Hydrogel-Based Skin Regeneration
Abstract
1. Introduction
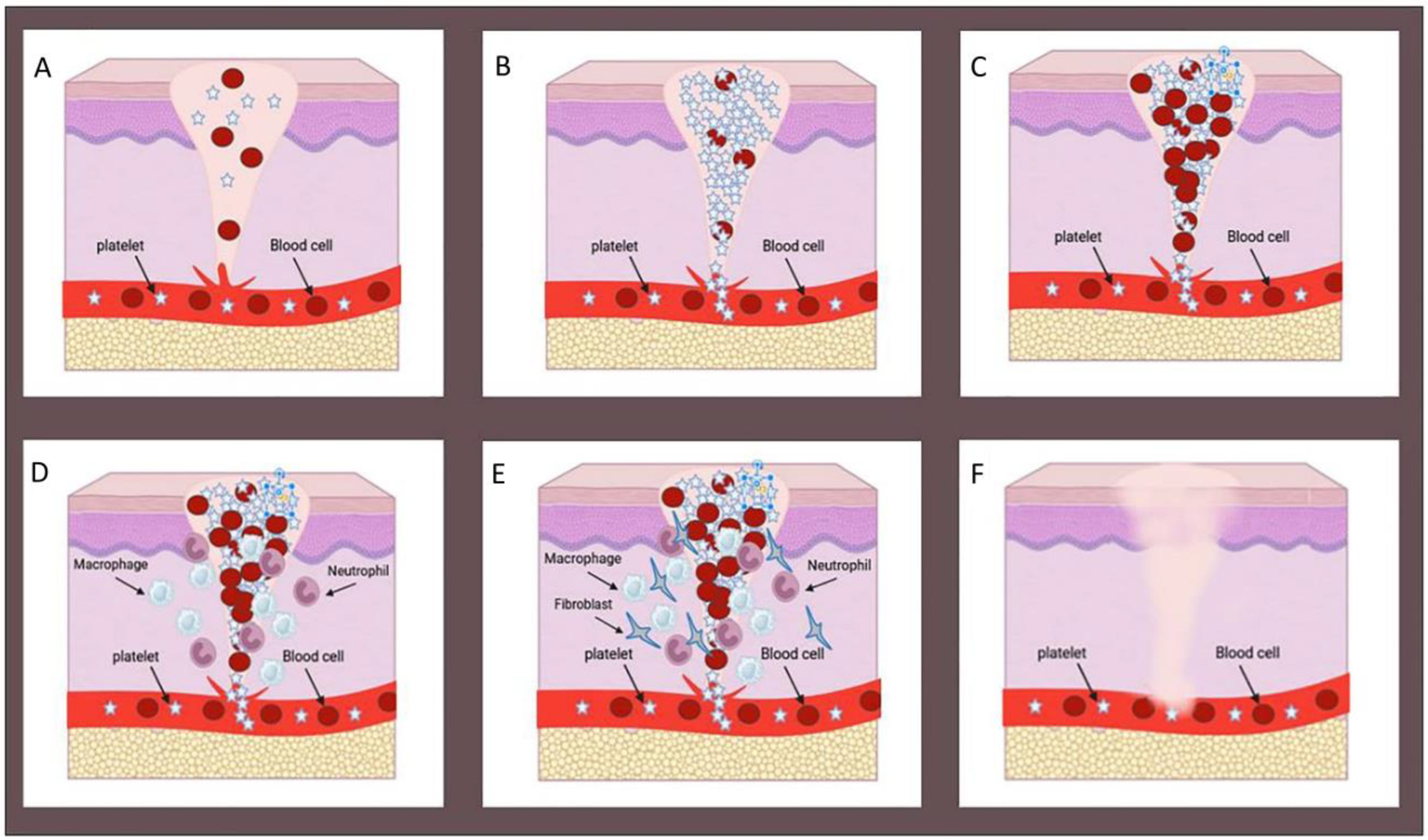
2. Wound Healing Process
3. Approaches for Skin Wounds
4. Recent Strategies for Wound Healing and Skin Regeneration
5. Hydrogels for Wound Healing
6. Polymer Selection for Hydrogel
7. Natural Polymers
7.1. Collagen
| Polymer Type | Disadvantages | Advantages | Ref. |
|---|---|---|---|
| Natural polymers | Poor Mechanical Strength | Biocompatibility | [31,32] |
| Rapid Degradation | |||
| Inflammatory Response | Biodegradability | ||
| Potential Allergic Reactions | Cell Affinity | ||
| Sourcing Challenges | Moisture Retention | ||
| Limited Control over Properties | Angiogenic Properties | ||
| Pathogen Contamination | |||
| Synthetic polymers | Biocompatibility Concerns | Tunable Properties | [33,34] |
| Tailored Biodegradability | |||
| Inflammatory Responses | Consistent Composition | ||
| Lack of Bioactivity | Mechanical Strength | ||
| Potential Toxicity | Absorption Capacity | ||
| Limited Water Retention | Pore Size Control | ||
| Mechanical Weakness | Long Shelf Life | ||
| Non-Natural Structure | High degree of sterility | ||
| Customized Drug Delivery |
7.2. Chitosan
7.3. Hyaluronic Acid (HA)
7.4. Gelatin
7.5. Alginate
7.6. Dextran
7.7. Fibrin
7.8. Silk
8. Synthetic Polymers
8.1. Polyethylene Glycol (PEG)
8.2. Polydopamine (PDA)
8.3. Polyacrylamide (PAM)
8.4. Polyvinyl Alcohol (PVA)
8.5. Carboxymethyl Cellulose (CMC)
8.6. Carbopol
9. Hybrid Hydrogels
10. Biomimetic Hydrogels
11. Preparation of Hydrogels
11.1. Physical Cross-Linking
11.2. Chemical Cross-Linking
12. Wound Healing Hydrogels Based on the Needs of the Skin Healing Process
12.1. Hemostasis
12.2. Antibacterial Properties
12.3. Anti-Inflammatory Properties
12.4. Antioxidant Properties
12.5. Angiogenesis
13. Wound Care Products in the Market
14. Summary and Outlook
- They must have advanced mechanical features so that they can withstand the dynamic nature of wound environments.
- Designing smart hydrogels can enable hydrogels to dynamically respond to changes in the wound environment and adapt properties based on specific healing stages.
- They must have advanced drug delivery capability and be effective with the controlled release of therapeutic agents to promote healing and prevent infections.
- The development of hydrogels with suitable degradation profiles can address concerns about their durability and possible removal methods.
- The hydrogel formulation must be tailored to the patient’s needs; considering factors such as skin type and wound characteristics can increase the formulation’s effectiveness and reduce side effects.
Author Contributions
Funding
Conflicts of Interest
References
- Monteiro-Riviere, N.A. Comparative anatomy, physiology, and biochemistry of mammalian skin. In Dermal and Ocular Toxicology; CRC Press: Boca Raton, FL, USA, 2020; pp. 3–71. [Google Scholar]
- Sultana, N.; Bandyopadhyay-Ghosh, S.; Soon, C.F. Tissue Engineering Strategies for Organ Regeneration; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Arabpour, Z.; Youseffi, M.; Soon, C.F.; Sultana, N.; Bazgeir, M.R.; Masoud, M.; Sefat, F. Designing biomaterials for regenerative medicine: State-of-the-art and future perspectives. In Tissue Engineering Strategies for Organ Regeneration; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–9. [Google Scholar]
- Fani, N.; Moradi, M.; Zavari, R.; Parvizpour, F.; Soltani, A.; Arabpour, Z.; Jafarian, A. Current advances in wound healing and regenerative medicine. Curr. Stem Cell Res. Ther. 2024, 19, 277–291. [Google Scholar] [CrossRef]
- Kus, K.J.; Ruiz, E.S. Wound dressings–a practical review. Curr. Dermatol. Rep. 2020, 9, 298–308. [Google Scholar] [CrossRef]
- Luneva, O.; Olekhnovich, R.; Uspenskaya, M. Bilayer Hydrogels for Wound Dressing and Tissue Engineering. Polymers 2022, 14, 3135. [Google Scholar] [CrossRef] [PubMed]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Kolahchi, A.R.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Long, L.; Hu, C.; Kong, Q.; Wang, Y. A spatiotemporal release platform based on pH/ROS stimuli-responsive hydrogel in wound repairing. J. Control. Release 2022, 341, 147–165. [Google Scholar] [CrossRef]
- Castano, O.; Pérez-Amodio, S.; Navarro-Requena, C.; Mateos-Timoneda, M.Á.; Engel, E. Instructive microenvironments in skin wound healing: Biomaterials as signal releasing platforms. Adv. Drug Deliv. Rev. 2018, 129, 95–117. [Google Scholar] [CrossRef]
- DiCarlo, A.L.; Bandremer, A.C.; Hollingsworth, B.A.; Kasim, S.; Laniyonu, A.; Todd, N.F.; Wang, S.-J.; Wertheimer, E.R.; Rios, C.I. Cutaneous radiation injuries: Models, assessment and treatments. Radiat. Res. 2020, 194, 315–344. [Google Scholar] [CrossRef]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of wound healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Chouhan, D.; Vijayakumar Sreelatha, H.; Arumugam, S.; Mandal, B.B.; Krishnan, L.K. Silk fibroin-based bioengineered scaffold for enabling hemostasis and skin regeneration of critical-size full-thickness heat-induced burn wounds. ACS Biomater. Sci. Eng. 2022, 8, 3856–3870. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, F.; Luo, H.; Xu, G.; Wang, D. Inflammatory microenvironment of skin wounds. Front. Immunol. 2022, 13, 789274. [Google Scholar] [CrossRef] [PubMed]
- Spielman, A.F.; Griffin, M.F.; Parker, J.; Cotterell, A.C.; Wan, D.C.; Longaker, M.T. Beyond the Scar: A Basic Science Review of Wound Remodeling. Adv. Wound Care 2023, 12, 57–67. [Google Scholar] [CrossRef]
- Song, J.; Hu, L.; Liu, B.; Jiang, N.; Huang, H.; Luo, J.; Wang, L.; Zeng, J.; Huang, F.; Huang, M. The emerging role of immune cells and targeted therapeutic strategies in diabetic wounds healing. J. Inflamm. Res. 2022, 15, 4119–4138. [Google Scholar] [CrossRef]
- Stetkevich, S.; Gupta, M.; Simman, R.; Jackson, S.E. How to Select an Extracellular Matrix for Wound Repair: A Comprehensive Review. Eplasty 2023, 23, e51. [Google Scholar]
- Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandal, B.B. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials 2019, 216, 119267. [Google Scholar] [CrossRef]
- Boateng, J.; Catanzano, O. Advanced therapeutic dressings for effective wound healing—A review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef]
- Amor, I.B.; Emran, T.B.; Hemmami, H.; Zeghoud, S.; Laouini, S.E. Nanomaterials based on chitosan for skin regeneration: An update. Int. J. Surg. 2023, 109, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Hou, Q.; Zhong, L.; Zhao, Y.; Li, M.; Fu, X. Bioactive molecules for skin repair and regeneration: Progress and perspectives. Stem Cells Int. 2019, 2019, 6789823. [Google Scholar] [CrossRef] [PubMed]
- Norahan, M.H.; Pedroza-González, S.C.; Sánchez-Salazar, M.G.; Álvarez, M.M.; de Santiago, G.T. Structural and biological engineering of 3D hydrogels for wound healing. Bioact. Mater. 2023, 24, 197–235. [Google Scholar] [CrossRef] [PubMed]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 1–22. [Google Scholar]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for wound healing applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural polymer-based hydrogels with enhanced mechanical performances: Preparation, structure, and property. Adv. Healthc. Mater. 2019, 8, 1900670. [Google Scholar] [CrossRef] [PubMed]
- Simorgh, S.; Milan, P.B.; Saadatmand, M.; Bagher, Z.; Gholipourmalekabadi, M.; Alizadeh, R.; Hivechi, A.; Arabpour, Z.; Hamidi, M.; Delattre, C. Human olfactory mucosa stem cells delivery using a collagen hydrogel: As a potential candidate for bone tissue engineering. Materials 2021, 14, 3909. [Google Scholar] [CrossRef] [PubMed]
- Abou Neel, E.A.; Bozec, L.; Knowles, J.C.; Syed, O.; Mudera, V.; Day, R.; Hyun, J.K. Collagen—Emerging collagen based therapies hit the patient. Adv. Drug Deliv. Rev. 2013, 65, 429–456. [Google Scholar] [CrossRef] [PubMed]
- Gaspar-Pintiliescu, A.; Anton, E.D.; Iosageanu, A.; Berger, D.; Matei, C.; Mitran, R.A.; Negreanu-Pirjol, T.; Craciunescu, O.; Moldovan, L. Enhanced wound healing activity of undenatured type I collagen isolated from discarded skin of Black Sea gilthead bream (Sparus aurata) conditioned as 3D porous dressing. Chem. Biodivers. 2021, 18, e2100293. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Zhou, J.; Wang, M.; Su, D.; Ma, Q.; Lv, G.; Chen, J. In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. Mater. Sci. Eng. C 2019, 101, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Li, J.; Liang, J.; Zhang, K.; Li, J. Hydrogel preparation methods and biomaterials for wound dressing. Life 2021, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, S.; Taghavi Shahraki, B.; Rabiee, N.; Fatahi, Y.; Dinarvand, R.; Tavakolizadeh, M.; Ahmadi, S.; Rabiee, M.; Bagherzadeh, M.; Pourjavadi, A. Burgeoning polymer nano blends for improved controlled drug release: A review. Int. J. Nanomed. 2020, 15, 4363–4392. [Google Scholar] [CrossRef]
- Prete, S.; Dattilo, M.; Patitucci, F.; Pezzi, G.; Parisi, O.I.; Puoci, F. Natural and Synthetic Polymeric Biomaterials for Application in Wound Management. J. Funct. Biomater. 2023, 14, 455. [Google Scholar] [CrossRef]
- Gobi, R.; Ravichandiran, P.; Babu, R.S.; Yoo, D.J. Biopolymer and synthetic polymer-based nanocomposites in wound dressing applications: A review. Polymers 2021, 13, 1962. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-based functional materials for skin wound repair: Mechanisms and applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. The implications of recent advances in carboxymethyl chitosan based targeted drug delivery and tissue engineering applications. J. Control. Release 2014, 186, 54–87. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Nair, S.; Tamura, H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv. 2010, 28, 142–150. [Google Scholar] [CrossRef]
- Wu, X.; Li, H. Incorporation of bioglass improved the mechanical stability and bioactivity of alginate/carboxymethyl chitosan hydrogel wound dressing. ACS Appl. Bio Mater. 2021, 4, 1677–1692. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Poverenov, E. Hydrophilic chitosan derivatives: Synthesis and applications. Chem. Eur. J. 2022, 28, e202202156. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, M.; Liu, Q.; Yan, S.; Feng, L.; Lan, Y.; Shan, G.; Xue, W.; Guo, R. Enhanced healing activity of burn wound infection by a dextran-HA hydrogel enriched with sanguinarine. Biomater. Sci. 2018, 6, 2472–2486. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- De Francesco, F.; Saparov, A.; Riccio, M. Hyaluronic acid accelerates re-epithelialization and healing of acute cutaneous wounds. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 37–45. [Google Scholar]
- Yang, H.; Song, L.; Zou, Y.; Sun, D.; Wang, L.; Yu, Z.; Guo, J. Role of hyaluronic acids and potential as regenerative biomaterials in wound healing. ACS Appl. Bio Mater. 2020, 4, 311–324. [Google Scholar] [CrossRef]
- Hu, D.; Wen, J.; Zhao, X.; Liu, K.; Zhang, Y.; Bu, Y.; Wang, K. A wound-friendly antibacterial hyaluronic acid dressing with on-demand removability for infected wound healing. Biomater. Res. 2023, 27, 38. [Google Scholar] [CrossRef]
- Garabet, W.; Shabes, P.; Wolters, K.H.; Rembe, J.-D.; Ibing, W.; Wagenhaeuser, M.U.; Simon, F.; Schelzig, H.; Oberhuber, A. Effect of Gelatin-Based Hemostats on Fibroblasts and Relevant Growth Factors in Wound Healing. Gels 2023, 9, 504. [Google Scholar] [CrossRef]
- He, Y.; Wang, C.; Wang, C.; Xiao, Y.; Lin, W. An overview on collagen and gelatin-based cryogels: Fabrication, classification, properties and biomedical applications. Polymers 2021, 13, 2299. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Z.; Jorgensen, A.M.; Yang, Y.; Jin, Q.; Zhang, G.; Cao, G.; Fu, Y.; Zhao, W.; Ju, J. Bioprinting a skin patch with dual-crosslinked gelatin (GelMA) and silk fibroin (SilMA): An approach to accelerating cutaneous wound healing. Mater. Today Bio 2023, 18, 100550. [Google Scholar] [CrossRef]
- Ahmad, F.; Mushtaq, B.; Ahmad, S.; Rasheed, A.; Nawab, Y. A novel composite of hemp fiber and alginate hydrogel for wound dressings. J. Polym. Environ. 2023, 31, 2294–2305. [Google Scholar] [CrossRef]
- Wang, T.; Yi, W.; Zhang, Y.; Wu, H.; Fan, H.; Zhao, J.; Wang, S. Sodium alginate hydrogel containing platelet-rich plasma for wound healing. Colloids Surf. B Biointerfaces 2023, 222, 113096. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guan, S.; Zhang, K.; Li, J. Benlysta-loaded sodium alginate hydrogel and its selective functions in promoting skin cell growth and inhibiting inflammation. ACS Omega 2020, 5, 10395–10400. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Liu, Y.; Wang, X.; Yan, H.; Wang, L.; Qu, L.; Kong, D.; Qiao, M.; Wang, L. Injectable hydrogel composed of hydrophobically modified chitosan/oxidized-dextran for wound healing. Mater. Sci. Eng. C 2019, 104, 109930. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, L.; Wei, C.; Guo, R. A bioactive dextran-based hydrogel promote the healing of infected wounds via antibacterial and immunomodulatory. Carbohydr. Polym. 2022, 291, 119558. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, M.; Musilkova, J.; Riedel, T.; Stranska, D.; Brynda, E.; Zaloudkova, M.; Bacakova, L. The potential applications of fibrin-coated electrospun polylactide nanofibers in skin tissue engineering. Int. J. Nanomed. 2016, 11, 771–789. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zou, D.; Dai, T.; Xu, H.; An, R.; Liu, Y.; Liu, B. Effects of incorporation of granule-lyophilised platelet-rich fibrin into polyvinyl alcohol hydrogel on wound healing. Sci. Rep. 2018, 8, 14042. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, D.M.; Roy, D.C.; Becerra, S.C.; Natesan, S.; Christy, R.J. In situ delivery of fibrin-based hydrogels prevents contraction and reduces inflammation. J. Burn. Care Res. 2017, 39, 40–53. [Google Scholar] [CrossRef]
- Natesan, S.; Zamora, D.O.; Wrice, N.L.; Baer, D.G.; Christy, R.J. Bilayer hydrogel with autologous stem cells derived from debrided human burn skin for improved skin regeneration. J. Burn. Care Res. 2013, 34, 18–30. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Hayati, F.; Ghamsari, S.M.; Dehghan, M.M.; Oryan, A. Effects of carbomer 940 hydrogel on burn wounds: An in vitro and in vivo study. J. Dermatol. Treat. 2018, 29, 593–599. [Google Scholar] [CrossRef]
- Grip, J.; Engstad, R.E.; Skjæveland, I.; Škalko-Basnet, N.; Holsæter, A.M. Sprayable Carbopol hydrogel with soluble beta-1, 3/1, 6-glucan as an active ingredient for wound healing–development and in-vivo evaluation. Eur. J. Pharm. Sci. 2017, 107, 24–31. [Google Scholar] [CrossRef]
- Zinov’ev, E.; Ivakhniuk, G.; Dadaian, K.; Lagvilava, T. Wound-healing effect of carbopol hydrogels in rats with alloxan diabetes model. Eksperimental’naia I Klin. Farmakol. 2014, 77, 20–25. [Google Scholar]
- Qian, Y.; Xu, C.; Xiong, W.; Jiang, N.; Zheng, Y.; He, X.; Ding, F.; Lu, X.; Shen, J. Dual cross-linked organic-inorganic hybrid hydrogels accelerate diabetic skin wound healing. Chem. Eng. J. 2021, 417, 129335. [Google Scholar] [CrossRef]
- Guo, Y.; An, X.; Fan, Z. Aramid nanofibers reinforced polyvinyl alcohol/tannic acid hydrogel with improved mechanical and antibacterial properties for potential application as wound dressing. J. Mech. Behav. Biomed. Mater. 2021, 118, 104452. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, X.; Li, S.; Zheng, Y.; Shi, X.; Li, F.; Guo, S.; Yang, J. Preparation of aminated fish scale collagen and oxidized sodium alginate hybrid hydrogel for enhanced full-thickness wound healing. Int. J. Biol. Macromol. 2020, 164, 626–637. [Google Scholar] [CrossRef]
- Lei, H.; Fan, D. Conductive, adaptive, multifunctional hydrogel combined with electrical stimulation for deep wound repair. Chem. Eng. J. 2021, 421, 129578. [Google Scholar] [CrossRef]
- Dou, C.; Li, Z.; Luo, Y.; Gong, J.; Li, Q.; Zhang, J.; Zhang, Q.; Qiao, C. Bio-based poly (γ-glutamic acid)-gelatin double-network hydrogel with high strength for wound healing. Int. J. Biol. Macromol. 2022, 202, 438–452. [Google Scholar] [CrossRef]
- Shi, L.; Yang, N.; Zhang, H.; Chen, L.; Tao, L.; Wei, Y.; Liu, H.; Luo, Y. A novel poly (γ-glutamic acid)/silk-sericin hydrogel for wound dressing: Synthesis, characterization and biological evaluation. Mater. Sci. Eng. C 2015, 48, 533–540. [Google Scholar] [CrossRef]
- Dutta, S.D.; Patel, D.K.; Lim, K.-T. Functional cellulose-based hydrogels as extracellular matrices for tissue engineering. J. Biol. Eng. 2019, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fang, H.; Zheng, S.; Li, L.; Jiao, Z.; Wang, H.; Nie, Y.; Liu, T.; Song, K. A biological functional hybrid scaffold based on decellularized extracellular matrix/gelatin/chitosan with high biocompatibility and antibacterial activity for skin tissue engineering. Int. J. Biol. Macromol. 2021, 187, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Bankoti, K.; Rameshbabu, A.P.; Datta, S.; Roy, M.; Goswami, P.; Roy, S.; Das, A.K.; Ghosh, S.K.; Dhara, S. Carbon nanodot decorated acellular dermal matrix hydrogel augments chronic wound closure. J. Mater. Chem. B 2020, 8, 9277–9294. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Jin, M.; Feng, Z.; Zhang, J.; Rong, Y.; Zhang, Y.; Zhang, S.; Yu, Y.; Yang, H.; Wang, T. Injectable decellularzied extracellular matrix hydrogel derived from human umbilical cord: A novel perspective to deal with refractory wound via medical wastes. Mater. Des. 2023, 229, 111877. [Google Scholar] [CrossRef]
- Nakayama, K.H.; Batchelder, C.A.; Lee, C.I.; Tarantal, A.F. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng. Part A 2010, 16, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, B.; Zhou, D.; Shao, M.; Xu, W.; Zhou, Y. Photocrosslinking maleilated hyaluronate/methacrylated poly (vinyl alcohol) nanofibrous mats for hydrogel wound dressings. Int. J. Biol. Macromol. 2020, 155, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, W.; Du, Y.; Xiao, Y.; Wang, X.; Zhang, S.; Wang, J.; Mao, C. Green gas-mediated cross-linking generates biomolecular hydrogels with enhanced strength and excellent hemostasis for wound healing. ACS Appl. Mater. Interfaces 2020, 12, 13622–13633. [Google Scholar] [CrossRef]
- Wang, X.-H.; Song, F.; Qian, D.; He, Y.-D.; Nie, W.-C.; Wang, X.-L.; Wang, Y.-Z. Strong and tough fully physically crosslinked double network hydrogels with tunable mechanics and high self-healing performance. Chem. Eng. J. 2018, 349, 588–594. [Google Scholar] [CrossRef]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, C.; Fan, D.; Fu, R.; Ma, P.; Duan, Z.; Li, X.; Lei, H.; Chi, L. Construction of porous sponge-like PVA-CMC-PEG hydrogels with pH-sensitivity via phase separation for wound dressing. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 505–515. [Google Scholar] [CrossRef]
- Ali, N.H.; Amin, M.C.I.M.; Ng, S.-F. Sodium carboxymethyl cellulose hydrogels containing reduced graphene oxide (rGO) as a functional antibiofilm wound dressing. J. Biomater. Sci. Polym. Ed. 2019, 30, 629–645. [Google Scholar] [CrossRef] [PubMed]
- Capanema, N.S.; Mansur, A.A.; de Jesus, A.C.; Carvalho, S.M.; de Oliveira, L.C.; Mansur, H.S. Superabsorbent crosslinked carboxymethyl cellulose-PEG hydrogels for potential wound dressing applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234. [Google Scholar] [CrossRef]
- Lv, X.; Ling, Y.; Tang, K.; Qiao, C.; Fu, L.; Xu, C.; Lin, B.; Wei, Y. Hybrid assembly based on nanomaterial reinforcement for multifunctionalized skin-like flexible sensors. Compos. Part A Appl. Sci. Manuf. 2024, 177, 107892. [Google Scholar] [CrossRef]
- Mohamadhoseini, M.; Mohamadnia, Z. Alginate-based self-healing hydrogels assembled by dual cross-linking strategy: Fabrication and evaluation of mechanical properties. Int. J. Biol. Macromol. 2021, 191, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-Y. Biocompatibility of chemically cross-linked gelatin hydrogels for ophthalmic use. J. Mater. Sci. Mater. Med. 2010, 21, 1899–1911. [Google Scholar] [CrossRef]
- Jiang, H.; Zheng, M.; Liu, X.; Zhang, S.; Wang, X.; Chen, Y.; Hou, M.; Zhu, J. Feasibility study of tissue transglutaminase for self-catalytic cross-linking of self-assembled collagen fibril hydrogel and its promising application in wound healing promotion. ACS Omega 2019, 4, 12606–12615. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, C.; Wang, B.; Yin, J.; Yan, S. Progress in hydrogels for skin wound repair. Macromol. Biosci. 2022, 22, 2100475. [Google Scholar] [CrossRef]
- Rezvanian, M.; Ahmad, N.; Amin, M.C.I.M.; Ng, S.-F. Optimization, characterization, and in vitro assessment of alginate–pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef]
- Jian, X.; Wang, H.; Jian, X.; Zou, Y.; Jiang, B.; Chen, C.; Guo, J.; Li, W.; Yu, B. A flexible adhesive hydrogel dressing of embedded structure with pro-angiogenesis activity for wound repair at moving parts inspired by commercial adhesive bandages. Mater. Today Adv. 2024, 21, 100452. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Y.; Huang, Y.; He, J.; Han, Y.; Guo, B. Physical double-network hydrogel adhesives with rapid shape adaptability, fast self-healing, antioxidant and NIR/pH stimulus-responsiveness for multidrug-resistant bacterial infection and removable wound dressing. Adv. Funct. Mater. 2020, 30, 1910748. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Guo, L.; Wang, X.; Chen, H.; Wang, X.; Liu, J.; Tredget, E.E.; Wu, Y. Self-assembling peptide hydrogel scaffolds support stem cell-based hair follicle regeneration. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2115–2125. [Google Scholar] [CrossRef]
- Giano, M.C.; Ibrahim, Z.; Medina, S.H.; Sarhane, K.A.; Christensen, J.M.; Yamada, Y.; Brandacher, G.; Schneider, J.P. Injectable bioadhesive hydrogels with innate antibacterial properties. Nat. Commun. 2014, 5, 4095. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhai, X.; Li, W.; Ji, S.; Dong, W.; Chen, W.; Wei, W.; Lu, Z. A photo-triggering double cross-linked adhesive, antibacterial, and biocompatible hydrogel for wound healing. iScience 2022, 25, 104619. [Google Scholar] [CrossRef]
- Zhou, C.; Sheng, C.; Gao, L.; Guo, J.; Li, P.; Liu, B. Engineering poly (ionic liquid) semi-IPN hydrogels with fast antibacterial and anti-inflammatory properties for wound healing. Chem. Eng. J. 2021, 413, 127429. [Google Scholar] [CrossRef]
- Gao, L.; Chen, J.; Feng, W.; Song, Q.; Huo, J.; Yu, L.; Liu, N.; Wang, T.; Li, P.; Huang, W. A multifunctional shape-adaptive and biodegradable hydrogel with hemorrhage control and broad-spectrum antimicrobial activity for wound healing. Biomater. Sci. 2020, 8, 6930–6945. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, J.; Li, M.; Liu, Z.; Wang, X.; Zhang, L.; Wang, Z. Hydrogel-based biomaterials engineered from natural-derived polysaccharides and proteins for hemostasis and wound healing. Front. Bioeng. Biotechnol. 2021, 9, 780187. [Google Scholar] [CrossRef]
- Cui, C.; Liu, W. Recent advances in wet adhesives: Adhesion mechanism, design principle and applications. Prog. Polym. Sci. 2021, 116, 101388. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small 2019, 15, 1900046. [Google Scholar] [CrossRef]
- Guo, S.; Ren, Y.; Chang, R.; He, Y.; Zhang, D.; Guan, F.; Yao, M. Injectable self-healing adhesive chitosan hydrogel with antioxidative, antibacterial, and hemostatic activities for rapid hemostasis and skin wound healing. ACS Appl. Mater. Interfaces 2022, 14, 34455–34469. [Google Scholar] [CrossRef]
- Preman, N.K.; ES, S.P.; Prabhu, A.; Shaikh, S.B.; Vipin, C.; Barki, R.R.; Bhandary, Y.P.; Rekha, P.; Johnson, R.P. Bioresponsive supramolecular hydrogels for hemostasis, infection control and accelerated dermal wound healing. J. Mater. Chem. B 2020, 8, 8585–8598. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, J.; Yu, F.; Zhao, Y.-x.; Mo, X.-M.; Pan, J.-F. In situ forming hydrogel of natural polysaccharides through Schiff base reaction for soft tissue adhesive and hemostasis. Int. J. Biol. Macromol. 2020, 147, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, H.; Wang, X.; Qiu, F.; Liu, H.; Lu, J.; Tong, L.; Yang, Y.; Wang, X.; Wu, H. Degradable and bioadhesive alginate-based composites: An effective hemostatic agent. ACS Biomater. Sci. Eng. 2019, 5, 5498–5505. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Fan, D.; Duan, Z.; Zhu, C.; Fu, R.; Li, X. Non-stick hemostasis hydrogels as dressings with bacterial barrier activity for cutaneous wound healing. Mater. Sci. Eng. C 2019, 105, 110118. [Google Scholar] [CrossRef]
- Bal-Öztürk, A.; Özkahraman, B.; Özbaş, Z.; Yaşayan, G.; Tamahkar, E.; Alarçin, E. Advancements and future directions in the antibacterial wound dressings–A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 703–716. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Y.; Song, T.; Zhang, B.; Li, D.; Xiao, Y.; Zhang, X. A chitosan-based multifunctional hydrogel containing in situ rapidly bioreduced silver nanoparticles for accelerating infected wound healing. J. Mater. Chem. B 2022, 10, 2135–2147. [Google Scholar] [CrossRef]
- Tian, R.; Qiu, X.; Yuan, P.; Lei, K.; Wang, L.; Bai, Y.; Liu, S.; Chen, X. Fabrication of self-healing hydrogels with on-demand antimicrobial activity and sustained biomolecule release for infected skin regeneration. ACS Appl. Mater. Interfaces 2018, 10, 17018–17027. [Google Scholar] [CrossRef]
- Singh, B.; Dhiman, A. Designing bio-mimetic moxifloxacin loaded hydrogel wound dressing to improve antioxidant and pharmacology properties. RSC Adv. 2015, 5, 44666–44678. [Google Scholar] [CrossRef]
- Du, X.; Wu, L.; Yan, H.; Qu, L.; Wang, L.; Ren, S.; Kong, D.; Wang, L. Multifunctional Hydrogel Patch with Toughness, Tissue Adhesiveness, and Antibacterial Activity for Sutureless Wound Closure. ACS Biomater. Sci. Eng. 2019, 5, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhao, J.; Li, H.; Fan, D. Paramylon hydrogel: A bioactive polysaccharides hydrogel that scavenges ROS and promotes angiogenesis for wound repair. Carbohydr. Polym. 2022, 289, 119467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hou, J.; Yuan, Q.; Xin, P.; Cheng, H.; Gu, Z.; Wu, J. Arginine derivatives assist dopamine-hyaluronic acid hybrid hydrogels to have enhanced antioxidant activity for wound healing. Chem. Eng. J. 2020, 392, 123775. [Google Scholar] [CrossRef]
- Asadi, N.; Pazoki-Toroudi, H.; Del Bakhshayesh, A.R.; Akbarzadeh, A.; Davaran, S.; Annabi, N. Multifunctional hydrogels for wound healing: Special focus on biomacromolecular based hydrogels. Int. J. Biol. Macromol. 2021, 170, 728–750. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Tong, X.; You, S.; Mao, R.; Cai, E.; Pan, W.; Zhang, C.; Hu, R.; Shen, J. Mild hyperthermia-assisted ROS scavenging hydrogels achieve diabetic wound healing. ACS Macro Lett. 2022, 11, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and impact of antioxidant hydrogel in chronic wound healing. Adv. Healthc. Mater. 2020, 9, 1901502. [Google Scholar] [CrossRef]
- Qi, X.; Cai, E.; Xiang, Y.; Zhang, C.; Ge, X.; Wang, J.; Lan, Y.; Xu, H.; Hu, R.; Shen, J. An Immunomodulatory Hydrogel by Hyperthermia-Assisted Self-Cascade Glucose Depletion and ROS Scavenging for Diabetic Foot Ulcer Wound Therapeutics. Adv. Mater. 2023, 35, 2306632. [Google Scholar] [CrossRef]
- Ahmadian, Z.; Gheybi, H.; Adeli, M. Efficient wound healing by antibacterial property: Advances and trends of hydrogels, hydrogel-metal NP composites and photothermal therapy platforms. J. Drug Deliv. Sci. Technol. 2022, 73, 103458. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Wang, J.; Zhang, Y.; Dong, M.; Bu, T.; Li, L.; Liu, Y.; Wang, L. Multifunctional injectable hydrogel dressings for effectively accelerating wound healing: Enhancing biomineralization strategy. Adv. Funct. Mater. 2021, 31, 2100093. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.; Zhang, Q.; Lu, H.; Qiu, X.; Zhou, D.; Qi, Y.; Huang, Y. Dual cross-linked HHA hydrogel supplies and regulates MΦ2 for synergistic improvement of immunocompromise and impaired angiogenesis to enhance diabetic chronic wound healing. Biomacromolecules 2020, 21, 3795–3806. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, H.; Zhou, L.; Cheng, F.; Liu, Z.; Zhang, H.; Wang, L.; Zhang, Q. Nanoenzyme-reinforced injectable hydrogel for healing diabetic wounds infected with multidrug resistant bacteria. Nano Lett. 2020, 20, 5149–5158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kankala, R.K.; Ou, C.; Chen, A.; Yang, Z. Advances in hydrogel-based vascularized tissues for tissue repair and drug screening. Bioact. Mater. 2022, 9, 198–220. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, R.; Zhao, X.; Zhang, Y.; Tam, A.; Yan, Y.; Shen, H.; Zhang, Y.S.; Qi, J.; Feng, Y. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019, 11, 3. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, X.; Shen, Y.-I.; Sebastian, R.; Dickinson, L.E.; Fox-Talbot, K.; Reinblatt, M.; Steenbergen, C.; Harmon, J.W.; Gerecht, S. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc. Natl. Acad. Sci. USA 2011, 108, 20976–20981. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.-Y.; Tao, L.; Wei, Y.; Hsu, S.-h. A novel biodegradable self-healing hydrogel to induce blood capillary formation. NPG Asia Mater. 2017, 9, e363. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, S.; Cai, B.; Wang, Y.; Deng, D.; Wang, X. An injectable and self-healing hydrogel with antibacterial and angiogenic properties for diabetic wound healing. Biomater. Sci. 2022, 10, 3480–3492. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, S.; Hu, Z.; Yang, W.; Lan, Y.; Zhu, J.; Hancharou, A.; Guo, R.; Tang, B. In situ formed anti-inflammatory hydrogel loading plasmid DNA encoding VEGF for burn wound healing. Acta Biomater. 2019, 100, 191–201. [Google Scholar] [CrossRef]
- Tan, J.; Li, L.; Wang, H.; Wei, L.; Gao, X.; Zeng, Z.; Liu, S.; Fan, Y.; Liu, T.; Chen, J. Biofunctionalized fibrin gel co-embedded with BMSCs and VEGF for accelerating skin injury repair. Mater. Sci. Eng. C 2021, 121, 111749. [Google Scholar] [CrossRef]
- Eke, G.; Mangir, N.; Hasirci, N.; MacNeil, S.; Hasirci, V. Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomaterials 2017, 129, 188–198. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2022 Compendium of Estimates; Mary Ann Liebert, Inc.: Larchmont, NY, USA, 2023; Volume 12, pp. 657–670. [Google Scholar]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef]
- Sim, W.; Barnard, R.T.; Blaskovich, M.; Ziora, Z.M. Antimicrobial Silver in Medicinal and Consumer Applications: A Patent Review of the Past Decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.E.; Loewen-Rodriguez, A.; Lessem, J. Dermagraft: Use in the treatment of chronic wounds. Adv. Wound Care 2012, 1, 138–141. [Google Scholar] [CrossRef]
- Garoufalis, M.G. The Importance of Wound Care Researchers and Manufactures Working with Medical Associations When Bringing New Products to the Marketplace; Mary Ann Liebert, Inc.: Larchmont, NY, USA, 2018. [Google Scholar]
- Sharma, A.; Sharma, D.; Zhao, F. Updates on Recent Clinical Assessment of Commercial Chronic Wound Care Products. Adv. Healthc. Mater. 2023, 12, e2300556. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.W.; Khachemoune, A. Skin substitutes for the management of mohs micrographic surgery wounds: A systematic review. Arch. Dermatol. Res. 2023, 315, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Abdo, J.; Ortman, H. Biologic and synthetic cellular and/or tissue-based products and smart wound dressings/coverings. Surg. Clin. 2020, 100, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Matar, D.Y.; Ng, B.; Darwish, O.; Wu, M.; Orgill, D.P.; Panayi, A.C. Skin inflammation with a focus on wound healing. Adv. Wound Care 2023, 12, 269–287. [Google Scholar] [CrossRef]
- Niknejad, H.; Peirovi, H.; Jorjani, M.; Ahmadiani, A.; Ghanavi, J.; Seifalian, A.M. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cells Mater. 2008, 15, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Koob, T.J.; Lim, J.J.; Massee, M.; Zabek, N.; Denoziere, G. Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1353–1362. [Google Scholar] [CrossRef]
- Sarkar, S.; Poundarik, A.A. Bioactive wound dressings for the management of chronic non healing ulcers (CNHU)—A review of clinical and translational studies. Materialia 2022, 21, 101269. [Google Scholar] [CrossRef]
- Dhall, S.; Coksaygan, T.; Hoffman, T.; Moorman, M.; Lerch, A.; Kuang, J.-Q.; Sathyamoorthy, M.; Danilkovitch, A. Viable cryopreserved umbilical tissue (vCUT) reduces post-operative adhesions in a rabbit abdominal adhesion model. Bioact. Mater. 2019, 4, 97–106. [Google Scholar] [CrossRef] [PubMed]

| Product | Company | Components | Ref. |
|---|---|---|---|
| NUSHIELD | Organogenesis in Canton, MA, USA | Comprising amnion, chorion, and a spongy layer | [130] |
| APLIGRAFT | Organogenesis in Canton, MA, USA | Incorporating living keratinocytes and stem cells | [131] |
| Pura PLay AM | Organogenesis in Canton, MA, USA | A collagen sheet treated with 0.1% poly hexamethylene biguanide hydrochloride | [132] |
| DERMAGRAFT | Organogenesis in Canton, MA, USA | Featuring living fibroblasts seeded on a bioabsorbable scaffold | [133] |
| AFFINITY | Organogenesis in Canton, MA, USA | Utilizing fresh amniotic membrane as a wound covering | [134] |
| AMNIOFIX | MIMEDX in Marietta, GA, USA | Allograft consisting of amnion/chorion membrane | [135] |
| GRAFIX and GRAFIX PL | OSIRIS (a part of Smith and Nephew), Columbia, MD, USA | Placental membrane, inclusive of mesenchymal stem cells | [136] |
| STRAVIX and STARVIX PL | OSIRIS (a part of Smith and Nephew), Columbia, MD, USA | Umbilical tissue | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arabpour, Z.; Abedi, F.; Salehi, M.; Baharnoori, S.M.; Soleimani, M.; Djalilian, A.R. Hydrogel-Based Skin Regeneration. Int. J. Mol. Sci. 2024, 25, 1982. https://doi.org/10.3390/ijms25041982
Arabpour Z, Abedi F, Salehi M, Baharnoori SM, Soleimani M, Djalilian AR. Hydrogel-Based Skin Regeneration. International Journal of Molecular Sciences. 2024; 25(4):1982. https://doi.org/10.3390/ijms25041982
Chicago/Turabian StyleArabpour, Zohreh, Farshad Abedi, Majid Salehi, Seyed Mahbod Baharnoori, Mohammad Soleimani, and Ali R. Djalilian. 2024. "Hydrogel-Based Skin Regeneration" International Journal of Molecular Sciences 25, no. 4: 1982. https://doi.org/10.3390/ijms25041982
APA StyleArabpour, Z., Abedi, F., Salehi, M., Baharnoori, S. M., Soleimani, M., & Djalilian, A. R. (2024). Hydrogel-Based Skin Regeneration. International Journal of Molecular Sciences, 25(4), 1982. https://doi.org/10.3390/ijms25041982






