Comprehensive Characterization of the C3HC4 RING Finger Gene Family in Potato (Solanum tuberosum L.): Insights into Their Involvement in Anthocyanin Biosynthesis
Abstract
1. Introduction
2. Results
2.1. Identification of StRING-HC Proteins
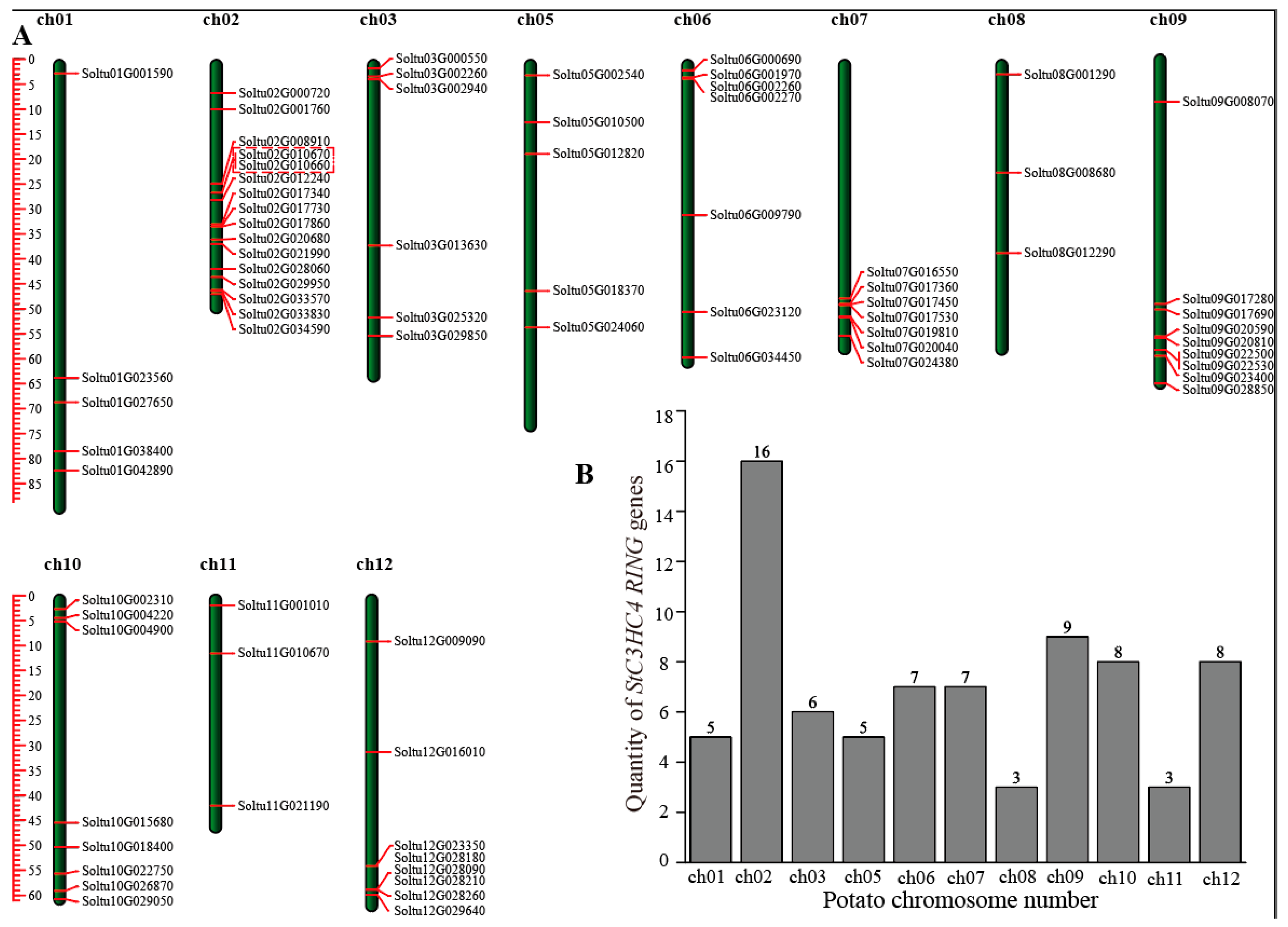
2.2. Chromosomal Distribution, Phylogenetic Analysis, and Classification of StRING-HCs
2.3. Gene Structure and Conserved Motifs of StRING-HC Members
2.4. Gene Duplication and Collinearity Analysis
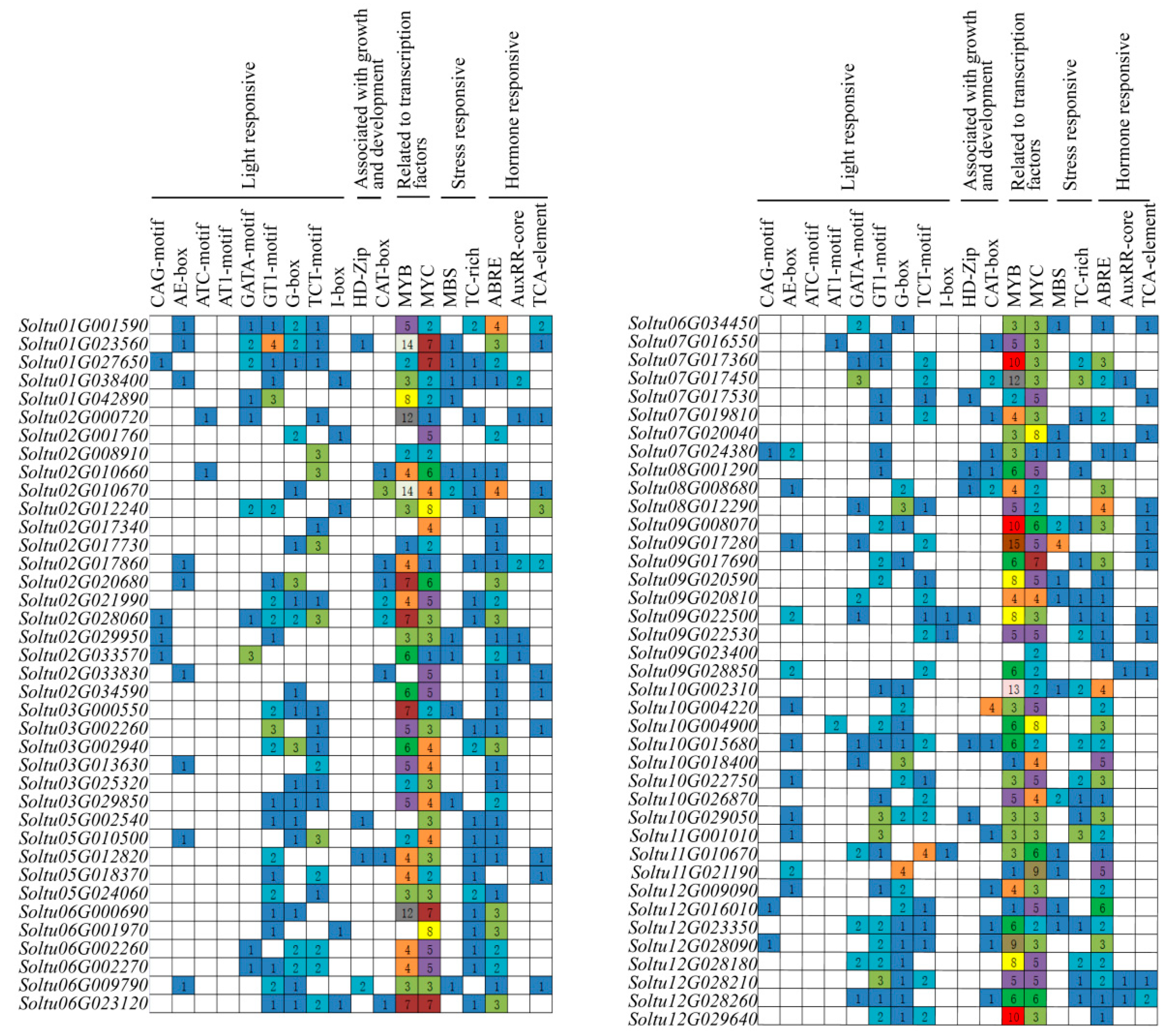
2.5. Promoter CIS-Acting Element Analysis
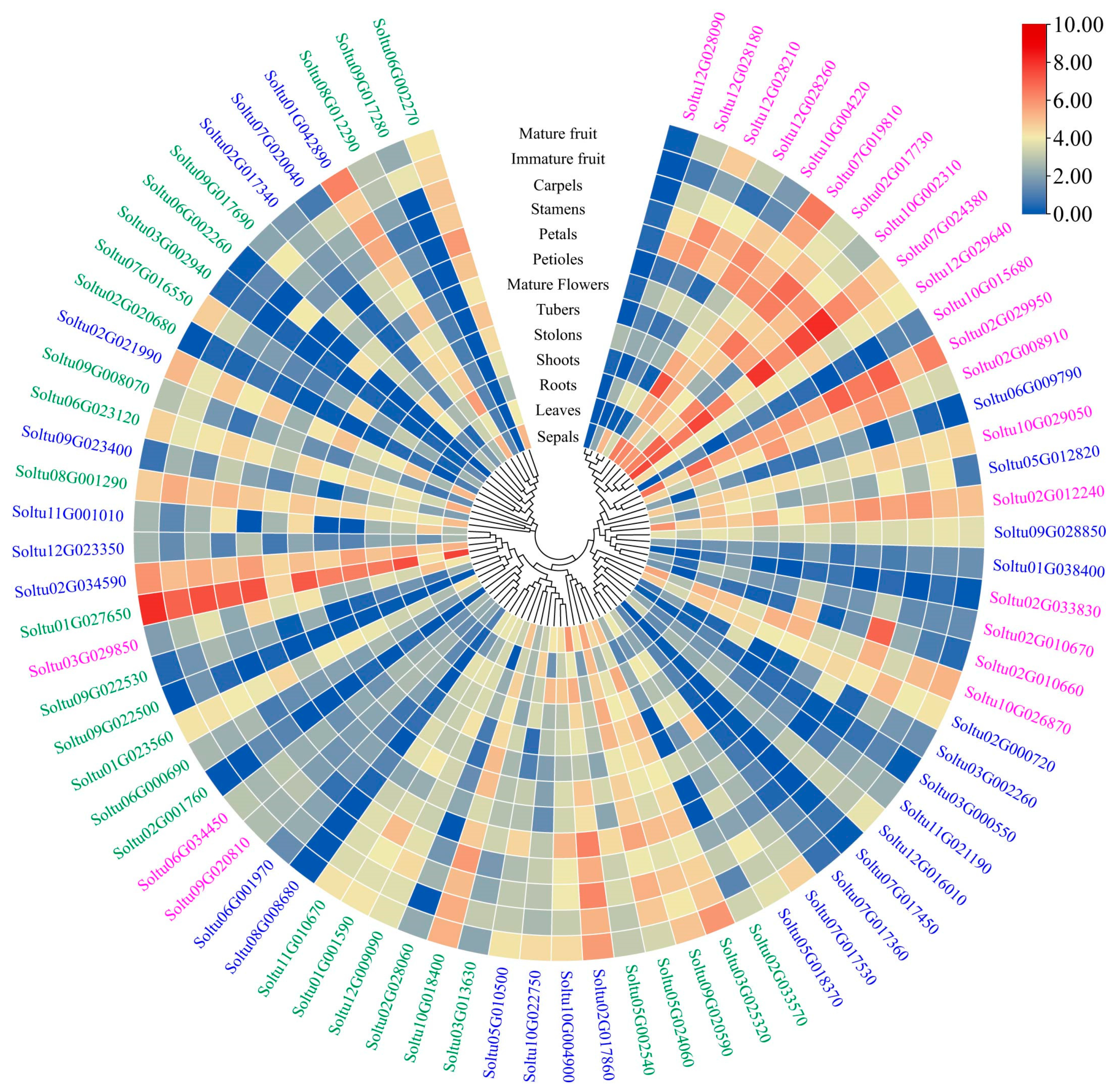
2.6. Expression Profiles of StRING-HC Genes in Different Tissues
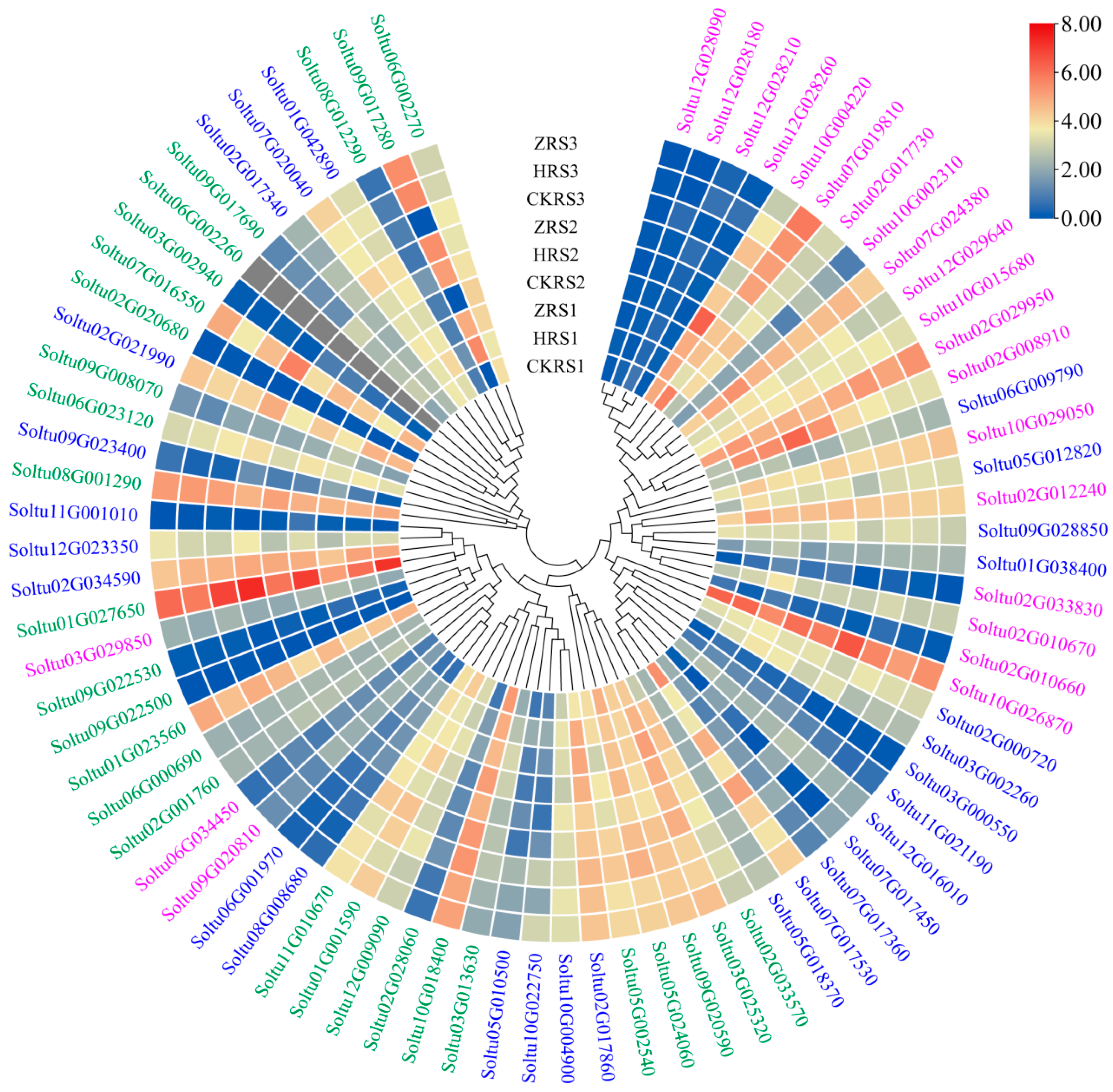
2.7. Expression Profiles of StRING-HC Genes under Abiotic Stresses and Hormone Treatments
2.8. Expression Profiles of StRING-HC Genes in Pigmented Potato Clones
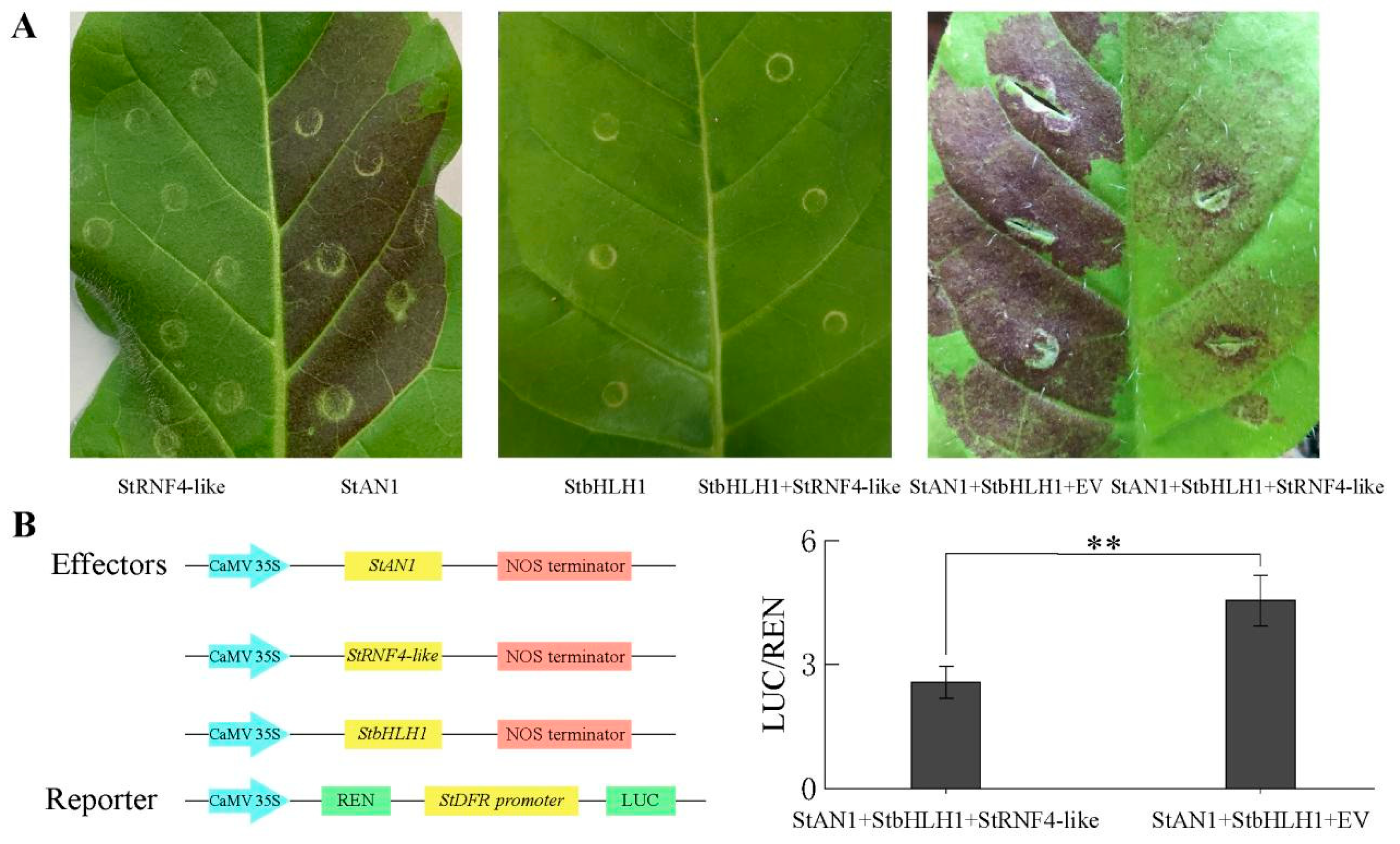
2.9. Cloning and Functional Analysis of the StRING-HC Gene
2.10. Validation of qPCR for StRNF4-like
3. Discussion
3.1. Phylogenetic Analysis and Evolution of StRING-HCs
3.2. Expansions of the StRING-HC Gene Family in Potato
3.3. The StRING-HCs Respond to Abiotic Stress
3.4. Identification of the StRING-HCs Associated with Anthocyanin Biosynthesis
4. Materials and Methods
4.1. Identification of StRING-HCs in Potato
4.2. Analysis of the Physicochemical Properties of StRING-HCs
4.3. Phylogenetic Analysis and Classification of RING-HCs
4.4. Sequence and Structural Characterization of StRING-HCs
4.5. Duplication of Genes and Chromosomal Localization
4.6. Analysis of Promoter Sequences of StRING-HCs
4.7. Plant Materials and Treatments
4.8. Extraction of RNA, Reverse Transcription of cDNA, and Quantitative Real-Time PCR
4.9. Expression Pattern Analysis of StRING-HCs in Potato
4.10. Cloning of the StRING-HC Gene
4.11. Subcellular Localization
4.12. Yeast Two-Hybrid Assays
4.13. Tobacco Transient Expression Experiment
4.14. Data Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berg, J.M.; Shi, Y. The galvanization of biology: A growing appreciation for the roles of zinc. Science 1996, 271, 1081–1085. [Google Scholar] [CrossRef]
- Gentile, A.; Da Cruz, P.; Tavares, R.G.; Krug-Baldacin, M.G.; Menossi, M. Molecular characterization of ScTFIIA gamma, encoding the putative TFIIA small subunit from sugarcane. Plant Cell Rep. 2010, 29, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Hui, S.; Zhang, M.; Li, P.; Xiao, J.; Li, X.; Yuan, M.; Wang, S. A Conserved Basal Transcription Factor Is Required for the Function of Diverse TAL Effectors in Multiple Plant Hosts. Front. Plant Sci. 2017, 8, 1919. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Ke, Y.; Huang, R.; Ma, L.; Yang, Z.; Chu, Z.; Xiao, J.; Li, X.; Wang, S. A host basal transcription factor is a key component for infection of rice by TALE-carrying bacteria. Elife 2016, 5, e19605. [Google Scholar] [CrossRef] [PubMed]
- Kosarev, P.; Mayer, K.F.; Hardtke, C.S. Evaluation and classification of RING-finger domains encoded by the Arabidopsis genome. Genome Biol. 2002, 3, RESEARCH0016. [Google Scholar] [CrossRef] [PubMed]
- Freemont, P.S. The RING finger. A novel protein sequence motif related to the zinc finger. Ann. N. Y. Acad. Sci. 1993, 684, 174–192. [Google Scholar] [CrossRef]
- Alam, I.; Yang, Y.Q.; Wang, Y.; Zhu, M.L.; Wang, H.B.; Chalhoub, B.; Lu, Y.H. Genome-wide identification, evolution and expression analysis of RING finger protein genes in Brassica rapa. Sci. Rep. 2017, 7, 40690. [Google Scholar] [CrossRef]
- Meng, X.; Liu, J.; Zhao, M. Genome-wide identification of RING finger genes in flax (Linum usitatissimum) and analyses of their evolution. PeerJ 2021, 9, e12491. [Google Scholar] [CrossRef]
- Stone, S.L.; Hauksdóttir, H.; Troy, A.; Herschleb, J.; Kraft, E.; Callis, J. Functional Analysis of the RING-Type Ubiquitin Ligase Family of Arabidopsis. Plant Physiol. 2005, 137, 13–30. [Google Scholar] [CrossRef]
- Sun, J.; Sun, Y.; Ahmed, R.I.; Ren, A.; Xie, A.M. Research Progress on Plant RING-Finger Proteins. Genes 2019, 10, 973. [Google Scholar] [CrossRef]
- Borden, K.L. RING domains: Master builders of molecular scaffolds? J. Mol. Biol. 2000, 295, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-López, D.; Muñóz-Belman, F.; González-Prieto, J.; Aguilar-Hernández, V.; Guzmán, P.; Massiah, M. Repertoire of plant RING E3 ubiquitin ligases revisited: New groups counting gene families and single genes. PLoS ONE 2018, 13, e0203442. [Google Scholar] [CrossRef] [PubMed]
- Guzman, P. ATLs and BTLs, plant-specific and general eukaryotic structurally-related E3 ubiquitin ligases. Plant Sci. 2014, 215, 69–75. [Google Scholar] [CrossRef]
- Hardtke, C.S.; Gohda, K.; Osterlund, M.T.; Oyama, T.; Okada, K.; Deng, X.W. HY5 stability and activity in Arabidopsis is regulated by phosphorylation in its COP1 binding domain. EMBO J. 2000, 19, 4997–5006. [Google Scholar] [CrossRef]
- Prasad, M.E.; Schofield, A.; Lyzenga, W.; Stone, L.S.L. Arabidopsis RING E3 Ligase XBAT32 Regulates Lateral Root Production through Its Role in Ethylene Biosynthesis. Plant Physiol. 2010, 153, 1587–1596. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Q.; Ren, J.; Qian, W.Q.; He, S.P.; Huang, K.W.; Yu, X.C.; Gao, Y.; Huang, P.; An, C.C. Two Novel RING-Type Ubiquitin Ligases, RGLG3 and RGLG4, Are Essential for Jasmonate-Mediated Responses in Arabidopsis. Plant Physiol. 2012, 160, 808–822. [Google Scholar] [CrossRef]
- Shen, L.; Thong, Z.; Gong, X.; Shen, Q.; Gan, Y.; Yu, H. The putative PRC1 RING-finger protein AtRING1A regulates flowering through repressing MADS AFFECTING FLOWERING genes in Arabidopsis. Development 2014, 141, 1303. [Google Scholar] [CrossRef] [PubMed]
- Peralta, D.A.; Araya, A.; Nardi, C.F.; Busi, M.V.; Gomez-Casati, D.F. Characterization of the Arabidopsis thaliana E3 ubiquitin-ligase AtSINAL7 and identification of the ubiquitination sites. PLoS ONE 2013, 8, e73104. [Google Scholar] [CrossRef]
- Kraft, E.; Bostick, M.; Jacobsen, S.E.; Callis, J. ORTH/VIM proteins that regulate DNA methylation are functional ubiquitin E3 ligases. Plant J. Cell Mol. Biol. 2008, 56, 704–715. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, D.; Zhu, C.; He, Y.; Zhang, H.; Liu, T.; Li, X.; Wu, C. The RING-Finger Ubiquitin Ligase HAF1 Mediates Heading date 1 Degradation during Photoperiodic Flowering in Rice. Plant Cell 2015, 27, 2455–2468. [Google Scholar] [CrossRef]
- Du, Y.; He, W.; Deng, C.; Chen, X.; Gou, L.; Zhu, F.; Guo, W.; Zhang, J.; Wang, T. Flowering-Related RING Protein 1 (FRRP1) Regulates Flowering Time and Yield Potential by Affecting Histone H2B Monoubiquitination in Rice (Oryza Sativa). PLoS ONE 2016, 11, e0150458. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, Z.; Liu, M.; Yang, X.; Qiu, D. C3HC4-type RING finger protein NbZFP1 is involved in growth and fruit development in Nicotiana benthamiana. PLoS ONE 2014, 9, e99352. [Google Scholar] [CrossRef]
- Klemm, T.; Mannuss, A.; Kobbe, D.; Knoll, A.; Trapp, O.; Dorn, A.; Puchta, H. The DNA translocase RAD5A acts independently of the other main DNA repair pathways, and requires both its ATPase and RING domain for activity in Arabidopsis thaliana. Plant J. 2017, 91, 725–740. [Google Scholar] [CrossRef]
- Chung, E.; Cho, C.W.; So, H.A.; Kang, J.S.; Chung, Y.S.; Lee, J.H. Overexpression of VrUBC1, a Mung Bean E2 Ubiquitin-Conjugating Enzyme, Enhances Osmotic Stress Tolerance in Arabidopsis. PLoS ONE 2013, 8, e66056. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, W.T. The Arabidopsis RING E3 ubiquitin ligase AtAIRP3/LOG2 participates in positive regulation of high-salt and drought stress responses. Plant Physiol. 2013, 162, 1733–1749. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lim, C.W.; Baek, W.; Lee, S.C. RING Type E3 Ligase CaAIR1 in Pepper Acts in the Regulation of ABA Signaling and Drought Stress Response. Plant Cell Physiol. 2015, 56, 1808–1819. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joo, H.; Lim, C.W.; Lee, S.C. Identification and functional expression of the pepper RING type E3 ligase, CaDTR1, involved in drought stress tolerance via ABA-mediated signalling. Sci. Rep. 2016, 6, 30097. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, X.; Ye, X.; Guo, P.; Hu, Z.; Qi, G.; Cui, F.; Liu, S. An S-ribonuclease binding protein EBS1 and brassinolide signaling are specifically required for Arabidopsis tolerance to bicarbonate. J. Exp. Bot. 2021, 72, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Lee, I.H.; Nou, I.S.; Lee, K.D.; Rashotte, A.M.; Kang, K.K. BrRZFP1 a Brassica rapa C3HC4-type RING zinc finger protein involved in cold, salt and dehydration stress. Plant Biol. 2013, 15, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Berrocal-Lobo, M.; Stone, S.; Yang, X.; Antico, J.; Callis, J.; Ramonell, K.M.; Somerville, S. ATL9, a RING zinc finger protein with E3 ubiquitin ligase activity implicated in chitin- and NADPH oxidase-mediated defense responses. PLoS ONE 2010, 5, e14426. [Google Scholar] [CrossRef]
- Cheung, M.-Y.; Zeng, N.-Y.; Tong, S.-W.; Li, F.W.-Y.; Zhao, K.-J.; Zhang, Q.; Sun, S.S.-M.; Lam, H.-M. Expression of a RING-HC protein from rice improves resistance to Pseudomonas syringae pv. tomato DC3000 in transgenic Arabidopsis thaliana. J. Exp. Bot. 2007, 58, 4147–4159. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Weng, Y.; Liu, B.; Gao, Q.; Ji, W.; Wang, Z.; Wang, Y.; Ma, X. Identification and Characterization of Regulatory Pathways Controlling Dormancy Under Lower Temperature in Alfalfa (Medicago sativa L.). Front. Plant Sci. 2022, 13, 872839. [Google Scholar] [CrossRef]
- Gu, Z.; Men, S.; Zhu, J.; Hao, Q.; Tong, N.; Liu, Z.-A.; Zhang, H.; Shu, Q.; Wang, L. Chalcone synthase is ubiquitinated and degraded via interactions with a RING-H2 protein in petals of Paeonia ‘He Xie’. J. Exp. Bot. 2019, 70, 4749–4762. [Google Scholar] [CrossRef]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Pfeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; Di Gaspero, G. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ. 2007, 30, 1381–1399. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. Cell Mol. Biol. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, J.L.; Cherono, S.; An, J.P.; Allan, A.C.; Han, Y.P. Colorful hues: Insight into the mechanisms of anthocyanin pigmentation in fruit. Plant Physiol. 2023, 192, 1718–1732. [Google Scholar] [CrossRef]
- Petroni, K.; Tonelli, C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 2011, 181, 219–229. [Google Scholar] [CrossRef]
- Li, Y.Q.; Shan, X.T.; Gao, R.F.; Han, T.T.; Zhang, J.; Wang, Y.N.; Kimani, S.; Wang, L.; Gao, X. MYB repressors and MBW activation complex collaborate to fine-tune flower coloration in Freesia hybrida. Commun. Biol. 2020, 3, 396. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lin-Wang, K.; Espley, R.V.; Wang, L.; Yang, H.Y.; Yu, B.; Dare, A.; Varkonyi-Gasic, E.; Wang, J.; Zhang, J.L.; et al. Functional diversification of the potato R2R3 MYB anthocyanin activators AN1, MYBA1, and MYB113 and their interaction with basic helix-loop-helix cofactors. J. Exp. Bot. 2016, 67, 2159–2176. [Google Scholar] [CrossRef]
- Vimolmangkang, S.; Han, Y.P.; Wei, G.C.; Korban, S.S. An apple MYB transcription factor, MdMYB3, is involved in regulation of anthocyanin biosynthesis and flower development. BMC Plant Biol. 2013, 13, 176. [Google Scholar] [CrossRef]
- Jin, H.; Cominelli, E.; Bailey, P.; Parr, A.; Mehrtens, F.; Jones, J.; Tonelli, C.; Weisshaar, B.; Martin, C. Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO J. 2000, 19, 6150–6161. [Google Scholar] [CrossRef]
- Li, Y.; Lin-Wang, K.; Liu, Z.; Allan, A.C.; Qin, S.; Zhang, J.; Liu, Y. Genome-wide analysis and expression profiles of the StR2R3-MYB transcription factor superfamily in potato (Solanum tuberosum L.). Int. J. Biol. Macromol. 2020, 148, 817–832. [Google Scholar] [CrossRef]
- Liu, Y.H.; Li, Y.M.; Liu, Z.; Wang, L.; Lin-Wang, K.; Zhu, J.Y.; Bi, Z.Z.; Sun, C.; Zhang, J.L.; Bai, J.P. Integrative analysis of metabolome and transcriptome reveals a dynamic regulatory network of potato tuber pigmentation. Iscience 2023, 26, 105903. [Google Scholar] [CrossRef]
- Pacin, M.; Semmoloni, M.; Legris, M.; Finlayson, S.A.; Casal, J.J. Convergence of Constitutive Photomorphogenesis 1 and Phytochrome Interacting Factor signalling during shade avoidance. New Phytol. 2016, 211, 967–979. [Google Scholar] [CrossRef]
- Qian, C.; Chen, Z.; Liu, Q.; Mao, W.; Chen, Y.; Tian, W.; Liu, Y.; Han, J.; Ouyang, X.; Huang, X. Coordinated Transcriptional Regulation by the UV-B Photoreceptor and Multiple Transcription Factors for Plant UV-B Responses. Mol. Plant 2020, 13, 777–792. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Mao, K.; Zhao, C.; Zhang, R.-F.; Zhao, X.-Y.; Zhang, H.-L.; Shu, H.-R.; Hao, Y.-J. Molecular cloning of cryptochrome 1 from apple and its functional characterization in Arabidopsis. Plant Physiol. Biochem. 2013, 67, 169–177. [Google Scholar] [CrossRef]
- Wu, M.; Si, M.; Li, X.; Song, L.; Liu, J.; Zhai, R.; Cong, L.; Yue, R.; Yang, C.; Ma, F.; et al. PbCOP1.1 Contributes to the Negative Regulation of Anthocyanin Biosynthesis in Pear. Plants 2019, 8, 39. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Mao, K.; Zhao, C.; Zhao, X.-Y.; Zhang, H.-L.; Shu, H.-R.; Hao, Y.-J. MdCOP1 Ubiquitin E3 Ligases Interact with MdMYB1 to Regulate Light-Induced Anthocyanin Biosynthesis and Red Fruit Coloration in Apple. Plant Physiol. 2012, 160, 1011–1022. [Google Scholar] [CrossRef]
- Jiang, M.; Ren, L.; Lian, H.; Liu, Y.; Chen, H. Novel insight into the mechanism underlying light-controlled anthocyanin accumulation in eggplant (Solanum melongena L.). Plant Sci. 2016, 249, 46–58. [Google Scholar] [CrossRef]
- Kim, B.; Piao, R.; Lee, G.; Koh, E.; Lee, Y.; Woo, S.; Reflinur; Jiang, W.; Septiningsih, E.M.; Thomson, M.J.; et al. OsCOP1 regulates embryo development and flavonoid biosynthesis in rice (Oryza Sativa L.). Theor. Appl. Genet. 2021, 134, 2587–2601. [Google Scholar] [CrossRef]
- Ma, A.N.; Wang, D.; Lu, H.L.; Wang, H.C.; Qin, Y.H.; Hu, G.B.; Zhao, J.T. LcCOP1 and LcHY5 control the suppression and induction of anthocyanin accumulation in bagging and debagging litchi fruit pericarp. Sci. Hortic. 2021, 287, 110281. [Google Scholar] [CrossRef]
- An, J.-P.; Liu, X.; Li, H.-H.; You, C.-X.; Wang, X.-F.; Hao, Y.-J. Apple RING E3 ligase MdMIEL1 inhibits anthocyanin accumulation by ubiquitinating and degrading MdMYB1 protein. Plant Cell Physiol. 2017, 58, 1953–1962. [Google Scholar] [CrossRef]
- Brown, C.R.; Wrolstad, R.; Durst, R.; Yang, C.P.; Clevidence, B. Breeding studies in potatoes containing high concentrations of anthocyanins. Am. J. Potato Res. 2003, 80, 241–249. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Sulc, M.; Orsák, M.; Pivec, V.; Hejtmánková, A.; Dvorák, P.; Cepl, J. Cultivar differences of total anthocyanins and anthocyanidins in red and purple-fleshed potatoes and their relation to antioxidant activity. Food Chem. 2009, 114, 836–843. [Google Scholar] [CrossRef]
- Reyes, L.F.; Miller, J.C.; Cisneros-Zevallos, L. Environmental conditions influence the content and yield of anthocyanins and total phenolics in purple- and red-flesh potatoes during tuber development. Am. J. Potato Res. 2004, 81, 187–193. [Google Scholar] [CrossRef]
- Bvenura, C.; Witbooi, H.; Kambizi, L. Pigmented Potatoes: A Potential Panacea for Food and Nutrition Security and Health? Foods 2022, 11, 175. [Google Scholar] [CrossRef]
- Qi, X.; Tang, X.; Liu, W.; Fu, X.; Luo, H.; Ghimire, S.; Zhang, N.; Si, H. A potato RING-finger protein gene StRFP2 is involved in drought tolerance. Plant Physiol. Biochem. 2020, 146, 438–446. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, Y.; Chen, S.; Hou, J.; Yu, Y.; Liu, T.; Du, J.; Song, B.; Xie, C. SbRFP1 regulates cold-induced sweetening of potato tubers by inactivation of StBAM1. Plant Physiol. Biochem. 2019, 136, 215–221. [Google Scholar] [CrossRef]
- Xu, D.; Zhu, D.; Deng, X.W. The role of COP1 in repression of photoperiodic flowering. F1000Research 2016, 5, 178. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, L.; Chang, W.; Li, Z.; Miao, M.; Li, Y.; Yang, J.; Liu, Z.; Tan, J. Overexpression of the maize E3 ubiquitin ligase gene ZmAIRP4 enhances drought stress tolerance in Arabidopsis. Plant Physiol. Biochem. 2018, 123, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ravichandran, S.; Teh, O.K.; McVey, S.; Lilley, C.; Teresinski, H.J.; Gonzalez-Ferrer, C.; Mullen, R.T.; Hofius, D.; Prithiviraj, B.; et al. The RING-Type E3 Ligase XBAT35.2 Is Involved in Cell Death Induction and Pathogen Response. Plant Physiol. 2017, 175, 1469–1483. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, J.; Wang, Z.; Xu, X.; Mao, X.; Li, D.; Hu, X.; Jin, D.; Li, C. Genome-wide Classification, Identification and Expression Profile of the C3HC4-type RING Finger Gene Family in Poplar (Populus trichocarpa). Plant Mol. Biol. Rep. 2015, 33, 1740–1754. [Google Scholar] [CrossRef]
- Qin, F.; Sakuma, Y.; Tran, L.-S.P.; Maruyama, K.; Kidokoro, S.; Fujita, Y.; Fujita, M.; Umezawa, T.; Sawano, Y.; Miyazono, K.-I.; et al. Arabidopsis DREB2A-interacting proteins function as RING E3 ligases and negatively regulate plant drought stress-responsive gene expression. Plant Cell 2008, 20, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-C.; Hsieh, E.-J.; Chen, J.-H.; Chen, H.-Y.; Lin, T.-P. Arabidopsis RGLG2, Functioning as a RING E3 Ligase, Interacts with AtERF53 and Negatively Regulates the Plant Drought Stress Response. Plant Physiol. 2012, 158, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Ryu, M.Y.; Seo, D.H.; Kang, B.G.; Kim, W.T. The Arabidopsis RING E3 Ubiquitin Ligase AtAIRP2 Plays Combinatory Roles with AtAIRP1 in Abscisic Acid-Mediated Drought Stress Responses. Plant Physiol. 2011, 157, 2240–2257. [Google Scholar] [CrossRef]
- Lawton-Rauh, A. Evolutionary dynamics of duplicated genes in plants. Mol. Phylogenet. Evol. 2003, 29, 396–409. [Google Scholar] [CrossRef]
- Singh, A.; Pandey, A.; Baranwal, V.; Kapoor, S.; Pandey, G.K. Comprehensive expression analysis of rice phospholipase D gene family during abiotic stresses and development. Plant Signal. Behav. 2012, 7, 847–855. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, R.; Wu, Y.; Wei, S.; Wang, Q.; Zheng, Y.; Xia, R.; Shang, X.; Yu, F.; Yang, X.; et al. ERAD-related E2 and E3 enzymes modulate the drought response by regulating the stability of PIP2 aquaporins. Plant Cell 2021, 33, 2883–2898. [Google Scholar] [CrossRef]
- Park, Y.C.; Chapagain, S.; Jang, C.S. A Negative Regulator in Response to Salinity in Rice: Oryza Sativa Salt-, ABA- and Drought-Induced RING Finger Protein 1 (OsSADR1). Plant Cell Physiol. 2018, 59, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-T.; Zhang, J.; Kang, Y.-H.; Chen, M.-C.; Song, T.-T.; Geng, H.; Tian, J.; Yao, Y.-C. McMYB10 Modulates the Expression of a Ubiquitin Ligase, McCOP1 During Leaf Coloration in Crabapple. Front. Plant Sci. 2018, 9, 704. [Google Scholar] [CrossRef]
- Hong, Y.; Lv, Y.; Zhang, J.; Ahmad, N.; Li, X.; Yao, N.; Liu, X.; Li, H. The safflower MBW complex regulates HYSA accumulation through degradation by the E3 ligase CtBB1. J. Integr. Plant Biol. 2023, 65, 1277–1296. [Google Scholar] [CrossRef] [PubMed]
- Ragland, R.L.; Patel, S.; Rivard, R.S.; Smith, K.; Peters, A.A.; Bielinsky, A.K.; Brown, E.J. RNF4 and PLK1 are required for replication fork collapse in ATR-deficient cells. Genes Dev. 2013, 27, 2259–2273. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Miao, M.; Lyu, H.; Cao, X.; Li, J.; Li, Y.; Li, Z.; Chang, W. Genome-Wide Identification, Evolution, and Expression Analysis of RING Finger Gene Family in Solanum lycopersicum. Int. J. Mol. Sci. 2019, 20, 4864. [Google Scholar] [CrossRef]
- Elisabeth, G.; Alexandre, G.; Christine, H.; Ivan, I.; Appel, R.D.; Amos, B. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar]
- Yu, C.-S.; Chen, Y.-C.; Lu, C.-H.; Hwang, J.-K. Prediction of protein subcellular localization. Proteins 2006, 64, 643–651. [Google Scholar] [CrossRef]
- Hall, B.G. Building Phylogenetic Trees from Molecular Data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Guo, A.-Y.; Zhu, Q.-H.; Chen, X.; Luo, J.-C. GSDS: A gene structure display server. Yi Chuan = Hered. 2007, 29, 1023–1026. [Google Scholar] [CrossRef]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.B.; Chen, S.S.; Sun, K.; Li, D.T.; Meng, J.J.; Tang, Y.F.; Song, S.Y. Sequencing and characterization the complete chloroplast genome of the potato, Solanum tuberosum L. Mitochondrial DNA Part B-Resour. 2019, 4, 953–954. [Google Scholar] [CrossRef]
- Cho, K.S.; Cho, J.H.; Im, J.S.; Choi, J.G.; Park, Y.E.; Hong, S.Y.; Kwon, M.; Kang, J.H.; Park, T.H. The complete mitochondrial genome sequences of potato (Solanum tuberosum L., Solanaceae). Mitochondrial DNA Part B-Resour. 2017, 2, 781–782. [Google Scholar] [CrossRef] [PubMed]
- Varré, J.S.; D’Agostino, N.; Touzet, P.; Gallina, S.; Tamburino, R.; Cantarella, C.; Ubrig, E.; Cardi, T.; Drouard, L.; Gualberto, J.M.; et al. Complete Sequence, Multichromosomal Architecture and Transcriptome Analysis of the Solanum tuberosum Mitochondrial Genome. Int. J. Mol. Sci. 2019, 20, 4788. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-h.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A toolkit incorporating gamma-series methods and sliding window strategies. Genom. Proteom. Bioinform. 2010, 8, 77–80. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Coulter, J.A.; Shen, B.; Li, Y.; Li, C.; Cao, Z.; Zhang, J. The WD40 Gene Family in Potato (Solanum tuberosum L.): Genome-Wide Analysis and Identification of Anthocyanin and Drought-Related WD40s. Agronomy 2020, 10, 401. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, N.; Si, H.J.; Calderón-Urrea, A. Selection and validation of reference genes for RT-qPCR analysis in potato under abiotic stress. Plant Methods 2017, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Zeng, Y.J.; Shen, W.T.; Tuo, D.C.; Li, X.Y.; Zhou, P. Nimble Cloning: A Simple, Versatile, and Efficient System for Standardized Molecular Cloning. Front. Bioeng. Biotechnol. 2020, 7, 460. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Li, Y.; Zhu, J.; Li, Z.; Wang, W.; Qi, Z.; Li, D.; Yao, P.; Bi, Z.; Sun, C.; et al. Comprehensive Characterization of the C3HC4 RING Finger Gene Family in Potato (Solanum tuberosum L.): Insights into Their Involvement in Anthocyanin Biosynthesis. Int. J. Mol. Sci. 2024, 25, 2082. https://doi.org/10.3390/ijms25042082
Chen L, Li Y, Zhu J, Li Z, Wang W, Qi Z, Li D, Yao P, Bi Z, Sun C, et al. Comprehensive Characterization of the C3HC4 RING Finger Gene Family in Potato (Solanum tuberosum L.): Insights into Their Involvement in Anthocyanin Biosynthesis. International Journal of Molecular Sciences. 2024; 25(4):2082. https://doi.org/10.3390/ijms25042082
Chicago/Turabian StyleChen, Limin, Yuanming Li, Jinyong Zhu, Zhitao Li, Weilu Wang, Zheying Qi, Dechen Li, Panfeng Yao, Zhenzhen Bi, Chao Sun, and et al. 2024. "Comprehensive Characterization of the C3HC4 RING Finger Gene Family in Potato (Solanum tuberosum L.): Insights into Their Involvement in Anthocyanin Biosynthesis" International Journal of Molecular Sciences 25, no. 4: 2082. https://doi.org/10.3390/ijms25042082
APA StyleChen, L., Li, Y., Zhu, J., Li, Z., Wang, W., Qi, Z., Li, D., Yao, P., Bi, Z., Sun, C., Liu, Y., & Liu, Z. (2024). Comprehensive Characterization of the C3HC4 RING Finger Gene Family in Potato (Solanum tuberosum L.): Insights into Their Involvement in Anthocyanin Biosynthesis. International Journal of Molecular Sciences, 25(4), 2082. https://doi.org/10.3390/ijms25042082






