The Role of Obesity in Type 2 Diabetes Mellitus—An Overview
Abstract
1. Introduction
2. Definitions and Epidemiology of Obesity and Type 2 DM
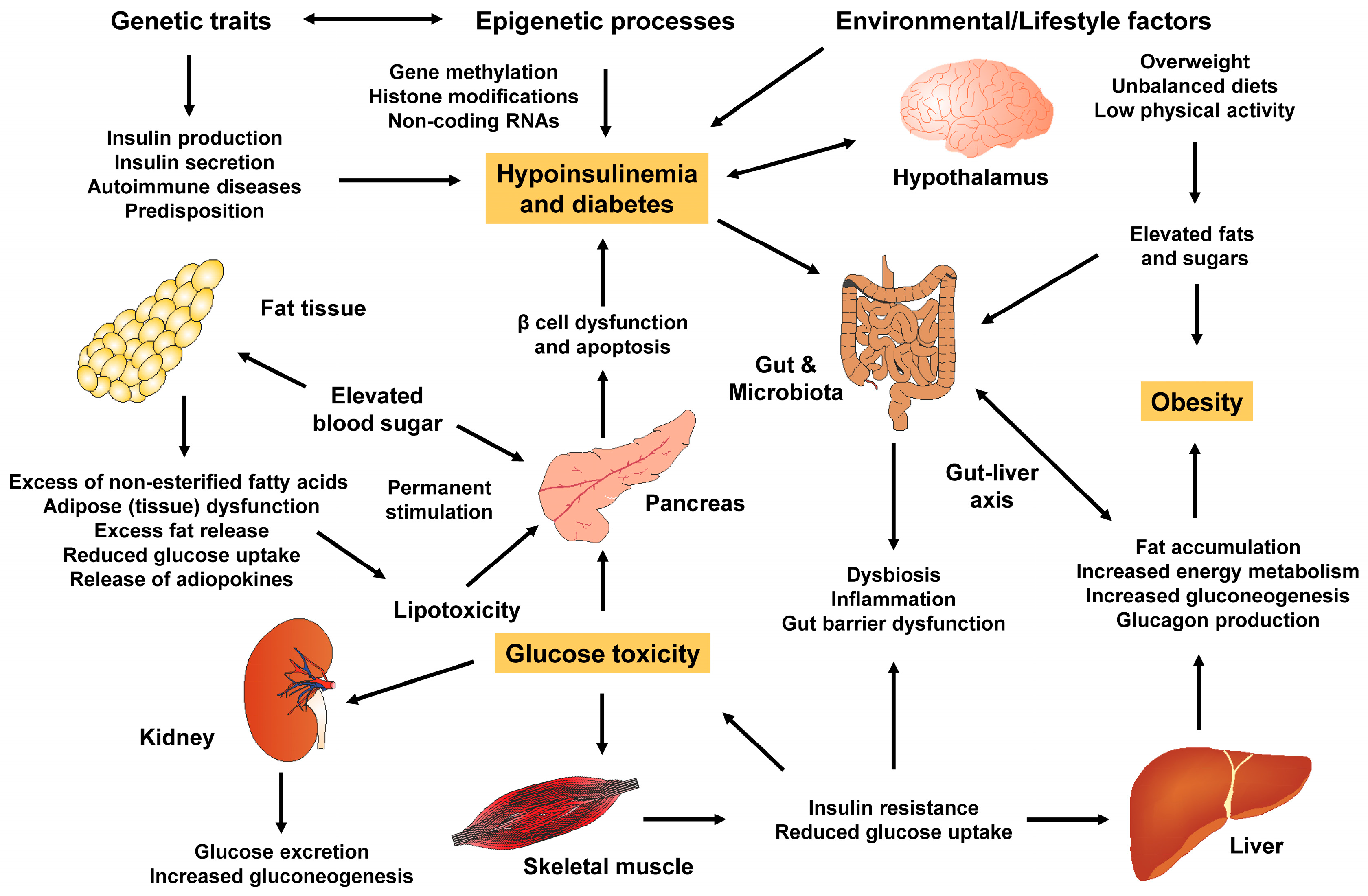
3. Mechanistic Link
3.1. White and Brown Adipose Tissue
3.2. Adipogenesis and Healthy Adipose Tissue
3.3. Dysfunctional Adipogenesis
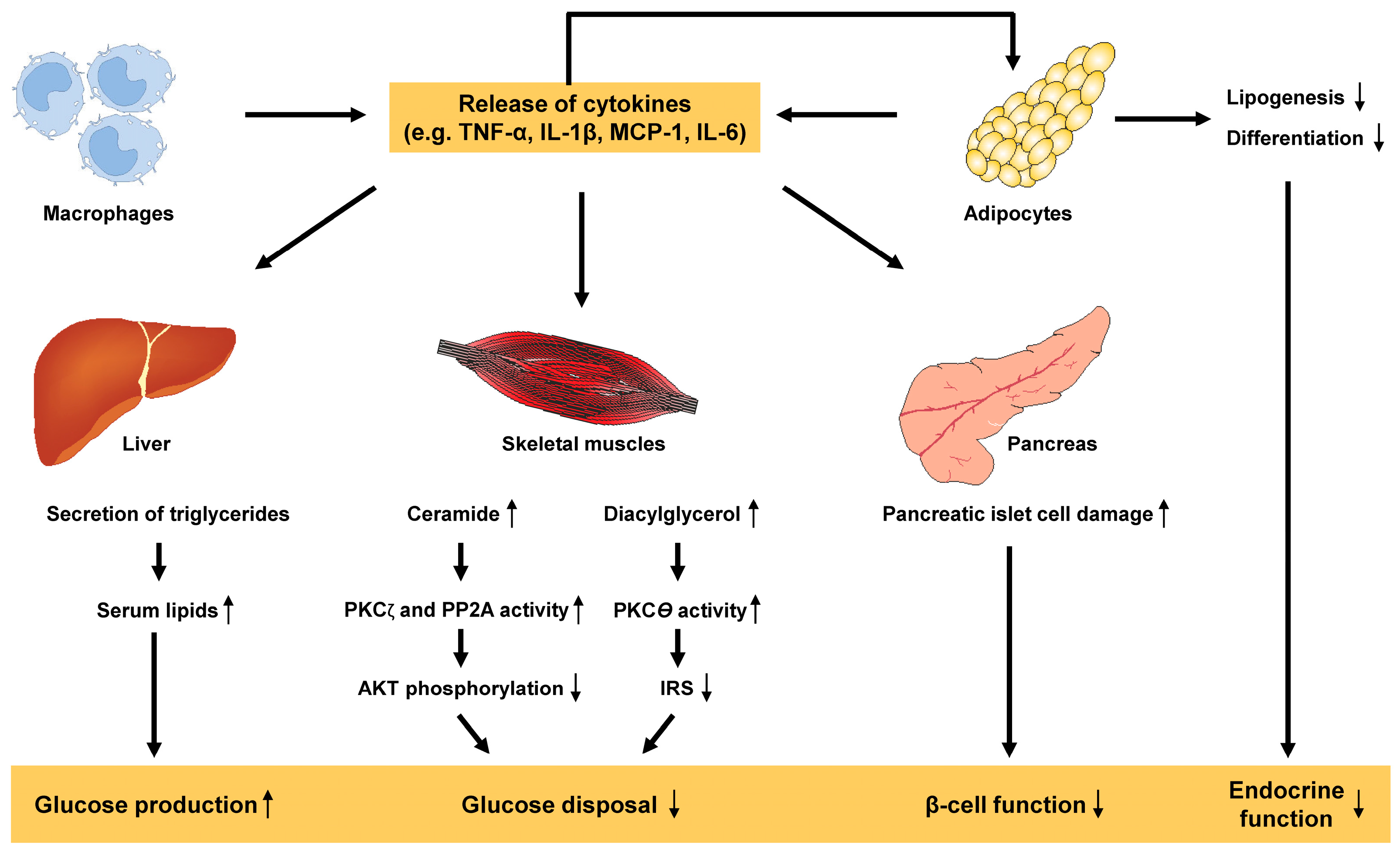
3.4. Adipose Tissue Dysfunction and Inflammation
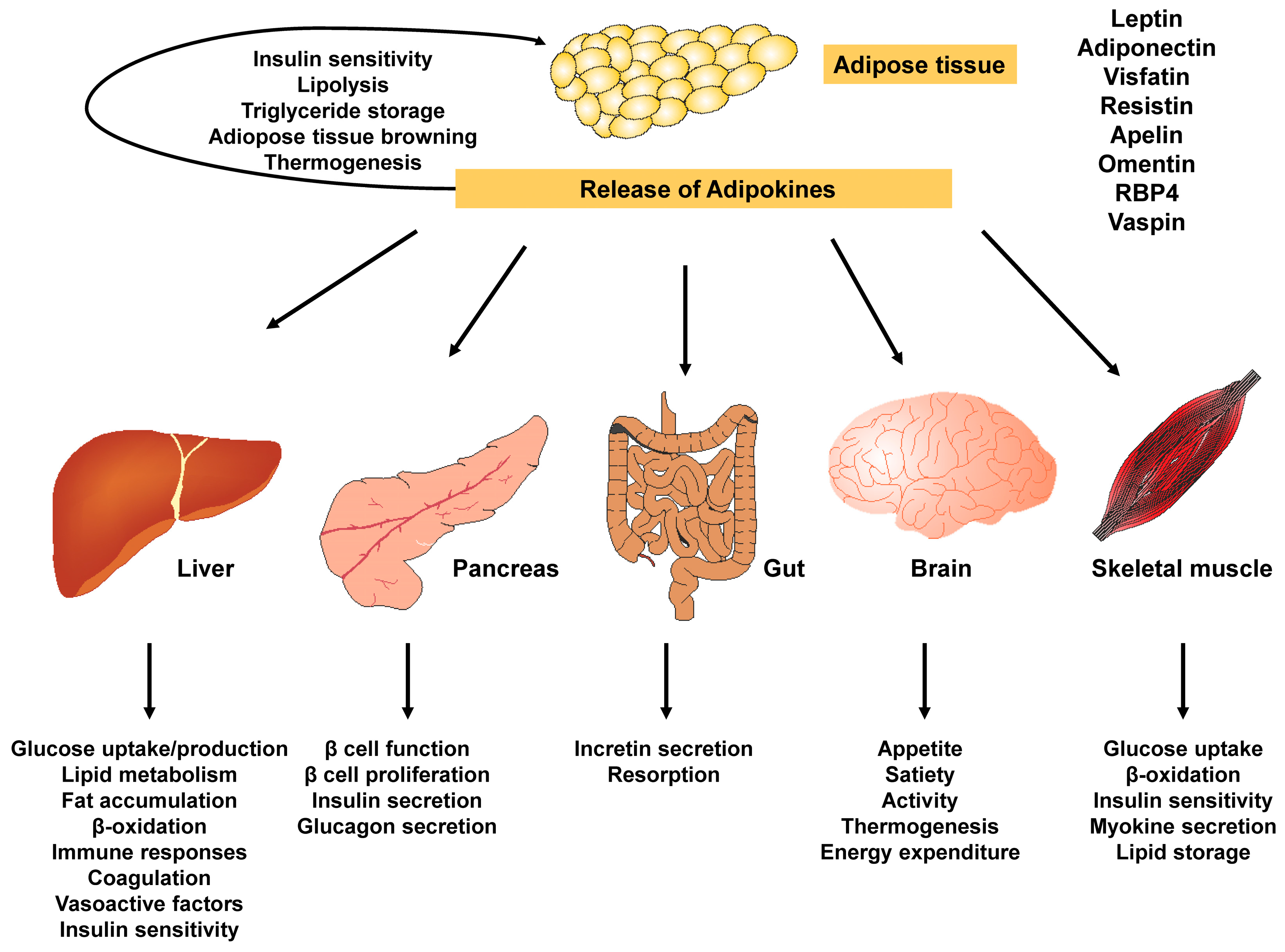
3.5. Adiponectin
3.6. Other Adipokines and Cortisol
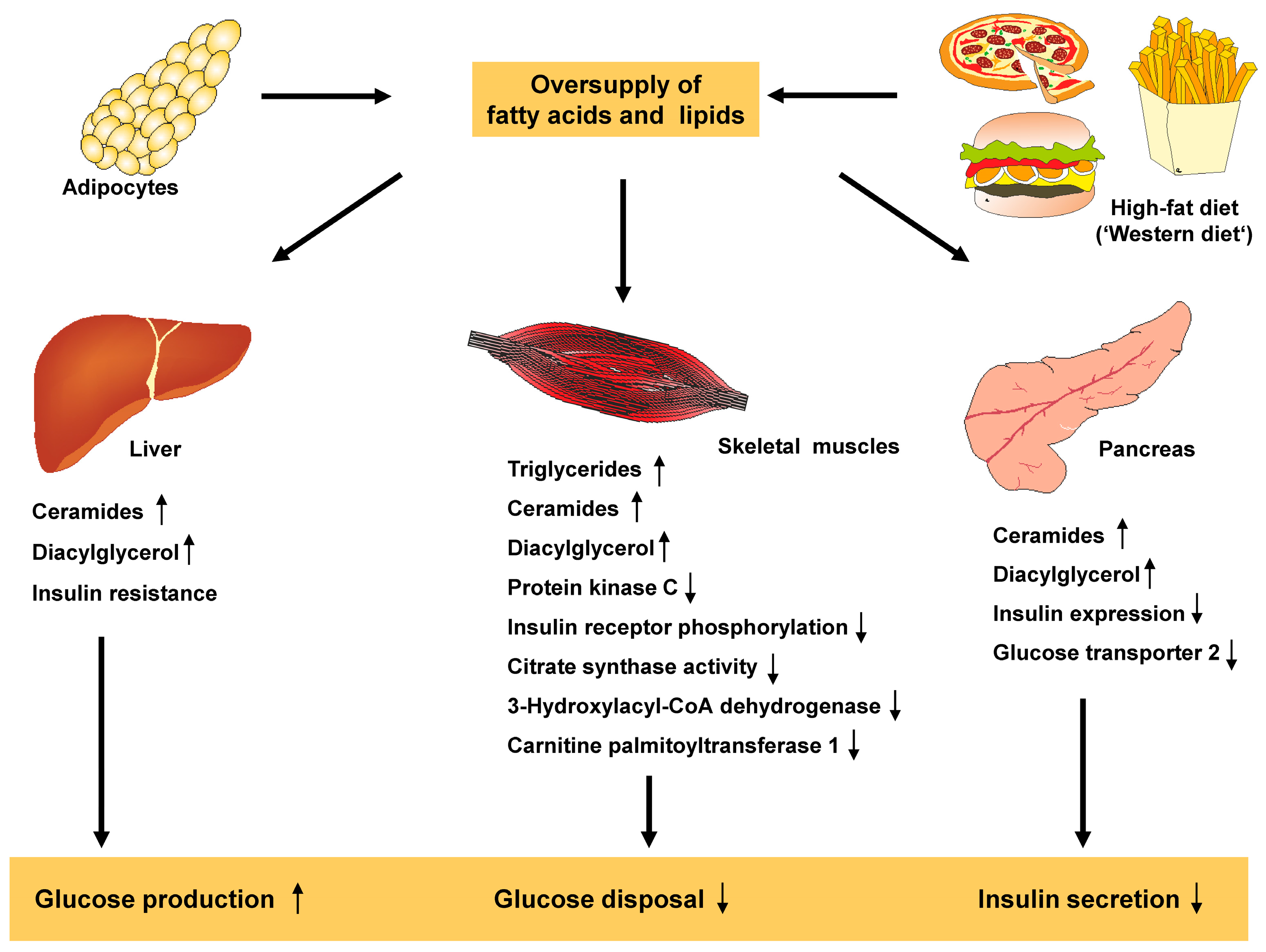
3.7. Lipids and Free Fatty Acids
3.8. Distribution of Fat and Ectopic Fat Storage
3.9. Disturbances in Lipid Homeostasis
4. In Vivo Models
5. Clinical Studies
6. Management
7. Summary
- Obesity is a significant and modifiable risk factor associated with the development and progression of type 2 DM, and the increase in obesity is the primary factor in the recent rise in the prevalence and incidence of type 2 DM.
- It is crucial to understand the role of obesity in the pathogenesis of type 2 DM, considering the various factors and complications associated with the condition.
- Obesity is a chronic progressive condition characterized by excessive and abnormal fat accumulation in the body, resulting from the consumption of more calories than the body can use, with a Body Mass Index ≥ 30 kg/m2.
- Type 2 DM is a chronic metabolic condition characterized by insulin resistance where the body is unable to effectively use insulin, leading to high blood glucose levels or hyperglycemia.
- Currently, over half a billion people worldwide have been diagnosed with diabetes, and this number is projected to more than double to 1.3 billion in the next 30 years.
- According to the World Obesity Federation 2023 atlas, it is predicted that over 51% of the global population will become overweight or obese in the next 12 years.
- Obesity and type 2 DM are intertwined in their pathophysiology and molecular mechanisms, influenced by various factors such as adipose tissue, homeostatic factors like adiponectin, body fat distribution, inflammation, free fatty acids, gut microbiome and dyslipidemia. Therefore, it is crucial to understand this close relationship in order to effectively manage and prevent these conditions as an urgent response to their alarming global rise.
- Numerous in vivo and clinical studies have highlighted the significance of a comprehensive management approach that addressed both obesity and type 2 DM simultaneously. This approach is essential for effectively handling these chronic and interconnected conditions.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxynonenal |
| ANT2 | adenine nucleotide translocase 2 |
| ApoE | apolipoprotein E |
| BAT | brown adipose tissue |
| BMI | Body mass index |
| DIO | diet-induced obesity |
| DM | diabetes mellitus |
| ER | endoplasmic reticulum |
| FGF 21 | fibroblast growth factor 21 |
| HbA1c | glycated hemoglobin |
| IDF | International Diabetes Federation |
| IL-1β | interleukin-1β |
| IMCL | intramyocellular lipids |
| IRS | insulin receptor substrate |
| LDL | low density lipoprotein(s) |
| MCP-1 | monocyte chemoattractant protein-1 |
| NEFA | non-esterified fatty acids |
| PAI-1 | Plasminogen activator inhibitor-1 |
| RBP4 | retinol binding protein 4 |
| ROS | reactive oxygen species |
| SOCS | suppressor of cytokine signaling |
| TNF-α | tumor necrosis factor-α |
| VLDL | very low density lipoprotein(s) |
| WAT | white adipose tissue |
References
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef]
- Expert Panel on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults (U.S.). Clinical Guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—The evidence report. Obes. Res. 1998, 6 (Suppl. S2), 51S–209S. [Google Scholar]
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Dyson, P.A. The therapeutics of lifestyle management on obesity. Diabetes Obes. Metab. 2010, 12, 941–946. [Google Scholar] [CrossRef]
- Hossain, P.; Kawar, B.; El Nahas, M. Obesity and diabetes in the developing world—A growing challenge. N. Engl. J. Med. 2007, 356, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, H. Obesity: Epidemiology, pathophysiology, and therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- Purnell, J.Q. Definitions, classification, and epidemiology of obesity. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- De Lorenzo, A.; Soldati, L.; Sarlo, F.; Calvani, M.; Di Lorenzo, N.; Di Renzo, L. New obesity classification criteria as a tool for bariatric surgery indication. World J. Gastroenterol. 2016, 22, 681–703. [Google Scholar] [CrossRef] [PubMed]
- World Obesity Atlas 2023. Available online: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2023 (accessed on 29 January 2024).
- Tsai, A.G.; Williamson, D.F.; Glick, H.A. Direct medical cost of overweight and obesity in the USA: A quantitative systematic review. Obes. Rev. 2011, 12, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief 2020, 360, 1–8. [Google Scholar]
- Hales, C.M.; Fryar, C.D.; Carroll, M.D.; Freedman, D.S.; Ogden, C.L. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA 2018, 319, 1723–1725. [Google Scholar] [CrossRef]
- Kirby, J.B.; Liang, L.; Chen, H.J.; Wang, Y. Race, place, and obesity: The complex relationships among community racial/ethnic composition, individual race/ethnicity, and obesity in the United States. Am. J. Public Health 2012, 102, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected US state-level prevalence of adult obesity and severe obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [CrossRef] [PubMed]
- Røder, M.E.; Porte, D., Jr.; Schwartz, R.S.; Kahn, S.E. Disproportionately elevated proinsulin levels reflect the degree of impaired B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1998, 83, 604–608. [Google Scholar] [CrossRef] [PubMed]
- IDF Diabetes Atlas. Available online: https://diabetesatlas.org/atlas-reports/ (accessed on 29 January 2024).
- Field, A.E.; Coakley, E.H.; Must, A.; Spadano, J.L.; Laird, N.; Dietz, W.H.; Rimm, E.; Colditz, G.A. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch. Intern. Med. 2001, 161, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, G.; Boelsen-Robinson, T.; Hart, A.C.; Pesantes, M.A.; Sameeha, M.J.; Phulkerd, S.; Alsukait, R.F.; Thow, A.M. A comparative policy analysis of the adoption and implementation of sugar-sweetened beverage taxes (2016-19) in 16 countries. Health Policy Plan. 2022, 37, 543–564. [Google Scholar] [CrossRef]
- Fujimoto, W.Y.; Bergstrom, R.W.; Boyko, E.J.; Leonetti, D.L.; Newell-Morris, L.L.; Wahl, P.W. Susceptibility to development of central adiposity among populations. Obes. Res. 1995, 3 (Suppl. S2), 179S–186S. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Huang, J.; Wang, J.; Chan, P.S.F.; Lok, V.; Chen, X.; Leung, C.; Wang, H.H.X.; Lao, X.Q.; Zheng, Z.J. Global, regional and time-trend prevalence of central obesity: A systematic review and meta-analysis of 13.2 million subjects. Eur. J. Epidemiol. 2020, 35, 673–683. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33 (Suppl. S1), S62–S69, Erratum in Diabetes Care 2010, 33, e57. [Google Scholar] [CrossRef]
- Goyal, R.; Singhal, M.; Jialal, I. Type 2 diabetes. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2022. Diabetes Care 2021, 45 (Suppl. S1), S17–S38. [Google Scholar] [CrossRef]
- 2021 Diabetes Atlas Numbers. Available online: https://diatribechange.org/news/2021-diabetes-atlas-numbers (accessed on 29 January 2024).
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Rodríguez, J.E.; Campbell, K.M. Racial and ethnic disparities in prevalence and care of patients with type 2 diabetes. Clin. Diabetes 2017, 35, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of type 2 diabetes—Global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef]
- Machado, S.A.; Pasquarelli-do-Nascimento, G.; da Silva, D.S.; Farias, G.R.; de Oliveira Santos, I.; Baptista, L.B.; Magalhães, K.G. Browning of the white adipose tissue regulation: New insights into nutritional and metabolic relevance in health and diseases. Nutr. Metab. 2022, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, K.; Kretowski, A. Brown adipose tissue and its role in insulin and glucose homeostasis. Int. J. Mol. Sci. 2021, 22, 1530. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Tang, Y.; Tang, Q.Q. Adipose tissue plasticity and the pleiotropic roles of BMP signaling. J. Biol. Chem. 2021, 296, 100678. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Ramos, D.; Mehta, R.; Aguilar-Salinas, C.A. Fibroblast growth factor 21 and browning of white adipose tissue. Front. Physiol. 2019, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Bertola, A.; Gallerand, A.; Ivanov, S. Immune cell involvement in brown adipose tissue function. Disc. Immunol. 2022, 1, kyac007. [Google Scholar] [CrossRef]
- Ma, X.; Wang, D.; Zhao, W.; Xu, L. Deciphering the roles of PPARγ in adipocytes via dynamic change of transcription complex. Front. Endocrinol. 2018, 9, 473. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.; Shen, C. Peroxisome proliferator-activated receptor gamma in white and brown adipocyte regulation and differentiation. Physiol. Res. 2020, 69, 759–773. [Google Scholar] [CrossRef]
- Cheng, C.F.; Ku, H.C.; Lin, H. PGC-1α as a pivotal factor in lipid and metabolic regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Ghosh, S.; Newman, S.; Salbaum, J.M. A map of the PGC-1α- and NT-PGC-1α-regulated transcriptional network in brown adipose tissue. Sci. Rep. 2018, 8, 7876. [Google Scholar] [CrossRef]
- Abraham, N.G.; Junge, J.M.; Drummond, G.S. Translational significance of heme oxygenase in obesity and metabolic syndrome. Trends Pharmacol. Sci. 2016, 37, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Greenberg, M.; Glick, Y.; Bellner, L.; Favero, G.; Rezzani, R.; Rodella, L.F.; Agostinucci, K.; Shapiro, J.I.; Abraham, N.G. Adipocyte specific HO-1 gene therapy is effective in antioxidant treatment of insulin resistance and vascular function in an obese mice model. Antioxidants 2020, 9, 40. [Google Scholar] [CrossRef]
- Modica, S.; Wolfrum, C. The dual role of BMP4 in adipogenesis and metabolism. Adipocyte 2017, 6, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Linnemann, A.K.; Baan, M.; Davis, D.B. Pancreatic β-cell proliferation in obesity. Adv. Nutr. 2014, 5, 278–288. [Google Scholar] [CrossRef]
- Scherer, P.E. The multifaceted roles of adipose tissue-therapeutic targets for diabetes and beyond: The 2015 Banting Lecture. Diabetes 2016, 65, 1452–1461. [Google Scholar] [CrossRef]
- Halberg, N.; Khan, T.; Trujillo, M.E.; Wernstedt-Asterholm, I.; Attie, A.D.; Sherwani, S.; Wang, Z.V.; Landskroner-Eiger, S.; Dineen, S.; Magalang, U.J.; et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol. 2009, 29, 4467–4483. [Google Scholar] [CrossRef] [PubMed]
- Crewe, C.; An, Y.A.; Scherer, P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Investig. 2017, 127, 74–82. [Google Scholar] [CrossRef]
- Straub, L.G.; Scherer, P.E. Metabolic messengers: Adiponectin. Nat. Metab. 2019, 1, 334–339. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sun, Y.C.; Cheng, P.; Shao, H.G. Adipose tissue macrophage-derived exosomal miR-29a regulates obesity-associated insulin resistance. Biochem. Biophys. Res. Commun. 2019, 515, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Calder, P.C. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef]
- Aguirre, V.; Uchida, T.; Yenush, L.; Davis, R.; White, M.F. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J. Biol. Chem. 2000, 275, 9047–9054. [Google Scholar] [CrossRef]
- Rui, L.; Yuan, M.; Frantz, D.; Shoelson, S.; White, M.F. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J. Biol. Chem. 2002, 277, 42394–42398. [Google Scholar] [CrossRef]
- Lemoine, A.Y.; Ledoux, S.; Quéguiner, I.; Caldérari, S.; Mechler, C.; Msika, S.; Corvol, P.; Larger, E. Link between adipose tissue angiogenesis and fat accumulation in severely obese subjects. J. Clin. Endocrinol. Metab. 2012, 97, E775–E780. [Google Scholar] [CrossRef]
- Li, N.; Zhao, S.; Zhang, Z.; Zhu, Y.; Gliniak, C.M.; Vishvanath, L.; An, Y.A.; Wang, M.Y.; Deng, Y.; Zhu, Q.; et al. Adiponectin preserves metabolic fitness during aging. eLife 2021, 10, e65108. [Google Scholar] [CrossRef]
- Holland, W.L.; Miller, R.A.; Wang, Z.V.; Sun, K.; Barth, B.M.; Bui, H.H.; Davis, K.E.; Bikman, B.T.; Halberg, N.; Rutkowski, J.M.; et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011, 17, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Guo, Z.; Johnson, C.M.; Hensrud, D.D.; Jensen, M.D. Splanchnic lipolysis in human obesity. J. Clin. Investig. 2004, 113, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Mertens, I.; Van Gaal, L.F. Obesity, haemostasis and the fibrinolytic system. Obes. Rev. 2002, 3, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Stith, R.D.; Luo, J. Endocrine and carbohydrate responses to interleukin-6 in vivo. Circ. Shock 1994, 44, 210–215. [Google Scholar] [PubMed]
- Seckl, J.R.; Morton, N.M.; Chapman, K.E.; Walker, B.R. Glucocorticoids and 11beta-hydroxysteroid dehydrogenase in adipose tissue. Recent Prog. Horm. Res. 2004, 59, 359–393. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Baldi, S.; Pettiti, M.; Toschi, E.; Camastra, S.; Natali, A.; Landau, B.R.; Ferrannini, E. Influence of obesity and type 2 diabetes on gluconeogenesis and glucose output in humans: A quantitative study. Diabetes 2000, 49, 1367–1373. [Google Scholar] [CrossRef]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef]
- Golay, A.; Munger, R.; Assimacopoulos-Jeannet, F.; Bobbioni-Harsch, E.; Habicht, F.; Felber, J.P. Progressive defect of insulin action on glycogen synthase in obesity and diabetes. Metabolism 2002, 51, 549–553. [Google Scholar] [CrossRef]
- Chadt, A.; Scherneck, S.; Joost, H.G.; Al-Hasani, H. Molecular links between obesity and diabetes: “Diabesity”. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholme, E.A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963, 1, 785–789. [Google Scholar] [CrossRef]
- Shulman, G.I. Cellular mechanisms of insulin resistance. J. Clin. Investig. 2000, 106, 171–176. [Google Scholar] [CrossRef]
- Manolopoulos, K.N.; Karpe, F.; Frayn, K.N. Gluteofemoral body fat as a determinant of metabolic health. Int. J. Obes. 2010, 34, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Hajri, T.; Han, X.X.; Bonen, A.; Abumrad, N.A. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J. Clin. Investig. 2002, 109, 1381–1389. [Google Scholar] [CrossRef]
- Russell, A.P.; Gastaldi, G.; Bobbioni-Harsch, E.; Arboit, P.; Gobelet, C.; Dériaz, O.; Golay, A.; Witztum, J.L.; Giacobino, J.P. Lipid peroxidation in skeletal muscle of obese as compared to endurance-trained humans: A case of good vs. bad lipids? FEBS Lett. 2003, 551, 104–106. [Google Scholar] [CrossRef]
- Unger, R.H.; Orci, L. Lipotoxic diseases of nonadipose tissues in obesity. Int. J. Obes. 2000, 24, S28–S32. [Google Scholar] [CrossRef] [PubMed]
- Rösen, P.; Nawroth, P.P.; King, G.; Möller, W.; Tritschler, H.J.; Packer, L. The role of oxidative stress in the onset and progression of diabetes and its complications: A summary of a congress series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab. Res. Rev. 2001, 17, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Wojtczak, L.; Schönfeld, P. Effect of fatty acids on energy coupling processes in mitochondria. Biochim. Biophys. Acta 1993, 1183, 41–57. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 2003, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Garcia, R.; Shulman, G.I. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med. 2004, 350, 664–671. [Google Scholar] [CrossRef]
- McMurray, F.; Patten, D.A.; Harper, M.E. Reactive oxygen species and oxidative stress in obesity-recent findings and empirical approaches. Obesity 2016, 24, 2301–2310. [Google Scholar] [CrossRef]
- Woo, C.Y.; Jang, J.E.; Lee, S.E.; Koh, E.H.; Lee, K.U. Mitochondrial dysfunction in adipocytes as a primary cause of adipose tissue inflammation. Diabetes Metab. J. 2019, 43, 247–256. [Google Scholar] [CrossRef]
- Ozcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Ozdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Liu, S.; Klionsky, D.J.; Lip, G.Y.H.; Tuomilehto, J.; Kavalakatt, S.; Pereira, D.M.; Samali, A.; Ren, J. ER stress in obesity pathogenesis and management. Trends Pharmacol. Sci. 2022, 43, 97–109. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and management of the metabolic syndrome. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.F.; Steiner, G. Acute effects of insulin in the control of VLDL production in humans. Implications for the insulin-resistant state. Diabetes Care 1996, 19, 390–393. [Google Scholar] [CrossRef]
- Apovian, C.M.; Okemah, J.; O’Neil, P.M. Body weight considerations in the management of type 2 diabetes. Adv. Ther. 2019, 36, 44–58. [Google Scholar] [CrossRef]
- Fuchs, A.; Samovski, D.; Smith, G.I.; Cifarelli, V.; Farabi, S.S.; Yoshino, J.; Pietka, T.; Chang, S.W.; Ghosh, S.; Myckatyn, T.M.; et al. Associations among adipose tissue immunology, inflammation, exosomes and insulin sensitivity in people with obesity and nonalcoholic fatty liver disease. Gastroenterology 2021, 161, 968–981.e12. [Google Scholar] [CrossRef]
- Alessi, M.C.; Poggi, M.; Juhan-Vague, I. Plasminogen activator inhibitor-1, adipose tissue and insulin resistance. Curr. Opin. Lipidol. 2007, 18, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Dobbins, R.L.; Szczepaniak, L.S.; Bentley, B.; Esser, V.; Myhill, J.; McGarry, J.D. Prolonged inhibition of muscle carnitine palmitoyltransferase-1 promotes intramyocellular lipid accumulation and insulin resistance in rats. Diabetes 2001, 50, 123–130. [Google Scholar] [CrossRef]
- Ikeda, H. KK mouse. Diabetes Res. Clin. Pract. 1994, 24, S313–S316. [Google Scholar] [CrossRef]
- Song, G.M.; Huan, Y.; Sun, S.J.; Chen, Y.T.; Liu, Q.; Shen, Z.F. Biological activity of EXf, a peptide analogue of exendin-4. Eur. J. Pharmacol. 2010, 628, 261–267. [Google Scholar] [CrossRef]
- Ingalls, A.M.; Dickie, M.M.; Snell, G.D. Obese, a new mutation in the house mouse. Obes. Res. 1996, 4, 101. [Google Scholar] [CrossRef] [PubMed]
- Day, C.; Bailey, C.J. Effect of the antiobesity agent sibutramine in obese-diabetic ob/ob mice. Int. J. Obes. Relat. Metab. Disord. 1998, 22, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.S.; Liu, Q.; Hammond, H.A.; Dugan, V.; Hey, P.J.; Caskey, C.J.; Hess, J.F. Leptin receptor missense mutation in the fatty Zucker rat. Nat. Genet. 1996, 13, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Zucker, L.M.; Antoniades, H.N. Insulin and obesity in the Zucker genetically obese rat “fatty”. Endocrinology 1972, 90, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Iijima, H. Sibutramine sensitivity assay revealed a unique phenotype of bombesin BB3 receptor-deficient mice. Eur. J. Pharmacol. 2003, 473, 41–46. [Google Scholar] [CrossRef]
- Clark, J.B.; Palmer, C.J.; Shaw, W.N. The diabetic Zucker fatty rat. Proc. Soc. Exp. Biol. Med. 1983, 173, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Surwit, R.S.; Feinglos, M.N.; Rodin, J.; Sutherland, A.; Petro, A.E.; Opara, E.C.; Kuhn, C.M.; Rebuffé-Scrive, M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism 1995, 44, 645–651. [Google Scholar] [CrossRef]
- Schemmel, R.; Mickelsen, O.; Motawi, K. Conversion of dietary to body energy in rats as affected by strain, sex and ration. J. Nutr. 1972, 102, 1187–1197. [Google Scholar] [CrossRef]
- Astrup, A.; Rössner, S.; Van Gaal, L.; Rissanen, A.; Niskanen, L.; Al Hakim, M.; Madsen, J.; Rasmussen, M.F.; Lean, M.E. Effects of liraglutide in the treatment of obesity: A randomised, double-blind, placebo-controlled study. Lancet 2009, 374, 1606–1616. [Google Scholar] [CrossRef]
- Cummings, B.P.; Digitale, E.K.; Stanhope, K.L.; Graham, J.L.; Baskin, D.G.; Reed, B.J.; Sweet, I.R.; Griffen, S.C.; Havel, P.J. Development and characterization of a novel rat model of type 2 diabetes mellitus: The UC Davis type 2 diabetes mellitus UCD-T2DM rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1782–R1793. [Google Scholar] [CrossRef]
- Cummings, B.P.; Stanhope, K.L.; Graham, J.L.; Baskin, D.G.; Griffen, S.C.; Nilsson, C.; Sams, A.; Knudsen, L.B.; Raun, K.; Havel, P.J. Chronic administration of the glucagon-like peptide-1 analog, liraglutide, delays the onset of diabetes and lowers triglycerides in UCD-T2DM rats. Diabetes 2010, 59, 2653–2661. [Google Scholar] [CrossRef]
- Cho, Y.R.; Kim, H.J.; Park, S.Y.; Ko, H.J.; Hong, E.G.; Higashimori, T.; Zhang, Z.; Jung, D.Y.; Ola, M.S.; Lanoue, K.F.; et al. Hyperglycemia, maturity-onset obesity, and insulin resistance in NONcNZO10/LtJ males, a new mouse model of type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E327–E336. [Google Scholar] [CrossRef]
- Koza, R.A.; Flurkey, K.; Graunke, D.M.; Braun, C.; Pan, H.J.; Reifsnyder, P.C.; Kozak, L.P.; Leiter, E.H. Contributions of dysregulated energy metabolism to type 2 diabetes development in NZO/H1Lt mice with polygenic obesity. Metabolism 2004, 53, 799–808. [Google Scholar] [CrossRef]
- Kim, J.H.; Sen, S.; Avery, C.S.; Simpson, E.; Chandler, P.; Nishina, P.M.; Churchill, G.A.; Naggert, J.K. Genetic analysis of a new mouse model for non-insulin-dependent diabetes. Genomics 2001, 74, 273–286. [Google Scholar] [CrossRef]
- Garber, A.; Henry, R.R.; Ratner, R.; Hale, P.; Chang, C.T.; Bode, B. Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes Obes. Metab. 2011, 13, 348–356. [Google Scholar] [CrossRef]
- Berg, A.H.; Combs, T.P.; Du, X.; Brownlee, M.; Scherer, P.E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001, 7, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, K.; Miyazaki, M.; Matsuhisa, M.; Takano, K.; Nakatani, Y.; Hatazaki, M.; Tamatani, T.; Yamagata, K.; Miyagawa, J.; Kitao, Y.; et al. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes 2005, 54, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Santomauro, A.T.; Boden, G.; Silva, M.E.; Rocha, D.M.; Santos, R.F.; Ursich, M.J.; Strassmann, P.G.; Wajchenberg, B.L. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 1999, 48, 1836–1841. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Miyazaki, Y.; Pettiti, M.; Matsuda, M.; Mahankali, S.; Santini, E.; DeFronzo, R.A.; Ferrannini, E. Metabolic effects of visceral fat accumulation in type 2 diabetes. J. Clin. Endocrinol. Metab. 2002, 87, 5098–5103. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, E.; Caprio, S. Type 2 diabetes in youth: Epidemiology and pathophysiology. Diabetes Care 2011, 34 (Suppl. S2), S161–S165. [Google Scholar] [CrossRef]
- Wilding, J.P. The importance of weight management in type 2 diabetes mellitus. Int. J. Clin. Pract. 2014, 68, 682–691. [Google Scholar] [CrossRef]
- Garvey, W.T.; Batterham, R.L.; Bhatta, M.; Buscemi, S.; Christensen, L.N.; Frias, J.P.; Jódar, E.; Kandler, K.; Rigas, G.; Wadden, T.A.; et al. Two-year effects of semaglutide in adults with overweight or obesity: The STEP 5 trial. Nat. Med. 2022, 28, 2083–2091. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.J.; Boucher, J.L.; Rutten-Ramos, S.; VanWormer, J.J. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: A systematic review and meta-analysis of randomized clinical trials. J. Acad. Nutr. Diet. 2015, 115, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Iglay, K.; Hannachi, H.; Joseph Howie, P.; Xu, J.; Li, X.; Engel, S.S.; Moore, L.M.; Rajpathak, S. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr. Med. Res. Opin. 2016, 32, 1243–1252. [Google Scholar] [CrossRef]
- Gaich, G.; Chien, J.Y.; Fu, H.; Glass, L.C.; Deeg, M.A.; Holland, W.L.; Kharitonenkov, A.; Bumol, T.; Schilske, H.K.; Moller, D.E. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013, 18, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Nahra, R.; Wang, T.; Gadde, K.M.; Oscarsson, J.; Stumvoll, M.; Jermutus, L.; Hirshberg, B.; Ambery, P. Effects of cotadutide on metabolic and hepatic parameters in adults with overweight or obesity and type 2 diabetes: A 54-week randomized phase 2b study. Diabetes Care 2021, 44, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, K.; Saito, M.; Park, J.; Murakami, H.; Shibata, N.; Ando, M.; Nagatake, T.; Konishi, K.; Ohno, H.; Tanisawa, K.; et al. Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota. Nat. Commun. 2022, 13, 4477. [Google Scholar] [CrossRef]
- Johnston, R.; Uthman, O.; Cummins, E.; Clar, C.; Royle, P.; Colquitt, J.; Tan, B.K.; Clegg, A.; Shantikumar, S.; Court, R.; et al. Canagliflozin, dapagliflozin and empagliflozin monotherapy for treating type 2 diabetes: Systematic review and economic evaluation. Health Technol. Assess. 2017, 21, 1–218. [Google Scholar] [CrossRef]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef]
- Poirier, P.; Després, J.P. Exercise in weight management of obesity. Cardiol. Clin. 2001, 19, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Leitner, D.R.; Frühbeck, G.; Yumuk, V.; Schindler, K.; Micic, D.; Woodward, E.; Toplak, H. Obesity and type 2 diabetes: Two diseases with a need for combined treatment strategies—EASO can lead the way. Obes. Facts 2017, 10, 483–492. [Google Scholar] [CrossRef]
- Van Gaal, L.; Scheen, A. Weight management in type 2 diabetes: Current and emerging approaches to treatment. Diabetes Care 2015, 38, 1161–1172. [Google Scholar] [CrossRef]
- Corcoran, C.; Jacobs, T.F. Metformin. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Fried, M.; Yumuk, V.; Oppert, J.M.; Scopinaro, N.; Torres, A.; Weiner, R.; Yashkov, Y.; Frühbeck, G. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes. Surg. 2014, 24, 42–55. [Google Scholar] [CrossRef]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Rastall, R.A.; Gibson, G.R.; Gill, H.S.; Guarner, F.; Klaenhammer, T.R.; Pot, B.; Reid, G.; Rowland, I.R.; Sanders, M.E. Modulation of the microbial ecology of the human colon by probiotics, prebiotics and synbiotics to enhance human health: An overview of enabling science and potential applications. FEMS Microbiol. Ecol. 2005, 52, 145–152. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Katz, R. n-3 long-chain polyunsaturated fatty acids in type 2 diabetes: A review. J. Am. Diet. Assoc. 2005, 105, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 2017, 26, 672–685.e4. [Google Scholar] [CrossRef]
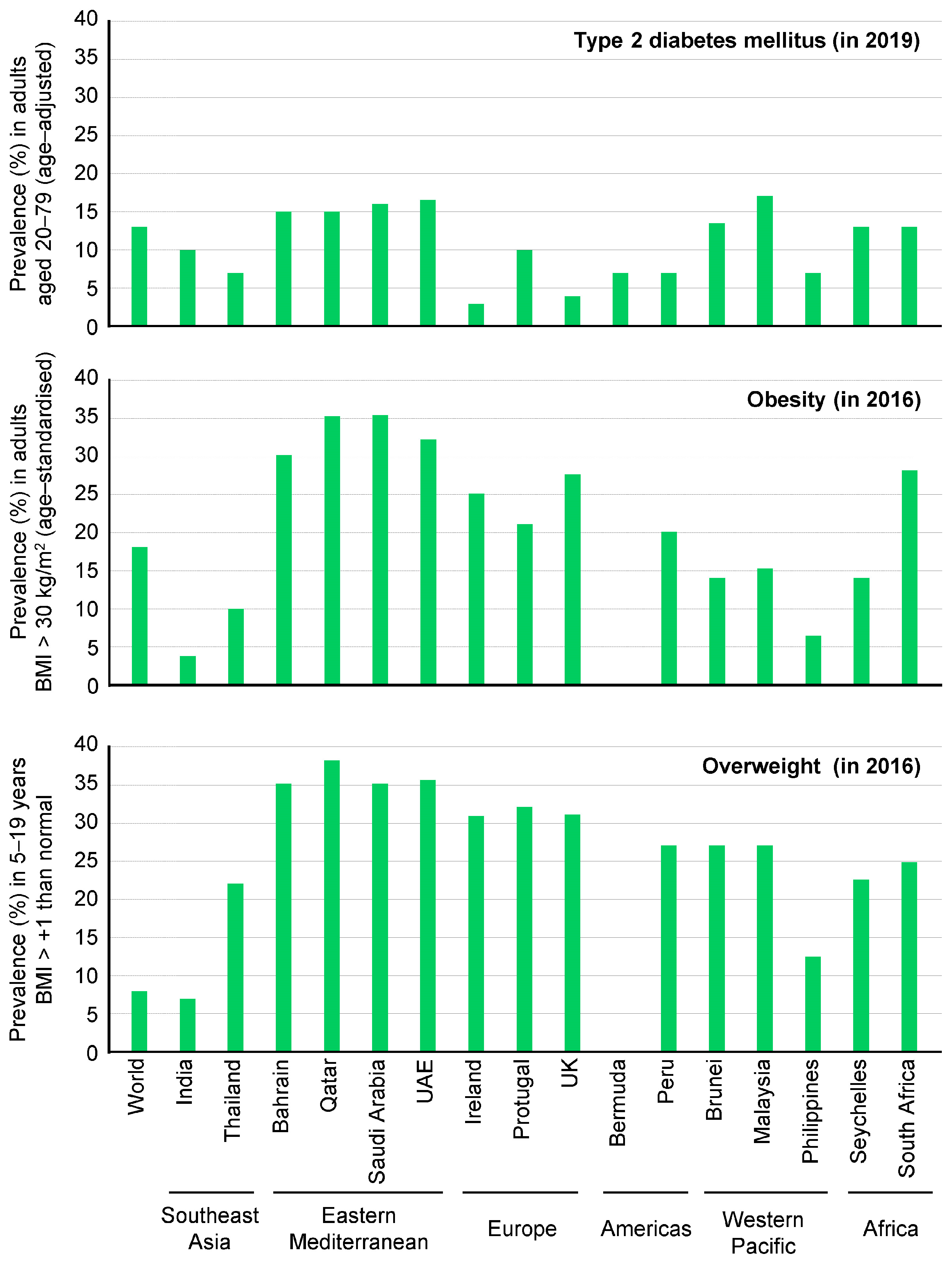
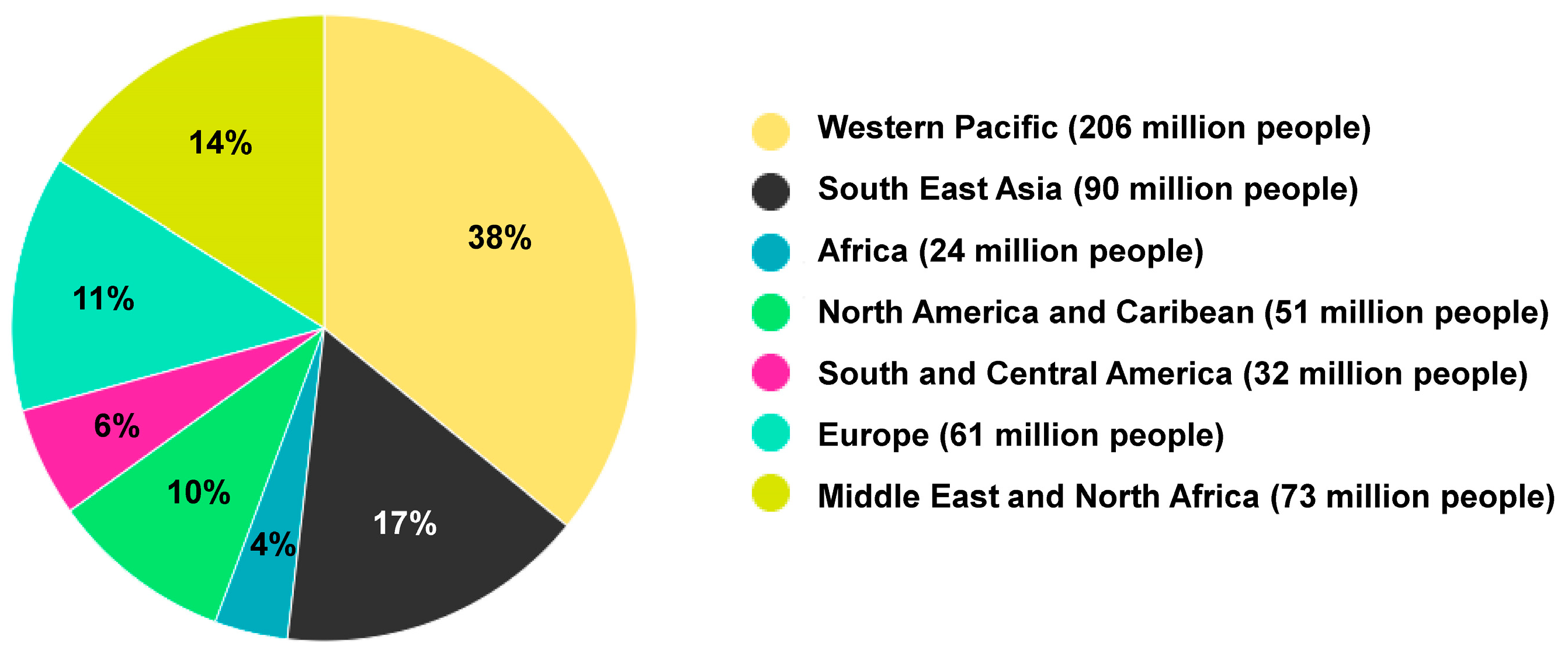
| Condition | BMI (kg/m2) | Disease Risk Relative to Normal Weight and Waist Circumference Men ≤ 40 inches (≤102 cm) Women ≤ 35 inches (≤88 cm) |
|---|---|---|
| Normal | 18.5–24.9 | data |
| Overweight | 25.0–29.9 | Increased |
| Obese | 30.0–34.9 (class 1) | High |
| 35.0–39.9 (class 2) | Very high | |
| Extremely Obese | ≥40 | Extremely high |
| Condition | Males | Females |
|---|---|---|
| Essential fat | <15% | <10% |
| Athletes | 15–19% | 10–14% |
| Fit | 20–24% | 15–19% |
| Acceptable | 25–29% | 20–24% |
| Pre-obesity | 30–34% | 25–29% |
| Obesity | >35% | >30% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrasekaran, P.; Weiskirchen, R. The Role of Obesity in Type 2 Diabetes Mellitus—An Overview. Int. J. Mol. Sci. 2024, 25, 1882. https://doi.org/10.3390/ijms25031882
Chandrasekaran P, Weiskirchen R. The Role of Obesity in Type 2 Diabetes Mellitus—An Overview. International Journal of Molecular Sciences. 2024; 25(3):1882. https://doi.org/10.3390/ijms25031882
Chicago/Turabian StyleChandrasekaran, Preethi, and Ralf Weiskirchen. 2024. "The Role of Obesity in Type 2 Diabetes Mellitus—An Overview" International Journal of Molecular Sciences 25, no. 3: 1882. https://doi.org/10.3390/ijms25031882
APA StyleChandrasekaran, P., & Weiskirchen, R. (2024). The Role of Obesity in Type 2 Diabetes Mellitus—An Overview. International Journal of Molecular Sciences, 25(3), 1882. https://doi.org/10.3390/ijms25031882








