DNA Barcoding and Fertilization Strategies in Sideritis syriaca subsp. syriaca, a Local Endemic Plant of Crete with High Medicinal Value
Abstract
1. Introduction
2. Results
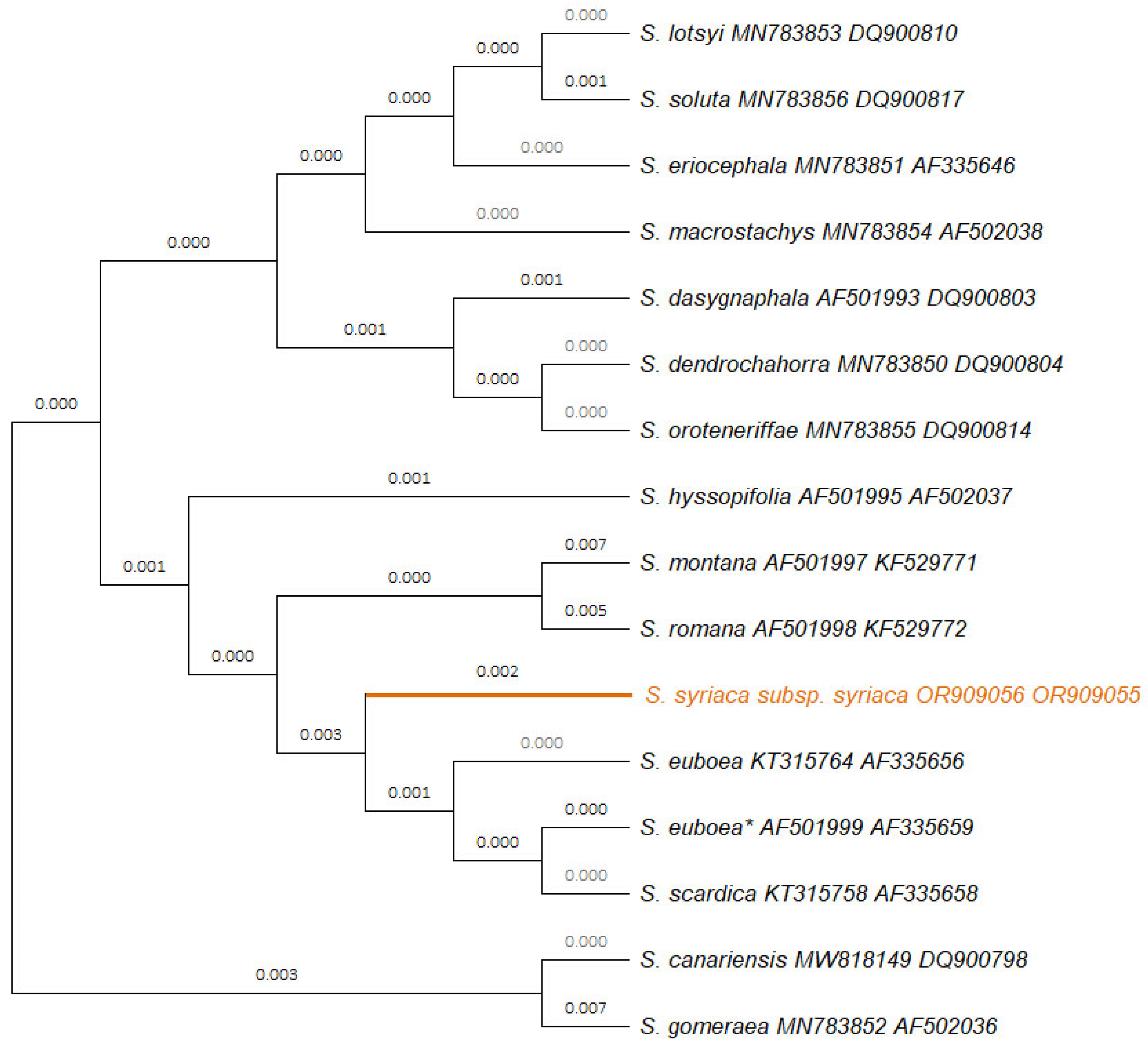
2.1. Molecular Characterization of the Studied Sideritis syriaca subsp. syriaca
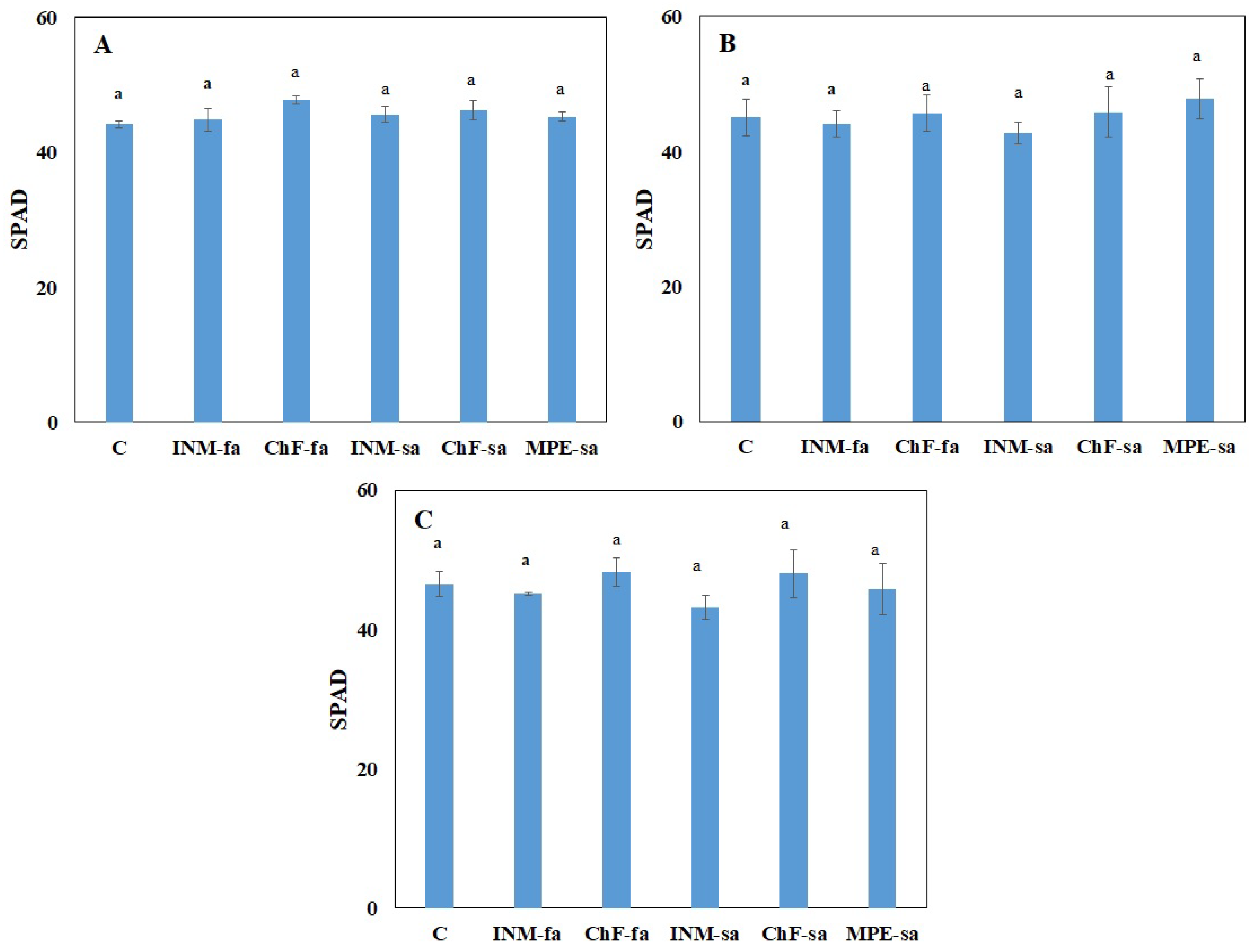
2.2. Fertilization Scheme Exerted Minor Effects on Leaf Color
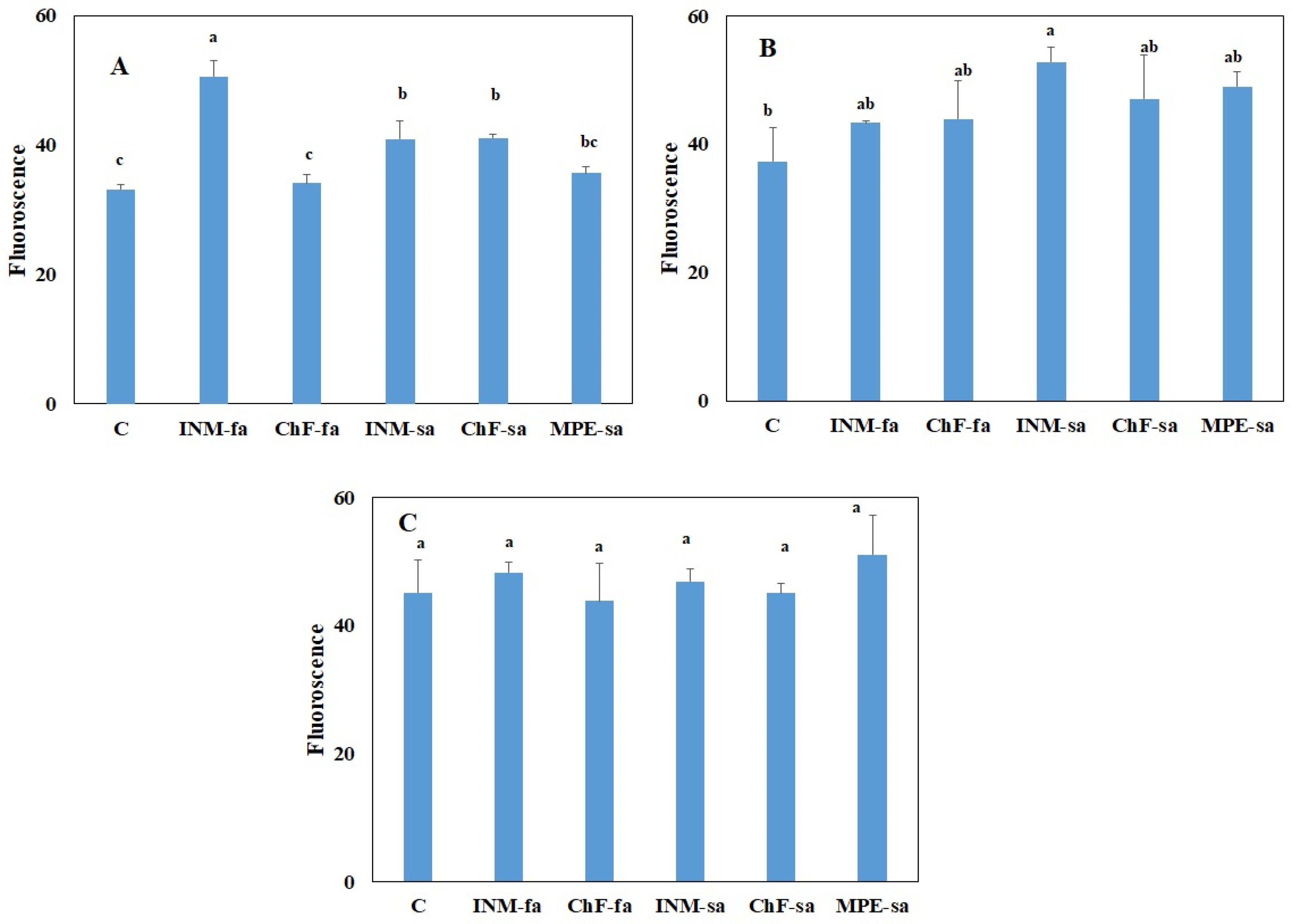
2.3. Fertilization Scheme Induced Limited Effects on Leaf Photosynthetic Performance
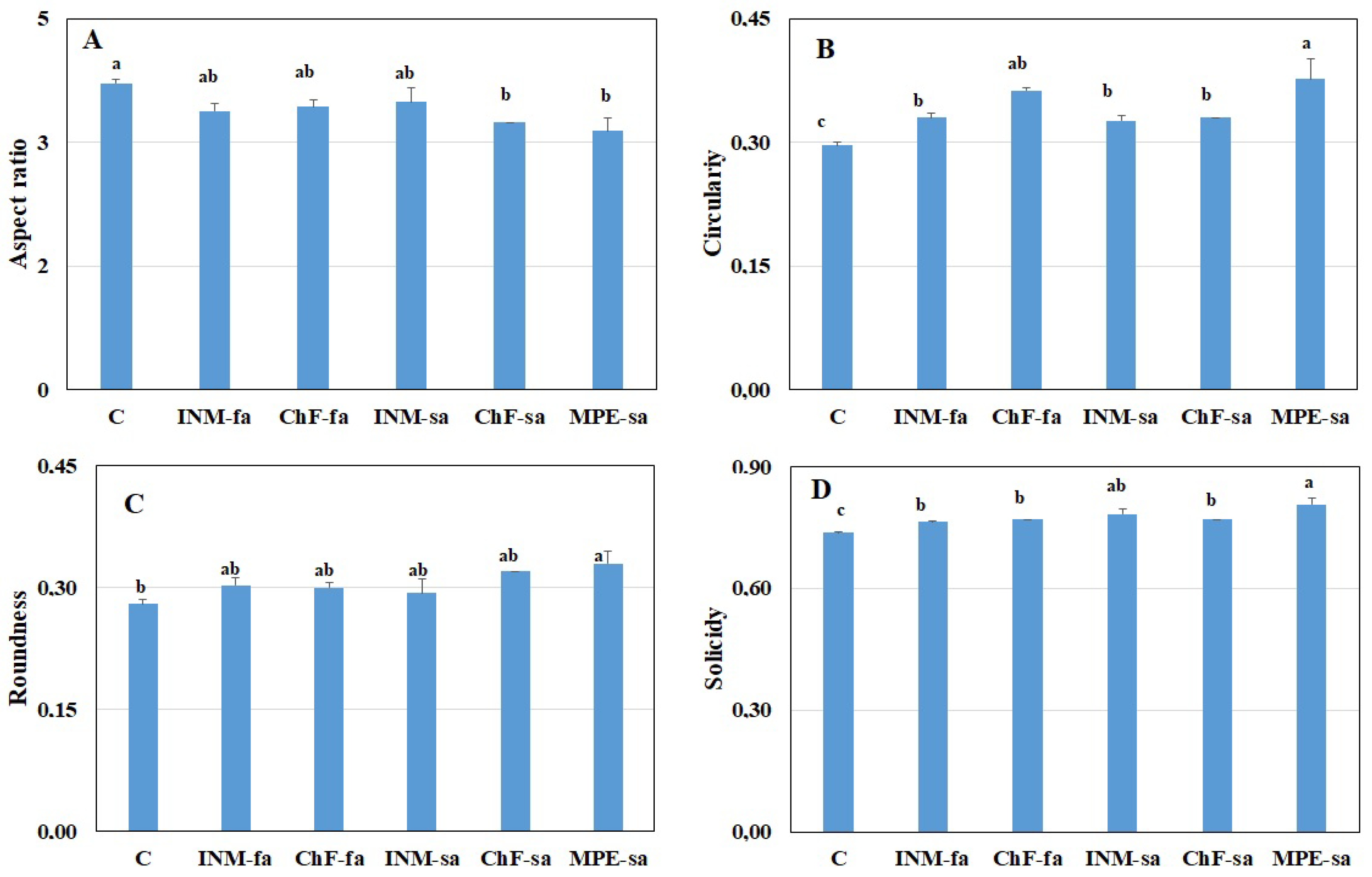
2.4. Fertilization Scheme Induced Distinct Leaf Shape Profiles
2.5. INM and ChF-sa Stimulated Plant Growth, without Affecting Biomass Allocation to Generative Organs
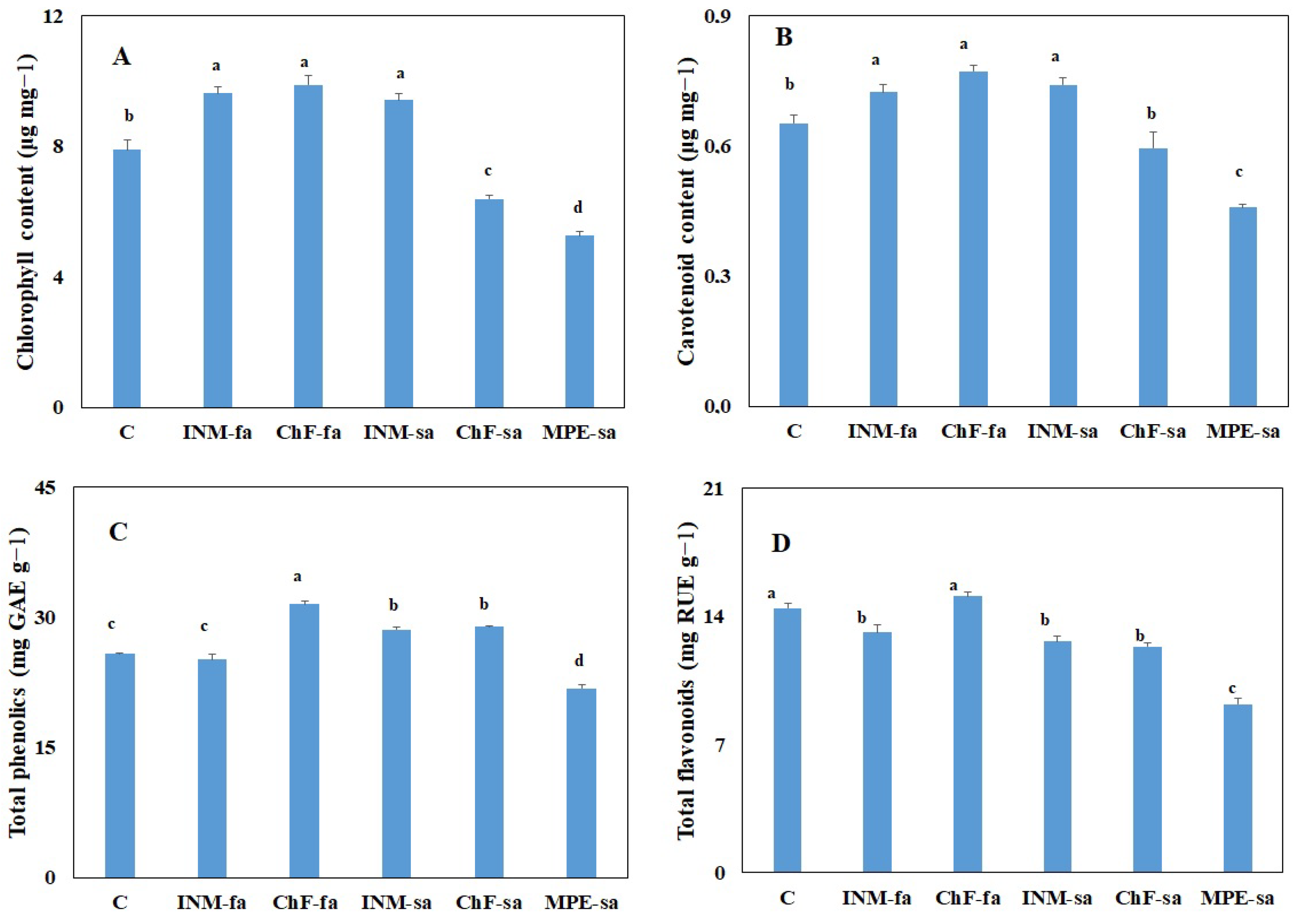
2.6. Fertilization Scheme Affected Leaf Chlorophyll Content
2.7. Fertilization Scheme Affected Leaf Antioxidant Compound Content
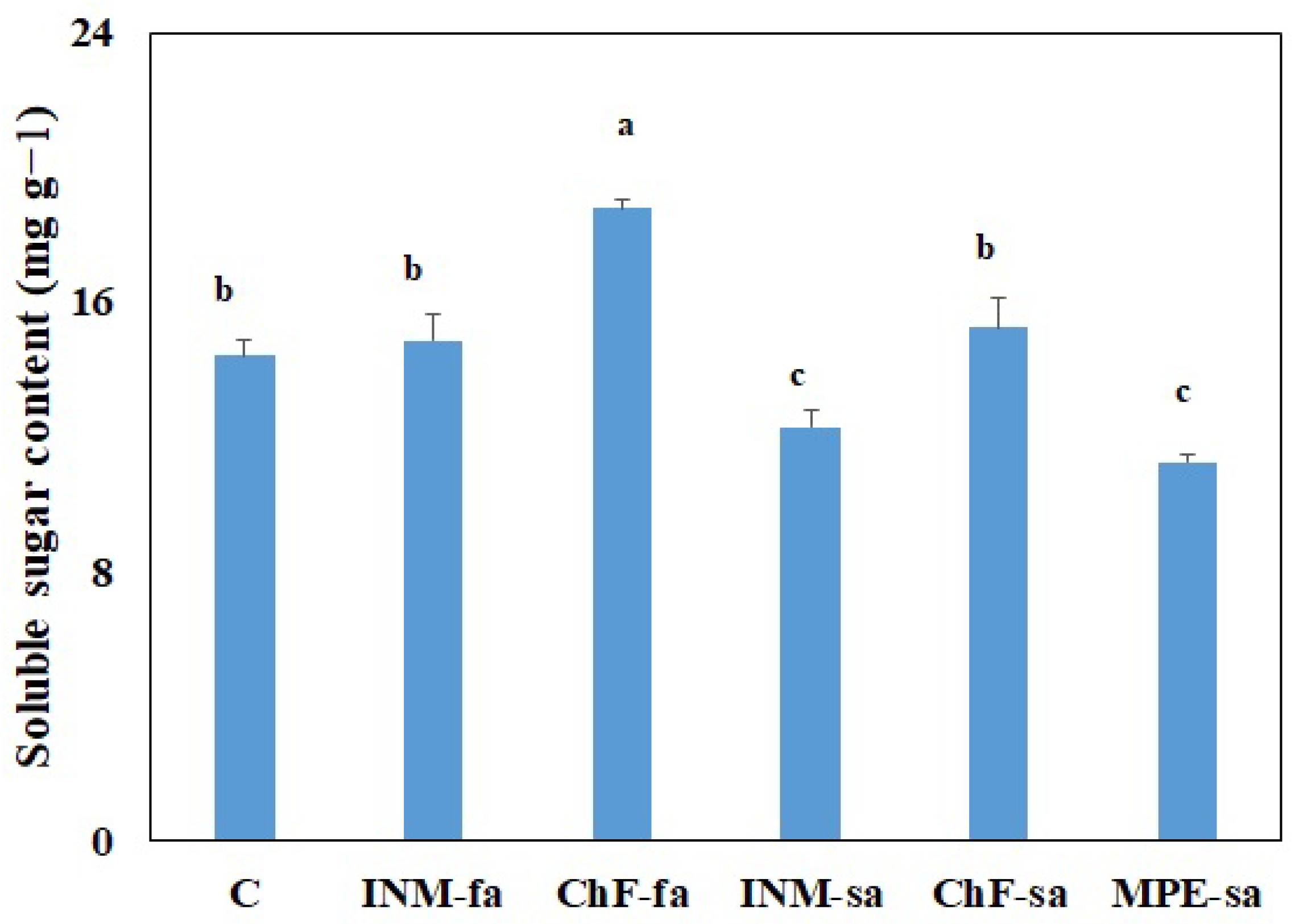
2.8. Fertilization Scheme Affected the Soluble Sugar Content of Leaves
2.9. Leaf and Inflorescence Nutrient Analysis
3. Discussion
3.1. Molecular Characterization of the Studied Sideritis syriaca subsp. syriaca
3.2. Fertilization Effects Supporting the Sustainable Exploitation of S. syriaca subsp. syriaca
4. Materials and Methods
4.1. Plant Material
4.2. DNA Barcoding and Molecular Analysis
4.3. Field Experiment and Experimental Design
4.4. Fertilization Treatments
4.5. Plant Measurements
4.5.1. Non-Invasive Evaluation of Leaf Coloration at Three Growth Stages
- (i)
- Leaf SPAD value, approximating chlorophyll content, was determined by using a SPAD-502 (Konica Minolta Corp., Solna, Sweden);
- (ii)
- Index of absorbance difference (IAD) accurately evaluated fruit ripeness since it is closely associated with outer mesocarp chlorophyll content [76]; IAD was computed as the difference between the absorbance values at 670 and 720 nm, near the chlorophyll absorbance peak [76]. The potential of IAD in reflecting respective differences in leaves had not been previously evaluated. However, the estimation of their chlorophyll index could provide an indication of their maturity state. Thus, the IAD index could help in providing the developmental stage of a measured leaf and could be worthwhile in estimating the steps of the leaf product chain and the potential marketing cycle of the product. In this investigation, IAD was determined in leaves by using the DA meter (tr DA Meter, T.R. Turoni, Italy);
- (iii)
- Leaf color was quantified by using a Chroma Meter (Model CR-400, Minolta Corp., Osaka, Japan); CIE L*a*b* coordinates were recorded using D65 illuminants and a 10° Standard Observer as a reference system: L* (a measure of lightness, ranging from 0 (black) to 100 (white)), a* (a measure of intensity in the green to red range, where negative values refer to green and positive to red), and b* (a measure of representing intensity in the blue to the yellow range, where negative values refer to blue and positive to yellow).
4.5.2. Non-Invasive Evaluation of Photosynthetic Performance in Growth Stages
4.5.3. Leaf Shape Indicators
4.5.4. Plant Growth and Biomass Partitioning to Generative Organs
4.5.5. Leaf Chlorophyll and Carotenoid Contents
4.5.6. Leaf Total Phenolic and Total Flavonoid Contents
4.5.7. Soluble Sugar Content in Leaves
4.5.8. Leaf and Inflorescence Nutrient Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Casiraghi, M.; Labra, M.; Ferri, E.; Galimberti, A.; De Mattia, F. DNA barcoding: A six-question tour to improve users’ awareness about the method. Brief. Bioinform. 2010, 11, 440–453. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Kress, W.J.; Erickson, D.L. DNA barcodes: Genes, genomics, and bioinformatics. Proc. Natl. Acad. Sci. USA 2008, 105, 2761–2762. [Google Scholar] [CrossRef]
- Kehie, M.; Kumaria, S.; Devi, K.S.; Tandon, P. Genetic diversity and molecular evolution of Naga King Chili inferred from internal transcribed spacer sequence of nuclear ribosomal DNA. Meta Gene 2016, 7, 56–63. [Google Scholar] [CrossRef]
- Vazquez, J.L.H.; Gómez-Mercado, F.; Guerrero, J.-L.G.; Rodriguez-García, I.; García-Maroto, F. Genetic relationships and population structure within taxa of the endemic Sideritis pusilla (Lamiaceae) assessed using RAPDs. Bot. J. Linn. Soc. 1999, 129, 345–358. [Google Scholar] [CrossRef]
- Lindqvist, C.; Albert, V.A. Origin of the Hawaiian endemic mints within North American Stachys (Lamiaceae). Am. J. Bot. 2002, 89, 1709–1724. [Google Scholar] [CrossRef]
- Patelou, E.; Chatzopoulou, P.; Polidoros, A.N.; Mylona, P.V. Genetic diversity and structure of Sideritis raeseri Boiss. & Heldr. (Lamiaceae) wild populations from Balkan Peninsula. J. Appl. Res. Med. Aromat. Plants 2020, 16, 100241. [Google Scholar] [CrossRef]
- Sarrou, E.; Doukidou, L.; Avramidou, E.V.; Martens, S.; Angeli, A.; Stagiopoulou, R.; Fyllas, N.M.; Tourvas, N.; Abraham, E.; Maloupa, E.; et al. Chemodiversity is closely linked to genetic and environmental diversity: Insights into the endangered populations of the local endemic plant Sideritis euboea Heldr. of Evia Island (Greece). J. Appl. Res. Med. Aromat. Plants 2022, 31, 100426. [Google Scholar] [CrossRef]
- Barber, J.C.; Finch, C.C.; Francisco-Ortega, J.; Santos-Guerra, A.; Jansen, R.K. Hybridization in Macaronesian Sideritis (Lamiaceae): Evidence from incongruence of multiple independent nuclear and chloroplast sequence datasets. Taxon 2007, 56, 74–88. [Google Scholar]
- Sevindik, E. Comparative and phylogenetic analysis of Rubisco large subunit (rbcL) proteins in some Sideritis L. (Lamiaceae) species: A bioinformatic approach. Genetica 2019, 51, 69–80. [Google Scholar] [CrossRef]
- Barber, J.C.; Ortega, J.F.; Santos-Guerra, A.; Marrero, A.; Jansen, R.K. Evolution of endemic Sideritis (Lamiaceae) in Macaronesia: Insights from a chloroplast DNA restriction site analysis. Syst. Bot. 2000, 25, 633–647. [Google Scholar] [CrossRef]
- Bendiksby, M.; Thorbek, L.; Scheen, A.C.; Lindqvist, C.; Ryding, O. An updated phylogeny and classification of Lamiaceae subfamily Lamioideae. Taxon 2011, 60, 471–484. [Google Scholar] [CrossRef]
- Theodoridis, S.; Stefanaki, A.; Tezcan, M.; Aki, C.; Kokkini, S.; Vlachonasios, K.E. DNA barcoding in native plants of the Labiatae (Lamiaceae) family from Chios Island (Greece) and the adjacent Çeşme-Karaburun Peninsula (Turkey). Mol. Ecol. Resour. 2012, 12, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, A.; Ganopoulos, I.; Xanthopoulou, A.; Chatzopoulou, P.; Tsaftaris, A.; Madesis, P. DNA barcode ITS2 coupled with high resolution melting (HRM) analysis for taxonomic identification of Sideritis species growing in Greece. Mol. Biol. Rep. 2014, 41, 5147–5155. [Google Scholar] [CrossRef] [PubMed]
- Salmaki, Y.; Heubl, G.; Weigend, M. Towards a new classification of tribe Stachydeae (Lamiaceae): Naming clades using molecular evidence. Bot. J. Linn. Soc. 2019, 190, 345–358. [Google Scholar] [CrossRef]
- Aneva, I.; Zhelev, P.; Bonchev, G. Sideritis elica, a new species of Lamiaceae from Bulgaria, revealed by morphology and molecular biology. Plants 2022, 11, 2900. [Google Scholar] [CrossRef] [PubMed]
- Kloukina, C.; Tomou, E.-M.; Krigas, N.; Sarropoulou, V.; Madesis, P.; Maloupa, E.; Skaltsa, H. Non-polar secondary metabolites and essential oil of ex situ propagated and cultivated Sideritis syriaca L. subsp. syriaca (Lamiaceae) with consolidated identity (DNA Barcoding): Towards a potential new industrial crop. Ind. Crops Prod. 2020, 158, 112957. [Google Scholar] [CrossRef]
- NCBI National Center for Biotechnology Information (NCBI) [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 15 November 2023).
- Grdiša, M.; Radosavljević, I.; Liber, Z.; Stefkov, G.; Ralli, P.; Chatzopoulou, P.S.; Carović-Stanko, K.; Šatović, Z. Divergent selection and genetic structure of Sideritis scardica populations from southern Balkan Peninsula as revealed by AFLP fingerprinting. Sci. Rep. 2019, 9, 12767. [Google Scholar] [CrossRef]
- Nemli, S.; Subaşi, Ü.; Eroglu, V.; Şenol, S.; Muhammed, B.; Tanyolac, B. High Levels of genetic variation as detected by AFLP in Sideritis tmolea from Western Turkey. Turkish J. Field Crops 2014, 192, 247–254. [Google Scholar] [CrossRef]
- Strid, A. Atlas of the Aegean Flora. Part 1: Text & Plates. Part 2: Maps; Botanic Garden and Botanical Museum Berlin, Freie Universität Berlin: Berlin, Germany, 2016; ISBN 978 392 180 097 3. [Google Scholar] [CrossRef]
- Dordas, C. Medicinal and Aromatic Plants; Μοdern Education Edition; Μοdern Education: Thessaloniki, Greece, 2012; ISBN 978-960-357-107-0. [Google Scholar]
- EMA/HMPC European Union Herbal Monograph on Sideritis scardica Griseb.; Sideritis clandestina (Bory & Chaub.) Hayek; Sideritis raeseri Boiss. & Heldr.; Sideritis syriaca L., herba (Final). Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-sideritis-scardica-griseb-sideritis-clandestina-bory-chaub_en.pdf (accessed on 30 November 2023).
- Bourgou, S.; Ben Haj Jilani, I.; Karous, O.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Greveniotis, V.; et al. Medicinal-Cosmetic Potential of the Local Endemic Plants of Crete (Greece), Northern Morocco and Tunisia: Priorities for Conservation and Sustainable Exploitation of Neglected and Underutilized Phytogenetic Resources. Biology 2021, 10, 1344. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction Risk Assessment of the Greek Endemic Flora. Biology 2021, 10, 195. [Google Scholar] [CrossRef]
- Bodeker, G.; Burford, G.; Volkov, A. Integrative, traditional and complementary medicine. In International Encyclopedia of Public Health; Academic Press: Cambridge, MA, USA, 2017; pp. 288–295. ISBN 9780128037089. [Google Scholar] [CrossRef]
- Jain, D.; Chaudhary, P.; Kotnala, A.; Hossain, R.; Hossain, M. Hepatoprotective activity of medicinal plants: A mini review. J. Med. Plants Stud. 2020, 8, 183–188. [Google Scholar] [CrossRef]
- Hoareau, L.; Dasilva, E.J. Medicinal plants: A re-emerging health aid. Electron. J. Biotechnol. 1999, 2, 3–4. [Google Scholar]
- Dutta, T.; Anand, U.; Saha, S.; Mane, A.; Prasanth, D.; Kandimalla, R.; Prockow, J.; Dey, A.; Cooke, S. Advancing urban ethnopharmacology: A modern concept of sustainability, conservation and cross-cultural adaptations of medicinal plant lore in the urban environment. Conserv. Physiol. 2022, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Hassanvand, F.; Rezaei Nejad, A.; Fanourakis, D. Morphological and physiological components mediating the silicon-induced enhancement of geranium essential oil yield under saline conditions. Ind. Crops Prod. 2019, 134, 19–25. [Google Scholar] [CrossRef]
- Thamkaew, G.; Sjöholm, I.; Galindo, F.G. A review of drying methods for improving the quality of dried herbs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Cheminal, A.; Kokkoris, I.P.; Strid, A.; Dimopoulos, P. Medicinal and aromatic Lamiaceae plants in Greece: Linking diversity and distribution patterns with ecosystem services. Forests 2020, 11, 661. [Google Scholar] [CrossRef]
- Malik, A.; Suryapani, S.; Ahmad, J. Chemical vs organic cultivation of medicinal and aromatic plants: The choice is clear. Int. J. Med. Aromat. Plants 2011, 1, 5–13. [Google Scholar]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Karimi, E.; Ghasemzadeh, A. Impact of organic and inorganic fertilizers application on the phytochemical and antioxidant activity of Kacip Fatimah (Labisia pumila Benth). Molecules 2013, 18, 10973–10988. [Google Scholar] [CrossRef] [PubMed]
- Kakar, K.; Xuan, T.D.; Noori, Z.; Aryan, S.; Gulab, G. Effects of organic and inorganic fertilizer application on growth, yield, and grain quality of rice. Agriculture 2020, 10, 544. [Google Scholar] [CrossRef]
- Rehim, A.; Amjad Bashir, M.; Raza, Q.-U.-A.; Gallagher, K.; Berlyn, G.P. Yield enhancement of biostimulants, vitamin B12, and CoQ10 compared to inorganic fertilizer in Radish. Agronomy 2021, 11, 697. [Google Scholar] [CrossRef]
- Selim, M. Introduction to the integrated nutrient management strategies and their contribution to yield and soil properties. Int. J. Agron. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Gezahegn, A.M. Role of Integrated Nutrient Management for sustainable maize production. Int. J. Agron. 2021, 2021, 9982884. [Google Scholar] [CrossRef]
- Jeandet, P.; Formela-Luboińska, M.; Labudda, M.; Morkunas, I. The role of sugars in plant responses to stress and their regulatory function during development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef]
- Asami, D.K.; Hong, Y.-J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, R.; Hallmann, E.; Rusaczonek, A.; Rembiałkowska, E. Antioxidant content in black currants from organic and conventional cultivation. Electron. J. Polish Agric. Univ. Ser. Food Sci. Technol. 2008, 11, 28–33. Available online: http://www.ejpau.media.pl/volume11/issue2/art-28.html (accessed on 12 November 2020).
- Wang, S.Y.; Chen, C.-T.; Sciarappa, W.; Wang, C.Y.; Camp, M.J. Fruit quality, antioxidant capacity, and flavonoid content of organically and conventionally grown blueberries. J. Agric. Food Chem. 2008, 56, 5788–5794. [Google Scholar] [CrossRef] [PubMed]
- Tõnutare, T.; Moor, U.; Mölder, K.; Põldma, P. Fruit composition of organically and conventionally cultivated strawberry “Polka”. Agron. Res. 2009, 7, 755–760. Available online: https://agronomy.emu.ee/vol07Spec2/p7sII27.pdf (accessed on 20 December 2020).
- Serri, F.; Souri, M.K.; Rezapanah, M. Growth, biochemical quality and antioxidant capacity of coriander leaves under organic and inorganic fertilization programs. Chem. Biol. Technol. Agric. 2021, 8, 33. [Google Scholar] [CrossRef]
- Mrid, R.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Sobeh, M.; Yasri, A. Secondary metabolites as biostimulant and bioprotectant agents: A review. Sci. Total Environ. 2021, 777, 146204. [Google Scholar] [CrossRef]
- Anestis, I.; Pipinis, E.; Kostas, S.; Papaioannou, E.; Karapatzak, E.; Dariotis, E.; Tsoulpha, P.; Koundourakis, E.; Chatzileontari, E.; Tsoktouridis, G.; et al. GIS-Facilitated germination of stored seeds from five wild-growing populations of Campanula pelviformis Lam. and fertilization effects on growth, nutrients, phenol content and antioxidant potential. Horticulturae 2023, 9, 877. [Google Scholar] [CrossRef]
- Amujoyegbe, B.; Opabode, J.; Olayinka, A. Effect of organic and inorganic fertilizer on yield and chlorophyll content of maize (Zea mays L.) and sorghum Sorghum bicolor (L.) Moench). Afr. J. Biotechnol. 2010, 6, 1869–1873. [Google Scholar] [CrossRef]
- Pavlović, D.; Nikolić, B.; Đurović, S.; Waisi, H.; Anđelković, A.; Marisavljević, D. Chlorophyll as a measure of plant health: Agroecological aspects. Pestic. Phytomed. 2014, 29, 21–34. [Google Scholar] [CrossRef]
- Hollingsworth, P.; Forrest, L.; Spouge, J.; Hajibabaei, M.; Ratnasingham, S.; Bank, M.; Chase, M.; Cowan, R.; Erickson, D.; Fazekas, A.J.; et al. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar]
- Hollingsworth, P.; Graham, S.; Little, D. Choosing and using a plant DNA barcode. PLoS ONE 2011, 6, e19254. [Google Scholar] [CrossRef] [PubMed]
- POWO. Plants of the World Online. Available online: http://www.plantsoftheworldonline.org/ (accessed on 2 July 2023).
- Arif, I.A.; Bakir, M.A.; Khan, H.A.; Al Farhan, A.H.A.; Al Homaidan, A.A.; Bahkali, A.H.; Sadoon, M.; Shobrak, M. A brief review of molecular techniques to assess plant diversity. Int. J. Mol. Sci. 2010, 11, 2079–2096. [Google Scholar] [CrossRef]
- Nazar, N.; Howard, C.; Slater, A.; Sgamma, T. Challenges in medicinal and aromatic plants DNA Barcoding; Lessons from the Lamiaceae. Plants 2022, 11, 137. [Google Scholar] [CrossRef]
- Tezcan, M.; Vlachonasios, K.; Aki, C. DNA barcoding study on Sideritis trojana Bornm. An endemic medicinal plant of IDA mountain, Turkey. Fresenius Environ. Bull. 2010, 19, 1352–1355. [Google Scholar]
- Pimm, S.; Russell, G.; Gittleman, J.; Brooks, T. The future of biodiversity. Science 1995, 269, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R. Medicinal Plants; Domville-Fife Press: London, UK, 2010. [Google Scholar]
- Ross, I. Medicinal Plants of the World: Chemical Constituents, Traditional and Modern Medicinal Uses; Humana Press Inc.: Totowa, NJ, USA, 2005. [Google Scholar]
- Laird, S.; Pierce, A. Promoting Sustainable and Ethical Botanicals. Strategies to Improve Commercial Raw Material Sourcing: Results from the Sustainable Botanicals Pilot Project Industry Surveys, Case Studies and Standards Collection; Rainforest Alliance: New York, NY, USA, 2002. [Google Scholar]
- Ledford, H. Botanical identities. Nature 2008, 451, 616. [Google Scholar] [CrossRef]
- Bilias, F.; Ipsilantis, I.; Samara, E.; Tsoktouridis, G.; Glavakis, E.; Grigoriadou, K.; Krigas, N.; Matsi, T. From the wild to the field: Effect of foliar or soil application of inorganic or semi-organic fertilizers on various parameters of four local endemic plant species of Crete (Greece). Braz. J. Bot. 2023, 46, 319–336. [Google Scholar] [CrossRef]
- Fageria, V.D. Nutrient interactions in crop plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Kalogiannakis, K.; Debouba, F.J.; Ipsilantis, I.; Tsoktouridis, G.; et al. Pilot cultivation of the vulnerable cretan endemic Verbascum arcturus L. (Scrophulariaceae): Effect of fertilization on growth and quality features. Sustainability 2021, 13, 14030. [Google Scholar] [CrossRef]
- Paschalidis, K.; Tsaniklidis, G.; Wang, B.Q.; Delis, C.; Trantas, E.; Loulakakis, K.; Makky, M.; Sarris, P.F.; Ververidis, F.; Liu, J.H. The interplay among polyamines and nitrogen in plant stress responses. Plants 2019, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Rosenthal, D.M.; Stinchcombe, J.R.; Baucom, R.S. The remarkable morphological diversity of leaf shape in sweetpotato (Ipomoea batatas): The influence of genetics, environment, and G×E. New Phytol. 2019, 225, 2183–2195. [Google Scholar] [CrossRef] [PubMed]
- Paschalidis, K.A.; Moschou, P.N.; Aziz, A.; Toumi, I.; Roubelakis-Angelakis, K.A. Polyamines in grapevine: An update. In Grapevine Molecular Physiology and Biotechnology; Roubelakis-Angelakis, K.A., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 207–228. ISBN 978-90-481-2305-6. [Google Scholar] [CrossRef]
- Tsoktouridis, G.; Krigas, N.; Sarropoulou, V.; Kampouropoulou, S.; Papanastasi, K.; Grigoriadou, K.; Menexes, G.; Maloupa, E. Micropropagation and molecular characterization of Thymus sibthorpii Benth. (Lamiaceae), an aromatic-medicinal thyme with ornamental value and conservation concern. In Vitr. Cell. Dev. Biol. Plant 2019, 55, 647–658. [Google Scholar] [CrossRef]
- Yamane, K.; Kawahara, T. Intra- and interspecific phylogenetic relationships among diploid Triticum–Aegilops species (Poaceae) based on base-pair substitutions, indels, and microsatellites in chloroplast noncoding sequences. Am. J. Bot. 2005, 92, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Löhne, C.; Borsch, T. Molecular evolution and phylogenetic utility of the petD group II intron: A case study in basal angiosperms. Mol. Biol. Evol. 2005, 22, 317–332. [Google Scholar] [CrossRef]
- Lee, H.-L.; Jansen, R.K.; Chumley, T.W.; Kim, K.-J. Gene relocations within chloroplast genomes of Jasminum and Menodora (Oleaceae) are due to multiple, overlapping inversions. Mol. Biol. Evol. 2007, 24, 1161–1180. [Google Scholar] [CrossRef]
- Kress, W.J.; Erickson, D.L. A two-locus global DNA barcode for land plants: The coding rbcL gene complements the non-coding trnH-psbA spacer region. PLoS ONE 2007, 2, e508. [Google Scholar] [CrossRef] [PubMed]
- Chase, M.W.; Cowan, R.S.; Hollingsworth, P.M.; van den Berg, C.; Madriñán, S.; Petersen, G.; Seberg, O.; Jørgsensen, T.; Cameron, K.M.; Carine, M.; et al. A proposal for a standardised protocol to barcode all land plants. Taxon 2007, 56, 295–299. [Google Scholar] [CrossRef]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Fanourakis, D.; Paschalidis, K.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Liapaki, E.; Jouini, M.; Ipsilantis, I.; Maloupa, E.; et al. Pilot cultivation of the local endemic Cretan marjoram Origanum microphyllum (Benth.) Vogel (Lamiaceae): Effect of fertilizers on growth and herbal quality features. Agronomy 2022, 12, 94. [Google Scholar] [CrossRef]
- Costa, G.; Fiori, G.; Torrigiani, P.; Noferini, M. Use of Vis/NIR spectroscopy to assess fruit ripening stage and improve management in post-harvest chain. In Fresh Produce; Global Science Books: UK, 2009; Volume 3, pp. 35–41. Available online: https://www.researchgate.net/publication/277217074_Use_of_VisNIR_Spectroscopy_to_Assess_Fruit_Ripening_Stage_and_Improve_Management_in_Post-Harvest_Chain#fullTextFileContent (accessed on 12 December 2023).
- Fanourakis, D.; Kazakos, F.; Nektarios, P.A. Allometric individual leaf area estimation in Chrysanthemum. Agronomy 2021, 11, 795. [Google Scholar] [CrossRef]
- Lichtenthaler, H.; Wellburn, A.R. Determination of total carotenoids and chlorophylls a and b of leaf in different solvents. Biochem. Soc. Trans. 1985, 11, 591–592. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bremmer, J.M. Part 3: Chemical methods. In Methods of Soil Analysis; Sparks, D.L., Ed.; SSSA, ASA: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]


| Treatment | N | P | K | Ca | Mg |
|---|---|---|---|---|---|
| (g kg−1) | |||||
| C | 13.4 ± 0.4 c* | 2.3 ± 0.1 b | 18.9 ± 0.3 b | 23.0 ± 1.4 a | 3.2 ± 0.1 a |
| INM-fa | 21.8 ± 1.7 ab | 3.4 ± 0.0 a | 18.7 ± 0.4 b | 18.7 ± 0.5 a | 2.7 ± 0.0 a |
| ChF-fa | 18.3 ± 0.3 abc | 3.5 ± 0.0 a | 22.4 ± 0.5 a | 22.9 ± 1.8 a | 3.0 ± 0.1 a |
| INM-sa | 22.8 ± 0.8 a | 2.5 ± 0.1 b | 18.7 ± 0.5 b | 22.1 ± 1.7 a | 2.9 ± 0.1 a |
| ChF-sa | 12.8 ± 1.6 c | 2.7 ± 0.0 b | 20.4 ± 0.4 ab | 23.3 ± 1.5 a | 4.2 ± 0.5 a |
| MPE-sa | 16.0 ± 1.5 bc | 2.3 ± 0.2 b | 19.1 ± 0.5 b | 25.6 ± 1.0 a | 3.2 ± 0.1 a |
| p F-test | 0.021 | <0.001 | 0.029 | NS # | NS |
| Treatment | Cu | Zn | Fe | Mn | B |
|---|---|---|---|---|---|
| (mg kg−1) | |||||
| C | 14.9 ± 0.1 abc* | 26.5 ± 1.4 a | 1058 ± 89 cd | 52.0 ± 1.6 c | 27.5 ± 0.8 b |
| INM-fa | 13.2 ± 0.4 c | 23.8 ± 0.5 a | 1057 ± 49 cd | 62.8 ± 1.3 bc | 19.7 ± 0.3 d |
| ChF-fa | 14.5 ± 0.5 bc | 27.5 ± 0.4 a | 1277 ± 79 bc | 83.0 ± 5.0 a | 26.9 ± 0.3 bc |
| INM-sa | 15.8 ± 0.3 ab | 24.7 ± 0.6 a | 1703 ± 111 a | 68.4 ± 1.1 b | 25.8 ± 0.3 bc |
| ChF-sa | 15.5 ± 0.6 ab | 23.3 ± 0.1 a | 871 ± 19 d | 37.0 ± 0.4 d | 24.1 ± 0.8 c |
| MPE-sa | 16.9 ± 0.2 a | 26.8 ± 1.0 a | 1578 ± 24 ab | 54.0 ± 1.5 c | 35.0 ± 0.6 a |
| p F-test | 0.033 | NS # | 0.003 | <0.001 | <0.001 |
| Treatment | N | P | K | Ca | Mg |
|---|---|---|---|---|---|
| (mg kg−1) | |||||
| C | 8.65 ± 0.00 c* | 1.31 ± 0.45 b | 12.67 ± 2.22 ab | 1.84 ± 0.19 bc | 1.04 ± 0.20 ab |
| INM-fa | 10.14 ± 0.36 bc | 2.40 ± 0.38 a | 13.95 ± 0.44 ab | 2.75 ± 0.31 ab | 1.28 ± 0.01 a |
| ChF-fa | 10.28 ± 2.07 bc | 1.72 ± 0.12 ab | 9.19 ± 1.24 b | 1.22 ± 0.01 c | 0.61 ± 0.12 b |
| INM-sa | 16.63 ± 1.68 a | 1.26 ± 0.31 b | 12.21 ± 1.16 ab | 3.20 ± 0.69 ab | 1.27 ± 0.29 a |
| ChF-sa | 10.46 ± 0.00 bc | 2.64 ± 0.00 a | 16.84 ± 0.00 a | 3.49 ± 0.00 a | 1.40 ± 0.00 a |
| MPE-sa | 13.38 ± 1.92 ab | 1.61 ± 0.53 ab | 12.52 ± 2.52 ab | 2.56 ± 1.04 ab | 1.36 ± 0.35 a |
| p F-test | 0.014 | 0.075 | 0.081 | 0.083 | NS # |
| Treatment | Β | Zn | Fe |
|---|---|---|---|
| mg kg−1 | |||
| C | 6.93 ± 0.12 a* | 14.81 ± 3.20 ab | 149.86 ± 43.79 ab |
| INM-fa | 5.60 ± 0.20 b | 15.32 ± 1.41 ab | 201.35 ± 42.26 a |
| ChF-fa | 6.60 ± 0.34 ab | 10.78 ± 2.93 b | 49.94 ± 23.79 b |
| INM-sa | 6.82 ± 0.78 a | 14.30 ± 1.20 ab | 135.70 ± 38.84 ab |
| ChF-sa | 5.49 ± 0.00 b | 20.20 ± 1.00 a | 157.32 ± 10.00 ab |
| MPE-sa | 6.29 ± 0.18 ab | 17.51 ± 4.47 ab | 148.68 ± 56.23 ab |
| p F-test | 0.068 | NS # | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tsichlas, I.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Ipsilantis, I.; Grigoriadou, K.; Samartza, I.; et al. DNA Barcoding and Fertilization Strategies in Sideritis syriaca subsp. syriaca, a Local Endemic Plant of Crete with High Medicinal Value. Int. J. Mol. Sci. 2024, 25, 1891. https://doi.org/10.3390/ijms25031891
Paschalidis K, Fanourakis D, Tsaniklidis G, Tsichlas I, Tzanakakis VA, Bilias F, Samara E, Ipsilantis I, Grigoriadou K, Samartza I, et al. DNA Barcoding and Fertilization Strategies in Sideritis syriaca subsp. syriaca, a Local Endemic Plant of Crete with High Medicinal Value. International Journal of Molecular Sciences. 2024; 25(3):1891. https://doi.org/10.3390/ijms25031891
Chicago/Turabian StylePaschalidis, Konstantinos, Dimitrios Fanourakis, Georgios Tsaniklidis, Ioannis Tsichlas, Vasileios A. Tzanakakis, Fotis Bilias, Eftihia Samara, Ioannis Ipsilantis, Katerina Grigoriadou, Ioulietta Samartza, and et al. 2024. "DNA Barcoding and Fertilization Strategies in Sideritis syriaca subsp. syriaca, a Local Endemic Plant of Crete with High Medicinal Value" International Journal of Molecular Sciences 25, no. 3: 1891. https://doi.org/10.3390/ijms25031891
APA StylePaschalidis, K., Fanourakis, D., Tsaniklidis, G., Tsichlas, I., Tzanakakis, V. A., Bilias, F., Samara, E., Ipsilantis, I., Grigoriadou, K., Samartza, I., Matsi, T., Tsoktouridis, G., & Krigas, N. (2024). DNA Barcoding and Fertilization Strategies in Sideritis syriaca subsp. syriaca, a Local Endemic Plant of Crete with High Medicinal Value. International Journal of Molecular Sciences, 25(3), 1891. https://doi.org/10.3390/ijms25031891











