Sex-Dependent Differences in the Ischemia/Reperfusion-Induced Expression of AMPA Receptors
Abstract
1. Introduction
2. Results
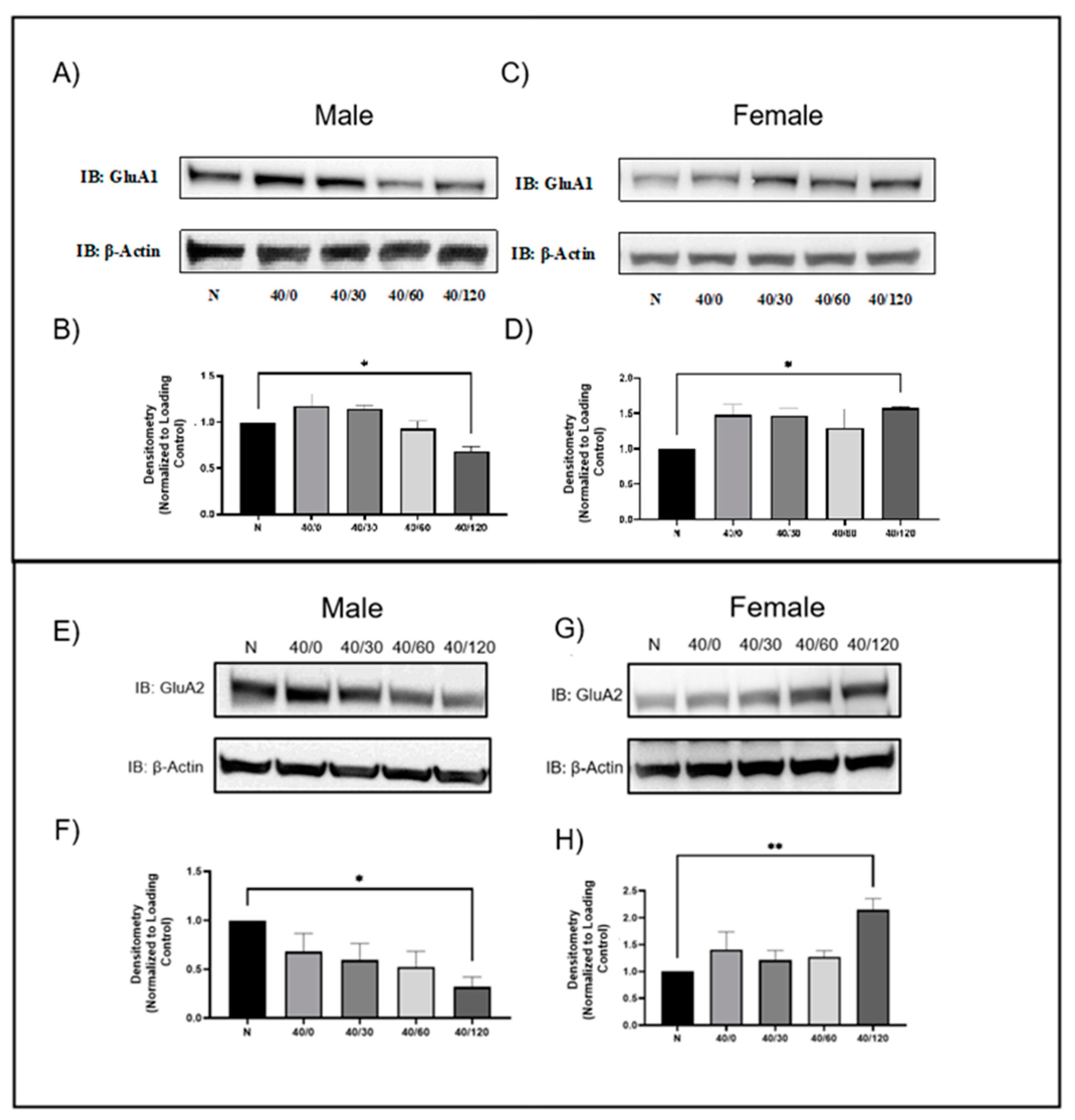
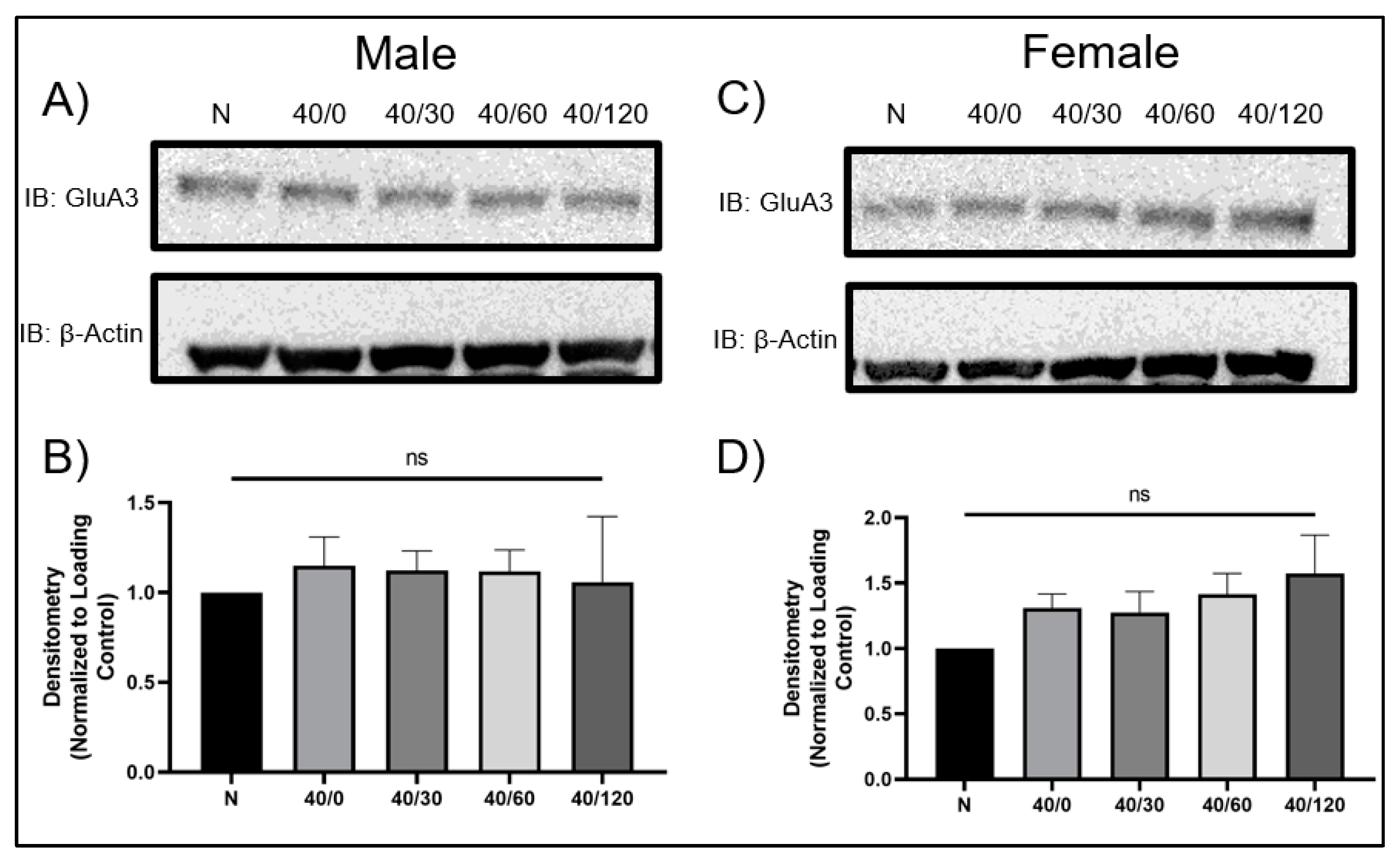
2.1. Sex Differences in GluA1 and GluA2 AMPAR Protein Levels with OGD/R
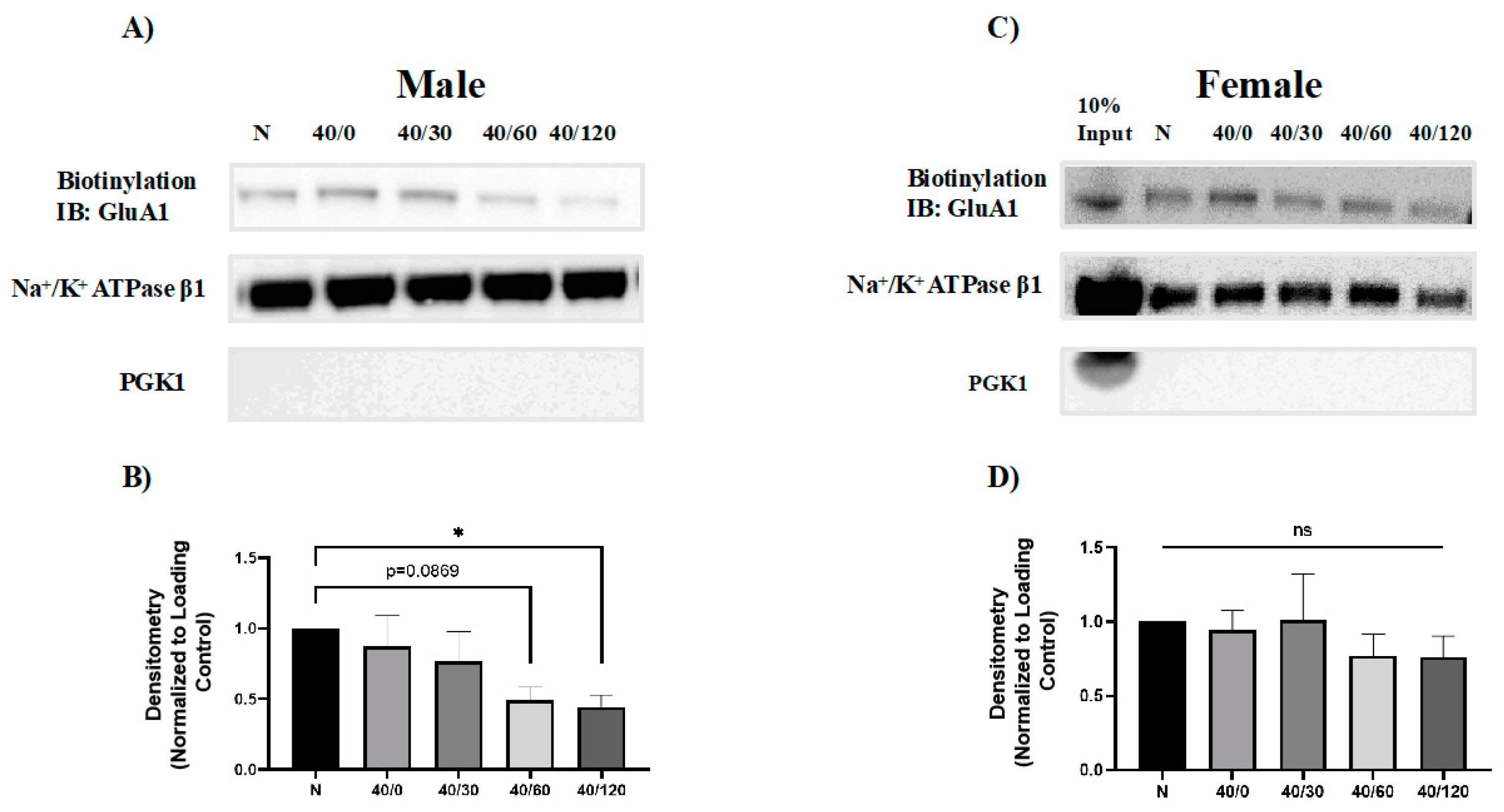
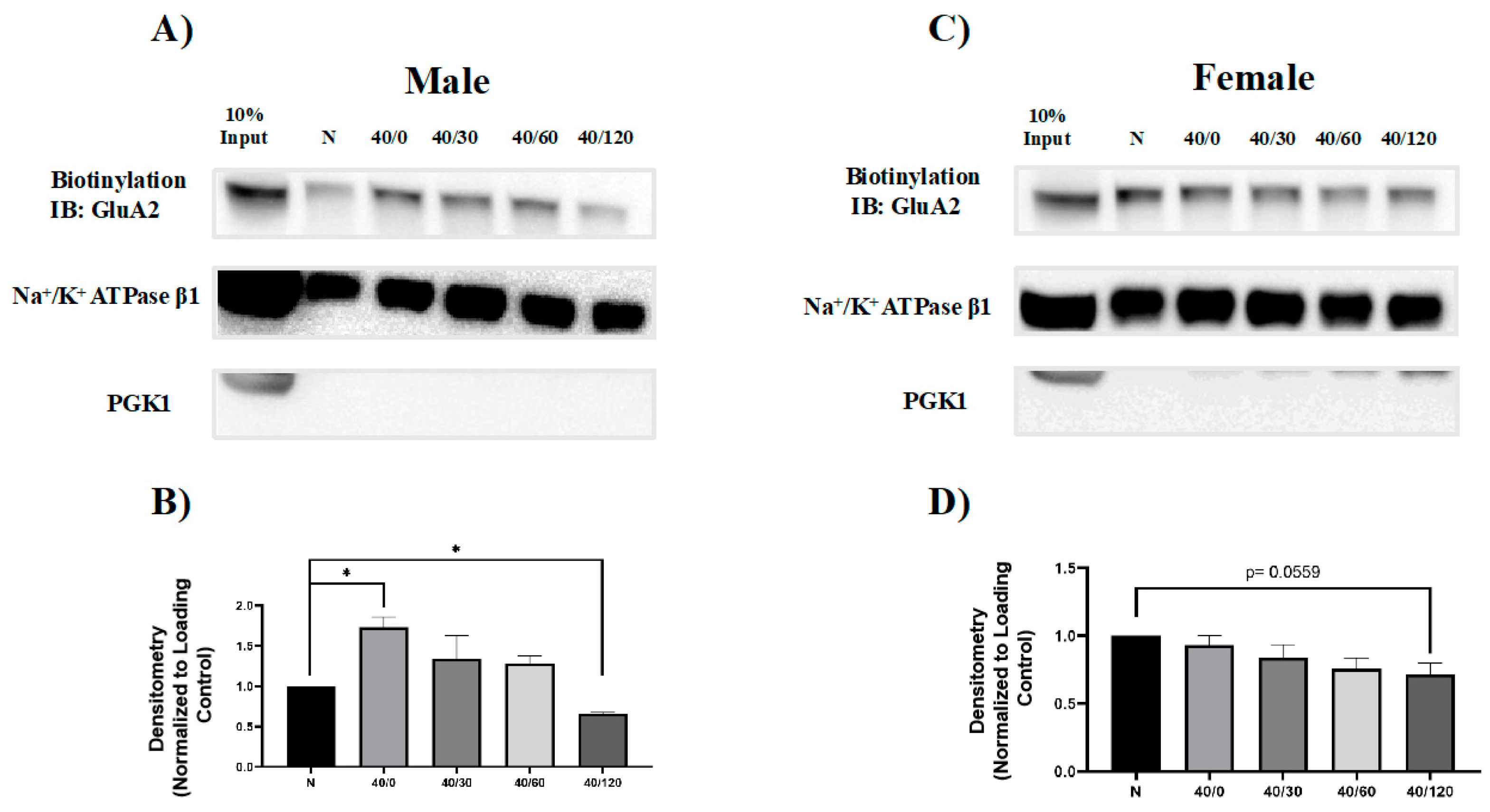
2.2. Sex Differences in the OGD/R-Induced Internalization of GluA1 and GluA2 AMPAR Subunits
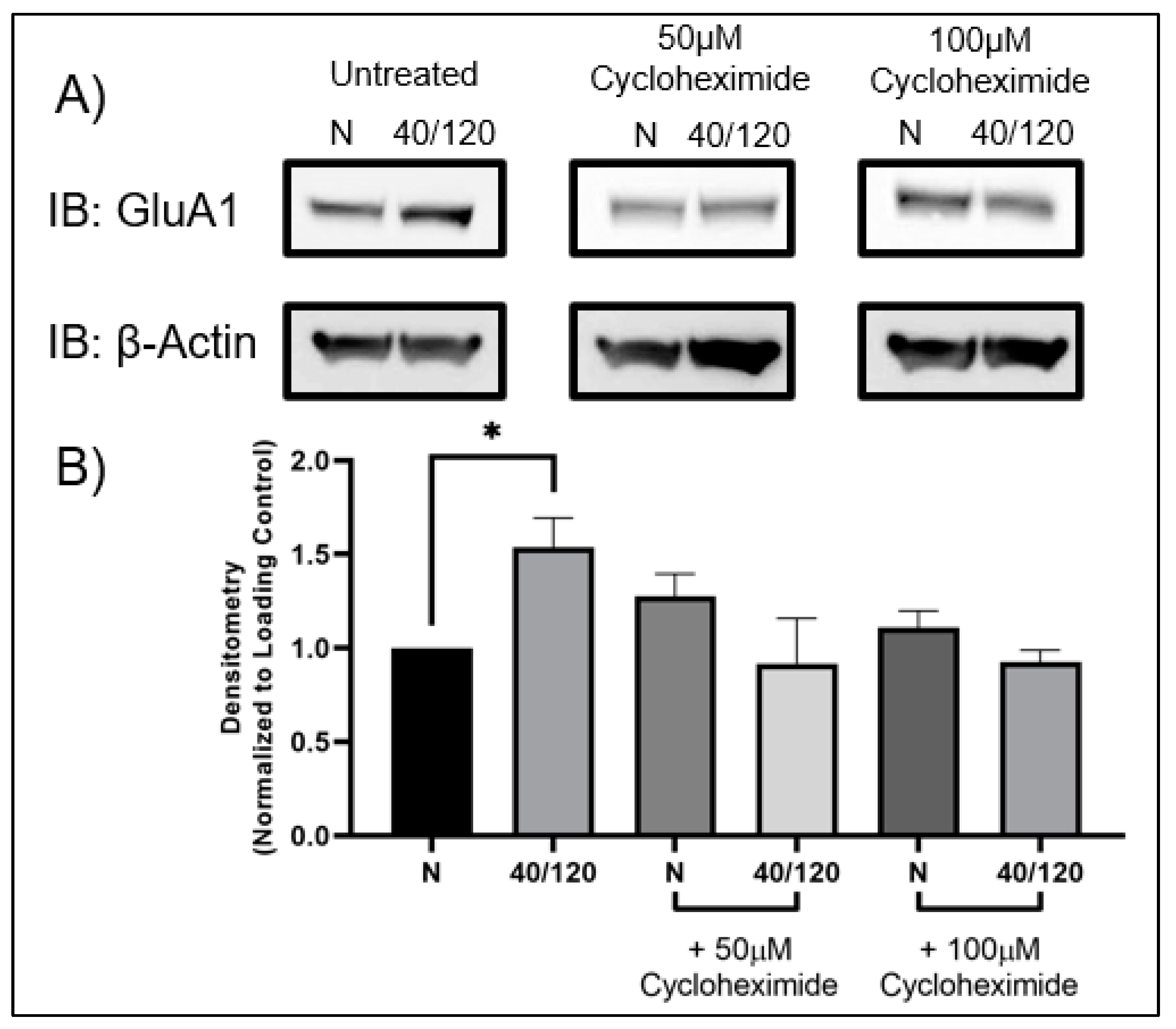
2.3. The OGD/R-Induced Increase in GluA1 and GluA2 in Females Occurs from Newly Synthesized AMPA Receptors
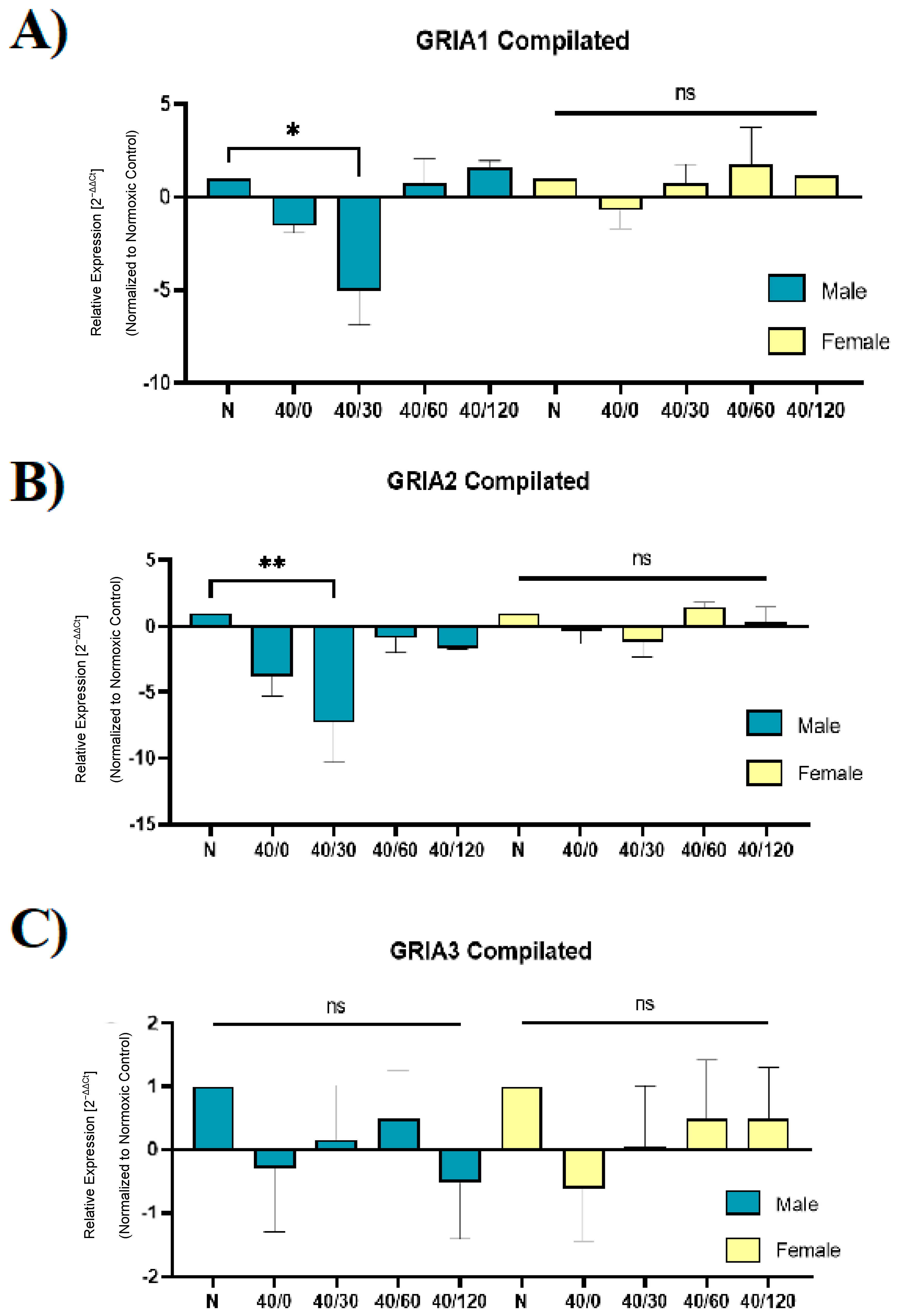
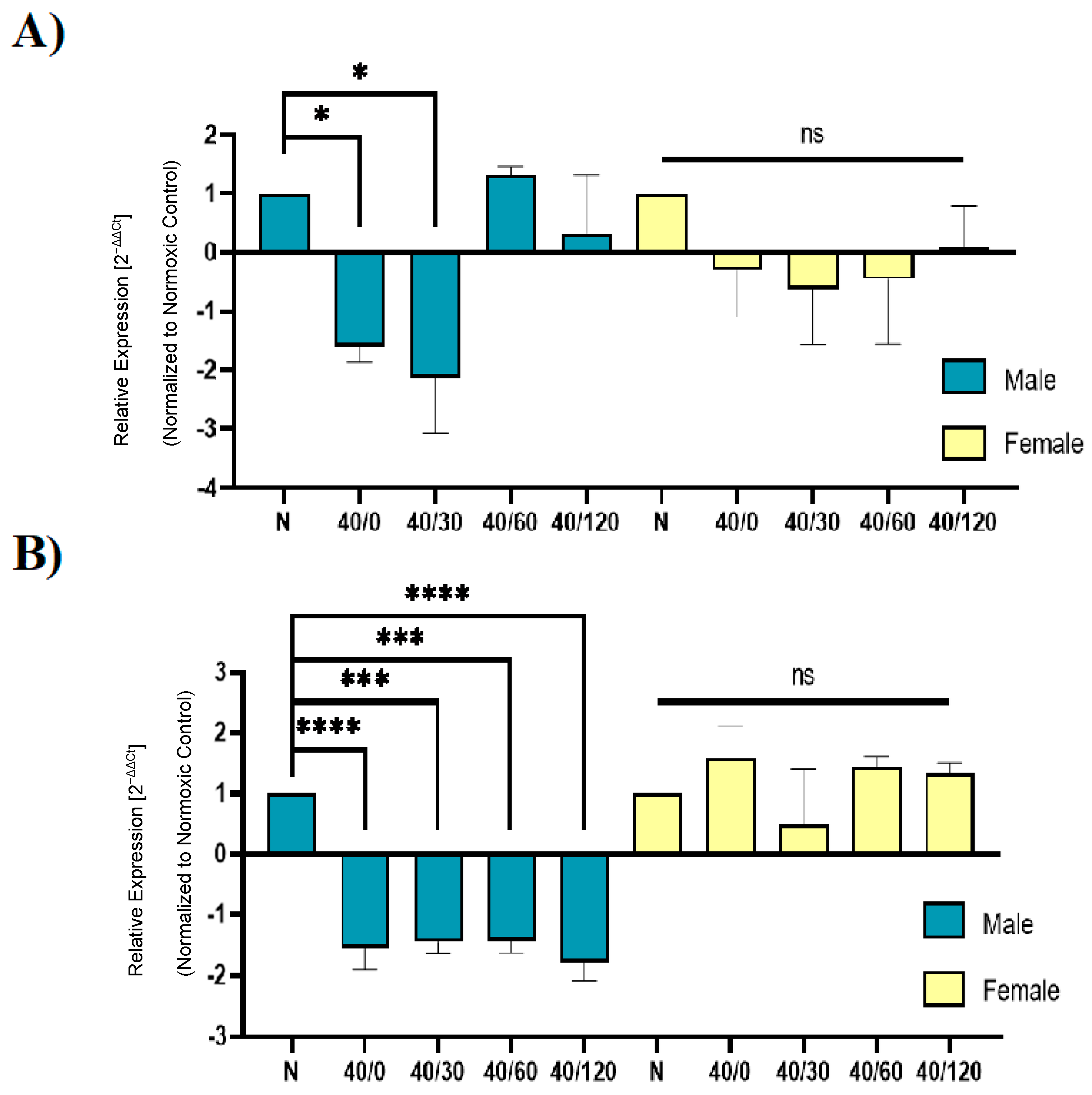
2.4. Sex Differences in the mRNA Expression of AMPA Receptors with OGD/R
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Reagents
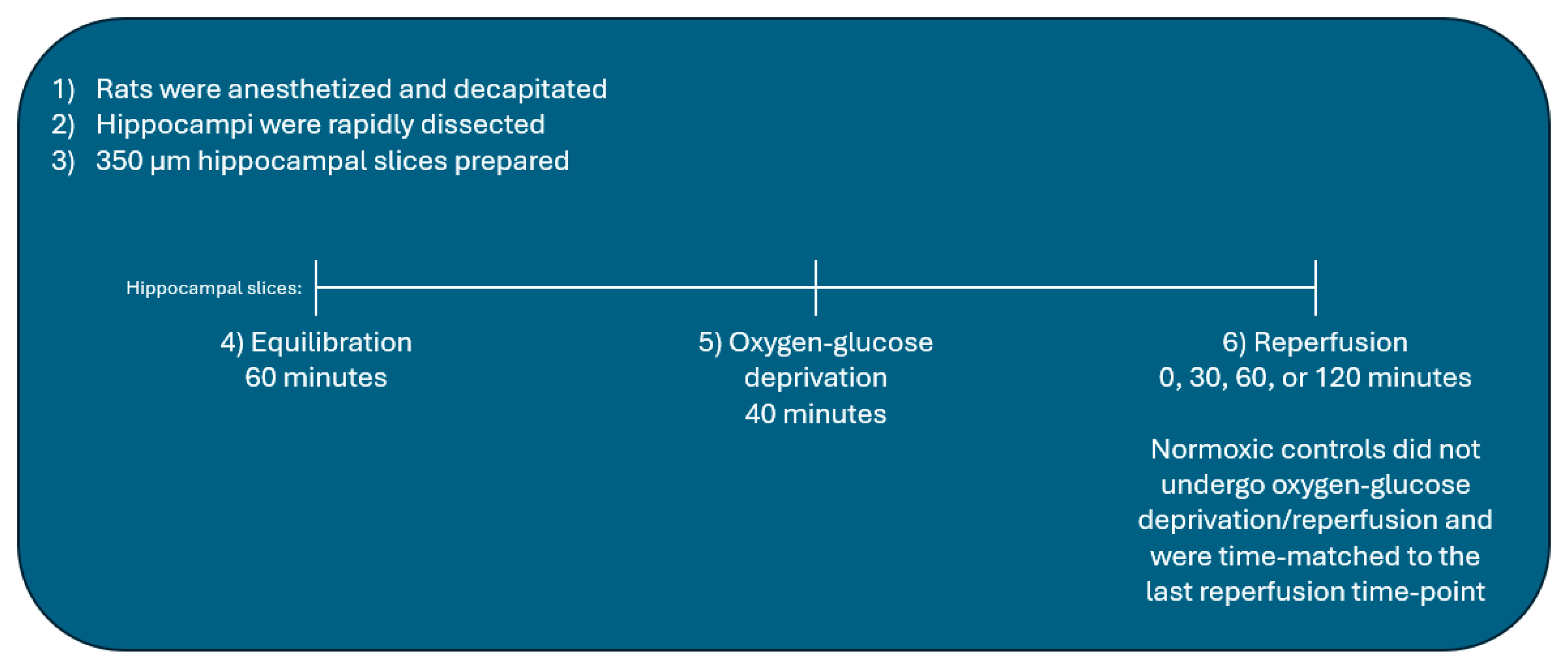
4.3. Preparation of Acute Rat Hippocampal Slices
4.4. Oxygen-Glucose Deprivation/Reperfusion of Hippocampal Slices
4.5. Lysate Preparation
4.6. Immunoblotting
4.7. Biotinylation of Hippocampal Slices
4.8. Reverse Transcription, Quantitative Polymerase Chain Reaction (RT-qPCR)
4.9. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Q.; Tong, X.; Shieb, L.; Vaughan, A.; Gillespie, C.; Wiltz, J.L.; King, S.C.; Odom, E.; Merritt, R.; Hong, Y. Recent trends in stroke death rates-United States, 2000–2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 933–939. [Google Scholar] [CrossRef]
- Marier, J. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995, 333, 1581–1587. [Google Scholar]
- Faden, A.; Demediuk, P.; Panter, S.S.; Vink, R. The role of excitatory amino acids in traumatic brain injury. Science 1989, 244, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Mcintosh, T.K.; Vink, R.; Soares, H.; Hayes, R.; Simon, R. Effects of the N-methyl-D-aspartate receptor blocker MK-801 on neurologic function after experimental brain injury. J. Neurotrauma 1989, 6, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Takagi, N.; Shinno, K.; Teves, L.; Bissoon, N.; Wallace, M.C.; Gurd, J.W. Transient ischemia differentially increases tyrosine phosphorylation of NMDAR receptor subunits 2A and 2B. J. Neurochem. 1997, 69, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lau, L.; Wei, J.; Zhu, D.; Zou, S.; Sun, H.S.; Fu, Y.; Liu, F.; Lu, Y. Expression of Ca2+-permeable AMPA receptor channels primes cell death in transient forebrain ischemia. Neuron 2004, 43, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wong, T.P.; Aarts, M.; Rooyakkers, A.; Liu, L.; Lai, T.W.; Wu, D.C.; Lu, J.; Tymianski, M.; Craig, A.M.; et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. 2007, 27, 2846–2857. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.Z.; Sensi, S.L.; Ogoshi, F.; Weiss, J.H. Blockade of Ca2+-permeable AMPA/Kainate channels decreases oxygen-glucose deprivation-induced Zn2+ accumulation and neuronal loss in hippocampal pyramidal neurons. J. Neurosci. 2002, 22, 1273–1279. [Google Scholar] [CrossRef]
- Anzai, T.; Tsuzuki, K.; Yamada, N.; Hayashi, T.; Iwakuma, M.; Inada, K.; Kameyama, K.; Hoka, S.; Saji, M. Overexpression of Ca2+-permeable AMPA receptors promotes delayed cell death of hippocampal CA1 neurons following transient forebrain ischemia. Neurosci. Res. 2003, 46, 41–51. [Google Scholar] [CrossRef]
- Calderone, A.; Jover, T.; Mashiko, T.; Noh, K.M.; Tanaka, H.; Bennett, M.V.; Zukin, R.S. Late calcium EDTA rescues hippocampal CA1 neurons from global ischemia-induced death. J. Neurosci. 2004, 24, 9903–9913. [Google Scholar] [CrossRef]
- Noh, K.M.; Yokota, H.; Mashiko, T.; Castillo, P.E.; Zukin, R.S.; Bennett, M.V. Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against global ischemia-induced death. Proc. Natl. Acad. Sci. USA 2005, 102, 12230–12235. [Google Scholar] [CrossRef]
- Liu, B.; Liao, M.; Mielke, J.G.; Ning, K.; Chen, Y.; Li, L.; El-Hayek, Y.H.; Gomez, E.; Zukin, R.S.; Fehlings, M.G.; et al. Ischemia insults direct glutamate receptor subunit 2-lacking AMPA receptors to synaptic sites. J. Neurosci. 2006, 26, 5309–5319. [Google Scholar] [CrossRef]
- Blanco-Suarez, E.; Fluza, M.; Liu, X.; Chakkarapani, E.; Hanley, J.G. Differential Tiam1/Rac1 activation in hippocampal and cortical neurons mediates differential spine shrinkage in response to oxygen/glucose deprivation. J. Cereb. Blood Flow Metab. 2014, 34, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini-Giampietry, D.E.; Gorter, J.A.; Bennett, M.V.L.; Zukin, R.S. The GluR2 (GluR-B) hypothesis: Ca2+-permeable AMPA receptors in neurological disorders. Trends Neurosci. 1997, 20, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Bass, B.L. RNA editing by adenosine deaminases that act on RNA. Annu. Rev. Biochem. 2002, 71, 817–846. [Google Scholar] [CrossRef] [PubMed]
- Terashima, A.; Pelkey, K.A.; Rah, J.C.; Such, Y.H.; Roche, K.W.; Collingridge, G.L. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron 2008, 57, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Arundine, M.; Tymianski, M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 2003, 34, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Rosenberg, P.A.; Volpe, J.J.; Jensen, F.E. Calcium-permeable AMPA/kainite receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc. Natl. Acad. Sci. USA 2003, 100, 6801–6806. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Simonetta, A.; Sheng, M. Subunit rules governing the sorting of internalized AMPA receptors in hippocampal neurons. Neuron 2004, 43, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Monreal, M.; Brown, T.C.; Royo, M.; Esteban, J.A. The balance between receptor recycling and trafficking toward lysosomes determines synaptic strength during long-term depression. J. Neurosci. 2012, 32, 13200–13205. [Google Scholar] [CrossRef]
- Koszegi, Z.; Fiuza, M.; Hanley, J.G. Endocytosis and lysosomal degradation of GluA2/3 AMPARs in response to oxygen/glucose deprivation in hippocampal but not cortical neurons. Sci. Rep. 2017, 7, 12318. [Google Scholar] [CrossRef]
- Beske, P.H.; Byrnes, N.M.; Astruc-Diaz, F.; Jackson, D.A. Identification of NADPH oxidase as a key mediator in the post-ischemia-induced sequestration and degradation of the GluA2 AMPA receptor subunit. J. Neurochem. 2015, 132, 504–519. [Google Scholar] [CrossRef]
- Jackson, D.A.; Beske, P.H.; Byrnes, N.M.; Astruc-Diaz, F. The post-ischemic increase in GluA2 ser880 phosphorylation involves NADPH oxidase. J. Pharm. Sci. Ther. 2018, 4, 170–181. [Google Scholar] [CrossRef]
- Achzet, L.M.; Astruc-Diaz, F.; Beske, P.H.; Natale, N.R.; Denton, T.T.; Jackson, D.A. Liposomal encapsulated FSC231, a PICK1 inhibitor, prevents the ischemia/reperfusion-induced degradation of GluA2-containing AMPA receptors. Pharmaceutics 2021, 13, 636. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, J.L.; Gorski, J.A.; Dell’Acqua, M.L. NMDA receptor-dependent LTD requires transient synaptic incorporation of Ca2+-permeable AMPARs mediated by AKAP150-anchored PKA and calcineurin. Neuron 2016, 89, 1000–1015. [Google Scholar] [CrossRef]
- Passafaro, M.; Piech, V.; Sheng, M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat. Neurosci. 2001, 4, 917–926. [Google Scholar] [CrossRef]
- Shi, S.-H.; Hayashi, Y.; Esteban, J.A.; Malinow, R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 2001, 105, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.G. Subunit-specific trafficking mechanisms regulating the synaptic expression of Ca2+-permeable AMPA receptors. Semin. Cell Dev. Biol. 2014, 27, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Holt, C.E.; Martin, K.C.; Schuman, E.M. Local translation in neurons: Visualization and function. Nat. Struct. Mol. Biol. 2019, 26, 557–566. [Google Scholar] [CrossRef]
- Peng, P.J.; Zhong, X.; Tu, W.; Soundarapandian, M.M.; Molner, P.; Zhu, D.; Lau, L.; Liu, S.; Liu, F.; Lu, Y. ADAR2-dependent RNA editing of AMPA receptor subunit GluA2 determines vulnerability of neurons in forebrain ischemia. Neuron 2006, 49, 719–733. [Google Scholar] [CrossRef]
- Noh, K.M.; Hwang, J.Y.; Follenzi, A.; Athanasiadou, R.; Miyawaki, T.; Greally, J.M.; Bennett, M.V.L.; Zukin, R.S. Repressor element-1 silencing transcription factor (REST)-dependent epigenetic remodeling is critical to ischemia-induced neuronal death. Proc. Natl. Acad. Sci. USA 2012, 109, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Broyles, H.V.; He, S.; McGrady, N.R.; Li, L.; Yorio, T. Involvement of AMPA receptors and its flip and flip isoforms in retinal ganglion cell death following oxygen/glucose deprivation. Physiol. Pharmacol. 2016, 57, 508–526. [Google Scholar] [CrossRef] [PubMed]

| Antibody | Description | Host | Source | Dilution | Product Number |
|---|---|---|---|---|---|
| GluA1 | AMPA Receptor subunit 1 | Rabbit | Cell Signaling | 1:1000 | 13185S |
| GluA2 | AMPA Receptor Subunit 2 | Rabbit | Cell Signaling | 1:1000 | 13607S |
| GluA3 | AMPA Receptor Subunit 3 | Rabbit | Cell Signaling | 1:1000 | 5117S |
| β-Actin | Beta-Actin | Rabbit | Cell Signaling | 1:1000 | 8457S |
| PGK1 | Phosphoglycerate kinase 1 | Rabbit | Cell Signaling | 1:1000 | 68540S |
| Na,K-ATPase β1 | Na, K-ATPase subunit β1 | Rabbit | Cell Signaling | 1:1000 | 44759S |
| # | Gene | Forward Primer | Reverse Primer | Product bps |
|---|---|---|---|---|
| 1 | Gria1 | GGACAACRCAAGCGTCCAGA | CACAGTAGCCCTCATAGCGG | 122 |
| 2 | Gria2 | TGGTTTTCCTTGGGTGCCTT | TCGATGGGAGACACCATCCT | 170 |
| 3 | Gria3 | CCATGCTCTTGTCAGCTTCG | TGTGCTCCTGAACCGTGTTT | 178 |
| 4 | Gria2 Q/R | CTACGAGTGGCACACTGAGG | AACCACCACACACCTCCAAC | 177 |
| 5 | Actb | GCAGGAGTACGATGAGTCCG | ACGCAGCTCAGTAACAGTCC | 74 |
| 6 | Adarb2 | GACGACACGCGGGAATATCT | GCCAGCAAGCACCTTCTCTA | 131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achzet, L.M.; Jackson, D.A. Sex-Dependent Differences in the Ischemia/Reperfusion-Induced Expression of AMPA Receptors. Int. J. Mol. Sci. 2024, 25, 2231. https://doi.org/10.3390/ijms25042231
Achzet LM, Jackson DA. Sex-Dependent Differences in the Ischemia/Reperfusion-Induced Expression of AMPA Receptors. International Journal of Molecular Sciences. 2024; 25(4):2231. https://doi.org/10.3390/ijms25042231
Chicago/Turabian StyleAchzet, Lindsay M., and Darrell A. Jackson. 2024. "Sex-Dependent Differences in the Ischemia/Reperfusion-Induced Expression of AMPA Receptors" International Journal of Molecular Sciences 25, no. 4: 2231. https://doi.org/10.3390/ijms25042231
APA StyleAchzet, L. M., & Jackson, D. A. (2024). Sex-Dependent Differences in the Ischemia/Reperfusion-Induced Expression of AMPA Receptors. International Journal of Molecular Sciences, 25(4), 2231. https://doi.org/10.3390/ijms25042231





