Eugenol Suppresses Platelet Activation and Mitigates Pulmonary Thromboembolism in Humans and Murine Models
Abstract
1. Introduction
2. Results
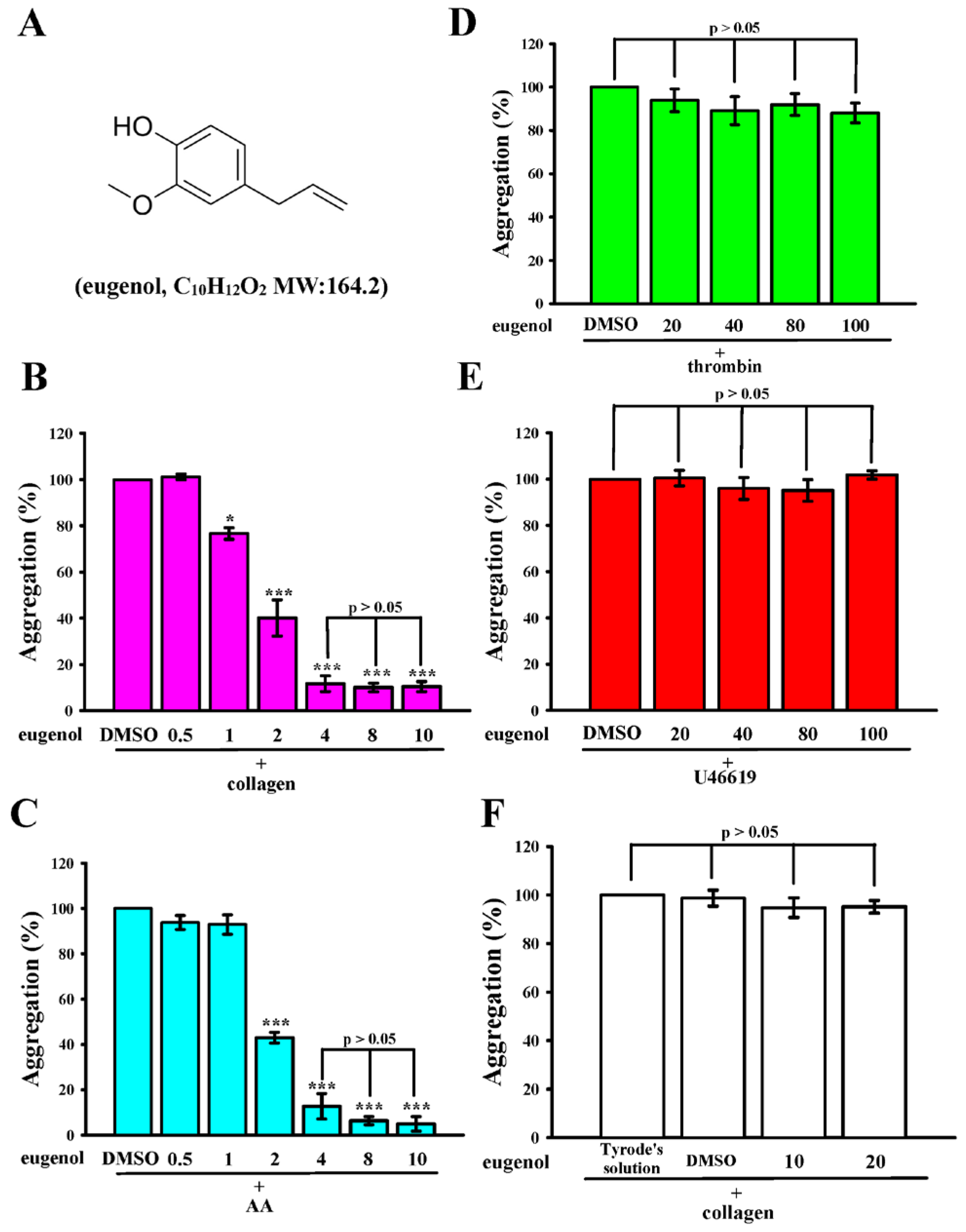
2.1. Eugenol Inhibits Collagen and AA-Induced Platelet Aggregation in Humans
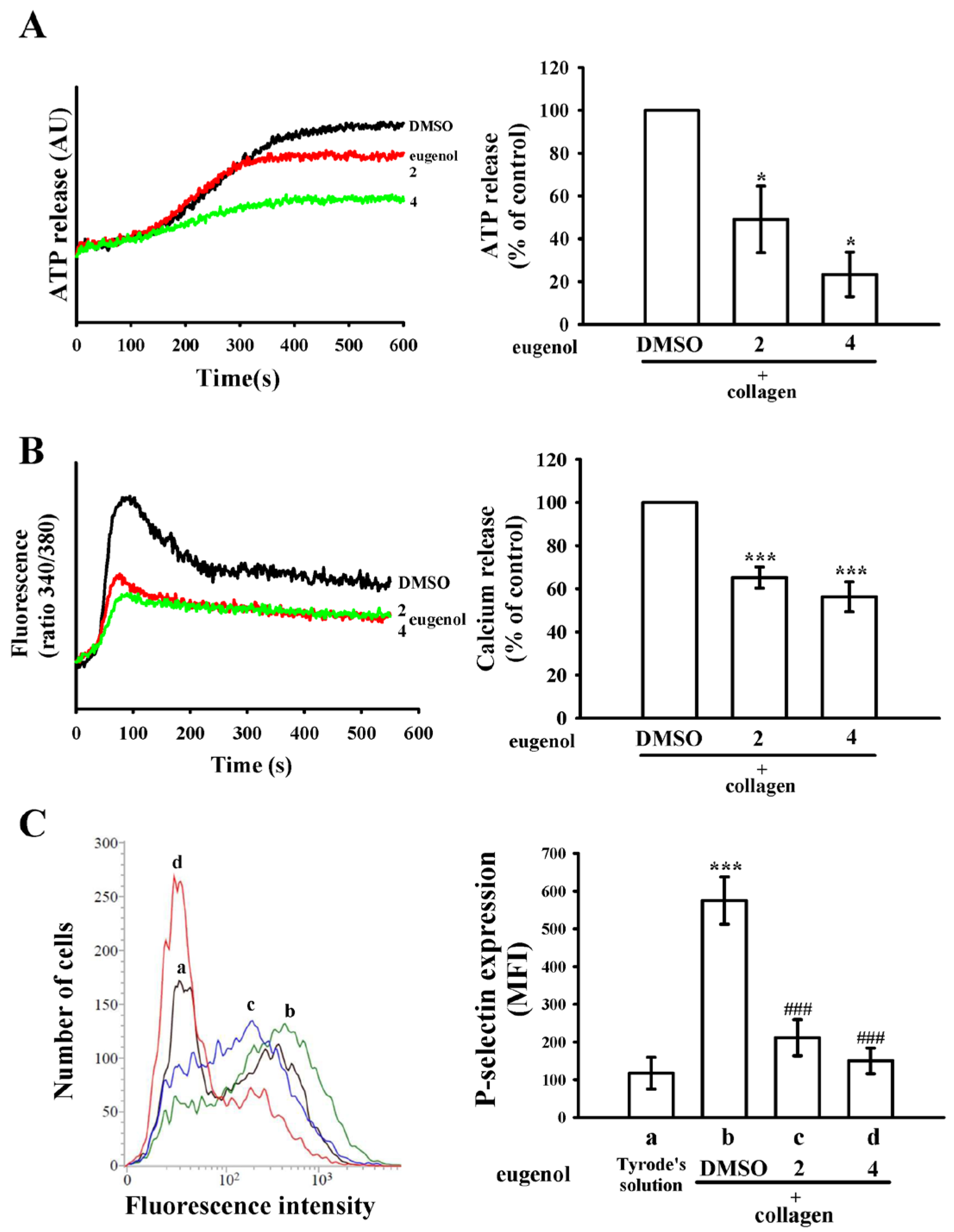
2.2. Eugenol Modulates ATP Release, [Ca2+]i Levels, and P-Selectin Surface Expression
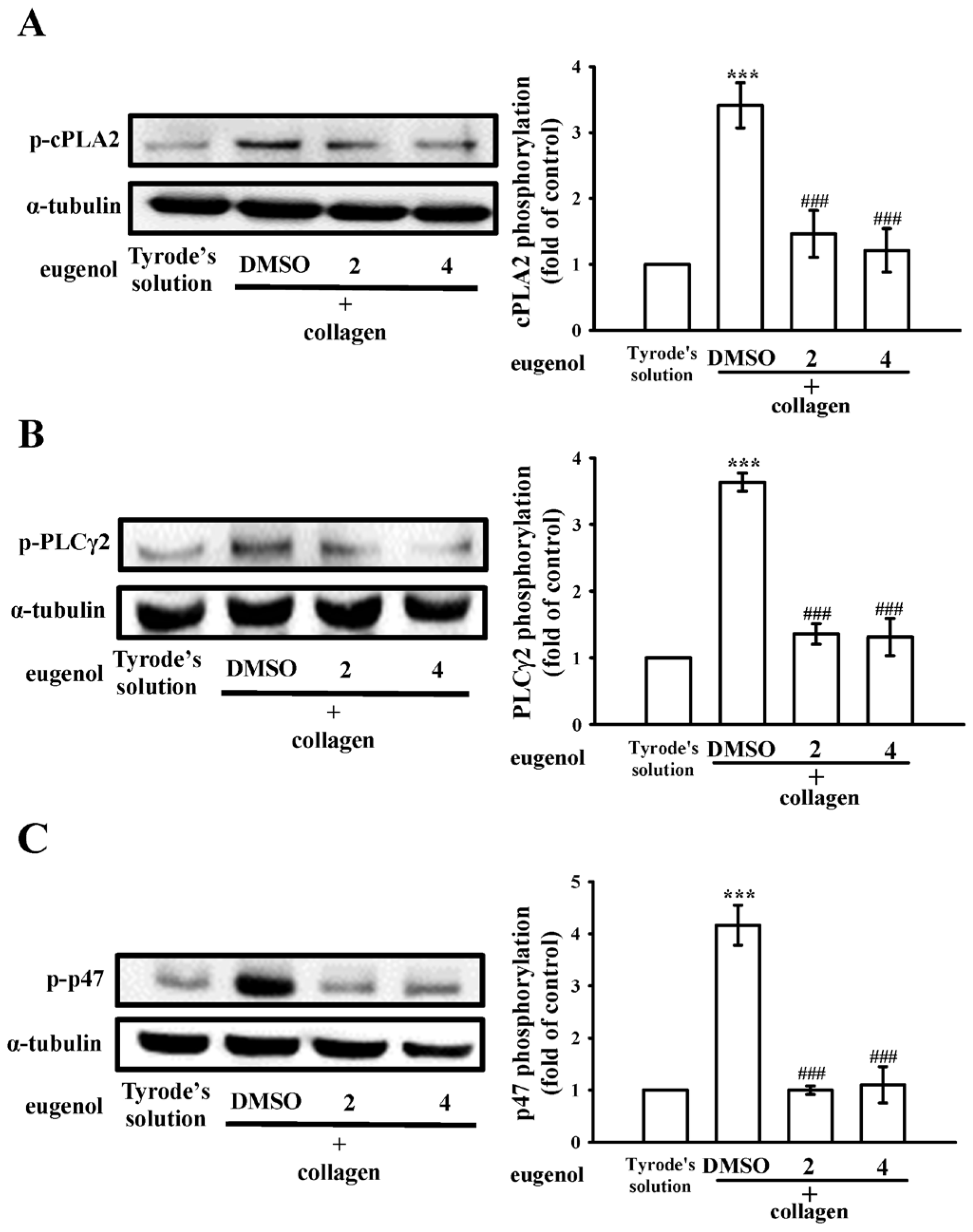
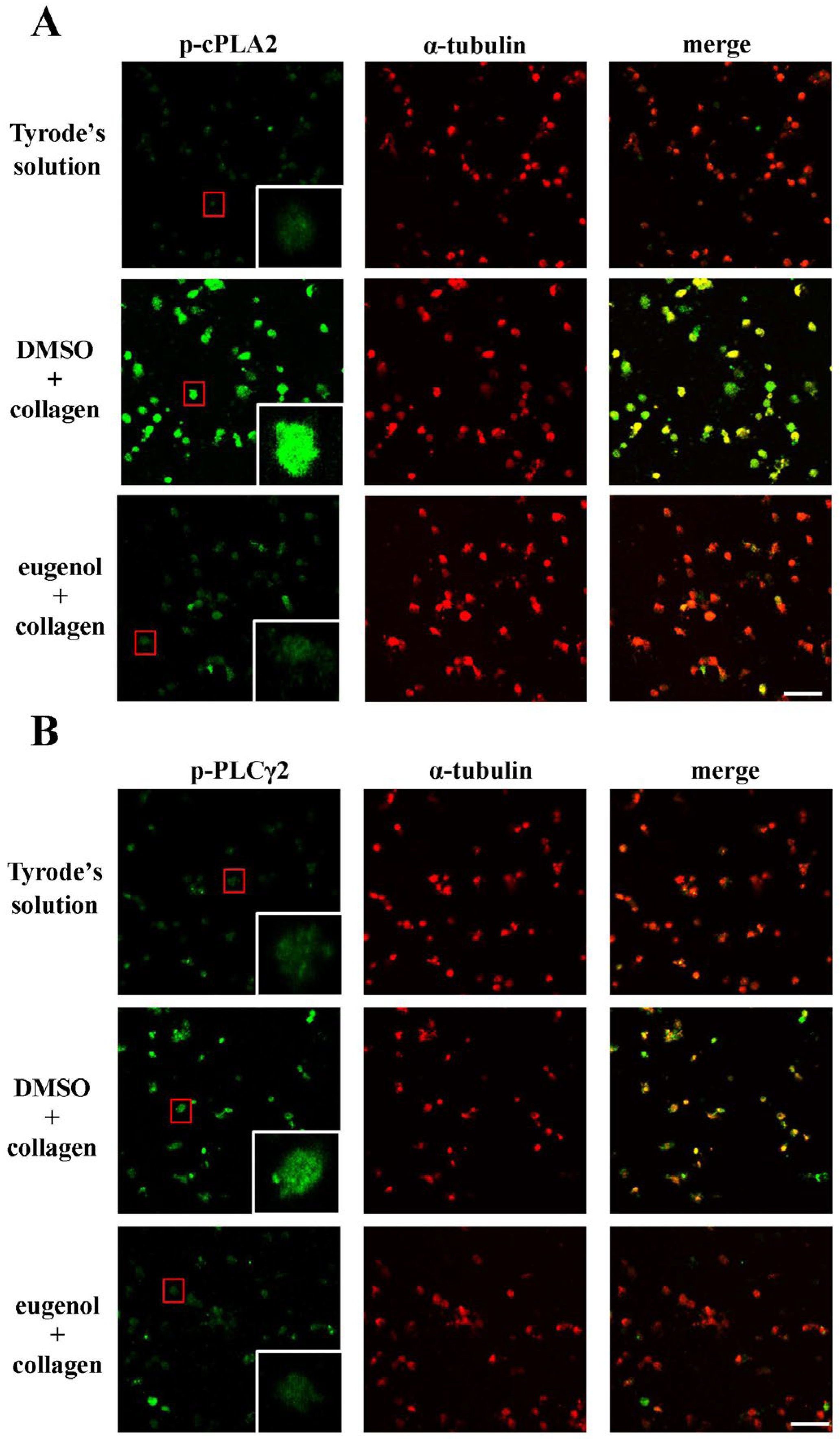
2.3. Evaluating Eugenol’s Impact on Platelet cPLA2, PLCγ2 Phosphorylation, and PKC Activation
2.4. Regulatory Effects of Eugenol on PI3K-Akt-GSK3β and MAPKs Activation
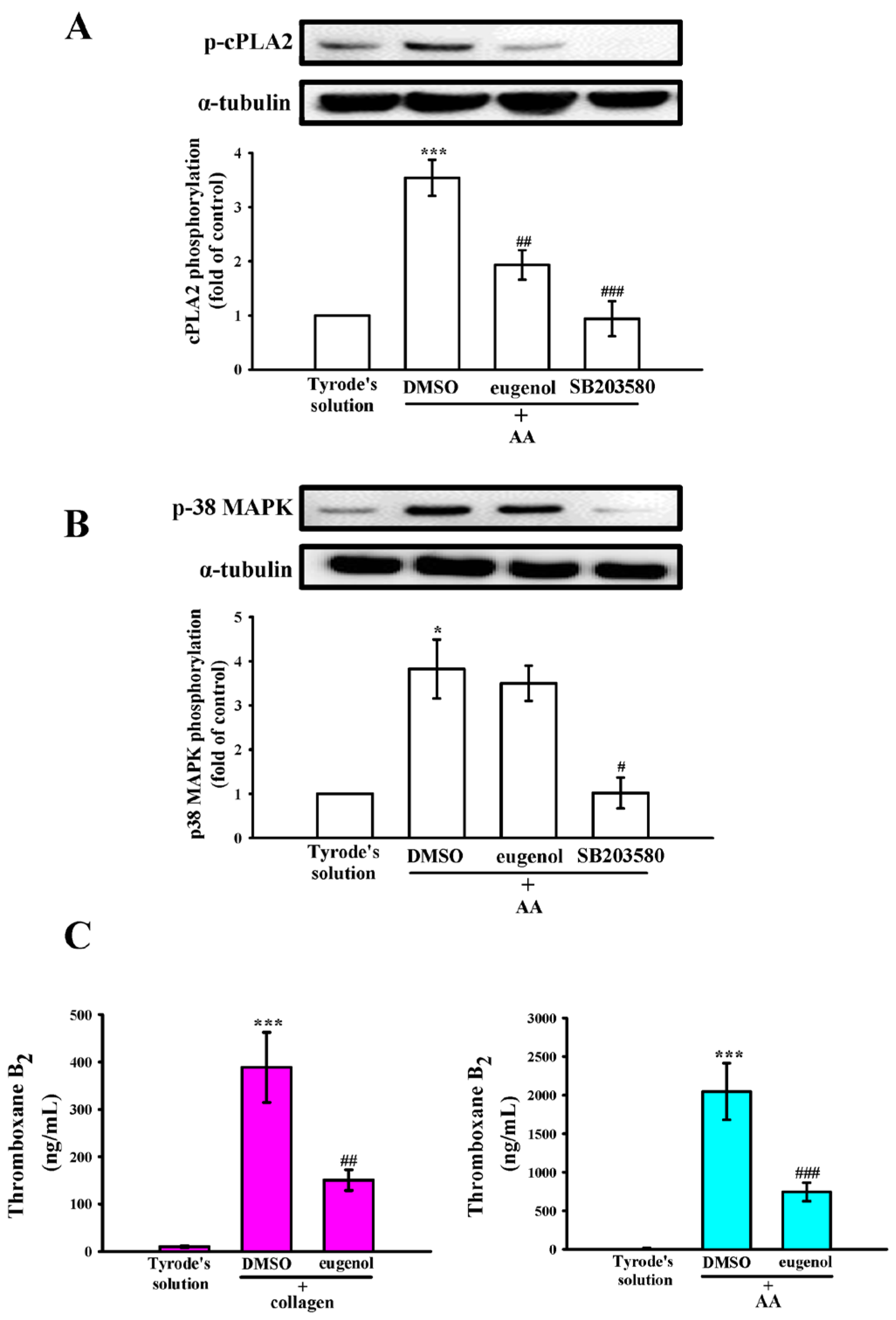
2.5. Interplay of p38 MAPK, cPLA2, and TxA2 in Eugenol’s Antiplatelet Mechanism
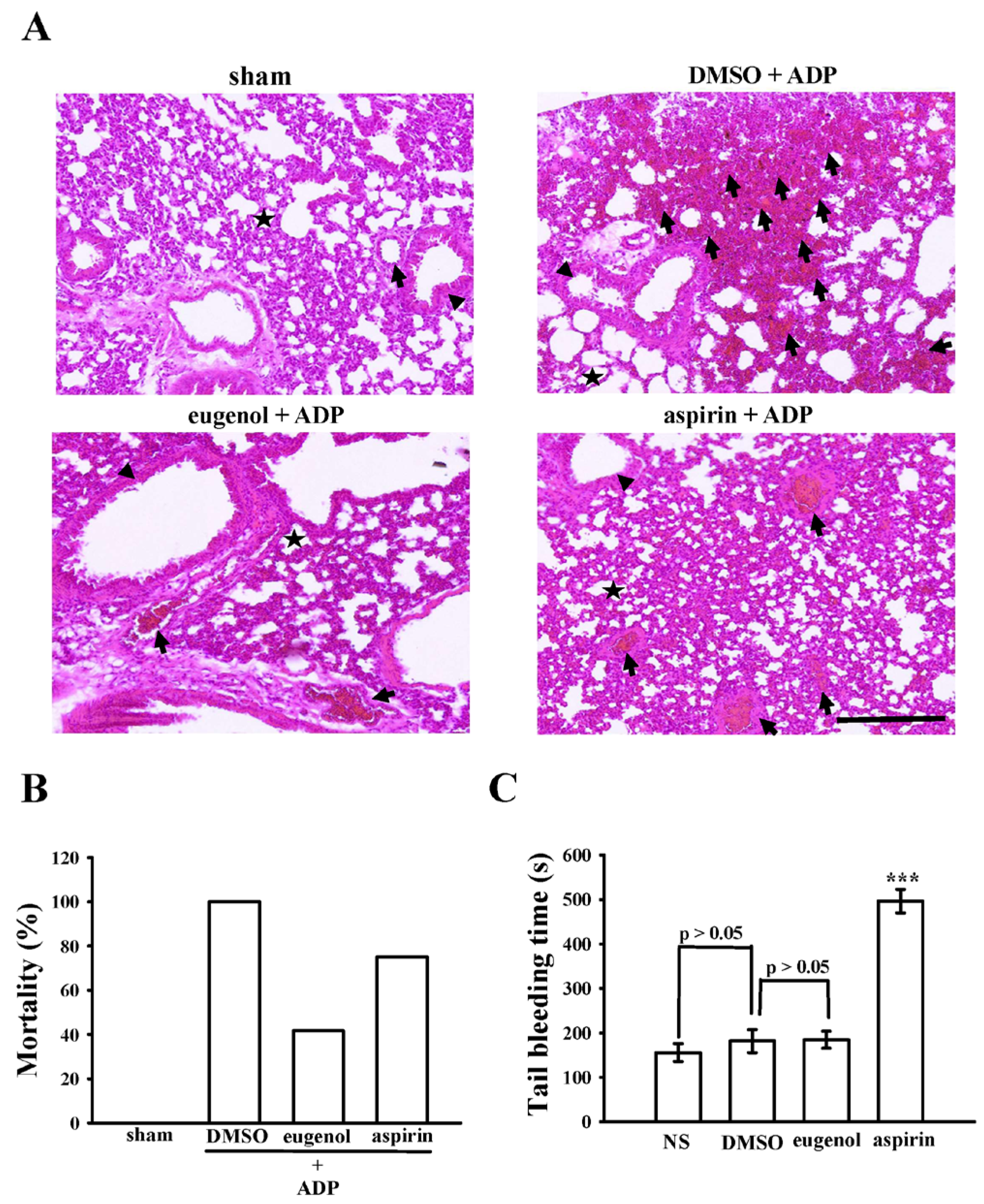
2.6. Anti-Thrombotic Efficacy of Eugenol in Acute Pulmonary Thromboembolism in Mice
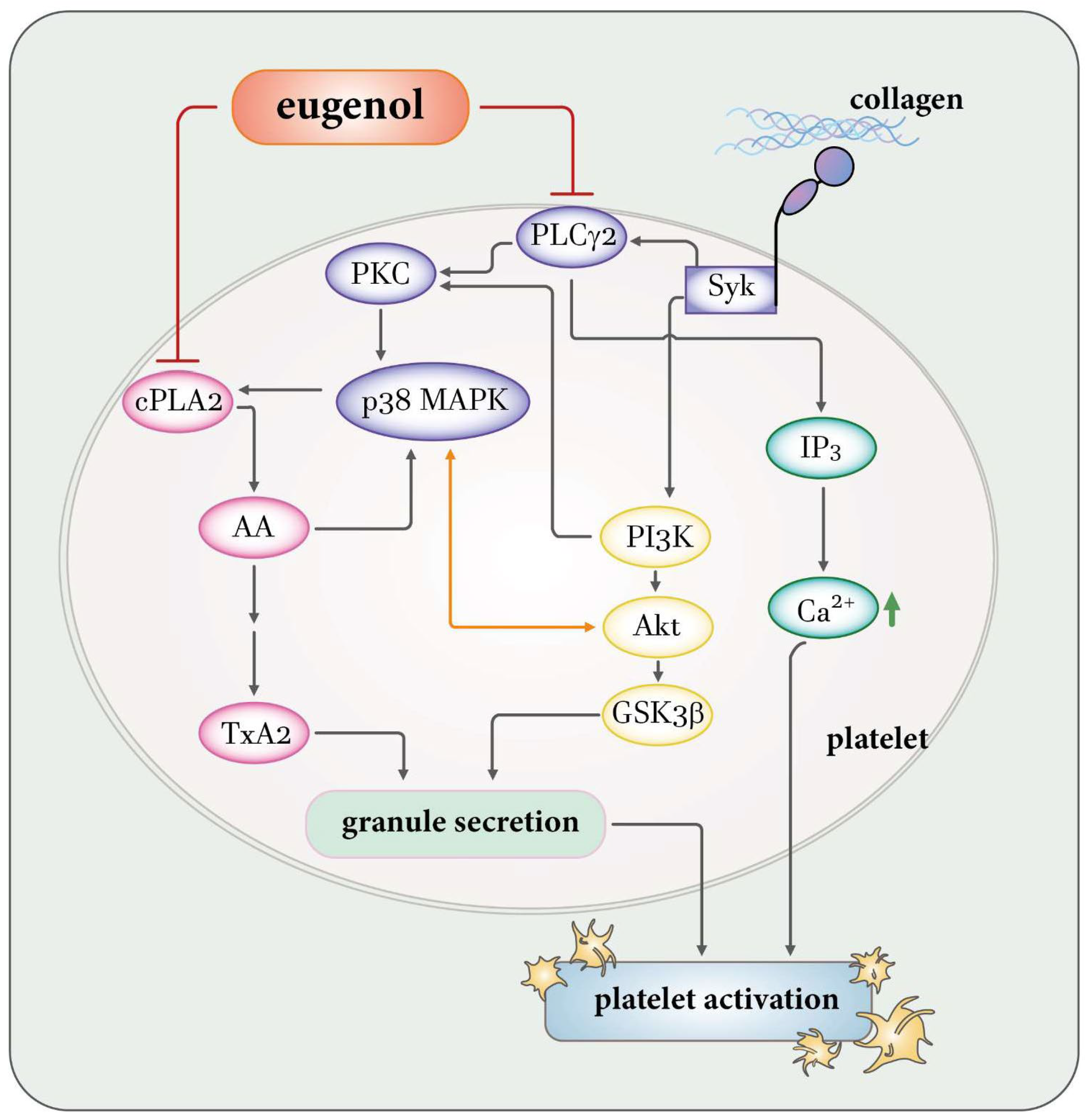
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents, and Antibodies
4.2. Isolation of Human Platelets Followed by Assessment of Aggregation Capability
4.3. Analysis of Change of [Ca2+]i Level and Surface Expression of P-Selectin
4.4. Measurement of TxB2 Formation
4.5. Immunoblotting
4.6. Utilization of Confocal Laser Fluorescence Microscopy
4.7. Acute Pulmonary Thromboembolism in Mice
4.8. Tail Bleeding Time in Mice
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrews, R.K.; Berndt, M.C. Platelet physiology and thrombosis. Thromb. Res. 2004, 114, 447–453. [Google Scholar] [CrossRef] [PubMed]
- George, J.N. Platelets. Lancet 2000, 355, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.R.; Yen, M.H.; Hung, W.C.; Lee, Y.M.; Su, C.H.; Huang, T.F. Triflavin inhibits platelet-induced vasoconstriction in de-endothelialized aorta. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3461–3468. [Google Scholar] [CrossRef] [PubMed]
- Ulanowska, M.; Olas, B. Biological properties and prospects for the application of eugenol-a review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef] [PubMed]
- Park, C.K.; Li, H.Y.; Yeon, K.Y.; Jung, S.J.; Choi, S.Y.; Lee, S.J.; Lee, S.; Park, K.; Kim, J.S.; Oh, S.B. Eugenol inhibits sodium currents in dental afferent neurons. J. Dent. Res. 2006, 85, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.M.; Lee, K.; Im, S.T.; Go, E.J.; Kim, Y.H.; Park, C.K. Co-application of eugenol and QX-314 elicits the prolonged blockade of voltage-gated sodium channels in nociceptive trigeminal ganglion neurons. Biomolecules 2020, 10, 1513. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Ostrowska, M.; Adamski, P.; Koziński, M.; Navarese, E.P.; Fabiszak, T.; Grześk, G.; Paciorek, P.; Kubica, J. Off-target effects of glycoprotein IIb/IIIa receptor inhibitors. Cardiol. J. 2014, 21, 458–464. [Google Scholar] [CrossRef]
- Gasparovic, H.; Petricevic, M.; Kopjar, T.; Djuric, Z.; Svetina, L.; Biocina, B. Impact of dual antiplatelet therapy on outcomes among aspirin-resistant patients following coronary artery bypass grafting. Am. J. Cardiol. 2014, 113, 1660–1667. [Google Scholar] [CrossRef]
- Olivier, C.B.; Diehl, P.; Schnabel, K.; Weik, P.; Zhou, Q.; Bode, C.; Moser, M. Third generation P2Y12 antagonists inhibit platelet aggregation more effectively than clopidogrel in a myocardial infarction registry. Thromb. Haemost. 2014, 111, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Barrett, N.E.; Holbrook, L.; Jones, S.; Kaiser, W.J.; Moraes, L.A.; Rana, R.; Sage, T.; Stanley, R.G.; Tucker, K.L.; Wright, B.; et al. Future innovations in anti-platelet therapies. Br. J. Pharmacol. 2008, 154, 918–939. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N. Triggers, targets and treatments for thrombosis. Nature 2008, 451, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, R.H.; Naidu, K.A. Spice active principles as the inhibitors of human platelet aggregation and thromboxane biosynthesis. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 73–78. [Google Scholar] [CrossRef]
- Cosemans, J.M.; Iserbyt, B.F.; Deckmyn, H.; Heemskerk, J.W. Multiple ways to switch platelet integrins on and off. J. Thromb. Haemost. 2008, 6, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Börsch-Haubold, A.G.; Ghomashchi, F.; Pasquet, S.; Goedert, M.; Cohen, P.; Gelb, M.H.; Watson, S.P. Phosphorylation of cytosolic phospholipase A2 in platelets is mediated by multiple stress-activated protein kinase pathways. Eur. J. Biochem. 1999, 265, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Varga-Szabo, D.; Braun, A.; Nieswandt, B. Calcium signaling in platelets. J. Thromb. Haemost. 2009, 7, 1057–1066. [Google Scholar] [CrossRef]
- Laurent, P.A.; Severin, S.; Gratacap, M.P.; Payrastre, B. Class I PI 3-kinases signaling in platelet activation and thrombosis: PDK1/Akt/GSK3 axis and impact of PTEN and SHIP1. Adv. Biol. Regul. 2014, 54, 162–174. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Fontana, P.; Zufferey, A.; Daali, Y.; Reny, J.L. Antiplatelet therapy: Targeting the TxA2 pathway. J. Cardiovasc. Transl. Res. 2014, 7, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ragab, A.; Séverin, S.; Gratacap, M.P.; Aguado, E.; Malissen, M.; Jandrot-Perrus, M.; Malissen, B.; Ragab-Thomas, J.; Payrastre, B. Roles of the C-terminal tyrosine residues of LAT in GPVI-induced platelet activation: Insights into the mechanism of PLC gamma 2 activation. Blood 2007, 110, 2466–2474. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.; Tourdot, B.E.; Fernandez-Perez, P.; Vesci, J.; Ren, J.; Smyrniotis, C.J.; Luci, D.K.; Jadhav, A.; Simeonov, A.; Maloney, D.J.; et al. Platelet 12-LOX is essential for FcγRIIa-mediated platelet activation. Blood 2014, 124, 2271–2279. [Google Scholar] [CrossRef]
- Grover, S.P.; Bergmeier, W.; Mackman, N. Platelet signaling pathways and new inhibitors. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e28–e35. [Google Scholar] [CrossRef] [PubMed]
- Estevez, B.; Du, X. New concepts and mechanisms of platelet activation signaling. Physiology 2017, 32, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Schoenwaelder, S.M.; Goncalves, I.; Nesbitt, W.S.; Yap, C.L.; Wright, C.E.; Kenche, V.; Anderson, K.E.; Dopheide, S.M.; Yuan, Y.; et al. PI 3-kinase p110beta: A new target for antithrombotic therapy. Nat. Med. 2005, 11, 507–514. [Google Scholar] [CrossRef]
- O’Brien, K.A.; Gartner, T.K.; Hay, N.; Du, X. ADP-stimulated activation of Akt during integrin outside-in signaling promotes platelet spreading by inhibiting glycogen synthase kinase-3β. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2232–2240. [Google Scholar] [CrossRef]
- Woulfe, D.S. Akt signaling in platelets and thrombosis. Expert. Rev. Hematol. 2010, 3, 81–91. [Google Scholar] [CrossRef]
- Jayakumar, T.; Chen, W.F.; Lu, W.J.; Chou, D.S.; Hsiao, G.; Hsu, C.Y.; Sheu, J.R.; Hsieh, C.Y. A novel antithrombotic effect of sulforaphane via activation of platelet adenylate cyclase: Ex vivo and in vivo studies. J. Nutr. Biochem. 2013, 24, 1086–1095. [Google Scholar] [CrossRef]
- Fan, X.; Wang, C.; Shi, P.; Gao, W.; Gu, J.; Geng, Y.; Yang, W.; Wu, N.; Wang, Y.; Xu, Y.; et al. Platelet MEKK3 regulates arterial thrombosis and myocardial infarct expansion in mice. Blood Adv. 2018, 2, 1439–1448. [Google Scholar] [CrossRef]
- Hughes, P.E.; Renshaw, M.W.; Pfaff, M.; Forsyth, J.; Keivens, V.M.; Schwartz, M.A.; Ginsberg, M.H. Suppression of integrin activation: A novel function of a Ras/Raf-initiated MAP kinase pathway. Cell 1997, 88, 521–530. [Google Scholar] [CrossRef]
- Adam, F.; Kauskot, A.; Rosa, J.P.; Bryckaert, M. Mitogen-activated protein kinases in hemostasis and thrombosis. J. Thromb. Haemost. 2008, 6, 2007–2016. [Google Scholar] [CrossRef]
- Momi, S.; Falcinelli, E.; Giannini, S.; Ruggeri, L.; Cecchetti, L.; Corazzi, T.; Libert, C.; Gresele, P. Loss of matrix metalloproteinase 2 in platelets reduces arterial thrombosis in vivo. J. Exp. Med. 2009, 206, 2365–2379. [Google Scholar] [CrossRef]
- Chen, W.F.; Lee, J.J.; Chang, C.C.; Lin, K.H.; Wang, S.H.; Sheu, J.R. Platelet protease-activated receptor (PAR)4, but not PAR1, associated with neutral sphingomyelinase responsible for thrombin-stimulated ceramide-NF-κB signaling in human platelets. Haematologica 2013, 98, 793–801. [Google Scholar] [CrossRef]
- Sheu, J.R.; Lee, C.R.; Lin, C.H.; Hsiao, G.; Ko, W.C.; Chen, Y.C.; Yen, M.H. Mechanisms involved in the antiplatelet activity of Staphylococcus aureus lipoteichoic acid in human platelets. Thromb. Haemost. 2000, 83, 777–784. [Google Scholar] [CrossRef]
- Sheu, J.R.; Hung, W.C.; Wu, C.H.; Lee, Y.M.; Yen, M.H. Antithrombotic effect of rutaecarpine, an alkaloid isolated from Evodia rutaecarpa, on platelet plug formation in in vivo experiments. Br. J. Haematol. 2000, 110, 110–115. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-C.; Shu, L.-H.; Kuo, Y.-J.; Lai, K.S.-L.; Hsia, C.-W.; Yen, T.-L.; Hsia, C.-H.; Jayakumar, T.; Yang, C.-H.; Sheu, J.-R. Eugenol Suppresses Platelet Activation and Mitigates Pulmonary Thromboembolism in Humans and Murine Models. Int. J. Mol. Sci. 2024, 25, 2098. https://doi.org/10.3390/ijms25042098
Huang W-C, Shu L-H, Kuo Y-J, Lai KS-L, Hsia C-W, Yen T-L, Hsia C-H, Jayakumar T, Yang C-H, Sheu J-R. Eugenol Suppresses Platelet Activation and Mitigates Pulmonary Thromboembolism in Humans and Murine Models. International Journal of Molecular Sciences. 2024; 25(4):2098. https://doi.org/10.3390/ijms25042098
Chicago/Turabian StyleHuang, Wei-Chieh, Lan-Hsin Shu, Yu-Ju Kuo, Kevin Shu-Leung Lai, Chih-Wei Hsia, Ting-Lin Yen, Chih-Hsuan Hsia, Thanasekaran Jayakumar, Chih-Hao Yang, and Joen-Rong Sheu. 2024. "Eugenol Suppresses Platelet Activation and Mitigates Pulmonary Thromboembolism in Humans and Murine Models" International Journal of Molecular Sciences 25, no. 4: 2098. https://doi.org/10.3390/ijms25042098
APA StyleHuang, W.-C., Shu, L.-H., Kuo, Y.-J., Lai, K. S.-L., Hsia, C.-W., Yen, T.-L., Hsia, C.-H., Jayakumar, T., Yang, C.-H., & Sheu, J.-R. (2024). Eugenol Suppresses Platelet Activation and Mitigates Pulmonary Thromboembolism in Humans and Murine Models. International Journal of Molecular Sciences, 25(4), 2098. https://doi.org/10.3390/ijms25042098










