The Ubiquitin-Associated and SH3 Domain-Containing Proteins (UBASH3) Family in Mammalian Development and Immune Response
Abstract
1. Introduction
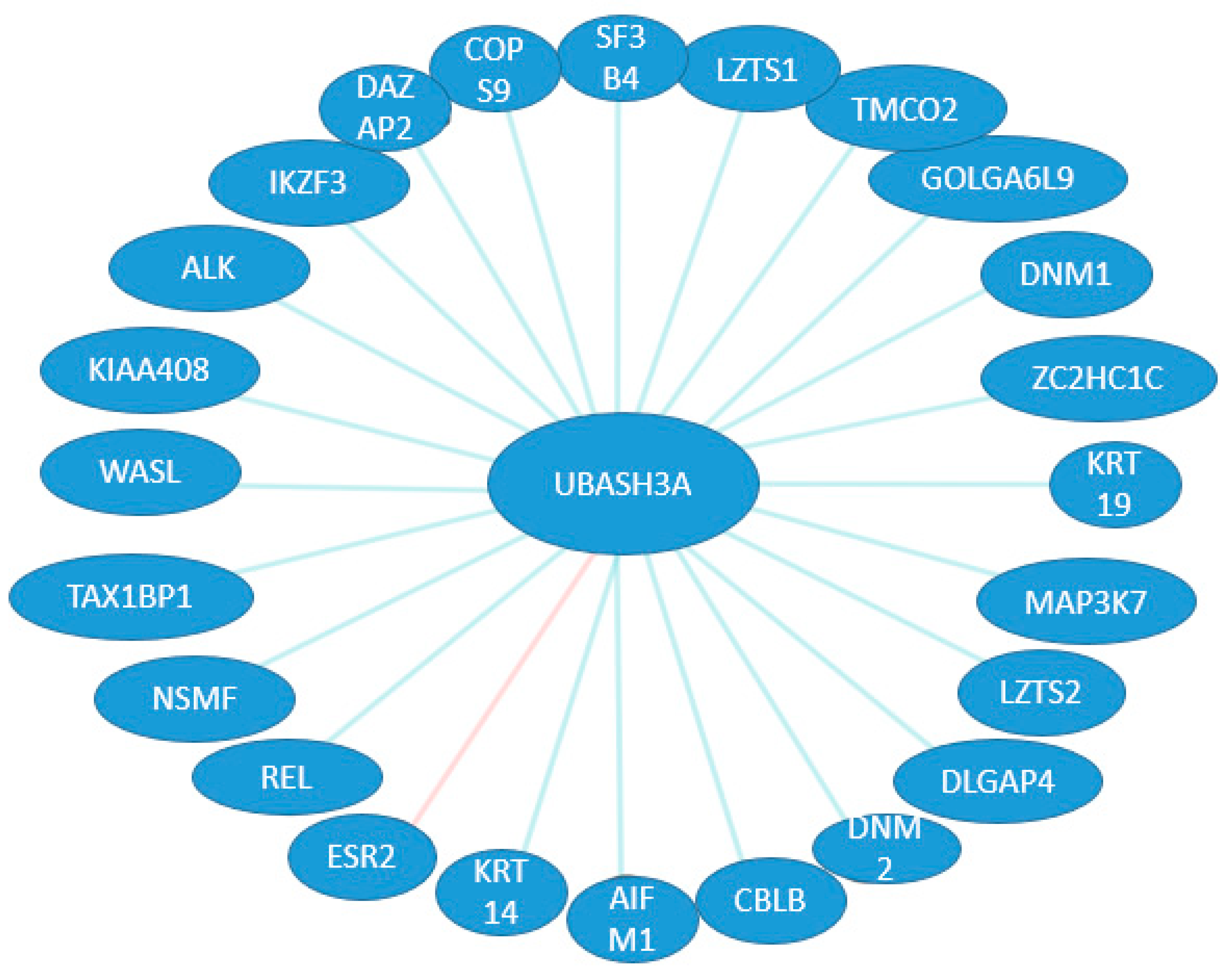
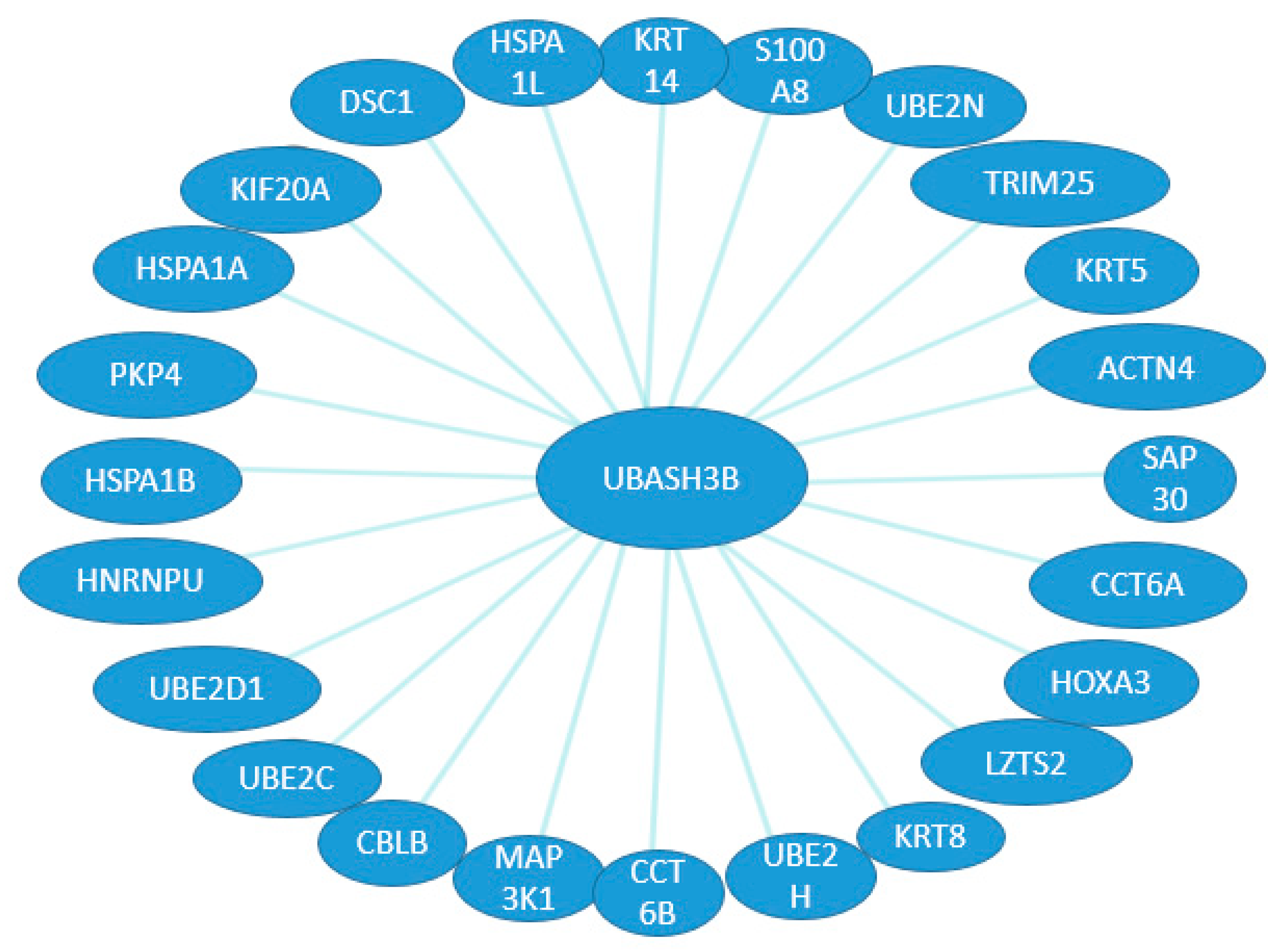
2. UBASH3 Homology and Structures
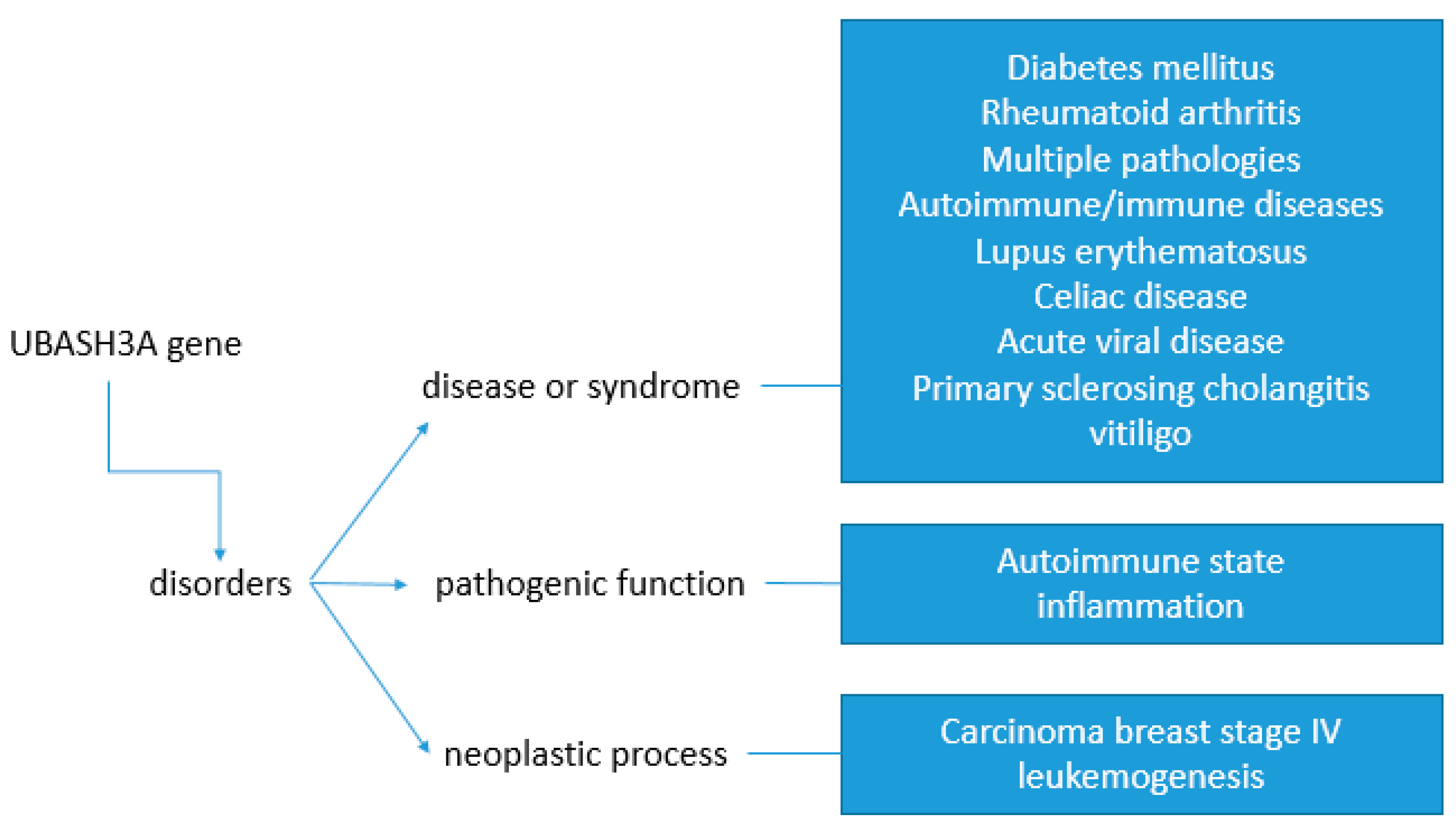
3. UBASH3 Human Disease Models
4. UBASH3 Gene Models
5. The UBASH3 Signalling Pathway in Mammalian Development
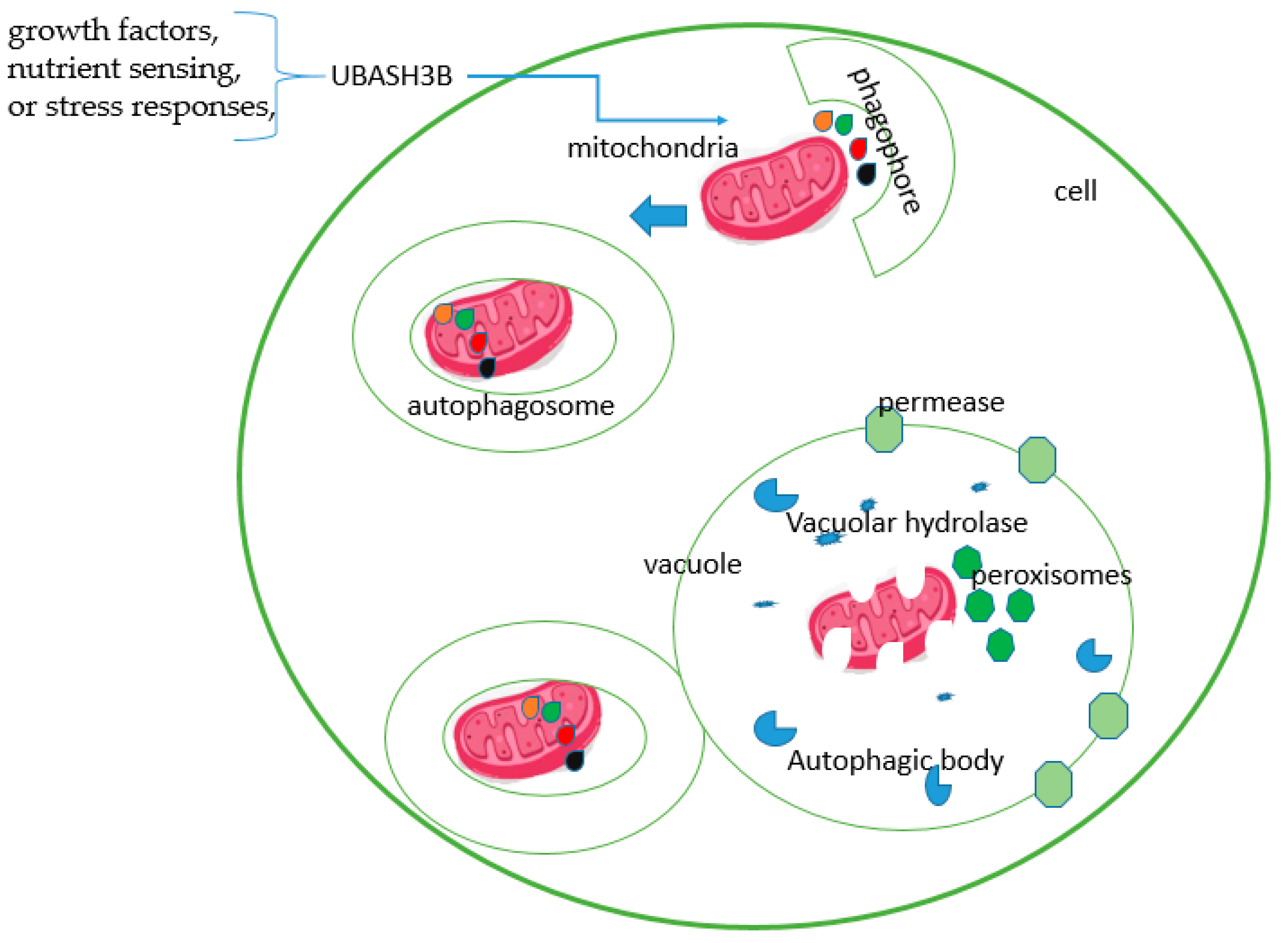
6. The Role of UBASH3 in Apoptosis and Autophagy
7. The UBASH3 Proteins in Inflammation
8. The UBASH3 Proteins in Autoimmunity
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsygankov, A.Y. TULA Proteins in Men, Mice, Hens, and Lice: Welcome to the Family. Int. J. Mol. Sci. 2023, 24, 9126. [Google Scholar] [CrossRef]
- Mehrabipour, M.; Jasemi, N.S.K.; Dvorsky, R.; Ahmadian, M.R. A Systematic Compilation of Human SH3 Domains: A Versatile Superfamily in Cellular Signaling. Cells 2023, 12, 2054. [Google Scholar] [CrossRef]
- Ge, Y.; Paisie Taylor, K.; Chen, S.; Concannon, P. UBASH3A Regulates the Synthesis and Dynamics of TCR-CD3 Complexes. J. Immunol. 2019, 203, 2827–2836. [Google Scholar] [CrossRef]
- Ge, Y.; Paisie Taylor, K.; Newman Jeremy, R.B.; McIntyre Lauren, M.; Concannon, P. UBASH3A Mediates Risk for Type 1 Diabetes Through Inhibition of T-Cell Receptor–Induced NF-κB Signaling. Diabetes 2017, 66, 2033–2043. [Google Scholar] [CrossRef]
- Hu, H.; Sun, S.C. Ubiquitin signaling in immune responses. Cell Res. 2016, 26, 457–483. [Google Scholar] [CrossRef]
- Lozic, M.; Minarik, L.; Racetin, A.; Filipovic, N.; Saraga Babic, M.; Vukojevic, K. CRKL, AIFM3, AIF, BCL2, and UBASH3A during Human Kidney Development. Int. J. Mol. Sci. 2021, 22, 9183. [Google Scholar] [CrossRef]
- Tsygankov, A.Y. TULA proteins as signaling regulators. Cell. Signal. 2020, 65, 109424. [Google Scholar] [CrossRef] [PubMed]
- Consortium, T.U. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2022, 51, D523–D531. [Google Scholar] [CrossRef] [PubMed]
- Todd John, A. Evidence that UBASH3 is a causal gene for type 1 diabetes. Eur. J. Hum. Genet. EJHG 2018, 26, 925–927. [Google Scholar] [CrossRef]
- Feshchenko, E.A.; Smirnova, E.V.; Swaminathan, G.; Teckchandani, A.M.; Agrawal, R.; Band, H.; Zhang, X.; Annan, R.S.; Carr, S.A.; Tsygankov, A.Y. TULA: An SH3- and UBA-containing protein that binds to c-Cbl and ubiquitin. Oncogene 2004, 23, 4690–4706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Vakhrusheva, O.; Bandi, S.R.; Demirel, O.; Kazi, J.U.; Fernandes, R.G.; Jakobi, K.; Eichler, A.; Ronnstrand, L.; Rieger, M.A.; et al. The Phosphatases STS1 and STS2 Regulate Hematopoietic Stem and Progenitor Cell Fitness. Stem Cell Rep. 2015, 5, 633–646. [Google Scholar] [CrossRef]
- Kunapuli, S.P.; Tsygankov, A.Y. TULA-Family Regulators of Platelet Activation. Int. J. Mol. Sci. 2022, 23, 14910. [Google Scholar] [CrossRef]
- Bertelsen, V.; Breen, K.; Sandvig, K.; Stang, E.; Madshus, I.H. The Cbl-interacting protein TULA inhibits dynamin-dependent endocytosis. Exp. Cell Res. 2007, 313, 1696–1709. [Google Scholar] [CrossRef]
- Agrawal, R.; Carpino, N.; Tsygankov, A. TULA proteins regulate activity of the protein tyrosine kinase Syk. J. Cell. Biochem. 2008, 104, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Concannon, P. Molecular-genetic characterization of common, noncoding UBASH3A variants associated with type 1 diabetes. Eur. J. Hum. Genet. 2018, 26, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, A.; Stahl Eli, A.; Trynka, G.; Raychaudhuri, S.; Festen Eleanora, A.; Franke, L.; Westra, H.-J.; Fehrmann Rudolf, S.N.; Kurreeman Fina, A.S.; Thomson, B.; et al. Meta-Analysis of Genome-Wide Association Studies in Celiac Disease and Rheumatoid Arthritis Identifies Fourteen Non-HLA Shared Loci. PLoS Genet. 2011, 7, e1002004. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Birlea Stanca, A.; Fain Pamela, R.; Gowan, K.; Riccardi Sheri, L.; Holland Paulene, J.; Mailloux Christina, M.; Sufit Alexandra, J.D.; Hutton Saunie, M.; Amadi-Myers, A.; et al. Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N. Engl. J. Med. 2010, 362, 1686–1697. [Google Scholar] [CrossRef] [PubMed]
- Plagnol, V.; Howson Joanna, M.M.; Smyth Deborah, J.; Walker, N.; Hafler Jason, P.; Wallace, C.; Stevens, H.; Jackson, L.; Simmonds Matthew, J.; Bingley Polly, J.; et al. Genome-Wide Association Analysis of Autoantibody Positivity in Type 1 Diabetes Cases. PLoS Genet. 2011, 7, e1002216. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Gallo, L.-M.; Sánchez, E.; Ortego-Centeno, N.; Sabio Jose, M.; García-Hernández Francisco, J.; de Ramón, E.; González-Gay Miguel, A.; Torsten Witte, N.; Anders, H.-J.; González-Escribano María, F.; et al. Evidence of New Risk Genetic Factor to Systemic Lupus Erythematosus: The UBASH3A Gene. PLoS ONE 2013, 8, e60646. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; Ni, J.; Leng, R.X.; Pan, H.F.; Ye, D.Q. Association of UBASH3A gene polymorphisms and systemic lupus erythematosus in a Chinese population. Gene 2015, 565, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ni, J.; Li, L.-J.; Leng, R.-X.; Pan, H.-F.; Ye, D.-Q. Decreased UBASH3A mRNA Expression Levels in Peripheral Blood Mononuclear Cells from Patients with Systemic Lupus Erythematosus. Inflammation 2015, 38, 1903–1910. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, H.; Xiao, F.L.; Liang, B.; Zhou, F.S.; Li, P.; Zheng, X.D.; Sun, L.D.; Yang, S.; Zhang, X.J. Association of UBASH3A gene polymorphism and atopic dermatitis in the Chinese Han population. Genes Immun. 2017, 18, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Howarth, S.; Sneddon, G.; Allinson, K.R.; Razvi, S.; Mitchell, A.L.; Pearce, S.H.S. Replication of association at the LPP and UBASH3A loci in a UK autoimmune Addison’s disease cohort. Eur. J. Endocrinol. 2023, 188, lvac010. [Google Scholar] [CrossRef] [PubMed]
- Tsygankov, A.Y. TULA-family proteins: An odd couple. Cell. Mol. Life Sci. 2009, 66, 2949–2952. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Peng, M.; Yi, L. UBASH3B Is a Novel Prognostic Biomarker and Correlated With Immune Infiltrates in Prostate Cancer. Front. Oncol. 2019, 9, 1517. [Google Scholar] [CrossRef] [PubMed]
- Soljic, V.; Perak, R.B.; Vukojevic, K.; Saraga-Babic, M.; Bubalo, P.; Karan, D.; Todorovic, J.; Batinic, D. ZAP-70 expression and proliferative activity in chronic lymphocytic leukemia. Leuk. Lymphoma 2013, 54, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- San Luis, B.; Sondgeroth, B.; Nassar, N.; Carpino, N. Sts-2 is a phosphatase that negatively regulates zeta-associated protein (ZAP)-70 and T cell receptor signaling pathways. J. Biol. Chem. 2011, 286, 15943–15954. [Google Scholar] [CrossRef] [PubMed]
- Pires-daSilva, A.; Sommer, R.J. The evolution of signalling pathways in animal development. Nat. Rev. Genet. 2003, 4, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef]
- Mundy, G.R. The effects of TGF-beta on bone. Ciba Found. Symp. 1991, 157, 137–143, discussion 143–151. [Google Scholar]
- Androutsellis-Theotokis, A.; Leker, R.R.; Soldner, F.; Hoeppner, D.J.; Ravin, R.; Poser, S.W.; Rueger, M.A.; Bae, S.K.; Kittappa, R.; McKay, R.D. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 2006, 442, 823–826. [Google Scholar] [CrossRef]
- Wagner, M.A.; Siddiqui, M.A. The JAK-STAT pathway in hypertrophic stress signaling and genomic stress response. Jak-Stat 2012, 1, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Kowanetz, K.; Crosetto, N.; Haglund, K.; Schmidt, M.H.H.; Heldin, C.H.; Dikic, I. Suppressors of T-cell receptor signaling Sts-1 and Sts-2 bind to Cbl and inhibit endocytosis of receptor tyrosine kinases. J. Biol. Chem. 2004, 279, 32786–32795. [Google Scholar] [CrossRef] [PubMed]
- Tauriello, D.V.; Maurice, M.M. The various roles of ubiquitin in Wnt pathway regulation. Cell Cycle 2010, 9, 3700–3709. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Tiwari, S. Mechanisms for the temporal regulation of substrate ubiquitination by the anaphase-promoting complex/cyclosome. Cell Div. 2019, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Reppschlager, K.; Gosselin, J.; Dangelmaier, C.A.; Thomas, D.H.; Carpino, N.; McKenzie, S.E.; Kunapuli, S.P.; Tsygankov, A.Y. TULA-2 Protein Phosphatase Suppresses Activation of Syk through the GPVI Platelet Receptor for Collagen by Dephosphorylating Tyr(P)346, a Regulatory Site of Syk. J. Biol. Chem. 2016, 291, 22427–22441. [Google Scholar] [CrossRef] [PubMed]
- Fragoza, R.; Das, J.; Wierbowski, S.D.; Liang, J.; Tran, T.N.; Liang, S.; Beltran, J.F.; Rivera-Erick, C.A.; Ye, K.; Wang, T.Y.; et al. Extensive disruption of protein interactions by genetic variants across the allele frequency spectrum in human populations. Nat. Commun. 2019, 10, 4141. [Google Scholar] [CrossRef] [PubMed]
- de Jong, R.; ten Hoeve, J.; Heisterkamp, N.; Groffen, J. Crkl is complexed with tyrosine-phosphorylated Cbl in Ph-positive leukemia. J. Biol. Chem. 1995, 270, 21468–21471. [Google Scholar] [CrossRef] [PubMed]
- Krupina, K.; Kleiss, C.; Metzger, T.; Fournane, S.; Schmucker, S.; Hofmann, K.; Fischer, B.; Paul, N.; Porter, I.M.; Raffelsberger, W.; et al. Ubiquitin Receptor Protein UBASH3B Drives Aurora B Recruitment to Mitotic Microtubules. Dev. Cell 2016, 36, 63–78. [Google Scholar] [CrossRef]
- Naujokat, C.; Saric, T. Concise review: Role and function of the ubiquitin-proteasome system in mammalian stem and progenitor cells. Stem Cells 2007, 25, 2408–2418. [Google Scholar] [CrossRef]
- Wattenhofer, M.; Shibuya, K.; Kudoh, J.; Lyle, R.; Michaud, J.; Rossier, C.; Kawasaki, K.; Asakawa, S.; Minoshima, S.; Berry, A.; et al. Isolation and characterization of the UBASH3A gene on 21q22.3 encoding a potential nuclear protein with a novel combination of domains. Hum. Genet. 2001, 108, 140–147. [Google Scholar] [CrossRef]
- Stamenova, S.D.; French, M.E.; He, Y.; Francis, S.A.; Kramer, Z.B.; Hicke, L. Ubiquitin binds to and regulates a subset of SH3 domains. Mol. Cell 2007, 25, 273–284. [Google Scholar] [CrossRef]
- Ahn, J.; Han, K.S.; Heo, J.H.; Bang, D.; Kang, Y.H.; Jin, H.A.; Hong, S.J.; Lee, J.H.; Ham, W.S. FOXC2 and CLIP4: A potential biomarker for synchronous metastasis of </=7-cm clear cell renal cell carcinomas. Oncotarget 2016, 7, 51423–51434. [Google Scholar]
- Rao, N.; Ghosh, A.K.; Ota, S.; Zhou, P.; Reddi, A.L.; Hakezi, K.; Druker, B.K.; Wu, J.; Band, H. The non-receptor tyrosine kinase Syk is a target of Cbl-mediated ubiquitylation upon B-cell receptor stimulation. EMBO J. 2001, 20, 7085–7095. [Google Scholar] [CrossRef]
- Lin, I.; Afshar, Y.; Goldstein, J.; Grossman, J.; Grody, W.W.; Quintero-Rivera, F. Central 22q11.2 deletion (LCR22 B-D) in a fetus with severe fetal growth restriction and a mother with severe systemic lupus erythematosus: Further evidence of CRKL haploinsufficiency in the pathogenesis of 22q11.2 deletion syndrome. Am. J. Med. Genet. Part A 2021, 185, 3042–3047. [Google Scholar] [CrossRef]
- Racetin, A.; Juric, M.; Filipovic, N.; Solic, I.; Kosovic, I.; Glavina Durdov, M.; Kunac, N.; Zekic Tomas, S.; Saraga, M.; Soljic, V.; et al. Expression and localization of DAB1 and Reelin during normal human kidney development. Croat. Med. J. 2019, 60, 521–531. [Google Scholar] [CrossRef]
- Collingwood, T.S.; Smirnova, E.V.; Bogush, M.; Carpino, N.; Annan, R.S.; Tsygankov, A.Y. T-cell ubiquitin ligand affects cell death through a functional interaction with apoptosis-inducing factor, a key factor of caspase-independent apoptosis. J. Biol. Chem. 2007, 282, 30920–30928. [Google Scholar] [CrossRef]
- Caric, A.; Poljicanin, A.; Tomic, S.; Vilovic, K.; Saraga-Babic, M.; Vukojevic, K. Apoptotic pathways in ovarian surface epithelium of human embryos during embryogenesis and carcinogenesis: Close relationship of developmental plasticity and neoplasm. Acta Histochem. 2014, 116, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Harrow, J.; Frankish, A.; Gonzalez, J.M.; Tapanari, E.; Diekhans, M.; Kokocinski, F.; Aken, B.L.; Barrell, D.; Zadissa, A.; Searle, S.; et al. GENCODE: The reference human genome annotation for The ENCODE Project. Genome Res. 2012, 22, 1760–1774. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, H.K.; Susin, S.A.; Penninger, J.; Kroemer, G. Apoptosis inducing factor (AIF): A phylogenetically old, caspase-independent effector of cell death. Cell Death Differ. 1999, 6, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Cutler, J.A.; Udainiya, S.; Madugundu, A.K.; Renuse, S.; Xu, Y.; Jung, J.; Kim, K.P.; Wu, X.; Pandey, A. Integrative phosphoproteome and interactome analysis of the role of Ubash3b in BCR-ABL signaling. Leukemia 2020, 34, 301–305. [Google Scholar] [CrossRef]
- Krupina, K.; Kleiss, C.; Awal, S.; Rodriguez-Hernandez, I.; Sanz-Moreno, V.; Sumara, I. UBASH3B-mediated silencing of the mitotic checkpoint: Therapeutic perspectives in cancer. Mol. Cell. Oncol. 2018, 5, e1271494. [Google Scholar] [CrossRef]
- Nedelsky, N.B.; Todd, P.K.; Taylor, J.P. Autophagy and the ubiquitin-proteasome system: Collaborators in neuroprotection. Biochim. Biophys. Acta 2008, 1782, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.; Kim, J. Autophagy: An Essential Degradation Program for Cellular Homeostasis and Life. Cells 2018, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Tsygankov, A.Y. TULA-family proteins: Jacks of many trades and then some. J. Cell. Physiol. 2018, 234, 274–288. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125 (Suppl. S2), S3–S23. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Nakayamada, S.; Zhang, T.; Nguyen, A.P.; Ohkubo, N.; Iwata, S.; Kato, S.; Tanaka, Y. IL-6 production through repression of UBASH3A gene via epigenetic dysregulation of super-enhancer in CD4(+) T cells in rheumatoid arthritis. Inflamm. Regen. 2022, 42, 46. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, W.; Gajendran, B.; Wang, C.; Zacksenhaus, E.; Sample, K.M.; Varier, K.M.; Hao, X.; Ben-David, Y. Ubash3b promotes TPA-mediated suppression of leukemogenesis through accelerated downregulation of PKCdelta protein. Biochimie 2021, 184, 8–17. [Google Scholar] [CrossRef]
- Chen, Y.G.; Ciecko, A.E.; Khaja, S.; Grzybowski, M.; Geurts, A.M.; Lieberman, S.M. UBASH3A deficiency accelerates type 1 diabetes development and enhances salivary gland inflammation in NOD mice. Sci. Rep. 2020, 10, 12019. [Google Scholar] [CrossRef]
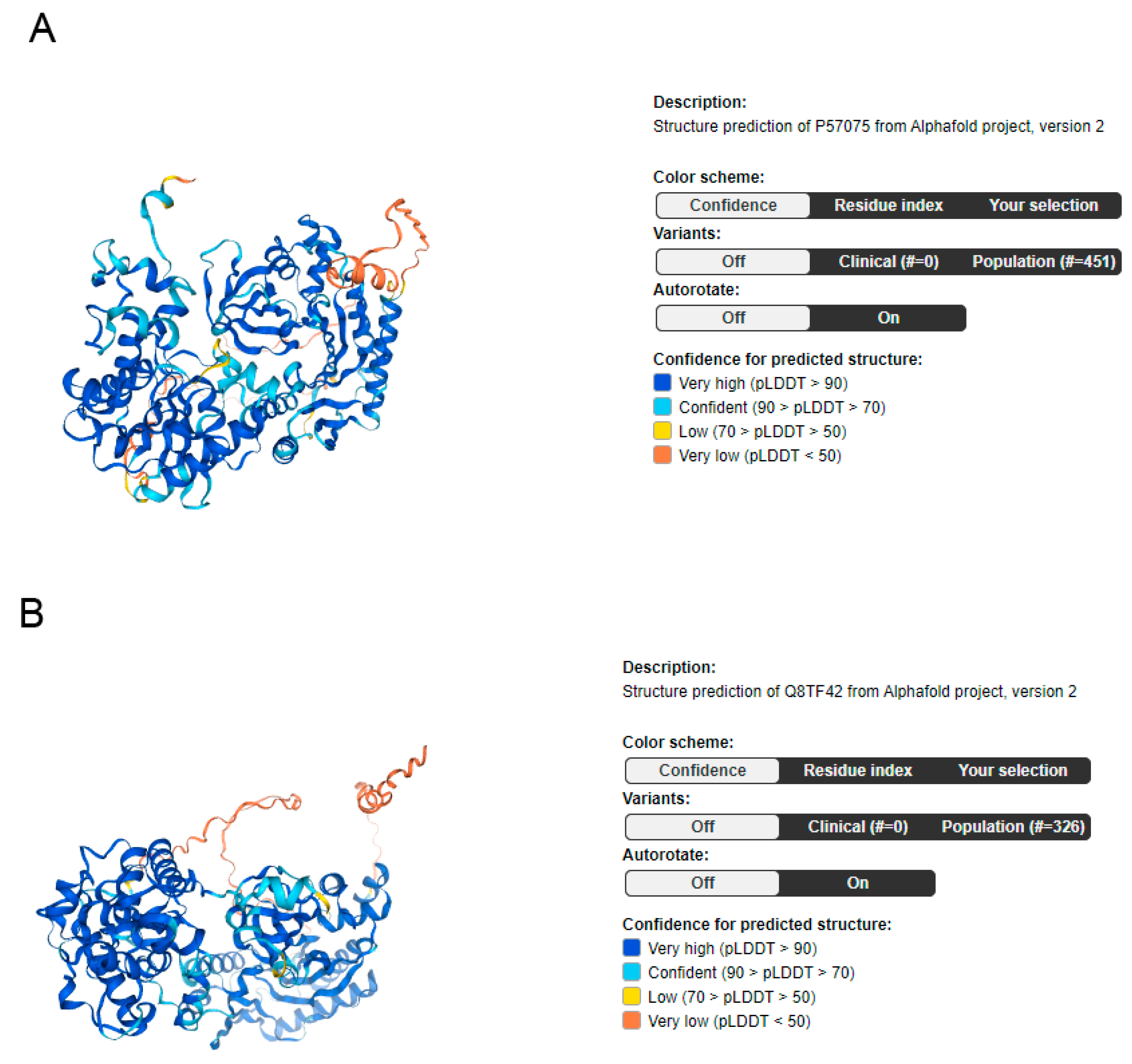


| Splice Variant | SwissProt | TrEMBL | Protein Class | Length and Mass | Signal Peptide (Predicted) | Transmembrane Regions (Predicted) |
|---|---|---|---|---|---|---|
| UBASH3B-201 | Q8TF42 | Enzymes Predicted intracellular proteins Mapped to neXtProt Protein evidence [9] Protein evidence [10] | 649 aa 72.7 kDa | No | 0 | |
| UBASH3A-201 | P57075 [Direct mapping] Ubiquitin-associated and SH3 domain-containing protein A | Predicted intracellular proteins Mapped to neXtProt Protein evidence [9] Protein evidence [10] | 623 aa 69.8 kDa | No | 0 | |
| UBASH3A-202 | P57075 [Direct mapping] Ubiquitin-associated and SH3 domain-containing protein A | Predicted intracellular proteins Mapped to neXtProt Protein evidence [9] Protein evidence [10] | 661 aa 74.1 kDa | No | 0 | |
| UBASH3A-203 | P57075 [Direct mapping] Ubiquitin-associated and SH3 domain-containing protein A | Predicted intracellular proteins Mapped to neXtProt Protein evidence [9] Protein evidence [10] | 526 aa 59.2 kDa | No | 0 | |
| UBASH3A-208 | A0A0U1RQL4 [Direct mapping] Ubiquitin-associated and SH3 domain-containing protein A | Predicted intracellular proteins Protein evidence [10] | 169 aa 18.7 kDa | No | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vukojević, K.; Šoljić, V.; Martinović, V.; Raguž, F.; Filipović, N. The Ubiquitin-Associated and SH3 Domain-Containing Proteins (UBASH3) Family in Mammalian Development and Immune Response. Int. J. Mol. Sci. 2024, 25, 1932. https://doi.org/10.3390/ijms25031932
Vukojević K, Šoljić V, Martinović V, Raguž F, Filipović N. The Ubiquitin-Associated and SH3 Domain-Containing Proteins (UBASH3) Family in Mammalian Development and Immune Response. International Journal of Molecular Sciences. 2024; 25(3):1932. https://doi.org/10.3390/ijms25031932
Chicago/Turabian StyleVukojević, Katarina, Violeta Šoljić, Vlatka Martinović, Fila Raguž, and Natalija Filipović. 2024. "The Ubiquitin-Associated and SH3 Domain-Containing Proteins (UBASH3) Family in Mammalian Development and Immune Response" International Journal of Molecular Sciences 25, no. 3: 1932. https://doi.org/10.3390/ijms25031932
APA StyleVukojević, K., Šoljić, V., Martinović, V., Raguž, F., & Filipović, N. (2024). The Ubiquitin-Associated and SH3 Domain-Containing Proteins (UBASH3) Family in Mammalian Development and Immune Response. International Journal of Molecular Sciences, 25(3), 1932. https://doi.org/10.3390/ijms25031932







