Quantitative MRI Evaluation of Ferritin Overexpression in Non-Small-Cell Lung Cancer
Abstract
1. Introduction
2. Results
2.1. Iron Metabolism Is Dysregulated in NSCLC
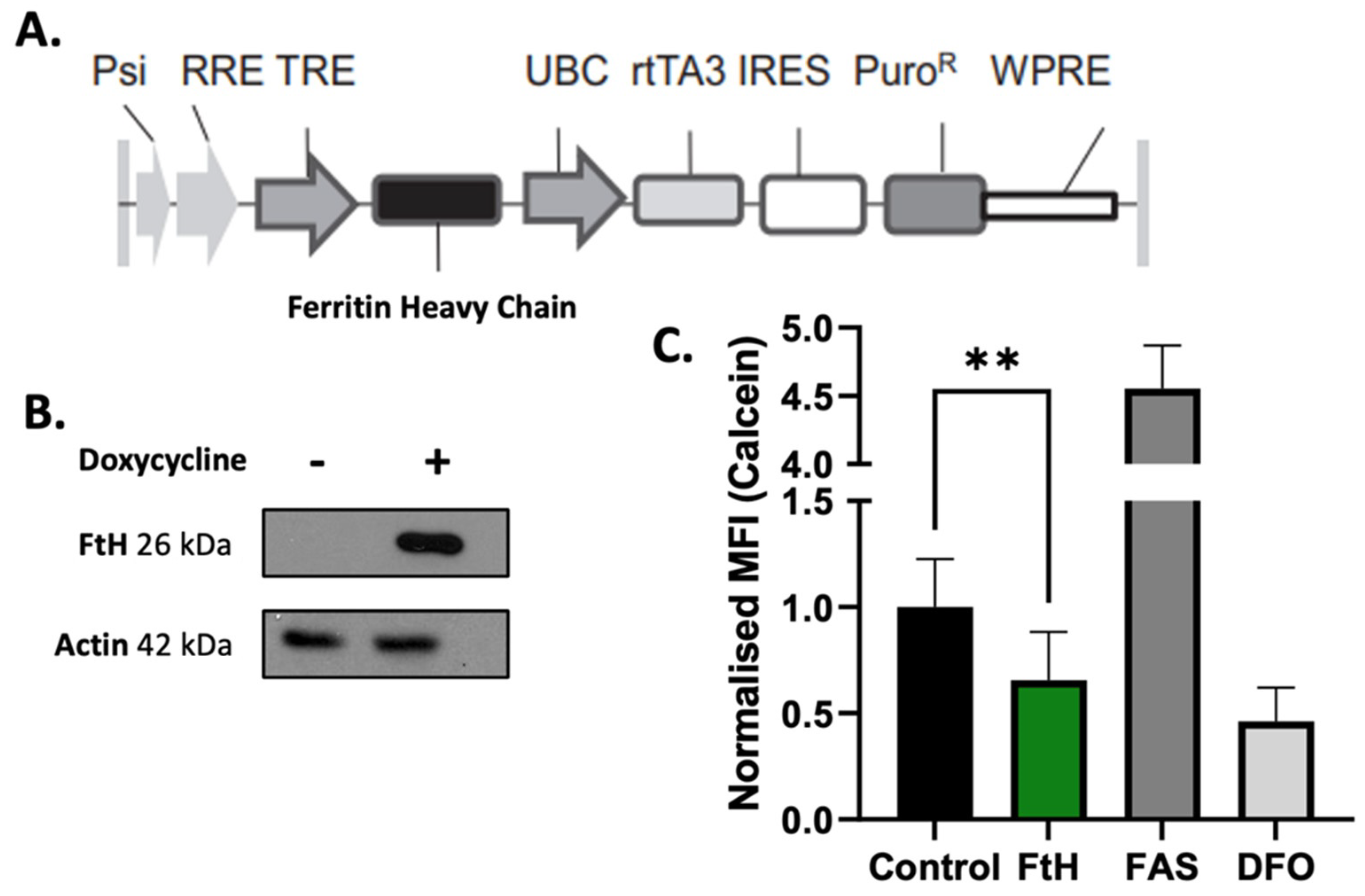
2.2. In Vitro Validation of Ferritin Overexpression in NSCLC Cells
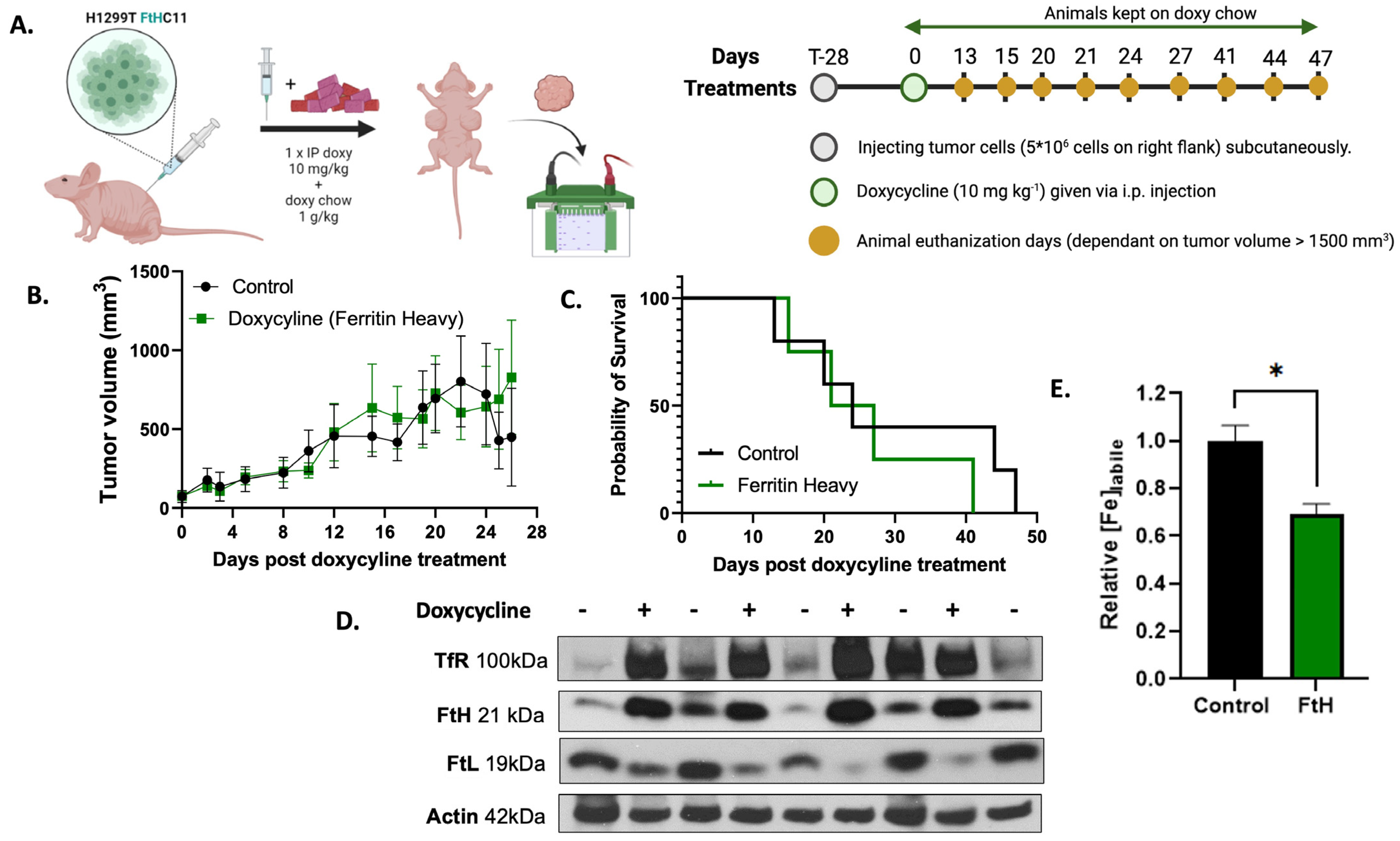
2.3. In Vivo Validation of H1299T FtH C11 Cells
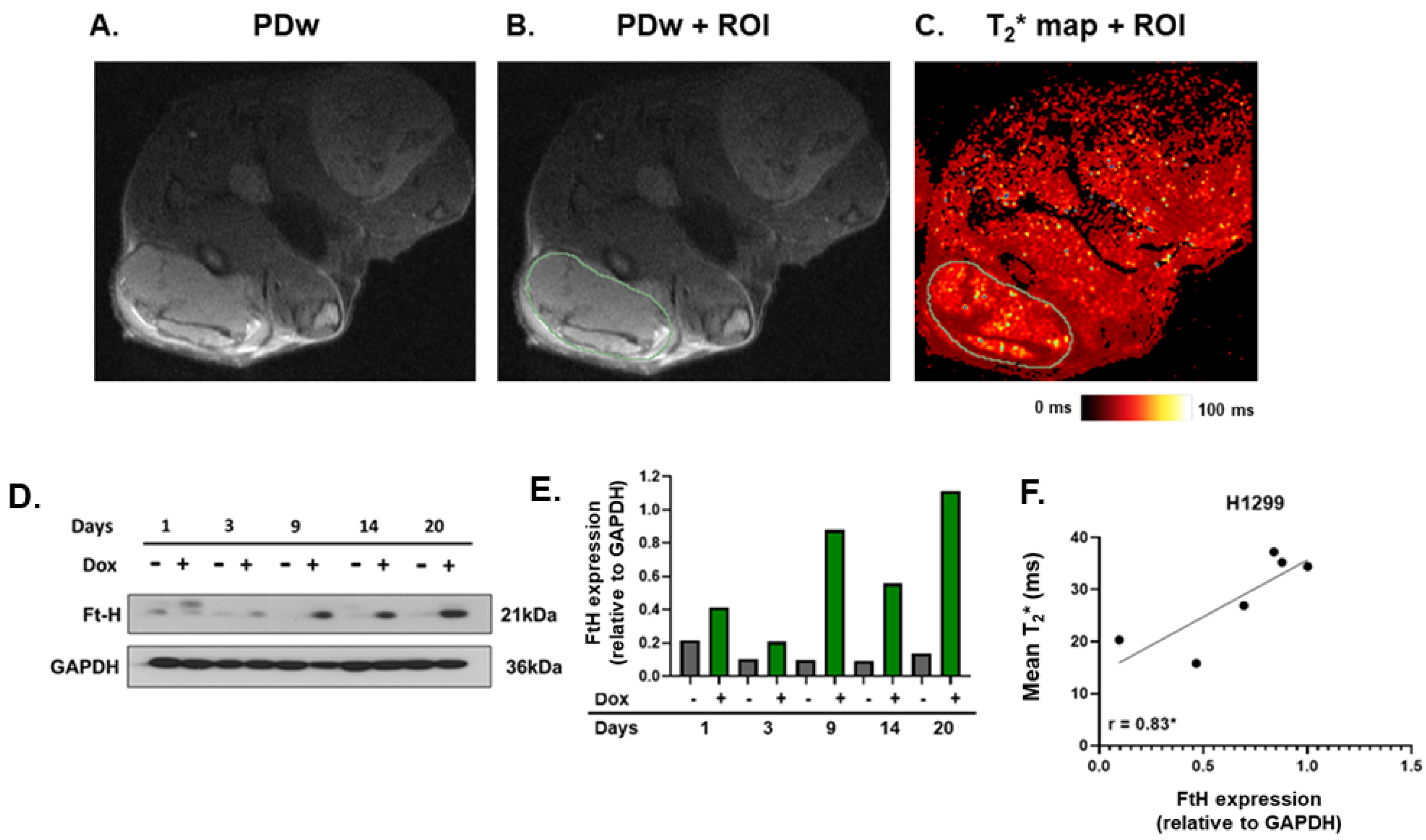
2.4. MRI T2* Relaxation Times Positively Correlated with Ferritin Overexpression
3. Discussion
3.1. Model Validation
3.2. Implications of T2* Detection of Ferritin Expression
3.3. Study Limitations: Technical Challenges and Future Perspectives
4. Conclusions
5. Methods
5.1. Cell Culture and Ferritin Overexpression Development
5.2. Western Blot Analysis
5.3. Animal Studies
5.3.1. Immune-Deficient Model
5.3.2. Immune-Competent Model
5.3.3. Procedure
5.4. Labile Iron Measurement in Cultured Cells
5.5. EPR Measurements
5.6. MRI Parameters
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torti, S.V.; Torti, F.M. Iron and Cancer: More Ore to Be Mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Petronek, M.S.; Spitz, D.R.; Buettner, G.R.; Allen, B.G. Linking Cancer Metabolic Dysfunction and Genetic Instability through the Lens of Iron Metabolism. Cancers 2019, 11, 1077. [Google Scholar] [CrossRef] [PubMed]
- Sukiennicki, G.M.; Marciniak, W.; Muszyńska, M.; Baszuk, P.; Gupta, S.; Białkowska, K.; Jaworska-Bieniek, K.; Durda, K.; Lener, M.; Pietrzak, S.; et al. Iron Levels, Genes Involved in Iron Metabolism and Antioxidative Processes and Lung Cancer Incidence. PLoS ONE 2019, 14, e0208610. [Google Scholar] [CrossRef] [PubMed]
- Alemán, M.R.; Santolaria, F.; Batista, N.; de La Vega, M.J.; González-Reimers, E.; Milena, A.; Llanos, M.; Luis Gómez-Sirvent, J. Leptin Role in Advanced Lung Cancer. A Mediator of the Acute Phase Response or a Marker of the Status of Nutrition? Cytokine 2002, 19, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Ferrigno, D.; Buccheri, G. Serum Ferritin Levels in Lung Cancer Patients. Eur. J. Cancer 1992, 28, 241. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Meral, M.; Kaynar, H.; Polat, H.; Ucar, E.Y. Relationship between Serum Levels of Some Acute-Phase Proteins and Stage of Disease and Performance Status in Patients with Lung Cancer. Med. Sci. Monit. 2007, 13, 195–200. [Google Scholar]
- Petronek, M.S.; Spitz, D.R.; Allen, B.G. Iron–Sulfur Cluster Biogenesis as a Critical Target in Cancer. Antioxidants 2021, 10, 1458. [Google Scholar] [CrossRef]
- Petronek, M.S.; Tomanek-Chalkley, A.M.; Monga, V.; Milhem, M.M.; Miller, B.J.; Magnotta, V.A.; Allen, B.G. Detection of Ferritin Expression in Soft Tissue Sarcomas With MRI: Potential Implications for Iron Metabolic Therapy. Iowa Orthop. J. 2022, 42, 255–262. [Google Scholar]
- Bayanbold, K.; Singhania, M.; Fath, M.A.; Searby, C.C.; Stolwijk, J.M.; Henrich, J.B.; Pulliam, C.F.; Schoenfeld, J.D.; Mapuskar, K.A.; Sho, S.; et al. Depletion of Labile Iron Induces Replication Stress and Enhances Responses to Chemoradiation in Non-Small-Cell Lung Cancer. Antioxidants 2023, 12, 2005. [Google Scholar] [CrossRef]
- Harrison, P.M.; Arosio, P. The Ferritins: Molecular Properties, Iron Storage Function and Cellular Regulation. Biochim. Biophys. Acta (BBA)-Bioenerg. 1996, 1275, 161–203. [Google Scholar] [CrossRef]
- Biamonte, F.; Battaglia, A.M.; Zolea, F.; Oliveira, D.M.; Aversa, I.; Santamaria, G.; Giovannone, E.D.; Rocco, G.; Viglietto, G.; Costanzo, F. Ferritin Heavy Subunit Enhances Apoptosis of Non-Small Cell Lung Cancer Cells through Modulation of miR-125b/P53 Axis. Cell Death Dis. 2018, 9, 1174. [Google Scholar] [CrossRef] [PubMed]
- Chavhan, G.B.; Babyn, P.S.; Thomas, B.; Shroff, M.M.; Haacke, E.M. Principles, Techniques, and Applications of T2*-Based MR Imaging and Its Special Applications. RadioGraphics 2009, 29, 1433–1449. [Google Scholar] [CrossRef]
- Petronek, M.S.; Monga, V.; Bodeker, K.L.; Kwofie, M.; Lee, C.-Y.; Mapuskar, K.A.; Stolwijk, J.M.; Zaher, A.; Wagner, B.A.; Smith, M.C.; et al. Magnetic Resonance Imaging of Iron Metabolism with T2* Mapping Predicts an Enhanced Clinical Response to Pharmacologic Ascorbate in Patients with GBM. Clin. Cancer Res. 2023, 30, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Petronek, M.S.; St-Aubin, J.J.; Lee, C.Y.; Spitz, D.R.; Gillan, E.G.; Allen, B.G.; Magnotta, V.A. Quantum Chemical Insight into the Effects of the Local Electron Environment on T2*-Based MRI. Sci. Rep. 2021, 11, 20817. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Á.; Munkácsy, G.; Győrffy, B. Pancancer Survival Analysis of Cancer Hallmark Genes. Sci. Rep. 2021, 11, 6047. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, B.; Chen, F.; Song, Z.; Han, B.; Meng, Y.; Hou, J.; Cao, P.; Chang, Y.; Tan, K. Hepcidin Upregulation in Lung Cancer: A Potential Therapeutic Target Associated With Immune Infiltration. Front. Immunol. 2021, 12, 612144. [Google Scholar] [CrossRef] [PubMed]
- Thielmann, C.M.; Costa da Silva, M.; Muley, T.; Meister, M.; Herpel, E.; Muckenthaler, M.U. Iron Accumulation in Tumor-Associated Macrophages Marks an Improved Overall Survival in Patients with Lung Adenocarcinoma. Sci. Rep. 2019, 9, 11326. [Google Scholar] [CrossRef]
- Westwood, M.; Anderson, L.J.; Firmin, D.N.; Gatehouse, P.D.; Charrier, C.C.; Wonke, B.; Pennell, D.J. A Single Breath-Hold Multiecho T2* Cardiovascular Magnetic Resonance Technique for Diagnosis of Myocardial Iron Overload. J. Magn. Reson. Imaging 2003, 18, 33–39. [Google Scholar] [CrossRef]
- Galimberti, S.; Trombini, P.; Bernasconi, D.P.; Redaelli, I.; Pelucchi, S.; Bovo, G.; Di Gennaro, F.; Zucchini, N.; Paruccini, N.; Piperno, A. Simultaneous Liver Iron and Fat Measures by Magnetic Resonance Imaging in Patients with Hyperferritinemia. Scand. J. Gastroenterol. 2015, 50, 429–438. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Petronek, M.S.; Monga, V.; Miller, B.J.; Milhem, M.M.; Magnotta, V.A.; Allen, B.G. T2* Imaging Assessment of Neoadjuvant Radiation Therapy Combined With Pharmacological Ascorbate in Extremity Soft-Tissue Sarcomas: A Pilot Study. Iowa Orthop. J. 2023, 43, 60–69. [Google Scholar]
- Yu, J.; Xue, Y.; Song, H.K. Comparison of Lung T2* During Free-Breathing at 1.5T and 3.0T with Ultrashort Echo Time (UTE) Imaging. Magn. Reson. Med. 2011, 66, 248–254. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Song, F.; Tian, M.; Shi, B.; Jiang, H.; Xu, W.; Wang, H.; Zhou, M.; Pan, X.; et al. EGFR Regulates Iron Homeostasis to Promote Cancer Growth through Redistribution of Transferrin Receptor 1. Cancer Lett. 2016, 381, 331–340. [Google Scholar] [CrossRef]
- Zamani, F.; Razmjou, S.; Akhlaghpoor, S.; Eslami, S.-M.; Azarkeivan, A.; Amiri, A. T2* Magnetic Resonance Imaging of the Liver in Thalassemic Patients in Iran. World J. Gastroenterol. 2011, 17, 522–525. [Google Scholar] [CrossRef]
- Alizadeh, K.; Sun, Q.; McGuire, T.; Thompson, T.; Prato, F.S.; Koropatnick, J.; Gelman, N.; Goldhawk, D.E. Hepcidin-Mediated Iron Regulation in P19 Cells Is Detectable by Magnetic Resonance Imaging. Sci. Rep. 2020, 10, 3163. [Google Scholar] [CrossRef]
- Mancias, J.D.; Wang, X.; Gygi, S.P.; Harper, J.W.; Kimmelman, A.C. Quantitative Proteomics Identifies NCOA4 as the Cargo Receptor Mediating Ferritinophagy. Nature 2014, 509, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.W.; Amante, J.J.; Chhoy, P.; Elaimy, A.L.; Liu, H.; Zhu, L.J.; Baer, C.E.; Dixon, S.J.; Mercurio, A.M. Prominin2 Drives Ferroptosis Resistance by Stimulating Multivesicular Body/Exosome-Mediated Iron Export. Dev. Cell 2019, 51, 575–586.e4. [Google Scholar] [CrossRef] [PubMed]
- Vignaud, A.; Maître, X.; Guillot, G.; Durand, E.; de Rochefort, L.; Robert, P.; Vivès, V.; Santus, R.; Darrasse, L. Magnetic Susceptibility Matching at the Air–Tissue Interface in Rat Lung by Using a Superparamagnetic Intravascular Contrast Agent: Influence on Transverse Relaxation Time of Hyperpolarized Helium-3. Magn. Reson. Med. 2005, 54, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Bergin, C.J.; Pauly, J.M.; Macovski, A. Lung Parenchyma: Projection Reconstruction MR Imaging. Radiology 1991, 179, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewski, M.R.; Dominguez-Viqueira, W.; Ortiz, A.; Shi, Y.; Costello, J.R.; Enderling, H.; Rosenberg, S.A.; Gillies, R.J. Heterogeneity Analysis of MRI T2 Maps for Measurement of Early Tumor Response to Radiotherapy. NMR Biomed. 2021, 34, e4454. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Liang, X.; Zhao, T.; Ding, C.; Zhang, M. Is the Standard Deviation of the Apparent Diffusion Coefficient a Potential Tool for the Preoperative Prediction of Tumor Grade in Endometrial Cancer? Acta Radiol. 2020, 61, 1724–1732. [Google Scholar] [CrossRef]
- Moser, J.C.; Rawal, M.; Wagner, B.A.; Du, J.; Cullen, J.J.; Buettner, G.R. Pharmacological Ascorbate and Ionizing Radiation (IR) Increase Labile Iron in Pancreatic Cancer. Redox Biol. 2014, 2, 22–27. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singhania, M.; Zaher, A.; Pulliam, C.F.; Bayanbold, K.; Searby, C.C.; Schoenfeld, J.D.; Mapuskar, K.A.; Fath, M.A.; Allen, B.G.; Spitz, D.R.; et al. Quantitative MRI Evaluation of Ferritin Overexpression in Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2024, 25, 2398. https://doi.org/10.3390/ijms25042398
Singhania M, Zaher A, Pulliam CF, Bayanbold K, Searby CC, Schoenfeld JD, Mapuskar KA, Fath MA, Allen BG, Spitz DR, et al. Quantitative MRI Evaluation of Ferritin Overexpression in Non-Small-Cell Lung Cancer. International Journal of Molecular Sciences. 2024; 25(4):2398. https://doi.org/10.3390/ijms25042398
Chicago/Turabian StyleSinghania, Mekhla, Amira Zaher, Casey F. Pulliam, Khaliunaa Bayanbold, Charles C. Searby, Joshua D. Schoenfeld, Kranti A. Mapuskar, Melissa A. Fath, Bryan G. Allen, Douglas R. Spitz, and et al. 2024. "Quantitative MRI Evaluation of Ferritin Overexpression in Non-Small-Cell Lung Cancer" International Journal of Molecular Sciences 25, no. 4: 2398. https://doi.org/10.3390/ijms25042398
APA StyleSinghania, M., Zaher, A., Pulliam, C. F., Bayanbold, K., Searby, C. C., Schoenfeld, J. D., Mapuskar, K. A., Fath, M. A., Allen, B. G., Spitz, D. R., & Petronek, M. S. (2024). Quantitative MRI Evaluation of Ferritin Overexpression in Non-Small-Cell Lung Cancer. International Journal of Molecular Sciences, 25(4), 2398. https://doi.org/10.3390/ijms25042398






