The Brown Sugar Mediated Carbon Quantum Dots as a Novel Fluorescence Sensor for Sensitive Detection of Gentamicin and Its Application in Foods
Abstract
1. Introduction
2. Results and Discussion
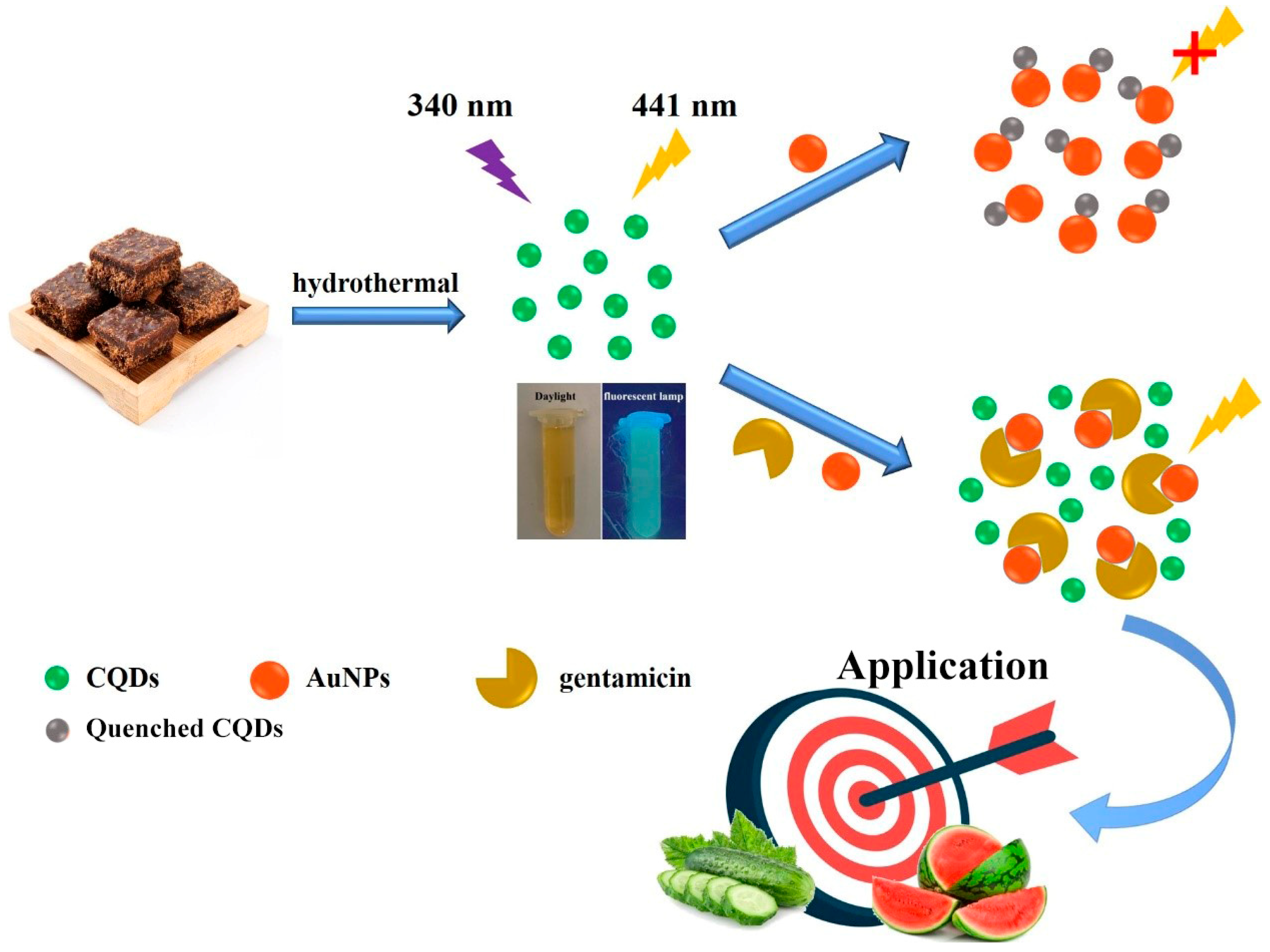
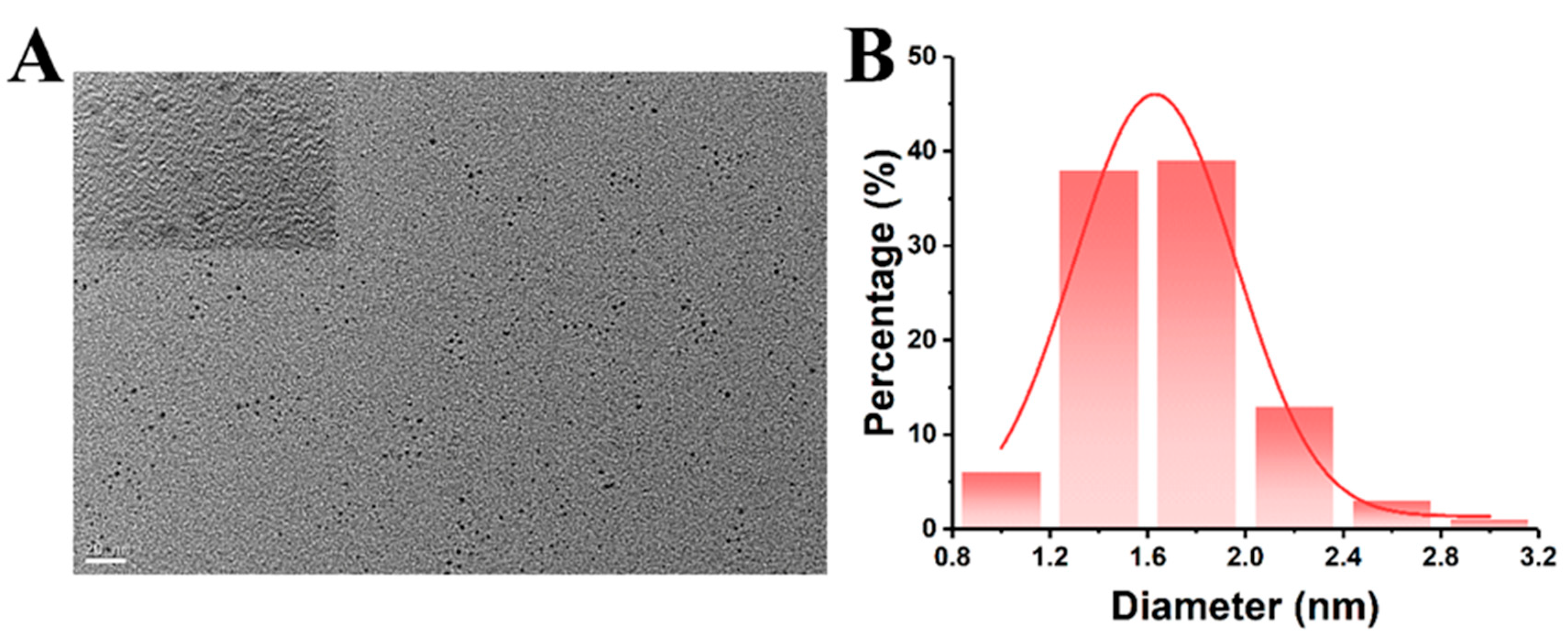
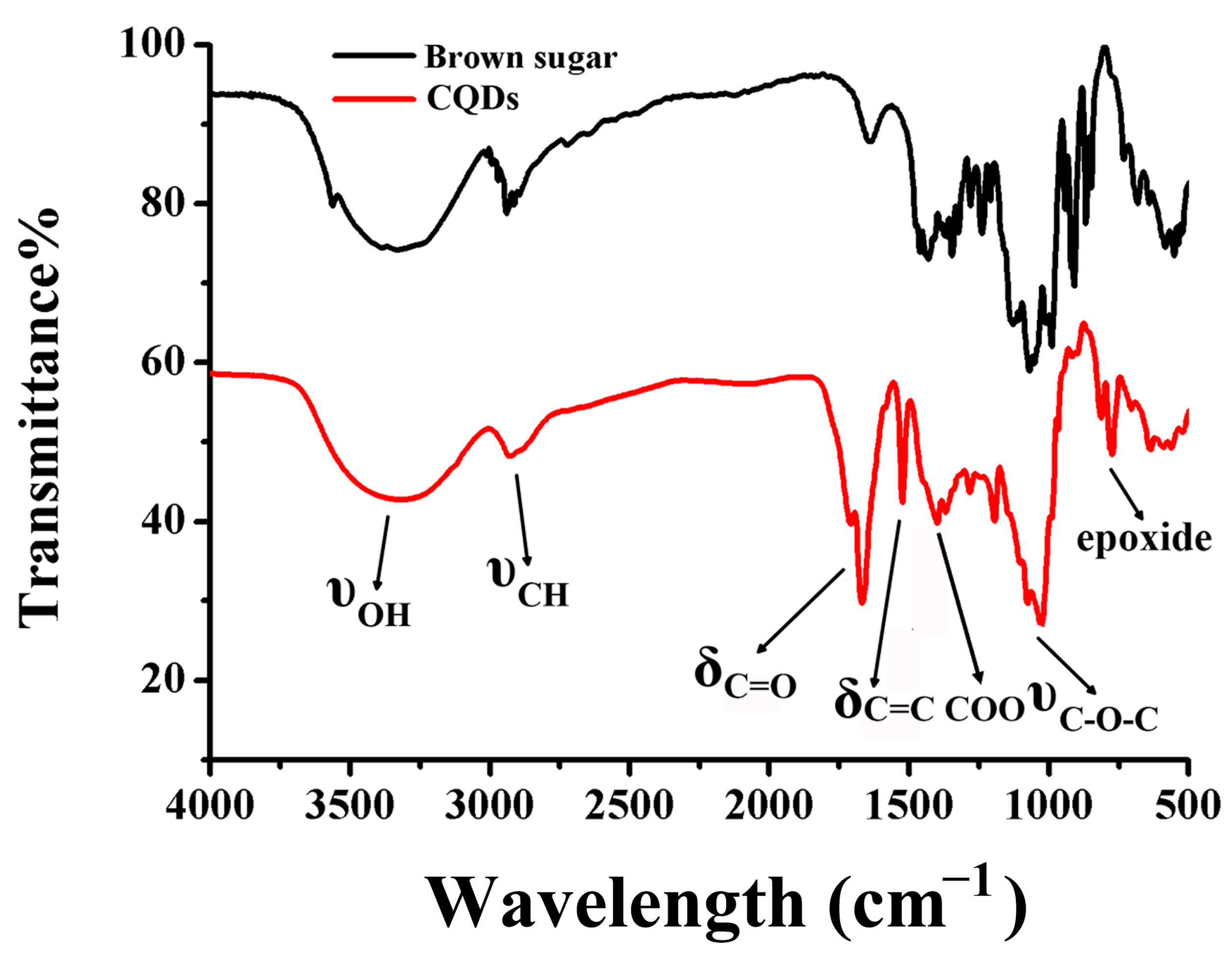
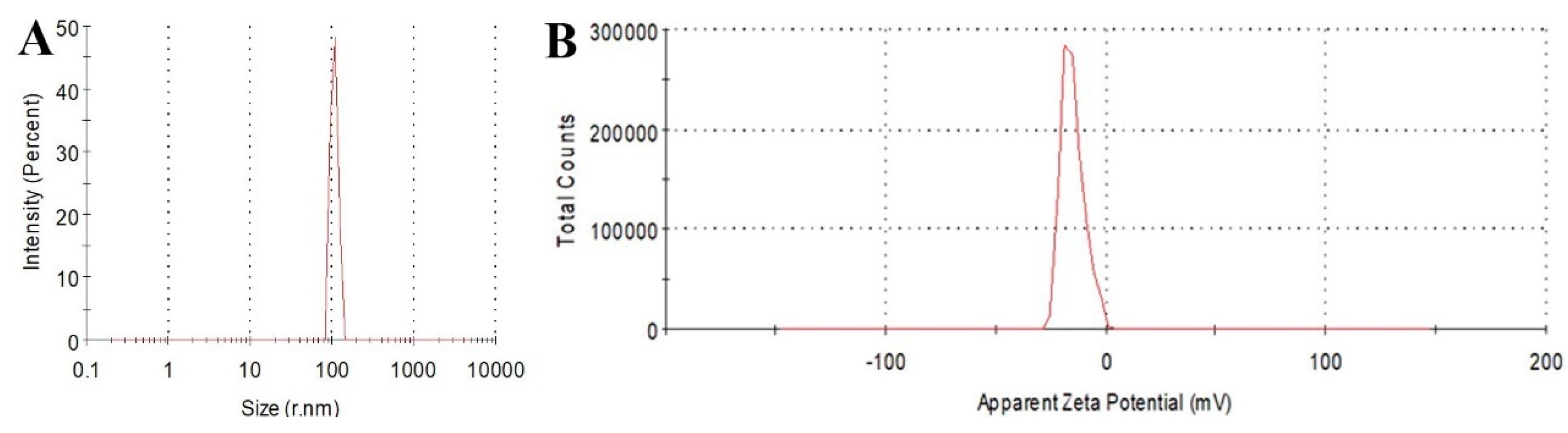
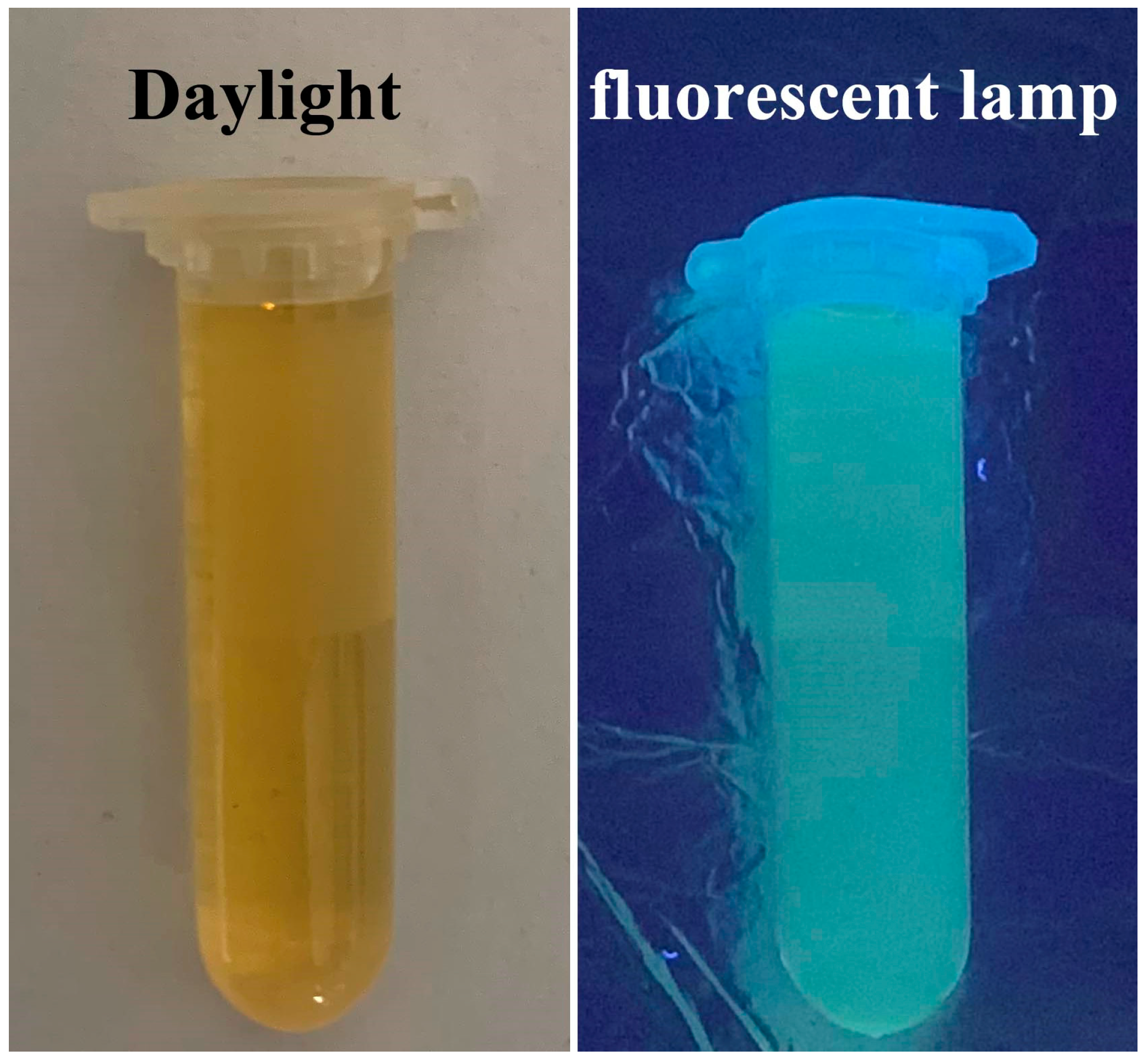
2.1. Characterization of CQDs
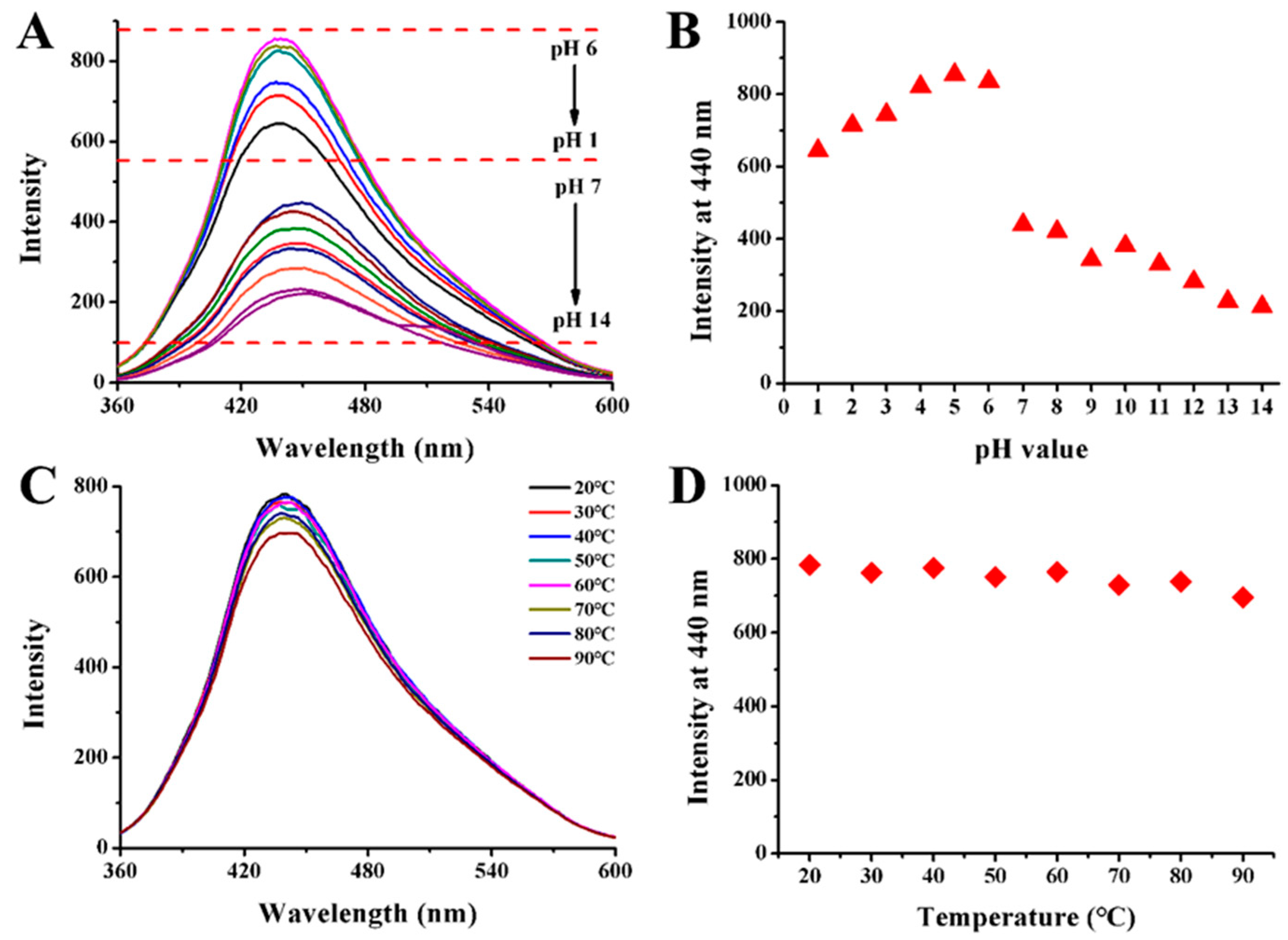
2.2. Stability Test of CQDs
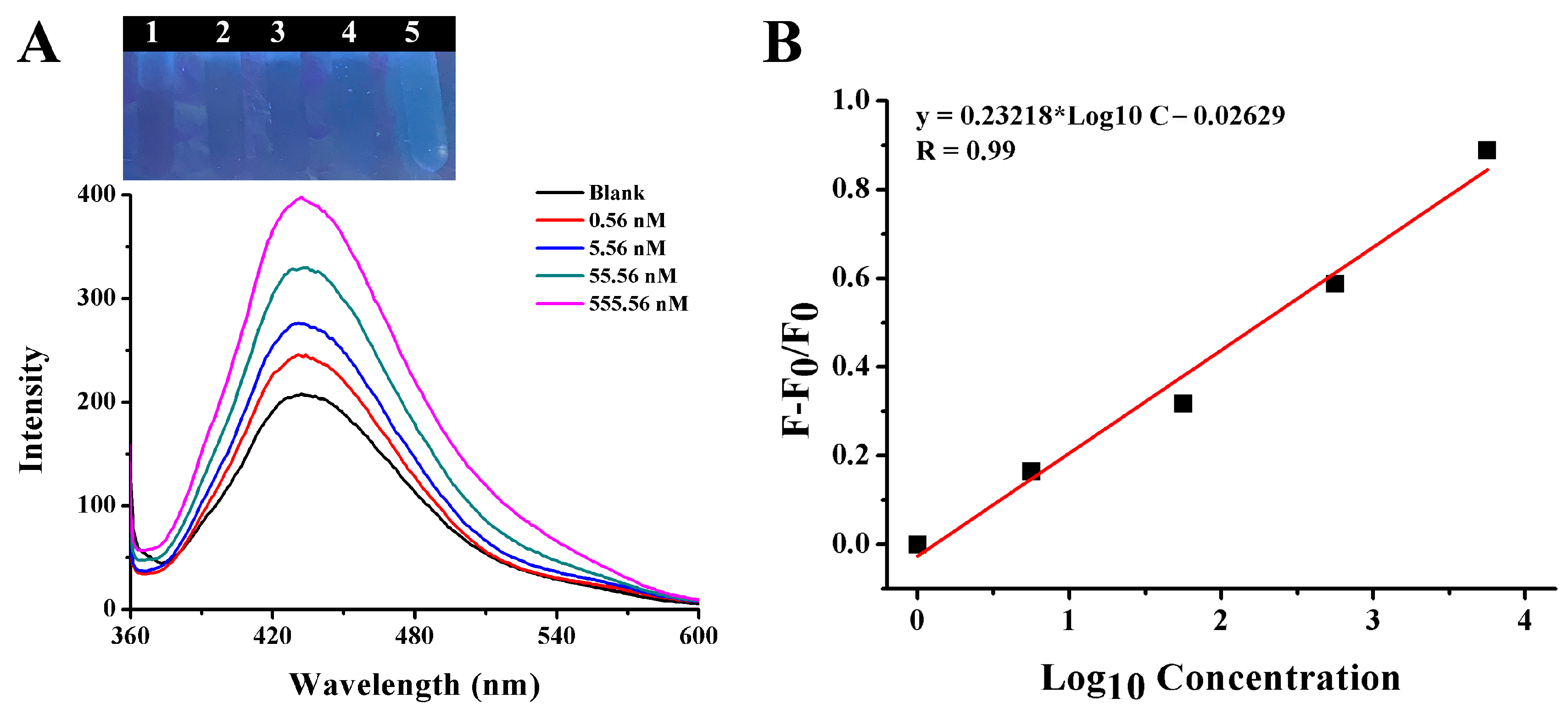
2.3. Fluorescence Detection of Gentamicin Based on CQDs and AuNPs
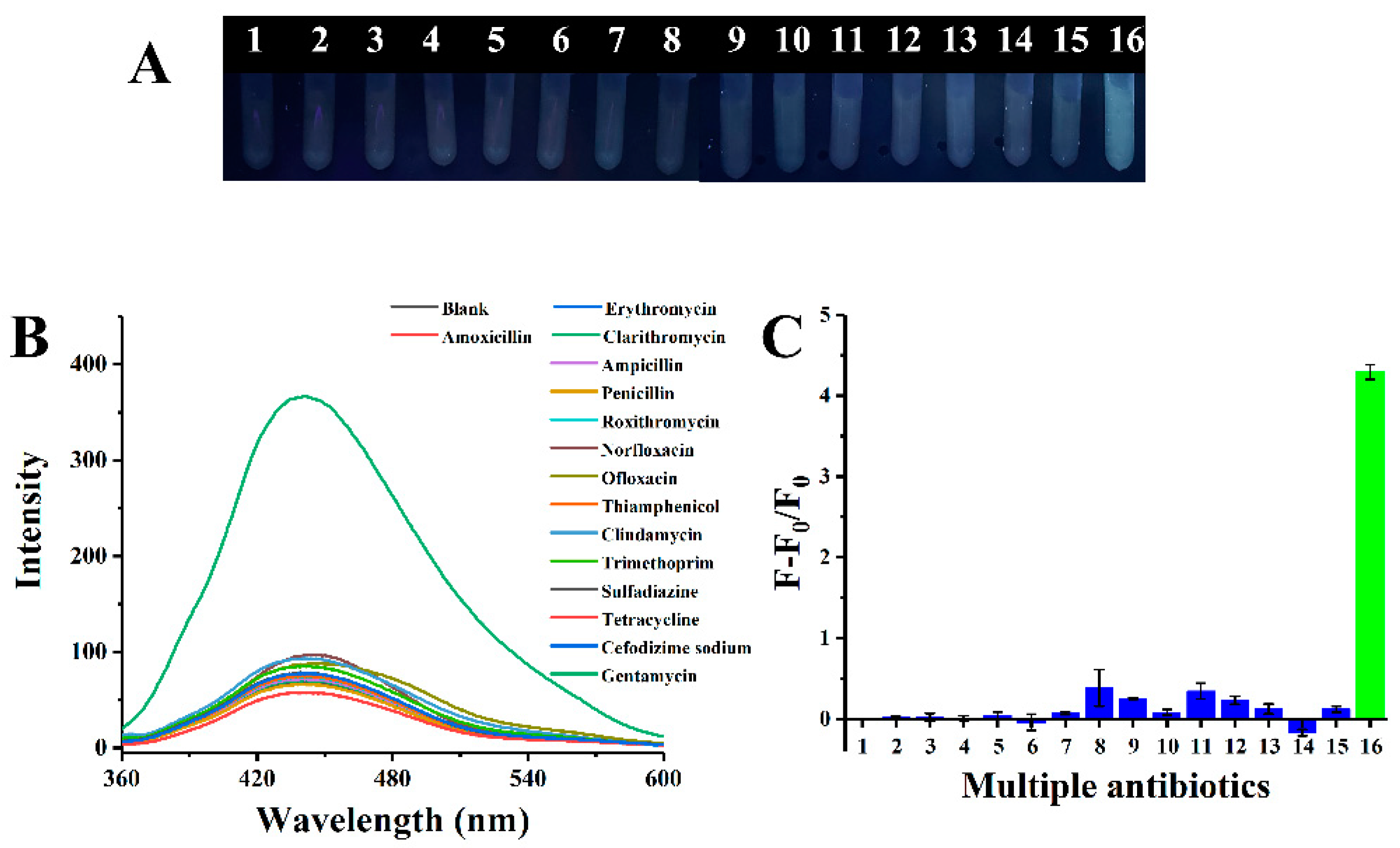
2.4. Selectivity Study
2.5. Determination of Gentamicin in Real Samples
3. Methods and Materials
3.1. Materials and Characterizations
3.2. Synthesis of CQDs and AuNPs
3.2.1. Green Synthesis of CQDs
3.2.2. Synthesis of AuNPs
3.3. Fluorescence Quenching by AuNPs
3.4. Fluorescence Detection of Gentamicin Based on CQDs and AuNPs
3.5. Selectivity Study
3.6. Detection of Gentamicin in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Geng, Y.; Ye, N.; Xiang, Y. A simple and sensitive colorimetric sensor for determination of gentamicin in milk based on lysine functionalized gold nanoparticles. Microchem. J. 2020, 158, 105190. [Google Scholar] [CrossRef]
- Gehring, R.; Haskell, S.R.; Payne, M.A.; Craigmill, A.L.; Webb, A.I.; Riviere, J.E. Aminoglycoside residues in food of animal origin. J. Am. Vet. Med. Assoc. 2005, 227, 63–66. [Google Scholar] [CrossRef]
- Maudonnet, E.N.; de Oliveira, J.A.A.; Rossato, M.; Hyppolito, M.A. Gentamicin attenuates gentamicin-induced ototoxicity–self-protection. Drug Chem. Toxicol. 2008, 31, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Blunston, M.A.; Yonovitz, A.; Woodahl, E.L.; Smolensky, M.H. Gentamicin-induced ototoxicity and nephrotoxicity vary with circadian time of treatment and entail separate mechanisms. Chronobiol. Int. 2015, 32, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mukherjee, S.; Hoffmann, M.; Kotewicz, M.L.; Young, S.; Abbott, J.; Luo, Y.; Davidson, M.K.; Allard, M.; McDermott, P. Whole-genome sequencing of gentamicin-resistant Campylobacter coli isolated from US retail meats reveals novel plasmid-mediated aminoglycoside resistance genes. Antimicrob. Agents Chemother. 2013, 57, 5398–5405. [Google Scholar] [CrossRef] [PubMed]
- No, C.R. On pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Communities 2010, 50, 1–72. [Google Scholar]
- Joseph, A.; Rustum, A. Development and validation of a RP-HPLC method for the determination of gentamicin sulfate and its related substances in a pharmaceutical cream using a short pentafluorophenyl column and a charged aerosol detector. J. Pharm. Biomed. Anal. 2010, 51, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Isoherranen, N.; Soback, S. Determination of gentamicins C1, C1a, and C2 in plasma and urine by HPLC. Clin. Chem. 2000, 46, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Lecaroz, C.; Campanero, M.A.; Gamazo, C.; Blanco-Prieto, M.J. Determination of gentamicin in different matrices by a new sensitive high-performance liquid chromatography-mass spectrometric method. J. Antimicrob. Chemother. 2006, 58, 557–563. [Google Scholar] [CrossRef]
- Preu, M.; Guyot, D.; Petz, M. Development of a gas chromatography–mass spectrometry method for the analysis of aminoglycoside antibiotics using experimental design for the optimisation of the derivatisation reactions. J. Chromatogr. A 1998, 818, 95–108. [Google Scholar] [CrossRef]
- Yadav, A.; Verma, D.; Solanki, P. Electrophoretically deposited L-cysteine functionalized MoS2@ MWCNT nanocomposite platform: A smart approach toward highly sensitive and label-free detection of gentamicin. Mater. Today Chem. 2021, 22, 100567. [Google Scholar] [CrossRef]
- Van Es, R.; Setford, S.; Blankwater, Y.; Meijer, D. Detection of gentamicin in milk by immunoassay and flow injection analysis with electrochemical measurement. Anal. Chim. Acta 2001, 429, 37–47. [Google Scholar] [CrossRef]
- Chen, Y.; Shang, Y.; Li, X.; Wu, X.; Xiao, X. Development of an enzyme-linked immunoassay for the detection of gentamicin in swine tissues. Food Chem. 2008, 108, 304–309. [Google Scholar] [CrossRef]
- Tuerhong, M.; Yang, X.; Xue-Bo, Y. Review on carbon dots and their applications. Chin. J. Anal. Chem. 2017, 45, 139–150. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, J. A review of carbon dots in biological applications. J. Mater. Sci. 2016, 51, 4728–4738. [Google Scholar] [CrossRef]
- Domena, J.B.; Celebic, E.; Ferreira, B.C.L.B.; Zhou, Y.; Zhang, W.; Chen, J.; Bartoli, M.; Tagliaferro, A.; Johnson, Q.; Chauhan, B.P.S.; et al. Investigation into Red Emission and Its Applications: Solvatochromic N-Doped Red Emissive Carbon Dots with Solvent Polarity Sensing and Solid-State Fluorescent Nanocomposite Thin Films. Molecules 2023, 28, 1755. [Google Scholar] [CrossRef] [PubMed]
- Molaei, M.J. Principles, mechanisms, and application of carbon quantum dots in sensors: A review. Anal. Methods 2020, 12, 1266–1287. [Google Scholar] [CrossRef]
- Mintz, K.J.; Bartoli, M.; Rovere, M.; Zhou, Y.; Hettiarachchi, S.D.; Paudyal, S.; Chen, J.; Domena, J.B.; Liyanage, P.Y.; Sampson, R.; et al. A deep investigation into the structure of carbon dots. Carbon 2021, 173, 433–447. [Google Scholar] [CrossRef]
- Azam, N.; Najabat Ali, M.; Javaid Khan, T. Carbon Quantum Dots for Biomedical Applications: Review and Analysis. Front. Mater. 2021, 8, 700403. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92. [Google Scholar] [CrossRef]
- Meng, W.; Bai, X.; Wang, B.; Liu, Z.; Lu, S.; Yang, B. Biomass-derived carbon dots and their applications. J. Nanobiotechnol. 2019, 2, 172–192. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, F.; Xu, L.; Liu, X.; Ma, P.; Sun, Y.; Wang, X.; Song, D. A fluorescence resonance energy transfer biosensor based on carbon dots and gold nanoparticles for the detection of trypsin. Sens. Actuators B 2018, 273, 1015–1021. [Google Scholar] [CrossRef]
- Li, J.; Rao, X.; Xiang, F.; Wei, J.; Yuan, M.; Liu, Z. A photoluminescence “switch-on” nanosensor composed of nitrogen and sulphur co-doped carbon dots and gold nanoparticles for discriminative detection of glutathione. Analyst 2018, 143, 2083–2089. [Google Scholar] [CrossRef]
- Fu, L.; Hou, Y.; Guo, H.; Liu, C.; Li, Y.; Jia, B.; Zhou, L.; Kong, W. Gold nanoparticles and nitrogen-doped carbon dots based fluorescent nanosensor for ultrasensitive detection of thiram in hawthorn. Microchem. J. 2022, 176, 107253. [Google Scholar] [CrossRef]
- Wang, B.; Chen, Y.; Wu, Y.; Weng, B.; Liu, Y.; Lu, Z.; Li, C.M.; Yu, C. Aptamer induced assembly of fluorescent nitrogen-doped carbon dots on gold nanoparticles for sensitive detection of AFB1. Biosens. Bioelectron. 2016, 78, 23–30. [Google Scholar] [CrossRef]
- Shi, J.; Tian, F.; Lyu, J.; Yang, M. Nanoparticle based fluorescence resonance energy transfer (FRET) for biosensing applications. J. Mater. Chem. B 2015, 3, 6989–7005. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Fourmy, D.; Puglisi, J.D. Structural origins of gentamicin antibiotic action. EMBO J. 1998, 17, 6437–6448. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Pu, P.; Zhao, J.; Dong, C.; Gao, C.; Chen, Y.; Chen, J.; Liu, Y.; Zhou, H. Preparation of highly photoluminescent sulfur-doped carbon dots for Fe (III) detection. J. Mater. Chem. A 2015, 3, 542–546. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.; Huang, C.; Xie, J.; Su, W.; Sheng, J.; Xiao, Q. A carbon dots based fluorescent probe for selective and sensitive detection of hemoglobin. Sens. Actuators B 2015, 221, 1215–1222. [Google Scholar] [CrossRef]
- Yan, F.; Zou, Y.; Wang, M.; Mu, X.; Yang, N.; Chen, L. Highly photoluminescent carbon dots-based fluorescent chemosensors for sensitive and selective detection of mercury ions and application of imaging in living cells. Sens. Actuators B 2014, 192, 488–495. [Google Scholar] [CrossRef]
- Zong, J.; Yang, X.; Trinchi, A.; Hardin, S.; Cole, I.; Zhu, Y.; Li, C.; Muster, T.; Wei, G. Carbon dots as fluorescent probes for “off–on” detection of Cu2+ and l-cysteine in aqueous solution. Biosens. Bioelectron. 2014, 51, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, X.; Li, G.; Zhao, C.; Liao, X. Green synthesis of fluorescent carbon dots for selective detection of tartrazine in food samples. J. Agric. Food Chem. 2015, 63, 6707–6714. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Li, L.; Cao, L.; Zan, M.; Yang, D.; Wang, Z.; Chang, Z.; Mei, Q.; Miao, P.; Dong, W.-F. Two-Step Hydrothermal Preparation of Carbon Dots for Calcium Ion Detection. ACS Appl. Mater. Interfaces 2019, 11, 44566–44572. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Yan, X.; Qu, D.; Li, D.; Tao, F.F.; Sun, Z. Red Emissive Sulfur, Nitrogen Codoped Carbon Dots and Their Application in Ion Detection and Theraonostics. ACS Appl. Mater. Interfaces 2017, 9, 18549–18556. [Google Scholar] [CrossRef]
- Campos, B.B.; Contreras-Cáceres, R.; Bandosz, T.J.; Jiménez-Jiménez, J.; Rodríguez-Castellón, E.; da Silva, J.C.E.; Algarra, M. Carbon dots as fluorescent sensor for detection of explosive nitrocompounds. Carbon 2016, 106, 171–178. [Google Scholar] [CrossRef]
- Ma, C.a.; Yin, C.; Fan, Y.; Yang, X.; Zhou, X. Highly efficient synthesis of N-doped carbon dots with excellent stability through pyrolysis method. J. Mater. Sci. 2019, 54, 9372–9384. [Google Scholar] [CrossRef]
- Han, Z.; Nan, D.; Yang, H.; Sun, Q.; Pan, S.; Liu, H.; Hu, X. Carbon quantum dots based ratiometric fluorescence probe for sensitive and selective detection of Cu2+ and glutathione. Sens. Actuators B 2019, 298, 126842. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Liu, Q.; Chen, M.; Chen, X. Facile synthesis of B, N-doped CQDs as versatile fluorescence probes for sensitive detection of cobalt ions in environmental water and biological samples. Microchem. J 2021, 163, 105888. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Park, S.-J.; Zhang, Y.; Kim, T.; Chae, S.; Park, M.; Kim, H.-Y. One-step synthesis of robust nitrogen-doped carbon dots: Acid-evoked fluorescence enhancement and their application in Fe3+ detection. J. Mater. Chem. A 2015, 3, 17747–17754. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Kim, Y.-P.; Oh, Y.-H.; Oh, E.; Ko, S.; Han, M.-K.; Kim, H.-S. Energy transfer-based multiplexed assay of proteases by using gold nanoparticle and quantum dot conjugates on a surface. Anal. Chem. 2008, 80, 4634–4641. [Google Scholar] [CrossRef]
- Dai, H.; Shi, Y.; Wang, Y.; Sun, Y.; Hu, J.; Ni, P.; Li, Z. A carbon dot based biosensor for melamine detection by fluorescence resonance energy transfer. Sens. Actuators B 2014, 202, 201–208. [Google Scholar] [CrossRef]
- Yoo, D.; Park, Y.; Cheon, B.; Park, M.-H. Carbon Dots as an Effective Fluorescent Sensing Platform for Metal Ion Detection. Nanoscale Res. Lett. 2019, 14, 272. [Google Scholar] [CrossRef]
- Clarot, I.; Chaimbault, P.; Hasdenteufel, F.; Netter, P.; Nicolas, A. Determination of gentamicin sulfate and related compounds by high-performance liquid chromatography with evaporative light scattering detection. J. Chromatogr. A 2004, 1031, 281–287. [Google Scholar] [CrossRef]
- Khaled, E.; Khalil, M.M.; Abed el Aziz, G.M. Calixarene/carbon nanotubes based screen printed sensors for potentiometric determination of gentamicin sulphate in pharmaceutical preparations and spiked surface water samples. Sens. Actuators B 2017, 244, 876–884. [Google Scholar] [CrossRef]
- ul Ain, N.; Aslam, Z.; Yousuf, M.; Waseem, W.A.; Bano, S.; Anis, I.; Ahmed, F.; Faizi, S.; Malik, M.I.; Shah, M.R. Green synthesis of methyl gallate conjugated silver nanoparticles: A colorimetric probe for gentamicin. New J. Chem. 2019, 43, 1972–1979. [Google Scholar] [CrossRef]
- Gukowsky, J.C.; Tan, C.; Han, Z.; He, L. Cysteamine-Modified Gold Nanoparticles as a Colorimetric Sensor for the Rapid Detection of Gentamicin. J. Food Sci. 2018, 83, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z.; Wang, S.; Qu, C.; Chen, L. On-Site Visual Detection of Hydrogen Sulfide in Air Based on Enhancing the Stability of Gold Nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 6300–6307. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Guo, Y.; Chen, X. The Brown Sugar Mediated Carbon Quantum Dots as a Novel Fluorescence Sensor for Sensitive Detection of Gentamicin and Its Application in Foods. Int. J. Mol. Sci. 2024, 25, 2143. https://doi.org/10.3390/ijms25042143
Guo X, Guo Y, Chen X. The Brown Sugar Mediated Carbon Quantum Dots as a Novel Fluorescence Sensor for Sensitive Detection of Gentamicin and Its Application in Foods. International Journal of Molecular Sciences. 2024; 25(4):2143. https://doi.org/10.3390/ijms25042143
Chicago/Turabian StyleGuo, Xinran, Yanxin Guo, and Xinyue Chen. 2024. "The Brown Sugar Mediated Carbon Quantum Dots as a Novel Fluorescence Sensor for Sensitive Detection of Gentamicin and Its Application in Foods" International Journal of Molecular Sciences 25, no. 4: 2143. https://doi.org/10.3390/ijms25042143
APA StyleGuo, X., Guo, Y., & Chen, X. (2024). The Brown Sugar Mediated Carbon Quantum Dots as a Novel Fluorescence Sensor for Sensitive Detection of Gentamicin and Its Application in Foods. International Journal of Molecular Sciences, 25(4), 2143. https://doi.org/10.3390/ijms25042143




