Hydroxypropyl-Beta Cyclodextrin Barrier Prevents Respiratory Viral Infections: A Preclinical Study
Abstract
1. Introduction
2. Results
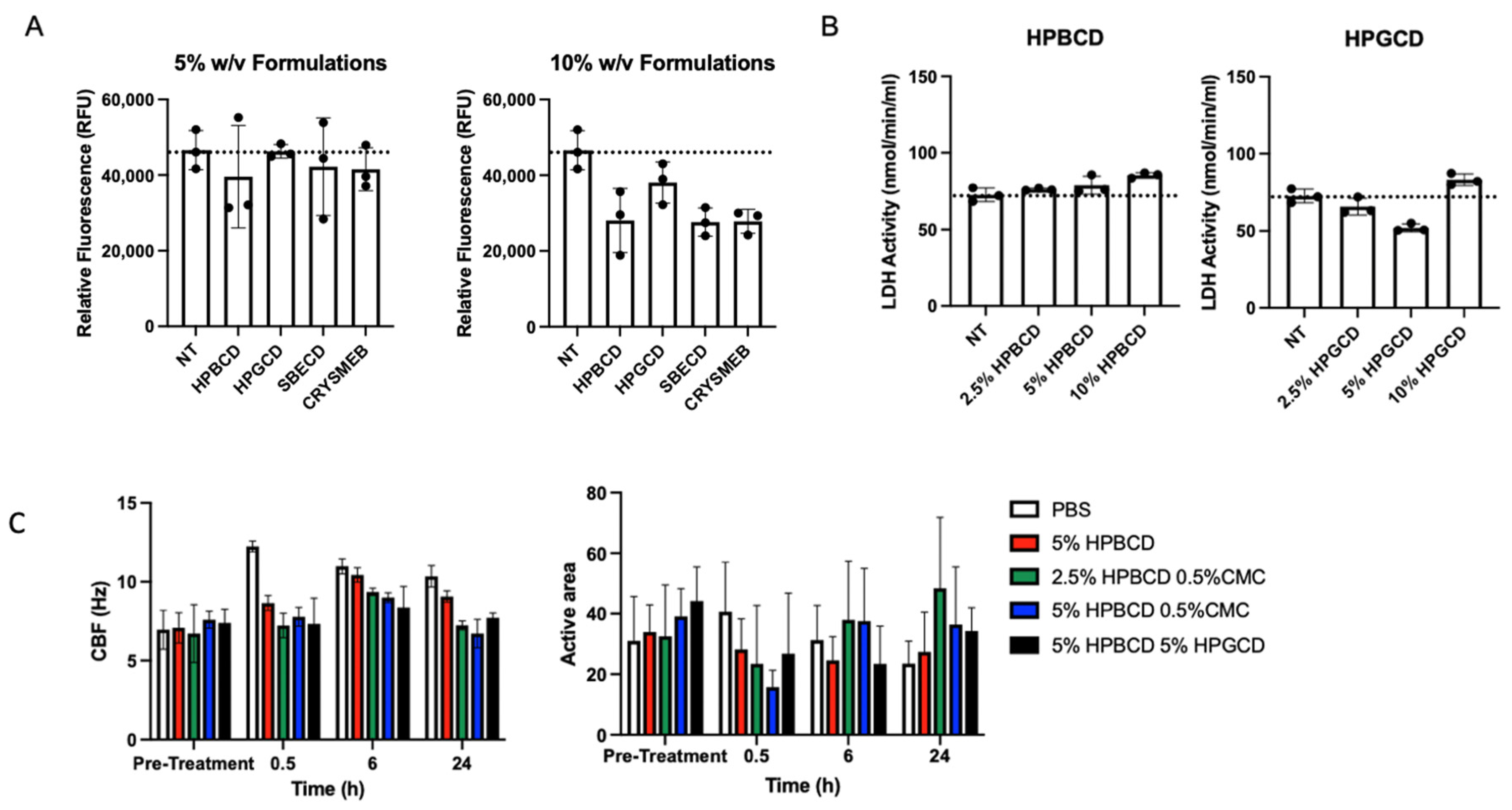
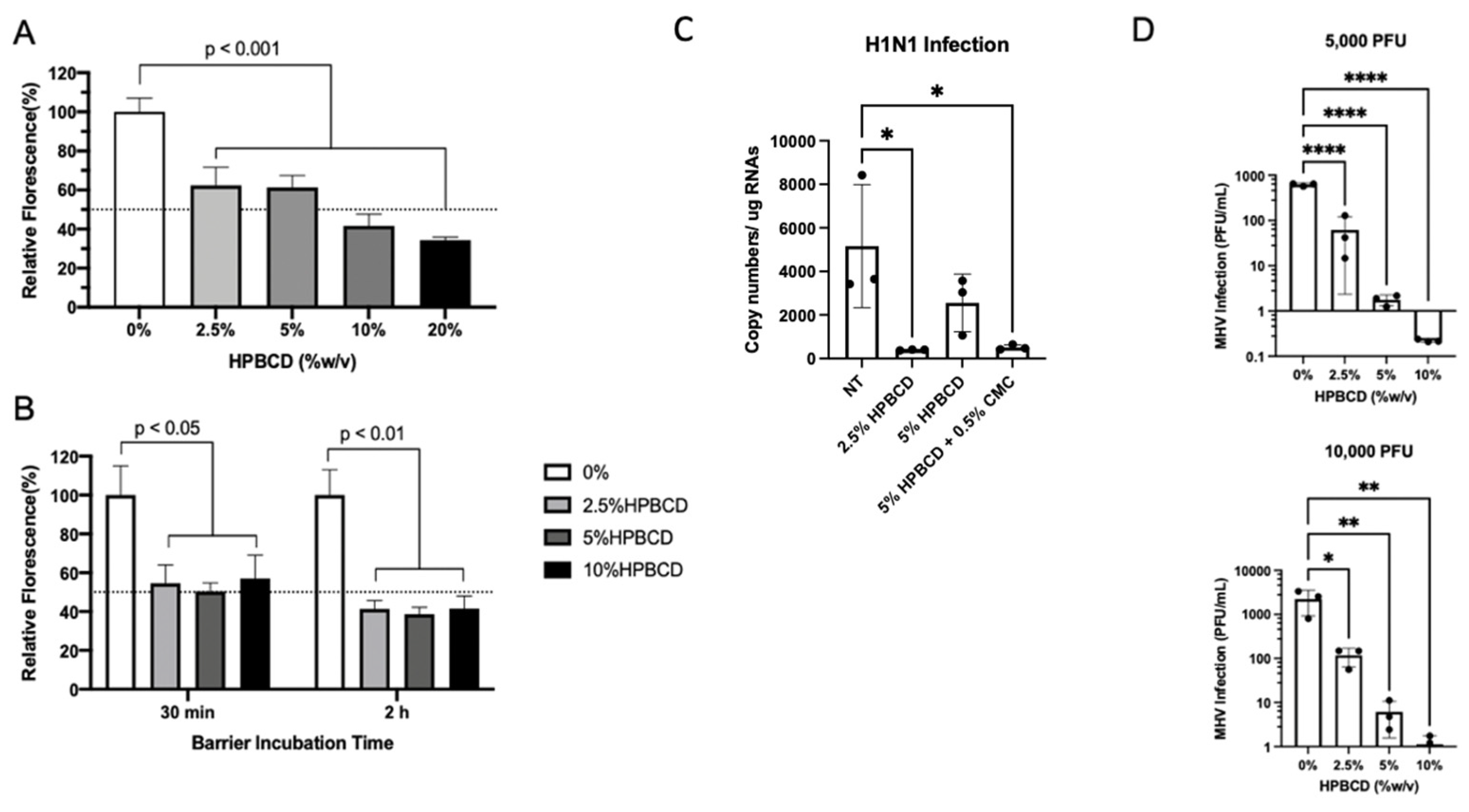
2.1. Cyclodextrin Safety and Efficacy Profiles
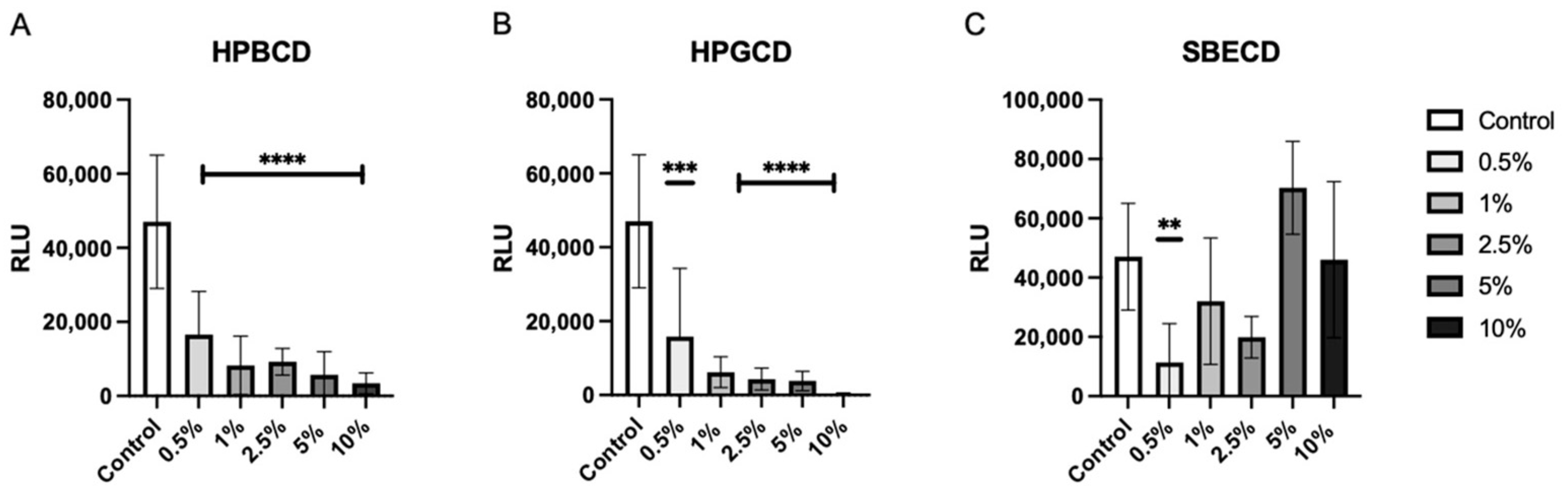
2.2. Antiviral Effects of Cyclodextrins
2.3. In Silico Modeling of CD Interaction with S Protein
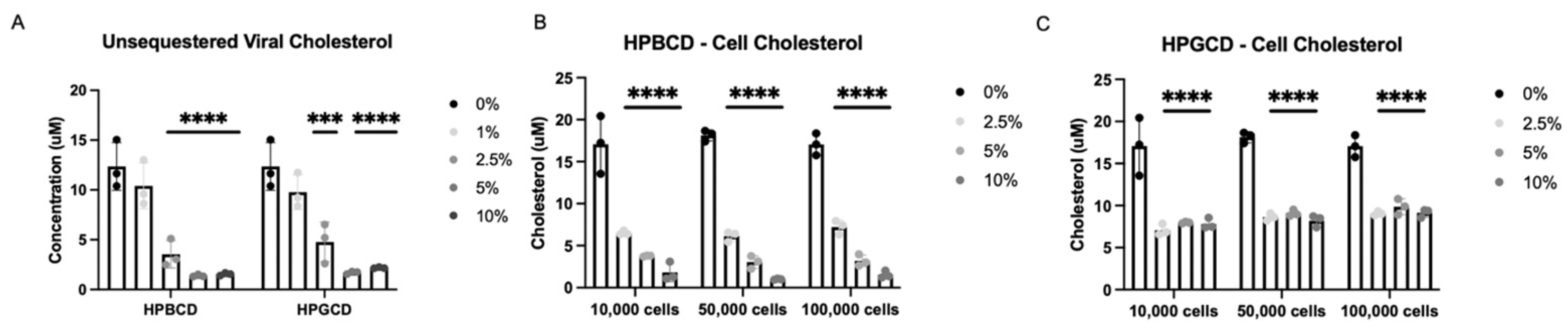
2.4. HPBCD and HPGCD Sequester Both Viral and Cellular Cholesterol
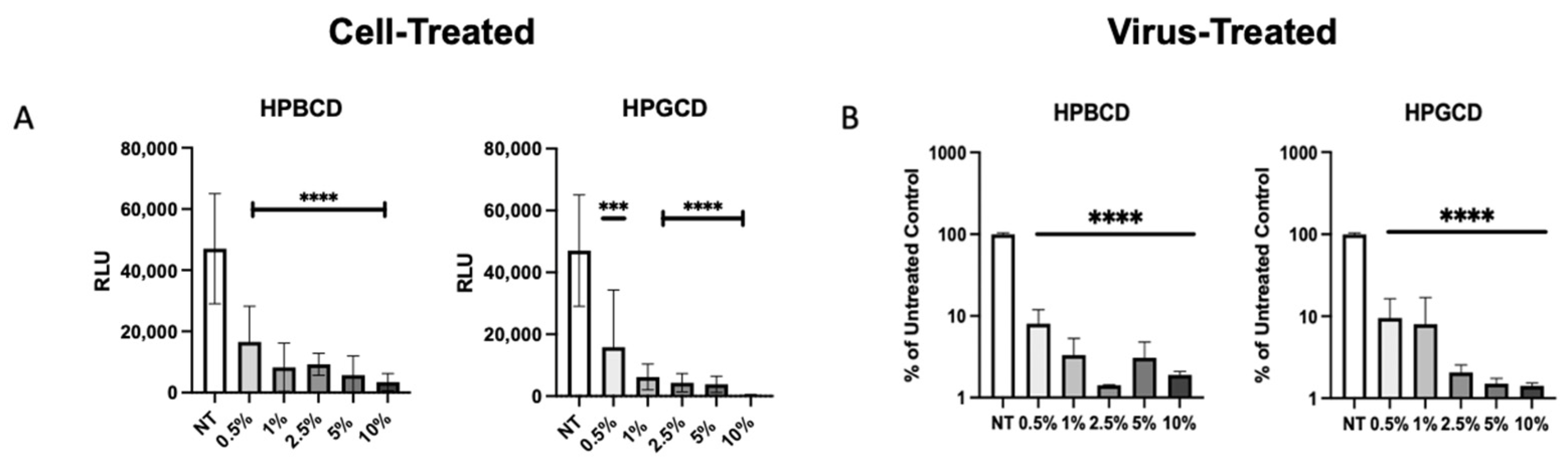
2.5. Cell-Treated and Virus-Treated Cyclodextrin Effect
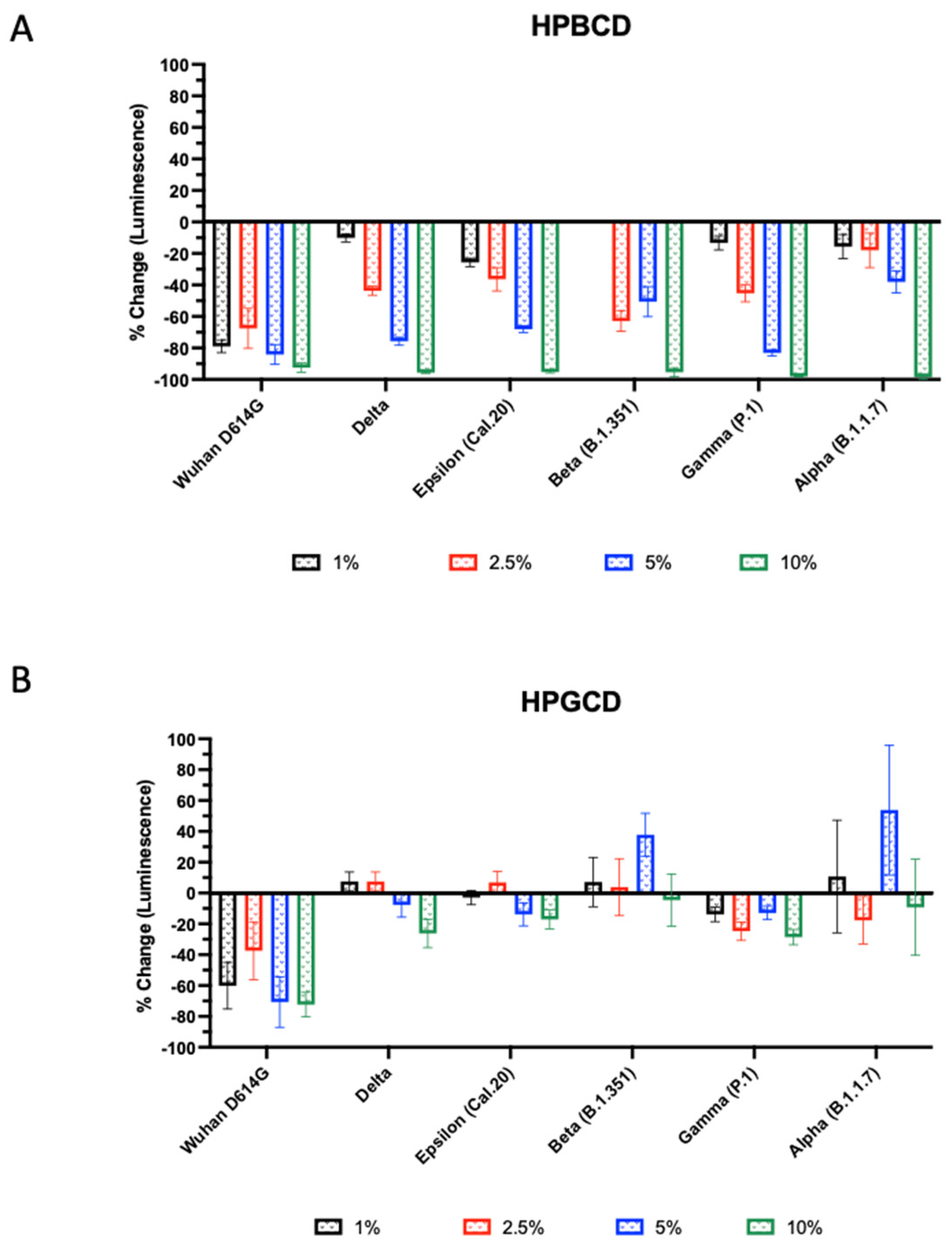
2.6. HPBCD Has Broad Effects across Different Enveloped Respiratory Viruses
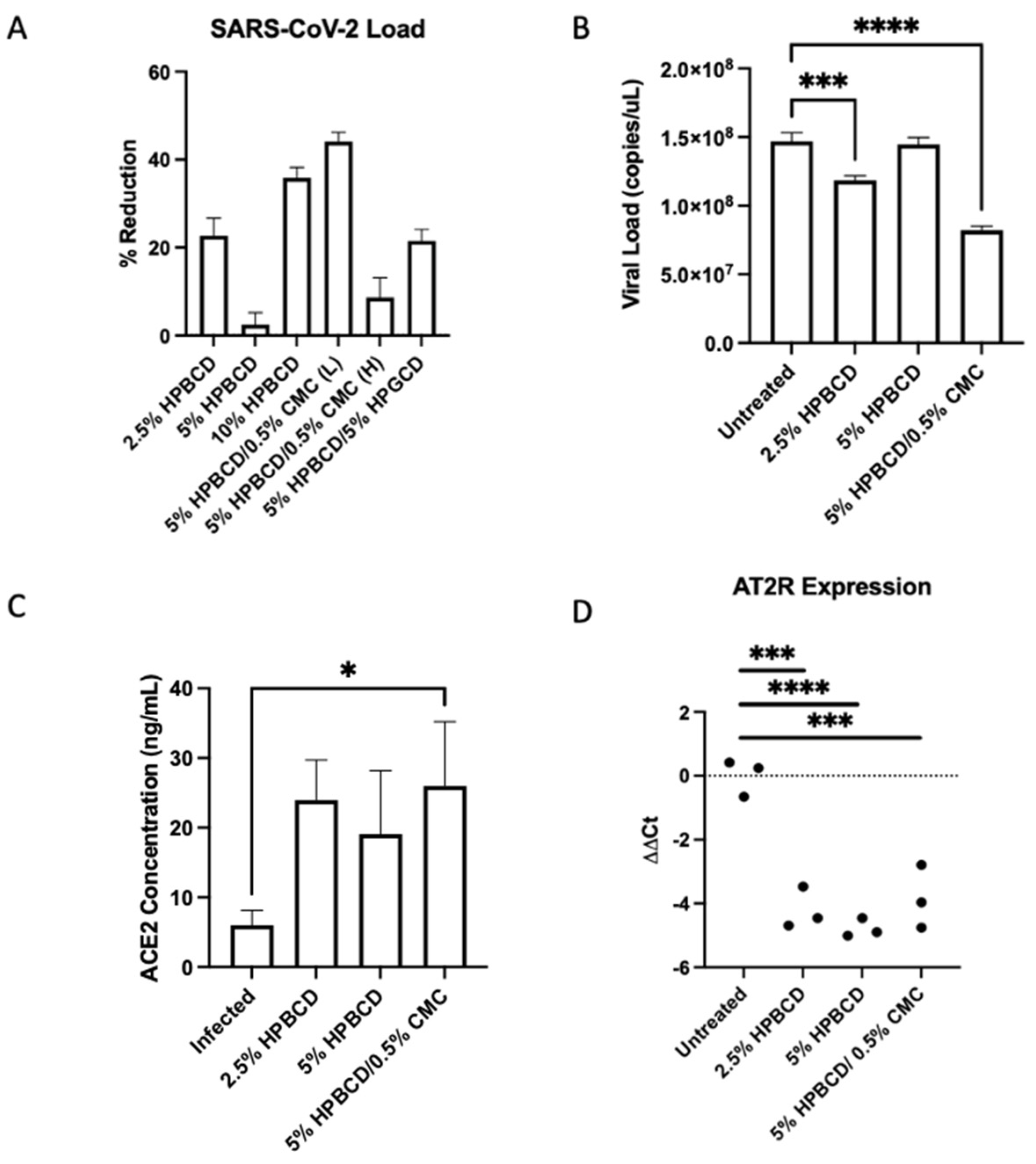
2.7. HPBCD Reduces SARS-CoV-2 Infection in Human Bronchial Cells
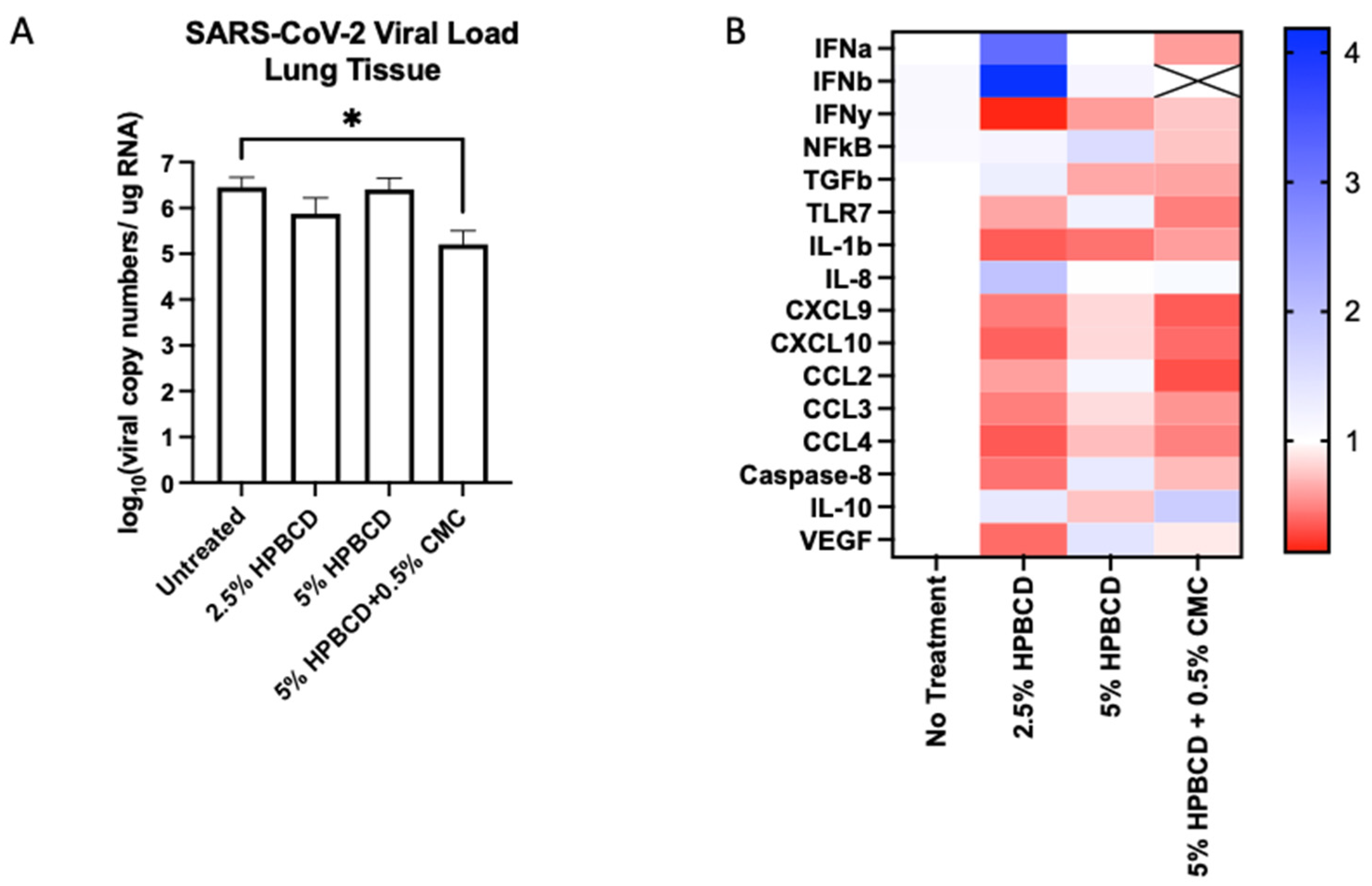
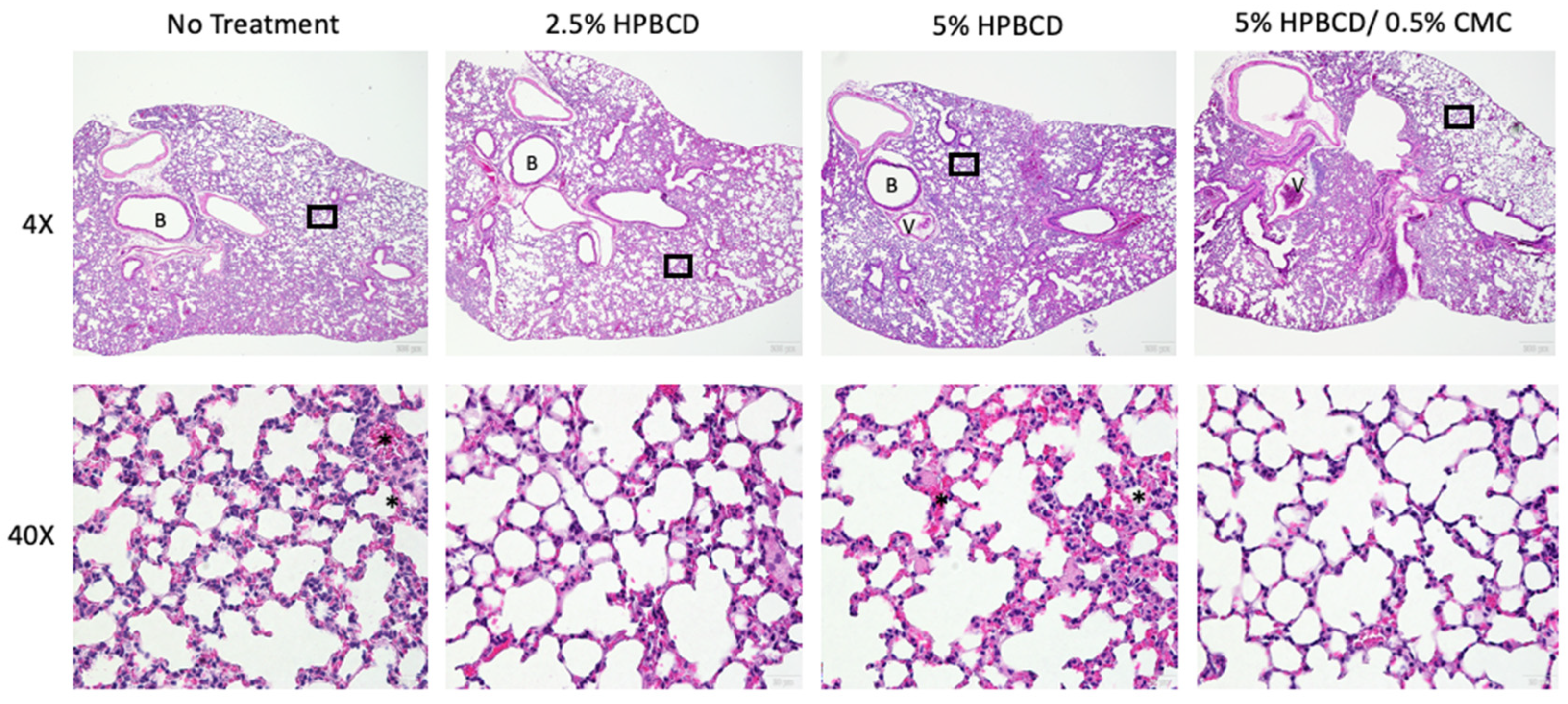
2.8. HPBCD Reduces SARS-CoV-2 Deposition in Mouse Lung Tissue
3. Discussion
4. Materials and Methods
4.1. Production of SARS-CoV-2 Pseudotypes
4.2. Cell Lines
4.3. Nasal Air–Liquid Interface (ALI) Cultures
4.4. VSV Pseudotype Transfection
4.5. Barrier Formulations
4.6. rVSV SARS-CoV-2 Pseudotype Infection Studies
4.7. Cholesterol Assay
4.8. Virus-Treated CD Studies
4.9. In Silico Molecular Docking
4.10. GFP Lentivirus Infection Studies
4.11. SARS-CoV-2 Culture
4.12. In Vitro/In Vivo Antiviral Activity Testing
4.13. Gene Expression RT-PCR
4.14. In Vivo K18-hACE2 Study
4.15. ACE2 ELISA
4.16. Hematoxylin and Eosin Staining
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molinari, N.A.; Ortega-Sanchez, I.R.; Messonnier, M.L.; Thompson, W.W.; Wortley, P.M.; Weintraub, E.; Bridges, C.B. The Annual Impact of Seasonal Influenza in the US: Measuring Disease Burden and Costs. Vaccine 2007, 25, 5086–5096. [Google Scholar] [CrossRef]
- DeMartino, J.M.N.; Foroughi, C.; Gaburo, K.; Radtke, T.; Kirson, N.; Krishnarajah, G. Annual Economic burden of respiratory syncytial virus infections among the 60+ population in the United States. In Proceedings of the AMCP Nexus, National Harbor, MD, USA, 11–14 October 2022. [Google Scholar]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls; StatPearls Publishing Copyright © 2022; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Rabaan, A.A.; Al-Ahmed, S.H.; Haque, S.; Sah, R.; Tiwari, R.; Malik, Y.S.; Dhama, K.; Yatoo, M.I.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. SARS-CoV-2, SARS-CoV, and MERS-COV: A comparative overview. Infez. Med. 2020, 28, 174–184. [Google Scholar]
- Szente, L.; Singhal, A.; Domokos, A.; Song, B. Cyclodextrins: Assessing the Impact of Cavity Size, Occupancy, and Substitutions on Cytotoxicity and Cholesterol Homeostasis. Molecules 2018, 23, 1228. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.T.; Serrano, M.L.; Pujol, F.H.; Rangel, H.R. Role of changes in SARS-CoV-2 spike protein in the interaction with the human ACE2 receptor: An in silico analysis. Excli J. 2020, 19, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Bridges, J.P.; Vladar, E.K.; Huang, H.; Mason, R.J. Respiratory epithelial cell responses to SARS-CoV-2 in COVID-19. Thorax 2022, 77, 203–209. [Google Scholar] [CrossRef]
- Bansil, R.; Turner, B.S. The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef]
- Sousa, I.P., Jr.; Carvalho, C.A.M.; Gomes, A.M.O. Current Understanding of the Role of Cholesterol in the Life Cycle of Alphaviruses. Viruses 2020, 13, 35. [Google Scholar] [CrossRef]
- Graham, D.R.; Chertova, E.; Hilburn, J.M.; Arthur, L.O.; Hildreth, J.E. Cholesterol depletion of human immunodeficiency virus type 1 and simian immunodeficiency virus with beta-cyclodextrin inactivates and permeabilizes the virions: Evidence for virion-associated lipid rafts. J. Virol. 2003, 77, 8237–8248. [Google Scholar] [CrossRef]
- Liao, Z.; Cimakasky, L.M.; Hampton, R.; Nguyen, D.H.; Hildreth, J.E. Lipid rafts and HIV pathogenesis: Host membrane cholesterol is required for infection by HIV type 1. AIDS Res. Hum. Retroviruses 2001, 17, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.; Scott, R.C. 2-Hydroxypropyl-beta-cyclodextrin (HP-beta-CD): A toxicology review. Food Chem. Toxicol. 2005, 43, 1451–1459. [Google Scholar] [CrossRef]
- di Cagno, M.P. The Potential of Cyclodextrins as Novel Active Pharmaceutical Ingredients: A Short Overview. Molecules 2016, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Salem, L.B.; Bosquillon, C.; Dailey, L.A.; Delattre, L.; Martin, G.P.; Evrard, B.; Forbes, B. Sparing methylation of beta-cyclodextrin mitigates cytotoxicity and permeability induction in respiratory epithelial cell layers in vitro. J. Control Release 2009, 136, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Otter, C.J.; Fausto, A.; Tan, L.H.; Khosla, A.S.; Cohen, N.A.; Weiss, S.R. Infection of primary nasal epithelial cells differentiates among lethal and seasonal human coronaviruses. Proc. Natl. Acad. Sci. USA 2023, 120, e2218083120. [Google Scholar] [CrossRef]
- Lee, R.J.; Workman, A.D.; Carey, R.M.; Chen, B.; Rosen, P.L.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Cohen, N.A. Fungal Aflatoxins Reduce Respiratory Mucosal Ciliary Function. Sci. Rep. 2016, 6, 33221. [Google Scholar] [CrossRef]
- Duadi, D.; Shabairou, N.; Primov-Fever, A.; Zalevsky, Z. Non-contact optical in-vivo sensing of cilia motion by analyzing speckle patterns. Sci. Rep. 2022, 12, 16614. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- McCallum, M.; Bassi, J.; De Marco, A.; Chen, A.; Walls, A.C.; Di Iulio, J.; Tortorici, M.A.; Navarro, M.J.; Silacci-Fregni, C.; Saliba, C.; et al. SARS-CoV-2 immune evasion by the B.1.427/B.1.429 variant of concern. Science 2021, 373, 648–654. [Google Scholar] [CrossRef]
- Mehra, R.; Kepp, K.P. Structure and Mutations of SARS-CoV-2 Spike Protein: A Focused Overview. ACS Infect. Dis. 2022, 8, 29–58. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Syed, A.A.S.; Fahira, A.; Shi, Y. Structural Analysis of the SARS-CoV-2 Omicron Variant Proteins. Research 2021, 2021, 9769586. [Google Scholar] [CrossRef]
- Chen, H.Y.; Huang, C.; Tian, L.; Huang, X.; Zhang, C.; Llewellyn, G.N.; Rogers, G.L.; Andresen, K.; O’Gorman, M.R.G.; Chen, Y.W.; et al. Cytoplasmic Tail Truncation of SARS-CoV-2 Spike Protein Enhances Titer of Pseudotyped Vectors but Masks the Effect of the D614G Mutation. J. Virol. 2021, 95, e0096621. [Google Scholar] [CrossRef] [PubMed]
- Fukushi, S.; Watanabe, R.; Taguchi, F. Pseudotyped vesicular stomatitis virus for analysis of virus entry mediated by SARS coronavirus spike proteins. Methods Mol. Biol. 2008, 454, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, X.E. Construction and applications of SARS-CoV-2 pseudoviruses: A mini review. Int. J. Biol. Sci. 2021, 17, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.J.; Liao, Q.W.; Xu, Y.C.; Zhao, Q.; Wu, B.L.; Ye, R.D. Anti-inflammatory signaling through G protein-coupled receptors. Acta Pharmacol. Sin. 2020, 41, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Sorice, M.; Misasi, R.; Riitano, G.; Manganelli, V.; Martellucci, S.; Longo, A.; Garofalo, T.; Mattei, V. Targeting Lipid Rafts as a Strategy Against Coronavirus. Front. Cell Dev. Biol. 2020, 8, 618296. [Google Scholar] [CrossRef]
- Dai, J.; Wang, H.; Liao, Y.; Tan, L.; Sun, Y.; Song, C.; Liu, W.; Qiu, X.; Ding, C. Coronavirus Infection and Cholesterol Metabolism. Front. Immunol. 2022, 13, 791267. [Google Scholar] [CrossRef]
- Pennington, A.K.; Ratcliffe, J.H.; Wilson, C.G.; Hardy, J.G. The influence of solution viscosity on nasal spray deposition and clearance. Int. J. Pharm. 1988, 43, 221–224. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, D.X.; Tam, J.P. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem. Biophys. Res. Commun. 2008, 369, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, W.; Fan, M.; Zhang, J.; Peng, Y.; Huang, F.; Wang, N.; He, L.; Zhang, L.; Holmdahl, R.; et al. Dependence of SARS-CoV-2 infection on cholesterol-rich lipid raft and endosomal acidification. Comput. Struct. Biotechnol. J. 2021, 19, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Rápalo, S.N.; De Jesús-González, L.A.; Cordero-Rivera, C.D.; Farfan-Morales, C.N.; Osuna-Ramos, J.F.; Martínez-Mier, G.; Quistián-Galván, J.; Muñoz-Pérez, A.; Bernal-Dolores, V.; del Ángel, R.M.; et al. Cholesterol-Rich Lipid Rafts as Platforms for SARS-CoV-2 Entry. Front. Immunol. 2021, 12, 796855. [Google Scholar] [CrossRef] [PubMed]
- Salvi, V.; Nguyen, H.O.; Sozio, F.; Schioppa, T.; Gaudenzi, C.; Laffranchi, M.; Scapini, P.; Passari, M.; Barbazza, I.; Tiberio, L.; et al. SARS-CoV-2-associated ssRNAs activate inflammation and immunity via TLR7/8. JCI Insight 2021, 6, e150542. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Ichikawa, N. The pivotal role of the angiotensin-II–NF-κB axis in the development of COVID-19 pathophysiology. Hypertens. Res. 2021, 44, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Moreau, G.B.; Burgess, S.L.; Sturek, J.M.; Donlan, A.N.; Petri, W.A.; Mann, B.J. Evaluation of K18-hACE2 Mice as a Model of SARS-CoV-2 Infection. Am. J. Trop. Med. Hyg. 2020, 103, 1215–1219. [Google Scholar] [CrossRef]
- Kutter, J.S.; Spronken, M.I.; Fraaij, P.L.; Fouchier, R.A.; Herfst, S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 2018, 28, 142–151. [Google Scholar] [CrossRef]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef]
- Hoffmann, M.; Krüger, N.; Schulz, S.; Cossmann, A.; Rocha, C.; Kempf, A.; Nehlmeier, I.; Graichen, L.; Moldenhauer, A.S.; Winkler, M.S.; et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell 2022, 185, 447–456.e411. [Google Scholar] [CrossRef]
- Meldrum, O.W.; Chotirmall, S.H. Mucus, Microbiomes and Pulmonary Disease. Biomedicines 2021, 9, 675. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Forier, K.; Steukers, L.; Van Vlierberghe, S.; Dubruel, P.; Braeckmans, K.; Glorieux, S.; Nauwynck, H.J. Immobilization of pseudorabies virus in porcine tracheal respiratory mucus revealed by single particle tracking. PLoS ONE 2012, 7, e51054. [Google Scholar] [CrossRef]
- CHMP. Cyclodextrins Used as Excipients; European Medicines Agency: London, UK, 2017; 16p. [Google Scholar]
- Bezerra, B.B.; Silva, G.P.D.d.; Coelho, S.V.A.; Correa, I.A.; Souza, M.R.M.d.; Macedo, K.V.G.; Matos, B.M.; Tanuri, A.; Matassoli, F.L.; Costa, L.J.d.; et al. Hydroxypropyl-beta-cyclodextrin (HP-BCD) inhibits SARS-CoV-2 replication and virus-induced inflammatory cytokines. Antivir. Res. 2022, 205, 105373. [Google Scholar] [CrossRef]
- Ma, D.Q.; Rajewski, R.A.; Vander Velde, D.; Stella, V.J. Comparative effects of (SBE)7m-beta-CD and HP-beta-CD on the stability of two anti-neoplastic agents, melphalan and carmustine. J. Pharm. Sci. 2000, 89, 275–287. [Google Scholar] [CrossRef]
- Du, J.; Shao, X.; Bouteiller, J.-M.C.; Lu, A.; Asante, I.; Louie, S.; Humayun, M.S.; Lazzi, G. Computational optimization of delivery parameters to guide the development of targeted Nasal spray. Sci. Rep. 2023, 13, 4099. [Google Scholar] [CrossRef]
- Irie, T.; Uekama, K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef]
- Zidovetzki, R.; Levitan, I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim. Biophys. Acta 2007, 1768, 1311–1324. [Google Scholar] [CrossRef]
- Danthi, P.; Chow, M. Cholesterol removal by methyl-beta-cyclodextrin inhibits poliovirus entry. J. Virol. 2004, 78, 33–41. [Google Scholar] [CrossRef]
- Vance, J.E.; Karten, B. Niemann-Pick C disease and mobilization of lysosomal cholesterol by cyclodextrin. J. Lipid Res. 2014, 55, 1609–1621. [Google Scholar] [CrossRef] [PubMed]
- Ory, D.S.; Ottinger, E.A.; Farhat, N.Y.; King, K.A.; Jiang, X.; Weissfeld, L.; Berry-Kravis, E.; Davidson, C.D.; Bianconi, S.; Keener, L.A.; et al. Intrathecal 2-hydroxypropyl-β-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: A non-randomised, open-label, phase 1-2 trial. Lancet 2017, 390, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Raïch-Regué, D.; Tenorio, R.; Fernández de Castro, I.; Tarrés-Freixas, F.; Sachse, M.; Perez-Zsolt, D.; Muñoz-Basagoiti, J.; Fernández-Sánchez, S.Y.; Gallemí, M.; Ortega-González, P.; et al. β-Cyclodextrins as affordable antivirals to treat coronavirus infection. Biomed. Pharmacother. 2023, 164, 114997. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Zhang, X.; Tian, B.R. Selective modifications at the different positions of cyclodextrins: A review of strategies. Turk. J. Chem. 2020, 44, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Gad, S.C.; Spainhour, C.B.; Shoemake, C.; Pallman, D.R.S.; Stricker-Krongrad, A.; Downing, P.A.; Seals, R.E.; Eagle, L.A.; Polhamus, K.; Daly, J. Tolerable Levels of Nonclinical Vehicles and Formulations Used in Studies by Multiple Routes in Multiple Species With Notes on Methods to Improve Utility. Int. J. Toxicol. 2016, 35, 95–178. [Google Scholar] [CrossRef] [PubMed]
- al-Nakib, W.; Higgins, P.G.; Barrow, G.I.; Tyrrell, D.A.; Andries, K.; Vanden Bussche, G.; Taylor, N.; Janssen, P.A. Suppression of colds in human volunteers challenged with rhinovirus by a new synthetic drug (R61837). Antimicrob. Agents Chemother. 1989, 33, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Ganjali Koli, M.; Fogolari, F. Exploring the role of cyclodextrins as a cholesterol scavenger: A molecular dynamics investigation of conformational changes and thermodynamics. Sci. Rep. 2023, 13, 21765. [Google Scholar] [CrossRef]
- Trotta, F.; Loftsson, T.; Gaud, R.S.; Trivedi, R.; Shende, P. Integration of cyclodextrins and associated toxicities: A roadmap for high quality biomedical applications. Carbohydr. Polym. 2022, 295, 119880. [Google Scholar] [CrossRef]
- Lee, R.J.; Hariri, B.M.; McMahon, D.B.; Chen, B.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Jiang, P.; Margolskee, R.F.; et al. Bacterial d-amino acids suppress sinonasal innate immunity through sweet taste receptors in solitary chemosensory cells. Sci. Signal 2017, 10, 495. [Google Scholar] [CrossRef]
- Patel, N.N.; Triantafillou, V.; Maina, I.W.; Workman, A.D.; Tong, C.C.L.; Kuan, E.C.; Papagiannopoulos, P.; Bosso, J.V.; Adappa, N.D.; Palmer, J.N.; et al. Fungal extracts stimulate solitary chemosensory cell expansion in noninvasive fungal rhinosinusitis. Int. Forum Allergy Rhinol. 2019, 9, 730–737. [Google Scholar] [CrossRef]
- Dong, T.; Dave, P.; Yoo, E.; Ebright, B.; Ahluwalia, K.; Zhou, E.; Asante, I.; Salimova, M.; Pei, H.; Lin, T.; et al. NAP1051, a Lipoxin A4 Biomimetic Analogue, Demonstrates Antitumor Activity Against the Tumor Microenvironment. Mol. Cancer Ther. 2021, 20, 2384–2397. [Google Scholar] [CrossRef]
| Cyclodextrins | Nominal Molecular Weight (g/mol) | Inner Cavity Diameter (A) | Inner Cavity Diameter (A) | Cavity Volume (A3) | Number of Subunits | R-Group(s) |
|---|---|---|---|---|---|---|
| HPBCD 1 | 1501 | 6.0–6.4 | 15.4 | 262 | 7 | R = -H or [CH2CH(CH3)O]nH |
| SBECD 2 | 2242 | R = -H or CH2CH2CH2CH2SO3Na | ||||
| CRYSMEB 3 | 1191 | R = -H or -CH3 | ||||
| HPGCD 4 | 1576 | 7.5–8.3 | 17.5 | 427 | 8 | R = [CH2CH(CH3)O]nH, n = 0, 1, 2 |
| Search Space Region | S1/S2 | S2 | ||||
|---|---|---|---|---|---|---|
| Variant | Ligand | Binding Score (Kcal/mol) | Kd (μM) | Ligand | Binding Score (Kcal/mol) | Kd (μM) |
| Wuhan | HPβCD | −6.9 | 8.70 | HPβCD | −7.4 | 3.74 |
| HPγCD | −7.1 | 6.21 | HPγCD | −6.0 | 39.79 | |
| Alpha | HPβCD | −6.6 | 14.45 | HPβCD | −6.9 | 8.70 |
| HPγCD | −6.6 | 14.45 | HPγCD | −5.8 | 55.77 | |
| Beta | HPβCD | −6.8 | 10.31 | HPβCD | −6.4 | 20.25 |
| HPγCD | −6.9 | 8.70 | HPγCD | −6.4 | 20.25 | |
| Delta | HPβCD | −6.8 | 10.31 | HPβCD | −7.6 | 2.67 |
| HPγCD | −6.8 | 10.31 | HPγCD | −6.0 | 39.79 | |
| Epsilon | HPβCD | −8.1 | 1.15 | HPβCD | −6.2 | 28.38 |
| HPγCD | −6.4 | 20.25 | HPγCD | −6.5 | 17.10 | |
| Gamma | HPβCD | −6.6 | 14.45 | HPβCD | −6.2 | 28.38 |
| HPγCD | −7.3 | 4.43 | HPγCD | −6.8 | 10.31 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, A.; Ebright, B.; Naik, A.; Tan, H.L.; Cohen, N.A.; Bouteiller, J.-M.C.; Lazzi, G.; Louie, S.G.; Humayun, M.S.; Asante, I. Hydroxypropyl-Beta Cyclodextrin Barrier Prevents Respiratory Viral Infections: A Preclinical Study. Int. J. Mol. Sci. 2024, 25, 2061. https://doi.org/10.3390/ijms25042061
Lu A, Ebright B, Naik A, Tan HL, Cohen NA, Bouteiller J-MC, Lazzi G, Louie SG, Humayun MS, Asante I. Hydroxypropyl-Beta Cyclodextrin Barrier Prevents Respiratory Viral Infections: A Preclinical Study. International Journal of Molecular Sciences. 2024; 25(4):2061. https://doi.org/10.3390/ijms25042061
Chicago/Turabian StyleLu, Angela, Brandon Ebright, Aditya Naik, Hui L. Tan, Noam A. Cohen, Jean-Marie C. Bouteiller, Gianluca Lazzi, Stan G. Louie, Mark S. Humayun, and Isaac Asante. 2024. "Hydroxypropyl-Beta Cyclodextrin Barrier Prevents Respiratory Viral Infections: A Preclinical Study" International Journal of Molecular Sciences 25, no. 4: 2061. https://doi.org/10.3390/ijms25042061
APA StyleLu, A., Ebright, B., Naik, A., Tan, H. L., Cohen, N. A., Bouteiller, J.-M. C., Lazzi, G., Louie, S. G., Humayun, M. S., & Asante, I. (2024). Hydroxypropyl-Beta Cyclodextrin Barrier Prevents Respiratory Viral Infections: A Preclinical Study. International Journal of Molecular Sciences, 25(4), 2061. https://doi.org/10.3390/ijms25042061







