Hydrogen Sulfide in the Oxidative Stress Response of Plants: Crosstalk with Reactive Oxygen Species
Abstract
1. Introduction
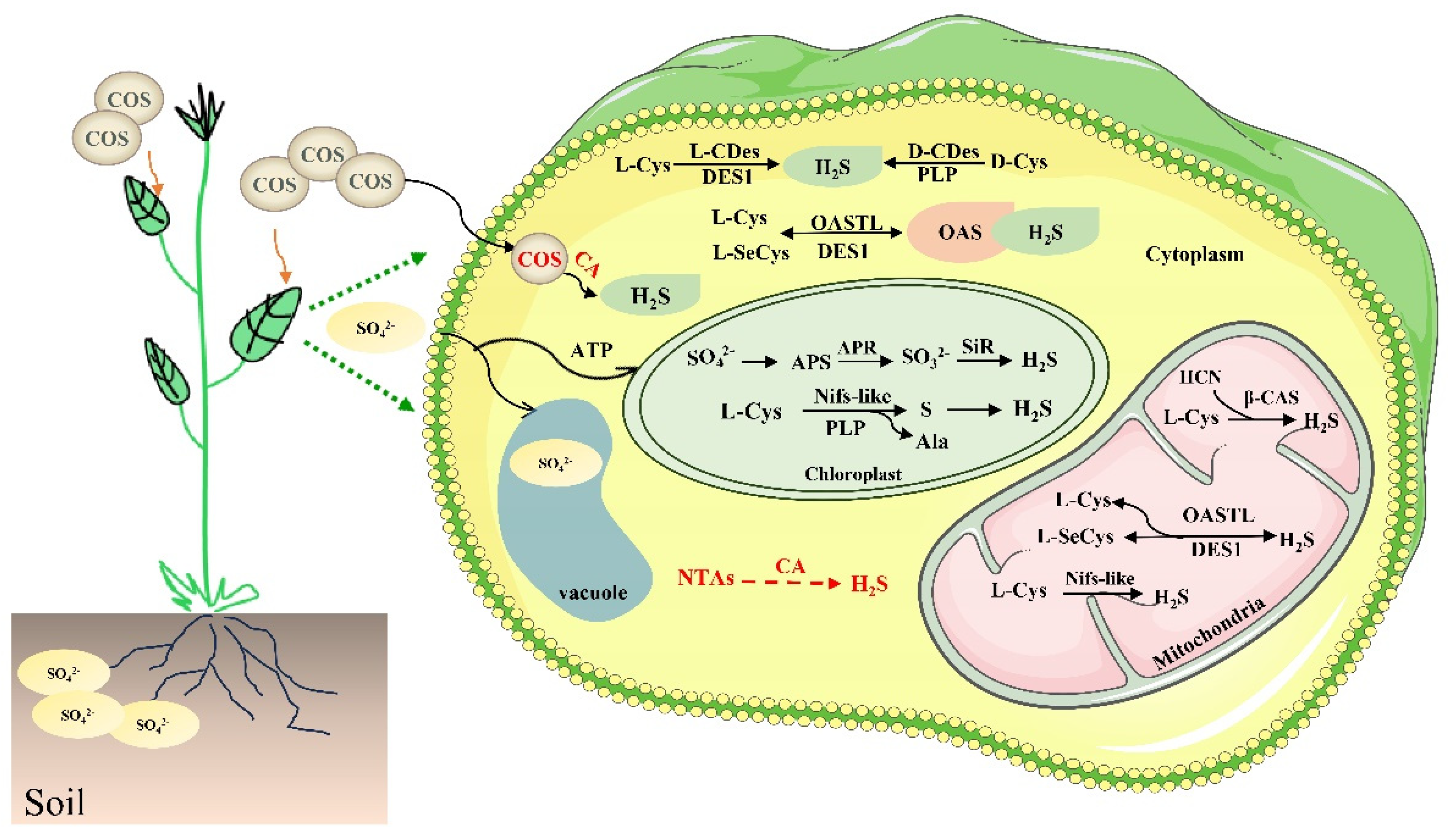
2. Biosynthesis of Endogenous H2S in Plants
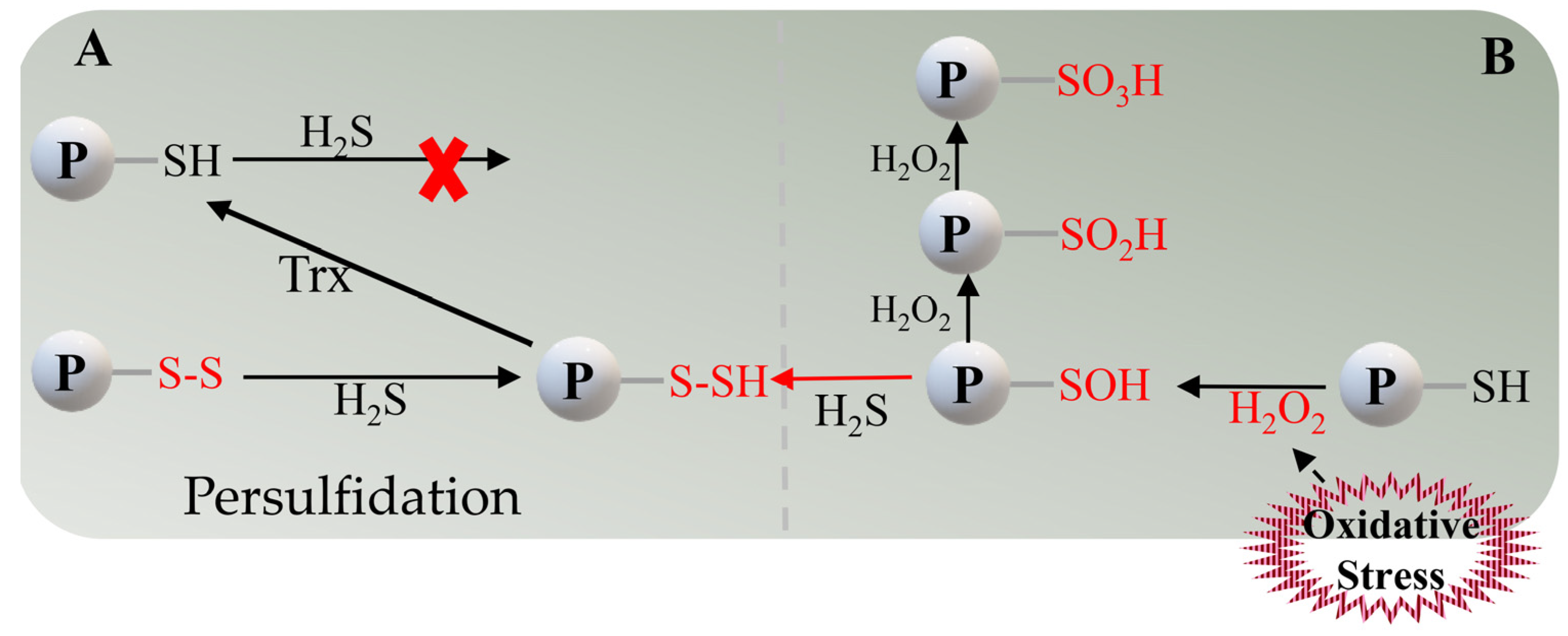
3. Post-Translational Modification (PTM) of Cysteine Residues by H2S under Oxidative Stress
| Proteins | Sites | Plant Species | Reference |
|---|---|---|---|
| Ascorbate peroxidase 1 | Cys32 | Arabidopsis thaliana | [56] |
| Cytosolic ascorbate peroxidase 1 | Cys234 | Solanum lycopersicum | [48] |
| Catalase 1 | Cys168 | Solanum lycopersicum | [48] |
| Percoxidase 5 | Cys46, 61 | Solanum lycopersicum | [48] |
| Plasma membrane H+-ATPase 1 | Cys446 | Arabidopsis thaliana | [11] |
| Open Stomata1 (Ost1)/Snf1-Related Protein Kinase 2.6 | Cys131, 137 | Arabidopsis thaliana | [57] |
| ABSCISIC ACID INSENSITIVE 4 (ABI4) | Cys250 | Arabidopsis thaliana | [58] |
| L-cysteine desulfhydrase | Cys44, 205 | Arabidopsis thaliana | [59] |
| Respiratory burst oxidase homolog protein | Cys825, 890 | Arabidopsis thaliana | [59] |
| His-Csa5G156220 | Unknown | Cucumis sativus L. | [12] |
| His-Csa5G157230 | Unknown | Cucumis sativus L. | [12] |
| Nitrate Reductase 2 | Unknown | Oryza sativa L. | [22] |
| Glucose-6-phosphate dehydrogenases 6 | Cys159 | Arabidopsis thaliana | [51] |
| Glucose-6-phosphate dehydrogenases C | Cys155 | Solanum lycopersicum | [51] |
| Autophagy-related (ATG18a) | Cys103 | Arabidopsis thaliana | [52] |
| Autophagy-related (ATG4) | Cys170 | Arabidopsis thaliana | [53] |
| 1-aminocyclopropane-1-carboxylic acid oxidases (ACOs) | Cys60 | Solanum lycopersicum | [60] |
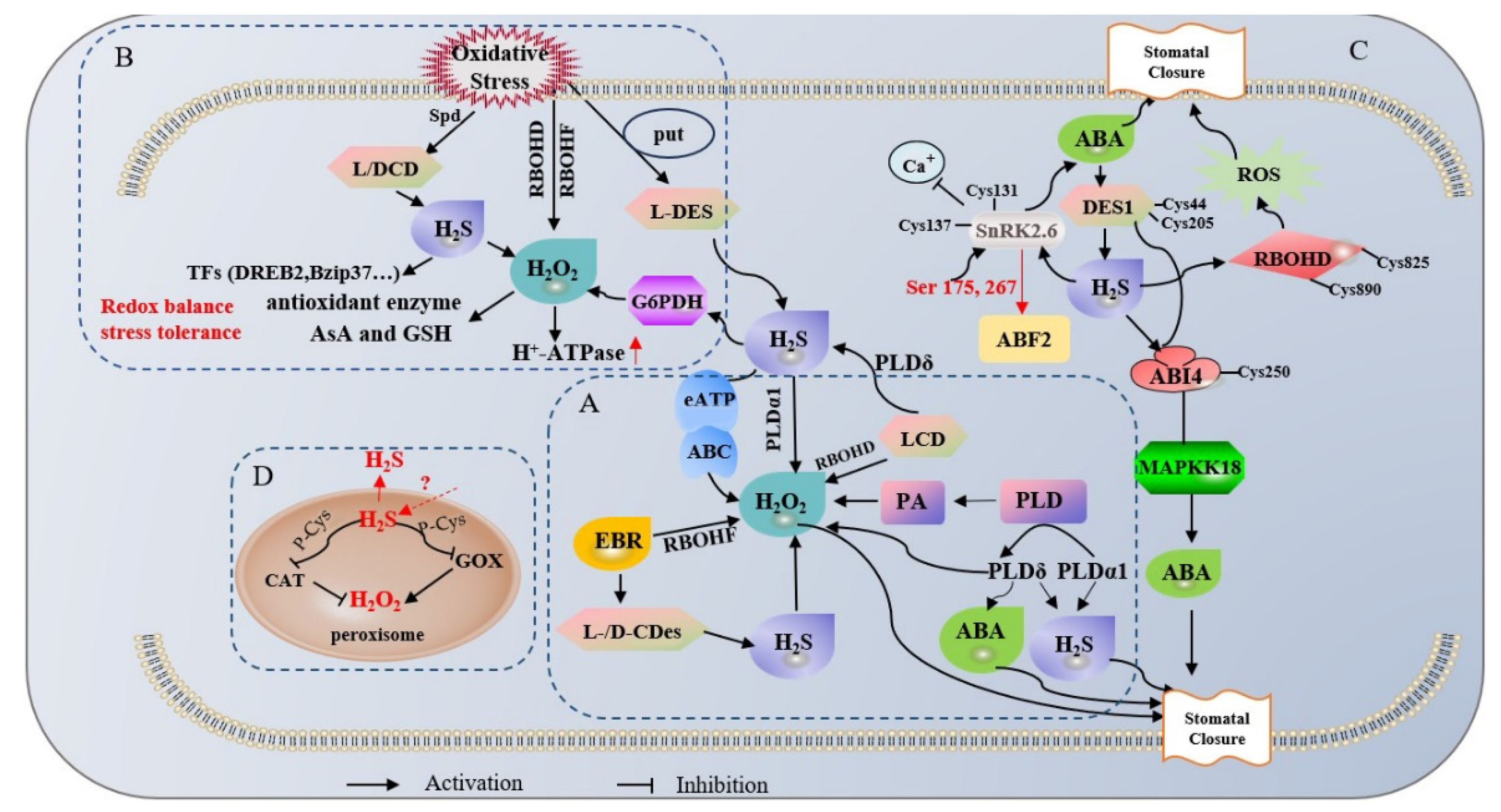
4. Biosynthesis of Endogenous H2S in Plants’ Interaction between H2S and ROS in the Regulation of Oxidative Stress
4.1. H2S Positively Regulates Oxidative Signal-Induced Stomatal Closure in Upstream and Downstream of H2O2
4.2. Positive Cooperation between H2S and H2O2 Signaling in the Stress Response
4.3. H2S Promotes ROS Bursts, Fine-Tuning Redox Homeostasis and ABA Signaling
5. Potential Tools for H2S and ROS Interactions: Peroxisomes and Dual Donor Systems
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Trx | Thioredoxin |
| APS | 5′-adenyl sulfate |
| APR | APS REDUCTASE |
| SiR | Sulfite reductase |
| L-CDes | L-cysteine dehydrogenase |
| D-CDes | D-cysteine dehydrogenase |
| PLP | 5′-pyridoxal phosphate |
| β-CAS | β-cyanoalanine synthetase |
| CA | Carbonic anhydrase |
| NTAs | Acid-derived N-thiocarboxylic anhydrides |
| RBOH | NADPH oxidase respiratory burst oxidase homolog |
| PLD | Phospholipase D |
| PA | Phosphatidic acid |
| eATP | Extracellular ATP |
| Put | Putrescine |
| AsA | Ascorbic acid |
| GSH | Glutathione |
| Spd | Spermidine |
| PM | Plasma membrane |
| GOx | Glycolate oxidase |
| G6PDH | Glucose-6-phosphate dehydrogenase |
| SnRK2.6 | Open Stomata1 (OST1)/Snf1-related Protein Kinase 2.6 |
| ABI4 | Abscisic Acid Insensitive 4 |
| MAPKKK18 | Mitogen-activated Protein Kinase Kinase Kinase 18 |
| ABF2 | ABA response element-binding factor 2 |
References
- Harrington, H.M.; Smith, I. Cysteine metabolism in cultured tobacco cells. Plant Physiol. 1980, 65, 151–155. [Google Scholar] [CrossRef]
- Shen, J.; Su, Y.; Zhou, C.; Zhang, F.; Zhou, H.; Liu, X.; Wu, D.; Yin, X.; Xie, Y.; Yuan, X. A putative rice L-cysteine desulfhydrase encodes a true L-cysteine synthase that regulates plant cadmium tolerance. Plant Growth Regul. 2019, 89, 217–226. [Google Scholar] [CrossRef]
- Xie, Y.; Lai, D.; Mao, Y.; Zhang, W.; Shen, W.; Guan, R. Molecular cloning, characterization, and expression analysis of a novel gene encoding L-cysteine desulfhydrase from Brassica napus. Mol. Biotechnol. 2013, 54, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Papenbrock, J.; Riemenschneider, A.; Kamp, A.; Schulz-Vogt, H.N.; Schmidt, A. Characterization of Cysteine-degrading and H2S-releasing enzymes of higher plants from the field to the test tube and back. Plant Biol. 2007, 9, 582–588. [Google Scholar] [CrossRef]
- Riemenschneider, A.; Wegele, R.; Schmidt, A.; Papenbrock, J. Isolation and characterization of a D-cysteine desulfhydrase protein from Arabidopsis thaliana. FEBS J. 2005, 272, 1291–1304. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Guan, W.; Zhou, M.; Shen, J.; Liu, X.; Wu, D.; Yin, X.; Xie, Y. Cloning and characterization of a gene encoding true D-cysteine desulfhydrase from Oryza sativa. Plant Mol. Biol. Rep. 2019, 38, 95–113. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, M.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; Shen, J.; Ge, Z.; Zhang, Z.; Shen, W. Hydrogen sulfide, a signaling molecule in plant stress responses. J. Integr. Plant Biol. 2021, 63, 146–160. [Google Scholar] [CrossRef]
- Arif, Y.; Hayat, S.; Yusuf, M.; Bajguz, A. Hydrogen sulfide: A versatile gaseous molecule in plants. Plant Physiol. Biochem. 2021, 158, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Palma, J.M. H2S signaling in plants and applications in agriculture. J. Adv. Res. 2020, 24, 131–137. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, J.; Zhou, H.; Zhao, D.; Duan, T.; Wang, S.; Yuan, X.; Xie, Y. Hydrogen sulfide-linked persulfidation maintains protein stability of ABSCISIC ACID-INSENSITIVE 4 and delays seed germination. Int. J. Mol. Sci. 2022, 23, 1389. [Google Scholar] [CrossRef]
- Ma, Y.; Li, F.; Yi, Y.; Wang, X.; Li, T.; Wang, X.; Sun, H.; Li, L.; Ren, M.; Han, S. Hydrogen sulfide improves salt tolerance through persulfidation of PMA1 in Arabidopsis. Plant Cell Rep. 2023, 42, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Cao, C.; Liang, S.; Ma, Y.; Liu, X.; Pei, Y. The role of H2S in low temperature-induced cucurbitacin C increases in cucumber. Plant Mol. Biol. 2019, 99, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Tabassum, J.; Mubarik, M.S.; Anwar, S.; Zahra, N.; Sharif, Y.; Hafeez, M.B.; Zhang, C.; Corpas, F.J.; Chen, H. Hydrogen sulfide: An emerging component against abiotic stress in plants. Plant Biol. 2022, 24, 540–558. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Tian, M.; Han, Y. Hydrogen sulfide: A multi-tasking signal molecule in the regulation of oxidative stress responses. J. Exp. Bot. 2020, 71, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Barroso, J.B.; Gonzalez-Gordo, S.; Munoz-Vargas, M.A.; Palma, J.M. Hydrogen sulfide: A novel component in Arabidopsis peroxisomes which triggers catalase inhibition. J. Integr. Plant Biol. 2019, 61, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Vellarino, F.L.; Garrido, I.; Ortega, A.; Casimiro, I.; Espinosa, F. Response to antimony toxicity in dittrichia viscosa plants: ROS, NO, H2S, and the antioxidant system. Antioxidants 2021, 10, 1698. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Gonzalez-Gordo, S.; Rodriguez-Ruiz, M.; Munoz-Vargas, M.A.; Palma, J.M. Thiol-based oxidative posttranslational modifications (OxiPTMs) of plant proteins. Plant Cell Physiol. 2022, 63, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, I.; Holzmeister, C.; Wirtz, M.; Geerlof, A.; Frohlich, T.; Romling, G.; Kuruthukulangarakoola, G.T.; Linster, E.; Hell, R.; Arnold, G.J. ROS-mediated inhibition of S-nitrosoglutathione reductase contributes to the activation of anti-oxidative mechanisms. Front. Plant Sci. 2016, 7, 1669. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Wang, J.; Cao, Q.; Wen, Z. Hydrogen sulfide is involved in maintaining ion homeostasis via regulating plasma membrane Na+/H+ antiporter system in the hydrogen peroxide-dependent manner in salt-stress Arabidopsis thaliana root. Protoplasma 2014, 251, 899–912. [Google Scholar] [CrossRef]
- Kabala, K.; Zboinska, M.; Glowiak, D.; Reda, M.; Jakubowska, D.; Janicka, M. Interaction between the signaling molecules hydrogen sulfide and hydrogen peroxide and their role in vacuolar H+-ATPase regulation in cadmium-stressed cucumber roots. Physiol. Plant 2019, 166, 688–704. [Google Scholar] [CrossRef]
- Wang, L.; Ma, X.; Che, Y.; Hou, L.; Liu, X.; Zhang, W. Extracellular ATP mediates H2S-regulated stomatal movements and guard cell K+ current in a H2O2-dependent manner in Arabidopsis. Sci. Bull. 2015, 60, 419–427. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, Y.; Zhang, F.; Guan, W.; Su, Y.; Yuan, X.; Xie, Y. Persulfidation of Nitrate Reductase 2 is involved in L-Cysteine desulfhydrase-regulated rice drought tolerance. Int. J. Mol. Sci. 2021, 22, 12119. [Google Scholar] [CrossRef]
- Zhang, W.; Zhi, W.; Qiao, H.; Huang, J.; Li, S.; Lu, Q.; Wang, N.; Li, Q.; Zhou, Q.; Sun, J. H2O2-dependent oxidation of the transcription factor GmNTL1 promotes salt tolerance in soybean. Plant Cell 2023, 36, 112–135. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Jia, H.; Li, F.; Ma, Y.; Liesche, J.; Liao, M.; Ding, X.; Liu, C.; Chen, Y. Persulfidation-induced structural change in SnRK2.6 establishes intramolecular interaction between phosphorylation and persulfidation. Mol. Plant 2021, 14, 1814–1830. [Google Scholar] [CrossRef]
- Aroca, A.; Zhang, J.; Xie, Y.; Romero, L.C.; Gotor, C. Hydrogen sulfide signaling in plant adaptations to adverse conditions: Molecular mechanisms. J. Exp. Bot. 2021, 72, 5893–5904. [Google Scholar] [CrossRef]
- Wilson, L.G.; Bressan, R.A.; Filner, P. Light-dependent emission of hydrogen sulfide from plants. Plant Physiol. 1978, 61, 184–189. [Google Scholar] [CrossRef]
- Gotor, C.; Garcia, I.; Aroca, A.; Laureano-Marin, A.M.; Arenas-Alfonseca, L.; Jurado-Flores, A.; Moreno, I.; Romero, L.C. Signaling by hydrogen sulfide and cyanide through post-translational modification. J. Exp. Bot. 2019, 70, 4251–4265. [Google Scholar] [CrossRef]
- Fu, Y.; Tang, J.; Yao, G.F.; Huang, Z.Q.; Li, Y.H.; Han, Z.; Chen, X.Y.; Hu, L.Y.; Hu, K.D.; Zhang, H. Central role of adenosine 5’-phosphosulfate reductase in the control of plant hydrogen sulfide metabolism. Front. Plant Sci. 2018, 9, 1404. [Google Scholar] [CrossRef]
- Nagasawa, T.; Ishii, T.; Kumagai, H.; Yamada, H. D-Cysteine desulfhydrase of Escherichia coli. purification and characterization. Eur. J. Biochem. 1985, 153, 541–551. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.; Garifullina, G.F.; Abdel-Ghany, S.; Kato, S.; Mihara, H.; Hale, K.L.; Burkhead, J.L.; Esaki, N.; Kurihara, T.; Pilon, M. Characterization of a Nifs-like chloroplast protein from Arabidopsis. Implications for its role in sulfur and selenium metabolism. Plant Physiol. 2002, 130, 1309–1318. [Google Scholar] [CrossRef]
- Van Hoewyk, D.; Pilon, M.; Pilon-Smits, E.A.H. The functions of Nifs-like proteins in plant sulfur and selenium metabolism. Plant Sci. 2008, 174, 117–123. [Google Scholar] [CrossRef]
- Alvarez, C.; Calo, L.; Romero, L.C.; Garcia, I.; Gotor, C. An O-acetylserine(thiol)lyase homolog with L-cysteine desulfhydrase activity regulates cysteine homeostasis in Arabidopsis. Plant Physiol. 2010, 152, 656–669. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Liu, J.; Liu, T.; Xue, S. Hydrogen sulfide (H2S) signaling in plant development and stress responses. aBIOTECH 2021, 2, 32–63. [Google Scholar] [CrossRef]
- Poor, P.; Patyi, G.; Takacs, Z.; Szekeres, A.; Bodi, N.; Bagyanszki, M.; Tari, I. Salicylic acid-induced ROS production by mitochondrial electron transport chain depends on the activity of mitochondrial hexokinases in tomato (Solanum lycopersicum L.). J. Plant Res. 2019, 132, 273–283. [Google Scholar] [CrossRef]
- Kurmanbayeva, A.; Bekturova, A.; Soltabayeva, A.; Oshanova, D.; Nurbekova, Z.; Srivastava, S.; Tiwari, P.; Dubey, A.K.; Sagi, M. Active O-acetylserine-(thiol) lyase A and B confer improved selenium resistance and degrade L-Cys and L-SeCys in Arabidopsis. J. Exp. Bot. 2022, 73, 2525–2539. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nakamura, T.; Kusano, T.; Sano, H. Three Arabidopsis genes encoding proteins with differential activities for cysteine synthase and beta-cyanoalanine synthase. Plant Cell Physiol. 2000, 41, 465–476. [Google Scholar] [CrossRef]
- Bloem, E.; Rubekin, K.; Haneklaus, S.; Banfalvi, Z.; Hesse, H.; Schnug, E. H2S and COS gas exchange of transgenic potato lines with modified expression levels of enzymes involved in sulphur metabolism. J. Agron. Crop Sci. 2011, 197, 311–321. [Google Scholar] [CrossRef]
- Chauhan, P.; Gupta, K.; Ravikumar, G.; Saini, D.K.; Chakrapani, H. Carbonyl Sulfide (COS) donor induced protein persulfidation protects against oxidative stress. Chem. Asian J. 2019, 14, 4717–4724. [Google Scholar] [CrossRef]
- Pintus, E.; Chinn, A.F.; Kadlec, M.; Garcia-Vazquez, F.A.; Novy, P.; Matson, J.B.; Ros-Santaella, J.L. N-thiocarboxyanhydrides, amino acid-derived enzyme-activated H2S donors, enhance sperm mitochondrial activity in presence and absence of oxidative stress. BMC Vet. Res. 2023, 19, 52. [Google Scholar] [CrossRef]
- Sun, C.; Yao, G.F.; Li, L.X.; Li, T.T.; Zhao, Y.Q.; Hu, K.D.; Zhang, C.; Zhang, H. E3 ligase BRG3 persulfidation delays tomato ripening by reducing ubiquitination of the repressor WRKY71. Plant Physiol. 2023, 192, 616–632. [Google Scholar] [CrossRef]
- Yao, G.; Gou, S.; Zhong, T.; Wei, S.; An, X.; Sun, H.; Sun, C.; Hu, K.; Zhang, H. Persulfidation of transcription factor MYB10 inhibits anthocyanin synthesis in red-skinned pear. Plant Physiol. 2023, 192, 2185–2202. [Google Scholar] [CrossRef]
- Wang, P.; Fang, H.; Gao, R.; Liao, W. Protein persulfidation in plants: Function and mechanism. Antioxidants 2021, 10, 1631. [Google Scholar] [CrossRef]
- Huang, J.; Xie, Y. Hydrogen sulfide signaling in plants. Antioxid. Redox Signal 2023, 39, 40–58. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, X.; Feng, J.; Zhu, S. Biological functions of hydrogen sulfide in plants. Int. J. Mol. Sci. 2022, 23, 15107. [Google Scholar] [CrossRef]
- He, B.; Zhang, Z.; Huang, Z.; Duan, X.; Wang, Y.; Cao, J.; Li, L.; He, K.; Nice, E.C.; He, W. Protein persulfidation: Rewiring the hydrogen sulfide signaling in cell stress response. Biochem. Pharmacol. 2023, 209, 115444. [Google Scholar] [CrossRef]
- Aroca, A.; Gotor, C.; Romero, L.C. Hydrogen sulfide signaling in plants: Emerging roles of protein persulfidation. Front. Plant Sci. 2018, 9, 1369. [Google Scholar] [CrossRef]
- Jurado-Flores, A.; Aroca, A.; Romero, L.C.; Gotor, C.; Kopriva, S. Sulfide promotes tolerance to drought through protein persulfidation in Arabidopsis. J. Exp. Bot. 2023, 74, 4654–4669. [Google Scholar] [CrossRef]
- Li, J.; Shi, C.; Wang, X.; Liu, C.; Ding, X.; Ma, P.; Wang, X.; Jia, H. Hydrogen sulfide regulates the activity of antioxidant enzymes through persulfidation and improves the resistance of tomato seedling to Copper Oxide nanoparticles (CuO NPs)-induced oxidative stress. Plant Physiol. Biochem. 2020, 156, 257–266. [Google Scholar] [CrossRef]
- Huang, J.; Willems, P.; Wei, B.; Tian, C.; Ferreira, R.B.; Bodra, N.; Martinez Gache, S.A.; Wahni, K.; Liu, K.; Vertommen, D. Mining for protein S-sulfenylation in Arabidopsis uncovers redox-sensitive sites. Proc. Natl. Acad. Sci. USA 2019, 116, 21256–21261. [Google Scholar] [CrossRef]
- Du, X.; Jin, Z.; Liu, Z.; Liu, D.; Zhang, L.; Ma, X.; Yang, G.; Liu, S.; Guo, Y.; Pei, Y. H2S persulfidated and increased kinase activity of MPK4 to response cold stress in Arabidopsis. Front. Mol. Biosci. 2021, 8, 635470. [Google Scholar] [CrossRef]
- Wang, X.; Shi, C.; Hu, Y.; Ma, Y.; Yi, Y.; Jia, H.; Li, F.; Sun, H.; Li, T.; Wang, X. Persulfidation maintains cytosolic G6PDs activity through changing tetrameric structure and competing cysteine sulfur oxidation under salt stress in Arabidopsis and tomato. New Phytol. 2023, 240, 626–643. [Google Scholar] [CrossRef]
- Aroca, A.; Yruela, I.; Gotor, C.; Bassham, D.C. Persulfidation of ATG18a regulates autophagy under ER stress in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2023604118. [Google Scholar] [CrossRef]
- Laureano-Marin, A.M.; Aroca, A.; Perez-Perez, M.E.; Yruela, I.; Jurado-Flores, A.; Moreno, I.; Crespo, J.L.; Romero, L.C.; Gotor, C. Abscisic acid-triggered persulfidation of the Cys protease ATG4 mediates regulation of autophagy by sulfide. Plant Cell 2020, 32, 3902–3920. [Google Scholar] [CrossRef]
- Aroca, A.; Garcia-Diaz, I.; Garcia-Calderon, M.; Gotor, C.; Marquez, A.J.; Betti, M. Photorespiration: Regulation and new insights on the potential role of persulfidation. J. Exp. Bot. 2023, 74, 6023–6039. [Google Scholar] [CrossRef]
- Garcia-Calderon, M.; Vignane, T.; Filipovic, M.R.; Ruiz, M.T.; Romero, L.C.; Marquez, A.J.; Gotor, C.; Aroca, A. Persulfidation protects from oxidative stress under nonphotorespiratory conditions in Arabidopsis. New Phytol. 2023, 238, 1431–1445. [Google Scholar] [CrossRef]
- Aroca, A.; Serna, A.; Gotor, C.; Romero, L.C. S-sulfhydration: A cysteine posttranslational modification in plant systems. Plant Physiol. 2015, 168, 334–342. [Google Scholar] [CrossRef]
- Chen, S.; Jia, H.; Wang, X.; Shi, C.; Wang, X.; Ma, P.; Wang, J.; Ren, M.; Li, J. Hydrogen sulfide positively regulates abscisic acid signaling through persulfidation of SnRK2.6 in guard cells. Mol. Plant 2020, 13, 732–744. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, J.; Shen, J.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; Fu, L.; Li, Z.; Yang, J. Hydrogen sulfide-linked persulfidation of ABI4 controls ABA responses through the transactivation of MAPKKK18 in Arabidopsis. Mol. Plant 2021, 14, 921–936. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, J.; Zhou, M.; Zhou, H.; Cui, B.; Gotor, C.; Romero, L.C.; Fu, L.; Yang, J.; Foyer, C.H. Persulfidation-based modification of Cysteine desulfhydrase and the NADPH oxidase RBOHD controls guard cell abscisic acid signaling. Plant Cell 2020, 32, 1000–1017. [Google Scholar] [CrossRef]
- Jia, H.; Chen, S.; Liu, D.; Liesche, J.; Shi, C.; Wang, J.; Ren, M.; Wang, X.; Yang, J.; Shi, W. Ethylene-induced hydrogen sulfide negatively regulates ethylene biosynthesis by persulfidation of ACO in tomato under osmotic stress. Front. Plant Sci. 2018, 9, 1517. [Google Scholar] [CrossRef]
- Liu, H.; Xue, S. Interplay between hydrogen sulfide and other signaling molecules in the regulation of guard cell signaling and abiotic/biotic stress response. Plant Commun. 2021, 2, 100179. [Google Scholar] [CrossRef]
- Song, Y.; Miao, Y.; Song, C.P. Behind the scenes: The roles of reactive oxygen species in guard cells. New Phytol. 2014, 201, 1121–1140. [Google Scholar] [CrossRef]
- Scuffi, D.; Nietzel, T.; Di Fino, L.M.; Meyer, A.J.; Lamattina, L.; Schwarzlander, M.; Laxalt, A.M.; Garcia-Mata, C. Hydrogen sulfide increases production of NADPH oxidase-dependent hydrogen peroxide and phospholipase d-derived phosphatidic acid in guard cell signaling. Plant Physiol. 2018, 176, 2532–2542. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, H.; Zhang, Q.; Li, M.; Yan, M.; Wang, R.; Wang, L.; Welti, R.; Zhang, W.; Wang, X. Phospholipase dalpha1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 2009, 21, 2357–2377. [Google Scholar] [CrossRef]
- Distefano, A.M.; Scuffi, D.; Garcia-Mata, C.; Lamattina, L.; Laxalt, A.M. Phospholipase Dδ is involved in nitric oxide-induced stomatal closure. Planta 2012, 236, 1899–1907. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Li, H.; Liu, R.; Wang, W.; Wu, W.; Yang, N.; Wang, S. Osmotic stress-triggered stomatal closure requires Phospholipase Dδ and hydrogen sulfide in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2021, 534, 914–920. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, R.; Zhou, Y.; Wang, W.; Wu, G.; Yang, N. Phospholipase Dδ and H2S increase the production of NADPH oxidase-dependent H2O2 to respond to osmotic stress-induced stomatal closure in Arabidopsis thaliana. J. Plant Physiol. 2022, 270, 153617. [Google Scholar] [CrossRef]
- Ma, Y.; Shao, L.; Zhang, W.; Zheng, F. Hydrogen sulfide induced by hydrogen peroxide mediates brassinosteroid-induced stomatal closure of Arabidopsis thaliana. Funct. Plant Biol. 2021, 48, 195–205. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Zhao, Y.; Zhang, X.; Zhang, S.; Bo, L.; Wang, Y.; Ding, Y.; An, L. Putrescine protects hulless barley from damage due to UV-B stress via H2S- and H2O2-mediated signaling pathways. Plant Cell Rep. 2016, 35, 1155–1168. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y.; He, X.; Yong, B.; Peng, Y.; Zhang, X.; Ma, X.; Yan, Y.; Huang, L.; Nie, G. The hydrogen sulfide, a downstream signaling molecule of hydrogen peroxide and nitric oxide, involves spermidine-regulated transcription factors and antioxidant defense in white clover in response to dehydration. Environ. Exp. Bot. 2019, 161, 255–264. [Google Scholar] [CrossRef]
- Kolupaev, Y.E.; Yemets, A.I.; Yastreb, T.O.; Blume, Y.B. The role of nitric oxide and hydrogen sulfide in regulation of redox homeostasis at extreme temperatures in plants. Front. Plant Sci. 2023, 14, 1128439. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Xu, C.; Liu, K.; Bi, H.; Ai, X. H2O2 Functions as a Downstream signal of IAA to mediate H2S-induced chilling tolerance in cucumber. Int. J. Mol. Sci. 2021, 22, 12910. [Google Scholar] [CrossRef]
- Xu, E.; Brosche, M. Salicylic acid signaling inhibits apoplastic reactive oxygen species signaling. BMC Plant Biol. 2014, 14, 155. [Google Scholar] [CrossRef]
- Fang, H.; Liu, Z.; Long, Y.; Liang, Y.; Jin, Z.; Zhang, L.; Liu, D.; Li, H.; Zhai, J.; Pei, Y. The Ca2+/calmodulin2-binding transcription factor TGA3 elevates LCD expression and H2S production to bolster Cr6+ tolerance in Arabidopsis. Plant J. 2017, 91, 1038–1050. [Google Scholar] [CrossRef]
- Du, X.; Jin, Z.; Zhang, L.; Liu, X.; Yang, G.; Pei, Y. H2S is involved in ABA-mediated stomatal movement through MPK4 to alleviate drought stress in Arabidopsis thaliana. Plant Soil. 2018, 435, 295–307. [Google Scholar] [CrossRef]
- Corpas, F.J.; Gonzalez-Gordo, S.; Palma, J.M. Plant peroxisomes: A factory of reactive species. Front. Plant Sci. 2020, 11, 853. [Google Scholar] [CrossRef]
- Palma, J.M.; Mateos, R.M.; Lopez-Jaramillo, J.; Rodriguez-Ruiz, M.; Gonzalez-Gordo, S.; Lechuga-Sancho, A.M.; Corpas, F.J. Plant catalases as NO and H2S targets. Redox Biol. 2020, 34, 101525. [Google Scholar] [CrossRef]
- Ni, X.; Li, X.; Shen, T.L.; Qian, W.J.; Xian, M. A Sweet H2S/H2O2 dual release system and specific protein S-persulfidation mediated by thioglucose/glucose oxidase. J. Am. Chem. Soc. 2021, 143, 13325–13332. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Liu, Y.; Liao, W. Hydrogen Sulfide in the Oxidative Stress Response of Plants: Crosstalk with Reactive Oxygen Species. Int. J. Mol. Sci. 2024, 25, 1935. https://doi.org/10.3390/ijms25031935
Liu Z, Liu Y, Liao W. Hydrogen Sulfide in the Oxidative Stress Response of Plants: Crosstalk with Reactive Oxygen Species. International Journal of Molecular Sciences. 2024; 25(3):1935. https://doi.org/10.3390/ijms25031935
Chicago/Turabian StyleLiu, Zhiya, Yayu Liu, and Weibiao Liao. 2024. "Hydrogen Sulfide in the Oxidative Stress Response of Plants: Crosstalk with Reactive Oxygen Species" International Journal of Molecular Sciences 25, no. 3: 1935. https://doi.org/10.3390/ijms25031935
APA StyleLiu, Z., Liu, Y., & Liao, W. (2024). Hydrogen Sulfide in the Oxidative Stress Response of Plants: Crosstalk with Reactive Oxygen Species. International Journal of Molecular Sciences, 25(3), 1935. https://doi.org/10.3390/ijms25031935







