NTRK Therapy among Different Types of Cancers, Review and Future Perspectives
Abstract
1. Introduction and Epidemiology
2. Different Methods for NTRK Gene Fusion Detection
3. TRK Biology and Ontogenesis
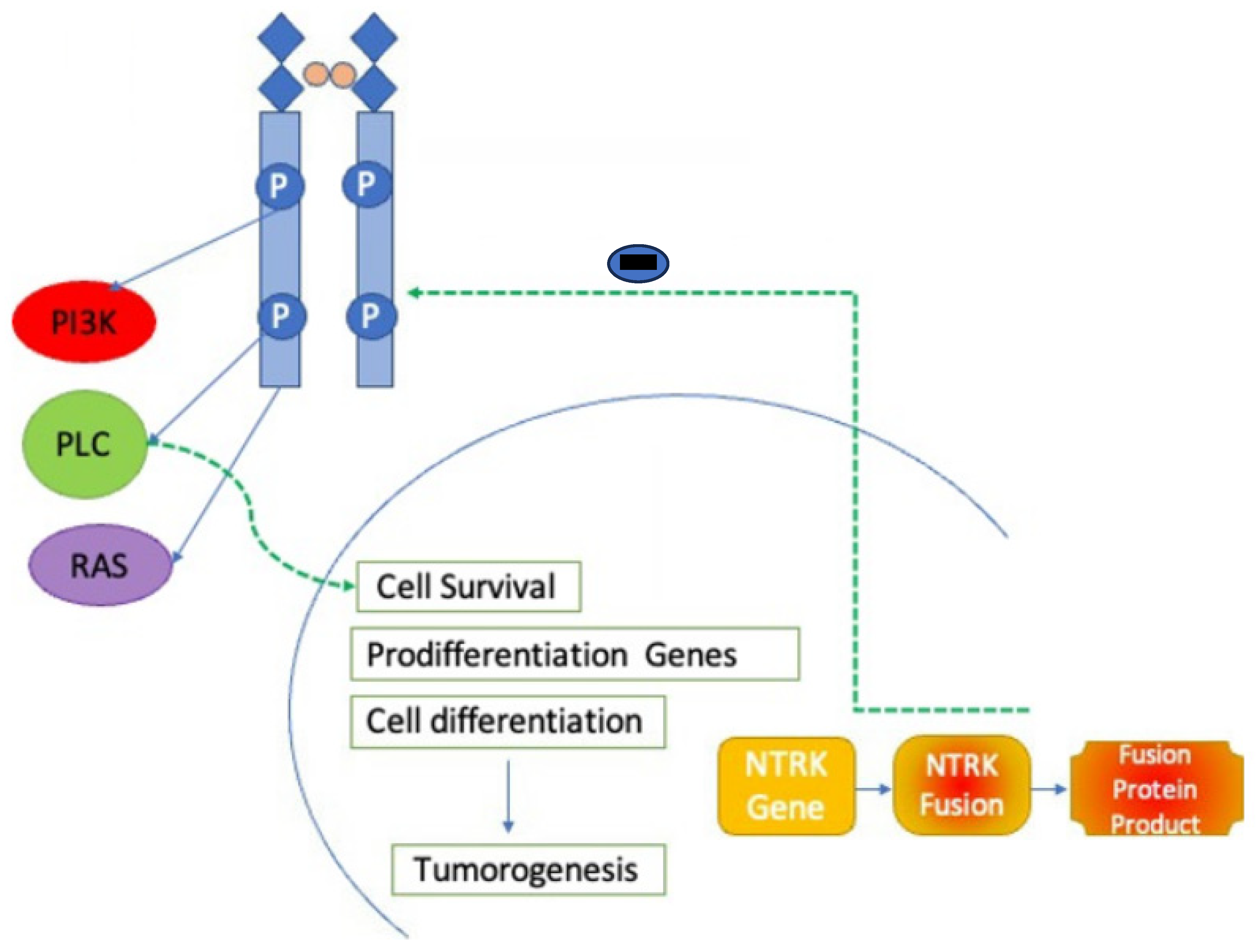
4. Mechanisms of Action
5. First-Generation NTRK Inhibitors
5.1. Larotrectinib
5.2. Entrectinib
6. Second-Generation NTRK Inhibitors
6.1. Repotrectinib
6.2. Selitrectinib
6.3. Taletrectinib
6.4. Other Agents
6.5. Mechanisms of Resistance to NTRK Inhibitors
6.6. Overall Side Effects and Effects of TRK Inhibition
7. Role of NTRK Inhibitors in Various Types of Cancers and Adverse Effects
7.1. NTRK Gene Fusion in Lung Cancer
7.2. NTRK Gene Fusion in Colorectal Cancer
7.3. NTRK Gene Fusion in Central Nervous System Malignancies
7.4. NTRK Gene Fusion in Thyroid Cancers
7.5. NTRK Gene Fusion in Hematological Malignancies
7.6. NTRK Gene Fusion in Sarcomas
7.7. NTRK Gene Fusion in Melanocytic Tumors
7.8. NTRK Gene Fusion in Salivary Gland Tumors
7.9. Other Malignancies
7.10. Future Directions and Challenges
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- National Cancer Institute. FDA Approves Entrectinib for NTRK Fusion Cancers. Cancer Currents Blog. 16 August 2019. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2019/fda-entrectinib-ntrk-fusion (accessed on 1 January 2020).
- Drilon, A.; Siena, S.; Ou, S.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Lannon, C.L.; Sorensen, P.H. ETV6-NTRK3: A chimeric protein tyrosine kinase with transformation activity in multiple cell lineages. Semin. Cancer Biol. 2005, 15, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Pulciani, S.; Santos, E.; Lauver, A.V.; Long, L.K.; Aaronson, S.A.; Barbacid, M. Oncogenes in solid human tumours. Nature 1982, 300, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cordero, R.; Ng, D.L. Neurotrophic receptor tyrosine kinase (NTRK) fusions and their role in cancer. Cancer Cytopathol. 2020, 128, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Marcus, L.; Donoghue, M.; Aungst, S.; Myers, C.E.; Helms, W.S.; Shen, G.; Zhao, H.; Stephens, O.; Keegan, P.; Pazdur, R. FDA Approval Summary: Entrectinib for the Treatment of NTRK gene Fusion Solid Tumors. Clin. Cancer Res. 2021, 27, 928–932. [Google Scholar] [CrossRef]
- Connor, A.; Perez-Ordoñez, B.; Shago, M.; Skálová, A.; Weinreb, I. Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: Expanded morphologic and immunohistochemical spectrum of a recently described entity. Am. J. Surg. Pathol. 2012, 36, 27–34. [Google Scholar] [CrossRef]
- Skálová, A.; Vanecek, T.; Simpson, R.H.; Laco, J.; Majewska, H.; Baneckova, M.; Steiner, P.; Michal, M. Mammary Analogue Secretory Carcinoma of Salivary Glands: Molecular Analysis of 25 ETV6 Gene Rearranged Tumors with Lack of Detection of Classical ETV6-NTRK3 Fusion Transcript by Standard RT-PCR: Report of 4 Cases Harboring ETV6-X Gene Fusion. Am. J. Surg. Pathol. 2016, 40, 3–13. [Google Scholar] [CrossRef]
- Murphy, D.A.; Ely, H.A.; Shoemaker, R.; Boomer, A.; Culver, B.P.; Hoskins, I.; Haimes, J.D.; Walters, R.D.; Fernandez, D.; Stahl, J.A.; et al. Detecting Gene Rearrangements in Patient Populations Through a 2-Step Diagnostic Test Comprised of Rapid IHC Enrichment Followed by Sensitive Next-Generation Sequencing. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 513–523. [Google Scholar] [CrossRef]
- Wong, D.; Yip, S.; Sorensen, P.H. Methods for Identifying Patients with Tropomyosin Receptor Kinase (TRK) Fusion Cancer. Pathol. Oncol. Res. 2020, 26, 1385–1399. [Google Scholar] [CrossRef] [PubMed]
- Martin-Zanca, D.; Oskam, R.; Mitra, G.; Copeland, T.; Barbacid, M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol. Cell Biol. 1989, 9, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Jing, S.Q.; Nanduri, V.; O’Rourke, E.; Barbacid, M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell 1991, 65, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Soppet, D.; Escandon, E.; Maragos, J.; Middlemas, D.S.; Reid, S.W.; Blair, J.; Burton, L.E.; Stanton, B.R.; Kaplan, D.R.; Hunter, T.; et al. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell 1991, 65, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Frade, J.M.; Barde, Y.A. Nerve growth factor: Two receptors, multiple functions. BioEssays: News and reviews in molecular, cellular and developmental biology. Bioessays 1998, 20, 137–145. [Google Scholar] [CrossRef]
- Patel, T.D.; Jackman, A.; Rice, F.L.; Kucera, J.; Snider, W.D. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron 2000, 25, 345–357, Erratum in Neuron 2003, 37, 183. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.K.; Teng, K.K.; Lee, R.; Wright, S.; Tevar, S.; Almeida, R.D.; Kermani, P.; Torkin, R.; Chen, Z.Y.; Lee, F.S.; et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 5455–5463. [Google Scholar] [CrossRef]
- Valent, A.; Danglot, G.; Bernheim, A. Mapping of the tyrosine kinase receptors trkA (NTRK1), trkB (NTRK2) and trkC(NTRK3) to human chromosomes 1q22, 9q22 and 15q25 by fluorescence in situ hybridization. Eur. J. Hum. Genet. 1997, 5, 102–104. [Google Scholar] [CrossRef]
- Bertrand, T.; Kothe, M.; Liu, J.; Dupuy, A.; Rak, A.; Berne, P.F.; Davis, S.; Gladysheva, T.; Valtre, C.; Crenne, J.Y.; et al. The crystal structures of TrkA and TrkB suggest key regions for achieving selective inhibition. J. Mol. Biol. 2012, 423, 439–453. [Google Scholar] [CrossRef]
- Rajagopal, R.; Chen, Z.Y.; Lee, F.S.; Chao, M.V. Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J. Neurosci. 2004, 24, 6650–6658. [Google Scholar] [CrossRef]
- Geiger, T.R.; Song, J.Y.; Rosado, A.; Peeper, D.S. Functional characterization of human cancer-derived TRKB mutations. PLoS ONE 2011, 6, e16871. [Google Scholar] [CrossRef]
- Harada, T.; Yatabe, Y.; Takeshita, M.; Koga, T.; Yano, T.; Wang, Y.; Giaccone, G. Role and relevance of TrkB mutations and expression in non-small cell lung cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 2638–2645. [Google Scholar] [CrossRef]
- Miranda, C.; Mazzoni, M.; Sensi, M.; Pierotti, M.A.; Greco, A. Functional characterization of NTRK1 mutations identified in melanoma. Genes Chromosomes Cancer 2014, 53, 875–880. [Google Scholar] [CrossRef]
- Tomasson, M.H.; Xiang, Z.; Walgren, R.; Zhao, Y.; Kasai, Y.; Miner, T.; Ries, R.E.; Lubman, O.; Fremont, D.H.; McLellan, M.D.; et al. Somatic mutations and germline sequence variants in the expressed tyrosine kinase genes of patients with de novo acute myeloid leukemia. Blood 2008, 111, 4797–4808. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Li, G.; Dogan, S.; Gounder, M.; Shen, R.; Arcila, M.; Wang, L.; Hyman, D.M.; Hechtman, J.; Wei, G.; et al. What hides behind the MASC: Clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 920–926. [Google Scholar] [CrossRef]
- Dunn, D.B. Larotrectinib and Entrectinib: TRK Inhibitors for the Treatment of Pediatric and Adult Patients with NTRK Gene Fusion. J. Adv. Pract. Oncol. 2020, 11, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Davis, L.E.; Vaishnavi, A.; Le, A.T.; Estrada-Bernal, A.; Keysar, S.; Jimeno, A.; Varella-Garcia, M.; Aisner, D.L.; Li, Y.; et al. An Oncogenic NTRK Fusion in a Patient with Soft-Tissue Sarcoma with Response to the Tropomyosin-Related Kinase Inhibitor LOXO-101. Cancer Discov. 2015, 5, 1049–1057. [Google Scholar] [CrossRef]
- Laetsch, T.W.; DuBois, S.G.; Mascarenhas, L.; Turpin, B.; Federman, N.; Albert, C.M.; Nagasubramanian, R.; Davis, J.L.; Rudzinski, E.; Feraco, A.M.; et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: Phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018, 19, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Kummar, S.; Tan, D.S.-W.; Lassen, U.N.; Leyvraz, S.; Liu, Y.; Moreno, V.; Patel, J.D.; Rosen, L.S.; Solomon, B.M.; et al. Long-term efficacy and safety of larotrectinib in patients with TRK fusion-positive lung cancer. J. Clin. Oncol. 2021, 39, 9109. [Google Scholar] [CrossRef]
- Rolfo, C.; Ruiz, R.; Giovannetti, E.; Gil-Bazo, I.; Russo, A.; Passiglia, F.; Giallombardo, M.; Peeters, M.; Raez, L. Entrectinib: A potent new TRK, ROS1, and ALK inhibitor. Expert Opin. Investig. Drugs 2015, 24, 1493–1500. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Barlesi, F.; Siena, S.; Ahn, M.J.; Drilon, A.; Conley, A.; Rolfo, C.; Wolf, J.; Seto, T.; Doebele, R.; et al. Patient-reported outcomes from STARTRK-2: A global phase II basket study of entrectinib for ROS1 fusion-positive non-small-cell lung cancer and NTRK fusion-positive solid tumours. ESMO Open 2021, 6, 100113. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.W.; Rogers, E.; Zhai, D.; Deng, W.; Chen, X.; Sprengeler, P.A.; Zhang, X.; Graber, A.; Reich, S.H.; Stopatschinskaja, S.; et al. Molecular Characteristics of Repotrectinib That Enable Potent Inhibition of TRK Fusion Proteins and Resistant Mutations. Mol. Cancer Ther. 2021, 20, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.; Drilon, A.E.; Doebele, R.C.; Kim, D.W.; Lin, J.J.; Lee, J.; Ahn, M.J.; Zhu, V.W.; Ejadi, S.; Camidge, D.R. Safety and preliminary clinical activity of repotrectinib in patients with advanced ROS1 fusion-positive non-small cell lung cancer (TRIDENT-1 study). J. Clin. Oncol. 2019, 37 (Suppl. 15), 9011. [Google Scholar] [CrossRef]
- Hyman, D.; Kummar, S.; Farago, A.; Geoerger, B.; Mau-Sorensen, M.; Taylor, M.; Garralda, E.; Nagasubramanian, R.; Natheson, M.; Song, L.; et al. Abstract CT127: Phase I and expanded access experience of LOXO-195 (BAY 2731954), a selective next-generation TRK inhibitor (TRKi). Cancer Res. 2019, 79 (Suppl. 13), CT127. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH). ClinicalTrials.gov. A Study to Test the Safety of the Investigational Drug Selitrectinib in Children and Adults that May Treat Cancer. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03215511?cond=LOXO-195&rank=1 (accessed on 11 April 2019).
- Florou, V.; Nevala-Plagemann, C.; Whisenant, J.; Maeda, P.; Gilcrease, G.W.; Garrido-Laguna, I. Clinical Activity of Selitrectinib in a Patient with Mammary Analogue Secretory Carcinoma of the Parotid Gland with Secondary Resistance to Entrectinib. J. Natl. Compr. Cancer Netw. 2021, 19, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Katayama, R.; Gong, B.; Togashi, N.; Miyamoto, M.; Kiga, M.; Iwasaki, S.; Kamai, Y.; Tominaga, Y.; Takeda, Y.; Kagoshima, Y.; et al. The new-generation selective ROS1/NTRK inhibitor DS-6051b overcomes crizotinib resistant ROS1-G2032R mutation in preclinical models. Nat. Commun. 2019, 10, 3604. [Google Scholar] [CrossRef]
- Zhou, C.; Fan, H.; Wang, Y.; Wu, H.; Yang, N.; Li, K.; Wang, X.; Qin, X.; Yu, Q.; Fang, Y.; et al. Taletrectinib (AB-106; DS-6051b) in metastatic non-small cell lung cancer (NSCLC) patients with ROS1 fusion: Preliminary results of TRUST. J. Clin. Oncol. 2021, 39 (Suppl. 15). [Google Scholar] [CrossRef]
- Kazandjian, D.; Blumenthal, G.M.; Luo, L.; He, K.; Fran, I.; Lemery, S.; Pazdur, R. Benefit-Risk Summary of Crizotinib for the Treatment of Patients with ROS1 Alteration-Positive, Metastatic Non-Small Cell Lung Cancer. Oncologist 2016, 21, 974–980. [Google Scholar] [CrossRef]
- Singh, H.; Brave, M.; Beaver, J.A.; Cheng, J.; Tang, S.; Zahalka, E.; Palmby, T.R.; Venugopal, R.; Song, P.; Liu, Q.I.; et al. Food and Drug Administration Approval: Cabozantinib for the Treatment of Advanced Renal Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 330–335. [Google Scholar] [CrossRef]
- Zou, H.Y.; Li, Q.; Lee, J.H.; Arango, M.E.; McDonnell, S.R.; Yamazaki, S.; Koudriakova, T.B.; Alton, G.; Cui, J.J.; Kung, P.P.; et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007, 67, 4408–4417. [Google Scholar] [CrossRef]
- Bowles, D.W.; Kessler, E.R.; Jimeno, A. Multi-targeted tyrosine kinase inhibitors in clinical development: Focus on XL-184 (cabozantinib). Drugs Today 2011, 47, 857–868. [Google Scholar] [CrossRef]
- Shamroe, C.L.; Comeau, J.M. Ponatinib: A new tyrosine kinase inhibitor for the treatment of chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. Ann. Pharmacother. 2013, 47, 1540–1546. [Google Scholar] [CrossRef]
- O’Hare, T.; Shakespeare, W.C.; Zhu, X.; Eide, C.A.; Rivera, V.M.; Wang, F.; Adrian, L.T.; Zhou, T.; Huang, W.S.; Xu, Q.; et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell 2009, 16, 401–412. [Google Scholar] [CrossRef]
- Karimi-Shah, B.A.; Chowdhury, B.A. Forced vital capacity in idiopathic pulmonary fibrosis--FDA review of pirfenidone and nintedanib. N. Engl. J. Med. 2015, 372, 1189–1191. [Google Scholar] [CrossRef]
- Hilberg, F.; Roth, G.J.; Krssak, M.; Kautschitsch, S.; Sommergruber, W.; Tontsch-Grunt, U.; Garin-Chesa, P.; Bader, G.; Zoephel, A.; Quant, J.; et al. BIBF 1120: Triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008, 68, 4774–4782. [Google Scholar] [CrossRef] [PubMed]
- Fuse, M.J.; Okada, K.; Oh-Hara, T.; Ogura, H.; Fujita, N.; Katayama, R. Mechanisms of Resistance to NTRK Inhibitors and Therapeutic Strategies in NTRK1-Rearranged Cancers. Mol. Cancer Ther. 2017, 16, 2130–2143. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Misale, S.; Wei, G.; Siravegna, G.; Crisafulli, G.; Lazzari, L.; Corti, G.; Rospo, G.; Novara, L.; Mussolin, B.; et al. Acquired Resistance to the TRK Inhibitor Entrectinib in Colorectal Cancer. Cancer Discov. 2016, 6, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Smeyne, R.J.; Klein, R.; Schnapp, A.; Long, L.K.; Bryant, S.; Lewin, A.; Lira, S.A.; Barbacid, M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature 1994, 368, 246–249. [Google Scholar] [CrossRef]
- Klein, R.; Smeyne, R.J.; Wurst, W.; Long, L.K.; Auerbach, B.A.; Joyner, A.L.; Barbacid, M. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell 1993, 75, 113–122. [Google Scholar] [CrossRef]
- Klein, R.; Silos-Santiago, I.; Smeyne, R.J.; Lira, S.A.; Brambilla, R.; Bryant, S.; Zhang, L.; Snider, W.D.; Barbacid, M. Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature 1994, 368, 249–251. [Google Scholar] [CrossRef]
- Tessarollo, L.; Tsoulfas, P.; Donovan, M.J.; Palko, M.E.; Blair-Flynn, J.; Hempstead, B.L.; Parada, L.F. Targeted deletion of all isoforms of the trkC gene suggests the use of alternate receptors by its ligand neurotrophin-3 in neuronal development and implicates trkC in normal cardiogenesis. Proc. Natl. Acad. Sci. USA 1997, 94, 14776–14781. [Google Scholar] [CrossRef]
- Qin, H.; Patel, M.R. The Challenge and Opportunity of NTRK Inhibitors in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2022, 23, 2916. [Google Scholar] [CrossRef]
- Okamura, R.; Boichard, A.; Kato, S.; Sicklick, J.K.; Bazhenova, L.; Kurzrock, R. Analysis of NTRK Alterations in Pan-Cancer Adult and Pediatric Malignancies: Implications for NTRK-Targeted Therapeutics. JCO Precis. Oncol. 2018, 2, 1–20. [Google Scholar] [CrossRef]
- Farago, A.F.; Taylor, M.S.; Doebele, R.C.; Zhu, V.W.; Kummar, S.; Spira, A.I.; Boyle, T.A.; Haura, E.B.; Arcila, M.E.; Benayed, R.; et al. Clinicopathologic Features of Non-Small-Cell Lung Cancer Harboring an NTRK Gene Fusion. JCO Precis. Oncol. 2018, 2, 1–12. [Google Scholar] [CrossRef]
- Drilon, A.; Tan, D.S.W.; Lassen, U.N.; Leyvraz, S.; Liu, Y.; Patel, J.D.; Rosen, L.; Solomon, B.; Norenberg, R.; Dima, L.; et al. Efficacy and Safety of Larotrectinib in Patients with Tropomyosin Receptor Kinase Fusion-Positive Lung Cancers. JCO Precis. Oncol. 2022, 6, e2100418. [Google Scholar] [CrossRef]
- Hoejgaard, M.; Drilon, A.; Lin, J.J.; Kummar, S.; Tan, D.S.W.; Patel, J.; Leyvraz, S.; Garcia, V.M.; Rosen, L.S.; Solomon, B.; et al. 15MO Efficacy and ctDNA analysis in an updated cohort of patients with TRK fusion lung cancer treated with larotrectinib. J. Thorac. Oncol. 2023, 18, S48–S49. [Google Scholar] [CrossRef]
- Sigal, D.S.; Bhangoo, M.S.; Hermel, J.A.; Pavlick, D.C.; Frampton, G.; Miller, V.A.; Ross, J.S.; Ali, S.M. Comprehensive genomic profiling identifies novel NTRK fusions in neuroendocrine tumors. Oncotarget 2018, 9, 35809–35812. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Z.W.; Ou, Q.; Wu, X.; Nagasaka, M.; Shao, Y.; Ou, S.I.; Yang, Y. NTRK fusion positive colorectal cancer is a unique subset of CRC with high TMB and microsatellite instability. Cancer Med. 2022, 11, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Kotch, C.; Fox, E.; Surrey, L.F.; Wertheim, G.B.; Baloch, Z.W.; Lin, F.; Pillai, V.; Luo, M.; Kreiger, P.A.; et al. NTRK Fusions Identified in Pediatric Tumors: The Frequency, Fusion Partners, and Clinical Outcome. JCO Precis. Oncol. 2021, 1, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Pietrantonio, F.; Di Nicolantonio, F.; Schrock, A.B.; Lee, J.; Tejpar, S.; Sartore-Bianchi, A.; Hechtman, J.F.; Christiansen, J.; Novara, L.; Tebbutt, N.; et al. ALK, ROS1, and NTRK Rearrangements in Metastatic Colorectal Cancer. J. Natl. Cancer Inst. 2017, 109, djx089. [Google Scholar] [CrossRef]
- Garralda, E.; Hong, D.S.; Xu, R.; Deeken, J.; Italiano, A.; Liu, T.; Ferrandiz, A.; Patel, J.; Lee, D.; Chung, H.; et al. Long-term efficacy and safety of larotrectinib in patients with tropomyosin receptor kinase (TRK) fusion gastrointestinal (GI) cancer: An expanded dataset. Ann. Oncol. 2022, 33 (Suppl. 4), S370. [Google Scholar] [CrossRef]
- Cohen, R.; Pudlarz, T.; Delattre, J.F.; Colle, R.; André, T. Molecular Targets for the Treatment of Metastatic Colorectal Cancer. Cancers 2020, 12, 2350. [Google Scholar] [CrossRef]
- Forsythe, A.; Zhang, W.; Phillip Strauss, U.; Fellous, M.; Korei, M.; Keating, K. A systematic review and meta-analysis of neurotrophic tyrosine receptor kinase gene fusion frequencies in solid tumors. Ther. Adv. Med. Oncol. 2020, 12, 1758835920975613. [Google Scholar] [CrossRef] [PubMed]
- Torre, M.; Vasudevaraja, V.; Serrano, J.; DeLorenzo, M.; Malinowski, S.; Blandin, A.F.; Pages, M.; Ligon, A.H.; Dong, F.; Meredith, D.M.; et al. Molecular and clinicopathologic features of gliomas harboring NTRK fusions. Acta Neuropathol. Commun. 2020, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Gambella, A.; Senetta, R.; Collemi, G.; Vallero, S.G.; Monticelli, M.; Cofano, F.; Zeppa, P.; Garbossa, D.; Pellerino, A.; Rudà, R.; et al. NTRK Fusions in Central Nervous System Tumors: A Rare, but Worthy Target. Int. J. Mol. Sci. 2020, 21, 753. [Google Scholar] [CrossRef]
- Aldape, K.; Brindle, K.M.; Chesler, L.; Chopra, R.; Gajjar, A.; Gilbert, M.R.; Gottardo, N.; Gutmann, D.H.; Hargrave, D.; Holland, E.C.; et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 2019, 16, 509–520. [Google Scholar] [CrossRef]
- LiVolsi, V.A.; Abrosimov, A.A.; Bogdanova, T.; Fadda, G.; Hunt, J.L.; Ito, M.; Rosai, J.; Thomas, G.A.; Williams, E.D. The Chernobyl thyroid cancer experience: Pathology. Clin. Oncol. 2011, 23, 261–267. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Lin, J.J.; Brose, M.S.; McDermott, R.S.; Almubarak, M.; Bauman, J.R.; Casanova, M.; Kummar, S.; Lee, S.H.; Leyvraz, S.; et al. Larotrectinib (laro) long-term efficacy and safety in patients (pts) with tropomyosin receptor kinase (TRK) fusion thyroid carcinoma (TC). J. Clin. Oncol. 2023, 41, 6091. [Google Scholar] [CrossRef]
- Taylor, J.; Pavlick, D.; Yoshimi, A.; Marcelus, C.; Chung, S.S.; Hechtman, J.F.; Benayed, R.; Cocco, E.; Durham, B.H.; Bitner, L.; et al. Oncogenic TRK fusions are amenable to inhibition in hematologic malignancies. J. Clin. Investig. 2018, 128, 3819–3825. [Google Scholar] [CrossRef]
- Qin, K.; Hou, H.; Liang, Y.; Zhang, X. Prognostic value of TP53 concurrent mutations for EGFR- TKIs and ALK-TKIs based targeted therapy in advanced non-small cell lung cancer: A meta-analysis. BMC Cancer 2020, 20, 328. [Google Scholar] [CrossRef]
- Knezevich, S.R.; McFadden, D.E.; Tao, W.; Lim, J.F.; Sorensen, P.H. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat. Genet. 1998, 18, 184–187. [Google Scholar] [CrossRef]
- Agaram, N.P.; Zhang, L.; Sung, Y.S.; Chen, C.L.; Chung, C.T.; Antonescu, C.R.; Fletcher, C.D. Recurrent NTRK1 Gene Fusions Define a Novel Subset of Locally Aggressive Lipofibromatosis-like Neural Tumors. Am. J. Surg. Pathol. 2016, 40, 1407–1416. [Google Scholar] [CrossRef]
- Kao, Y.C.; Fletcher, C.D.M.; Alaggio, R.; Wexler, L.; Zhang, L.; Sung, Y.S.; Orhan, D.; Chang, W.C.; Swanson, D.; Dickson, B.C.; et al. Recurrent BRAF Gene Fusions in a Subset of Pediatric Spindle Cell Sarcomas: Expanding the Genetic Spectrum of Tumors with Overlapping Features with Infantile Fibrosarcoma. Am. J. Surg. Pathol. 2018, 42, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.; Cotzia, P.; Hyman, D.M.; Drilon, A.; Tap, W.D.; Zhang, L.; Hechtman, J.F.; Frosina, D.; Jungbluth, A.A.; Murali, R.; et al. NTRK Fusions Define a Novel Uterine Sarcoma Subtype with Features of Fibrosarcoma. Am. J. Surg. Pathol. 2018, 42, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Mori, T.; Motoi, T.; Kobayashi, E.; Yoshida, M.; Yatabe, Y.; Ichikawa, H.; Kawai, A.; Yonemori, K.; Antonescu, C.R.; et al. Frequent CD30 Expression in an Emerging Group of Mesenchymal Tumors with NTRK, BRAF, RAF1, or RET Fusions. Mod. Pathol. 2023, 36, 100083. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Suurmeijer, A.J.H.; Agaram, N.P.; Antonescu, C.R. Head and Neck Mesenchymal Tumors with Kinase Fusions: A Report of 15 Cases with Emphasis on Wide Anatomic Distribution and Diverse Histologic Appearance. Am. J. Surg. Pathol. 2023, 47, 248–258. [Google Scholar] [CrossRef]
- Demetri, G.D.; Antonescu, C.R.; Bjerkehagen, B.; Bovée, J.V.M.G.; Boye, K.; Chacón, M.; Dei Tos, A.P.; Desai, J.; Fletcher, J.A.; Gelderblom, H.; et al. Diagnosis and management of tropomyosin receptor kinase (TRK) fusion sarcomas: Expert recommendations from the World Sarcoma Network. Ann. Oncol. 2020, 31, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Kiuru, M.; Jungbluth, A.; Kutzner, H.; Wiesner, T.; Busam, K.J. Spitz Tumors: Comparison of Histological Features in Relationship to Immunohistochemical Staining for ALK and NTRK1. Int. J. Surg. Pathol. 2016, 24, 200–206. [Google Scholar] [CrossRef]
- Uguen, A. Spitz Tumors with NTRK1 Fusions: TRK-A and pan-TRK Immunohistochemistry as Ancillary Diagnostic Tools. Am. J. Surg. Pathol. 2019, 43, 1438–1439. [Google Scholar] [CrossRef] [PubMed]
- Yeh, I.; Tee, M.K.; Botton, T.; Shain, A.H.; Sparatta, A.J.; Gagnon, A.; Vemula, S.S.; Garrido, M.C.; Nakamaru, K.; Isoyama, T.; et al. NTRK3 kinase fusions in Spitz tumours. J. Pathol. 2016, 240, 282–290. [Google Scholar] [CrossRef]
- Yeh, I.; Busam, K.J.; McCalmont, T.H.; LeBoit, P.E.; Pissaloux, D.; Alberti, L.; de la Fouchardière, A.; Bastian, B.C. Filigree-like Rete Ridges, Lobulated Nests, Rosette-like Structures, and Exaggerated Maturation Characterize Spitz Tumors with NTRK1 Fusion. Am. J. Surg. Pathol. 2019, 43, 737–746. [Google Scholar] [CrossRef]
- Cappellesso, R.; Nozzoli, F.; Zito Marino, F.; Simi, S.; Castiglione, F.; De Giorgi, V.; Cota, C.; Senetta, R.; Scognamiglio, G.; Anniciello, A.M.; et al. NTRK Gene Fusion Detection in Atypical Spitz Tumors. Int. J. Mol. Sci. 2021, 22, 12332. [Google Scholar] [CrossRef]
- Forschner, A.; Forchhammer, S.; Bonzheim, I. NTRK gene fusions in melanoma: Detection, prevalence and potential therapeutic implications. J. Dtsch. Dermatol. Ges. 2020, 18, 1387–1392. [Google Scholar] [CrossRef]
- Lezcano, C.; Shoushtari, A.N.; Ariyan, C.; Hollmann, T.J.; Busam, K.J. Primary and Metastatic Melanoma with NTRK Fusions. Am. J. Surg. Pathol. 2018, 42, 1052–1058. [Google Scholar] [CrossRef]
- Wang, M.; Banik, I.; Shain, A.H.; Yeh, I.; Bastian, B.C. Integrated genomic analyses of acral and mucosal melanomas nominate novel driver genes. Genome Med. 2022, 14, 65. [Google Scholar] [CrossRef]
- Silvertown, J.D.; Lisle, C.; Semenuk, L.; Knapp, C.; Jaynes, J.; Berg, D.; Kaul, N.; Lachapelle, J.; Richardson, L.; Speevak, M.; et al. Prevalence of NTRK Fusions in Canadian Solid Tumour Cancer Patients. Mol. Diagn. Ther. 2023, 27, 87–103. [Google Scholar] [CrossRef]
- Laé, M.; Fréneaux, P.; Sastre-Garau, X.; Chouchane, O.; Sigal-Zafrani, B.; Vincent-Salomon, A. Secretory breast carcinomas with ETV6-NTRK3 fusion gene belong to the basal-like carcinoma spectrum. Mod. Pathol. 2009, 22, 291–298. [Google Scholar] [CrossRef]
- Cao, Z.; Li, J.; Sun, L.; Xu, Z.; Ke, Y.; Shao, B.; Guo, Y.; Sun, Y. GISTs with NTRK Gene Fusions: A Clinicopathological, Immunophenotypic, and Molecular Study. Cancers 2022, 15, 105. [Google Scholar] [CrossRef]
| 1982 | Identification of NTRK as an oncogene in patient with colorectal carcinoma |
| 1989 | Isolation of cDNA of the NTRK1 proto-oncogene |
| 1997 | Gene mapping of NTRK1, NTRK2, and NTRK3 to human chromosomes, 1q22, 9q22, and 15q25 by FISH |
| 2015 | First-generation NTRK inhibitors entered clinical trials |
| 2017 | Second-generation NTRK inhibitors entered clinical trials |
| 2018 | FDA granted accelerated approval for larotrectinib for adult and pediatric patients with NTRK fusion-positive solid tumors |
| 2019 | FDA granted accelerated approval for entrectinib for adult and pediatric patients with solid tumors with NTRK gene fusion without a known acquired resistance mutation |
| 2020 | FDA granted Fast Track to repotrectinib in NTRK-positive advanced solid tumors |
| 2021 | Phase 2 basket trial of taletrectinib for solid tumors with NTRK initiated |
| Medication | Target Genes | Related Trials | Findings | Side Effects |
|---|---|---|---|---|
| Larotrectinib | TRKA/B/C | NCT02576431 NCT02122913 NCT02637687 | ORR 75% (95% CI 68–81) CR 22% PR 109% Stable 16% PD 6% | 18% Grade 3–4 treatment-related side effects |
| Entrectinib | TRKA/B/C, ROS1, ALK | ALKA-372-001 STARTRK-1 STARTRK-2 | ORR 57% CR 7% PR 50% Stable 17% PD 7% | 10% weight gain, 12% anemia, 4% CNS manifestation |
| Repotrectinib | TRKA/B/C, ROS1, ALK | NCT03093116 (TRIDENT-1) | ORR 41–62% | Grade 1 CNS-related side effects |
| Selitrectinib | TRKA/B/C | NCT03215511 NCT03206931 | N/A | N/A |
| Taletrectinib | TRKA/B/C, ALK | NCT04395677 | N/A | GI-related side effects—nausea, diarrhea, vomiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theik, N.W.Y.; Muminovic, M.; Alvarez-Pinzon, A.M.; Shoreibah, A.; Hussein, A.M.; Raez, L.E. NTRK Therapy among Different Types of Cancers, Review and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 2366. https://doi.org/10.3390/ijms25042366
Theik NWY, Muminovic M, Alvarez-Pinzon AM, Shoreibah A, Hussein AM, Raez LE. NTRK Therapy among Different Types of Cancers, Review and Future Perspectives. International Journal of Molecular Sciences. 2024; 25(4):2366. https://doi.org/10.3390/ijms25042366
Chicago/Turabian StyleTheik, Nyein Wint Yee, Meri Muminovic, Andres M. Alvarez-Pinzon, Ahmed Shoreibah, Atif M. Hussein, and Luis E. Raez. 2024. "NTRK Therapy among Different Types of Cancers, Review and Future Perspectives" International Journal of Molecular Sciences 25, no. 4: 2366. https://doi.org/10.3390/ijms25042366
APA StyleTheik, N. W. Y., Muminovic, M., Alvarez-Pinzon, A. M., Shoreibah, A., Hussein, A. M., & Raez, L. E. (2024). NTRK Therapy among Different Types of Cancers, Review and Future Perspectives. International Journal of Molecular Sciences, 25(4), 2366. https://doi.org/10.3390/ijms25042366







