A Comprehensive Retrospective Study on the Mechanisms of Cyclic Mechanical Stretch-Induced Vascular Smooth Muscle Cell Death Underlying Aortic Dissection and Potential Therapeutics for Preventing Acute Aortic Aneurysm and Associated Ruptures
Abstract
1. Introduction
2. CMS-Induced Cell Death (Including Apoptosis) and Proliferation in VSMCs
3. CMS-Induced MAPK Activation in RASMCs
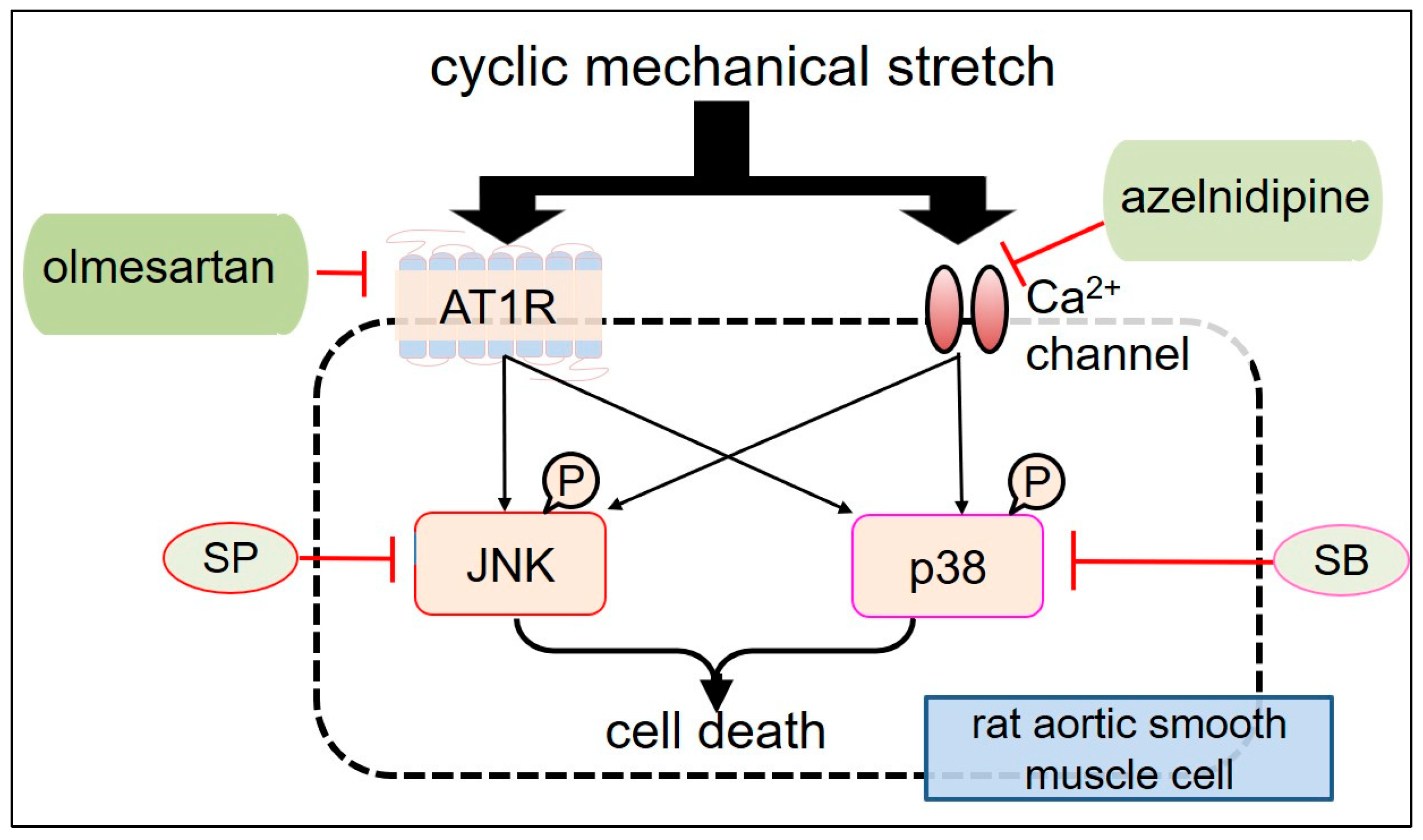
4. Azelnidipine (CS905) (CS) and Olmesartan (Olm) Protect CMS-Induced RASMC Death
5. Exploring CMS-Induced Cell Death Mechanisms
5.1. Various Mechanisms Underlying VSMC Death Induced by Bio-MS
5.2. Research on CMS-Induced Cell Death Mechanisms in Our Laboratory
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Nedkoff, L.; Briffa, T.; Zemedikun, D.; Herrington, S.; Wright, F.L. Global Trends in Atherosclerotic Cardiovascular Disease. Clin. Ther. 2023, 45, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 20, 3346. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Tian, F.; Xu, J.; Du, X.; Zhang, S.; Liu, L. New insight into dyslipidemia-induced cellular senescence in atherosclerosis. Biol. Rev. Camb. Philos. Soc. 2022, 97, 1844–1867. [Google Scholar] [CrossRef]
- Bäck, M.; Yurdagul, A., Jr.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Sayed, A.; Munir, M.; Bahbah, E.I. Aortic Dissection: A Review of the Pathophysiology, Management and Prospective Advances. Curr. Cardiol. Rev. 2021, 17, e230421186875. [Google Scholar] [CrossRef]
- McClure, R.S.; Brogly, S.B.; Lajkosz, K.; Payne, D.; Hall, S.F.; Johnson, A.P. Epidemiology and management of thoracic aortic dissections and thoracic aortic aneurysms in Ontario, Canada: A population-based study. J. Thorac. Cardiovasc. Surg. 2018, 155, 2254–2264.e4. [Google Scholar] [CrossRef]
- Fukui, T. Management of acute aortic dissection and thoracic aortic rupture. J. Intensive Care. 2018, 6, 15. [Google Scholar] [CrossRef]
- Melvinsdottir, I.H.; Lund, S.H.; Agnarsson, B.A.; Sigvaldason, K.; Gudbjartsson, T.; Geirsson, A. The incidence and mortality of acute thoracic aortic dissection: Results from a whole nation study. Eur. J. Cardiothorac. Surg. 2016, 50, 1111–1117. [Google Scholar] [CrossRef]
- Yuan, X.; Mitsis, A.; Nienaber, C.A. Current Understanding of Aortic Dissection. Life 2022, 12, 1606. [Google Scholar] [CrossRef]
- Evangelista, A.; Isselbacher, E.M.; Bossone, E.; Gleason, T.G.; Eusanio, M.D.; Sechtem, U.; Ehrlich, M.P.; Trimarchi, S.; Braverman, A.C.; Myrmel, T.; et al. IRAD Investigators. Insights From the International Registry of Acute Aortic Dissection: A 20-Year Experience of Collaborative Clinical Research. Circulation 2018, 137, 1846–1860. [Google Scholar] [CrossRef]
- Uchida, T.; Sadahiro, M. Thoracic Endovascular Aortic Repair for Acute Aortic Dissection. Ann. Vasc. Dis. 2018, 11, 464–472. [Google Scholar] [CrossRef]
- Reutersberg, B.; Salvermoser, M.; Trenner, M.; Geisbüsch, S.; Zimmermann, A.; Eckstein, H.H.; Kuehnl, A. Hospital Incidence and In-Hospital Mortality of Surgically and Interventionally Treated Aortic Dissections: Secondary Data Analysis of the Nationwide German Diagnosis-Related Group Statistics from 2006 to 2014. J. Am. Heart Assoc. 2019, 8, e011402. [Google Scholar] [CrossRef]
- Zhou, Z.; Cecchi, A.C.; Prakash, S.K.; Milewicz, D.M. Risk Factors for Thoracic Aortic Dissection. Genes 2022, 13, 1814. [Google Scholar] [CrossRef]
- Hibino, M.; Yanagawa, B.; Pandey, A.K.; Verma, S. Ambient temperature and aortic dissection: Do pipes burst in freezing weather? Eur. Heart J. 2022, 43, 236–238. [Google Scholar] [CrossRef]
- Mantella, L.E.; Quan, A.; Verma, S. Variability in vascular smooth muscle cell stretch-induced responses in 2D culture. Vasc. Cell 2015, 7, 7. [Google Scholar] [CrossRef]
- Khanafer, K.; Berguer, R. Fluid-structure interaction analysis of turbulent pulsatile flow within a layered aortic wall as related to aortic dissection. J. Biomech. 2009, 42, 2642–2648. [Google Scholar] [CrossRef]
- Sherifova, S.; Holzapfel, G.A. Biomechanics of aortic wall failure with a focus on dissection and aneurysm: A review. Acta Biomater. 2019, 99, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.J.; Dev, V.; Strauss, B.H.; Fedak, P.W.; Butany, J. Variation in the histopathological features of patients with ascending aortic aneurysms: A study of 111 surgically excised cases. J. Clin. Pathol. 2008, 61, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Wernig, F.; Mayr, M.; Xu, Q. Mechanical stretch-induced apoptosis in smooth muscle cells is mediated by beta1-integrin signaling pathways. Hypertension 2003, 41, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Mayr, M.; Hu, Y.; Hainaut, H.; Xu, Q. Mechanical stress-induced DNA damage and rac-p38MAPK signal pathways mediate p53-dependent apoptosis in vascular smooth muscle cells. FASEB J. 2002, 16, 1423–1425. [Google Scholar] [CrossRef]
- Cheng, W.P.; Wang, B.W.; Chen, S.C.; Chang, H.; Shyu, K.G. Mechanical stretch induces the apoptosis regulator PUMA in vascular smooth muscle cells. Cardiovasc. Res. 2012, 93, 181–189. [Google Scholar] [CrossRef]
- Cattaruzza, M.; Dimigen, C.; Ehrenreich, H.; Hecker, M. Stretch-induced endothelin B receptor-mediated apoptosis in vascular smooth muscle cells. FASEB J. 2000, 14, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Irani, K. Oxidant signaling in vascular cell growth, death, and survival: A review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ. Res. 2000, 87, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.B.; Zhong, J.C.; Fan, D.; Basu, R.; Morton, J.S.; Parajuli, N.; McMurtry, M.S.; Davidge, S.T.; Kassiri, Z.; Oudit, G.Y. Angiotensin-converting enzyme 2 is a critical determinant of angiotensin II-induced loss of vascular smooth muscle cells and adverse vascular remodeling. Hypertension 2014, 64, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Pollman, M.J.; Yamada, T.; Horiuchi, M.; Gibbons, G.H. Vasoactive substances regulate vascular smooth muscle cell apoptosis. Countervailing influences of nitric oxide and angiotensin II. Circ. Res. 1996, 79, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.J.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.R.; De Meyer, G.R.Y. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef]
- Bennett, M.; Yu, H.; Clarke, M. Signalling from dead cells drives inflammation and vessel remodelling. Vascul. Pharmacol. 2012, 56, 187–192. [Google Scholar] [CrossRef]
- Kanno, T.; Takahashi, T.; Tsujisawa, T.; Ariyoshi, W.; Nishihara, T. Mechanical stress-mediated Runx2 activation is dependent on Ras/ERK1/2 MAPK signaling in osteoblasts. J. Cell. Biochem. 2007, 101, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Kluge, A.; Rangrez, A.Y.; Kilian, L.S.; Pott, J.; Bernt, A.; Frauen, R.; Rohrbeck, A.; Frey, N.; Frank, D. Rho-family GTPase 1 (Rnd1) is a biomechanical stress-sensitive activator of cardiomyocyte hypertrophy. J. Mol. Cell. Cardiol. 2019, 129, 130–143. [Google Scholar] [CrossRef]
- Yamazaki, T.; Komuro, I.; Kudoh, S.; Zou, Y.; Shiojima, I.; Mizuno, T.; Takano, H.; Hiroi, Y.; Ueki, K.; Tobe, K. Mechanical stress activates protein kinase cascade of phosphorylation in neonatal rat cardiac myocytes. J. Clin. Investig. 1995, 961, 438–446. [Google Scholar] [CrossRef]
- Wei, B.; Chen, Z.; Zhang, X.; Feldman, M.; Dong, X.Z.; Doran, R.; Zhao, B.L.; Yin, W.X.; Kotlikoff, M.I.; Ji, G.U. Nitric oxide mediates stretch-induced Ca2+ release via activation of phosphatidylinositol 3-kinase-Akt pathway in smooth muscle. PLoS ONE 2008, 3, e2526. [Google Scholar] [CrossRef]
- Zhao, J.; Ozawa, K.; Kyotani, Y.; Nagayama, K.; Ito, S.; Komatsubara, A.T.; Tsuji, Y.; Yoshizumi, M. Azelnidipine inhibits cultured rat aortic smooth muscle cell death induced by cyclic mechanical stretch. PLoS ONE 2014, 9, e102813. [Google Scholar] [CrossRef]
- Ito, S.; Ozawa, K.; Zhao, J.; Kyotani, Y.; Nagayama, K.; Yoshizumi, M. Olmesartan inhibits cultured rat aortic smooth muscle cell death induced by cyclic mechanical stretch through the inhibition of the c-Jun N-terminal kinase and p38 signaling pathways. J. Pharmacol. Sci. 2015, 127, 69–74. [Google Scholar] [CrossRef]
- Zhao, J.; Nishimura, Y.; Kimura, A.; Ozawa, K.; Kondo, T.; Tanaka, T.; Yoshizumi, M. Chemokines protect vascular smooth muscle cells from cell death induced by cyclic mechanical stretch. Sci. Rep. 2017, 7, 16128. [Google Scholar] [CrossRef]
- Zhao, J.; Nakahira, K.; Kimura, A.; Kyotani, Y.; Yoshizumi, M. Upregulation of iNOS Protects Cyclic Mechanical Stretch-Induced Cell Death in Rat Aorta Smooth Muscle Cells. Int. J. Mol. Sci. 2020, 21, 8660. [Google Scholar] [CrossRef] [PubMed]
- Intengan, H.D.; Schiffrin, E.L. Vascular remodeling in hypertension: Roles of apoptosis, inflammation, and fibrosis. Hypertension 2001, 38, 581–587. [Google Scholar] [CrossRef]
- Chakraborty, R.; Chatterjee, P.; Dave, J.M.; Ostriker, A.C.; Greif, D.M.; Rzucidlo, E.M.; Martin, K.A. Targeting smooth muscle cell phenotypic switching in vascular disease. JVS Vasc. Sci. 2021, 2, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.T.; Solanki, R.; Warren, D.T. Mechanical programming of arterial smooth muscle cells in health and ageing. Biophys. Rev. 2021, 13, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.D. Mechanisms of Vascular Remodeling in Hypertension. Am. J. Hypertens. 2021, 34, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.M.; Schiffrin, E.L. Apoptosis in aorta of deoxycorticosterone acetate-salt hypertensive rats: Effect of endothelin receptor antagonism. J. Hypertens. 1997, 15, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.; Smith, R.C.; Kim, H.S. Vascular cell apoptosis in remodeling, restenosis, and plaque rupture. Circ. Res. 2000, 87, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Clarke, M.C.; Figg, N.; Littlewood, T.D.; Bennett, M.R. Smooth muscle cell apoptosis promotes vessel remodeling and repair via activation of cell migration, proliferation, and collagen synthesis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2402–2409. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Ge, S.; Zhang, L.; Che, H.; Liang, C. Aortic dissection is associated with reduced polycystin-1 expression, an abnormality that leads to increased ERK phosphorylation in vascular smooth muscle cells. Eur. J. Histochem. 2016, 60, 2711. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Guo, D.C.; Estrera, A.L.; Safi, H.J.; Huynh, T.T.; Yin, Z.; Cao, S.N.; Lin, J.; Kurian, T.; Buja, L.M.; et al. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J. Thorac. Cardiovasc. Surg. 2006, 131, 671–678. [Google Scholar] [CrossRef]
- Morrow, D.; Sweeney, C.; Birney, Y.A.; Guha, S.; Collins, N.; Cummins, P.M.; Murphy, R.; Walls, D.; Redmond, E.M.; Cahill, P.A. Biomechanical regulation of hedgehog signaling in vascular smooth muscle cells in vitro and in vivo. Am. J. Physiol. Cell Physiol. 2007, 292, C488–C496. [Google Scholar] [CrossRef]
- Cheng, W.P.; Hung, H.F.; Wang, B.W.; Shyu, K.G. The molecular regulation of GADD153 in apoptosis of cultured vascular smooth muscle cells by cyclic mechanical stretch. Cardiovasc. Res. 2008, 77, 551–559. [Google Scholar] [CrossRef]
- Bao, H.; Li, H.P.; Shi, Q.; Huang, K.; Chen, X.H.; Chen, Y.X.; Han, Y.; Xiao, Q.; Yao, Q.P.; Qi, Y.X. Lamin A/C negatively regulated by miR-124-3p modulates apoptosis of vascular smooth muscle cells during cyclic stretch application in rats. Acta Physiol. 2020, 228, e13374. [Google Scholar] [CrossRef]
- Morrow, D.; Sweeney, C.; Birney, Y.A.; Cummins, P.M.; Walls, D.; Redmond, E.M.; Cahill, P.A. Cyclic strain inhibits Notch receptor signaling in vascular smooth muscle cells in vitro. Circ. Res. 2005, 96, 567–575. [Google Scholar] [CrossRef]
- Wang, X.; Liang, X.; Zhou, L.; Liu, S.; Fang, Z.; Hu, C.; Hou, Y.; Guo, Z. Yes-associated protein reacts differently in vascular smooth muscle cells under different intensities of mechanical stretch. Aging 2022, 14, 286–296. [Google Scholar] [CrossRef]
- Liu, T.; Xu, J.; Guo, J.L.; Lin, C.Y.; Luo, W.M.; Yuan, Y.; Liu, H.; Zhang, J. YAP1 up-regulation inhibits apoptosis of aortic dissection vascular smooth muscle cells. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4632–4639. [Google Scholar]
- Wang, L.; Deng, L.; Lin, N.; Shi, Y.; Chen, J.; Zhou, Y.; Chen, D.; Liu, S.; Li, C. Berberine inhibits proliferation and apoptosis of vascular smooth muscle cells induced by mechanical stretch via the PDI/ERS and MAPK pathways. Life Sci. 2020, 259, 118253. [Google Scholar] [CrossRef]
- Wang, J.; Liu, K.; Wang, H.; Li, Z.; Li, Y.; Ping, S.; Bardeesi, A.S.A.; Guo, Y.; Zhou, Y.; Pei, T.; et al. Role of nifedipine and hydrochlorothiazide in MAPK activation and vascular smooth muscle cell proliferation and apoptosis. Herz 2017, 42, 573–584. [Google Scholar] [CrossRef]
- Ping, S.; Liu, S.; Zhou, Y.; Li, Z.; Li, Y.; Liu, K.; Bardeesi, A.S.; Wang, L.; Chen, J.; Deng, L.; et al. Protein disulfide isomerase-mediated apoptosis and proliferation of vascular smooth muscle cells induced by mechanical stress and advanced glycosylation end products result in diabetic mouse vein graft atherosclerosis. Cell Death Dis. 2017, 8, e2818. [Google Scholar] [CrossRef] [PubMed]
- Ping, S.; Li, Y.; Liu, S.; Zhang, Z.; Wang, J.; Zhou, Y.; Liu, K.; Huang, J.; Chen, D.; Wang, J.; et al. Simultaneous Increases in Proliferation and Apoptosis of Vascular Smooth Muscle Cells Accelerate Diabetic Mouse Venous Atherosclerosis. PLoS ONE 2015, 10, e0141375. [Google Scholar] [CrossRef]
- Zhong, H.Y.; Yuan, C.; Liu, X.L.; Wang, Q.Q.; Li, X.; Zhao, Y.C.; Li, X.; Liu, D.D.; Zheng, T.F.; Zhang, M. Mechanical stretch aggravates vascular smooth muscle cell apoptosis and vascular remodeling by downregulating EZH2. Int. J. Biochem. Cell Biol. 2022, 151, 106278. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Li, M.; Zhang, Y.; Chen, W.; Zhang, M.; Zhang, C.; Zhang, M. Mechanical Stretch Induces Smooth Muscle Cell Dysfunction by Regulating ACE2 via P38/ATF3 and Post-transcriptional Regulation by miR-421. Front. Physiol. 2021, 11, 540591. [Google Scholar] [CrossRef]
- Song, J.t.; Hu, B.; Qu, H.y.; Bi, C.l.; Huang, X.z.; Zhang, M. Mechanical stretch modulates microRNA 21 expression, participating in proliferation and apoptosis in cultured human aortic smooth muscle cells. PLoS ONE 2012, 7, e47657. [Google Scholar] [CrossRef]
- Sotoudeh, M.; Li, Y.S.; Yajima, N.; Chang, C.C.; Tsou, T.C.; Wang, Y.; Usami, S.; Ratcliffe, A.; Chien, S.; Shyy, J.Y. Induction of apoptosis in vascular smooth muscle cells by mechanical stretch. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1709–H1716. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.; Xu, Q. Biomechanical stress-induced signaling in smooth muscle cells: An update. Curr. Vasc. Pharmacol. 2003, 1, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Chapman, G.B.; Durante, W.; Hellums, J.D.; Schafer, A.I. Physiological cyclic stretch causes cell cycle arrest in cultured vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H748–H754. [Google Scholar] [CrossRef]
- Schad, J.F.; Meltzer, K.R.; Hicks, M.R.; Beutler, D.S.; Cao, T.V.; Standley, P.R. Cyclic strain upregulates VEGF and attenuates proliferation of vascular smooth muscle cells. Vasc. Cell 2011, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, Q. Mechanical stress-initiated signal transductions in vascular smooth muscle cells. Cell Signal. 2000, 12, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.H.; Nguyen, H.T. Molecular mechanism of apoptosis induced by mechanical forces. Int. Rev. Cytol. 2005, 245, 45–90. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cheng, L.; Hochleitner, B.W.; Xu, Q. Activation of mitogen-activated protein kinases (ERK/JNK) and AP-1 tran-scription factor in rat carotid arteries after balloon injury. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2808–2816. [Google Scholar] [CrossRef]
- Yamashita, A.; Hanna, A.K.; Hirata, S.; Dardik, A.; Sumpio, B.E. Antisense basic fibroblast growth factor alters the time course of mitogen-activated protein kinase in arterialized vein graft remodeling. J. Vasc. Surg. 2003, 37, 866–873. [Google Scholar] [CrossRef] [PubMed][Green Version]
- López-Candales, A.; Holmes, D.R.; Liao, S.; Scott, M.J.; Wickline, S.A.; Thompson, R.W. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am. J. Pathol. 1997, 150, 993–1007. [Google Scholar] [PubMed]
- Yoshimura, K.; Aoki, H.; Ikeda, Y.; Fujii, K.; Akiyama, N.; Furutani, A.; Hoshii, Y.; Tanaka, N.; Ricci, R.; Ishihara, T.; et al. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat. Med. 2005, 11, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, J.; Armstrong, J.; Holt, C.M. Mechanical stretch induces phosphorylation of p38-MAPK and apoptosis in human saphenous vein. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 451–456. [Google Scholar] [CrossRef]
- Yoshimura, K.; Aoki, H.; Ikeda, Y.; Furutani, A.; Hamano, K.; Matsuzaki, M. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase in mice. Ann. N. Y. Acad. Sci. 2006, 1085, 74–81. [Google Scholar] [CrossRef]
- Oizumi, K.; Nishino, H.; Koike, H.; Sada, T.; Miyamoto, M.; Kimura, T. Antihypertensive effects of CS-905, a novel di-hydropyridine Ca++ channel blocker. Jpn. J. Pharmacol. 1989, 51, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ram, C.V.S. Therapeutic Usefulness of a Novel Calcium Channel Blocker Azelnidipine in the Treatment of Hypertension: A Narrative Review. Cardiol. Ther. 2022, 11, 473–489. [Google Scholar] [CrossRef]
- Kurobe, H.; Matsuoka, Y.; Hirata, Y.; Sugasawa, N.; Maxfield, M.W.; Sata, M.; Kitagawa, T. Azelnidipine suppresses the progression of aortic aneurysm in wild mice model through anti-inflammatory effects. J. Thorac. Cardiovasc. Surg. 2013, 146, 1501–1508. [Google Scholar] [CrossRef]
- Pinheiro, L.C.; Oliveira-Paula, G.H. Sources and Effects of Oxidative Stress in Hypertension. Curr. Hypertens. Rev. 2020, 16, 166–180. [Google Scholar] [CrossRef]
- Wang, B.W.; Chang, H.; Shyu, K.G. Regulation of resistin by cyclic mechanical stretch in cultured rat vascular smooth muscle cells. Clin. Sci. 2009, 118, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Kyaw, M.; Yoshizumi, M.; Tsuchiya, K.; Kirima, K.; Tamaki, T. Antioxidants inhibit JNK and p38 MAPK activation but not ERK 1/2 activation by angiotensin II in rat aortic smooth muscle cells. Hypertens. Res. 2001, 24, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, T.; Sato, K.; Kishimoto, K.; Yamazaki, Y.; Horiguchi, N.; Ichikawa, T.; Kakizaki, S.; Takagi, H.; Izumi, T.; Mori, M. Azelnidipine is a calcium blocker that attenuates liver fibrosis and may increase antioxidant defence. Br. J. Pharmacol. 2012, 165, 1173–1187. [Google Scholar] [CrossRef]
- Kurashiki, T.; Miyake, T.; Nakagami, H.; Nishimura, M.; Morishita, R. Prevention of Progression of Aortic Aneurysm by Peptide Vaccine Against Ang II (Angiotensin II) in a Rat Model. Hypertension 2020, 76, 1879–1888. [Google Scholar] [CrossRef]
- Zou, Y.; Akazawa, H.; Qin, Y.; Sano, M.; Takano, H.; Minamino, T.; Makita, N.; Iwanaga, K.; Zhu, W.; Kudoh, S.; et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat. Cell Biol. 2004, 6, 499–506. [Google Scholar] [CrossRef]
- Schleifenbaum, J.; Kassmann, M.; Szijártó, I.A.; Hercule, H.C.; Tano, J.Y.; Weinert, S.; Heidenreich, M.; Pathan, A.R.; Anistan, Y.M.; Alenina, N.; et al. Stretch-activation of angiotensin II type 1a receptors contributes to the myogenic response of mouse mesenteric and renal arteries. Circ. Res. 2014, 115, 263–272. [Google Scholar] [CrossRef]
- Werner, C.; Baumhäkel, M.; Teo, K.K.; Schmieder, R.; Mann, J.; Unger, T.; Yusuf, S.; Böhm, M. RAS blockade with ARB and ACE inhibitors: Current perspective on rationale and patient selection. Clin. Res. Cardiol. 2008, 97, 418–431. [Google Scholar] [CrossRef]
- Silverstein, R.L.; Fenves, A.Z.; Ram, C.V. ARBs and target organ protection. Exploring benefits beyond their antihypertensive effects. Postgrad. Med. 2004, 116, 31–38+41. [Google Scholar] [CrossRef]
- Deedwania, P.; Weber, M.; Reimitz, P.E.; Bakris, G. Olmesartan-based monotherapy vs combination therapy in hypertension: A meta-analysis based on age and chronic kidney disease status. J. Clin. Hypertens. 2017, 19, 1309–1318. [Google Scholar] [CrossRef]
- Qin, Y.; Yasuda, N.; Akazawa, H.; Ito, K.; Kudo, Y.; Liao, C.H.; Yamamoto, R.; Miura, S.; Saku, K.; Komuro, I. Multivalent ligand-receptor interactions elicit inverse agonist activity of AT(1) receptor blockers against stretch-induced AT(1) receptor activation. Hypertens. Res. 2009, 32, 875–883. [Google Scholar] [CrossRef]
- Kyotani, Y.; Zhao, J.; Tomita, S.; Nakayama, H.; Isosaki, M.; Uno, M.; Yoshizumi, M. Olmesartan inhibits angiotensin II-Induced migration of vascular smooth muscle cells through Src and mitogen-activated protein kinase pathways. J. Pharmacol. Sci. 2010, 113, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Kjeldsen, S.E.; Kreutz, R.; Pathak, A.; Grassi, G.; Esler, M. Individualized Beta-Blocker Treatment for High Blood Pressure Dictated by Medical Comorbidities: Indications Beyond the 2018 European Society of Cardiology/European Society of Hypertension Guidelines. Hypertension 2022, 79, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.W.; Lee, S.J.; Kim, Y.H.; Bae, J.U.; Park, S.Y.; Bae, S.S.; Kim, C.D. Mechanical stretch increases MMP-2 production in vascular smooth muscle cells via activation of PDGFR-β/Akt signaling pathway. PLoS ONE 2013, 8, e70437. [Google Scholar] [CrossRef] [PubMed]
- Li, H.P.; Liu, J.T.; Chen, Y.X.; Wang, W.B.; Han, Y.; Yao, Q.P.; Qi, Y.X. Suppressed nuclear envelope proteins activate autophagy of vascular smooth muscle cells during cyclic stretch application. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118855. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, T.; Yamada, S.; Guo, X.; Tanimoto, A.; Wang, K.Y.; Nabeshima, A.; Kitada, S.; Noguchi, H.; Kimura, S.; Shimajiri, S.; et al. Apoptosis signal-regulating kinase 1 deficiency attenuates vascular injury-induced neointimal hyperplasia by suppressing apoptosis in smooth muscle cells. Am. J. Pathol. 2013, 182, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.X.; Zhang, W.M.; Zhang, H.J.; Li, T.T.; Wang, Y.L.; Qin, Y.W.; Gu, H.; Du, J. Mechanical stretch-induced endoplasmic reticulum stress.; apoptosis and inflammation contribute to thoracic aortic aneurysm and dissection. J. Pathol. 2015, 236, 373–383. [Google Scholar] [CrossRef]
- Takaguri, A.; Kubo, T.; Mori, M.; Satoh, K. The protective role of YAP1 on ER stress-induced cell death in vascular smooth muscle cells. Eur. J. Pharmacol. 2017, 815, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal. Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Schober, A.; Weber, C. Chemokines and microRNAs in atherosclerosis. Cell. Mol. Life Sci. 2015, 72, 3253–3266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Subramanian, P.; Sevilmis, G.; Globke, B.; Soehnlein, O.; Karshovska, E.; Megens, R.; Heyll, K.; Chun, J.; Saulnier-Blache, J.S.; et al. Lipoprotein-derived lysophosphatidic acid promotes atherosclerosis by releasing CXCL1 from the endothelium. Cell Metab. 2011, 13, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Landsman, L.; Bar-On, L.; Zernecke, A.; Kim, K.W.; Krauthgamer, R.; Shagdarsuren, E.; Lira, S.A.; Weissman, I.L.; Weber, C.; Jung, S. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood 2009, 113, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Moatti, D.; Faure, S.; Fumeron, F.; Amara, M.-W.; Seknadji, P.; McDermott, D.H.; Debré, P.; Aumont, M.C.; Murphy, P.M.; de Prost, D.; et al. Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood 2001, 97, 1925–1928. [Google Scholar] [CrossRef] [PubMed]
- Lesnik, P.; Haskell, C.A.; Charo, I.F. Decreased atherosclerosis in CX3CR1-/- mice reveals a role for fractalkine in atherogenesis. J. Clin. Investig. 2003, 111, 333–340. [Google Scholar] [CrossRef]
- Boisvert, W.A.; Rose, D.M.; Johnson, K.A.; Fuentes, M.E.; Lira, S.A.; Curtiss, L.K.; Terkeltaub, R.A. Up-regulated expression of the CXCR2 ligand KC/GRO-alpha in atherosclerotic lesions plays a central role in macrophage accumulation and lesion progression. Am. J. Pathol. 2006, 168, 1385–1395. [Google Scholar] [CrossRef]
- Roy, R.; Wilcox, J.; Webb, A.J.; O’Gallagher, K. Dysfunctional and Dysregulated Nitric Oxide Synthases in Cardiovascular Disease: Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 15200. [Google Scholar] [CrossRef]
- Uijl, E.; Ye, D.; Ren, L.; Mirabito Colafella, K.M.; van Veghel, R.; Garrelds, I.M.; Lu, H.S.; Daugherty, A.; Hoorn, E.J.; Nioi, P.; et al. Conventional Vasopressor and Vasopressor-Sparing Strategies to Counteract the Blood Pres-sure-Lowering Effect of Small Interfering RNA Targeting Angiotensinogen. J. Am. Heart Assoc. 2022, 11, e026426. [Google Scholar] [CrossRef] [PubMed]
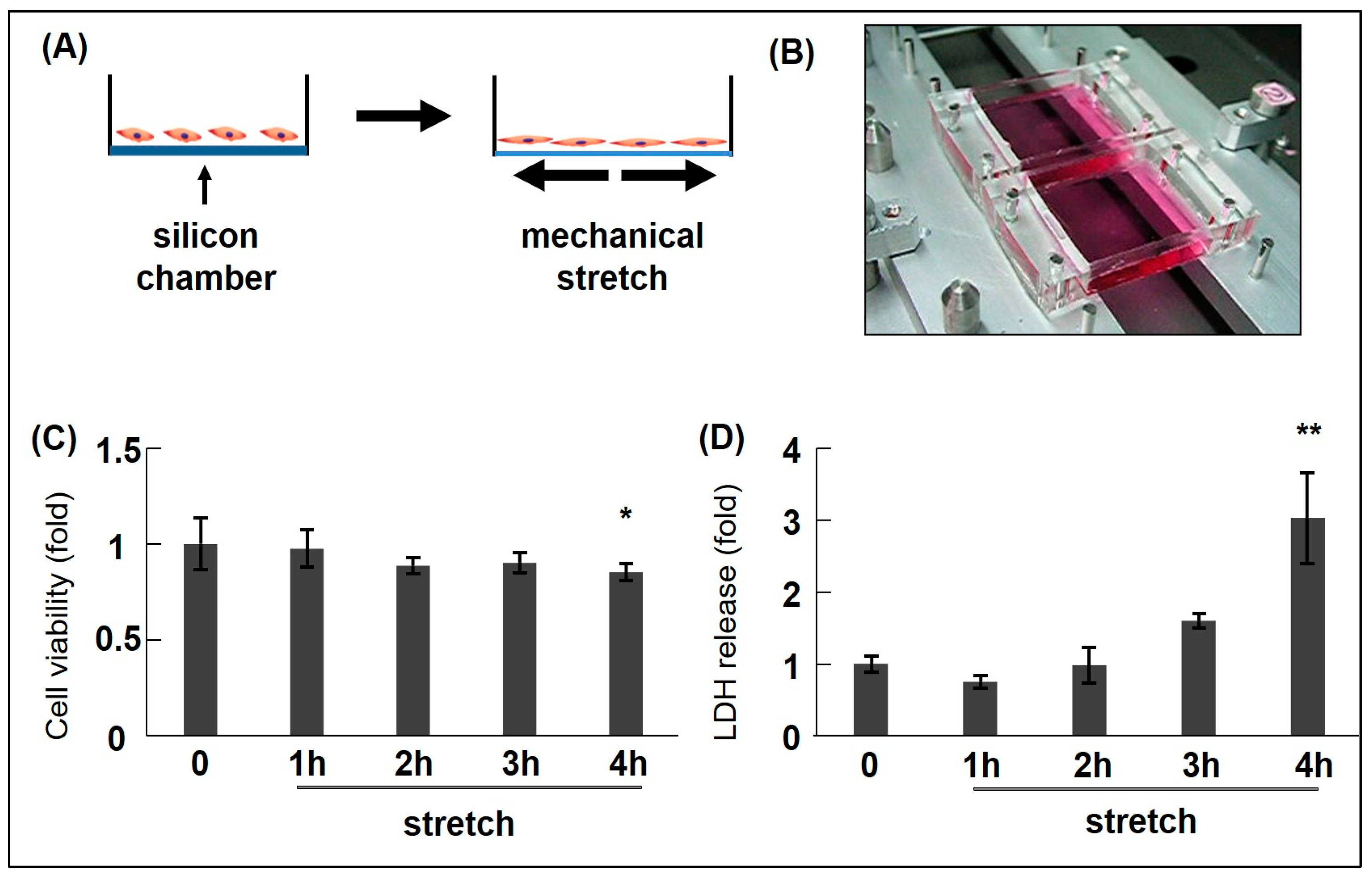

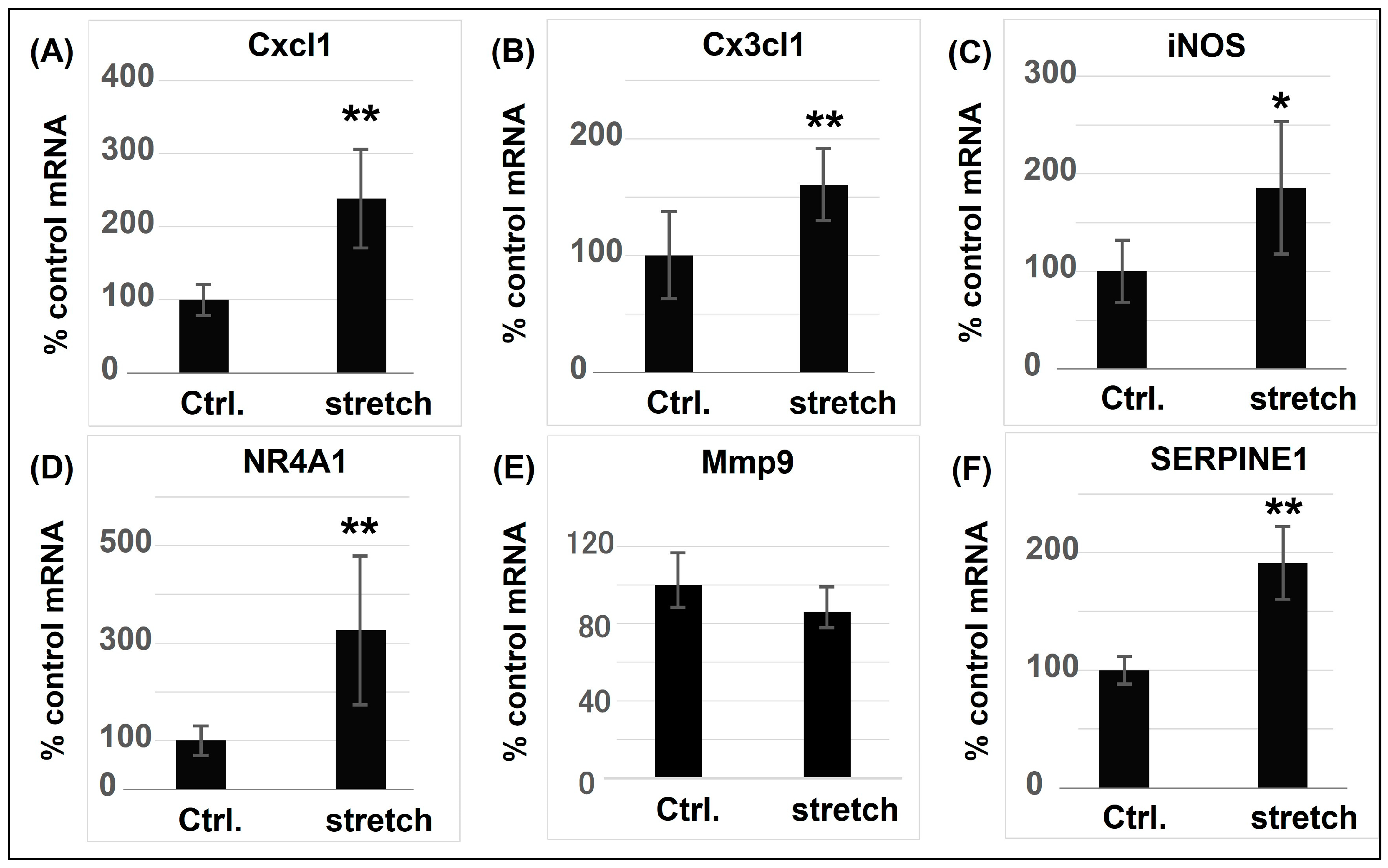

| Cell Type (ASMC) | Type of Stretch Frequency, Intensity, Duration | Functions | Related Molecules | Study |
|---|---|---|---|---|
| Rat | uniaxial model, 1 Hz, 15%, 4 h | cell death ↑ | iNOS, p38, STAT1 | 36 |
| Rat | FX-5000T, 1.25 Hz, 15%, 24 h | apoptosis ↑ | Lamin A/C, miR-124-3p, TP53, CREB1, MYC, STAT1/5/6, JUN | 48 |
| Rat | uniaxial model, 1 Hz, 15%, 4 h | cell death ↑ | Cxcl1, Cx3cl1, JNK, STAT1 | 35 |
| Rat | Flexcell, 1 Hz, 18%, 48 h | apoptosis ↑ | YAP1 | 51 |
| Rat | uniaxial model, 1 Hz, 15%, 4 h | cell death ↑ | JNK, p38, ERK1/2 | 34 |
| Rat | FX-2000, 1 Hz, 10–20%, 6 h up to 18 h | apoptosis ↑ | GADD153, TNF-α, JNK, AP-1, caspase-3 | 47 |
| Rat | FX-4000T, 1 Hz, 0–10%, 0–24 h | apoptosis ↑ | Notch 3, sonic hedgehog, patched 1 | 46 |
| Rat | FX-4000T, 1 Hz, 0–15%, 0–24 h | apoptosis ↑ | Notch 3 receptor, MAPK, Gi-protein, caspase-3, Bax, Bcl-xL | 49 |
| Rat | FX4000, 1 Hz, 7% or 15%, 6 h | apoptosis ↑ | β1-integrin, Rac, p38, p53 | 20 |
| Rat | FX-3000, 0.5 Hz, 20%, 6 h | apoptosis ↑ | ET-1, endothelin B receptor | 23 |
| Mouse | FX-5000, 1 Hz, 12%, 24 h | apoptosis ↑ | EZH2, YAP, TEAD1 | 56 |
| Human | FX-2000, 1 Hz, 10–20%, 18 h | apoptosis ↑ | PUMA, JNK, IFN-γ, IRF-1 | 22 |
| Porcine | Equi-biaxial dynamic stretch, 1 Hz, 7% or 25%, 24 h up to 72 h | apoptosis ↑ | TNF-α receptor-1, TRAF-2, JNK, p38, TNF-α | 59 |
| Rat, Mouse, Human | FX-3000, 1 Hz, 5% to 25%, 10 min up to 6 h | apoptosis ↑ | Rac, p38, p53, Bcl-2, Bcl-xL, Bax | 21 |
| Cell Type (ASMC) | Type of Stretch Frequency, Intensity, Duration | Functions | Related Molecules | Study |
|---|---|---|---|---|
| Rat | FX-5000T, 1 Hz, 15%, 24 h FX-5000T, 1 Hz 10% or 20%, 24 h | apoptosis ↓, proliferation ↑ apoptosis ↑, proliferation ↓ | YAP | 50 |
| Mouse | FX-3000, 1 Hz, 10%, 1 h | apoptosis ↑, proliferation ↑ | PDI, ERK, JNK, p38, caspase-3/12 | 52 |
| Mouse | FX-3000, 1 Hz, 0–10%, 1 h | apoptosis ↑, proliferation ↑ | ERK, JNK, p38 | 53 |
| Mouse | FX-3000, 1 Hz, 10%, 1 h | apoptosis ↑, proliferation ↑ | ERK, JNK, p38, NF-κB/p65, caspase-3 | 55 |
| Mouse | FX-3000, 1 Hz, 10%, 1 h | apoptosis ↑, proliferation ↑ | PDI, NOX1, ROS, caspase-3 | 54 |
| Human | FX-5000T, 1 Hz,18%, 12 h | apoptosis ↑, proliferation ↑ | p38, ATF3, ACE2, miR-421 | 57 |
| Human | FX-5000, 1 Hz, 10–16%, 12 h | apoptosis ↑, proliferation ↑ | miR-21, NF-κB, AP-1, PDCD4 | 58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Yoshizumi, M. A Comprehensive Retrospective Study on the Mechanisms of Cyclic Mechanical Stretch-Induced Vascular Smooth Muscle Cell Death Underlying Aortic Dissection and Potential Therapeutics for Preventing Acute Aortic Aneurysm and Associated Ruptures. Int. J. Mol. Sci. 2024, 25, 2544. https://doi.org/10.3390/ijms25052544
Zhao J, Yoshizumi M. A Comprehensive Retrospective Study on the Mechanisms of Cyclic Mechanical Stretch-Induced Vascular Smooth Muscle Cell Death Underlying Aortic Dissection and Potential Therapeutics for Preventing Acute Aortic Aneurysm and Associated Ruptures. International Journal of Molecular Sciences. 2024; 25(5):2544. https://doi.org/10.3390/ijms25052544
Chicago/Turabian StyleZhao, Jing, and Masanori Yoshizumi. 2024. "A Comprehensive Retrospective Study on the Mechanisms of Cyclic Mechanical Stretch-Induced Vascular Smooth Muscle Cell Death Underlying Aortic Dissection and Potential Therapeutics for Preventing Acute Aortic Aneurysm and Associated Ruptures" International Journal of Molecular Sciences 25, no. 5: 2544. https://doi.org/10.3390/ijms25052544
APA StyleZhao, J., & Yoshizumi, M. (2024). A Comprehensive Retrospective Study on the Mechanisms of Cyclic Mechanical Stretch-Induced Vascular Smooth Muscle Cell Death Underlying Aortic Dissection and Potential Therapeutics for Preventing Acute Aortic Aneurysm and Associated Ruptures. International Journal of Molecular Sciences, 25(5), 2544. https://doi.org/10.3390/ijms25052544






