Deciphering Molecular Mechanisms Involved in the Modulation of Human Aquaporins’ Water Permeability by Zinc Cations: A Molecular Dynamics Approach
Abstract
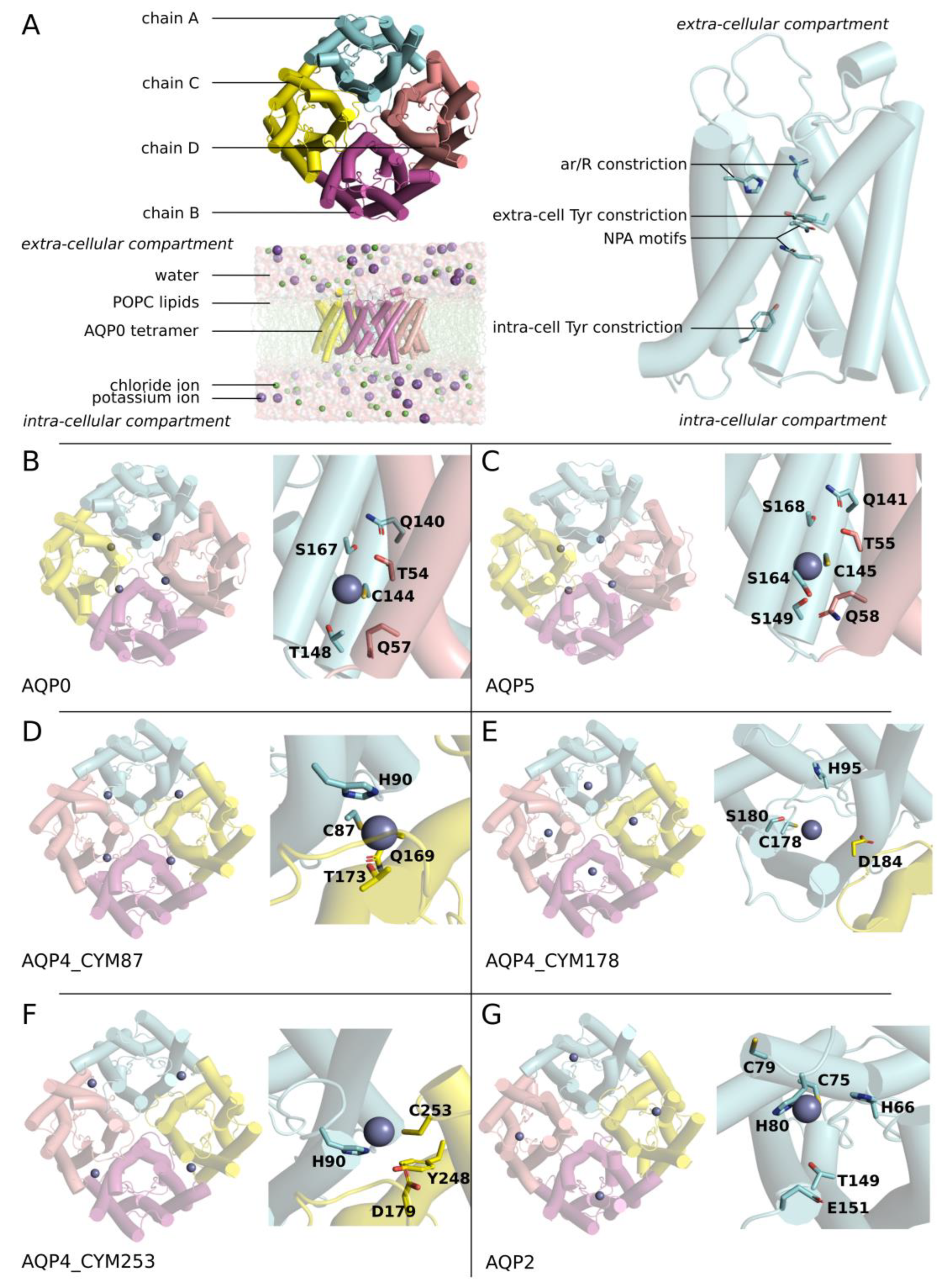
1. Introduction
2. Results
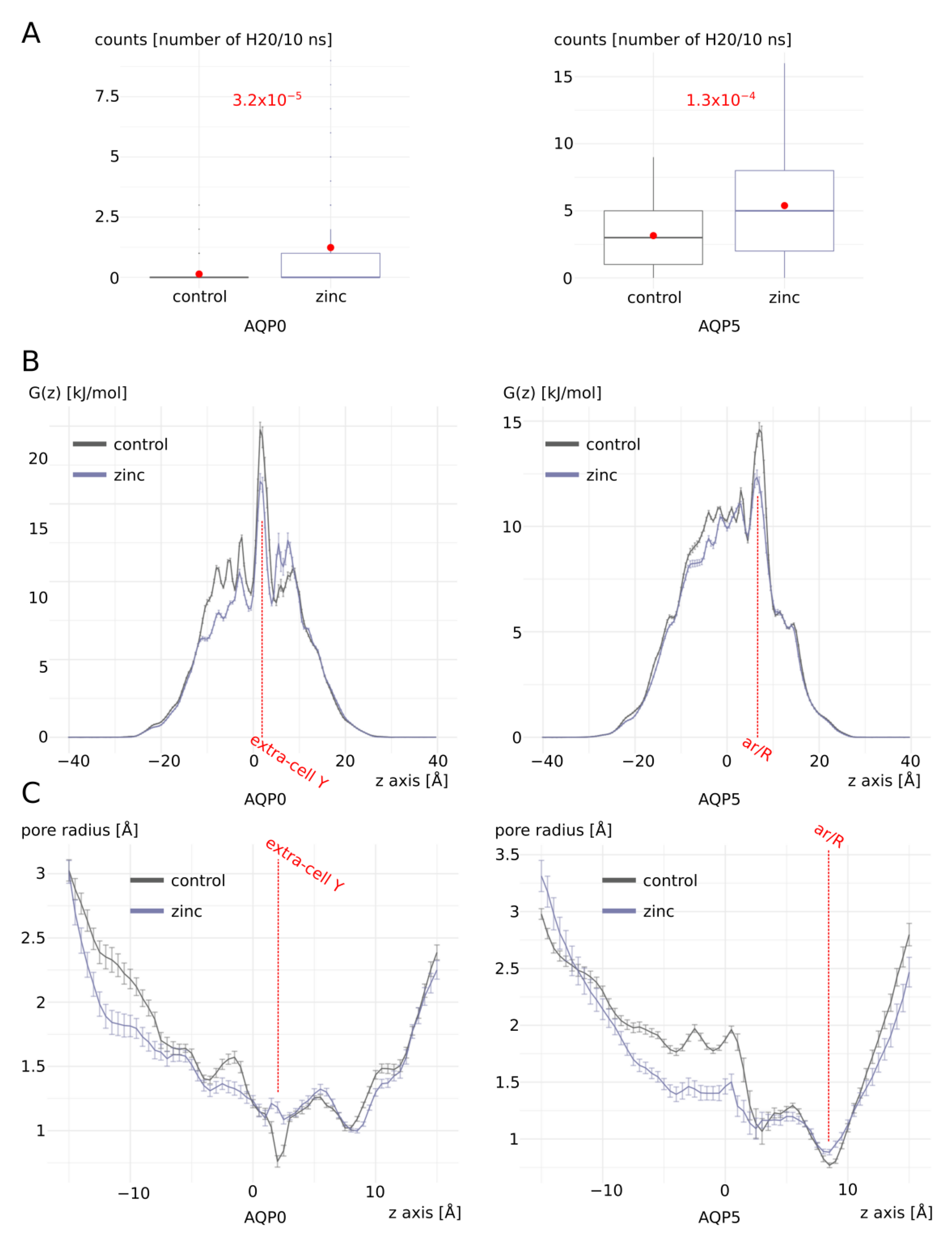
2.1. Zinc Increases AQP0 and AQP5 Water Permeability
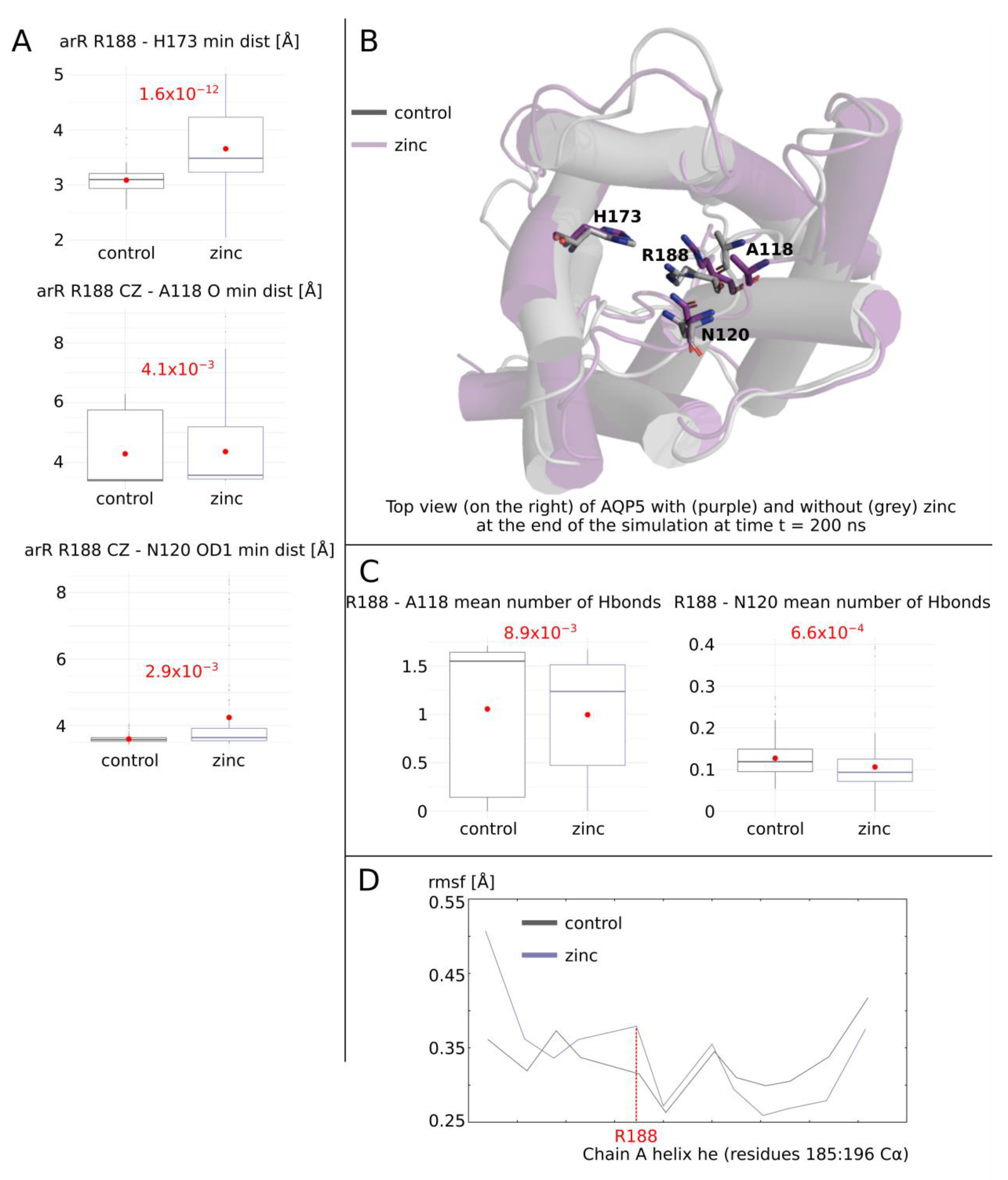
2.2. Molecular Mechanism of AQP0 and AQP5 Water Permeability Enhancement by Zinc
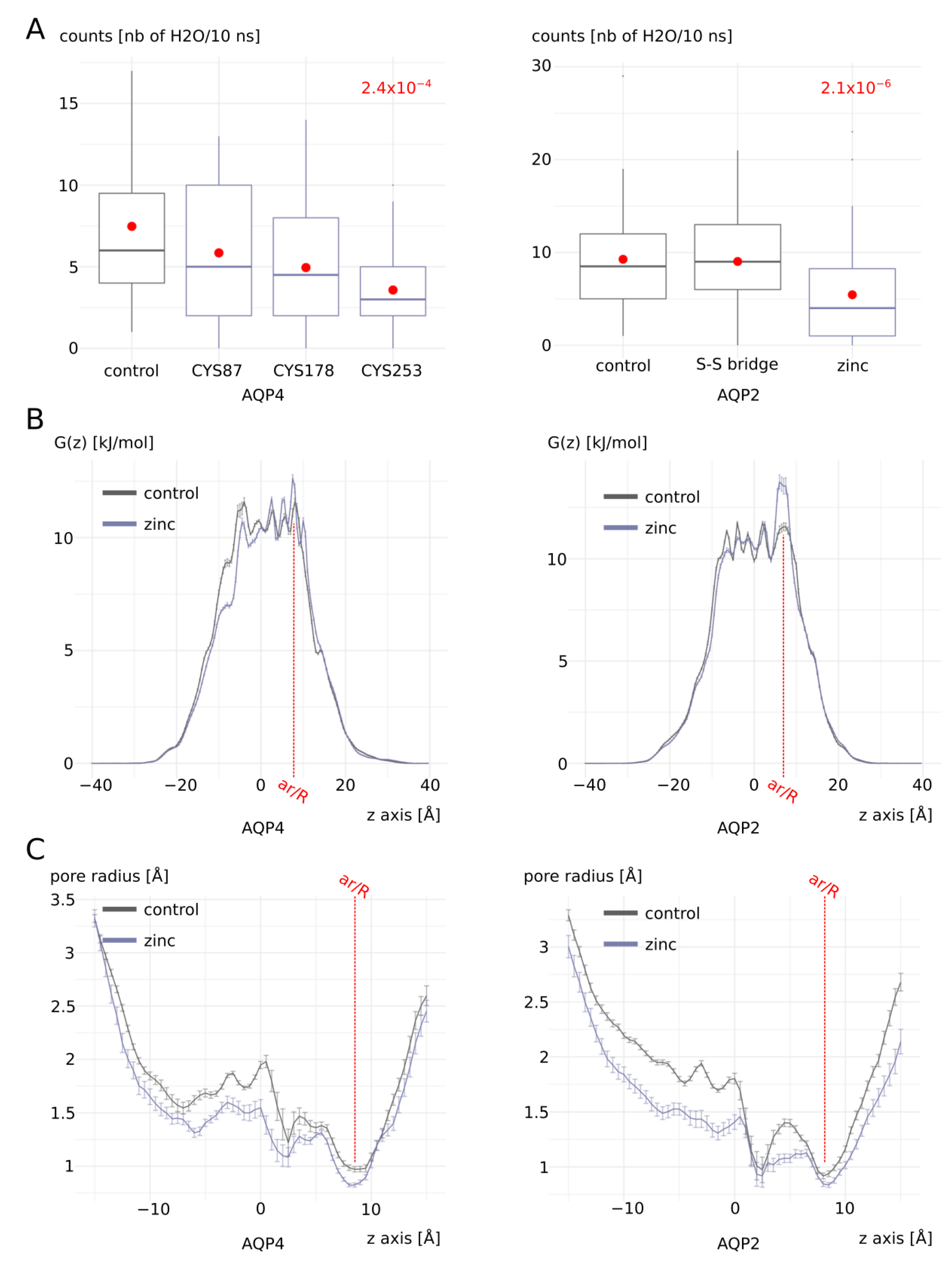
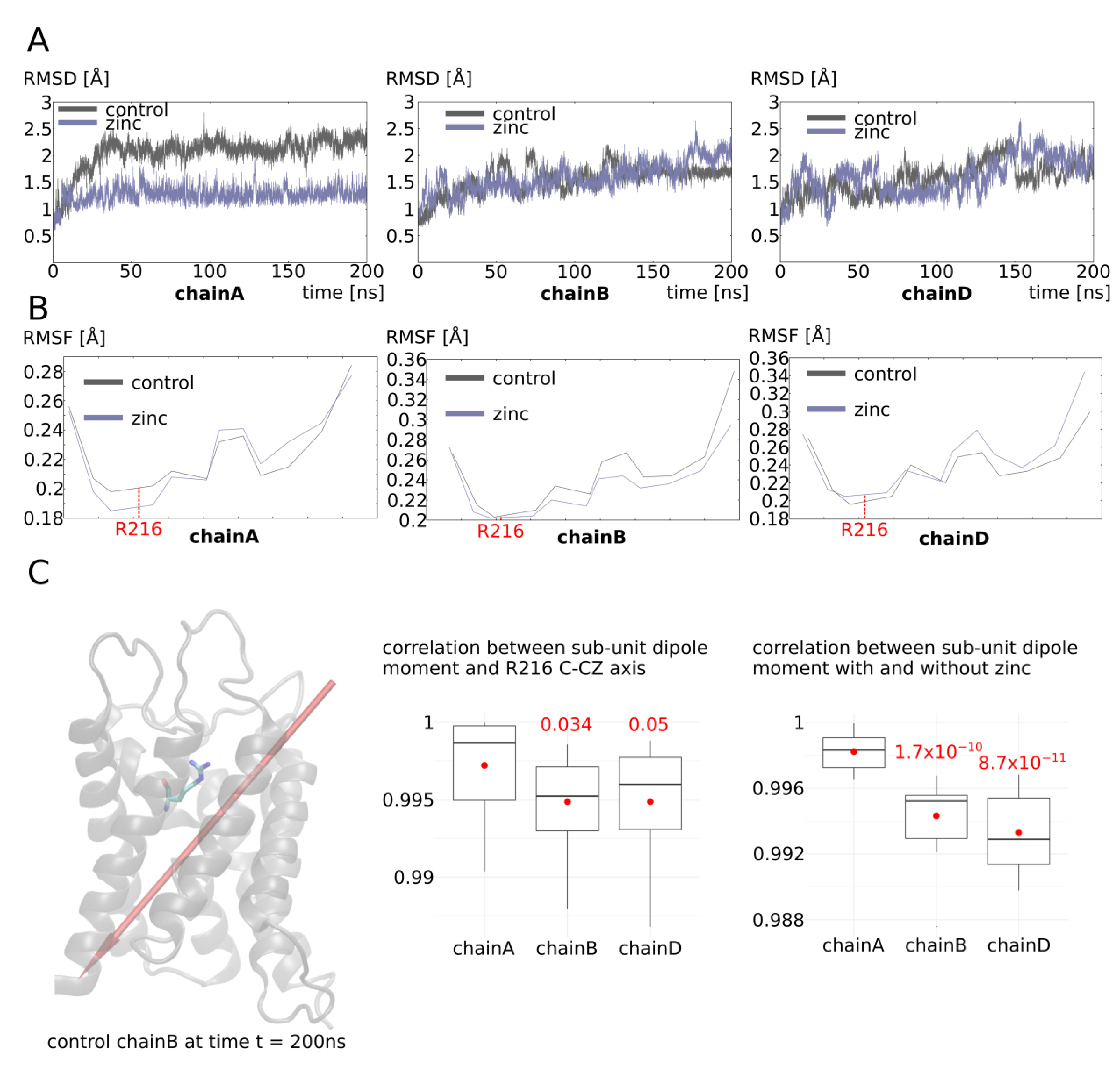
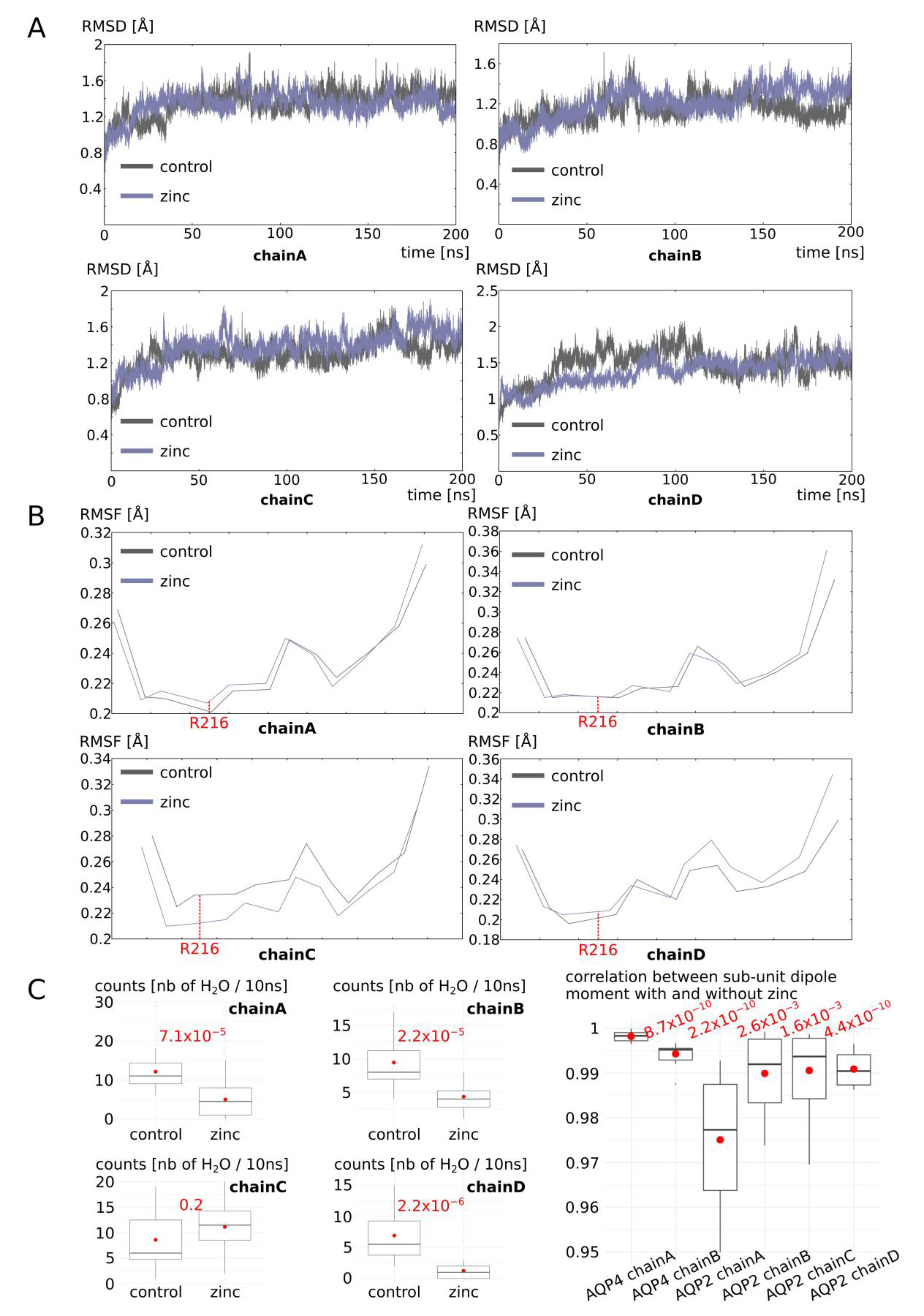
2.3. Zinc Decreases AQP4 and AQP2 Water Permeability
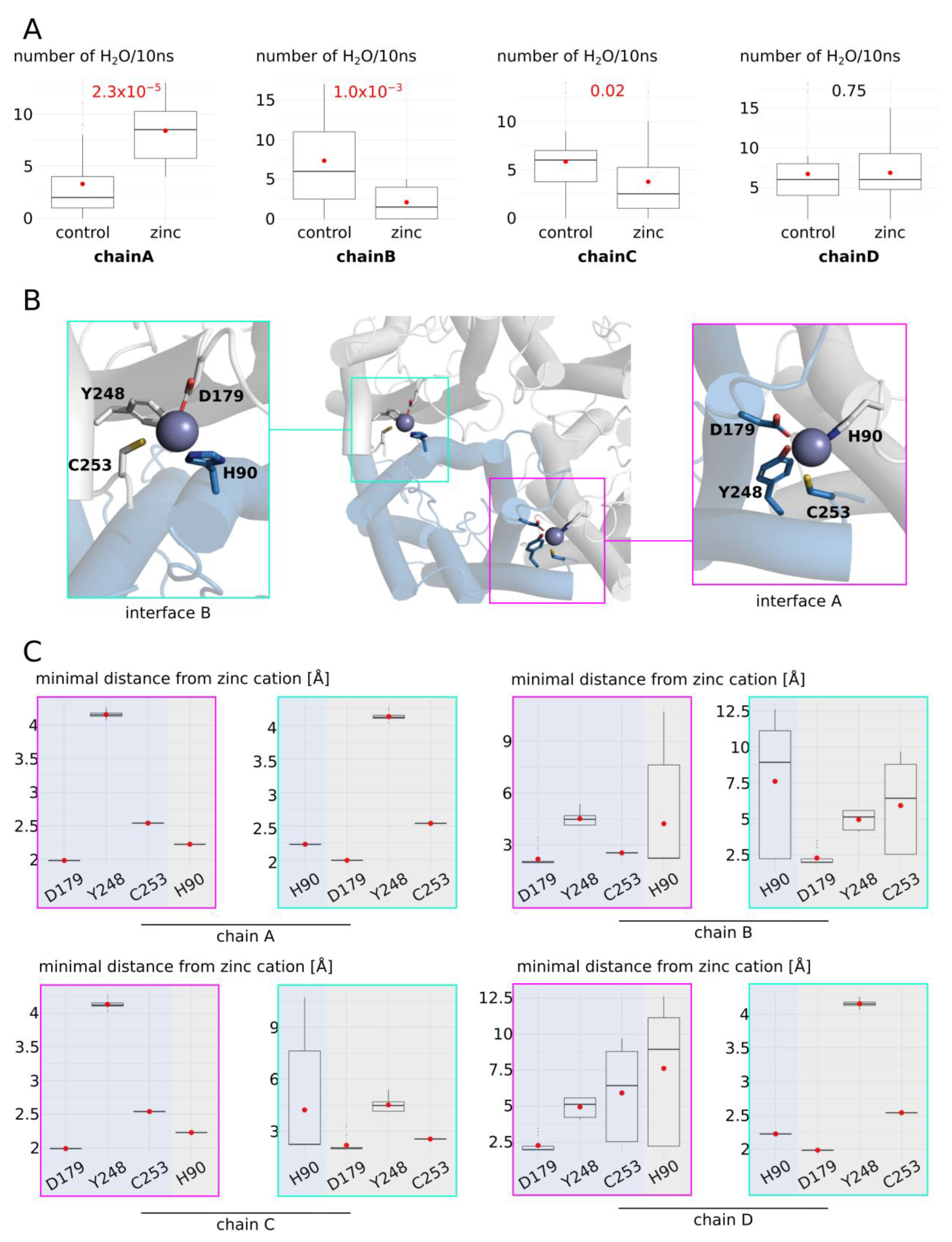
2.4. Molecular Mechanism of AQP4 and AQP2 Water Permeability Reduction by Zinc
3. Discussion
4. Materials and Methods
4.1. Molecular Dynamics Simulations
4.2. Analysis
4.2.1. Water Permeability
4.2.2. Free-Energy Profiles
4.2.3. Other Properties
4.2.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Livingstone, C. Zinc. Nutr. Clin. Pract. 2015, 30, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Hambidge, K.M.; Krebs, N.F. Zinc Deficiency: A Special Challenge1,2. J. Nutr. 2007, 137, 1101–1105. [Google Scholar] [CrossRef]
- Shankar, A.H.; Prasad, A.S. Zinc and Immune Function: The Biological Basis of Altered Resistance to Infection. Am. J. Clin. Nutr. 1998, 68, 447S–463S. [Google Scholar] [CrossRef] [PubMed]
- Cassandri, M.; Smirnov, A.; Novelli, F.; Pitolli, C.; Agostini, M.; Malewicz, M.; Melino, G.; Raschellà, G. Zinc-Finger Proteins in Health and Disease. Cell Death Discov. 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- McCall, K.A.; Huang, C.; Fierke, C.A. Function and Mechanism of Zinc Metalloenzymes. J. Nutr. 2000, 130, 1437S–1446S. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, B.L. Role of Zinc in Plasma Membrane Function. J. Nutr. 2000, 130, 1432S–1436S. [Google Scholar] [CrossRef] [PubMed]
- Klug, A.; Schwabe, J.W.R. Zinc Fingers. FASEB J. 1995, 9, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Beitz, E. Aquaporins; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-79885-9. [Google Scholar]
- Abascal, F.; Irisarri, I.; Zardoya, R. Diversity and Evolution of Membrane Intrinsic Proteins. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 1468–1481. [Google Scholar] [CrossRef]
- Tajkhorshid, E.; Nollert, P.; Jensen, M.Ø.; Miercke, L.J.W.; O’Connell, J.; Stroud, R.M.; Schulten, K. Control of the Selectivity of the Aquaporin Water Channel Family by Global Orientational Tuning. Science 2002, 296, 525–530. [Google Scholar] [CrossRef]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of Water Channels in Xenopus Oocytes Expressing Red Cell CHIP28 Protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef]
- Tradtrantip, L.; Jin, B.-J.; Yao, X.; Anderson, M.O.; Verkman, A.S. Aquaporin-Targeted Therapeutics: State-of-the-Field. In Aquaporins; Advances in Experimental Medicine and Biology; Yang, B., Ed.; Springer: Dordrecht, The Netherlands, 2017; pp. 239–250. ISBN 978-94-024-1057-0. [Google Scholar]
- Verkman, A.S.; Smith, A.J.; Phuan, P.; Tradtrantip, L.; Anderson, M.O. The Aquaporin-4 Water Channel as a Potential Drug Target in Neurological Disorders. Expert Opin. Ther. Targets 2017, 21, 1161–1170. [Google Scholar] [CrossRef]
- Takeda, T.; Taguchi, D. Aquaporins as Potential Drug Targets for Meniere’s Disease and Its Related Diseases. Handb. Exp. Pharmacol. 2009, 190, 171–184. [Google Scholar] [CrossRef]
- Yang, B.; Verkman, A.S. Water and Glycerol Permeabilities of Aquaporins 1–5 and MIP Determined Quantitatively by Expression of Epitope-Tagged Constructs inXenopus Oocytes *. J. Biol. Chem. 1997, 272, 16140–16146. [Google Scholar] [CrossRef] [PubMed]
- Németh-Cahalan, K.L.; Kalman, K.; Froger, A.; Hall, J.E. Zinc Modulation of Water Permeability Reveals That Aquaporin 0 Functions as a Cooperative Tetramer. J. Gen. Physiol. 2007, 130, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Yukutake, Y.; Hirano, Y.; Suematsu, M.; Yasui, M. Rapid and Reversible Inhibition of Aquaporin-4 by Zinc. Biochemistry 2009, 48, 12059–12061. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Jo, Y.; Lee, Y.-H.; Park, K.; Park, H.-K.; Choi, S.-Y. Zn2+ Stimulates Salivary Secretions via Metabotropic Zinc Receptor ZnR/GPR39 in Human Salivary Gland Cells. Sci. Rep. 2019, 9, 17648. [Google Scholar] [CrossRef] [PubMed]
- Day, R.E.; Kitchen, P.; Owen, D.S.; Bland, C.; Marshall, L.; Conner, A.C.; Bill, R.M.; Conner, M.T. Human Aquaporins: Regulators of Transcellular Water Flow. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 1492–1506. [Google Scholar] [CrossRef] [PubMed]
- Grahn, B.H.; Paterson, P.G.; Gottschall-Pass, K.T.; Zhang, Z. Zinc and the Eye. J. Am. Coll. Nutr. 2001, 20, 106–118. [Google Scholar] [CrossRef]
- Ma, T.; Song, Y.; Gillespie, A.; Carlson, E.J.; Epstein, C.J.; Verkman, A.S. Defective Secretion of Saliva in Transgenic Mice Lacking Aquaporin-5 Water Channels *. J. Biol. Chem. 1999, 274, 20071–20074. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin Water Channels in the Nervous System. Nat. Rev. Neurosci. 2013, 14, 265–277. [Google Scholar] [CrossRef]
- Sensi, S.L.; Paoletti, P.; Bush, A.I.; Sekler, I. Zinc in the Physiology and Pathology of the CNS. Nat. Rev. Neurosci. 2009, 10, 780–791. [Google Scholar] [CrossRef]
- Mahajan, S.K. Zinc in Kidney Disease. J. Am. Coll. Nutr. 1989, 8, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Mom, R.; Robert-Paganin, J.; Mom, T.; Chabbert, C.; Réty, S.; Auguin, D. A Perspective for Ménière’s Disease: In Silico Investigations of Dexamethasone as a Direct Modulator of AQP2. Biomolecules 2022, 12, 511. [Google Scholar] [CrossRef] [PubMed]
- Mom, R.; Réty, S.; Auguin, D. Cortisol Interaction with Aquaporin-2 Modulates Its Water Permeability: Perspectives for Non-Genomic Effects of Corticosteroids. Int. J. Mol. Sci. 2023, 24, 1499. [Google Scholar] [CrossRef] [PubMed]
- Hub, J.S.; Aponte-Santamaría, C.; Grubmüller, H.; de Groot, B.L. Voltage-Regulated Water Flux through Aquaporin Channels In Silico. Biophys. J. 2010, 99, L97–L99. [Google Scholar] [CrossRef]
- Mom, R.; Muries, B.; Benoit, P.; Robert-Paganin, J.; Réty, S.; Venisse, J.-S.; Padua, A.; Label, P.; Auguin, D. Voltage-Gating of Aquaporins, a Putative Conserved Safety Mechanism during Ionic Stresses. FEBS Lett. 2021, 595, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, H.; Kamali, R.; Binesh, A. Investigation of the Aquaporin-2 Gating Mechanism with Molecular Dynamics Simulations. Proteins: Struct. Funct. Bioinform. 2021, 89, 819–831. [Google Scholar] [CrossRef]
- Abir-Awan, M.; Kitchen, P.; Salman, M.M.; Conner, M.T.; Conner, A.C.; Bill, R.M. Inhibitors of Mammalian Aquaporin Water Channels. Int. J. Mol. Sci. 2019, 20, 1589. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.F.; Stroud, R.M. Structural Basis of Aquaporin Inhibition by Mercury. J. Mol. Biol. 2007, 368, 607–617. [Google Scholar] [CrossRef]
- Xie, H.; Ma, S.; Zhao, Y.; Zhou, H.; Tong, Q.; Chen, Y.; Zhang, Z.; Yu, K.; Lin, Q.; Kai, L.; et al. Molecular Mechanisms of Mercury-Sensitive Aquaporins. J. Am. Chem. Soc. 2022, 144, 22229–22241. [Google Scholar] [CrossRef]
- Hirano, Y.; Okimoto, N.; Kadohira, I.; Suematsu, M.; Yasuoka, K.; Yasui, M. Molecular Mechanisms of How Mercury Inhibits Water Permeation through Aquaporin-1: Understanding by Molecular Dynamics Simulation. Biophys. J. 2010, 98, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Catalano, C.; AL Mughram, M.H.; Guo, Y.; Kellogg, G.E. 3D Interaction Homology: Hydropathic Interaction Environments of Serine and Cysteine Are Strikingly Different and Their Roles Adapt in Membrane Proteins. Curr. Res. Struct. Biol. 2021, 3, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Dajani, S.; Saripalli, A.; Sharma-Walia, N. Water Transport Proteins–Aquaporins (AQPs) in Cancer Biology. Oncotarget 2018, 9, 36392–36405. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Hara-Chikuma, M.; Papadopoulos, M.C. Aquaporins—New Players in Cancer Biology. J. Mol. Med. 2008, 86, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Kucuk, O. Zinc in Cancer Prevention. Cancer Metastasis Rev. 2002, 21, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.B.; Costello, L.C. Zinc as an Anti-Tumor Agent in Prostate Cancer and in Other Cancers. Arch. Biochem. Biophys. 2007, 463, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.P.; Choi, D.W. Ions, Cell Volume, and Apoptosis. Proc. Natl. Acad. Sci. USA 2000, 97, 9360–9362. [Google Scholar] [CrossRef] [PubMed]
- Benfenati, V.; Caprini, M.; Dovizio, M.; Mylonakou, M.N.; Ferroni, S.; Ottersen, O.P.; Amiry-Moghaddam, M. An Aquaporin-4/Transient Receptor Potential Vanilloid 4 (AQP4/TRPV4) Complex Is Essential for Cell-Volume Control in Astrocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 2563–2568. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Saadoun, S.; Verkman, A.S. Aquaporins and Cell Migration. Pflugers Arch.—Eur. J. Physiol. 2008, 456, 693–700. [Google Scholar] [CrossRef]
- Bründl, J.; Wallinger, S.; Breyer, J.; Weber, F.; Evert, M.; Georgopoulos, N.T.; Rosenhammer, B.; Burger, M.; Otto, W.; Rubenwolf, P. Expression, Localisation and Potential Significance of Aquaporins in Benign and Malignant Human Prostate Tissue. BMC Urol. 2018, 18, 75. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmüller, H.; MacKerell, A.D. CHARMM36m: An Improved Force Field for Folded and Intrinsically Disordered Proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.L.; Cheng, X.; Jo, S.; Rui, H.; Song, K.C.; Dávila-Contreras, E.M.; Qi, Y.; Lee, J.; Monje-Galvan, V.; Venable, R.M.; et al. CHARMM-GUI Membrane Builder toward Realistic Biological Membrane Simulations. J. Comput. Chem. 2014, 35, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A Smooth Particle Mesh Ewald Method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Frick, A.; Eriksson, U.K.; de Mattia, F.; Öberg, F.; Hedfalk, K.; Neutze, R.; de Grip, W.J.; Deen, P.M.T.; Törnroth-Horsefield, S. X-Ray Structure of Human Aquaporin 2 and Its Implications for Nephrogenic Diabetes Insipidus and Trafficking. Proc. Natl. Acad. Sci. USA 2014, 111, 6305–6310. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.D.; Yeh, R.; Sandstrom, A.; Chorny, I.; Harries, W.E.C.; Robbins, R.A.; Miercke, L.J.W.; Stroud, R.M. Crystal Structure of Human Aquaporin 4 at 1.8 Å and Its Mechanism of Conductance. Proc. Natl. Acad. Sci. USA 2009, 106, 7437–7442. [Google Scholar] [CrossRef] [PubMed]
- Horsefield, R.; Nordén, K.; Fellert, M.; Backmark, A.; Törnroth-Horsefield, S.; Terwisscha van Scheltinga, A.C.; Kvassman, J.; Kjellbom, P.; Johanson, U.; Neutze, R. High-Resolution x-Ray Structure of Human Aquaporin 5. Proc. Natl. Acad. Sci. USA 2008, 105, 13327–13332. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Reichow, S.L.; Clemens, D.M.; Freites, J.A.; Németh-Cahalan, K.L.; Heyden, M.; Tobias, D.J.; Hall, J.E.; Gonen, T. Allosteric Mechanism of Water-Channel Gating by Ca2+–Calmodulin. Nat. Struct. Mol. Biol. 2013, 20, 1085–1092. [Google Scholar] [CrossRef]
- Roche, J.V.; Törnroth-Horsefield, S. Aquaporin Protein-Protein Interactions. Int. J. Mol. Sci. 2017, 18, 2255. [Google Scholar] [CrossRef]
- Mark, P.; Nilsson, L. Structure and Dynamics of the TIP3P, SPC, and SPC/E Water Models at 298 K. J. Phys. Chem. A 2001, 105, 9954–9960. [Google Scholar] [CrossRef]
- Michaud-Agrawal, N.; Denning, E.J.; Woolf, T.B.; Beckstein, O. MDAnalysis: A Toolkit for the Analysis of Molecular Dynamics Simulations. J. Comput. Chem. 2011, 32, 2319–2327. [Google Scholar] [CrossRef]
- Gowers, R.J.; Linke, M.; Barnoud, J.; Reddy, T.J.E.; Melo, M.N.; Seyler, S.L.; Domański, J.; Dotson, D.L.; Buchoux, S.; Kenney, I.M.; et al. MDAnalysis: A Python Package for the Rapid Analysis of Molecular Dynamics Simulations. In Proceedings of the 15th Python in Science Conference 2016, Austin, TX, USA, 11–17 July 2016; pp. 98–105. [Google Scholar] [CrossRef]
- Hub, J.; de Groot, B.L. Mechanism of Selectivity in Aquaporins and Aquaglyceroporins. Proc. Natl. Acad. Sci. USA 2008, 105, 1198–1203. [Google Scholar] [CrossRef]
- Smart, O.S.; Neduvelil, J.G.; Wang, X.; Wallace, B.A.; Sansom, M.S.P. HOLE: A Program for the Analysis of the Pore Dimensions of Ion Channel Structural Models. J. Mol. Graph. 1996, 14, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, LLC. The PyMOL Molecular Graphics System, Version 1.8 2015; Schrödinger, LLC.: New York, NY, USA, 2015. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mom, R.; Réty, S.; Mocquet, V.; Auguin, D. Deciphering Molecular Mechanisms Involved in the Modulation of Human Aquaporins’ Water Permeability by Zinc Cations: A Molecular Dynamics Approach. Int. J. Mol. Sci. 2024, 25, 2267. https://doi.org/10.3390/ijms25042267
Mom R, Réty S, Mocquet V, Auguin D. Deciphering Molecular Mechanisms Involved in the Modulation of Human Aquaporins’ Water Permeability by Zinc Cations: A Molecular Dynamics Approach. International Journal of Molecular Sciences. 2024; 25(4):2267. https://doi.org/10.3390/ijms25042267
Chicago/Turabian StyleMom, Robin, Stéphane Réty, Vincent Mocquet, and Daniel Auguin. 2024. "Deciphering Molecular Mechanisms Involved in the Modulation of Human Aquaporins’ Water Permeability by Zinc Cations: A Molecular Dynamics Approach" International Journal of Molecular Sciences 25, no. 4: 2267. https://doi.org/10.3390/ijms25042267
APA StyleMom, R., Réty, S., Mocquet, V., & Auguin, D. (2024). Deciphering Molecular Mechanisms Involved in the Modulation of Human Aquaporins’ Water Permeability by Zinc Cations: A Molecular Dynamics Approach. International Journal of Molecular Sciences, 25(4), 2267. https://doi.org/10.3390/ijms25042267






