The Complement Component 4 Binding Protein α Gene: A Versatile Immune Gene That Influences Lipid Metabolism in Bovine Mammary Epithelial Cell Lines
Abstract
1. Introduction
2. Results
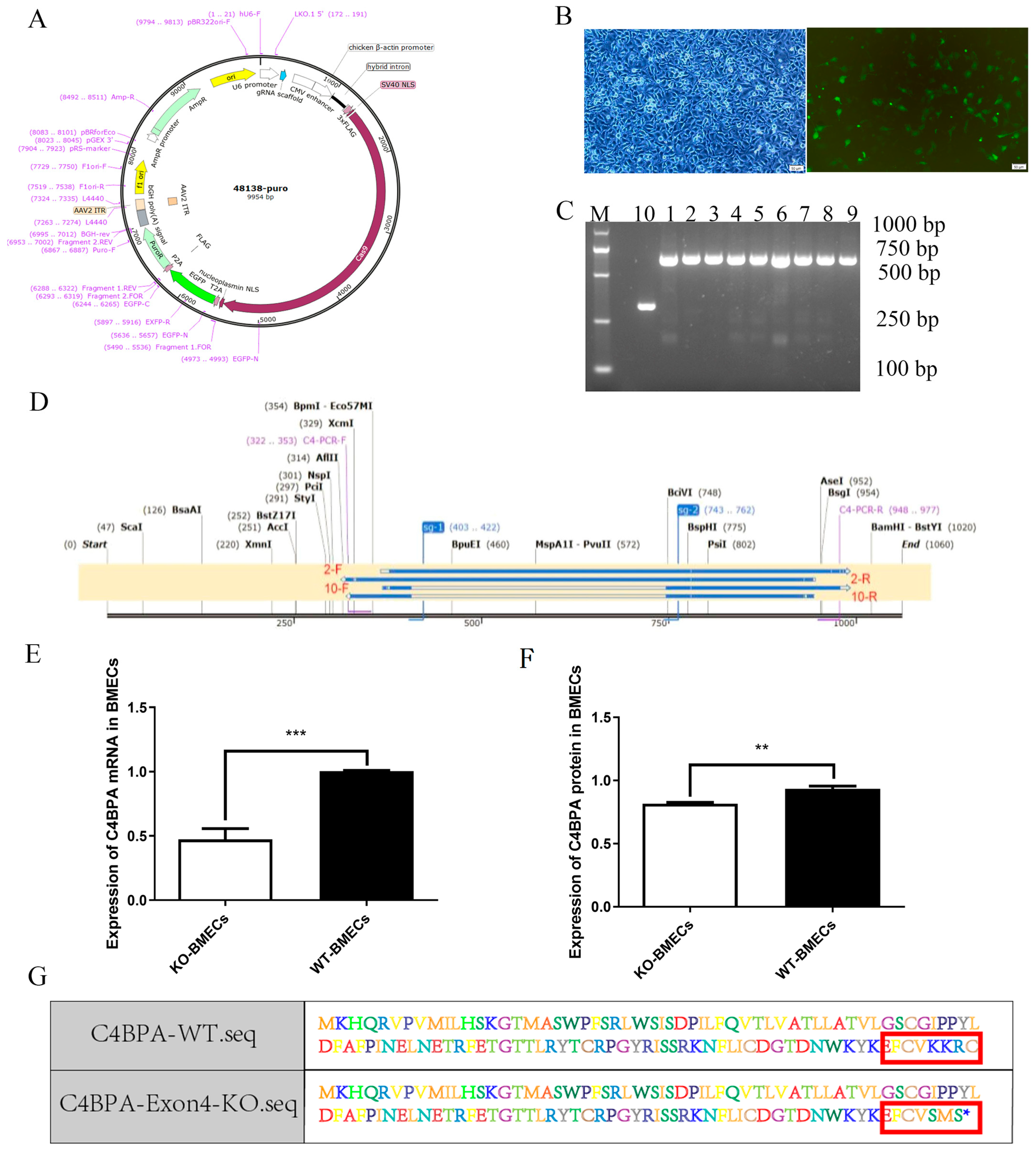
2.1. Construction of C4BPA Knockout BMECs Line Model
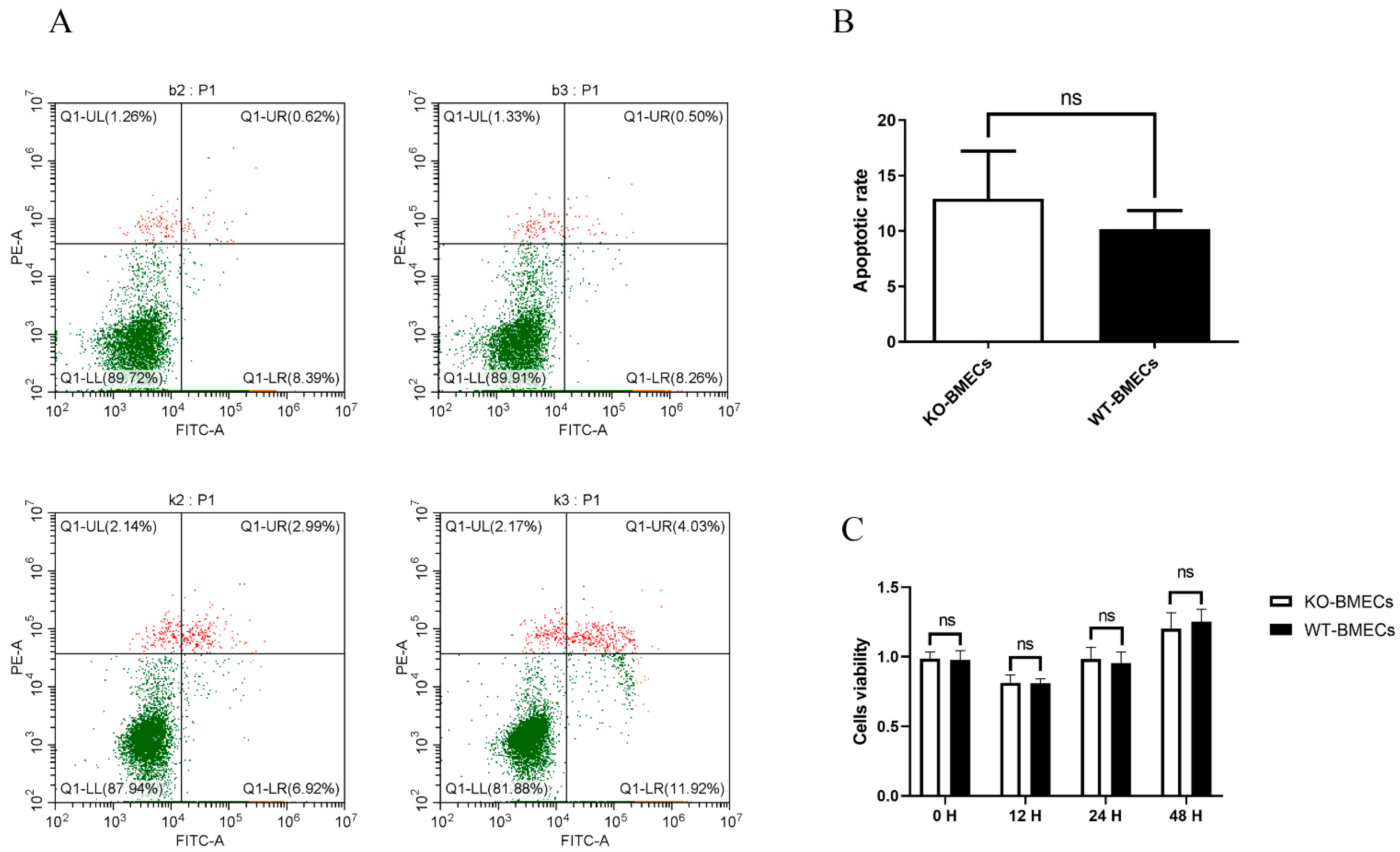
2.2. The Proliferation Rate and Apoptosis Rate of KO-BMECs and WT Cell Line
2.3. Lipid Accumulation in BMECs of C4BPA Gene KO or WT Cell Line
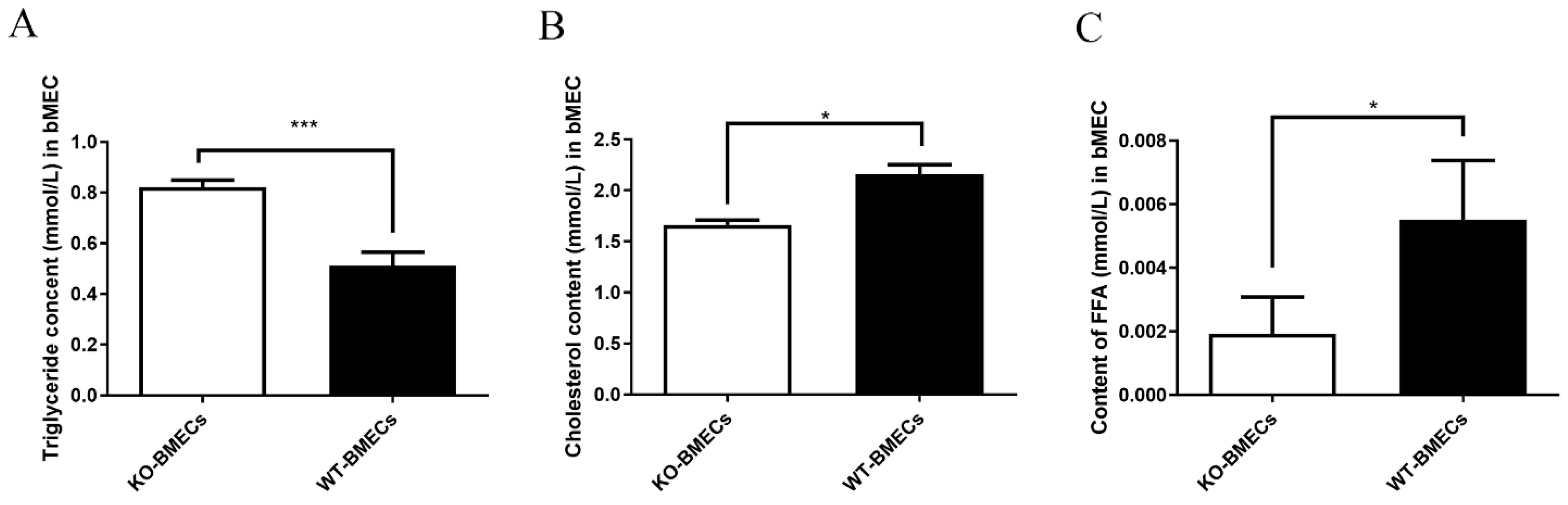
2.4. Triacylglycerol (TG), Cholesterol (CHOL), and Free Fatty Acid (FFA) Contents in BMECs of C4BPA Gene KO or WT Cell Line
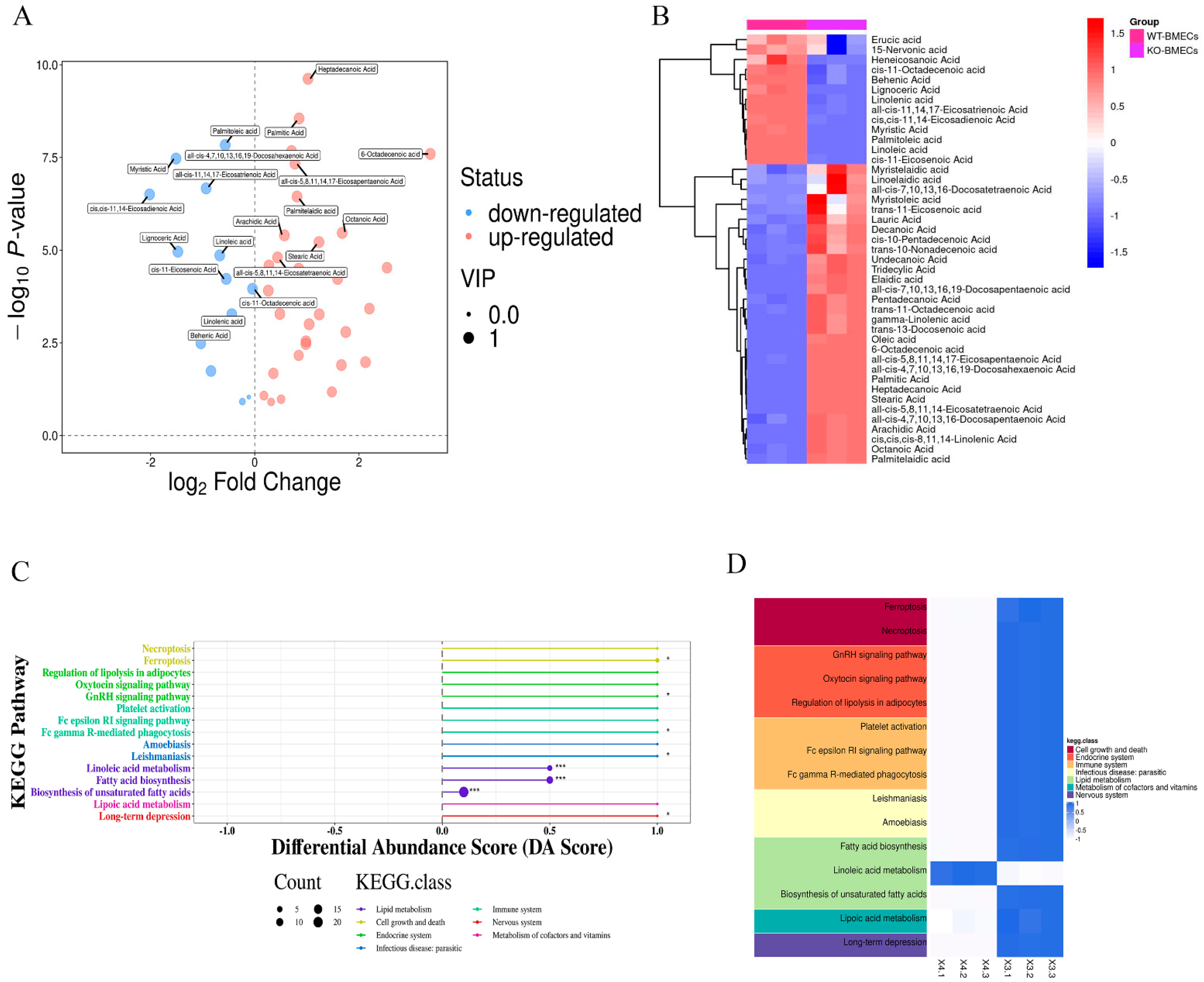
2.5. Results of Fatty Acid Contents in BMECs of C4BPA Gene KO or WT Cell Line Based on the GC-MS
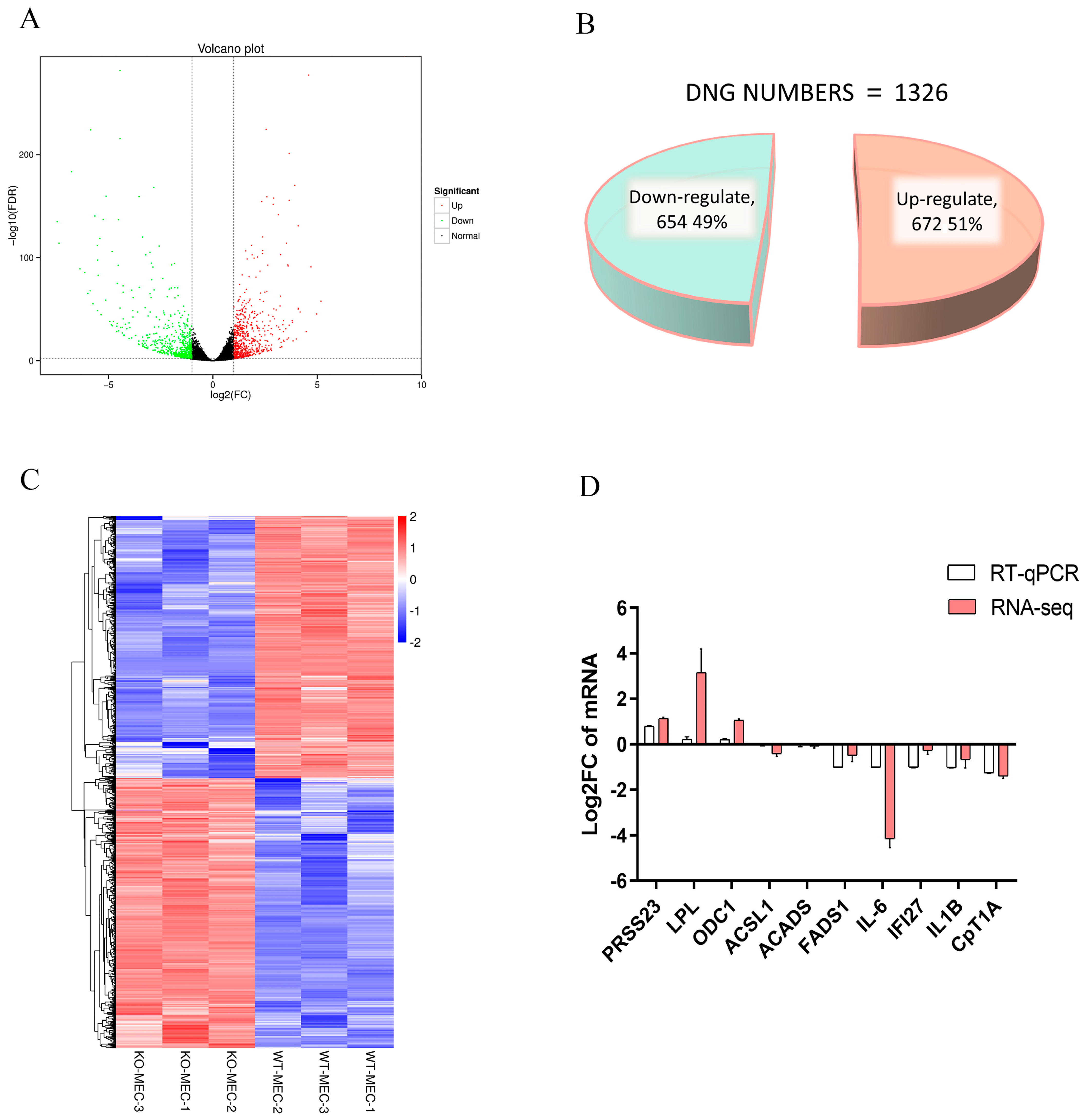
2.6. Identification of DEGs in BMECs of C4BPA Gene KO or WT Cell Line
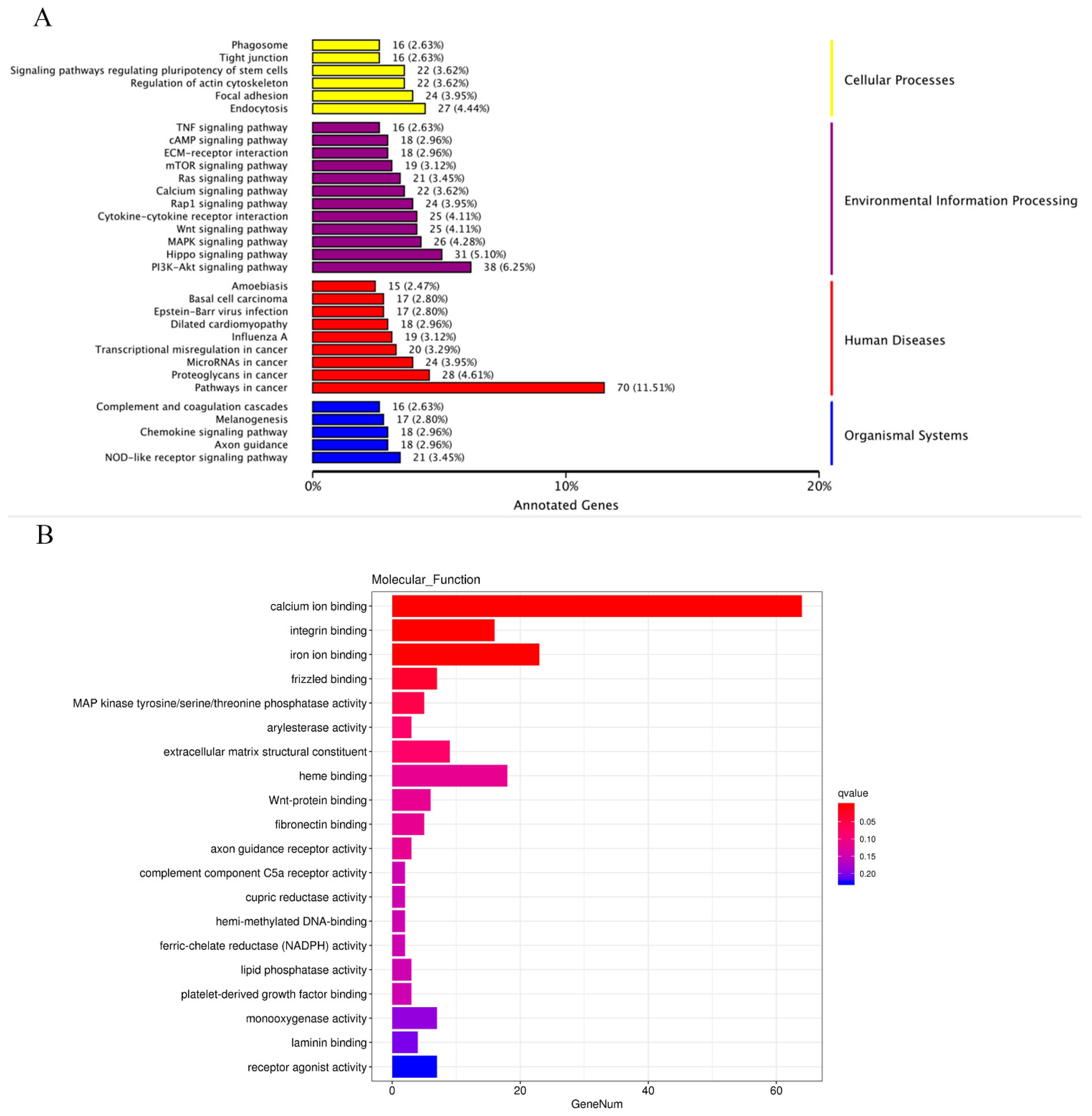
2.7. Functional Enrichment Analysis of the DEGs in BMECs of C4BPA Gene KO or WT Cell Line
2.8. Expression of Different Genes Related to Fat Metabolism
3. Discussion
4. Materials and Methods
4.1. Cell Culture and the Construction of C4BPA Knockout BMECs Line Model
4.2. Construction of C4BPA Gene BMECs KO Cell Line
4.3. Analysis of mRNA and Protein Levels in BMECs of C4BPA Gene KO or WT Cell Line
4.4. Cell Apoptosis in BMECs of C4BPA Gene KO or WT Cell Line
4.5. Lipid Droplet Accumulation in BMECs of C4BPA Gene KO or WT Cell Line
4.6. Triglycerides, Cholesterol, and FFA Content in BMECs of C4BPA Gene KO or WT Cell Line
4.7. Intracellular Fatty Acids Content in BMECs of C4BPA Gene KO or WT Cell Line
4.8. GC-MS Analysis in BMECs of C4BPA Gene KO or WT Cell Line
4.9. DEGs and RNA Sequencing Mapping in BMECs of C4BPA Gene KO or WT Cell Line
4.10. GO and KEGG Analysis of DEGs
4.11. Western Blotting
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Neil, C.; Nicklas, T.; Fulgoni, V. Food Sources of Energy and Nutrients of Public Health Concern and Nu trients to Limit with a Focus on Milk and other Dairy Foods in Children 2 to 18 Years of Age: National Health and Nutrition Examination Survey, 2011–2014. Nutrients 2018, 10, 1050. [Google Scholar] [CrossRef]
- Lopes, T.S.; Fontoura, P.S.; Oliveira, A.; Rizzo, F.A.; Silveira, S.; Streck, A.F. Use of plant extracts and essential oils in the control of bovine mastitis. Res. Vet. Sci. 2020, 131, 186–193. [Google Scholar] [CrossRef]
- Shen, B.L.; Zhang, L.; Lian, C.; Lu, C.; Zhang, Y.; Pan, Q.; Yang, R.; Zhao, Z. Deep sequencing and screening of differentially expressed microRNAs related to milk fat metabolism in bovine primary mammary epithelial cells. Int. J. Mol. Sci. 2016, 17, 200. [Google Scholar] [CrossRef]
- Blom, A.M.; Villoutreix, B.O.; Dahlbäck, B. Complement inhibitor C4b-binding protein—Friend or foe in the innate immune system. Mol. Immunol. 2004, 40, 1333–1346. [Google Scholar] [CrossRef]
- Blom, A.M.; Villoutreix, B.O.; Dahlbäck, B. Functions of human complement. Arch. Immunol. Ther. Exp. 2004, 52, 83–95. [Google Scholar]
- De Frutos, P.G.; Alim, R.; Hardig, Y.; Zoller, B.; Dahlback, B. Differential regulation of alpha and beta chains of C4b-binding protein during acute-phase response resulting in stable plasma levels of free anticoagulant protein S. Blood 1994, 84, 815–822. [Google Scholar] [CrossRef]
- Buil, A.; Trégouët, D.-A.; Souto, J.C.; Saut, N.; Germain, M.; Rotival, M.; Tiret, L.; Cambien, F.; Lathrop, M.; Zeller, T.; et al. C4BPB/C4BPA is a new susceptibility locus for venous thrombosis with unknown protein S–independent mechanism: Results from genome-wide association and gene expression analyses followed by case-control studies. Blood 2010, 115, 4644–4650. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, S.R.; Angelini, F.; Bacharier, L.B.; Blom, A.M.; Mizoguchi, E.; Fujiwara, H.; Plebani, A.; Notaran gelo, L.D.; Dahlback, B.; Tsitsikov, E.; et al. C4b-Binding Protein (C4BP) Activates B Cells through the CD40 Receptor. Immunity 2003, 18, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Dahlb, B. Genes for C4b-Binding Protein a- and B-chains (C4BPAand C4BPB)Are Located on Chromosome 1, Band lq32, in Humans and on Chromosome 13 in Rats. Somat. Cell Mol. Genet. 1990, 16, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Ziyi, P.; Yu, H.; Jialing, L.; Haochen, W.; Jing, F.; Ping, J.; Zhihui, Z. C4BPA: A Novel Co-Regulator of Immunity and Fat Metabolism in the Bovine Mammary Epithelial Cells. Front. Genet. 2022, 12, 830566. [Google Scholar] [CrossRef]
- Sogawa, K.; Takano, S.; Iida, F.; Satoh, M.; Tsuchida, S.; Kawashima, Y.; Yoshitomi, H.; Sanda, A.; Kodera, Y.; Takizawa, H.R.; et al. Identification of a novel serum biomarker for pancreatic cancer, C4b-binding protein α-chain (C4BPA) by quantitative proteomic analysis using tandem mass tags. Br. J. Cancer 2016, 115, 949–956. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Ceron, J.J.; De Torre, C.; Ljubić, B.B.; Holden, S.L.; Queau, Y.; Morris, P.J.; Pastor, J.; German, A.J. Obese dogs with and without obesity-related metabolic dysfunction—A proteomic approach. BMC Vet. Res. 2016, 12, 211. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, X.; Wang, Y.; Li, A.; Hang, X.; Guo, P.; Yang, R.; Lu, C.; Zhao, Z. Difference analysis of C4BPA gene expression in mammary tissue of dairy cows. Chin. J. Vet. Sci. 2016, 36, 1032–1043. [Google Scholar]
- Abebe, R.; Hatiya, H.; Abera, M.; Megersa, B.; Asmare, K. Bovine mastitis: Prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet. Res. 2016, 12, 270. [Google Scholar] [CrossRef]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Yatoo, M.I.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in therapeutic and managemental approaches of bovine mastitis: A comprehensive review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef]
- Gupta, D.; Bhattacharjee, O.; Mandal, D.; Sen, M.K.; Dey, D.; Dasgupta, A.; Kazi, T.A.; Gupta, R.; Sinharoy, S.; Acharya, K.; et al. CRISPR-Cas9 system: A new-fangled dawn in gene editing. Life Sci. 2019, 232, 116636. [Google Scholar] [CrossRef]
- Jin, S.; Yong, H.; Liu, Y.; Bao, W. CRISPR/Cas9-mediated high-mobility group A2 knockout inhibits cell proliferation and invasion in papillary thyroid carcinoma cells. Adv. Med. Sci. 2023, 68, 409–416. [Google Scholar] [CrossRef]
- Wu, S.; Deng, H.; Lu, B.; Yuan, L.; Wang, X. Generation of CD16A gene knockout human embryonic stem cell line using CRISPR/Cas9. Stem Cell Res. 2022, 64, 102935. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Zhang, Z.; Chen, J.; Dong, G. Effects of Peptidoglycan, Lipoteichoic Acid and Lipopolysaccha ride on Inflammation, Proliferation and Milk Fat Synthesis in Bovine Mammary Epithelial Cells. Toxins 2020, 12, 497. [Google Scholar] [CrossRef]
- Ma, X.; Bai, Y.; Liu, K.; Han, Y.; Zhang, J.; Liu, Y.; Hou, X.; Hao, E.; Hou, Y.; Bai, G. Ursolic acid inhibits the cholesterol biosynthesis and alleviates high fat diet-induced hypercholesterolemia via irreversible inhibition of HMGCS1 in vivo. Phytomedicine 2022, 103, 154233. [Google Scholar] [CrossRef]
- Welte, M.A. Expanding Roles for Lipid Droplets. Curr. Biol. 2015, 25, R470–R481. [Google Scholar] [CrossRef]
- Santos, S.D.; Delattre, A.-I.; De Longueville, F.; Bult, H.; Raes, M. Gene Expression Profiling of LPS-Stimu lated Murine Macrophages and Role of the NF-κB and PI3K/mTOR Signaling Pathways. Ann. N. Y. Acad. Sci. 2007, 1096, 70–77. [Google Scholar] [CrossRef]
- Jiang, K.; Guo, S.; Yang, C.; Yang, J.; Chen, Y.; Shaukat, A.; Zhao, G.; Wu, H.; Deng, G. Barbaloin protects against lipopolysaccharide (LPS)-induced acute lung injury by inhibiting the ROS-mediated PI3K/AKT/NF-κB pathway. Int. Immunopharmacol. 2018, 64, 140–150. [Google Scholar] [CrossRef]
- McCurdy, C.E.; Klemm, D.J. Adipose tissue insulin sensitivity and macrophage recruitment: Does PI3K pick the pathway. Adipocyte 2013, 2, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.T.; Stephens, L.R. PI3K Signalling in Inflammation, Biochimica et Biophysica Acta (BBA)—Molecular and Cell Biology of Lipids; Elsevier: Amsterdam, The Netherlands, 2015; Volume 1851, pp. 882–897. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, N.; Zhang, X.; Zhu, Y.; Liu, S.; Chang, Y. Chrysin Improves Glucose and Lipid Metabolism Disorders by Regulating the AMPK/PI3K/AKT Signaling Pathway in Insulin-Resistant HepG2 Cells and HFD/STZ-Induced C57BL/6J Mice. J. Agric. Food Chem. 2021, 69, 5618–5627. [Google Scholar] [CrossRef]
- Savova, M.S.; Mihaylova, L.V.; Tews, D.; Wabitsch, M.; Georgiev, M.I. Targeting PI3K/AKT signaling pathway in obesity. Biomed. Pharmacother. 2023, 159, 114244. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, J.; Jiang, C.; Song, X.; Wu, H.; Zhang, J.; Raza, S.H.A.; Zhang, L.; Zhang, L.; Cai, B.X.; et al. FOXO1 regulates the formation of bovine fat by targeting CD36 and STEAP4. Int. J. Biol. Macromol. 2023, 248, 126025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, C.; Wan, J.; Zhang, X.; Jia, Y.; Zhou, C. Compartmentalized activities of HMGCS1 control cer vical cancer radiosensitivity. Cell. Signal. 2023, 101, 110507. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, W.; Wang, Y.; Li, H.; Zhang, C.; Wang, Y.; Lin, Y.; Shi, H.; Xiang, H.; Huang, L.; et al. Expression Variation of CPT1A Induces Lipid Reconstruction in Goat Intramuscular Precursor Adipocytes. Int. J. Mol. Sci. 2023, 24, 13415. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, H.; Ren, Z.; Yi, X.; Zhang, Q.; Yang, Z.; Kuang, Y.; Zhu, Y. Overexpression CPT1A reduces lipid accumulation via PPARα/CD36 axis to suppress the cell proliferation in ccRCC. Acta Biochim. Et Biophys. Sin. 2022, 54, 220–231. [Google Scholar] [CrossRef]
- Stefanovic-Racic, M.; Perdomo, G.; Mantell, B.S.; Sipula, I.J.; Brown, N.F.; O’Doherty, R.M. A moderate increase in carnitine palmitoyltransferase 1a activity is sufficient to substantially reduce hepatic triglyceride levels. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E969–E977. [Google Scholar] [CrossRef] [PubMed]
- Illig, T.; Gieger, C.; Zhai, G.; Römisch-Margl, W.; Wang-Sattler, R.; Prehn, C.; Altmaier, E.; Kastenmüller, G.; Kato, B.S.; Mewes, H.-W.; et al. A genome-wide perspective of genetic variation in human metabolism. Nat. Genet. 2010, 42, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Ropka-Molik, K.; Knapik, J.; Pieszka, M.; Szmatoła, T. The expression of the SCD1 gene and its correlation with fattening and carcass traits in sheep. Arch. Anim. Breed. 2016, 59, 37–43. [Google Scholar] [CrossRef]
- Li, Z.; Lu, S.; Cui, K.; Shafique, L.; Rehman, S.U.; Luo, C.; Wang, Z.; Ruan, J.; Qian, Q.; Liu, Q. Fatty acid biosyn thesis and transcriptional regulation of Stearoyl-CoA Desaturase 1 (SCD1) in buffalo milk. BMC Genet. 2020, 21, 23. [Google Scholar] [CrossRef]


| SgRNA Strand | Target-Seq | Location | Gene ID |
|---|---|---|---|
| C4BPA-sgRNA1-F | CACCGAGAGAGCTGATGGATCATTG | Intron 3 | 281651 |
| C4BPA-sgRNA1-R | AAACCAATGATCCATCAGCTCTCTC | Intron 3 | 281651 |
| C4BPA-sgRNA2-F | CACCGAGTGTATCCCTGTTTAAAGA | Intron 5 | 281651 |
| C4BPA-sgRNA2-R | AAACTCTTTAAACAGGGATACACTC | Intron 5 | 281651 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhao, Z.; Jiang, X.; Li, J.; Miao, F.; Yu, H.; Lin, Z.; Jiang, P. The Complement Component 4 Binding Protein α Gene: A Versatile Immune Gene That Influences Lipid Metabolism in Bovine Mammary Epithelial Cell Lines. Int. J. Mol. Sci. 2024, 25, 2375. https://doi.org/10.3390/ijms25042375
Chen X, Zhao Z, Jiang X, Li J, Miao F, Yu H, Lin Z, Jiang P. The Complement Component 4 Binding Protein α Gene: A Versatile Immune Gene That Influences Lipid Metabolism in Bovine Mammary Epithelial Cell Lines. International Journal of Molecular Sciences. 2024; 25(4):2375. https://doi.org/10.3390/ijms25042375
Chicago/Turabian StyleChen, Xuanxu, Zhihui Zhao, Xinyi Jiang, Jing Li, Fengshuai Miao, Haibin Yu, Ziwei Lin, and Ping Jiang. 2024. "The Complement Component 4 Binding Protein α Gene: A Versatile Immune Gene That Influences Lipid Metabolism in Bovine Mammary Epithelial Cell Lines" International Journal of Molecular Sciences 25, no. 4: 2375. https://doi.org/10.3390/ijms25042375
APA StyleChen, X., Zhao, Z., Jiang, X., Li, J., Miao, F., Yu, H., Lin, Z., & Jiang, P. (2024). The Complement Component 4 Binding Protein α Gene: A Versatile Immune Gene That Influences Lipid Metabolism in Bovine Mammary Epithelial Cell Lines. International Journal of Molecular Sciences, 25(4), 2375. https://doi.org/10.3390/ijms25042375






