Extracellular Vesicles in Flaviviridae Pathogenesis: Their Roles in Viral Transmission, Immune Evasion, and Inflammation
Abstract
1. Introduction
2. Biology of EVs
2.1. EV Classification and Functions
- -
- -
- Ectosomes, which comprise microvesicles and some other variants of EVs such as oncosomes. Their characteristic feature is that they are formed in the plasma membrane directly from outward budding, and their size typically ranges from 100 nm up to 1 µm in diameter, more commonly >200 nm [20]. They must be centrifuged at 10,000–15,000× g for sedimentation.
- -
- Exosomes, generated in the cell during the endocytic pathway due to inward budding of the endosomal membrane, with a typical size of 30–150 nm in diameter, thus requiring high-speed centrifugation (100,000× g) for sedimentation [16,21]. The process of exosome generation is as follows [22]: after invagination of the plasma membrane, some extracellular components and cell membrane proteins are wrapped together to form early endosomes. These early endosomes can exchange substances with other organelles or fuse to form late endosomes and intracellular multivesicular bodies (MVB), which contain numerous intraluminal vesicles (ILV). MVBs can be degraded by autophagosome/lysosome pathways or fuse with the plasma membrane to release endogenous substances and also ILVs, which, at this stage, are regarded as exosomes [22].
2.2. Current Challenges in EV Studies
2.3. EVs and Viruses
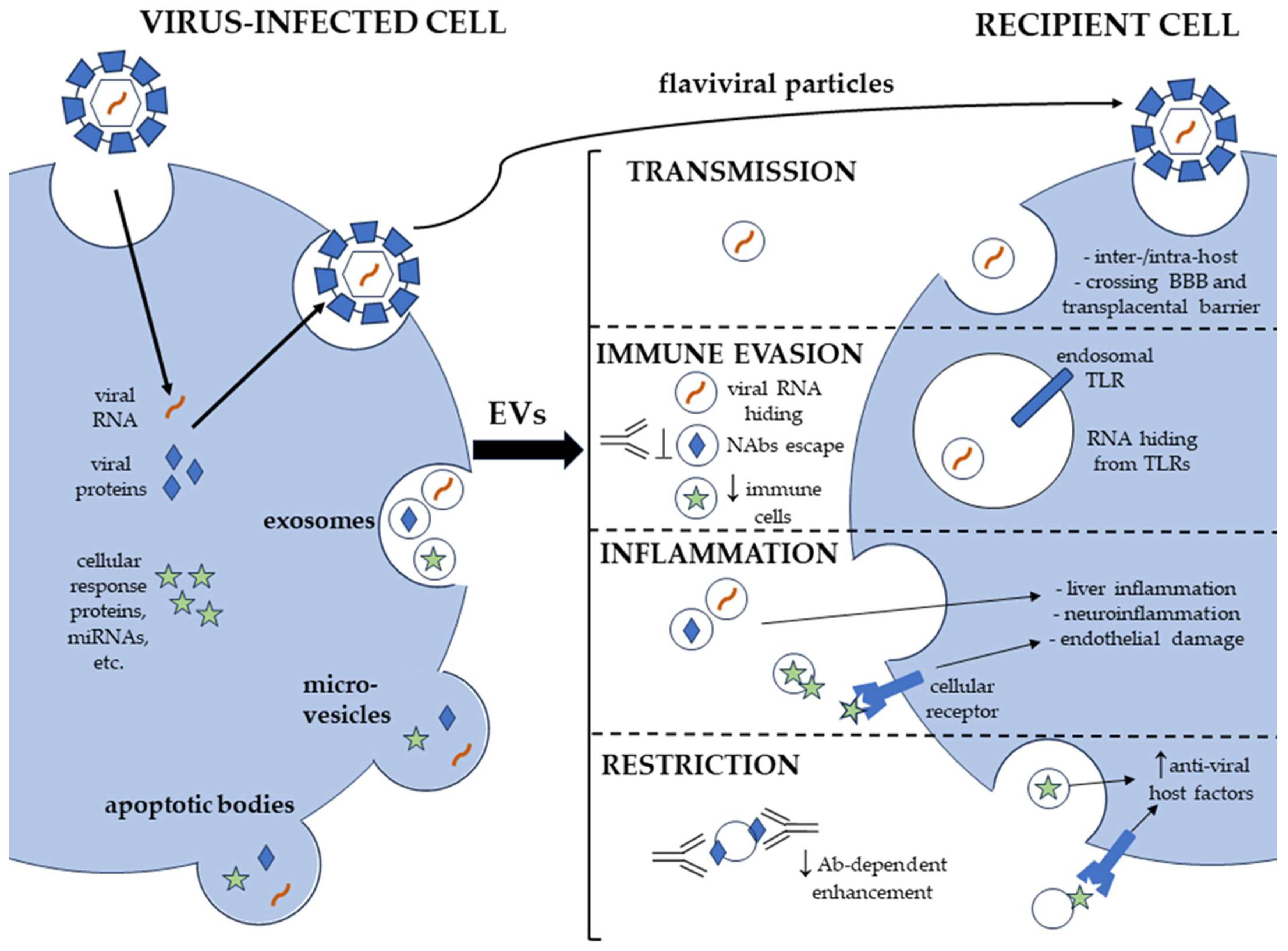
3. EV Roles in the Transmission of Flaviviridae
3.1. EVs in the Transmission of Blood-Borne HCV
3.2. EVs in the Transmission of Arthropod-Borne Flaviviruses
3.3. EVs in Flaviviral CNS Invasion
3.4. EVs in ZIKV Crossing the Transplacental Barrier
4. EVs Favor Immune Evasion by Flaviviridae
4.1. EVs Favor the Evasion of Innate Immune Recognition and Neutralizing Antibodies
4.2. EVs Carry Effector Molecules Targeting the Host Immune System
5. EV Roles in the Inflammatory Pathogenesis of Flaviviridae
5.1. EVs in Liver Inflammation
5.2. EVs in Endothelial Disfunction
5.2.1. DENV
5.2.2. ZIKV
5.2.3. NS1 Protein Associated with EVs
5.3. EVs in Neuroinflammation
5.4. EVs and Inflammasomes
6. EVs Help Restrict Flaviviridae Infections
6.1. EVs Stimulate Host Innate Immunity
6.2. EVs Attenuate Antibody-Dependent Enhancement
6.3. EVs May Favor Antigen Presentation
7. Clinical Application of EVs for the Treatment of Flaviviridae Infections
7.1. EVs in Diagnostics and in the Therapy of Flaviviridae Infections
7.2. EVs as Delivery Platforms
7.3. EVs as a Target for Inhibitors
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Blach, S.; Terrault, N.A.; Tacke, F.; Gamkrelidze, I.; Craxi, A.; Tanaka, J.; Waked, I.; Dore, G.J.; Abbas, Z.; Abdallah, A.R. Global Change in Hepatitis C Virus Prevalence and Cascade of Care between 2015 and 2020: A Modelling Study. Lancet Gastroenterol. Hepatol. 2022, 7, 396–415. [Google Scholar] [CrossRef] [PubMed]
- Godói, I.P.; Lemos, L.L.P.; De Araújo, V.E.; Bonoto, B.C.; Godman, B.; Guerra Junior, A.A. CYD-TDV Dengue Vaccine: Systematic Review and Meta-Analysis of Efficacy, Immunogenicity and Safety. J. Comp. Eff. Res. 2017, 6, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Satchidanandam, V. Japanese Encephalitis Vaccines. Curr. Treat. Options Infect. Dis. 2020, 12, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B. Learning Immunology from the Yellow Fever Vaccine: Innate Immunity to Systems Vaccinology. Nat. Rev. Immunol. 2009, 9, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Kubinski, M.; Beicht, J.; Gerlach, T.; Volz, A.; Sutter, G.; Rimmelzwaan, G.F. Tick-Borne Encephalitis Virus: A Quest for Better Vaccines against a Virus on the Rise. Vaccines 2020, 8, 451. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Ramasamy, V.; Shanmugam, R.K.; Ahuja, R.; Khanna, N. Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine. Front. Cell. Infect. Microbiol. 2020, 10, 572681. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.P.; Maasoumy, B. Breakthroughs in Hepatitis C Research: From Discovery to Cure. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 533–550. [Google Scholar] [CrossRef]
- Pellett, P.E.; Mitra, S.; Holland, T.C. Basics of Virology. Handb. Clin. Neurol. 2014, 123, 45–66. [Google Scholar]
- Anderson, M.R.; Kashanchi, F.; Jacobson, S. Exosomes in Viral Disease. Neurotherapeutics 2016, 13, 535–546. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 2021, 8, 2003505. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and Secretion of Exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Raposo, G. As We Wait: Coping with an Imperfect Nomenclature for Extracellular Vesicles. J. Extracell. Vesicles 2013, 2, 20389. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Aloi, N.; Drago, G.; Ruggieri, S.; Cibella, F.; Colombo, P.; Longo, V. Extracellular Vesicles and Immunity: At the Crossroads of Cell Communication. Int. J. Mol. Sci. 2024, 25, 1205. [Google Scholar] [CrossRef]
- Caruso, S.; Poon, I.K.H. Apoptotic Cell-Derived Extracellular Vesicles: More Than Just Debris. Front. Immunol. 2018, 9, 1486. [Google Scholar] [CrossRef]
- Santavanond, J.P.; Rutter, S.F.; Atkin-Smith, G.K.; Poon, I.K.H. Apoptotic Bodies: Mechanism of Formation, Isolation and Functional Relevance. Subcell. Biochem. 2021, 97, 61–88. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Yakubovich, E.I.; Polischouk, A.G.; Evtushenko, V.I. Principles and Problems of Exosome Isolation from Biological Fluids. Biochem. Mosc. Suppl. Ser. Membr. Cell Biol. 2022, 16, 115–126. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Tavano, S.; Heisenberg, C.-P. Migrasomes Take Center Stage. Nat. Cell Biol. 2019, 21, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, C.; Migliorino, R.; Leone, A.; Budillon, A. Large Extracellular Vesicles: Size Matters in Tumor Progression. Cytokine Growth Factor Rev. 2020, 51, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Sheta, M.; Taha, E.A.; Lu, Y.; Eguchi, T. Extracellular Vesicles: New Classification and Tumor Immunosuppression. Biology 2023, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular Vesicles and Nanoparticles: Emerging Complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and Directions in Studying Cell-Cell Communication by Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Shurtleff, M.J.; Temoche-Diaz, M.M.; Schekman, R. Extracellular Vesicles and Cancer: Caveat Lector. Annu. Rev. Cancer Biol. 2018, 2, 395–411. [Google Scholar] [CrossRef]
- Stam, J.; Bartel, S.; Bischoff, R.; Wolters, J.C. Isolation of Extracellular Vesicles with Combined Enrichment Methods. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2021, 1169, 122604. [Google Scholar] [CrossRef]
- Gandham, S.; Su, X.; Wood, J.; Nocera, A.L.; Alli, S.C.; Milane, L.; Zimmerman, A.; Amiji, M.; Ivanov, A.R. Technologies and Standardization in Research on Extracellular Vesicles. Trends Biotechnol. 2020, 38, 1066–1098. [Google Scholar] [CrossRef]
- Hartjes, T.A.; Mytnyk, S.; Jenster, G.W.; van Steijn, V.; Royen, M.E. van Extracellular Vesicle Quantification and Characterization: Common Methods and Emerging Approaches. Bioengineering 2019, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lin, S.; Zhou, C.; Cui, D.; Haick, H.; Tang, N. From Conventional to Microfluidic: Progress in Extracellular Vesicle Separation and Individual Characterization. Adv. Healthc. Mater. 2023, 12, e2202437. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Liu, X.; Zhu, L.; Luo, L.; Sun, N.; Pei, R. Current Advances in Technologies for Single Extracellular Vesicle Analysis and Its Clinical Applications in Cancer Diagnosis. Biosensors 2023, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Ligat, G.; Malnou, C.E. The Yin and the Yang of Extracellular Vesicles during Viral Infections. Biomed. J. 2023, 100659. [Google Scholar] [CrossRef] [PubMed]
- Raab-Traub, N.; Dittmer, D.P. Viral Effects on the Content and Function of Extracellular Vesicles. Nat. Rev. Microbiol. 2017, 15, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Vora, A.; Zhou, W.; Londono-Renteria, B.; Woodson, M.; Sherman, M.B.; Colpitts, T.M.; Neelakanta, G.; Sultana, H. Arthropod EVs Mediate Dengue Virus Transmission through Interaction with a Tetraspanin Domain Containing Glycoprotein Tsp29Fb. Proc. Natl. Acad. Sci. USA 2018, 115, E6604–E6613. [Google Scholar] [CrossRef]
- Zhou, W.; Woodson, M.; Neupane, B.; Bai, F.; Sherman, M.B.; Choi, K.H.; Neelakanta, G.; Sultana, H. Exosomes Serve as Novel Modes of Tick-Borne Flavivirus Transmission from Arthropod to Human Cells and Facilitates Dissemination of Viral RNA and Proteins to the Vertebrate Neuronal Cells. PLoS Pathog. 2018, 14, e1006764. [Google Scholar] [CrossRef]
- Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; De Jesús-González, L.A.; Palacios-Rápalo, S.N.; Cordero-Rivera, C.D.; Farfan-Morales, C.N.; Hurtado-Monzón, A.M.; Gallardo-Flores, C.E.; Alcaraz-Estrada, S.L.; Salas-Benito, J.S.; et al. The Regulation of Flavivirus Infection by Hijacking Exosome-Mediated Cell-Cell Communication: New Insights on Virus-Host Interactions. Viruses 2020, 12, 765. [Google Scholar] [CrossRef]
- Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; De Jesús-González, L.A.; Hurtado-Monzón, A.M.; Farfan-Morales, C.N.; Cervantes-Salazar, M.; Bolaños, J.; Cigarroa-Mayorga, O.E.; Martín-Martínez, E.S.; Medina, F.; et al. Isolation and Characterization of Exosomes Released from Mosquito Cells Infected with Dengue Virus. Virus Res. 2019, 266, 1–14. [Google Scholar] [CrossRef]
- Yang, C.-F.; Tu, C.-H.; Lo, Y.-P.; Cheng, C.-C.; Chen, W.-J. Involvement of Tetraspanin C189 in Cell-to-Cell Spreading of the Dengue Virus in C6/36 Cells. PLoS Negl. Trop. Dis. 2015, 9, e0003885. [Google Scholar] [CrossRef]
- Martínez-Rojas, P.P.; Quiroz-García, E.; Monroy-Martínez, V.; Agredano-Moreno, L.T.; Jiménez-García, L.F.; Ruiz-Ordaz, B.H. Participation of Extracellular Vesicles from Zika-Virus-Infected Mosquito Cells in the Modification of Naïve Cells’ Behavior by Mediating Cell-to-Cell Transmission of Viral Elements. Cells 2020, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- York, S.B.; Sun, L.; Cone, A.S.; Duke, L.C.; Cheerathodi, M.R.; Meckes, D.G. Zika Virus Hijacks Extracellular Vesicle Tetraspanin Pathways for Cell-to-Cell Transmission. mSphere 2021, 6, e0019221. [Google Scholar] [CrossRef]
- Sultana, H.; Neelakanta, G. Arthropod Exosomes as Bubbles with Message(s) to Transmit Vector-Borne Diseases. Curr. Opin. Insect Sci. 2020, 40, 39–47. [Google Scholar] [CrossRef]
- Bukong, T.N.; Momen-Heravi, F.; Kodys, K.; Bala, S.; Szabo, G. Exosomes from Hepatitis C Infected Patients Transmit HCV Infection and Contain Replication Competent Viral RNA in Complex with Ago2-miR122-HSP90. PLoS Pathog. 2014, 10, e1004424. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnaiah, V.; Thumann, C.; Fofana, I.; Habersetzer, F.; Pan, Q.; de Ruiter, P.E.; Willemsen, R.; Demmers, J.A.A.; Stalin Raj, V.; Jenster, G.; et al. Exosome-Mediated Transmission of Hepatitis C Virus between Human Hepatoma Huh7.5 Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 13109–13113. [Google Scholar] [CrossRef]
- Longatti, A.; Boyd, B.; Chisari, F.V. Virion-Independent Transfer of Replication-Competent Hepatitis C Virus RNA between Permissive Cells. J. Virol. 2014, 89, 2956–2961. [Google Scholar] [CrossRef] [PubMed]
- Grünvogel, O.; Colasanti, O.; Lee, J.-Y.; Klöss, V.; Belouzard, S.; Reustle, A.; Esser-Nobis, K.; Hesebeck-Brinckmann, J.; Mutz, P.; Hoffmann, K.; et al. Secretion of Hepatitis C Virus Replication Intermediates Reduces Activation of Toll-Like Receptor 3 in Hepatocytes. Gastroenterology 2018, 154, 2237–2251.e16. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Q.; Chen, H.; He, P.; Li, Y.; Si, M.; Jiao, X. Circulating microRNAs as a Biomarker to Predict Therapy Efficacy in Hepatitis C Patients with Different Genotypes. Microb. Pathog. 2017, 112, 320–326. [Google Scholar] [CrossRef]
- Catanese, M.T.; Uryu, K.; Kopp, M.; Edwards, T.J.; Andrus, L.; Rice, W.J.; Silvestry, M.; Kuhn, R.J.; Rice, C.M. Ultrastructural Analysis of Hepatitis C Virus Particles. Proc. Natl. Acad. Sci. USA 2013, 110, 9505–9510. [Google Scholar] [CrossRef]
- Deng, L.; Jiang, W.; Wang, X.; Merz, A.; Hiet, M.-S.; Chen, Y.; Pan, X.; Jiu, Y.; Yang, Y.; Yu, B.; et al. Syntenin Regulates Hepatitis C Virus Sensitivity to Neutralizing Antibody by Promoting E2 Secretion through Exosomes. J. Hepatol. 2019, 71, 52–61. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Yu, Q.; He, J.J. Exosome-Associated Hepatitis C Virus in Cell Cultures and Patient Plasma. Biochem. Biophys. Res. Commun. 2014, 455, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-W.; Mettling, C.; Wu, S.-R.; Yu, C.-Y.; Perng, G.-C.; Lin, Y.-S.; Lin, Y.-L. Autophagy-Associated Dengue Vesicles Promote Viral Transmission Avoiding Antibody Neutralization. Sci. Rep. 2016, 6, 32243. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.-C.; Strilets, T.; Tan, W.-L.; Castillo, D.; Medkour, H.; Rey-Cadilhac, F.; Serrato-Pomar, I.M.; Rachenne, F.; Chowdhury, A.; Chuo, V.; et al. The Anti-Immune Dengue Subgenomic Flaviviral RNA Is Present in Vesicles in Mosquito Saliva and Is Associated with Increased Infectivity. PLoS Pathog. 2023, 19, e1011224. [Google Scholar] [CrossRef]
- Punyadee, N.; Mairiang, D.; Thiemmeca, S.; Komoltri, C.; Pan-ngum, W.; Chomanee, N.; Charngkaew, K.; Tangthawornchaikul, N.; Limpitikul, W.; Vasanawathana, S.; et al. Microparticles Provide a Novel Biomarker To Predict Severe Clinical Outcomes of Dengue Virus Infection. J. Virol. 2014, 89, 1587–1607. [Google Scholar] [CrossRef] [PubMed]
- Safadi, D.E.; Lebeau, G.; Lagrave, A.; Mélade, J.; Grondin, L.; Rosanaly, S.; Begue, F.; Hoareau, M.; Veeren, B.; Roche, M.; et al. Extracellular Vesicles Are Conveyors of the NS1 Toxin during Dengue Virus and Zika Virus Infection. Viruses 2023, 15, 364. [Google Scholar] [CrossRef]
- Velandia-Romero, M.L.; Calderón-Peláez, M.A.; Balbás-Tepedino, A.; Márquez-Ortiz, R.A.; Madroñero, L.J.; Barreto Prieto, A.; Castellanos, J.E. Extracellular Vesicles of U937 Macrophage Cell Line Infected with DENV-2 Induce Activation in Endothelial Cells EA.Hy926. PLoS ONE 2020, 15, e0227030. [Google Scholar] [CrossRef]
- Zhao, F.; Xu, Y.; Liu, N.; Lv, D.; Chen, Y.; Liu, Z.; Jin, X.; Xiao, M.; Lavillette, D.; Zhong, J.; et al. Extracellular Vesicles from Zika Virus-Infected Cells Display Viral E Protein That Binds ZIKV-Neutralizing Antibodies to Prevent Infection Enhancement. EMBO J. 2023, 42, e112096. [Google Scholar] [CrossRef]
- Karamichali, E.; Chihab, H.; Kakkanas, A.; Marchio, A.; Karamitros, T.; Pogka, V.; Varaklioti, A.; Kalliaropoulos, A.; Martinez-Gonzales, B.; Foka, P.; et al. HCV Defective Genomes Promote Persistent Infection by Modulating the Viral Life Cycle. Front. Microbiol. 2018, 9, 2942. [Google Scholar] [CrossRef]
- Zhou, W.; Tahir, F.; Wang, J.C.-Y.; Woodson, M.; Sherman, M.B.; Karim, S.; Neelakanta, G.; Sultana, H. Discovery of Exosomes From Tick Saliva and Salivary Glands Reveals Therapeutic Roles for CXCL12 and IL-8 in Wound Healing at the Tick–Human Skin Interface. Front. Cell Dev. Biol. 2020, 8, 554. [Google Scholar] [CrossRef]
- Clé, M.; Desmetz, C.; Barthelemy, J.; Martin, M.-F.; Constant, O.; Maarifi, G.; Foulongne, V.; Bolloré, K.; Glasson, Y.; De Bock, F.; et al. Zika Virus Infection Promotes Local Inflammation, Cell Adhesion Molecule Upregulation, and Leukocyte Recruitment at the Blood-Brain Barrier. mBio 2020, 11, e01183-20. [Google Scholar] [CrossRef]
- Ashraf, U.; Ding, Z.; Deng, S.; Ye, J.; Cao, S.; Chen, Z. Pathogenicity and Virulence of Japanese Encephalitis Virus: Neuroinflammation and Neuronal Cell Damage. Virulence 2021, 12, 968–980. [Google Scholar] [CrossRef]
- Meyding-Lamadé, U.; Craemer, E.; Schnitzler, P. Emerging and Re-Emerging Viruses Affecting the Nervous System. Neurol. Res. Pract. 2019, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Turtle, L.; Griffiths, M.J.; Solomon, T. Encephalitis Caused by Flaviviruses. QJM Mon. J. Assoc. Physicians 2012, 105, 219–223. [Google Scholar] [CrossRef]
- Salimi, H.; Cain, M.D.; Klein, R.S. Encephalitic Arboviruses: Emergence, Clinical Presentation, and Neuropathogenesis. Neurother. J. Am. Soc. Exp. Neurother. 2016, 13, 514–534. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R.; Muthukumaravel, S.; Jambulingam, P. The Involvement of Neuroinflammation in Dengue Viral Disease: Importance of Innate and Adaptive Immunity. Neuroimmunomodulation 2019, 26, 111–118. [Google Scholar] [CrossRef]
- Chaturvedi, U.C.; Dhawan, R.; Khanna, M.; Mathur, A. Breakdown of the Blood-Brain Barrier during Dengue Virus Infection of Mice. J. Gen. Virol. 1991, 72, 859–866. [Google Scholar] [CrossRef] [PubMed]
- McMinn, P.C. The Molecular Basis of Virulence of the Encephalitogenic Flaviviruses. J. Gen. Virol. 1997, 78, 2711–2722. [Google Scholar] [CrossRef]
- Ramos-Zaldívar, H.M.; Polakovicova, I.; Salas-Huenuleo, E.; Corvalán, A.H.; Kogan, M.J.; Yefi, C.P.; Andia, M.E. Extracellular Vesicles through the Blood-Brain Barrier: A Review. Fluids Barriers CNS 2022, 19, 60. [Google Scholar] [CrossRef]
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells 2020, 9, 851. [Google Scholar] [CrossRef]
- Ruan, J.; Miao, X.; Schlüter, D.; Lin, L.; Wang, X. Extracellular Vesicles in Neuroinflammation: Pathogenesis, Diagnosis, and Therapy. Mol. Ther. 2021, 29, 1946–1957. [Google Scholar] [CrossRef]
- Fikatas, A.; Dehairs, J.; Noppen, S.; Doijen, J.; Vanderhoydonc, F.; Meyen, E.; Swinnen, J.V.; Pannecouque, C.; Schols, D. Deciphering the Role of Extracellular Vesicles Derived from ZIKV-Infected hcMEC/D3 Cells on the Blood-Brain Barrier System. Viruses 2021, 13, 2363. [Google Scholar] [CrossRef]
- Zhou, W.; Woodson, M.; Sherman, M.B.; Neelakanta, G.; Sultana, H. Exosomes Mediate Zika Virus Transmission through SMPD3 Neutral Sphingomyelinase in Cortical Neurons. Emerg. Microbes Infect. 2019, 8, 307–326. [Google Scholar] [CrossRef]
- Chen, T.; Tu, S.; Ding, L.; Jin, M.; Chen, H.; Zhou, H. The Role of Autophagy in Viral Infections. J. Biomed. Sci. 2023, 30, 5. [Google Scholar] [CrossRef]
- Xing, H.; Tan, J.; Miao, Y.; Lv, Y.; Zhang, Q. Crosstalk between Exosomes and Autophagy: A Review of Molecular Mechanisms and Therapies. J. Cell. Mol. Med. 2021, 25, 2297–2308. [Google Scholar] [CrossRef] [PubMed]
- Ponpuak, M.; Mandell, M.; Kimura, T.; Chauhan, S.; Cleyrat, C.; Deretic, V. Secretory Autophagy. Curr. Opin. Cell Biol. 2015, 35, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Murrow, L.; Malhotra, R.; Debnath, J. ATG12-ATG3 Interacts with Alix to Promote Basal Autophagic Flux and Late Endosome Function. Nat. Cell Biol. 2015, 17, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Leidal, A.M.; Huang, H.H.; Marsh, T.; Solvik, T.; Zhang, D.; Ye, J.; Kai, F.; Goldsmith, J.; Liu, J.Y.; Huang, Y.-H.; et al. The LC3-Conjugation Machinery Specifies the Loading of RNA-Binding Proteins into Extracellular Vesicles. Nat. Cell Biol. 2020, 22, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Delorme-Axford, E.; Bayer, A.; Sadovsky, Y.; Coyne, C.B. Autophagy as a Mechanism of Antiviral Defense at the Maternal–Fetal Interface. Autophagy 2013, 9, 2173–2174. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.T. Autophagy as a Broad Antiviral at the Placental Interface. Autophagy 2013, 9, 1905–1907. [Google Scholar] [CrossRef]
- Zhang, S.; Yi, C.; Li, C.; Zhang, F.; Peng, J.; Wang, Q.; Liu, X.; Ye, X.; Li, P.; Wu, M.; et al. Chloroquine Inhibits Endosomal Viral RNA Release and Autophagy-Dependent Viral Replication and Effectively Prevents Maternal to Fetal Transmission of Zika Virus. Antivir. Res. 2019, 169, 104547. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Parnell, L.A.; Diamond, M.S.; Mysorekar, I.U. Inhibition of Autophagy Limits Vertical Transmission of Zika Virus in Pregnant Mice. J. Exp. Med. 2017, 214, 2303–2313. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, R.; Hildt, E.; Ploen, D. Look Who’s Talking-the Crosstalk between Oxidative Stress and Autophagy Supports Exosomal-Dependent Release of HCV Particles. Cell Biol. Toxicol. 2017, 33, 211–231. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Devhare, P.; Sujijantarat, N.; Steele, R.; Kwon, Y.-C.; Ray, R.; Ray, R.B. Knockdown of Autophagy Inhibits Infectious Hepatitis C Virus Release by the Exosomal Pathway. J. Virol. 2016, 90, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhu, B.; Fu, Z.F.; Chen, H.; Cao, S. Immune Evasion Strategies of Flaviviruses. Vaccine 2013, 31, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, Z.; Wang, M.; Cheng, A. Innate Immune Evasion Mediated by Flaviviridae Non-Structural Proteins. Viruses 2017, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Cai, W.; Cheng, A.; Wang, M.; Yin, Z.; Jia, R. Flaviviruses: Innate Immunity, Inflammasome Activation, Inflammatory Cell Death, and Cytokines. Front. Immunol. 2022, 13, 829433. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.F.; Voon, G.Z.; Lim, H.X.; Chua, M.L.; Poh, C.L. Innate and Adaptive Immune Evasion by Dengue Virus. Front. Cell. Infect. Microbiol. 2022, 12, 1004608. [Google Scholar] [CrossRef]
- Xie, X.; Zeng, J. Neuroimmune Evasion of Zika Virus to Facilitate Viral Pathogenesis. Front. Cell. Infect. Microbiol. 2021, 11, 662447. [Google Scholar] [CrossRef]
- Hu, H.; Feng, Y.; He, M.-L. Targeting Type I Interferon Induction and Signaling: How Zika Virus Escapes from Host Innate Immunity. Int. J. Biol. Sci. 2023, 19, 3015–3028. [Google Scholar] [CrossRef]
- Latanova, A.; Starodubova, E.; Karpov, V. Flaviviridae Nonstructural Proteins: The Role in Molecular Mechanisms of Triggering Inflammation. Viruses 2022, 14, 1808. [Google Scholar] [CrossRef]
- Santangelo, L.; Bordoni, V.; Montaldo, C.; Cimini, E.; Zingoni, A.; Battistelli, C.; D’Offizi, G.; Capobianchi, M.R.; Santoni, A.; Tripodi, M.; et al. Hepatitis C Virus Direct-Acting Antivirals Therapy Impacts on Extracellular Vesicles microRNAs Content and on Their Immunomodulating Properties. Liver Int. Off. J. Int. Assoc. Study Liver 2018, 38, 1741–1750. [Google Scholar] [CrossRef]
- Martins, S.d.T.; Kuczera, D.; Lötvall, J.; Bordignon, J.; Alves, L.R. Characterization of Dendritic Cell-Derived Extracellular Vesicles During Dengue Virus Infection. Front. Microbiol. 2018, 9, 1792. [Google Scholar] [CrossRef]
- Cobb, D.A.; Kim, O.-K.; Golden-Mason, L.; Rosen, H.R.; Hahn, Y.S. Hepatocyte-Derived Exosomes Promote T Follicular Regulatory Cell Expansion during Hepatitis C Virus Infection. Hepatology 2018, 67, 71–85. [Google Scholar] [CrossRef]
- Harwood, N.M.K.; Golden-Mason, L.; Cheng, L.; Rosen, H.R.; Mengshol, J.A. HCV-infected Cells and Differentiation Increase Monocyte Immunoregulatory Galectin-9 Production. J. Leukoc. Biol. 2016, 99, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Essandoh, K.; Yang, L.; Wang, X.; Huang, W.; Qin, D.; Hao, J.; Wang, Y.; Zingarelli, B.; Peng, T.; Fan, G.-C. Blockade of Exosome Generation with GW4869 Dampens the Sepsis-Induced Inflammation and Cardiac Dysfunction. Biochim. Biophys. Acta 2015, 1852, 2362–2371. [Google Scholar] [CrossRef] [PubMed]
- Macrophage-Derived Extracellular Vesicles: Diverse Mediators of Pathology and Therapeutics in Multiple Diseases|Cell Death & Disease. Available online: https://www.nature.com/articles/s41419-020-03127-z (accessed on 7 December 2023).
- Cai, C.; Koch, B.; Morikawa, K.; Suda, G.; Sakamoto, N.; Rueschenbaum, S.; Akhras, S.; Dietz, J.; Hildt, E.; Zeuzem, S.; et al. Macrophage-Derived Extracellular Vesicles Induce Long-Lasting Immunity Against Hepatitis C Virus Which Is Blunted by Polyunsaturated Fatty Acids. Front. Immunol. 2018, 9, 723. [Google Scholar] [CrossRef] [PubMed]
- Skokos, D.; Botros, H.G.; Demeure, C.; Morin, J.; Peronet, R.; Birkenmeier, G.; Boudaly, S.; Mécheri, S. Mast Cell-Derived Exosomes Induce Phenotypic and Functional Maturation of Dendritic Cells and Elicit Specific Immune Responses in Vivo. J. Immunol. 2003, 170, 3037–3045. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bréchard, S. Neutrophil Extracellular Vesicles: A Delicate Balance between Pro-Inflammatory Responses and Anti-Inflammatory Therapies. Cells 2022, 11, 3318. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, J.; Karlson, T.D.L.; Glader, P.; Telemo, E.; Valadi, H. Activated Human T Cells Secrete Exosomes That Participate in IL-2 Mediated Immune Response Signaling. PLoS ONE 2012, 7, e49723. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.D.; Wong, W.-Y.; Lee, M.M.-L.; Cho, W.C.-S.; Yee, B.K.; Kwan, Y.W.; Tai, W.C.-S. Exosomes in Inflammation and Inflammatory Disease. Proteomics 2019, 19, e1800149. [Google Scholar] [CrossRef]
- Basit, H.; Tyagi, I.; Koirala, J.; Hepatitis, C. StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hora, S.; Wuestefeld, T. Liver Injury and Regeneration: Current Understanding, New Approaches, and Future Perspectives. Cells 2023, 12, 2129. [Google Scholar] [CrossRef] [PubMed]
- Shojaie, L.; Iorga, A.; Dara, L. Cell Death in Liver Diseases: A Review. Int. J. Mol. Sci. 2020, 21, 9682. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Cheng, X.; Pan, X.; Li, J. Emerging Role of Exosomes in Liver Physiology and Pathology. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2017, 47, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Kodys, K.; Adejumo, A.; Szabo, G. Circulating and Exosome Packaged Single-Stranded Hepatitis C RNA Induce Monocyte Differentiation via TLR7/8 to Polarized Macrophages and Fibrocytes. J. Immunol. 2017, 198, 1974–1984. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, T.; Kimura, K.; Tokunaga, Y.; Tsukiyama-Kohara, K.; Tateno, C.; Hayashi, Y.; Hishima, T.; Kohara, M. M2 Macrophages Play Critical Roles in Progression of Inflammatory Liver Disease in Hepatitis C Virus Transgenic Mice. J. Virol. 2015, 90, 300–307. [Google Scholar] [CrossRef]
- Devhare, P.B.; Sasaki, R.; Shrivastava, S.; Di Bisceglie, A.M.; Ray, R.; Ray, R.B. Exosome-Mediated Intercellular Communication between Hepatitis C Virus-Infected Hepatocytes and Hepatic Stellate Cells. J. Virol. 2017, 91, e02225-16. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, C.H.; Lee, S.-W. Exosomal Transmission of MicroRNA from HCV Replicating Cells Stimulates Transdifferentiation in Hepatic Stellate Cells. Mol. Ther. Nucleic Acids 2019, 14, 483–497. [Google Scholar] [CrossRef]
- Liao, T.-L.; Chen, Y.-M.; Hsieh, S.-L.; Tang, K.-T.; Chen, D.-Y.; Yang, Y.-Y.; Liu, H.-J.; Yang, S.-S. Hepatitis C Virus-Induced Exosomal MicroRNAs and Toll-Like Receptor 7 Polymorphism Regulate B-Cell Activating Factor. mBio 2021, 12, e0276421. [Google Scholar] [CrossRef] [PubMed]
- Vitoria, W.O.; Thomé, L.S.; Kanashiro-Galo, L.; de Carvalho, L.V.; Penny, R.; Santos, W.L.C.; Vasconcelos, P.F.d.C.; Sotto, M.N.; Duarte, M.I.S.; Quaresma, J.A.S.; et al. Upregulation of Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1 in Renal Tissue in Severe Dengue in Humans: Effects on Endothelial Activation/Dysfunction. Rev. Soc. Bras. Med. Trop. 2019, 52, e20180353. [Google Scholar] [CrossRef]
- Vervaeke, P.; Vermeire, K.; Liekens, S. Endothelial Dysfunction in Dengue Virus Pathology. Rev. Med. Virol. 2015, 25, 50–67. [Google Scholar] [CrossRef]
- Basu, A.; Chaturvedi, U.C. Vascular Endothelium: The Battlefield of Dengue Viruses. Fems Immunol. Med. Microbiol. 2008, 53, 287–299. [Google Scholar] [CrossRef]
- Beatty, P.R.; Puerta-Guardo, H.; Killingbeck, S.S.; Glasner, D.R.; Hopkins, K.; Harris, E. Dengue Virus NS1 Triggers Endothelial Permeability and Vascular Leak That Is Prevented by NS1 Vaccination. Sci. Transl. Med. 2015, 7, 304ra141. [Google Scholar] [CrossRef] [PubMed]
- Fosse, J.H.; Haraldsen, G.; Falk, K.; Edelmann, R. Endothelial Cells in Emerging Viral Infections. Front. Cardiovasc. Med. 2021, 8, 619690. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Leon, M.; Lerman, L.O.; Lerman, A. Viral Endothelial Dysfunction: A Unifying Mechanism for COVID-19. Mayo Clin. Proc. 2021, 96, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Amraei, R.; Rahimi, N. COVID-19, Renin-Angiotensin System and Endothelial Dysfunction. Cells 2020, 9, 1652. [Google Scholar] [CrossRef] [PubMed]
- Mandel, J.; Casari, M.; Stepanyan, M.; Martyanov, A.; Deppermann, C. Beyond Hemostasis: Platelet Innate Immune Interactions and Thromboinflammation. Int. J. Mol. Sci. 2022, 23, 3868. [Google Scholar] [CrossRef]
- Buffolo, F.; Monticone, S.; Camussi, G.; Aikawa, E. Role of Extracellular Vesicles in the Pathogenesis of Vascular Damage. Hypertension 2022, 79, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Lata, S.; Ali, A.; Banerjea, A.C. Dengue Haemorrhagic Fever: A Job Done via Exosomes? Emerg. Microbes Infect. 2019, 8, 1626–1635. [Google Scholar] [CrossRef]
- Perez-Toledo, M.; Beristain-Covarrubias, N. A New Player in the Game: Platelet-Derived Extracellular Vesicles in Dengue Hemorrhagic Fever. Platelets 2020, 31, 412–414. [Google Scholar] [CrossRef]
- Sung, P.-S.; Hsieh, S.-L. CLEC2 and CLEC5A: Pathogenic Host Factors in Acute Viral Infections. Front. Immunol. 2019, 10, 2867. [Google Scholar] [CrossRef]
- Sung, P.-S.; Huang, T.-F.; Hsieh, S.-L. Extracellular Vesicles from CLEC2-Activated Platelets Enhance Dengue Virus-Induced Lethality via CLEC5A/TLR2. Nat. Commun. 2019, 10, 2402. [Google Scholar] [CrossRef]
- Srikiatkhachorn, A.; Mathew, A.; Rothman, A.L. Immune Mediated Cytokine Storm and Its Role in Severe Dengue. Semin. Immunopathol. 2017, 39, 563–574. [Google Scholar] [CrossRef]
- Anfasa, F.; Goeijenbier, M.; Widagdo, W.; Siegers, J.Y.; Mumtaz, N.; Okba, N.; van Riel, D.; Rockx, B.; Koopmans, M.P.G.; Meijers, J.C.M.; et al. Zika Virus Infection Induces Elevation of Tissue Factor Production and Apoptosis on Human Umbilical Vein Endothelial Cells. Front. Microbiol. 2019, 10, 817. [Google Scholar] [CrossRef]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef]
- Wang, K.; Zou, S.; Chen, H.; Higazy, D.; Gao, X.; Zhang, Y.; Cao, S.; Cui, M. Zika Virus Replication on Endothelial Cells and Invasion into the Central Nervous System by Inhibiting Interferon β Translation. Virology 2023, 582, 23–34. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; Glasner, D.R.; Espinosa, D.A.; Biering, S.B.; Patana, M.; Ratnasiri, K.; Wang, C.; Beatty, P.R.; Harris, E. Flavivirus NS1 Triggers Tissue-Specific Vascular Endothelial Dysfunction Reflecting Disease Tropism. Cell Rep. 2019, 26, 1598–1613.e8. [Google Scholar] [CrossRef]
- Rastogi, M.; Singh, S.K. Zika Virus NS1 Affects the Junctional Integrity of Human Brain Microvascular Endothelial Cells. Biochimie 2020, 176, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Lo, N.T.N.; Roodsari, S.Z.; Tin, N.L.; Wong, M.P.; Biering, S.B.; Harris, E. Molecular Determinants of Tissue Specificity of Flavivirus Nonstructural Protein 1 Interaction with Endothelial Cells. J. Virol. 2022, 96, e0066122. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Akbar, I.; Kumari, B.; Vrati, S.; Basu, A.; Banerjee, A. Japanese Encephalitis Virus-Induced Let-7a/b Interacted with the NOTCH-TLR7 Pathway in Microglia and Facilitated Neuronal Death via Caspase Activation. J. Neurochem. 2019, 149, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Lahon, A.; Banerjea, A.C. Dengue Virus Degrades USP33-ATF3 Axis via Extracellular Vesicles to Activate Human Microglial Cells. J. Immunol. 2020, 205, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Mezzasoma, L.; Bellezza, I.; Orvietani, P.; Manni, G.; Gargaro, M.; Sagini, K.; Llorente, A.; Scarpelli, P.; Pascucci, L.; Cellini, B.; et al. Amniotic Fluid Stem Cell-Derived Extracellular Vesicles Are Independent Metabolic Units Capable of Modulating Inflammasome Activation in THP-1 Cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22218. [Google Scholar] [CrossRef]
- Välimäki, E.; Miettinen, J.J.; Lietzén, N.; Matikainen, S.; Nyman, T.A. Monosodium Urate Activates Src/Pyk2/PI3 Kinase and Cathepsin Dependent Unconventional Protein Secretion From Human Primary Macrophages. Mol. Cell. Proteom. 2013, 12, 749–763. [Google Scholar] [CrossRef]
- Si, X.-L.; Fang, Y.-J.; Li, L.-F.; Gu, L.-Y.; Yin, X.-Z.; Jun-Tian; Yan, Y.-P.; Pu, J.-L.; Zhang, B.-R. From Inflammasome to Parkinson’s Disease: Does the NLRP3 Inflammasome Facilitate Exosome Secretion and Exosomal Alpha-Synuclein Transmission in Parkinson’s Disease? Exp. Neurol. 2021, 336, 113525. [Google Scholar] [CrossRef]
- Välimäki, E.; Cypryk, W.; Virkanen, J.; Nurmi, K.; Turunen, P.M.; Eklund, K.K.; Åkerman, K.E.; Nyman, T.A.; Matikainen, S. Calpain Activity Is Essential for ATP-Driven Unconventional Vesicle-Mediated Protein Secretion and Inflammasome Activation in Human Macrophages. J. Immunol. 2016, 197, 3315–3325. [Google Scholar] [CrossRef]
- Cypryk, W.; Czernek, L.; Horodecka, K.; Chrzanowski, J.; Stańczak, M.; Nurmi, K.; Bilicka, M.; Gadzinowski, M.; Walczak-Drzewiecka, A.; Stensland, M.; et al. Lipopolysaccharide Primes Human Macrophages for Noncanonical Inflammasome-Induced Extracellular Vesicle Secretion. J. Immunol. 2023, 210, 322–334. [Google Scholar] [CrossRef]
- Budden, C.F.; Gearing, L.J.; Kaiser, R.; Standke, L.; Hertzog, P.J.; Latz, E. Inflammasome-Induced Extracellular Vesicles Harbour Distinct RNA Signatures and Alter Bystander Macrophage Responses. J. Extracell. Vesicles 2021, 10, e12127. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.; Yuan, Y.; Jin, C.; Chang, C.; Zhu, Y.; Zhang, X.; Tian, C.; He, F.; Wang, J. Inflammasome-Derived Exosomes Activate NF-κB Signaling in Macrophages. J. Proteome Res. 2017, 16, 170–178. [Google Scholar] [CrossRef]
- Negash, A.A.; Ramos, H.J.; Crochet, N.; Lau, D.T.Y.; Doehle, B.; Papic, N.; Delker, D.A.; Jo, J.; Bertoletti, A.; Hagedorn, C.H.; et al. IL-1β Production through the NLRP3 Inflammasome by Hepatic Macrophages Links Hepatitis C Virus Infection with Liver Inflammation and Disease. PLoS Pathog. 2013, 9, e1003330. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Y.; Li, H.; Tao, W.; Xiang, Y.; Huang, B.; Niu, J.; Zhong, J.; Meng, G. HCV Genomic RNA Activates the NLRP3 Inflammasome in Human Myeloid Cells. PLoS ONE 2014, 9, e84953. [Google Scholar] [CrossRef]
- Hottz, E.D.; Lopes, J.F.; Freitas, C.; Valls-de-Souza, R.; Oliveira, M.F.; Bozza, M.T.; Da Poian, A.T.; Weyrich, A.S.; Zimmerman, G.A.; Bozza, F.A.; et al. Platelets Mediate Increased Endothelium Permeability in Dengue through NLRP3-Inflammasome Activation. Blood 2013, 122, 3405–3414. [Google Scholar] [CrossRef]
- Lien, T.-S.; Sun, D.-S.; Chang, C.-M.; Wu, C.-Y.; Dai, M.-S.; Chan, H.; Wu, W.-S.; Su, S.-H.; Lin, Y.-Y.; Chang, H.-H. Dengue Virus and Antiplatelet Autoantibodies Synergistically Induce Haemorrhage through Nlrp3-Inflammasome and FcγRIII. Thromb. Haemost. 2015, 113, 1060–1070. [Google Scholar] [CrossRef]
- He, Z.; Chen, J.; Zhu, X.; An, S.; Dong, X.; Yu, J.; Zhang, S.; Wu, Y.; Li, G.; Zhang, Y.; et al. NLRP3 Inflammasome Activation Mediates Zika Virus-Associated Inflammation. J. Infect. Dis. 2018, 217, 1942–1951. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, G.; Wu, D.; Luo, Z.; Pan, P.; Tian, M.; Wang, Y.; Xiao, F.; Li, A.; Wu, K.; et al. Zika Virus Infection Induces Host Inflammatory Responses by Facilitating NLRP3 Inflammasome Assembly and Interleukin-1β Secretion. Nat. Commun. 2018, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.J.; Lanteri, M.C.; Blahnik, G.; Negash, A.; Suthar, M.S.; Brassil, M.M.; Sodhi, K.; Treuting, P.M.; Busch, M.P.; Norris, P.J.; et al. IL-1β Signaling Promotes CNS-Intrinsic Immune Control of West Nile Virus Infection. PLoS Pathog. 2012, 8, e1003039. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.K.; Gupta, M.; Kumawat, K.L.; Basu, A. NLRP3 Inflammasome: Key Mediator of Neuroinflammation in Murine Japanese Encephalitis. PLoS ONE 2012, 7, e32270. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.; Afroz, S.; Minhas, G.; Battu, S.; Khan, N. Dengue Virus Envelope Protein Domain III Induces Pro-Inflammatory Signature and Triggers Activation of Inflammasome. Cytokine 2019, 123, 154780. [Google Scholar] [CrossRef] [PubMed]
- Lien, T.-S.; Sun, D.-S.; Wu, C.-Y.; Chang, H.-H. Exposure to Dengue Envelope Protein Domain III Induces Nlrp3 Inflammasome-Dependent Endothelial Dysfunction and Hemorrhage in Mice. Front. Immunol. 2021, 12, 617251. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, G.; Visoso-Carvajal, G.; Garcia-Cordero, J.; Leon-Juarez, M.; Chavez-Munguia, B.; Lopez, T.; Nava, P.; Villegas-Sepulveda, N.; Cedillo-Barron, L. Dengue Virus Serotype 2 and Its Non-Structural Proteins 2A and 2B Activate NLRP3 Inflammasome. Front. Immunol. 2020, 11, 352. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Q.; Wu, Y.; Ma, L.; Zhang, Z.; Liu, T.; Jin, S.; She, Y.; Li, Y.-P.; Cui, J. Zika Virus Elicits Inflammation to Evade Antiviral Response by Cleaving cGAS via NS1-Caspase-1 Axis. EMBO J. 2018, 37, e99347. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent Advances in the Mechanisms of NLRP3 Inflammasome Activation and Its Inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef]
- Lu, A.; Li, Y.; Schmidt, F.I.; Yin, Q.; Chen, S.; Fu, T.-M.; Tong, A.B.; Ploegh, H.L.; Mao, Y.; Wu, H. Molecular Basis of Caspase-1 Polymerization and Its Inhibition by a New Capping Mechanism. Nat. Struct. Mol. Biol. 2016, 23, 416–425. [Google Scholar] [CrossRef]
- Gram, A.M.; Frenkel, J.; Ressing, M.E. Inflammasomes and Viruses: Cellular Defence versus Viral Offence. J. Gen. Virol. 2012, 93, 2063–2075. [Google Scholar] [CrossRef]
- Cai, X.; Chen, J.; Xu, H.; Liu, S.; Jiang, Q.-X.; Halfmann, R.; Chen, Z.J. Prion-like Polymerization Underlies Signal Transduction in Antiviral Immune Defense and Inflammasome Activation. Cell 2014, 156, 1207–1222. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of Assembly, Regulation and Signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 Inflammasome in Inflammatory Diseases. Nat. Rev. Drug Discov. 2018, 17, 688. [Google Scholar] [CrossRef]
- Rathinam, V.A.K.; Fitzgerald, K.A. Inflammasome Complexes: Emerging Mechanisms and Effector Functions. Cell 2016, 165, 792. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by Inflammatory Caspases Determines Pyroptotic Cell Death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- He, W.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.-H.; Zhong, C.-Q.; Han, J. Gasdermin D Is an Executor of Pyroptosis and Required for Interleukin-1β Secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Novick, D.; Kim, S.; Kaplanski, G. Interleukin-18 and IL-18 Binding Protein. Front. Immunol. 2013, 4, 289. [Google Scholar] [CrossRef]
- Joosten, L.A.B.; Netea, M.G.; Dinarello, C.A. Interleukin-1β in Innate Inflammation, Autophagy and Immunity. Semin. Immunol. 2013, 25, 416–424. [Google Scholar] [CrossRef]
- Wozniak, A.L.; Adams, A.; King, K.E.; Dunn, W.; Christenson, L.K.; Hung, W.-T.; Weinman, S.A. The RNA Binding Protein FMR1 Controls Selective Exosomal miRNA Cargo Loading during Inflammation. J. Cell Biol. 2020, 219, e201912074. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, Y.; Liang, C.; Liu, B.; Ding, F.; Wang, Y.; Zhu, B.; Zhao, R.; Yu, X.-Y.; Li, Y. Stem Cell-Derived Exosomes Prevent Pyroptosis and Repair Ischemic Muscle Injury through a Novel Exosome/circHIPK3/FOXO3a Pathway. Theranostics 2020, 10, 6728–6742. [Google Scholar] [CrossRef]
- Wu, M.-F.; Chen, S.-T.; Yang, A.-H.; Lin, W.-W.; Lin, Y.-L.; Chen, N.-J.; Tsai, I.-S.; Li, L.; Hsieh, S.-L. CLEC5A Is Critical for Dengue Virus-Induced Inflammasome Activation in Human Macrophages. Blood 2013, 121, 95–106. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Sun, L.; Zhou, L.; Ma, T.-C.; Song, L.; Wu, J.-G.; Li, J.-L.; Ho, W.-Z. Toll-like Receptor 3-Activated Macrophages Confer Anti-HCV Activity to Hepatocytes through Exosomes. FASEB J. 2016, 30, 4132–4140. [Google Scholar] [CrossRef]
- Giugliano, S.; Kriss, M.; Golden-Mason, L.; Dobrinskikh, E.; Stone, A.E.L.; Soto-Gutierrez, A.; Mitchell, A.; Khetani, S.R.; Yamane, D.; Stoddard, M.; et al. Hepatitis C Virus Infection Induces Autocrine Interferon Signaling by Human Liver Endothelial Cells and Release of Exosomes, Which Inhibits Viral Replication. Gastroenterology 2015, 148, 392–402.e13. [Google Scholar] [CrossRef]
- Qian, X.; Xu, C.; Fang, S.; Zhao, P.; Wang, Y.; Liu, H.; Yuan, W.; Qi, Z. Exosomal MicroRNAs Derived From Umbilical Mesenchymal Stem Cells Inhibit Hepatitis C Virus Infection. Stem Cells Transl. Med. 2016, 5, 1190–1203. [Google Scholar] [CrossRef]
- Dreux, M.; Garaigorta, U.; Boyd, B.; Décembre, E.; Chung, J.; Whitten-Bauer, C.; Wieland, S.; Chisari, F.V. Short-Range Exosomal Transfer of Viral RNA from Infected Cells to Plasmacytoid Dendritic Cells Triggers Innate Immunity. Cell Host Microbe 2012, 12, 558–570. [Google Scholar] [CrossRef]
- Zhu, X.; He, Z.; Yuan, J.; Wen, W.; Huang, X.; Hu, Y.; Lin, C.; Pan, J.; Li, R.; Deng, H.; et al. IFITM3-Containing Exosome as a Novel Mediator for Anti-Viral Response in Dengue Virus Infection. Cell. Microbiol. 2015, 17, 105–118. [Google Scholar] [CrossRef]
- Slonchak, A.; Clarke, B.; Mackenzie, J.; Amarilla, A.A.; Setoh, Y.X.; Khromykh, A.A. West Nile Virus Infection and Interferon Alpha Treatment Alter the Spectrum and the Levels of Coding and Noncoding Host RNAs Secreted in Extracellular Vesicles. BMC Genom. 2019, 20, 474. [Google Scholar] [CrossRef]
- Conzelmann, C.; Groß, R.; Zou, M.; Krüger, F.; Görgens, A.; Gustafsson, M.O.; El Andaloussi, S.; Münch, J.; Müller, J.A. Salivary Extracellular Vesicles Inhibit Zika Virus but Not SARS-CoV-2 Infection. J. Extracell. Vesicles 2020, 9, 1808281. [Google Scholar] [CrossRef]
- Roche, P.A.; Furuta, K. The Ins and Outs of MHC Class II-Mediated Antigen Processing and Presentation. Nat. Rev. Immunol. 2015, 15, 203–216. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B Lymphocytes Secrete Antigen-Presenting Vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Wheway, J.; Latham, S.L.; Combes, V.; Grau, G.E. Endothelial Microparticles Interact with and Support the Proliferation of T Cells. J. Immunol. 2014, 193, 3378–3387. [Google Scholar] [CrossRef]
- Théry, C.; Duban, L.; Segura, E.; Véron, P.; Lantz, O.; Amigorena, S. Indirect Activation of Naïve CD4+ T Cells by Dendritic Cell-Derived Exosomes. Nat. Immunol. 2002, 3, 1156–1162. [Google Scholar] [CrossRef]
- Muntasell, A.; Berger, A.C.; Roche, P.A. T Cell-Induced Secretion of MHC Class II–Peptide Complexes on B Cell Exosomes. EMBO J. 2007, 26, 4263–4272. [Google Scholar] [CrossRef]
- Tkach, M.; Kowal, J.; Zucchetti, A.E.; Enserink, L.; Jouve, M.; Lankar, D.; Saitakis, M.; Martin-Jaular, L.; Théry, C. Qualitative Differences in T-Cell Activation by Dendritic Cell-Derived Extracellular Vesicle Subtypes. EMBO J. 2017, 36, 3012–3028. [Google Scholar] [CrossRef]
- Wolfers, J.; Lozier, A.; Raposo, G.; Regnault, A.; Théry, C.; Masurier, C.; Flament, C.; Pouzieux, S.; Faure, F.; Tursz, T.; et al. Tumor-Derived Exosomes Are a Source of Shared Tumor Rejection Antigens for CTL Cross-Priming. Nat. Med. 2001, 7, 297–303. [Google Scholar] [CrossRef]
- Walker, J.D.; Maier, C.L.; Pober, J.S. Cytomegalovirus-Infected Human Endothelial Cells Can Stimulate Allogeneic CD4+ Memory T Cells by Releasing Antigenic Exosomes. J. Immunol. 2009, 182, 1548–1559. [Google Scholar] [CrossRef]
- Testa, J.S.; Apcher, G.S.; Comber, J.D.; Eisenlohr, L.C. Exosome-Driven Antigen Transfer for MHC Class II Presentation Facilitated by the Receptor Binding Activity of Influenza Hemagglutinin. J. Immunol. 2010, 185, 6608–6616. [Google Scholar] [CrossRef]
- Ghosh, S.; Bhowmik, S.; Majumdar, S.; Goswami, A.; Chakraborty, J.; Gupta, S.; Aggarwal, S.; Ray, S.; Chatterjee, R.; Bhattacharyya, S.; et al. The Exosome Encapsulated microRNAs as Circulating Diagnostic Marker for Hepatocellular Carcinoma with Low Alpha-Fetoprotein. Int. J. Cancer 2020, 147, 2934–2947. [Google Scholar] [CrossRef]
- Wang, S.-C.; Li, C.-Y.; Chang, W.-T.; Cheng, W.-C.; Yen, C.-H.; Tu, W.-Y.; Lin, Z.-Y.; Lin, C.-C.; Yeh, M.-L.; Huang, C.-F.; et al. Exosome-Derived Differentiation Antagonizing Non-Protein Coding RNA with Risk of Hepatitis C Virus-Related Hepatocellular Carcinoma Recurrence. Liver Int. Off. J. Int. Assoc. Study Liver 2021, 41, 956–968. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, X.; Qi, Q.; Gao, Y.; Wei, Q.; Han, S. lncRNA-HEIH in Serum and Exosomes as a Potential Biomarker in the HCV-Related Hepatocellular Carcinoma. Cancer Biomark. Sect. Dis. Markers 2018, 21, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Silveira, G.F.; Meyer, F.; Delfraro, A.; Mosimann, A.L.P.; Coluchi, N.; Vasquez, C.; Probst, C.M.; Báfica, A.; Bordignon, J.; Dos Santos, C.N.D. Dengue Virus Type 3 Isolated from a Fatal Case with Visceral Complications Induces Enhanced Proinflammatory Responses and Apoptosis of Human Dendritic Cells. J. Virol. 2011, 85, 5374–5383. [Google Scholar] [CrossRef] [PubMed]
- Block, L.N.; Schmidt, J.K.; Keuler, N.S.; McKeon, M.C.; Bowman, B.D.; Wiepz, G.J.; Golos, T.G. Zika Virus Impacts Extracellular Vesicle Composition and Cellular Gene Expression in Macaque Early Gestation Trophoblasts. Sci. Rep. 2022, 12, 7348. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Fan, Z.; Chen, H.; He, P.; Li, Y.; Zhang, Q.; Ke, C. Serum and Exosomal miR-122 and miR-199a as a Biomarker to Predict Therapeutic Efficacy of Hepatitis C Patients. J. Med. Virol. 2017, 89, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Song, X.; Yang, F.; Wu, S.; Wang, J.; Chen, Z.; Liu, Y. Exosomes Derived from miR-122-Modified Adipose Tissue-Derived MSCs Increase Chemosensitivity of Hepatocellular Carcinoma. J. Hematol. Oncol. 2015, 8, 122. [Google Scholar] [CrossRef]
- Zou, X.; Yuan, M.; Zhang, T.; Zheng, N.; Wu, Z. EVs Containing Host Restriction Factor IFITM3 Inhibited ZIKV Infection of Fetuses in Pregnant Mice through Trans-Placenta Delivery. Mol. Ther. 2021, 29, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Almeida, F. Exosome-Based Vaccines: History, Current State, and Clinical Trials. Front. Immunol. 2021, 12, 711565. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.J.; Atai, N.A.; Cacciottolo, M.; Nice, J.; Salehi, A.; Guo, C.; Sedgwick, A.; Kanagavelu, S.; Gould, S.J. Exosome-Mediated mRNA Delivery in Vivo Is Safe and Can Be Used to Induce SARS-CoV-2 Immunity. J. Biol. Chem. 2021, 297, 101266. [Google Scholar] [CrossRef]
- Hu, K.; McKay, P.F.; Samnuan, K.; Najer, A.; Blakney, A.K.; Che, J.; O’Driscoll, G.; Cihova, M.; Stevens, M.M.; Shattock, R.J. Presentation of Antigen on Extracellular Vesicles Using Transmembrane Domains from Viral Glycoproteins for Enhanced Immunogenicity. J. Extracell. Vesicles 2022, 11, e12199. [Google Scholar] [CrossRef]
- Anticoli, S.; Manfredi, F.; Chiozzini, C.; Arenaccio, C.; Olivetta, E.; Ferrantelli, F.; Capocefalo, A.; Falcone, E.; Ruggieri, A.; Federico, M. An Exosome-Based Vaccine Platform Imparts Cytotoxic T Lymphocyte Immunity Against Viral Antigens. Biotechnol. J. 2018, 13, e1700443. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Neelakanta, G.; Sultana, H. Tetraspanins as Potential Therapeutic Candidates for Targeting Flaviviruses. Front. Immunol. 2021, 12, 630571. [Google Scholar] [CrossRef] [PubMed]
| Virus | Components | Links |
|---|---|---|
| HCV | Viral RNA | [44,45,46,47,48] |
| E protein (inside) | [45] | |
| E protein (surface) | [49,50] | |
| Viral particles | [45,51] | |
| DENV | Viral RNA | [36,52,53] |
| E protein (inside) | [36,40,52] | |
| E protein (surface) | [54] | |
| prM/M protein (inside) | [52] | |
| NS1 protein (inside) | [52] | |
| NS1 protein (surface) | [54,55] | |
| NS3 protein (inside) | [56] | |
| ZIKV | Viral RNA | [41,42] |
| E protein (inside) | [41] | |
| E protein (surface) | [41,57] | |
| NS1 protein (surface) | [55] | |
| WNV | Viral RNA | [37] |
| Langat virus (LGTV) | Viral RNA | [37] |
| E protein (inside) | [37] | |
| NS1 protein (inside) | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latanova, A.; Karpov, V.; Starodubova, E. Extracellular Vesicles in Flaviviridae Pathogenesis: Their Roles in Viral Transmission, Immune Evasion, and Inflammation. Int. J. Mol. Sci. 2024, 25, 2144. https://doi.org/10.3390/ijms25042144
Latanova A, Karpov V, Starodubova E. Extracellular Vesicles in Flaviviridae Pathogenesis: Their Roles in Viral Transmission, Immune Evasion, and Inflammation. International Journal of Molecular Sciences. 2024; 25(4):2144. https://doi.org/10.3390/ijms25042144
Chicago/Turabian StyleLatanova, Anastasia, Vadim Karpov, and Elizaveta Starodubova. 2024. "Extracellular Vesicles in Flaviviridae Pathogenesis: Their Roles in Viral Transmission, Immune Evasion, and Inflammation" International Journal of Molecular Sciences 25, no. 4: 2144. https://doi.org/10.3390/ijms25042144
APA StyleLatanova, A., Karpov, V., & Starodubova, E. (2024). Extracellular Vesicles in Flaviviridae Pathogenesis: Their Roles in Viral Transmission, Immune Evasion, and Inflammation. International Journal of Molecular Sciences, 25(4), 2144. https://doi.org/10.3390/ijms25042144







