.; Delgado-Lara, D.L.; Pacheco-Moisés, F.P.; Mireles-Ramírez, M.A.; Torres-Mendoza, B.M.; Moreno-Cih, R.I.; Velázquez-Brizuela, I.E. Cognitive disorder and dementia in type 2 diabetes mellitus. World J. Diabetes 2022, 13, 319–337. [Google Scholar] [CrossRef]
- Vigneri, P.; Frasca, F.; Sciacca, L.; Pandini, G.; Vigneri, R. Diabetes and cancer. Endocr. Relat. Cancer 2009, 16, 1103–1123. [Google Scholar] [CrossRef]
- Napoli, N.; Chandran, M.; Pierroz, D.D.; Abrahamsen, B.; Schwartz, A.V.; Ferrari, S.L.; IOF Bone and Diabetes Working Group. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 2017, 13, 208–219. [Google Scholar] [CrossRef]
- Christensen, M.; Vedtofte, L.; Holst, J.J.; Vilsbøll, T.; Knop, F.K. Glucose-dependent insulinotropic polypeptide: A bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes 2011, 60, 3103–3109. [Google Scholar] [CrossRef]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef]
- Tsukiyama, K.; Yamada, Y.; Yamada, C.; Harada, N.; Kawasaki, Y.; Ogura, M.; Bessho, K.; Li, M.; Amizuka, N.; Sato, M.; et al. Gastric inhibitory polypeptide as an endogenous factor promoting new bone formation after food ingestion. Mol. Endocrinol. 2006, 20, 1644–1651. [Google Scholar] [CrossRef]
- Mentlein, R.; Gallwitz, B.; Schmidt, W.E. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like Peptide-1(7–36)Amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 1993, 214, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, T.J.; McIntosh, C.H.; Pederson, R.A. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 1995, 136, 3585–3596. [Google Scholar] [CrossRef] [PubMed]
- Kühn-Wache, K.; Manhart, S.; Hoffmann, T.; Hinke, S.A.; Gelling, R.; Pederson, R.A.; McIntosh, C.H.; Demuth, H.U. Analogs of glucose-dependent insulinotropic polypeptide with increased dipeptidyl peptidase IV resistance. Adv. Exp. Med. Biol. 2000, 477, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, R.; Drucker, D.J. Beyond the pancreas: Contrasting cardiometabolic actions of GIP and GLP1. Nat. Rev. Endocrinol. 2023, 19, 201–216. [Google Scholar] [CrossRef]
- Bollag, R.J.; Zhong, Q.; Phillips, P.; Min, L.; Zhong, L.; Cameron, R.; Mulloy, A.L.; Rasmussen, H.; Qin, F.; Ding, K.H.; et al. Osteoblast-derived cells express functional glucose- dependent insulinotropic peptide receptors. Endocrinology 2000, 141, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Mieczkowska, A.; Bouvard, B.; Chappard, D.; Mabilleau, G. Glucose-dependent insulinotropic polypeptide (GIP) directly affects collagen fibril diameter and collagen cross-linking in osteoblast cultures. Bone 2015, 74, 29–36. [Google Scholar] [CrossRef]
- Zhong, Q.; Itokawa, T.; Sridhar, S.; Ding, K.H.; Xie, D.; Kang, B.; Bollag, W.B.; Bollag, R.J.; Hamrick, M.; Insogna, K.; et al. Effects of glucose-dependent insulinotropic peptide on osteoclast function. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E543–E548. [Google Scholar] [CrossRef]
- Tsai, J.; Kaneko, K.; Suh, A.J.; Bockman, R.; Park-Min, K.H. Origin of osteoclasts: Osteoclast precursor cells. J. Bone Metab. 2023, 30, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Kotake, S.; Yago, T.; Kawamoto, M.; Nanke, Y. Role of osteoclasts and interleukin-17 in the pathogenesis of rheumatoid arthritis: Crucial ‘human Osteoclastology’. J. Bone Miner. Metab. 2012, 30, 125–135. [Google Scholar] [CrossRef]
- Usui, M.; Onizuka, S.; Sato, T.; Kokabu, S.; Ariyoshi, W.; Nakashima, K. Mechanism of alveolar bone destruction in periodontitis—Periodontal bacteria and inflammation. Jpn. Dent. Sci. Rev. 2021, 57, 201–208. [Google Scholar] [CrossRef]
- Tanaka, S.; Nakamura, I.; Inoue, J.-I.; Oda, H.; Nakamura, K. Signal transduction pathways regulating osteoclast differentiation and function. J. Bone Miner. Metab. 2003, 21, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL–RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Azuma, Y.; Kaji, K.; Katogi, R.; Takeshita, S.; Kudo, A. Tumor necrosis factor-α induces differentiation of and bone resorption by osteoclasts. J. Biol. Chem. 2000, 275, 4858–4864. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Takahashi, N.; Jimi, E.; Udagawa, N.; Takami, M.; Kotake, S.; Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; et al. Tumor necrosis factor α stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL–RANK interaction. J. Exp. Med. 2000, 191, 275–286. [Google Scholar] [CrossRef]
- Kitaura, H.; Sands, M.S.; Aya, K.; Zhou, P.; Hirayama, T.; Uthgenannt, B.; Wei, S.; Takeshita, S.; Novack, D.V.; Silva, M.J.; et al. Marrow stromal cells and osteoclast precursors differentially contribute to TNF-α-induced osteoclastogenesis in vivo. J. Immunol. 2004, 173, 4838–4846. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, H.; Zhou, P.; Kim, H.J.; Novack, D.V.; Ross, F.P.; Teitelbaum, S.L. M-CSF mediates TNF-induced inflammatory osteolysis. J. Clin. Investig. 2005, 115, 3418–3427. [Google Scholar] [CrossRef]
- Nissen, A.; Christensen, M.; Knop, F.K.; Vilsbøll, T.; Holst, J.J.; Hartmann, B. Glucose-dependent insulinotropic polypeptide inhibits bone resorption in humans. J. Clin. Endocrinol. Metab. 2014, 99, E2325–E2329. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.B.; Lund, A.; Calanna, S.; Jørgensen, N.R.; Holst, J.J.; Vilsbøll, T.; Knop, F.K. Glucose-dependent insulinotropic polypeptide (GIP) inhibits bone resorption independently of insulin and glycemia. J. Clin. Endocrinol. Metab. 2018, 103, 288–294. [Google Scholar] [CrossRef]
- Christensen, M.B.; Lund, A.B.; Jørgensen, N.R.; Holst, J.J.; Vilsbøll, T.; Knop, F.K. Glucose-dependent insulinotropic polypeptide (GIP) reduces bone resorption in patients with Type 2 diabetes. J. Endocr. Soc. 2020, 4, bvaa097. [Google Scholar] [CrossRef]
- Bollag, R.J.; Zhong, Q.; Ding, K.H.; Phillips, P.; Zhong, L.; Qin, F.; Cranford, J.; Mulloy, A.L.; Cameron, R.; Isales, C.M. Glucose-dependent insulinotropic peptide is an integrative hormone with osteotropic effects. Mol. Cell. Endocrinol. 2001, 177, 35–41. [Google Scholar] [CrossRef]
- Mabilleau, G.; Perrot, R.; Mieczkowska, A.; Boni, S.; Flatt, P.R.; Irwin, N.; Chappard, D. Glucose-dependent insulinotropic polypeptide (GIP) dose-dependently reduces osteoclast differentiation and resorption. Bone 2016, 91, 102–112. [Google Scholar] [CrossRef]
- Chen, S.; Okahara, F.; Osaki, N.; Shimotoyodome, A. Increased GIP signaling induces adipose inflammation via a HIF-1α-dependent pathway and impairs insulin sensitivity in mice. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E414–E425. [Google Scholar] [CrossRef] [PubMed]
- Varol, C.; Zvibel, I.; Spektor, L.; Mantelmacher, F.D.; Vugman, M.; Thurm, T.; Khatib, M.; Elmaliah, E.; Halpern, Z.; Fishman, S. Long-acting glucose-dependent insulinotropic polypeptide ameliorates obesity-induced adipose tissue inflammation. J. Immunol. 2014, 193, 4002–4009. [Google Scholar] [CrossRef]
- Kahles, F.; Liberman, A.; Halim, C.; Rau, M.; Möllmann, J.; Mertens, R.W.; Rückbeil, M.; Diepolder, I.; Walla, B.; Diebold, S.; et al. The incretin hormone GIP is upregulated in patients with atherosclerosis and stabilizes plaques in ApoE−/− mice by blocking monocyte/macrophage activation. Mol. Metab. 2018, 14, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nakamura, N.; Miyabe, M.; Nishikawa, T.; Miyajima, S.; Adachi, K.; Mizutani, M.; Kikuchi, T.; Miyazawa, K.; Goto, S.; et al. Anti-inflammatory role of glucose-dependent insulinotropic polypeptide in periodontitis. J. Diabetes Investig. 2016, 7, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amer, Y.; Ross, F.P.; Edwards, J.; Teitelbaum, S.L. Lipopolysaccharide-stimulated osteoclastogenesis is mediated by tumor necrosis factor via its P55 receptor. J. Clin. Investig. 1997, 100, 1557–1565. [Google Scholar] [CrossRef]
- Islam, S.; Hassan, F.; Tumurkhuu, G.; Dagvadorj, J.; Koide, N.; Naiki, Y.; Mori, I.; Yoshida, T.; Yokochi, T. Bacterial lipopolysaccharide induces osteoclast formation in RAW 264.7 macrophage cells. Biochem. Biophys. Res. Commun. 2007, 360, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, A.L.; Abd-El-Aleem, S.; Morales-Aza, B.; Donaldson, L.F. A model of periodontitis in the rat: Effect of lipopolysaccharide on bone resorption, osteoclast activity, and local peptidergic innervation. J. Clin. Periodontol. 2004, 31, 596–603. [Google Scholar] [CrossRef]
- Hong, C.Y.; Lin, S.K.; Kok, S.H.; Cheng, S.J.; Lee, M.S.; Wang, T.M.; Chen, C.S.; Lin, L.D.; Wang, J.S. The role of lipopolysaccharide in infectious bone resorption of periapical lesion. J. Oral Pathol. Med. 2004, 33, 162–169. [Google Scholar] [CrossRef]
- Mörmann, M.; Thederan, M.; Nackchbandi, I.; Giese, T.; Wagner, C.; Hänsch, G.M. Lipopolysaccharides (LPS) induce the differentiation of human monocytes to osteoclasts in a tumour necrosis factor (TNF) α-dependent manner: A link between infection and pathological bone resorption. Mol. Immunol. 2008, 45, 3330–3337. [Google Scholar] [CrossRef]
- Chiang, C.Y.; Kyritsis, G.; Graves, D.T.; Amar, S. Interleukin-1 and tumor necrosis factor activities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect. Immun. 1999, 67, 4231–4236. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Matsuguchi, T.; Tsuboi, N.; Mitani, A.; Tanaka, S.; Matsuoka, M.; Yamamoto, G.; Hishikawa, T.; Noguchi, T.; Yoshikai, Y. Gene expression of osteoclast differentiation factor is induced by lipopolysaccharide in mouse osteoblasts via toll-like receptors. J. Immunol. 2001, 166, 3574–3579. [Google Scholar] [CrossRef] [PubMed]
- Stensen, S.; Gasbjerg, L.S.; Helsted, M.M.; Hartmann, B.; Christensen, M.B.; Knop, F.K. GIP and the gut-bone axis—Physiological, pathophysiological and potential therapeutic implications. Peptides 2020, 125, 170197. [Google Scholar] [CrossRef] [PubMed]
- Gaudin-Audrain, C.; Irwin, N.; Mansur, S.; Flatt, P.R.; Thorens, B.; Baslé, M.; Chappard, D.; Mabilleau, G. Glucose-dependent insulinotropic polypeptide receptor deficiency leads to modifications of trabecular bone volume and quality in mice. Bone 2013, 53, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Cheng, H.; Hamrick, M.; Zhong, Q.; Ding, K.H.; Correa, D.; Williams, S.; Mulloy, A.; Bollag, W.; Bollag, R.J.; et al. Glucose-dependent insulinotropic polypeptide receptor knockout mice have altered bone turnover. Bone 2005, 37, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Park, S.J.; Kwon, M.J.; Jeong, T.S.; Bok, S.H.; Choi, W.Y.; Jeong, W.I.; Ryu, S.Y.; Do, S.H.; Lee, C.S.; et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-κB pathway in lipopolysaccharide-stimulated macrophage. Mol. Cell. Biochem. 2003, 243, 153–160. [Google Scholar] [CrossRef]
- Ma, P.; Liu, H.-T.; Wei, P.; Xu, Q.-S.; Bai, X.-F.; Du, Y.-G.; Yu, C. Chitosan oligosaccharides inhibit LPS-induced over-expression of IL-6 and TNF-α in RAW264.7 macrophage cells through blockade of mitogen-activated protein kinase (MAPK) and PI3K/Akt signaling pathways. Carbohydr. Polym. 2011, 84, 1391–1398. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, F.; Li, X.; Zhou, Y.; Yin, S.; Zhou, X.; Endodontalis, P. Porphyromonas endodontalis Lipopolysaccharides induce RANKL by mouse osteoblast in a way different from that of Escherichia coli lipopolysaccharide. J. Endod. 2011, 37, 1653–1658. [Google Scholar] [CrossRef]
- Leite, F.R.M.; Aquino, S.G.D.; Guimarães, M.R.; Cirelli, J.A.; Junior, C.R. RANKL expression is differentially modulated by TLR2 and TLR4 signaling in fibroblasts and osteoblasts. Immunol. Innov. 2014, 2, 1. [Google Scholar] [CrossRef][Green Version]
- Pacios, S.; Kang, J.; Galicia, J.; Gluck, K.; Patel, H.; Ovaydi-Mandel, A.; Petrov, S.; Alawi, F.; Graves, D.T. Diabetes aggravates periodontitis by limiting repair through enhanced inflammation. FASEB J. 2012, 26, 1423–1430. [Google Scholar] [CrossRef]
- Mahamed, D.A.; Marleau, A.; Alnaeeli, M.; Singh, B.; Zhang, X.; Penninger, J.M.; Teng, Y.T. G(-) anaerobes-reactive CD4+ T-cells trigger RANKL-mediated enhanced alveolar bone loss in diabetic NOD mice. Diabetes 2005, 54, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Hu, K.S.; Yang, H.L. Roles of TNF-α, GSK-3β and RANKL in the occurrence and development of diabetic osteoporosis. Int. J. Clin. Exp. Pathol. 2015, 8, 11995–12004. [Google Scholar] [PubMed]
- Takeshita, S.; Kaji, K.; Kudo, A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J. Bone Miner. Res. 2000, 15, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Shen, W.R.; Kimura, K.; Kishikawa, A.; Shima, K.; Ogawa, S.; Qi, J.; Ohori, F.; Noguchi, T.; Marahleh, A.; et al. DPP-4 inhibitor impedes lipopolysaccharide-induced osteoclast formation and bone resorption in vivo. Biomed. Pharmacother. 2019, 109, 242–253. [Google Scholar] [CrossRef]
- Ma, J.; Kitaura, H.; Ogawa, S.; Ohori, F.; Noguchi, T.; Marahleh, A.; Nara, Y.; Pramusita, A.; Kinjo, R.; Kanou, K.; et al. Docosahexaenoic acid inhibits TNF-α-induced osteoclast formation and orthodontic tooth movement through GPR120. Front. Immunol. 2022, 13, 929690. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, A.; Kitaura, H.; Ohori, F.; Noguchi, T.; Marahleh, A.; Ma, J.; Ren, J.; Miura, M.; Fan, Z.; Narita, K.; et al. (D-Ala2)GIP Inhibits Inflammatory Bone Resorption by Suppressing TNF-α and RANKL Expression and Directly Impeding Osteoclast Formation. Int. J. Mol. Sci. 2024, 25, 2555. https://doi.org/10.3390/ijms25052555
Lin A, Kitaura H, Ohori F, Noguchi T, Marahleh A, Ma J, Ren J, Miura M, Fan Z, Narita K, et al. (D-Ala2)GIP Inhibits Inflammatory Bone Resorption by Suppressing TNF-α and RANKL Expression and Directly Impeding Osteoclast Formation. International Journal of Molecular Sciences. 2024; 25(5):2555. https://doi.org/10.3390/ijms25052555
Chicago/Turabian StyleLin, Angyi, Hideki Kitaura, Fumitoshi Ohori, Takahiro Noguchi, Aseel Marahleh, Jinghan Ma, Jiayi Ren, Mariko Miura, Ziqiu Fan, Kohei Narita, and et al. 2024. "(D-Ala2)GIP Inhibits Inflammatory Bone Resorption by Suppressing TNF-α and RANKL Expression and Directly Impeding Osteoclast Formation" International Journal of Molecular Sciences 25, no. 5: 2555. https://doi.org/10.3390/ijms25052555
APA StyleLin, A., Kitaura, H., Ohori, F., Noguchi, T., Marahleh, A., Ma, J., Ren, J., Miura, M., Fan, Z., Narita, K., & Mizoguchi, I. (2024). (D-Ala2)GIP Inhibits Inflammatory Bone Resorption by Suppressing TNF-α and RANKL Expression and Directly Impeding Osteoclast Formation. International Journal of Molecular Sciences, 25(5), 2555. https://doi.org/10.3390/ijms25052555




