Are TaNAC Transcription Factors Involved in Promoting Wheat Yield by cis-Regulation of TaCKX Gene Family?
Abstract
1. Introduction
2. Results
2.1. Identification of TaNAC Transcription Factor Binding Sites in TaCKX GFMs cis-Regulatory Sequences
2.2. Sequencing of Selected TaCKX GFM cis-Regulatory Regions from Kontesa and Ostka Cultivars
2.3. Mapping of Conserved Motifs in the Kontesa and Ostka Cultivars
2.4. Identification of TaNAC Transcription Factor Binding Sites in Selected TaCKX GFMs cis-Regulatory Sequences of Kontesa and Ostka cvs. and Selection of TaNAC Genes
2.5. NAC Domain Identification, Prediction of Protein Structure, and Various Attributes of Selected TaNACs
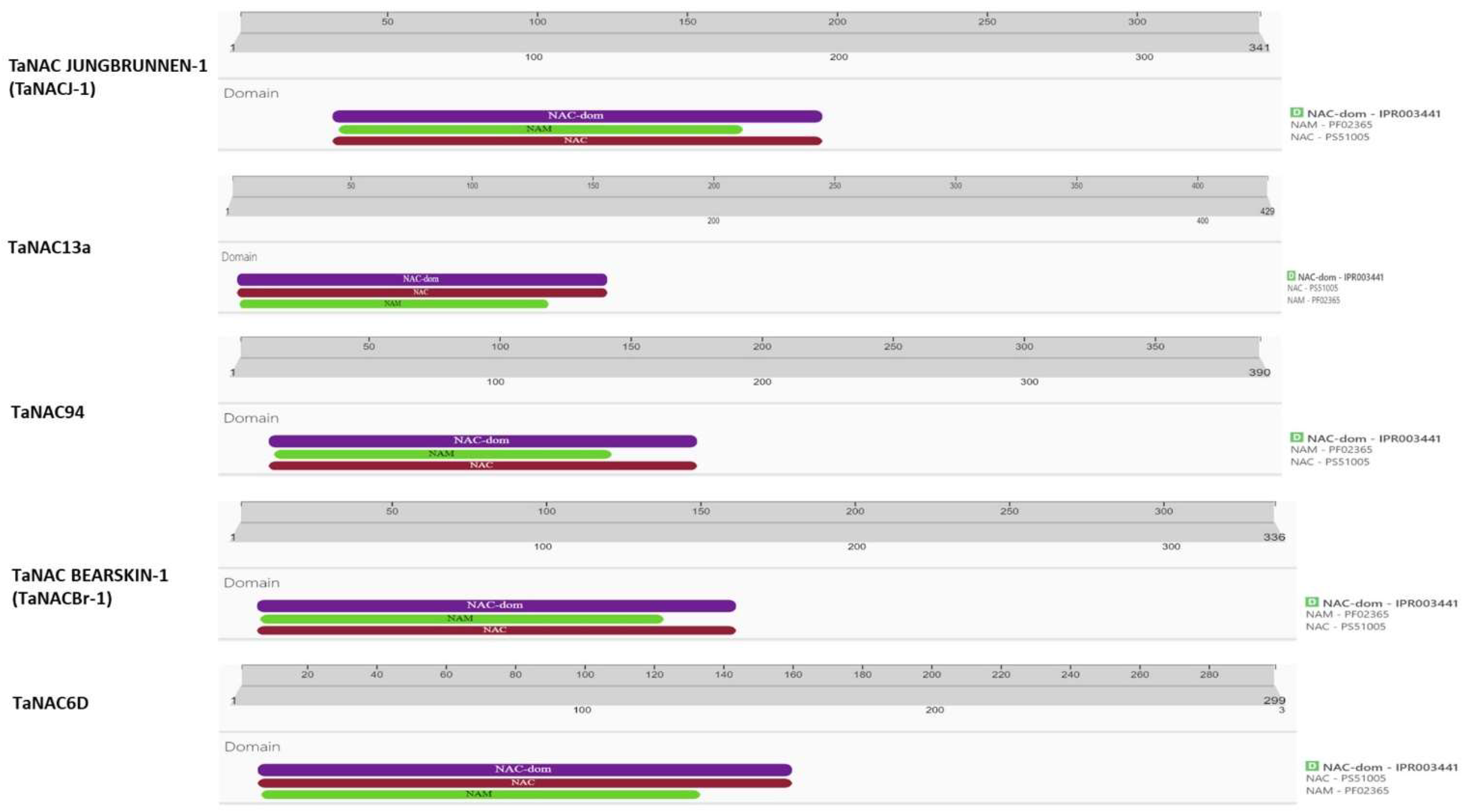
2.5.1. Identification of the TaNAC Domain in Selected TaNAC Proteins
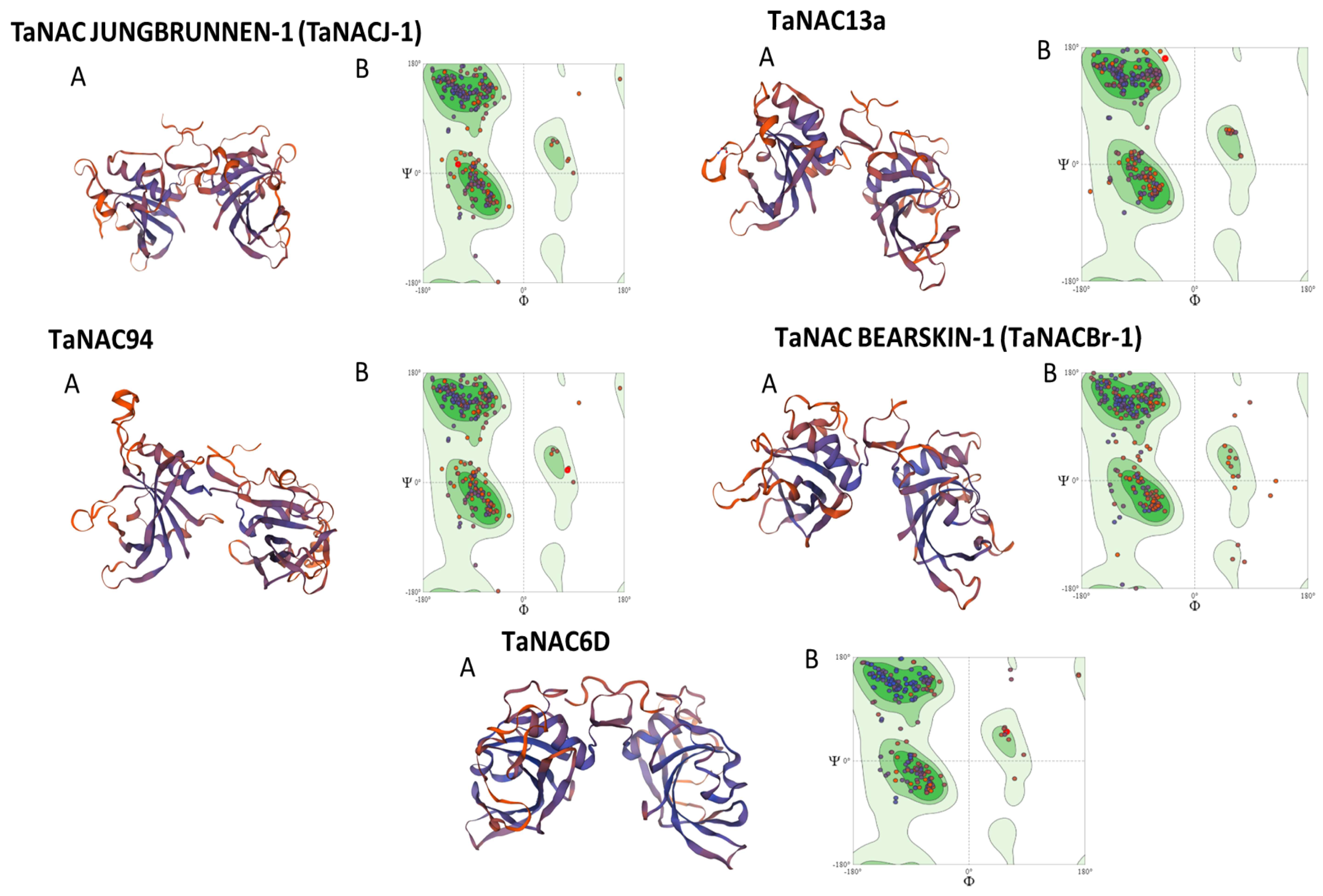
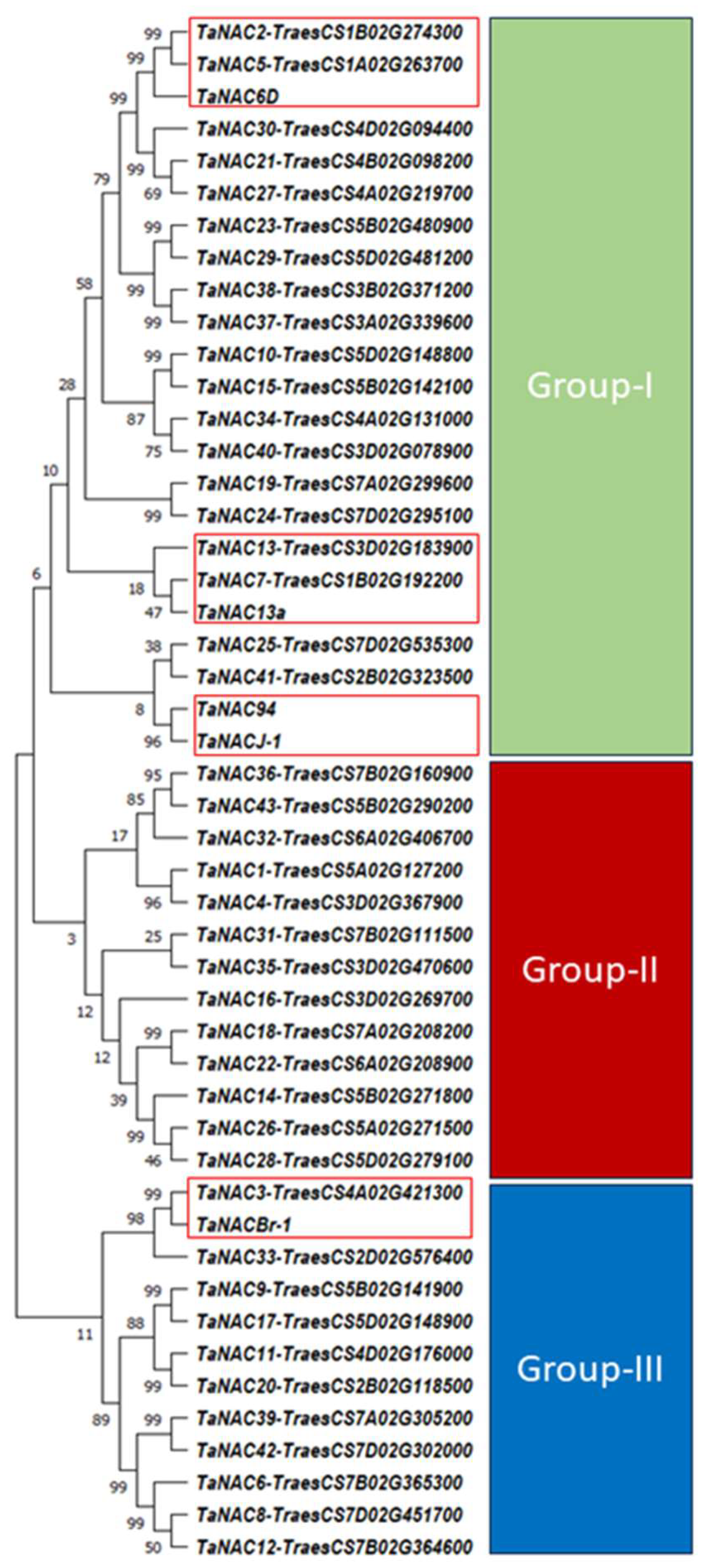
2.5.2. Structure Prediction and Phylogenetic Analysis of Selected TaNACs
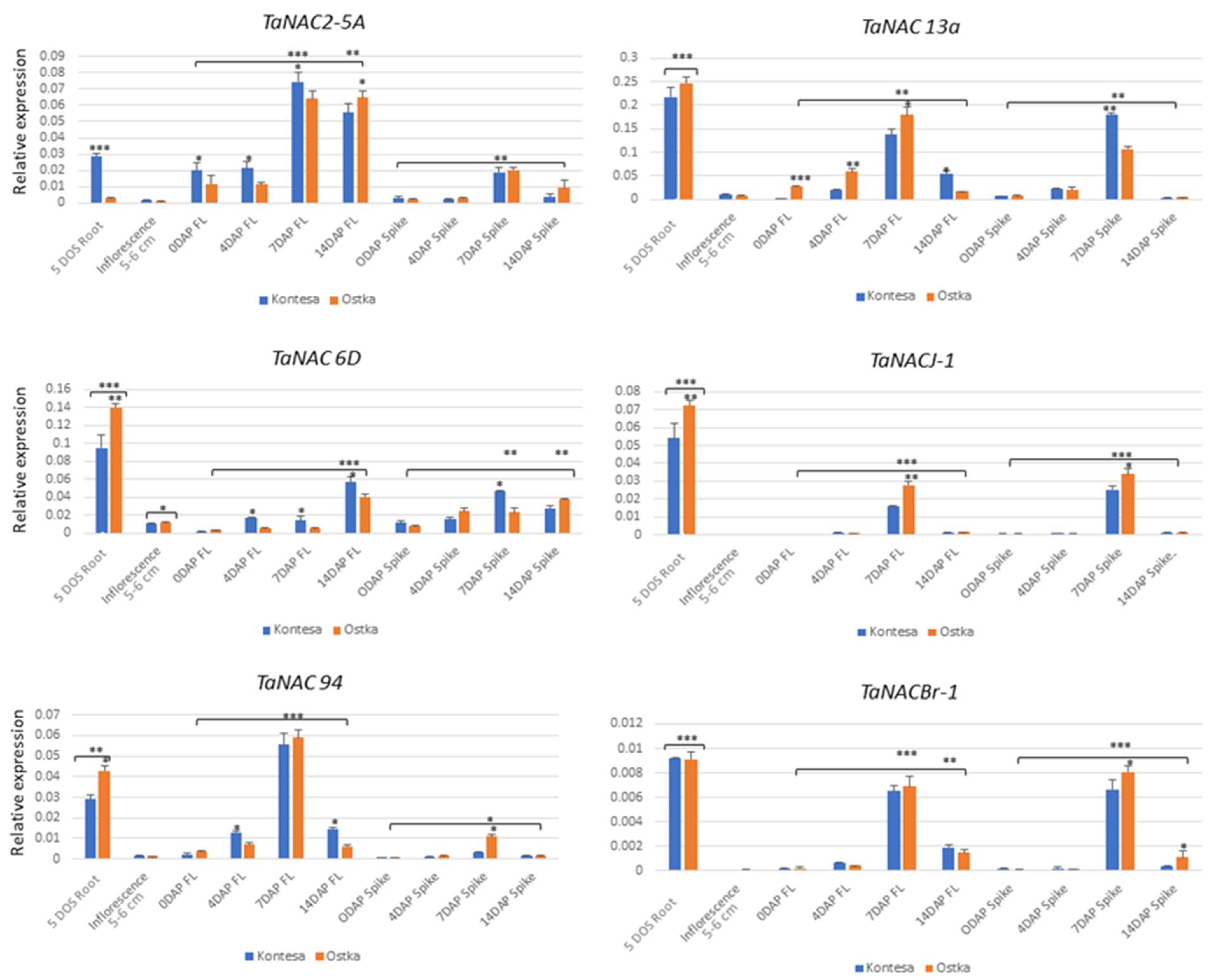
2.6. Analysis of Expression of Selected TaNACs in Different Tissues of Developing Wheat Plants
3. Discussion
3.1. TaNAC TFs Orchestrate cis-Regulation of TaCKX GFMs
3.2. Structural Characterization and Various Attributes Reveal the Recognizable Pattern of Selected TaNACs
3.3. Selected TaNACs Are Preferentially Expressed in the Roots
3.4. Inflorescence Is Not the Primary Site for the Expression of Our Selected TaNAC GFMs
3.5. The Selected TaNACs Showed a Distinct Preference for Expression in Flag Leaves and Spikes during the 7 and 14 DAP Periods
4. Materials and Methods
4.1. Acquisition of TaCKX GFMs’ cis-Regulatory Sequences and Identification of TaNAC Transcription Factor Binding Sites
4.2. Plant Materials and Growth Conditions
4.3. Genomic DNA Extraction and PCR Amplification
4.4. Cloning and Sequencing of Selected TaCKX cis-Regulatory Sequences of the Kontesa and Ostka Cultivars
4.5. Motif Conservation Analysis
4.6. Identification of TaNAC Transcription Factor Binding Sites in Selected TaCKX GFMs
4.7. NAC Domain Identification, Prediction of Protein Structure, and Phylogenetic Analysis
4.8. RNA Extraction and cDNA Synthesis
4.9. Quantitative RT-qPCR
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dvorak, J.; Akhunov, E.D. Tempos of Gene Locus Deletions and Duplications and Their Relationship to Recombination Rate During Diploid and Polyploid Evolution in the Aegilops-Triticum Alliance. Genetics 2005, 171, 323–332. [Google Scholar] [CrossRef]
- Griffiths, S.; Sharp, R.; Foote, T.N.; Bertin, I.; Wanous, M.; Reader, S.; Colas, I.; Moore, G. Molecular characterization of Ph1 as a major chromosome pairing locus in polyploid wheat. Nature 2006, 439, 749–752. [Google Scholar] [CrossRef]
- Paux, E.; Roger, D.; Badaeva, E.; Gay, G.; Bernard, M.; Sourdille, P.; Feuillet, C. Characterizing the composition and evolution of homoeologous genomes in hexaploid wheat through BAC-end sequencing on chromosome 3B. Plant J. 2006, 48, 463–474. [Google Scholar] [CrossRef]
- Nadolska-Orczyk, A.; Rajchel, I.K.; Orczyk, W.; Gasparis, S. Major genes determining yield-related traits in wheat and barley. Theor. Appl. Genet. 2017, 130, 1081–1098. [Google Scholar] [CrossRef]
- Shewry, P.R.; Underwood, C.; Wan, Y.; Lovegrove, A.; Bhandari, D.; Toole, G.; Mills, E.C.; Denyer, K.; Mitchell, R.A. Storage product synthesis and accumulation in developing grains of wheat. J. Cereal Sci. 2009, 50, 106–112. [Google Scholar] [CrossRef]
- Dowla, M.N.U.; Edwards, I.; O’Hara, G.; Islam, S.; Ma, W. Developing Wheat for Improved Yield and Adaptation Under a Changing Climate: Optimization of a Few Key Genes. Engineering 2018, 4, 514–522. [Google Scholar] [CrossRef]
- Hochman, Z.; Gobbett, D.L.; Horan, H. Climate trends account for stalled wheat yields in Australia since 1990. Glob. Chang. Biol. 2017, 23, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Jameson, P.E.; Song, J. Cytokinin: A key driver of seed yield. J. Exp. Bot. 2015, 67, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Yamburenko, M.V.; Kieber, J.J.; Schaller, G.E. Dynamic patterns of expression for genes regulating cytokinin metabolism and signaling during rice inflorescence development. PLoS ONE 2017, 12, e0176060. [Google Scholar] [CrossRef] [PubMed]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T. Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ. 2018, 42, 998–1018. [Google Scholar] [CrossRef]
- Gao, S.; Xiao, Y.; Xu, F.; Gao, X.; Cao, S.; Zhang, F.; Wang, G.; Sanders, D.; Chu, C. Cytokinin-dependent regulatory module underlies the maintenance of zinc nutrition in rice. New Phytol. 2019, 224, 202–215. [Google Scholar] [CrossRef]
- Pavlů, J.; Novák, J.; Koukalová, V.; Luklová, M.; Brzobohatý, B.; Černý, M. Cytokinin at the Crossroads of Abiotic Stress Signalling Pathways. Int. J. Mol. Sci. 2018, 19, 2450. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.E.; Street, I.H.; Kieber, J.J. Cytokinin and the cell cycle. Curr. Opin. Plant Biol. 2014, 21, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, J.; Song, J.C.; Jameson, P.E. Cytokinin dehydrogenase: A genetic target for yield improvement in wheat. Plant Biotechnol. J. 2019, 18, 614–630. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Perry, L.; Kisiala, A.; Olechowski, H.; Emery, R.J.N. Cytokinin activity during early kernel development corresponds positively with yield potential and later stage ABA accumulation in field-grown wheat (Triticum aestivum L.). Planta 2020, 252, 76. [Google Scholar] [CrossRef]
- Brandizzi, F. Divide, expand, differentiate–new insights on plant organ growth through cytokinin signaling. Plant J. 2019, 97, 803–804. [Google Scholar] [CrossRef]
- Kudo, T.; Kiba, T.; Sakakibara, H. Metabolism and Long-distance Translocation of Cytokinins. J. Integr. Plant Biol. 2010, 52, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, B.; Bajguz, A.; Bocian, J.; Orczyk, W.; Nadolska-Orczyk, A. Genotype-Dependent Effect of Silencing of TaCKX1 and TaCKX2 on Phytohormone Crosstalk and Yield-Related Traits in Wheat. Int. J. Mol. Sci. 2021, 22, 11494. [Google Scholar] [CrossRef]
- Jabłoński, B.; Ogonowska, H.; Szala, K.; Bajguz, A.; Orczyk, W.; Nadolska-Orczyk, A. Silencing of TaCKX1 Mediates Expression of Other TaCKX Genes to Increase Yield Parameters in Wheat. Int. J. Mol. Sci. 2020, 21, 4809. [Google Scholar] [CrossRef]
- Jablonski, B.; Szala, K.; Przyborowski, M.; Bajguz, A.; Chmur, M.; Gasparis, S.; Orczyk, W.; Nadolska-Orczyk, A. TaCKX2.2 Genes Coordinate Expression of Other TaCKX Family Members, Regulate Phytohormone Content and Yield-Related Traits of Wheat. Int. J. Mol. Sci. 2021, 22, 4142. [Google Scholar] [CrossRef]
- Szala, K.; Ogonowska, H.; Lugowska, B.; Zmijewska, B.; Wyszynska, R.; Dmochowska-Boguta, M.; Orczyk, W.; Nadolska-Orczyk, A. Different sets of TaCKX genes affect yield-related traits in wheat plants grown in a controlled environment and in field conditions. BMC Plant Biol. 2020, 20, 496. [Google Scholar] [CrossRef] [PubMed]
- Ogonowska, H.; Barchacka, K.; Gasparis, S.; Jablonski, B.; Orczyk, W.; Dmochowska-Boguta, M.; Nadolska-Orczyk, A. Specificity of expression of TaCKX family genes in developing plants of wheat and their co-operation within and among organs. PLoS ONE 2019, 14, e0214239. [Google Scholar] [CrossRef]
- Luo, G.; Shen, L.; Zhao, S.; Li, R.; Song, Y.; Song, S.; Yu, K.; Yang, W.; Li, X.; Sun, J.; et al. Genome-wide identification of seed storage protein gene regulators in wheat through coexpression analysis. Plant J. 2021, 108, 1704–1720. [Google Scholar] [CrossRef] [PubMed]
- Szala, K.; Dmochowska-Boguta, M.; Bocian, J.; Orczyk, W.; Nadolska-Orczyk, A. Transgenerational Paternal Inheritance of TaCKX GFMs Expression Patterns Indicate a Way to Select Wheat Lines with Better Parameters for Yield-Related Traits. Int. J. Mol. Sci. 2023, 24, 8196. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Bocian, J.; Hameed, A.; Orczyk, W.; Nadolska-Orczyk, A. Cis-Regulation by NACs: A Promising Frontier in Wheat Crop Improvement. Int. J. Mol. Sci. 2022, 23, 15431. [Google Scholar] [CrossRef]
- Strader, L.; Weijers, D.; Wagner, D. Plant transcription factors—Being in the right place with the right company. Curr. Opin. Plant Biol. 2021, 65, 102136. [Google Scholar] [CrossRef]
- Li, J.; Xie, L.; Tian, X.; Liu, S.; Xu, D.; Jin, H.; Song, J.; Dong, Y.; Zhao, D.; Li, G.; et al. TaNAC100 acts as an integrator of seed protein and starch synthesis exerting pleiotropic effects on agronomic traits in wheat. Plant J. 2021, 108, 829–840. [Google Scholar] [CrossRef]
- Shen, L.; Luo, G.; Song, Y.; Xu, J.; Ji, J.; Zhang, C.; Gregová, E.; Yang, W.; Li, X.; Sun, J.; et al. A novel NAC family transcription factor SPR suppresses seed storage protein synthesis in wheat. Plant Biotechnol. J. 2020, 19, 992–1007. [Google Scholar] [CrossRef]
- A Chapman, E.; Orford, S.; Lage, J.; Griffiths, S. Delaying or delivering: Identification of novel NAM-1 alleles that delay senescence to extend wheat grain fill duration. J. Exp. Bot. 2021, 72, 7710–7728. [Google Scholar] [CrossRef]
- Mao, C.; He, J.; Liu, L.; Deng, Q.; Yao, X.; Liu, C.; Qiao, Y.; Li, P.; Ming, F. OsNAC2 integrates auxin and cytokinin pathways to modulate rice root development. Plant Biotechnol. J. 2019, 18, 429–442. [Google Scholar] [CrossRef]
- He, X.; Qu, B.; Li, W.; Zhao, X.; Teng, W.; Ma, W.; Ren, Y.; Li, B.; Li, Z.; Tong, Y. The nitrate inducible NAC transcription factor TaNAC2-5A controls nitrate response and increases wheat yield. Plant Physiol. 2015, 169, 1991–2005. [Google Scholar] [CrossRef]
- Li, W.; He, X.; Chen, Y.; Jing, Y.; Shen, C.; Yang, J.; Teng, W.; Zhao, X.; Hu, W.; Hu, M.; et al. A wheat transcription factor positively sets seed vigour by regulating the grain nitrate signal. New Phytol. 2019, 225, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, G.; Goossens, A.; Pauwels, L. Lessons from Domestication: Targeting Cis-Regulatory Elements for Crop Improvement. Trends Plant Sci. 2016, 21, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Pompili, V.; Piazza, S.; Li, M.; Varotto, C.; Malnoy, M. Transcriptional regulation of MdmiR285N microRNA in apple (Malus x domestica) and the heterologous plant system Arabidopsis thaliana. Hortic. Res. 2020, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Yokotani, N.; Hasegawa, Y.; Sato, M.; Hirakawa, H.; Kouzai, Y.; Nishizawa, Y.; Yamamoto, E.; Naito, Y.; Isobe, S. Transcriptome analysis of Clavibacter michiganensis subsp. michiganensis-infected tomatoes: A role of salicylic acid in the host response. BMC Plant Biol. 2021, 21, 476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, H.; Wu, J.; Jin, Y.; Xiao, S.; Li, T.; Liu, X.; Zhang, H.; Zhang, Z.; Su, J.; et al. Comprehensive transcriptome analysis of stem-differentiating xylem upon compression stress in Cunninghamia lanceolata. Front. Genet. 2022, 13, 843269. [Google Scholar] [CrossRef] [PubMed]
- Ooka, H.; Satoh, K.; Doi, K.; Nagata, T.; Otomo, Y.; Murakami, K.; Matsubara, K.; Osato, N.; Kawai, J.; Carninci, P.; et al. Comprehensive Analysis of NAC Family Genes in Oryza sativa and Arabidopsis thaliana. DNA Res. 2003, 10, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Quevillon, E.; Silventoinen, V.; Pillai, S.; Harte, N.; Mulder, N.; Apweiler, R.; Lopez, R. InterProScan: Protein domains identifier. Nucleic Acids Res. 2005, 33, W116–W120. [Google Scholar] [CrossRef]
- Guérin, C.; Dupuits, C.; Mouzeyar, S.; Roche, J. Insights into Four NAC Transcription Factors Involved in Grain Development and in Response to Moderate Heat in the Triticeae Tribe. Int. J. Mol. Sci. 2022, 23, 11672. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Li, S.; Wang, Z.; Cheng, X.; Li, F.; Mei, F.; Chen, N.; Kang, Z. Regulatory changes in TaSNAC8-6A are associated with drought tolerance in wheat seedlings. Plant Biotechnol. J. 2019, 18, 1078–1092. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Zhang, H.; Qian, X.; Li, A.; Zhao, G.; Jing, R. TaNAC2, a NAC-type wheat transcription factor conferring enhanced multiple abiotic stress tolerances in Arabidopsis. J. Exp. Bot. 2012, 63, 2933–2946. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Zhang, G.; Sun, Y.-F.; Zhu, L.; Xu, L.S.; Chen, X.M.; Liu, B.; Yu, Y.-T.; Wang, X.-J.; Huang, L.L.; et al. TaNAC8, a novel NAC transcription factor gene in wheat, responds to stripe rust pathogen infection and abiotic stresses. Physiol. Mol. Plant Pathol. 2010, 74, 394–402. [Google Scholar] [CrossRef]
- Zhou, W.; Qian, C.; Li, R.; Zhou, S.; Zhang, R.; Xiao, J.; Wang, X.; Zhang, S.; Xing, L.; Cao, A. TaNAC6s are involved in the basal and broad-spectrum resistance to powdery mildew in wheat. Plant Sci. 2018, 277, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, M.; Gao, S.; Zhang, Z.; Zhao, X.; Zhao, C.; Zhang, F.; Chen, X. Molecular characterization of novel TaNAC genes in wheat and overexpression of TaNAC2a confers drought tolerance in tobacco. Physiol. Plant. 2011, 144, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wei, J.; Song, N.; Wang, N.; Zhao, J.; Kang, Z. A novel wheat NAC transcription factor, TaNAC30, negatively regulates resistance of wheat to stripe rust. J. Integr. Plant Biol. 2017, 60, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yuan, S.; Zhao, C.; Park, R.F.; Wen, X.; Yang, W.; Liu, D. TaNAC35 acts as a negative regulator for leaf rust resistance in a compatible interaction between common wheat and Puccinia triticina. Mol. Genet. Genom. 2020, 296, 279–287. [Google Scholar] [CrossRef]
- Klopfenstein, D.V.; Zhang, L.; Pedersen, B.S.; Ramírez, F.; Vesztrocy, A.W.; Naldi, A.; Mungall, C.J.; Yunes, J.M.; Botvinnik, O.; Weigel, M.; et al. GOATOOLS: A Python library for Gene Ontology analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef]
- Chen, D.; Chai, S.; McIntyre, C.L.; Xue, G.-P. Overexpression of a predominantly root-expressed NAC transcription factor in wheat roots enhances root length, biomass and drought tolerance. Plant Cell Rep. 2017, 37, 225–237. [Google Scholar] [CrossRef]
- Wasson, A.P.; Richards, R.A.; Chatrath, R.; Misra, S.C.; Prasad, S.V.S.; Rebetzke, G.J.; Kirkegaard, J.A.; Christopher, J.; Watt, M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 2012, 63, 3485–3498. [Google Scholar] [CrossRef]
- Qing, C.H.I.; Du, L.Y.; Wen, M.A.; Niu, R.Y.; Wu, B.W.; Guo, L.J.; Meng, M.A.; Liu, X.L.; Zhao, H.X. MiR164-TaNAC14 module regulates root development and abiotic-stress tolerance of wheat seedlings. J. Integr. Agric. 2022, 22, 981–998. [Google Scholar]
- Long, S.P.; Ort, D.R. More than taking the heat: Crops and global change. Curr. Opin. Plant Biol. 2010, 13, 240–247. [Google Scholar] [CrossRef]
- Tucker, S.C.; Grimes, J. The inflorescence: Introduction. Bot. Rev. 1999, 65, 303–316. [Google Scholar] [CrossRef]
- Sultana, N. Characterization of TaNAC-S Gene in Australian Wheat Cultivars in Relation to Senescence and Nitrogen Stress Response. Ph.D. Thesis, Murdoch University, Perth, Australia, 2020. [Google Scholar]
- Sultana, N.; Islam, S.; Juhasz, A.; Ma, W. Wheat leaf senescence and its regulatory gene network. Crop. J. 2021, 9, 703–717. [Google Scholar] [CrossRef]
- Xia, N.; Zhang, G.; Liu, X.-Y.; Deng, L.; Cai, G.-L.; Zhang, Y.; Wang, X.-J.; Zhao, J.; Huang, L.-L.; Kang, Z.-S. Characterization of a novel wheat NAC transcription factor gene involved in defense response against stripe rust pathogen infection and abiotic stresses. Mol. Biol. Rep. 2010, 37, 3703–3712. [Google Scholar] [CrossRef]
- Harrington, S.A.; Overend, L.E.; Cobo, N.; Borrill, P.; Uauy, C. Conserved residues in the wheat (Triticum aestivum) NAM-A1 NAC domain are required for protein binding and when mutated lead to delayed peduncle and flag leaf senescence. BMC Plant Biol. 2019, 19, 407. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, Y.; Li, B.; Chang, J.; Chen, M.; Li, K.; Yang, G.; He, G. TaNAC29, a NAC transcription factor from wheat, enhances salt and drought tolerance in transgenic Arabidopsis. BMC Plant Biol. 2015, 15, 268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, C.; Guo, Y. Wheat Transcription Factor TaSNAC11-4B Positively Regulates Leaf Senescence through Promoting ROS Production in Transgenic Arabidopsis. Int. J. Mol. Sci. 2020, 21, 7672. [Google Scholar] [CrossRef] [PubMed]
- International Wheat Genome Sequencing Consortium (IWGSC); Appels, R.; Eversole, K.; Stein, N.; Feuillet, C.; Keller, B.; Rogers, J.; Pozniak, C.J.; Choulet, F.; Distelfeld, A.; et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar]
- Yates, A.D.; Allen, J.; Amode, R.M.; Azov, A.G.; Barba, M.; Becerra, A.; Bhai, J.; I Campbell, L.; Martinez, M.C.; Chakiachvili, M.; et al. Ensembl Genomes 2022: An expanding genome resource for non-vertebrates. Nucleic Acids Res. 2021, 50, D996–D1003. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2016, 45, D1040–D1045. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Hatta, A.; Periyannan, S.; Lagudah, E.; Wulff, B.B. Isolation of wheat genomic DNA for gene mapping and cloning. In Wheat Rust Diseases: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2017; pp. 207–213. [Google Scholar]
- Chung, C.T.; Niemela, S.L.; Miller, R.H. One-step preparation of competent Escherichia coli: Transformation and storage of bacterial cells in the same solution. Proc. Natl. Acad. Sci. USA 1989, 86, 2172–2175. [Google Scholar] [CrossRef] [PubMed]

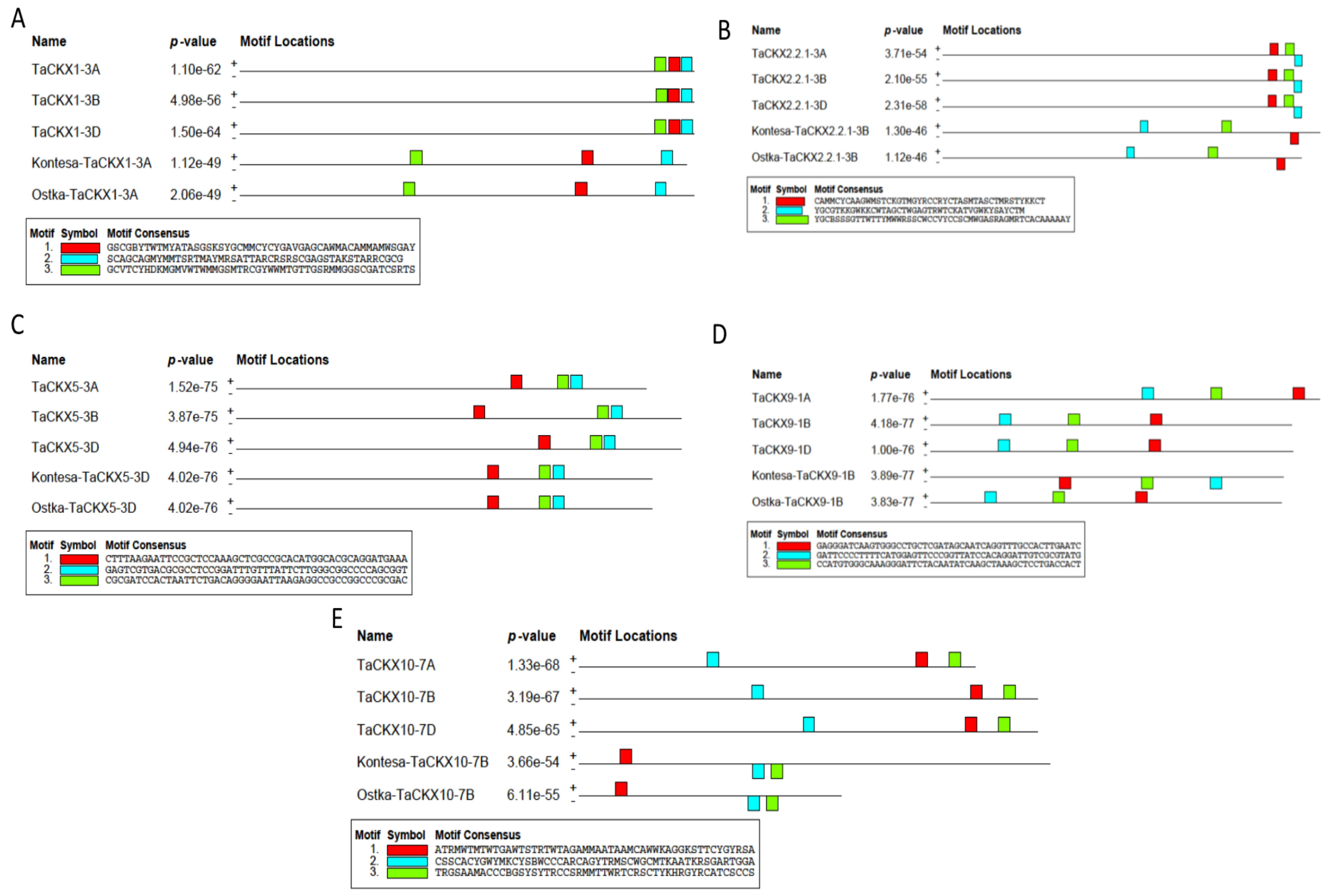
| Selected TaCKX | TaNAC TF ID | Family | Start | Stop | Strand | Score | p-Value | q-Value | Transcription Factor Binding Sites |
|---|---|---|---|---|---|---|---|---|---|
| TaCKX1 (Threshold p-value 10−4) | |||||||||
| TaCKX1-3A (TraesCS3A02G109500) | |||||||||
| Traes_5DL_0924913F8 | NAC | 552 | 571 | + | 14.4721 | 3.05 × 105 | 0.0668 | TACCGTCTTGATCCCGTC | |
| Traes_6BL_A169A3ECB | NAC | 707 | 724 | + | 14.1667 | 1.04 × 105 | 0.0402 | TACCGTCTTGATCCCGTC | |
| Traes_6AL_0D7866801 | NAC | 707 | 724 | + | 14.1667 | 1.04 × 105 | 0.0402 | TACCGTCTTGATCCCGTC | |
| TRAES3BF114300070CFD_t1 | NAC | 1492 | 1510 | − | 11.8333 | 4.54 × 105 | 0.173 | CTTCTCCGGTAGGGCACCC | |
| TaCKX2.2.1 (Threshold p-value 10−4) | |||||||||
| TaCKX2.2.1-3B (TraesCS3B02G161000) | |||||||||
| Traes_1AL_C4FA8404A | NAC | 1113 | 1133 | + | 18.7576 | 3.38 × 107 | 0.00133 | TGTAACTTGGGAGACAAGACA | |
| Traes_4BL_D592DBAAF | NAC | 1115 | 1130 | − | 16.4545 | 1.45 × 106 | 0.00573 | CTTGTCTCCCAAGTTA | |
| Traes_4AS_B95A1C3A1 | NAC | 1115 | 1135 | + | 15.8636 | 2.57 × 106 | 0.0101 | TAACTTGGGAGACAAGACATT | |
| Traes_1AL_C4FA8404A | NAC | 1115 | 1135 | − | 14.3182 | 4.29 × 106 | 0.00848 | AATGTCTTGTCTCCCAAGTTA | |
| Traes_4BS_373BDBA94 | NAC | 1115 | 1130 | − | 14.9242 | 5.50 × 106 | 0.0218 | CTTGTCTCCCAAGTTA | |
| Traes_5BL_657BF1497 | NAC | 1115 | 1132 | + | 14.4394 | 6.70 × 106 | 0.0265 | TAACTTGGGAGACAAGAC | |
| Traes_1BL_8925B27BC | NAC | 1116 | 1130 | + | 14.3333 | 8.20 × 106 | 0.0325 | AACTTGGGAGACAAG | |
| Traes_7AL_46F73E667 | NAC | 1113 | 1133 | + | 13.7727 | 9.26 × 106 | 0.0366 | TGTAACTTGGGAGACAAGACA | |
| Traes_2BL_209C14A8F | NAC | 1113 | 1133 | + | 12.6061 | 1.61 × 105 | 0.0636 | TGTAACTTGGGAGACAAGACA | |
| Traes_4AS_B95A1C3A1 | NAC | 1113 | 1133 | − | 11.7273 | 1.84 × 105 | 0.0364 | TGTCTTGTCTCCCAAGTTACA | |
| Traes_4BS_373BDBA94 | NAC | 1118 | 1133 | + | 12.5 | 2.39 × 105 | 0.0474 | CTTGGGAGACAAGACA | |
| Traes_5DL_B69423A67 | NAC | 1116 | 1132 | + | 12.7121 | 2.47 × 105 | 0.0979 | AACTTGGGAGACAAGAC | |
| Traes_4AL_99942CBEA | NAC | 1115 | 1131 | + | 12.0758 | 3.72 × 105 | 0.147 | TAACTTGGGAGACAAGA | |
| TaCKX5 (Threshold p-value 10−4) | |||||||||
| TaCKX5-3D (TraesCS3D02G310200) | |||||||||
| Traes_6BL_A169A3ECB | NAC | 1213 | 1230 | − | 14.2273 | 9.92 × 106 | 0.0386 | TGCCGTATCTTGACCGGC | |
| Traes_6AL_0D7866801 | NAC | 1213 | 1230 | − | 14.2273 | 9.92 × 106 | 0.0386 | TGCCGTATCTTGACCGGC | |
| Traes_5DL_0924913F8 | NAC | 1211 | 1230 | − | 13.7727 | 1.16 × 105 | 0.0445 | TGCCGTATCTTGACCGGCTT | |
| Traes_5BL_39FF03C5D | NAC | 1211 | 1230 | − | 13.7727 | 1.16 × 105 | 0.0445 | TGCCGTATCTTGACCGGCTT | |
| TaCKX9 (Threshold p-value 10−4) | |||||||||
| TaCKX9-1B (TraesCS1B02G248700) | |||||||||
| Traes_2BL_209C14A8F | NAC | 1197 | 1217 | + | 16.4394 | 1.71 × 106 | 0.00463 | GGATGCTTAAAACATAAGCCA | |
| Traes_7AL_46F73E667 | NAC | 1197 | 1217 | + | 16.2424 | 1.94 × 106 | 0.00536 | GGATGCTTAAAACATAAGCCA | |
| Traes_4AL_99942CBEA | NAC | 1199 | 1215 | + | 14.8939 | 5.19 × 106 | 0.0142 | ATGCTTAAAACATAAGC | |
| Traes_4AL_99942CBEA | NAC | 1201 | 1217 | − | 14.0606 | 1.01 × 105 | 0.0142 | TGGCTTATGTTTTAAGC | |
| Traes_2AL_82C3E7E14 | NAC | 1200 | 1214 | − | 13.3485 | 1.80 × 105 | 0.0328 | CTTATGTTTTAAGCA | |
| Traes_6AL_8BA1FF8B2 | NAC | 882 | 893 | − | 11.798 | 2.03 × 105 | 0.062 | GGACAAGCCAAG | |
| Traes_2AL_82C3E7E14 | NAC | 1202 | 1216 | + | 12.9848 | 2.32 × 105 | 0.0328 | CTTAAAACATAAGCC | |
| TaCKX10 (Threshold p-value 10−4) | |||||||||
| TaCKX10-7B (TraesCS7B02G264400) | |||||||||
| Traes_1AL_C4FA8404A | NAC | 468 | 488 | + | 12.7273 | 9.05 × 106 | 0.0318 | GAAAACTTGCTGATCACTACT | |
| TRAES3BF002300010CFD_t1 | NAC | 406 | 427 | − | 12.5152 | 2.33 × 105 | 0.0856 | TTCGTGTTTGTATTGGCCACGT | |
| TRAES3BF114300070CFD_t1 | NAC | 160 | 178 | + | 12.2576 | 3.46 × 105 | 0.133 | CCTGTATTTCACGGAGTCG | |
| Traes_5DL_0924913F8 | NAC | 1023 | 1042 | − | 11.5455 | 4.64 × 105 | 0.178 | CTCCGTTTTATTTACTCCGC | |
| Traes_5BL_39FF03C5D | NAC | 1023 | 1042 | − | 11.5455 | 4.64 × 105 | 0.178 | CTCCGTTTTATTTACTCCGC | |
| Traes_2DS_5DF921ABB | NAC | 1527 | 1534 | − | 12.0303 | 6.67 × 105 | 0.258 | TACGTAAT | |
| * TaCKX2.1 (Threshold p-value 10−4) | |||||||||
| TaCKX2.1-3D TraesCS3D02G143600 | |||||||||
| Traes_5DL_0924913F8 | NAC | 653 | 672 | + | 12.2727 | 3.05 × 105 | 0.0668 | CGTCGTGCTCATCCCGGAGC | |
| Traes_6BL_A169A3ECB | NAC | 653 | 670 | + | 12.5152 | 3.10 × 105 | 0.0952 | CGTCGTGCTCATCCCGGA | |
| Traes_5DL_0924913F8 | NAC | 537 | 556 | + | 11.697 | 4.26 × 105 | 0.0668 | CGGCGTGGGCGTCAGGGCAC | |
| * TaCKX11 (Threshold p-value 10−4) | |||||||||
| TaCKX11-7B TraesCS7B02G455000 | |||||||||
| Traes_2BL_209C14A8F | NAC | 687 | 707 | − | 9.48485 | 6.29 × 105 | 0.115 | CATAAATTCAAATTTAATCAA | |
| Traes_2BL_209C14A8F | NAC | 685 | 705 | + | 8.98485 | 7.61 × 105 | 0.115 | AATTGATTAAATTTGAATTTA |
| Attributes | Selected TaNACs | ||||
|---|---|---|---|---|---|
| Transcription Factor JUNGBRUNNEN 1-like [Triticum aestivum] | NAC Domain-Containing Protein 13-like [Triticum aestivum] | Putative NAC Domain-Containing Protein 94 [Triticum aestivum] | Protein BEARSKIN1-like [Triticum aestivum] | NAC Domain-Containing Protein 48-like [Triticum aestivum] | |
| TaCKX GFMs Regulated by TaNACs | TaCKX1-3A TraesCS3A02G109500 TaCKX2.1-3D TraesCS3D02G143600 TaCKX5-3D TraesCS3D02G310200 | TaCKX 2.2.1-3B TraesCS3B02G161000 TaCKX10-7B TraesCS7B02G264400 | TaCKX5-3D TraesCS3D02G310200 TaCKX1-3A TraesCS3A02G109500 TaCKX2.1-3D TraesCS3D02G143600 | TaCKX9-1B TraesCS1B02G248700 TaCKX 2.2.1-3B TraesCS3B02G161000 TaCKX11-7B TraesCS7B02G455000 | TaCKX10-7B TraesCS7B02G264400 |
| Rename of selected TaNACs | TaNAC JUNGBRUNNEN-1 (TaNAC J-1) | TaNAC13a | TaNAC94 | TaNAC BEARSKIN-1 (TaNAC Br-1) | TaNAC6D |
| TaNAC TF ID | Traes_5DL_0924913F8 | Traes_1AL_C4FA8404A | Traes_6BL_A169A3ECB | Traes_2BL_209C14A8F | TRAES3BF002300010CFD_t1 |
| TaNAC Protein ID | XP_044402035.1 | XP_044450494.1 | XP_044416817.1 | XP_044328275.1 | XP_044358948.1 |
| Protein size | 341 aa | 429 aa | 390 aa | 336 aa | 299 aa |
| NAC domain size | 35-194 aa | 6-156 aa | 16-175 aa | 10-161 aa | 9-159 aa |
| NCBI Gene ID | XM_044546100.1 | XM_044594559.1 | XM_044560882.1 | XM_044472340 | XM_044503013.1 |
| Ensemble Gene ID | TraesCS5D02G421100 | TraesCS1D02G266500 | TraesCS6D02G286300 | TraesCS2D02G309800 | TraesCS3D02G401200 |
| Gene Description at Plant Ensemble | n/a | n/a | n/a | n/a | NAC6D |
| Gene Ontology Analysis |
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, A.; Bocian, J.; Przyborowski, M.; Orczyk, W.; Nadolska-Orczyk, A. Are TaNAC Transcription Factors Involved in Promoting Wheat Yield by cis-Regulation of TaCKX Gene Family? Int. J. Mol. Sci. 2024, 25, 2027. https://doi.org/10.3390/ijms25042027
Iqbal A, Bocian J, Przyborowski M, Orczyk W, Nadolska-Orczyk A. Are TaNAC Transcription Factors Involved in Promoting Wheat Yield by cis-Regulation of TaCKX Gene Family? International Journal of Molecular Sciences. 2024; 25(4):2027. https://doi.org/10.3390/ijms25042027
Chicago/Turabian StyleIqbal, Adnan, Joanna Bocian, Mateusz Przyborowski, Wacław Orczyk, and Anna Nadolska-Orczyk. 2024. "Are TaNAC Transcription Factors Involved in Promoting Wheat Yield by cis-Regulation of TaCKX Gene Family?" International Journal of Molecular Sciences 25, no. 4: 2027. https://doi.org/10.3390/ijms25042027
APA StyleIqbal, A., Bocian, J., Przyborowski, M., Orczyk, W., & Nadolska-Orczyk, A. (2024). Are TaNAC Transcription Factors Involved in Promoting Wheat Yield by cis-Regulation of TaCKX Gene Family? International Journal of Molecular Sciences, 25(4), 2027. https://doi.org/10.3390/ijms25042027






