Autocrine Regulation of Interleukin-6 via the Activation of STAT3 and Akt in Cardiac Myxoma Cells
Abstract
1. Introduction
2. Results
2.1. Patient Profile
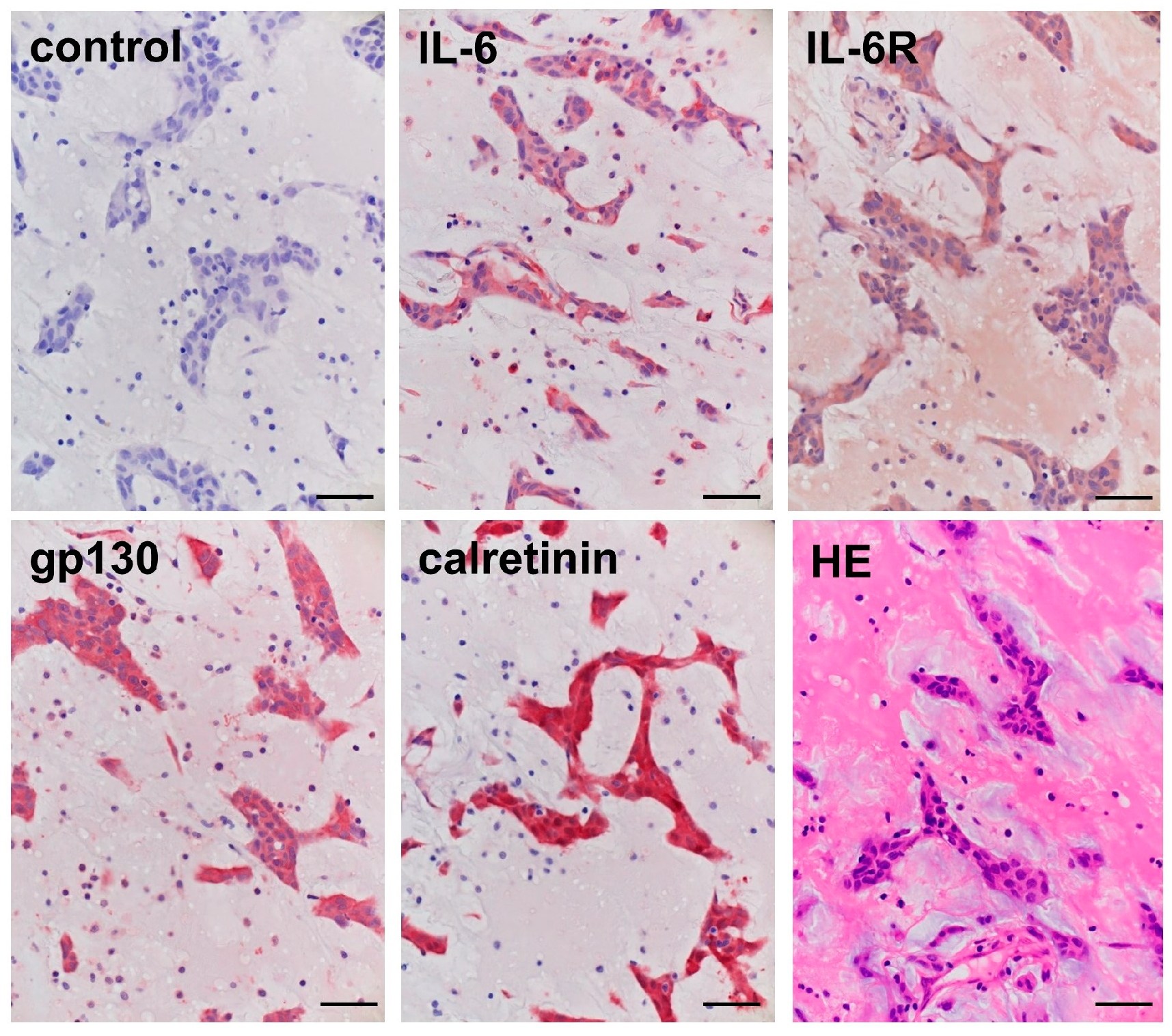
2.2. Immunohistochemical Staining in the Cardiac Myxoma Tissue
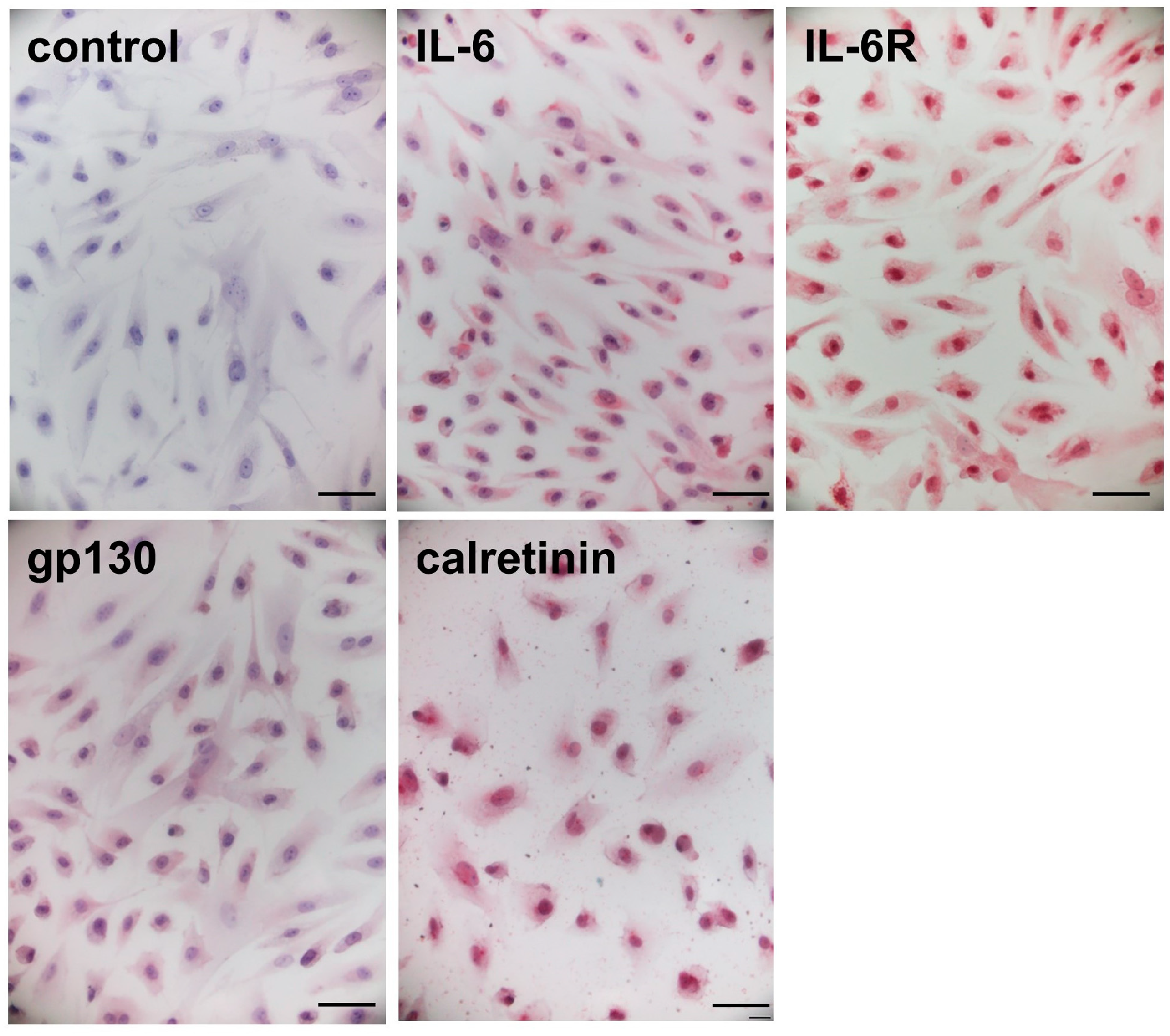
2.3. Immunocytochemical Staining in the Cardiac Myxoma Cells
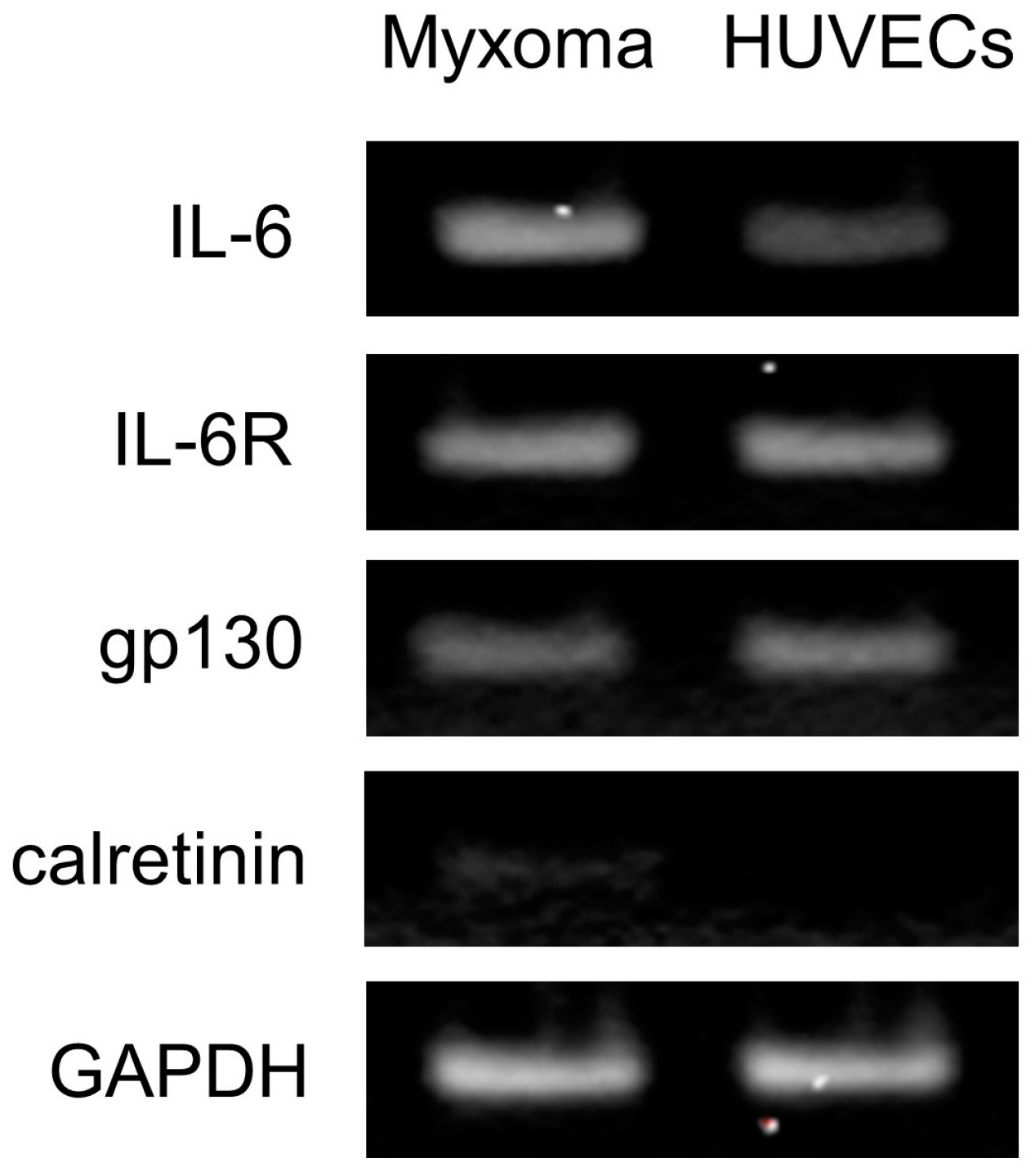
2.4. Gene Expression of IL-6 and Its Receptor Complexes in the Cardiac Myxoma Cells
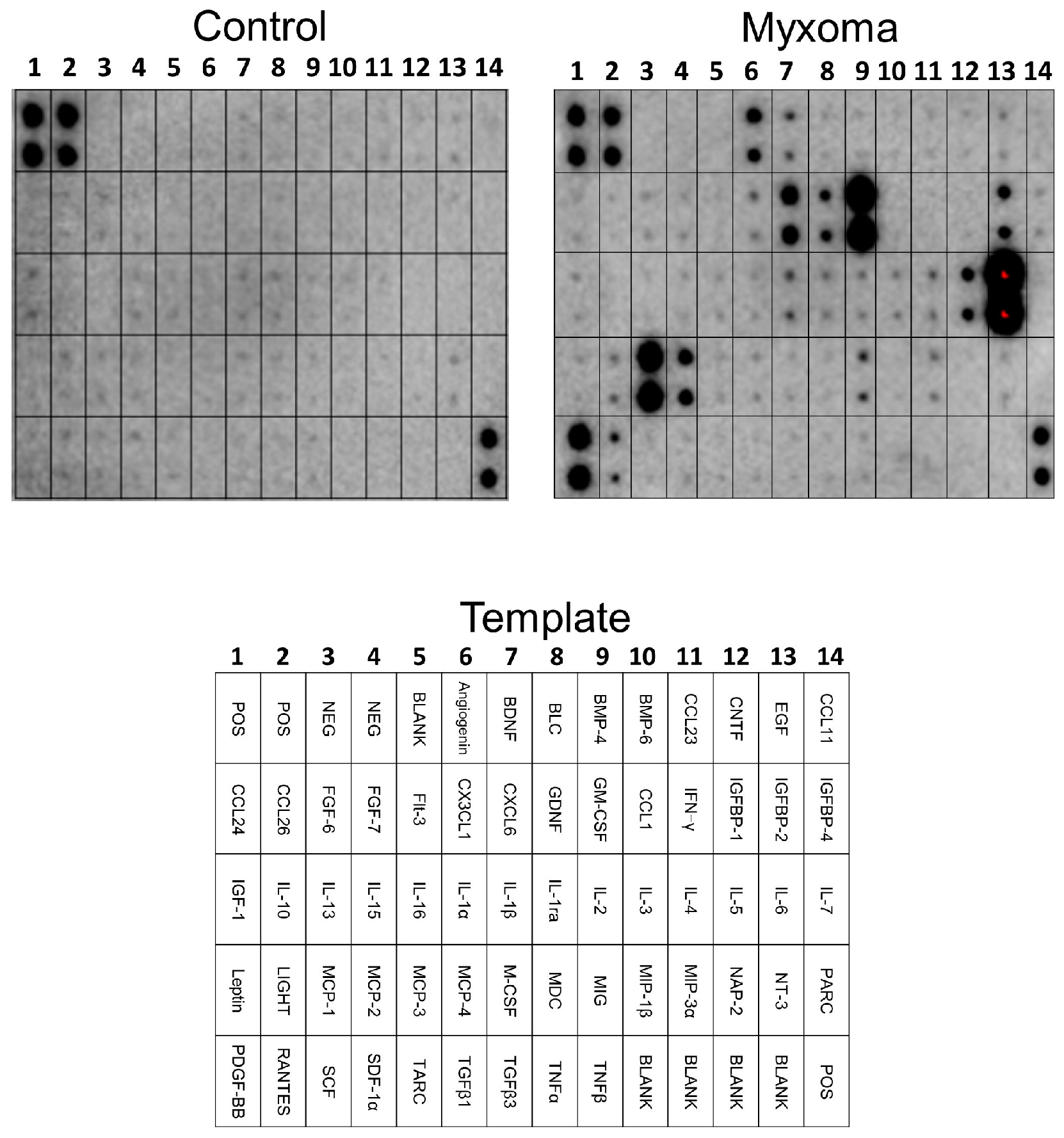
2.5. Antibody Array Assay
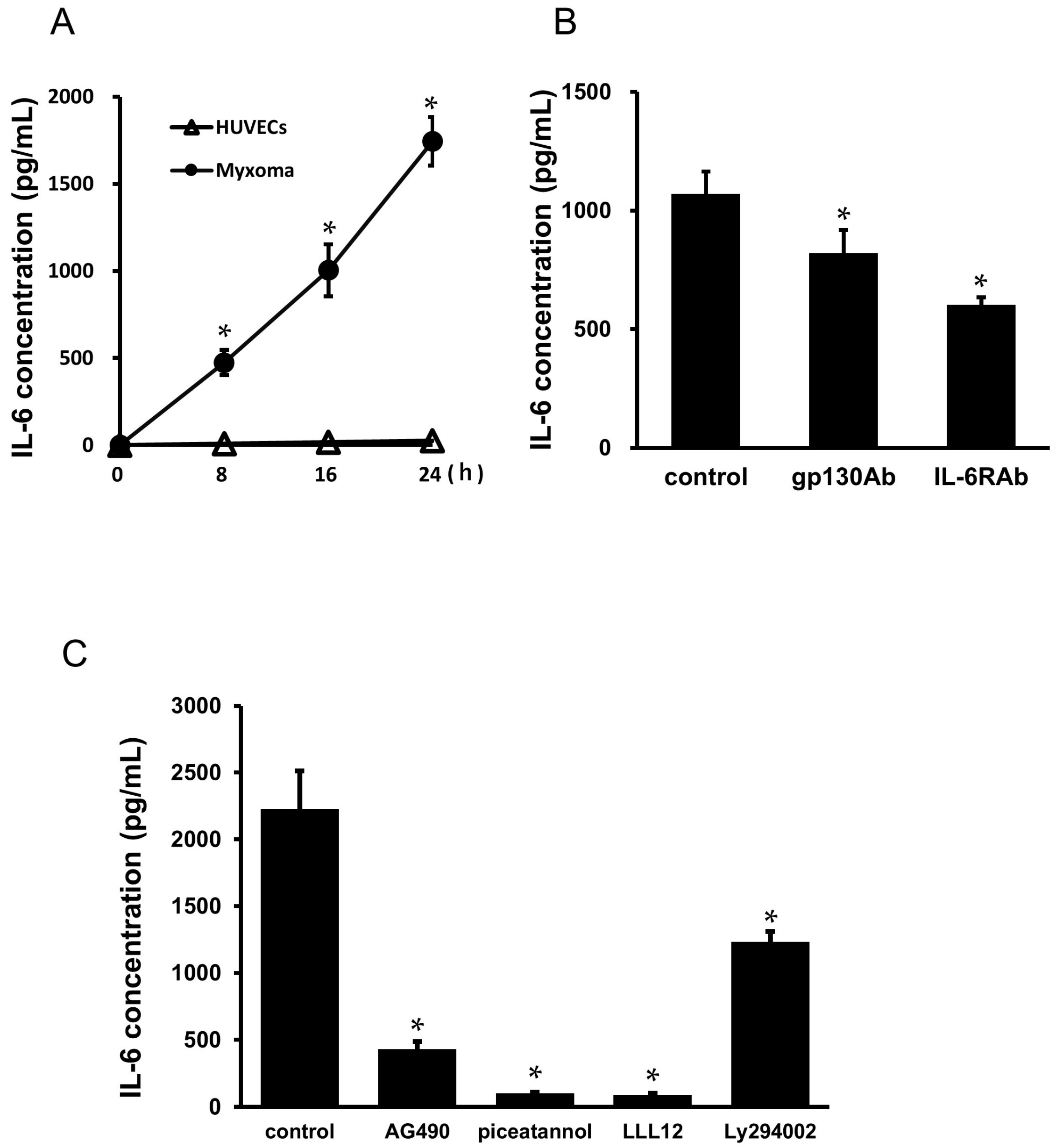
2.6. Culture Supernatant of the Myxoma Cells Induced STAT3 Phosphorylation in HUVECs
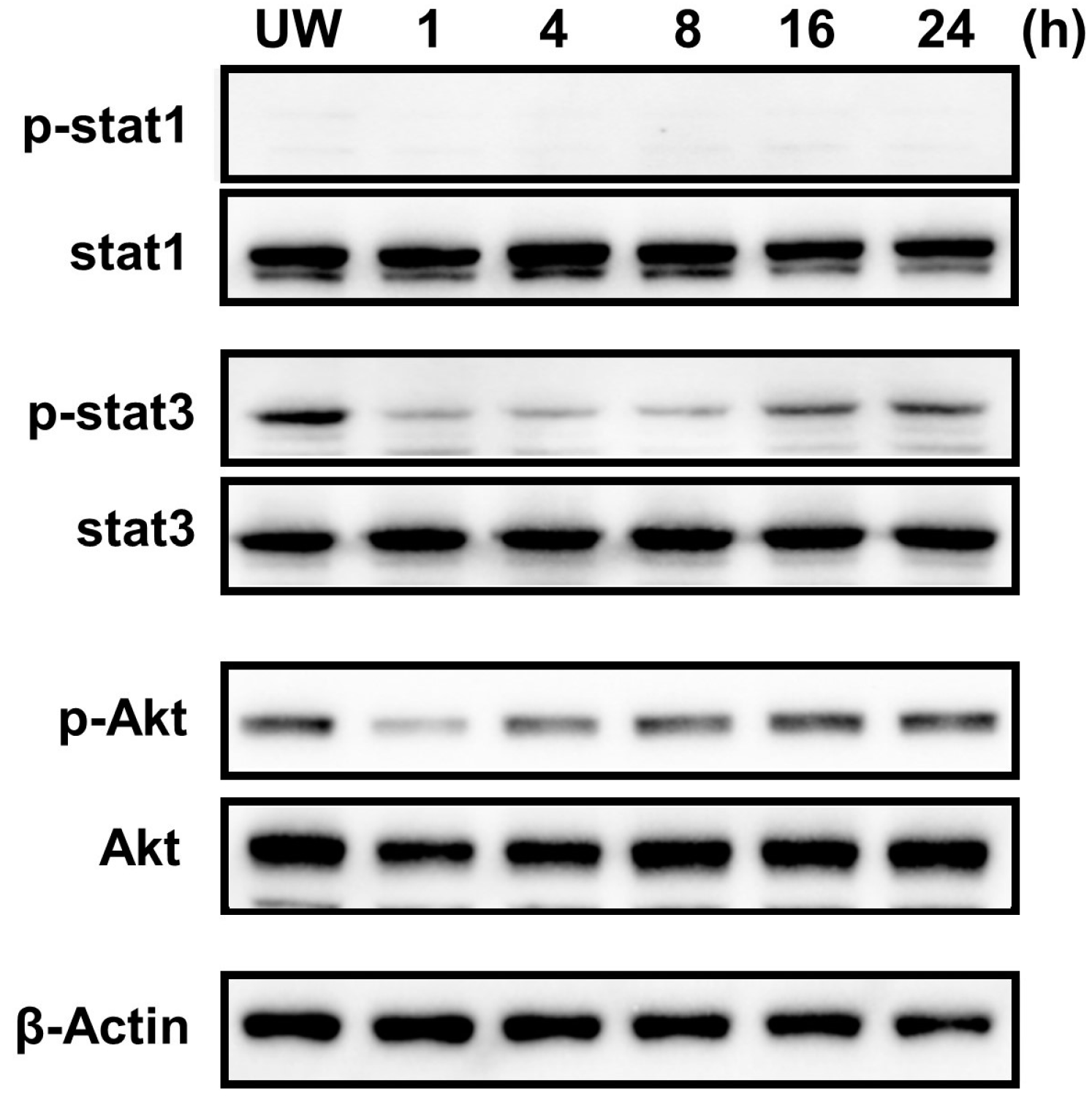
2.7. Constitutive Phosphorylation of STAT3 and Akt in the Cardiac Myxoma Cells
2.8. Secretion of IL-6 from the Cardiac Myxoma Cells
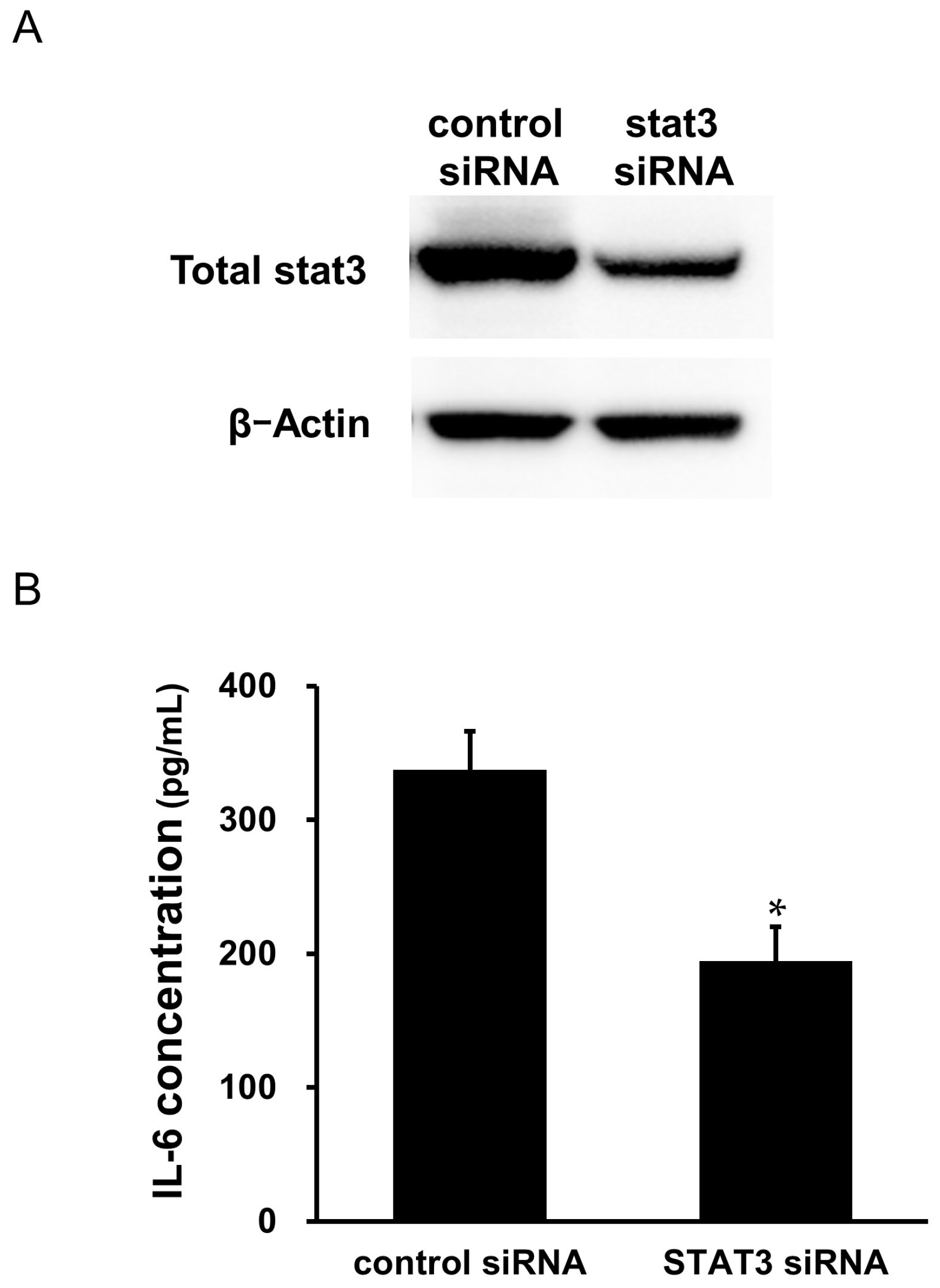
2.9. Effects of STAT3 siRNA Transfection on the Secretion of IL-6 from the Cardiac Myxoma Cells
2.10. Effects of IL-6 + sIL-6R on the Gene Expression of IL-6 in the Cardiac Myxoma Cells
2.11. Effects of IL-6 + sIL-6R on the Phosphorylation of STAT3 and Akt in the Cardiac Myxoma Cells
2.12. Immunofluorescence Staining
3. Discussion
4. Materials and Methods
4.1. Reagent
4.2. Culture of Cardiac Myxoma Cells
4.3. Culture of Human Umbilical Vein Endothelial Cells (HUVECs)
4.4. Immunohistochemical Staining in the Cardiac Myxoma Tissue
4.5. Immunocytochemical Staining in the Cardiac Myxoma Cells
4.6. Antibody Array Assay
4.7. Western Immunoblot Analysis
4.8. RT-PCR
4.9. Real-Time PCR
4.10. Transfection with Small Interfering RNA (siRNA)
4.11. Enzyme-Linked Immunosorbent Assay (ELISA)
4.12. Immunofluorescence Staining
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishihara, K.; Hirano, T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor. Rev. 2002, 13, 357–368. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. IL-6 trans-signaling via the soluble IL-6 receptor: Importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef]
- Campbell, I.L.; Erta, M.; Lim, S.L.; Frausto, R.; May, U.; Rose-John, S.; Scheller, J.; Hidalgo, J. Trans-signaling is a dominant mechanism for the pathogenic actions of interleukin-6 in the brain. J. Neurosci. 2014, 34, 2503–2513. [Google Scholar] [CrossRef] [PubMed]
- Naka, T.; Nishimoto, N.; Kishimoto, T. The paradigm of IL-6: From basic science to medicine. Arthritis Res. 2002, 4 (Suppl. S3), S233–S242. [Google Scholar] [CrossRef] [PubMed]
- Pinede, L.; Duhaut, P.; Loire, R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine 2001, 80, 159–172. [Google Scholar] [CrossRef]
- Islam, A. Cardiac myxomas: A narrative review. World J. Cardiol. 2022, 14, 206–219. [Google Scholar] [CrossRef]
- Hirano, T.; Taga, T.; Yasukawa, K.; Nakajima, K.; Nakano, N.; Takatsuki, F.; Shimizu, M.; Murashima, A.; Tsunasawa, S.; Sakiyama, F.; et al. Human B-cell differentiation factor defined by an anti-peptide antibody and its possible role in autoantibody production. Proc. Natl. Acad. Sci. USA 1987, 84, 228–231. [Google Scholar] [CrossRef]
- Jourdan, M.; Bataille, R.; Seguin, J.; Zhang, X.G.; Chaptal, P.A.; Klein, B. Constitutive production of interleukin-6 and immunologic features in cardiac myxomas. Arthritis Rheum. 1990, 33, 398–402. [Google Scholar] [CrossRef]
- Saji, T.; Yanagawa, E.; Matsuura, H.; Yamamoto, S.; Ishikita, T.; Matsuo, N.; Yoshirwara, K.; Takanashi, Y. Increased serum interleukin-6 in cardiac myxoma. Am. Heart J. 1991, 122, 579–580. [Google Scholar] [CrossRef]
- Wiedermann, C.J.; Reinisch, N.; Fischer-Colbrie, R.; Vollmar, A.M.; Herold, M.; Knapp, E. Proinflammatory cytokines in cardiac myxomas. J. Intern. Med. 1992, 232, 263–265. [Google Scholar] [CrossRef]
- Seino, Y.; Ikeda, U.; Shimada, K. Increased expression of interleukin 6 mRNA in cardiac myxomas. Br. Heart J. 1993, 69, 565–567. [Google Scholar] [CrossRef]
- Sakamoto, H.; Sakamaki, T.; Wada, A.; Nakajima, T.; Kanda, T.; Murata, K. Dexamethasone inhibits production of interleukin-6 by cultured cardiac myxoma cells. Am. Heart J. 1994, 127, 704–705. [Google Scholar] [CrossRef]
- Kanda, T.; Sakamaki, T.; Murata, K. A cardiac myxoma with interleukin-6 production and cerebral metastasis. Int. J. Cardiol. 1994, 45, 144–146. [Google Scholar] [CrossRef]
- Soeparwata, R.; Poeml, P.; Schmid, C.; Neuhof, H.; Scheld, H.H. Interleukin-6 plasma levels and tumor size in cardiac myxoma. J. Thorac. Cardiovasc. Surg. 1996, 112, 1675–1677. [Google Scholar] [CrossRef]
- Parissis, J.T.; Mentzikof, D.; Georgopoulou, M.; Gikopoulos, M.; Kanapitsas, A.; Merkouris, K.; Kefalas, C. Correlation of interleukin-6 gene expression to immunologic features in patients with cardiac myxomas. J. Interferon Cytokine Res. 1996, 16, 589–593. [Google Scholar] [CrossRef]
- Acebo, E.; Val-Bernal, J.F.; Gómez-Román, J.J.; Revuelta, J.M. Clinicopathologic study and DNA analysis of 37 cardiac myxomas: A 28-year experience. Chest 2003, 123, 1379–1385. [Google Scholar] [CrossRef]
- Kanda, T.; Umeyama, S.; Sasaki, A.; Nakazato, Y.; Morishita, Y.; Imai, S.; Suzuki, T.; Murata, K. Interleukin-6 and cardiac myxoma. Am. J. Cardiol. 1994, 74, 965–967. [Google Scholar] [CrossRef]
- Mendoza, C.E.; Rosado, M.F.; Bernal, L. The role of interleukin-6 in cases of cardiac myxoma. Clinical features, immunologic abnormalities, and a possible role in recurrence. Tex. Heart Inst. J. 2001, 28, 3–7. [Google Scholar]
- Yokomuro, H.; Yoshihara, K.; Watanabe, Y.; Shiono, N.; Koyama, N.; Takanashi, Y. The variations in the immunologic features and interleukin-6 levels for the surgical treatment of cardiac myxomas. Surg. Today 2007, 37, 750–753. [Google Scholar] [CrossRef]
- Endo, A.; Ohtahara, A.; Kinugawa, T.; Ogino, K.; Hisatome, I.; Shigemasa, C. Characteristics of cardiac myxoma with constitutional signs: A multicenter study in Japan. Clin. Cardiol. 2002, 25, 367–370. [Google Scholar] [CrossRef]
- García-Zubiri, C.; Citores, M.J.; Yebra-Bango, M. Contribution of monocytes to overproduction of interleukin-6 in a case of cardiac myxoma. Am. J. Med. Sci. 2009, 338, 336–337. [Google Scholar] [CrossRef]
- Rogers, J.H. Calretinin: A gene for a novel calcium-binding protein expressed principally in neurons. J. Cell Biol. 1987, 105, 1343–1353. [Google Scholar] [CrossRef]
- Orlandi, A.; Ciucci, A.; Ferlosio, A.; Genta, R.; Spagnoli, L.G.; Gabbiani, G. Cardiac myxoma cells exhibit embryonic endocardial stem cell features. J. Pathol. 2006, 209, 231–239. [Google Scholar] [CrossRef]
- Skamrov, A.V.; Nechaenko, M.A.; Goryunova, L.E.; Feoktistova, E.S.; Khaspekov, G.L.; Kovalevsky, D.A.; Vinnitsky, L.I.; Sheremeteva, G.F.; Beabealashvilli, R. Gene expression analysis to identify mRNA markers of cardiac myxoma. J. Mol. Cell. Cardiol. 2004, 37, 717–733. [Google Scholar] [CrossRef]
- Terracciano, L.M.; Mhawech, P.; Suess, K.; D’Armiento, M.; Lehmann, F.S.; Jundt, G.; Moch, H.; Sauter, G.; Mihatsch, M.J. Calretinin as a marker for cardiac myxoma. Diagnostic and histogenetic considerations. Am. J. Clin. Pathol. 2000, 114, 754–759. [Google Scholar] [CrossRef]
- Yuan, S.M.; Lin, H.Z. Interleukin-6 in Patients with Cardiac Myxoma. J. Coll. Physicians Surg. Pak. 2020, 30, 849–852. [Google Scholar]
- Sakamoto, H.; Sakamaki, T.; Kanda, T.; Hirao, Y.; Ohyama, Y.; Ogishi, K.; Negishi, M.; Masuda, H.; Sumino, H.; Sawada, Y.; et al. Immunosuppressive drugs inhibit the production of interleukin-6 and interleukin-8 in cultured cardiac myxoma cells. Res. Commun. Mol. Pathol. Pharmacol. 1997, 97, 60–66. [Google Scholar]
- Sakamoto, H.; Sakamaki, T.; Sumino, H.; Sawada, Y.; Sato, H.; Sato, M.; Fujita, K.; Kanda, T.; Tamura, J.; Kurabayashi, M. Production of endothelin-1 and big endothelin-1 by human cardiac myxoma cells—Implications of the origin of myxomas. Circ. J. 2004, 68, 1230–1232. [Google Scholar] [CrossRef]
- Morishima, A.; Marui, A.; Shimamoto, T.; Saji, Y.; Nishina, T.; Komeda, M. A case of interleukin-6-producing cardiac myxoma resembling multicentric Castleman’s disease. J. Thorac. Cardiovasc. Surg. 2009, 138, 499–501. [Google Scholar] [CrossRef]
- Kuroki, S.; Naitoh, K.; Katoh, O.; Yamada, H.; Itoh, T. Increased interleukin-6 activity in cardiac myxoma with mediastinal lymphadenopathy. Intern. Med. 1992, 31, 1207–1209. [Google Scholar] [CrossRef]
- Takizawa, T.; Sumino, H.; Kanda, T.; Kobayashi, I.; Nagai, R.; Ichikawa, S. An interleukin-6-producing cardiac myxoma associated with mediastinal lymphadenopathy. Cardiology 1999, 92, 275–277. [Google Scholar] [CrossRef]
- Kanda, T.; Nakajima, T.; Sakamoto, H.; Suzuki, T.; Murata, K. An interleukin-6 secreting myxoma in a hypertrophic left ventricle. Chest 1994, 105, 962–963. [Google Scholar] [CrossRef]
- Sakamoto, H.; Sakamaki, T.; Kanda, T.; Tsuchiya, Y.; Sato, M.; Sato, H.; Oyama, Y.; Sawada, Y.; Tamura, J.; Nagai, R.; et al. Vascular endothelial growth factor is an autocrine growth factor for cardiac myxoma cells. Circ. J. 2004, 68, 488–493. [Google Scholar] [CrossRef]
- Gaumann, A.; Strubel, G.; Bode-Lesniewska, B.; Schmidtmann, I.; Kriegsmann, J.; Kirkpatrick, C.J. The role of tumor vascularisation in benign and malignant cardiovascular neoplasms: A comparison of cardiac myxoma and sarcomas of the pulmonary artery. Oncol. Rep. 2008, 20, 309–318. [Google Scholar] [CrossRef]
- Zhang, T.; Koide, N.; Wada, Y.; Tsukioka, K.; Takayama, K.; Kono, T.; Kitahara, H.; Amano, J. Significance of monocyte chemotactic protein-1 and thymidine phosphorylase in angiogenesis of human cardiac myxoma. Circ. J. 2003, 67, 54–60. [Google Scholar] [CrossRef]
- Jougasaki, M.; Ichiki, T.; Takenoshita, Y.; Setoguchi, M. Statins suppress interleukin-6-induced monocyte chemo-attractant protein-1 by inhibiting Janus kinase/signal transducers and activators of transcription pathways in human vascular endothelial cells. Br. J. Pharmacol. 2010, 159, 1294–1303. [Google Scholar] [CrossRef]
- Zegeye, M.M.; Lindkvist, M.; Fälker, K.; Kumawat, A.K.; Paramel, G.; Grenegård, M.; Sirsjö, A.; Ljungberg, L.U. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Commun. Signal 2018, 16, 55. [Google Scholar] [CrossRef]
- Biswas, P.; Delfanti, F.; Bernasconi, S.; Mengozzi, M.; Cota, M.; Polentarutti, N.; Mantovani, A.; Lazzarin, A.; Sozzani, S.; Poli, G. Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in the U937 cell line. Blood 1998, 91, 258–265. [Google Scholar] [CrossRef]
- Viedt, C.; Vogel, J.; Athanasiou, T.; Shen, W.; Orth, S.R.; Kübler, W.; Kreuzer, J. Monocyte chemoattractant protein-1 induces proliferation and interleukin-6 production in human smooth muscle cells by differential activation of nuclear factor-kappaB and activator protein-1. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 914–920. [Google Scholar] [CrossRef]
- Franchimont, N.; Durant, D.; Rydziel, S.; Canalis, E. Platelet-derived growth factor induces interleukin-6 transcription in osteoblasts through the activator protein-1 complex and activating transcription factor-2. J. Biol. Chem. 1999, 274, 6783–6789. [Google Scholar] [CrossRef]
- Griffin, J.D.; Cannistra, S.A.; Sullivan, R.; Demetri, G.D.; Ernst, T.J.; Kanakura, Y. The biology of GM-CSF: Regulation of production and interaction with its receptor. Int. J. Cell Cloning 1990, 8 (Suppl. S1), 35–44; discussion 44–45. [Google Scholar] [CrossRef]
- Farzam-Kia, N.; Moratalla, A.C.; Lemaître, F.; Levert, A.; Da Cal, S.; Margarido, C.; Carpentier Solorio, Y.; Arbour, N. GM-CSF distinctly impacts human monocytes and macrophages via ERK1/2-dependent pathways. Immunol. Lett. 2023, 261, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Franchimont, N.; Rydziel, S.; Canalis, E. Interleukin 6 is autoregulated by transcriptional mechanisms in cultures of rat osteoblastic cells. J. Clin. Investig. 1997, 100, 1797–1803. [Google Scholar] [CrossRef][Green Version]
- Huang, W.L.; Yeh, H.H.; Lin, C.C.; Lai, W.W.; Chang, J.Y.; Chang, W.T.; Su, W.C. Signal transducer and activator of transcription 3 activation up-regulates interleukin-6 autocrine production: A biochemical and genetic study of established cancer cell lines and clinical isolated human cancer cells. Mol. Cancer 2010, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Schuringa, J.J.; Wierenga, A.T.; Kruijer, W.; Vellenga, E. Constitutive Stat3, Tyr705, and Ser727 phosphorylation in acute myeloid leukemia cells caused by the autocrine secretion of interleukin-6. Blood 2000, 95, 3765–3770. [Google Scholar] [CrossRef]
- Yeh, H.H.; Lai, W.W.; Chen, H.H.; Liu, H.S.; Su, W.C. Autocrine IL-6-induced Stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion. Oncogene 2006, 25, 4300–4309. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Woo, S.U.; Kang, J.H.; Kim, K.; Kwon, M.H.; Park, S.; Shin, H.J.; Gwak, H.S.; Chwae, Y.J. STAT3 transcriptional factor activated by reactive oxygen species induces IL6 in starvation-induced autophagy of cancer cells. Autophagy 2010, 6, 1125–1138. [Google Scholar] [CrossRef]
- Faruqi, T.R.; Gomez, D.; Bustelo, X.R.; Bar-Sagi, D.; Reich, N.C. Rac1 mediates STAT3 activation by autocrine IL-6. Proc. Natl. Acad. Sci. USA 2001, 98, 9014–9019. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Lee, B.S.; Yang, Y.; Park, C.W.; Ha, H.J.; Pyun, K.H.; Choi, I. Roles of protein phosphatase 1 and 2A in an IL-6-mediated autocrine growth loop of human myeloma cells. Cell. Immunol. 1996, 168, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, T.; Jougasaki, M.; Setoguchi, M.; Imamura, J.; Nakashima, H.; Matsuoka, T.; Sonoda, M.; Nakamura, K.; Minagoe, S.; Tei, C. Cardiotrophin-1 stimulates intercellular adhesion molecule-1 and monocyte chemoattractant protein-1 in human aortic endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H750–H763. [Google Scholar] [CrossRef] [PubMed]




| Gene Name | Sequence5′-3′ | Accession Number |
|---|---|---|
| IL-6 | (Forward) ACRCACCTCTTCAGAACGAATTG (Reverse) CCATCTTTGGAAGGTTCAGGTTG | NM_000600.3 |
| IL-6R | (Forward) CACGCCTTGGACAGAATCC (Reverse) GCTTGTCGCATTTGCAGAATC | NM_181359 |
| gp130 | (Forward) TCAAATCCCTACTCCTTCACTTAC (Reverse) TGGTGAGGAAAATAAACAAGGC | NM_175767 |
| Calretinin | (Forward) TGCCTGTCCAGGAAAACTTC (Reverse) TCATGCTCGTCAATGTAGCC | NM_001740.4 |
| GAPDH | (Forward) GCACCGTCAAGGCTGAGAAC (Reverse) TGGTGAAGACGCCAGTGGA | NM_002046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jougasaki, M.; Takenoshita, Y.; Umebashi, K.; Yamamoto, M.; Sudou, K.; Nakashima, H.; Sonoda, M.; Kinjo, T. Autocrine Regulation of Interleukin-6 via the Activation of STAT3 and Akt in Cardiac Myxoma Cells. Int. J. Mol. Sci. 2024, 25, 2232. https://doi.org/10.3390/ijms25042232
Jougasaki M, Takenoshita Y, Umebashi K, Yamamoto M, Sudou K, Nakashima H, Sonoda M, Kinjo T. Autocrine Regulation of Interleukin-6 via the Activation of STAT3 and Akt in Cardiac Myxoma Cells. International Journal of Molecular Sciences. 2024; 25(4):2232. https://doi.org/10.3390/ijms25042232
Chicago/Turabian StyleJougasaki, Michihisa, Yoko Takenoshita, Katsuyuki Umebashi, Masayoshi Yamamoto, Ku Sudou, Hitoshi Nakashima, Masahiro Sonoda, and Tamahiro Kinjo. 2024. "Autocrine Regulation of Interleukin-6 via the Activation of STAT3 and Akt in Cardiac Myxoma Cells" International Journal of Molecular Sciences 25, no. 4: 2232. https://doi.org/10.3390/ijms25042232
APA StyleJougasaki, M., Takenoshita, Y., Umebashi, K., Yamamoto, M., Sudou, K., Nakashima, H., Sonoda, M., & Kinjo, T. (2024). Autocrine Regulation of Interleukin-6 via the Activation of STAT3 and Akt in Cardiac Myxoma Cells. International Journal of Molecular Sciences, 25(4), 2232. https://doi.org/10.3390/ijms25042232






