Three-Dimensionally Cultured Jaw Periosteal Cells Attenuate Macrophage Activation of CD4+ T Cells and Inhibit Osteoclastogenesis
Abstract
1. Introduction
2. Results
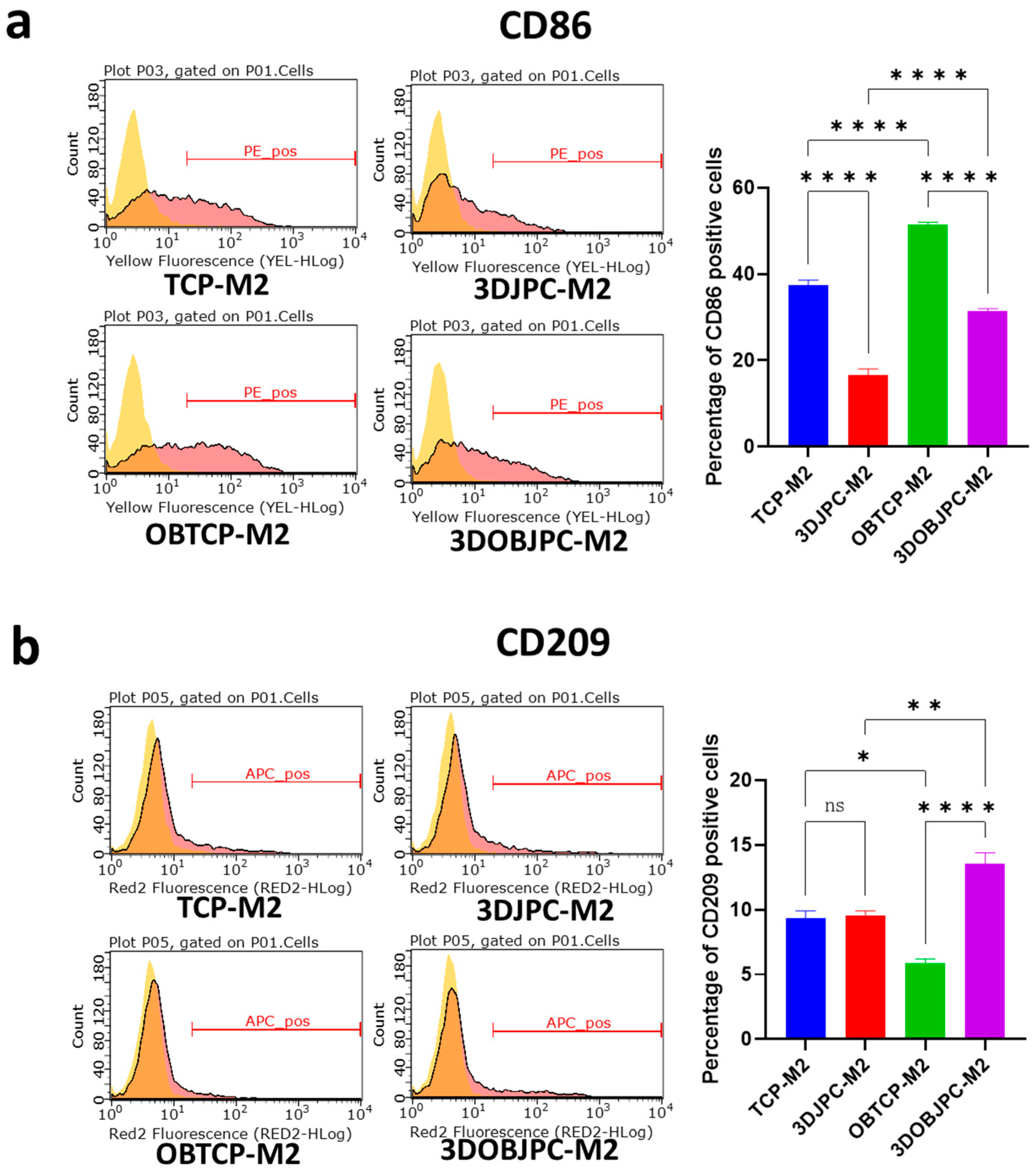
2.1. Phenotypic Changes of THP-1-Derived M1/M2 Macrophages Co-Cultured with 3D-Cultured JPCs
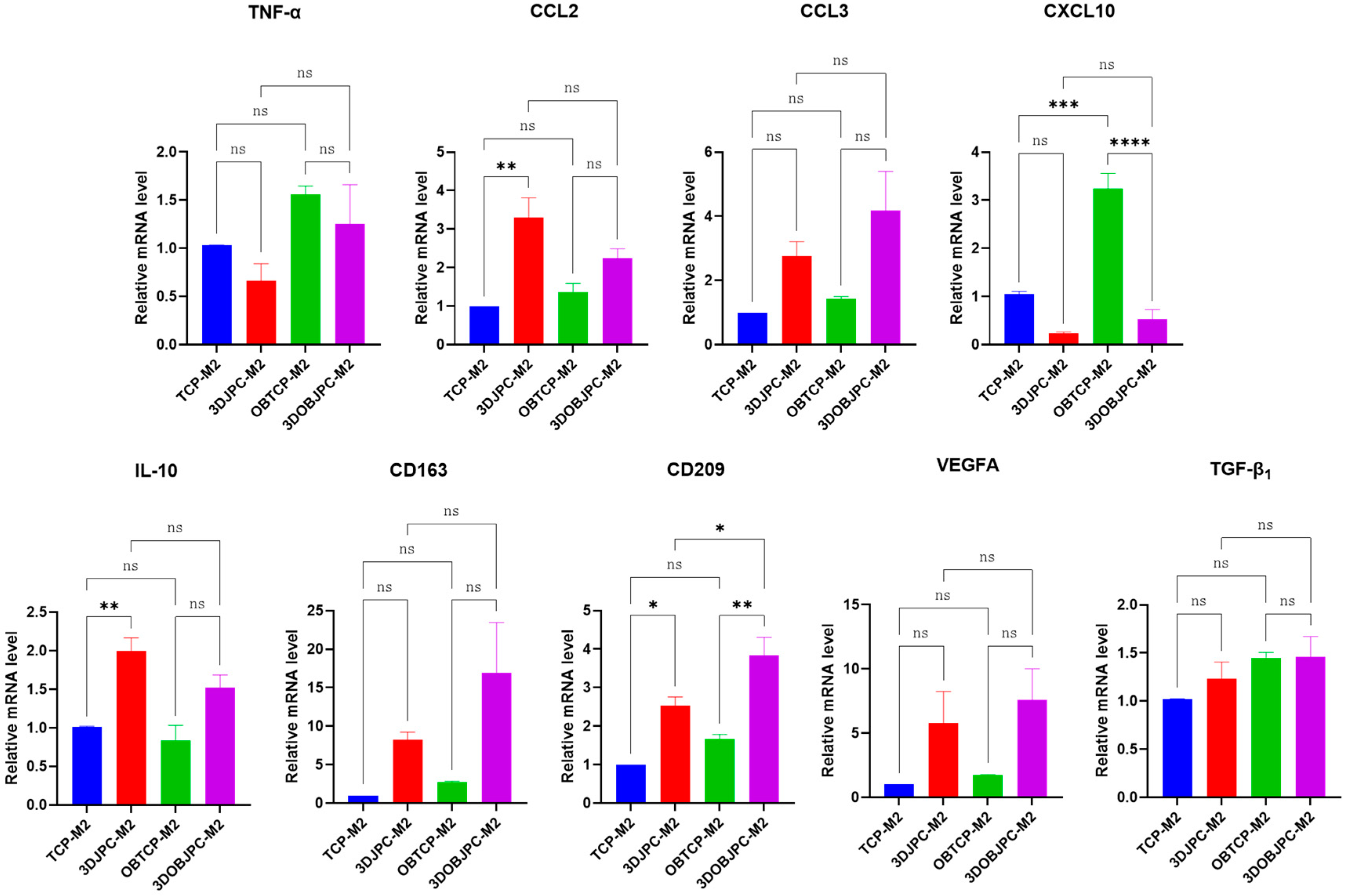
2.2. Quantitative PCR Analysis of Polarization-Related Genes in M1/M2 Macrophages Co-Cultured with 3DJPCs/3DOBJPCs
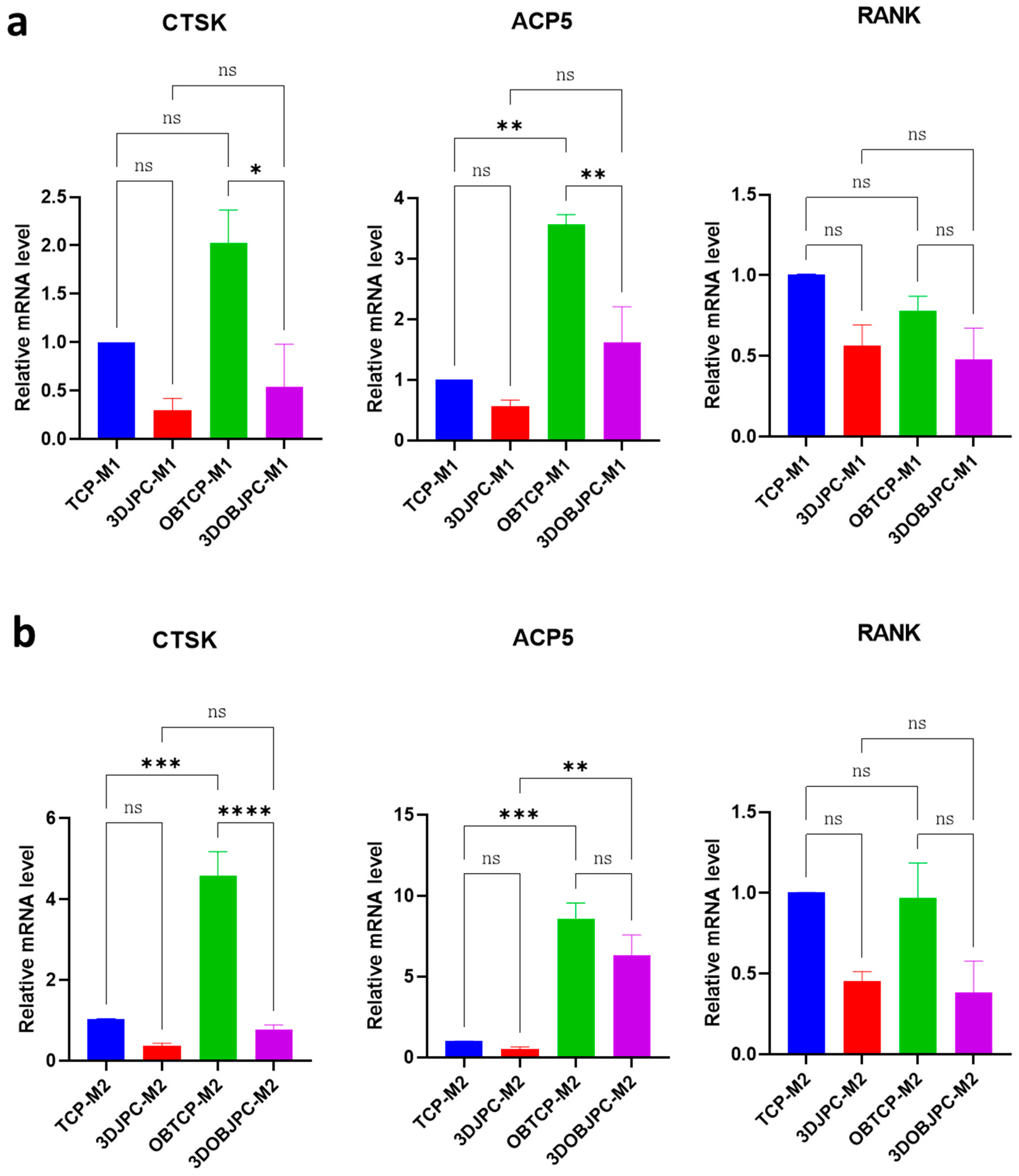
2.3. Quantitative PCR Analysis of Osteoclastogenesis-Related Genes in M1/M2 Macrophages Co-Cultured with 3DJPCs/3DOBJPCs
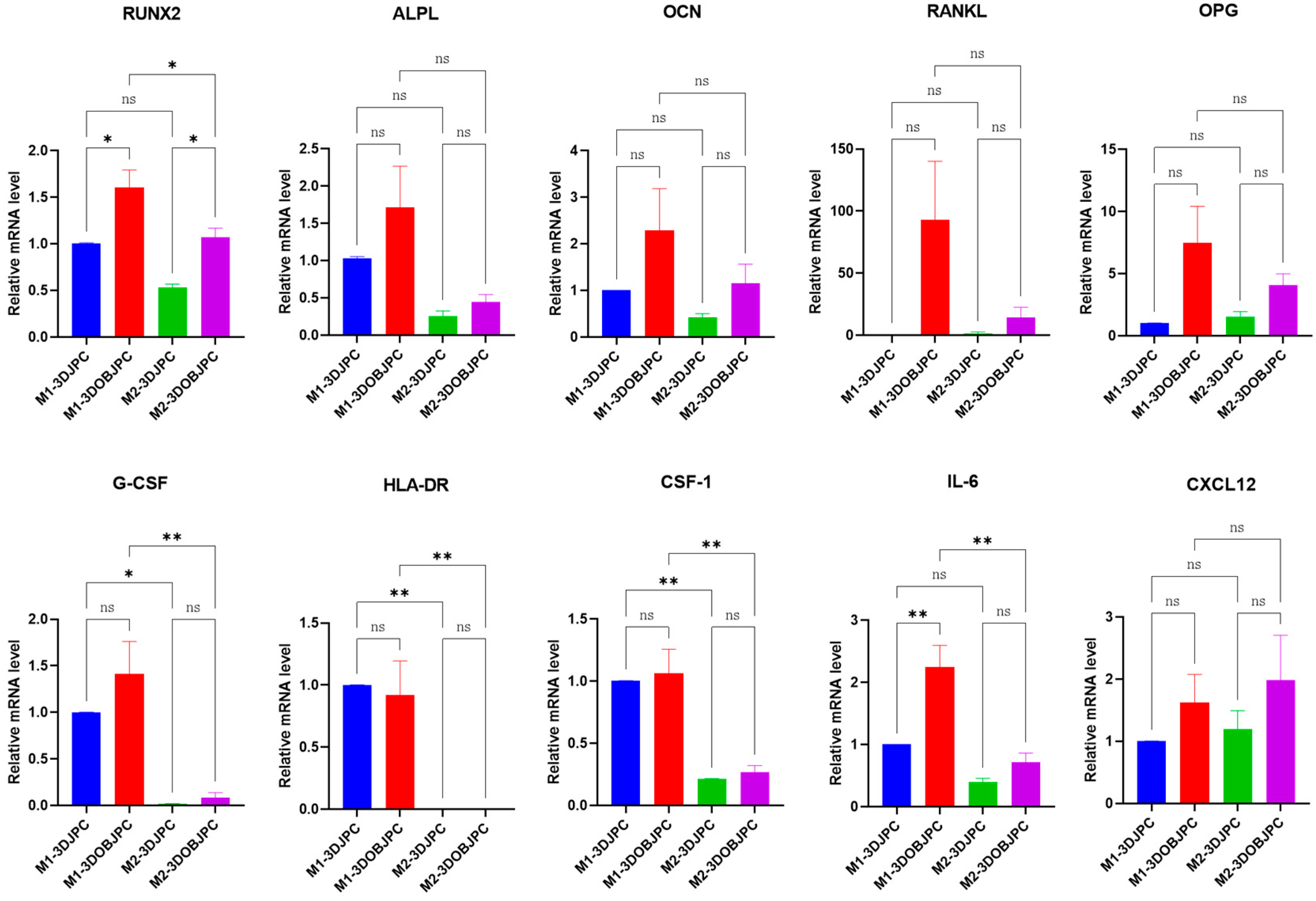
2.4. Quantitative PCR Analysis of Osteogenesis and Immunomodulation-Related Genes in 3DJPCs/3DOBJPCs Co-Cultured with M1/M2 Macrophages
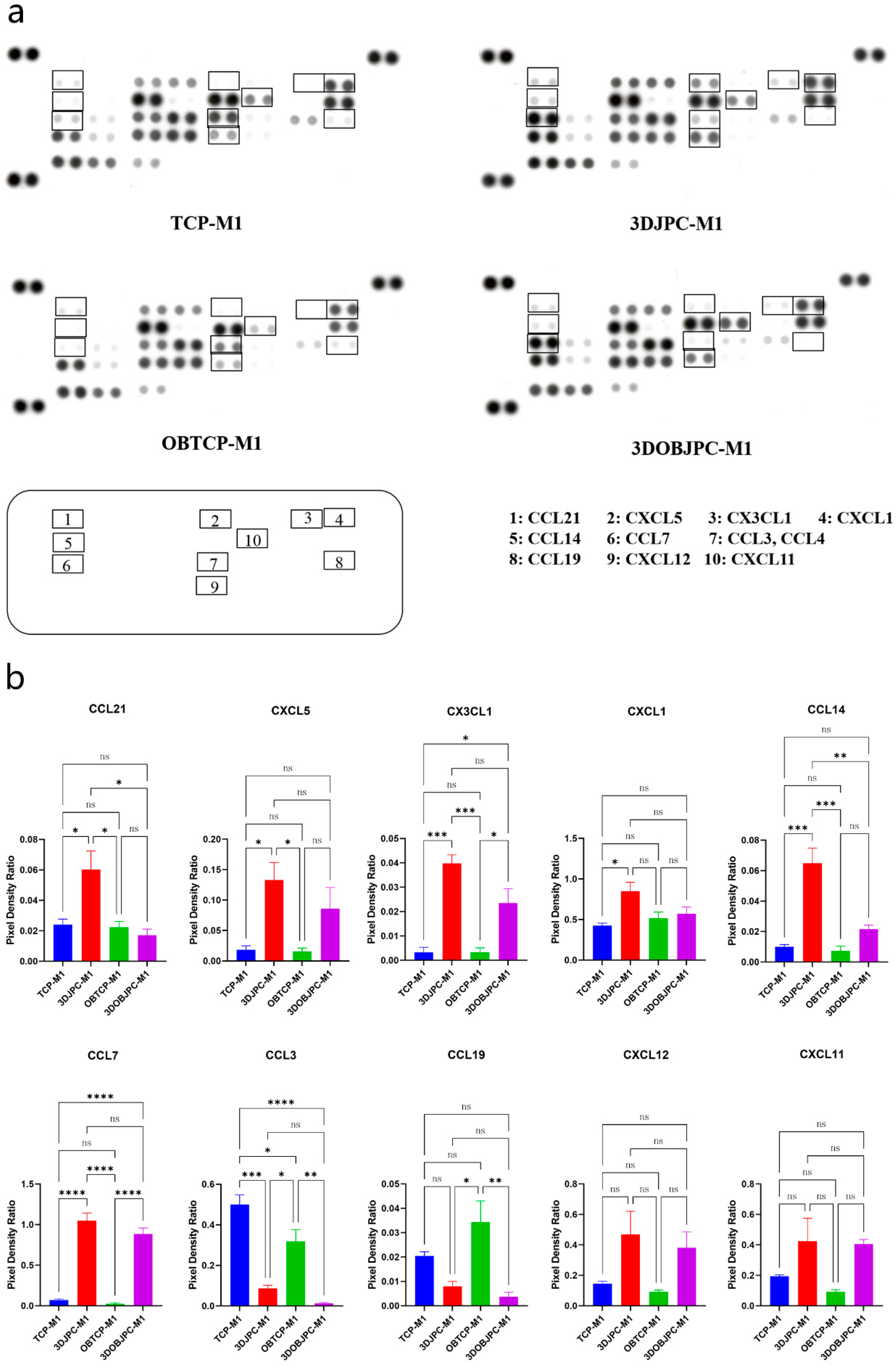
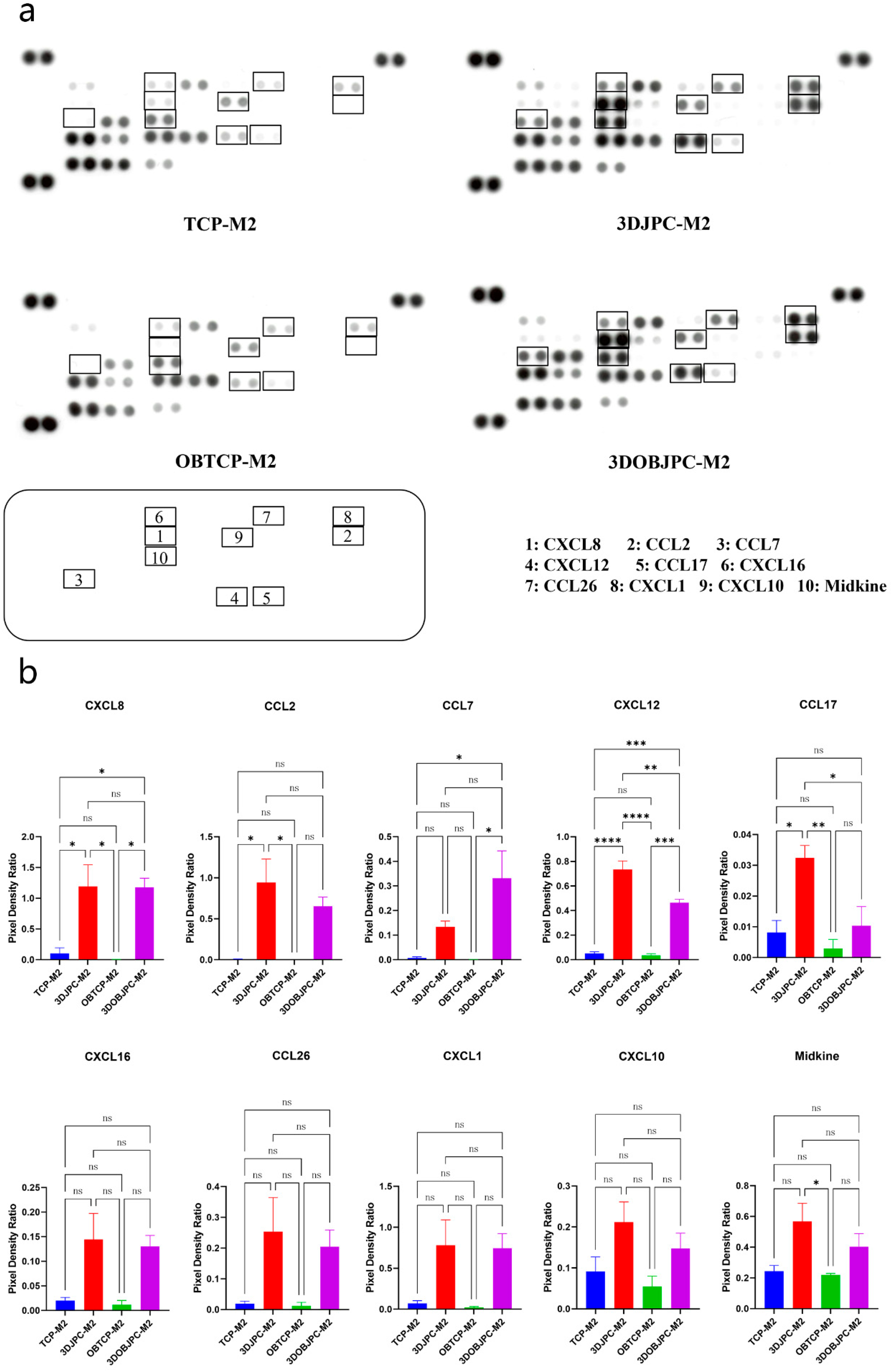
2.5. Chemokine Secretion by M1/M2 Macrophages Co-Cultured with 3DJPCs/3DOBJPCs Ana-lyzed by Proteome Profiler Arrays
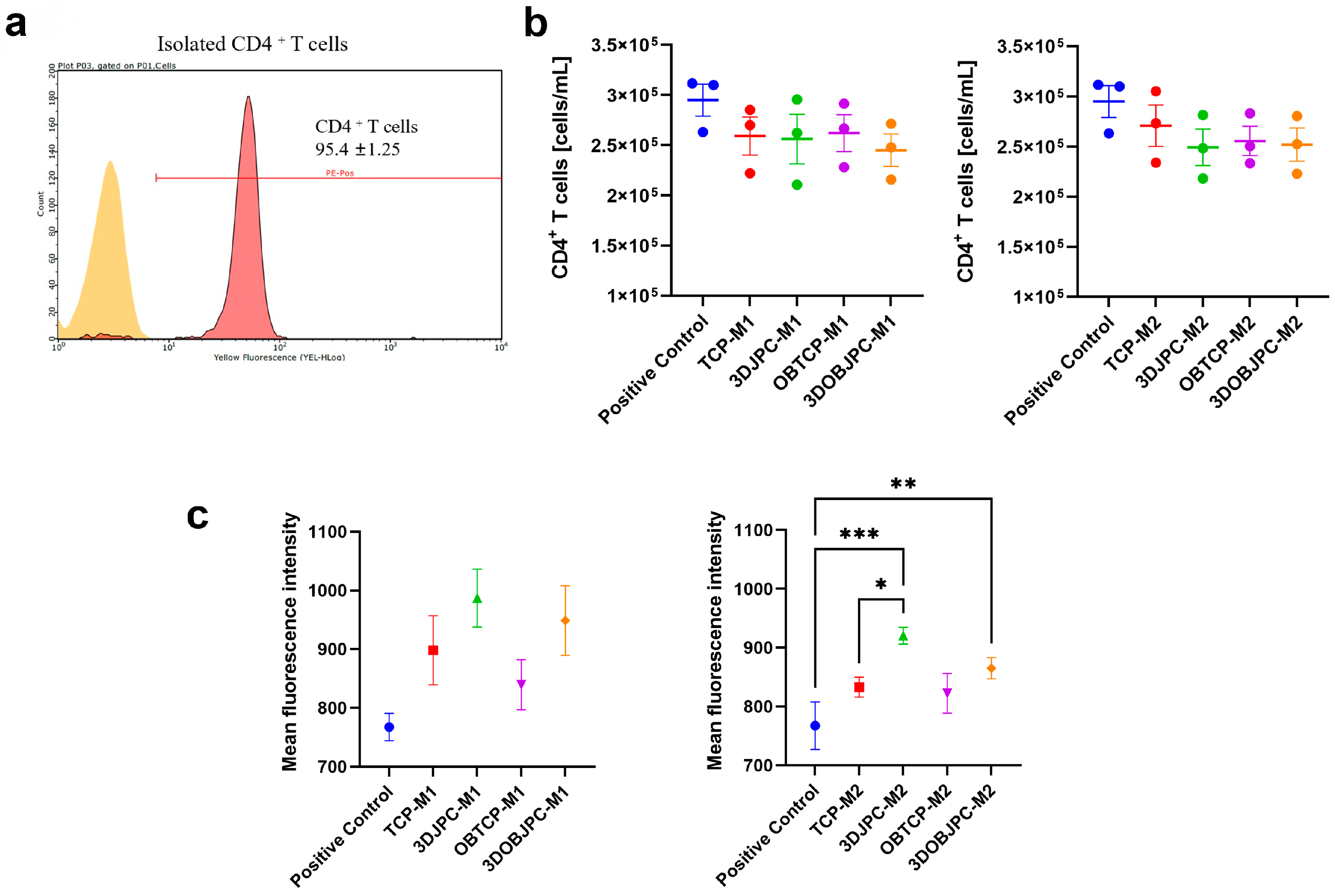
2.6. Functional Assay: Effects of M1/M2 Macrophage Secretomes from Co-Cultures with 3DJPCs/3DOBJPCs on the Proliferation Activity of CD4+ T Cells
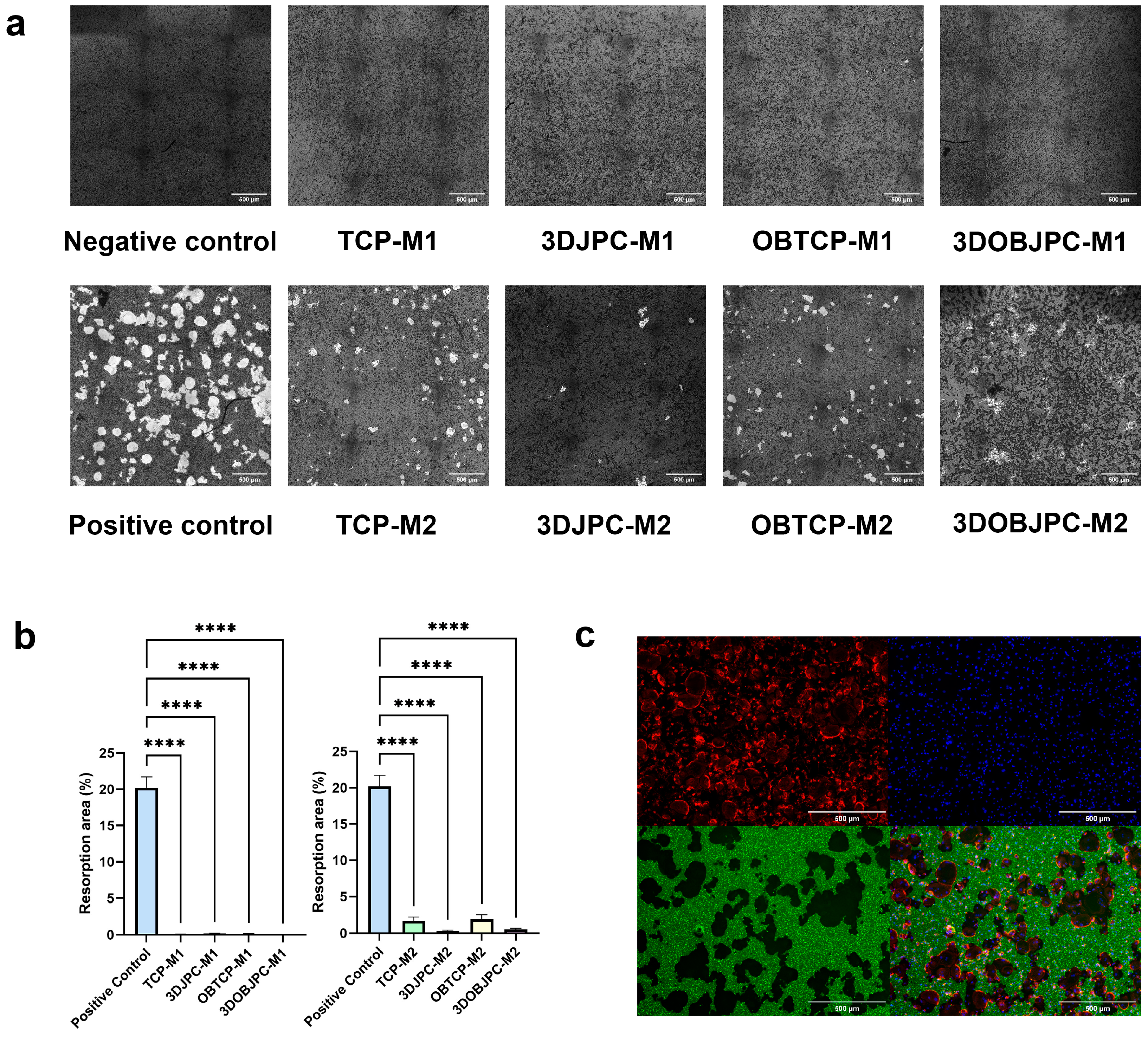
2.7. Functional Assay: Effects of 3DJPCs/3DOBJPCs Secretomes Collected from Co-Cultures with M1/M2 Macrophages on the Differentiation of Osteoclasts
3. Discussion
4. Materials and Methods
4.1. THP-1 and JPC Cell Culture
4.2. Preparation of JPC-Seeded β-TCP Scaffolds (3DJPCs) and Osteogenic Differentiation of 3DJPCs (3DOBJPCs)
4.3. Co-Culture of 3DJPCs/3DOBJPCs and M1/M2 Macrophages
4.4. Detection of Surface Markers on M1/M2 Macrophages by Flow Cytometry
4.5. Quantitative PCR for Macrophage and 3DJPCs Gene Expression Analysis
4.6. Chemokine Detection in Supernatants from Co-Cultured M1/M2 Macrophages by Proteome Profiler Arrays
4.7. Functional Assay: Influence of M1/M2 Secretomes on CD4+ T Cell Proliferation
4.8. Functional Assay: Effect of JPC Supernatants on Osteoclasts Differentiation
4.9. Statistical Analysis
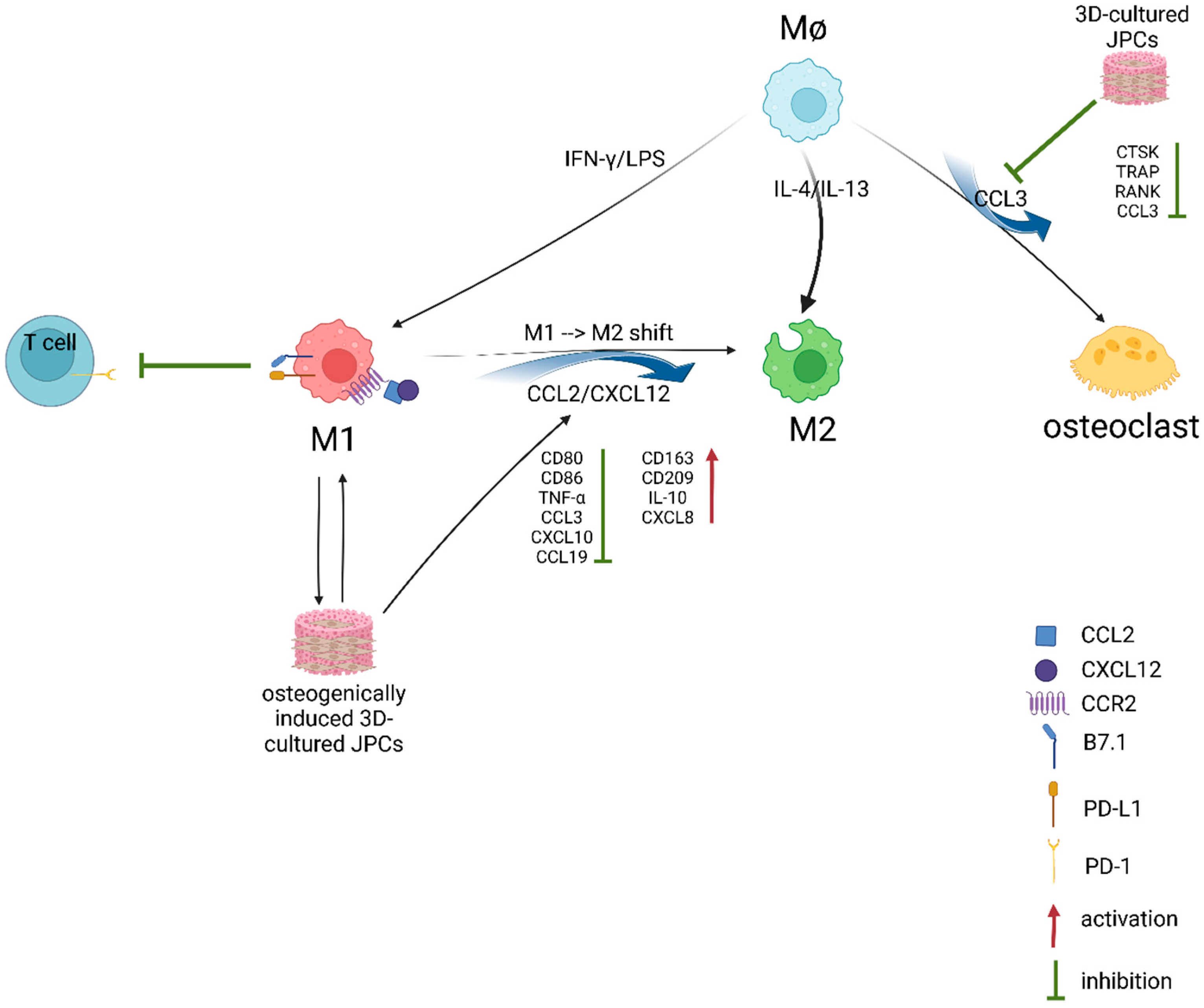
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Titsinides, S.; Agrogiannis, G.; Karatzas, T. Bone grafting materials in dentoalveolar reconstruction: A comprehensive review. Jpn. Dent. Sci. Rev. 2019, 55, 26–32. [Google Scholar] [CrossRef]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Marion, N.W.; Mao, J.J. Mesenchymal stem cells and tissue engineering. Methods Enzymol. 2006, 420, 339–361. [Google Scholar]
- He, F.; Umrath, F.; von Ohle, C.; Reinert, S.; Alexander, D. Analysis of the Influence of Jaw Periosteal Cells on Macrophages Phenotype Using an Innovative Horizontal Coculture System. Biomedicines 2021, 9, 1753. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Umrath, F.; Reinert, S.; Alexander, D. Jaw Periosteum-Derived Mesenchymal Stem Cells Regulate THP-1-Derived Macrophage Polarization. Int. J. Mol. Sci. 2021, 22, 4310. [Google Scholar] [CrossRef]
- Brauchle, E.; Carvajal Berrio, D.; Rieger, M.; Schenke-Layland, K.; Reinert, S.; Alexander, D. Raman spectroscopic analyses of jaw periosteal cell mineralization. Stem Cells Int. 2017, 2017, 1651376. [Google Scholar] [CrossRef]
- Danalache, M.; Kliesch, S.-M.; Munz, M.; Naros, A.; Reinert, S.; Alexander, D. Quality Analysis of Minerals Formed by Jaw Periosteal Cells under Different Culture Conditions. Int. J. Mol. Sci. 2019, 20, 4193. [Google Scholar] [CrossRef]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage polarization: Different gene signatures in M1 (LPS+) vs. classically and M2 (LPS−) vs. alternatively activated macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Mahon, O.R.; Browe, D.C.; Gonzalez-Fernandez, T.; Pitacco, P.; Whelan, I.T.; Von Euw, S.; Hobbs, C.; Nicolosi, V.; Cunningham, K.T.; Mills, K.H.G.; et al. Nano-particle mediated M2 macrophage polarization enhances bone formation and MSC osteogenesis in an IL-10 dependent manner. Biomaterials 2020, 239, 119833. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Thippabhotla, S.; Zhong, C.; He, M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci. Rep. 2019, 9, 13012. [Google Scholar] [CrossRef]
- Kotani, S.; Fujita, Y.; Kitsugi, T.; Nakamura, T.; Yamamuro, T.; Ohtsuki, C.; Kokubo, T. Bone bonding mechanism of β-tricalcium phosphate. J. Biomed. Mater. Res. 1991, 25, 1303–1315. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Alvarez, M.M.; Liu, J.C.; Trujillo-de Santiago, G.; Cha, B.H.; Vishwakarma, A.; Ghaemmaghami, A.M.; Khademhosseini, A. Delivery strategies to control inflammatory response: Modulating M1-M2 polarization in tissue engineering applications. J. Control. Release Off. J. Control. Release Soc. 2016, 240, 349–363. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Schliefsteiner, C.; Peinhaupt, M.; Kopp, S.; Logl, J.; Lang-Olip, I.; Hiden, U.; Heinemann, A.; Desoye, G.; Wadsack, C. Human Placental Hofbauer Cells Maintain an Anti-inflammatory M2 Phenotype despite the Presence of Gestational Diabetes Mellitus. Front. Immunol. 2017, 8, 888. [Google Scholar] [CrossRef]
- Durafourt, B.A.; Moore, C.S.; Zammit, D.A.; Johnson, T.A.; Zaguia, F.; Guiot, M.C.; Bar-Or, A.; Antel, J.P. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia 2012, 60, 717–727. [Google Scholar] [CrossRef]
- Chui, R.; Dorovini-Zis, K. Regulation of CCL2 and CCL3 expression in human brain endothelial cells by cytokines and lipopolysaccharide. J. Neuroinflamm. 2010, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef]
- Lotinun, S.; Kiviranta, R.; Matsubara, T.; Alzate, J.A.; Neff, L.; Luth, A.; Koskivirta, I.; Kleuser, B.; Vacher, J.; Vuorio, E.; et al. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J. Clin. Investig. 2013, 123, 666–681. [Google Scholar] [CrossRef]
- Minkin, C. Bone acid phosphatase: Tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif. Tissue Int. 1982, 34, 285–290. [Google Scholar] [CrossRef] [PubMed]
- van Megen, K.M.; van ‘t Wout, E.T.; Lages Motta, J.; Dekker, B.; Nikolic, T.; Roep, B.O. Activated Mesenchymal Stromal Cells Process and Present Antigens Regulating Adaptive Immunity. Front. Immunol. 2019, 10, 694. [Google Scholar] [CrossRef]
- Noronha, N.C.; Mizukami, A.; Caliari-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef]
- Kim, D.S.; Jang, I.K.; Lee, M.W.; Ko, Y.J.; Lee, D.H.; Lee, J.W.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Enhanced Immunosuppressive Properties of Human Mesenchymal Stem Cells Primed by Interferon-gamma. EBioMedicine 2018, 28, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.E.; Nibbs, R.J. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Qu, Q.; Zheng, B.; Xiong, S.; Fan, G.H. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J. Leukoc. Biol. 2015, 97, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Giri, J.; Das, R.; Nylen, E.; Chinnadurai, R.; Galipeau, J. CCL2 and CXCL12 Derived from Mesenchymal Stromal Cells Cooperatively Polarize IL-10+ Tissue Macrophages to Mitigate Gut Injury. Cell Rep. 2020, 30, 1923–1934.e4. [Google Scholar] [CrossRef] [PubMed]
- Jordan, L.A.; Erlandsson, M.C.; Fenner, B.F.; Davies, R.; Harvey, A.K.; Choy, E.H.; Errington, R.; Bokarewa, M.I.; Williams, A.S. Inhibition of CCL3 abrogated precursor cell fusion and bone erosions in human osteoclast cultures and murine collagen-induced arthritis. Rheumatology 2018, 57, 2042–2052. [Google Scholar] [CrossRef]
- Lin, C.; He, H.; Liu, H.; Li, R.; Chen, Y.; Qi, Y.; Jiang, Q.; Chen, L.; Zhang, P.; Zhang, H.; et al. Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut 2019, 68, 1764–1773. [Google Scholar] [CrossRef]
- Mayoux, M.; Roller, A.; Pulko, V.; Sammicheli, S.; Chen, S.; Sum, E.; Jost, C.; Fransen, M.F.; Buser, R.B.; Kowanetz, M.; et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaav7431. [Google Scholar] [CrossRef]
- Pandey, V.; Fleming-Martinez, A.; Bastea, L.; Doeppler, H.R.; Eisenhauer, J.; Le, T.; Edenfield, B.; Storz, P. CXCL10/CXCR3 signaling contributes to an inflammatory microenvironment and its blockade enhances progression of murine pancreatic precancerous lesions. Elife 2021, 10, e60646. [Google Scholar] [CrossRef]
- Cole, K.E.; Strick, C.A.; Paradis, T.J.; Ogborne, K.T.; Loetscher, M.; Gladue, R.P.; Lin, W.; Boyd, J.G.; Moser, B.; Wood, D.E.; et al. Interferon-inducible T cell alpha chemoattractant (I-TAC): A novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J. Exp. Med. 1998, 187, 2009–2021. [Google Scholar] [CrossRef]
- Colvin, R.A.; Campanella, G.S.; Sun, J.; Luster, A.D. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J. Biol. Chem. 2004, 279, 30219–30227. [Google Scholar] [CrossRef]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—A target for novel cancer therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Umrath, F.; Schmitt, L.F.; Kliesch, S.M.; Schille, C.; Geis-Gerstorfer, J.; Gurewitsch, E.; Bahrini, K.; Peters, F.; Reinert, S.; Alexander, D. Mechanical and Functional Improvement of beta-TCP Scaffolds for Use in Bone Tissue Engineering. J. Funct. Biomater. 2023, 14, 427. [Google Scholar] [CrossRef] [PubMed]



| Abbreviation | Group | Cells | Media |
|---|---|---|---|
| TCP | β-TCP scaffold control | - | control medium |
| OBTCP | osteogenic β-TCP scaffold control | - | osteogenic medium |
| 3DJPC | co-cultured untreated JPC-seeded β-TCP scaffold | JPCs | control medium |
| 3DOBJPC | co-cultured osteogenically induced JPC-seeded β-TCP scaffold | JPCs | osteogenic medium |
| Gene | TCP-M1 | 3DJPC-M1 | OBTCP-M1 | 3DOBJPC-M1 |
|---|---|---|---|---|
| TNF-α | 1.00 ± 0.00 | 0.08 ± 0.01 a | 1.18 ± 0.11 | 0.07 ± 0.01 b |
| CCL2 | 1.03 ± 0.05 | 0.90 ± 0.02 | 1.08 ± 0.09 | 0.56 ± 0.04 b |
| CCL3 | 1.01 ± 0.00 | 0.27 ± 0.09 a | 0.83 ± 0.12 | 0.14 ± 0.07 b |
| CXCL10 | 1.01 ± 0.00 | 0.20 ± 0.03 a | 1.10 ± 0.27 | 0.18 ± 0.11 b |
| IL-10 | 1.00 ± 0.00 | 1.16 ± 0.04 | 1.98 ± 0.09 | 1.65 ± 0.54 |
| CD163 | 1.01 ± 0.00 | 2.21 ± 0.48 a | 0.57 ± 0.06 | 0.86 ± 0.03 |
| CD209 | 1.01 ± 0.00 | 1.67 ± 0.46 | 0.66 ± 0.07 | 0.99 ± 0.23 |
| VEGFA | 1.01 ± 0.01 | 1.48 ± 0.43 | 1.13 ± 0.34 | 2.17 ± 0.56 |
| TGF-β1 | 1.00 ± 0.00 | 0.72 ± 0.08 | 0.82 ± 0.11 | 0.89 ± 0.17 |
| Gene | TCP-M2 | 3DJPC-M2 | OBTCP-M2 | 3DOBJPC-M2 |
|---|---|---|---|---|
| TNF-α | 1.03 ± 0.00 | 0.66 ± 0.18 | 1.56 ± 0.09 | 1.25 ± 0.41 |
| CCL2 | 1.00 ± 0.00 | 3.31 ± 0.51 a | 1.36 ± 0.23 | 2.25 ± 0.24 |
| CCL3 | 1.00 ± 0.00 | 2.76 ± 0.45 | 1.44 ± 0.06 | 4.17 ± 1.23 |
| CXCL10 | 1.06 ± 0.05 | 0.24 ± 0.03 | 3.24 ± 0.31 | 0.53 ± 0.20 b |
| IL-10 | 1.01 ± 0.01 | 1.99 ± 0.17 a | 0.84 ± 0.20 | 1.52 ± 0.17 |
| CD163 | 1.00 ± 0.00 | 8.26 ± 0.98 | 2.74 ± 0.12 | 16.96 ± 6.49 |
| CD209 | 1.00 ± 0.00 | 2.54 ± 0.22 a | 1.66 ± 0.12 | 3.84 ± 0.46 b |
| VEGFA | 1.00 ± 0.00 | 5.77 ± 2.47 | 1.71 ± 0.07 | 7.59 ± 2.42 |
| TGF-β1 | 1.02 ± 0.01 | 1.23 ± 0.17 | 1.45 ± 0.06 | 1.46 ± 0.21 |
| Chemokine | TCP-M1 | 3DJPC-M1 | OBTCP-M1 | 3DOBJPC-M1 |
|---|---|---|---|---|
| CCL21 | 0.02 ± 0.00 | 0.06 ± 0.01 a | 0.02 ± 0.00 | 0.02 ± 0.00 |
| CXCL5 | 0.02 ± 0.01 | 0.13 ± 0.03 a | 0.02 ± 0.01 | 0.09 ± 0.03 |
| CX3CL1 | 0.00 ± 0.00 | 0.04 ± 0.00 a | 0.00 ± 0.00 | 0.02 ± 0.01 b |
| CXCL1 | 0.43 ± 0.03 | 0.85 ± 0.11 a | 0.52 ± 0.08 | 0.57 ± 0.08 |
| CCL14 | 0.01 ± 0.00 | 0.06 ± 0.01 a | 0.01 ± 0.00 | 0.02 ± 0.00 |
| CCL7 | 0.07 ± 0.01 | 1.05 ± 0.09 a | 0.03 ± 0.01 | 0.89 ± 0.07 b |
| CCL3/CCL4 | 0.50 ± 0.05 | 0.09 ± 0.01 a | 0.32 ± 0.06 | 0.01 ± 0.00 b |
| CCL19 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.01 | 0.00 ± 0.00 b |
| CXCL12 | 0.14 ± 0.02 | 0.47 ± 0.15 | 0.09 ± 0.01 | 0.38 ± 0.10 |
| CXCL11 | 0.19 ± 0.01 | 0.43 ± 0.15 | 0.09 ± 0.01 | 0.41 ± 0.03 |
| Chemokine | TCP-M2 | 3DJPC-M2 | OBTCP-M2 | 3DOBJPC-M2 |
|---|---|---|---|---|
| CXCL8 | 0.10 ± 0.09 | 1.20 ± 0.35 a | 0.00 ± 0.00 | 1.18 ± 0.15 b |
| CCL2 | 0.00 ± 0.00 | 0.94 ± 0.29 a | 0.00 ± 0.00 | 0.65 ± 0.11 |
| CCL7 | 0.01 ± 0.00 | 0.13 ± 0.02 | 0.00 ± 0.00 | 0.33 ± 0.11 b |
| CXCL12 | 0.05 ± 0.01 | 0.73 ± 0.07 a | 0.04 ± 0.01 | 0.47 ± 0.03 b |
| CCL17 | 0.01 ± 0.00 | 0.03 ± 0.00 a | 0.00 ± 0.00 | 0.01 ± 0.01 |
| CXCL16 | 0.02 ± 0.01 | 0.14 ± 0.05 | 0.01 ± 0.01 | 0.13 ± 0.02 |
| CCL26 | 0.02 ± 0.01 | 0.25 ± 0.11 | 0.01 ± 0.01 | 0.20 ± 0.05 |
| CXCL1 | 0.07 ± 0.03 | 0.78 ± 0.31 | 0.02 ± 0.01 | 0.74 ± 0.18 |
| CXCL10 | 0.09 ± 0.04 | 0.21 ± 0.05 | 0.05 ± 0.02 | 0.15 ± 0.04 |
| Midkine | 0.24 ± 0.04 | 0.57 ± 0.12 | 0.22 ± 0.01 | 0.40 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, F.; Wang, L.; Umrath, F.; Naros, A.; Reinert, S.; Alexander, D. Three-Dimensionally Cultured Jaw Periosteal Cells Attenuate Macrophage Activation of CD4+ T Cells and Inhibit Osteoclastogenesis. Int. J. Mol. Sci. 2024, 25, 2355. https://doi.org/10.3390/ijms25042355
He F, Wang L, Umrath F, Naros A, Reinert S, Alexander D. Three-Dimensionally Cultured Jaw Periosteal Cells Attenuate Macrophage Activation of CD4+ T Cells and Inhibit Osteoclastogenesis. International Journal of Molecular Sciences. 2024; 25(4):2355. https://doi.org/10.3390/ijms25042355
Chicago/Turabian StyleHe, Fang, Liuran Wang, Felix Umrath, Andreas Naros, Siegmar Reinert, and Dorothea Alexander. 2024. "Three-Dimensionally Cultured Jaw Periosteal Cells Attenuate Macrophage Activation of CD4+ T Cells and Inhibit Osteoclastogenesis" International Journal of Molecular Sciences 25, no. 4: 2355. https://doi.org/10.3390/ijms25042355
APA StyleHe, F., Wang, L., Umrath, F., Naros, A., Reinert, S., & Alexander, D. (2024). Three-Dimensionally Cultured Jaw Periosteal Cells Attenuate Macrophage Activation of CD4+ T Cells and Inhibit Osteoclastogenesis. International Journal of Molecular Sciences, 25(4), 2355. https://doi.org/10.3390/ijms25042355







