Overexpression of Alpha-1 Antitrypsin Increases the Proliferation of Mesenchymal Stem Cells by Upregulation of Cyclin D1
Abstract
1. Introduction
2. Results
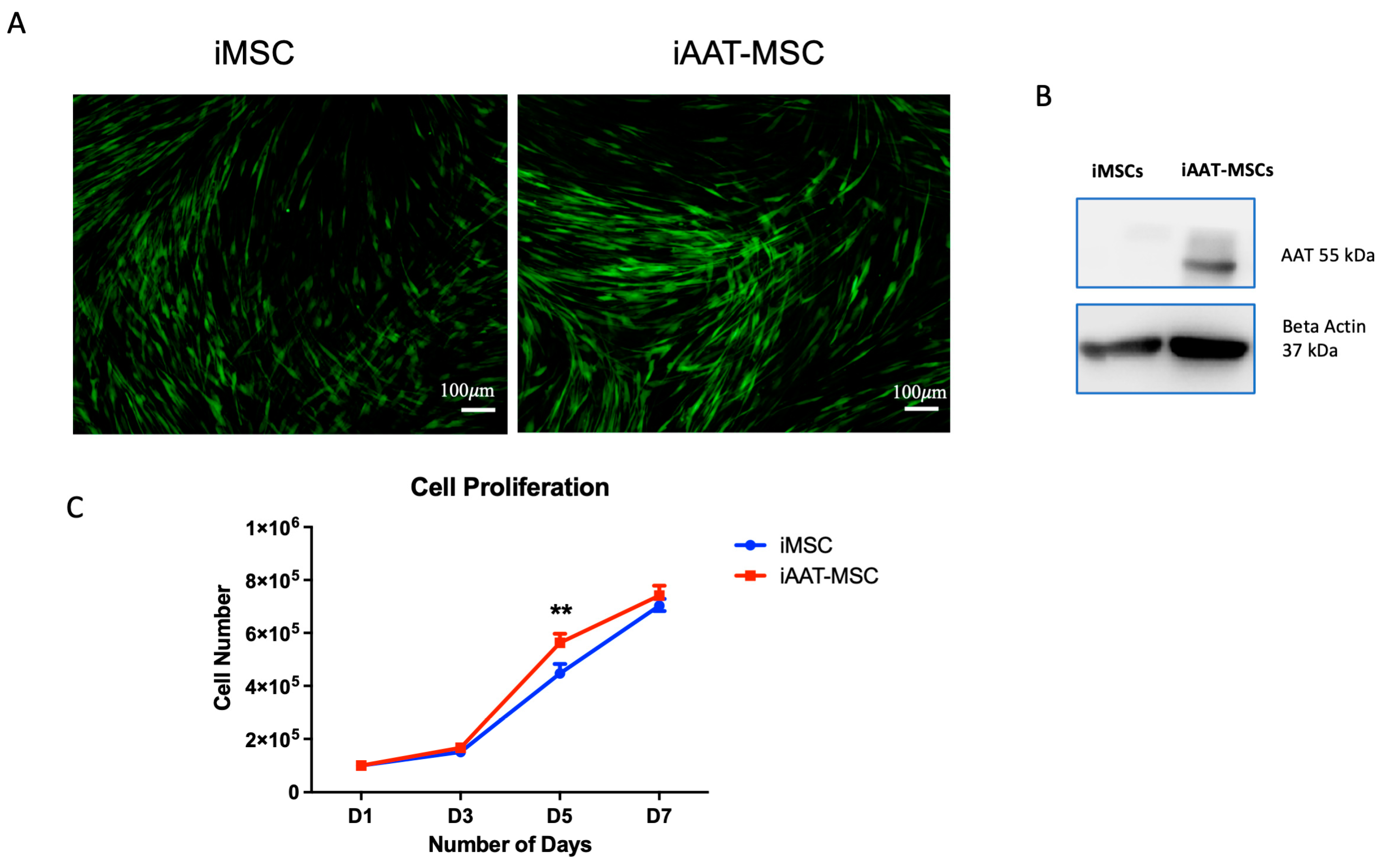
2.1. iAAT-MSCs Showed Higher Proliferation Compared to Control iMSCs
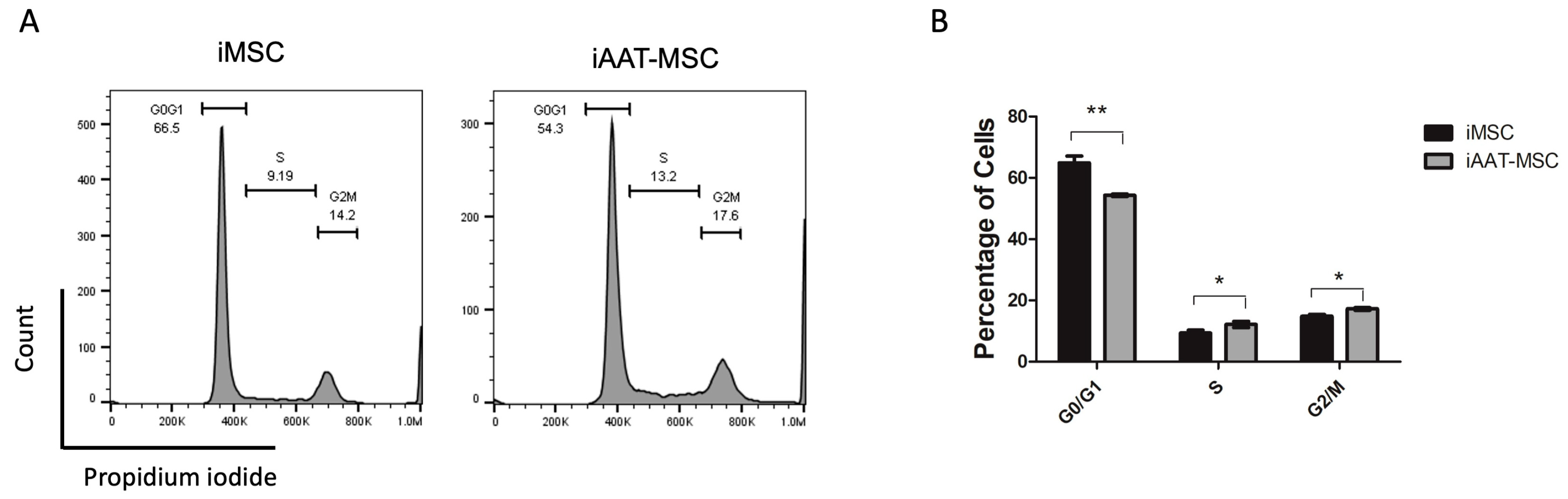
2.2. Cell Cycle Analysis Showed a Difference between iAAT-MSCs and iMSCs
2.3. Upregulation of Cyclin D1 in iAAT-MSCs Compared to iMSCs
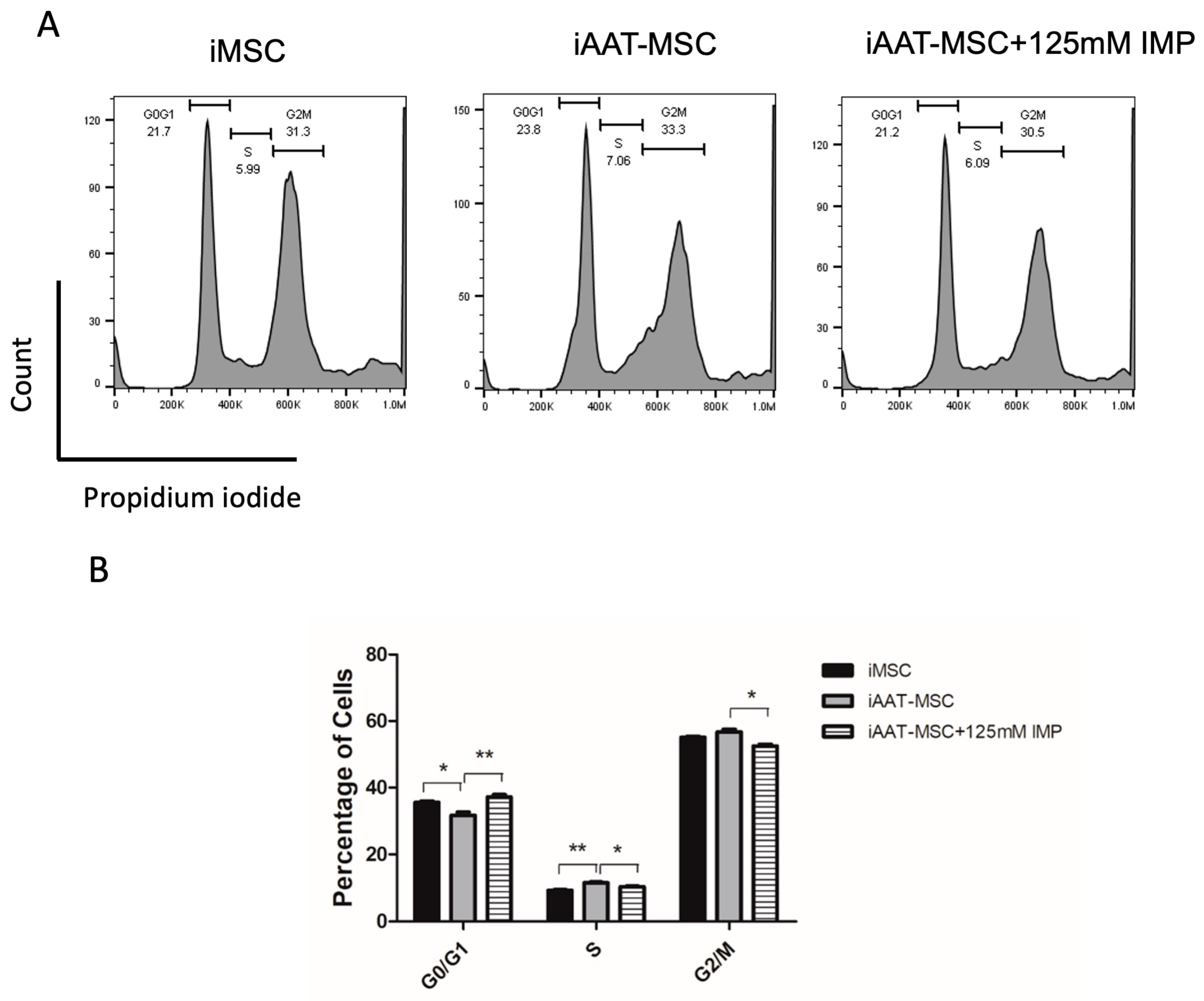
2.4. Inhibition of Cyclin D1 Expression with Imperatorin Reduces AAT’s Pro-Proliferation Effect
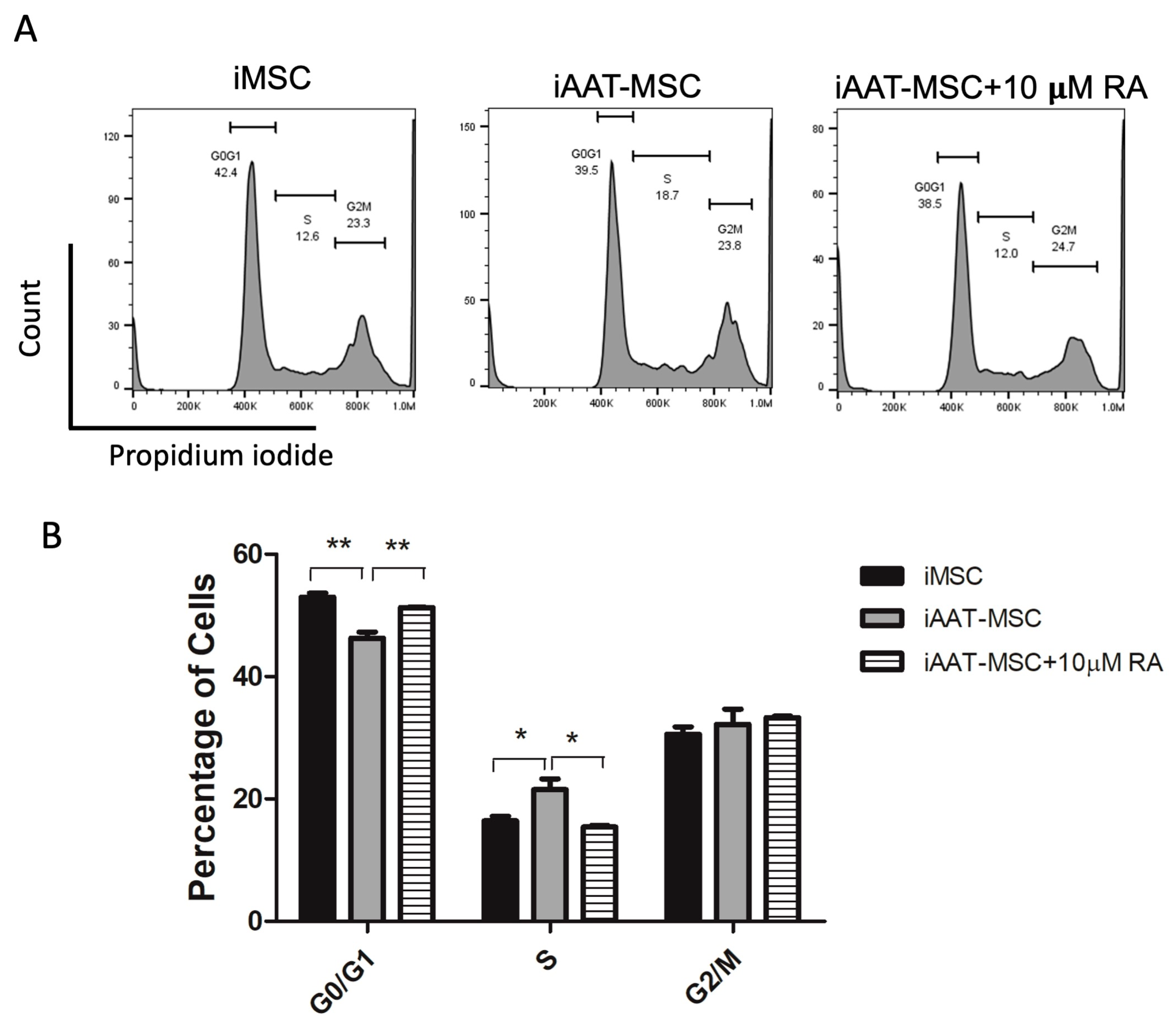
2.5. Inhibition of Cyclin D1 with Retinoic Acid Further Confirms the Role of Cyclin D1 in Enhanced Cell Proliferation in iAAT-MSCs
2.6. AAT-MSCs Expressed a Similar Amount of GSK3β and β-Catenin Compared to iMSCs
3. Discussion
4. Materials and Methods
4.1. Cell Preparation
4.2. Cell Culture and Cell Counting
4.3. Cell Cycle Analysis by Flow Cytometry
4.4. Gene Expression by qPCR Analysis
4.5. Treatment of AAT-MSCs with Cyclin D Inhibitors, Imperatorin and Retinoic Acid
4.6. Western Blot
4.7. Immunofluorescence Staining
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gronthos, S.; Zannettino, A.C. Methods for the purification and characterization of human adipose-derived stem cells. Methods Mol. Biol. 2011, 702, 109–120. [Google Scholar]
- Marquez-Curtis, L.A.; Janowska-Wieczorek, A.; McGann, L.E.; Elliott, J.A. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology 2015, 71, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzynski, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, J.; Sensebe, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef]
- Schwarz, N.; Tumpara, S.; Wrenger, S.; Ercetin, E.; Hamacher, J.; Welte, T.; Janciauskiene, S. Alpha1-antitrypsin protects lung cancer cells from staurosporine-induced apoptosis: The role of bacterial lipopolysaccharide. Sci. Rep. 2020, 10, 9563. [Google Scholar] [CrossRef]
- Lim, J.H.; Park, S.M.; Yook, J.M.; Ahn, J.S.; Choi, S.Y.; Oh, S.H.; Jung, H.Y.; Choi, J.Y.; Cho, J.H.; Park, S.H.; et al. Alpha-1 antitrypsin inhibits formaldehyde-induced apoptosis of human peritoneal mesothelial cells. Perit. Dial. Int. 2020, 40, 124–131. [Google Scholar] [CrossRef]
- Magnier-Gaubil, C.; Herbert, J.M.; Quarck, R.; Papp, B.; Corvazier, E.; Wuytack, F.; Lévy-Tolédano, S.; Enouf, J. Smooth muscle cell cycle and proliferation. Relationship between calcium influx and sarco-endoplasmic reticulum Ca2+ATPase regulation. J. Biol. Chem. 1996, 271, 27788–27794. [Google Scholar] [CrossRef]
- Song, L.; Gou, W.; Wang, J.; Wei, H.; Lee, J.; Strange, C.; Wang, H. Overexpression of alpha-1 antitrypsin in mesenchymal stromal cells improves their intrinsic biological properties and therapeutic effects in nonobese diabetic mice. Stem Cells Transl. Med. 2021, 10, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Shoeibi, S.; Green, E.; Wei, H.; Gou, W.; Strange, C.; Wang, H. Immortalized Mesenchymal Stromal Cells Overexpressing Alpha-1 Antitrypsin Protect Acinar Cells from Apoptotic and Ferroptotic Cell Death. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Geiger, S.; Ozay, E.I.; Geumann, U.; Hereth, M.K.; Magnusson, T.; Shanthalingam, S.; Hirsch, D.; Kalin, S.; Gunther, C.; Osborne, B.A.; et al. Alpha-1 Antitrypsin-Expressing Mesenchymal Stromal Cells Confer a Long-Term Survival Benefit in a Mouse Model of Lethal GvHD. Mol. Ther. 2019, 27, 1436–1451. [Google Scholar] [CrossRef]
- Gou, W.; Hua, W.; Swaby, L.; Cui, W.; Green, E.; Morgan, K.A.; Strange, C.; Wang, H. Stem Cell Therapy Improves Human Islet Graft Survival in Mice via Regulation of Macrophages. Diabetes 2022, 71, 2642–2655. [Google Scholar] [CrossRef]
- Wei, H.; Green, E.; Ball, L.; Fan, H.; Lee, J.; Strange, C.; Wang, H. Proteomic Analysis of Exosomes Secreted from Human Alpha-1 Antitrypsin Overexpressing Mesenchymal Stromal Cells. Biology 2021, 11, 9. [Google Scholar] [CrossRef]
- Diehl, J.A.; Cheng, M.; Roussel, M.F.; Sherr, C.J. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998, 12, 3499–3511. [Google Scholar] [CrossRef]
- Alao, J.P. The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol. Cancer 2007, 6, 24. [Google Scholar] [CrossRef]
- Montalto, F.I.; De Amicis, F. Cyclin D1 in Cancer: A Molecular Connection for Cell Cycle Control, Adhesion and Invasion in Tumor and Stroma. Cells 2020, 9, 2648. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Li, W.; Zhang, H. Activation of Wnt/beta-catenin signalling via GSK3 inhibitors direct differentiation of human adipose stem cells into functional hepatocytes. Sci. Rep. 2017, 7, 40716. [Google Scholar] [CrossRef]
- Kafri, P.; Hasenson, S.E.; Kanter, I.; Sheinberger, J.; Kinor, N.; Yunger, S.; Shav-Tal, Y. Quantifying beta-catenin subcellular dynamics and cyclin D1 mRNA transcription during Wnt signaling in single living cells. Elife 2016, 5, e16748. [Google Scholar] [CrossRef]
- Boyle, W.J.; Smeal, T.; Defize, L.H.; Angel, P.; Woodgett, J.R.; Karin, M.; Hunter, T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell 1991, 64, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Koziol, E.; Skalicka-Wozniak, K. Imperatorin-pharmacological meaning and analytical clues: Profound investigation. Phytochem. Rev. 2016, 15, 627–649. [Google Scholar] [CrossRef] [PubMed]
- Spinella, M.J.; Freemantle, S.J.; Sekula, D.; Chang, J.H.; Christie, A.J.; Dmitrovsky, E. Retinoic acid promotes ubiquitination and proteolysis of cyclin D1 during induced tumor cell differentiation. J. Biol. Chem. 1999, 274, 22013–22018. [Google Scholar] [CrossRef] [PubMed]
- Shtutman, M.; Zhurinsky, J.; Simcha, I.; Albanese, C.; D’Amico, M.; Pestell, R.; Ben-Ze’ev, A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 5522–5527. [Google Scholar] [CrossRef]
- Singh, A.M.; Dalton, S. The cell cycle and Myc intersect with mechanisms that regulate pluripotency and reprogramming. Cell Stem Cell 2009, 5, 141–149. [Google Scholar] [CrossRef]
- Herrera, R.E.; Sah, V.P.; Williams, B.O.; Makela, T.P.; Weinberg, R.A.; Jacks, T. Altered cell cycle kinetics, gene expression, and G1 restriction point regulation in Rb-deficient fibroblasts. Mol. Cell Biol. 1996, 16, 2402–2407. [Google Scholar] [CrossRef] [PubMed]
- Sancho, R.; Marquez, N.; Gomez-Gonzalo, M.; Calzado, M.A.; Bettoni, G.; Coiras, M.T.; Alcami, J.; Lopez-Cabrera, M.; Appendino, G.; Munoz, E. Imperatorin inhibits HIV-1 replication through an Sp1-dependent pathway. J. Biol. Chem. 2004, 279, 37349–37359. [Google Scholar] [CrossRef] [PubMed]
- Lecarpentier, Y.; Schussler, O.; Hebert, J.L.; Vallee, A. Multiple Targets of the Canonical WNT/beta-Catenin Signaling in Cancers. Front. Oncol. 2019, 9, 1248. [Google Scholar] [CrossRef] [PubMed]
- Skarn, M.; Noordhuis, P.; Wang, M.Y.; Veuger, M.; Kresse, S.H.; Egeland, E.V.; Micci, F.; Namlos, H.M.; Hakelien, A.M.; Olafsrud, S.M.; et al. Generation and characterization of an immortalized human mesenchymal stromal cell line. Stem Cells Dev. 2014, 23, 2377–2389. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolf, B.; Muralidharan, P.; Lee, M.Y.; Hua, W.; Green, E.; Wang, H.; Strange, C. Overexpression of Alpha-1 Antitrypsin Increases the Proliferation of Mesenchymal Stem Cells by Upregulation of Cyclin D1. Int. J. Mol. Sci. 2024, 25, 2015. https://doi.org/10.3390/ijms25042015
Wolf B, Muralidharan P, Lee MY, Hua W, Green E, Wang H, Strange C. Overexpression of Alpha-1 Antitrypsin Increases the Proliferation of Mesenchymal Stem Cells by Upregulation of Cyclin D1. International Journal of Molecular Sciences. 2024; 25(4):2015. https://doi.org/10.3390/ijms25042015
Chicago/Turabian StyleWolf, Bryan, Prasanth Muralidharan, Michael Y. Lee, Wei Hua, Erica Green, Hongjun Wang, and Charlie Strange. 2024. "Overexpression of Alpha-1 Antitrypsin Increases the Proliferation of Mesenchymal Stem Cells by Upregulation of Cyclin D1" International Journal of Molecular Sciences 25, no. 4: 2015. https://doi.org/10.3390/ijms25042015
APA StyleWolf, B., Muralidharan, P., Lee, M. Y., Hua, W., Green, E., Wang, H., & Strange, C. (2024). Overexpression of Alpha-1 Antitrypsin Increases the Proliferation of Mesenchymal Stem Cells by Upregulation of Cyclin D1. International Journal of Molecular Sciences, 25(4), 2015. https://doi.org/10.3390/ijms25042015







